Abstract
Entrectinib, a multikinase inhibitor of ROS1 and tropomyosin receptor kinases, is recommended to treat ROS1-positive metastatic non-small cell lung cancer (NSCLC). In a previous study, entrectinib-related cardiotoxicity occurred in 2% of patients; however, lethal arrhythmias remain understudied. We encountered a case of fatal arrhythmia due to drug-induced Brugada syndrome caused by entrectinib. An 81-year-old Japanese male with lung adenocarcinoma harboring ROS1-fusion gene was treated with entrectinib. The patient developed lethal arrhythmias three days after drug initiation, including ventricular tachycardia with Brugada-like electrocardiogram changes. Echocardiography and coronary angiography revealed no evidence of acute coronary syndrome or myocarditis. Following the termination of entrectinib, the electrocardiogram abnormality improved within 12 days. Hence, paying special attention to and monitoring electrocardiogram changes is necessary. In addition, it is also necessary to consider early therapeutic interventions and discontinuation of the drug in cases of drug-induced Brugada syndrome.
Keywords: Entrectinib, Non-small cell lung cancer, Ventricular tachycardia, Brugada syndrome
Introduction
Selecting targeted therapies based on oncogenic drivers is essential for treating metastatic non-small cell lung cancer (NSCLC) [1]. Entrectinib is a potent inhibitor of ROS1 and tropomyosin receptor kinase (TRK) A/B/C or anaplastic lymphoma kinase [2]. One of the serious treatment-related adverse events associated with this agent is cardiac disorder, which occurred in 2% of patients with ROS1 fusion-positive NSCLC in a previous study [2]. Herein, we report a novel case of lethal ventricular arrhythmia due to entrectinib-induced Brugada syndrome (BrS).
Case report
An 81-year-old Japanese male was referred to the Department of Respiratory Medicine of our hospital after an abnormal chest radiographic finding during a physical examination. He had a history of bilateral iliac aneurysms and had not regularly been taking any medication. The patient had no family history of cancer or arrhythmia, and the baseline electrocardiogram (ECG) showed no apparent abnormality (Fig. 1A). The performance status, based on the Eastern Cooperative Oncology Group criteria, was 0. According to the bronchoscopic biopsy and imaging test results, the patient was diagnosed with stage IIIA (cT2aN2M0) lung adenocarcinoma with the ROS1 fusion gene. Approximately 18 months after chemoradiotherapy, the disease recurred, crizotinib treatment was initiated, and a partial response was achieved.
Fig. 1.

Changes in electrocardiogram (ECG). ECGs recorded at baseline a, admission to the intensive care unit b, and 15 h c, three days d, and 12 days e after admission to the intensive care unit. Corrected QT = 0.425 (a), 0.471 (b), 0.391 (c), 0.428 (d), and 0.431 (e). Baseline ECG showed no apparent abnormality (a). At the admission to the intensive care unit, ECG showed ST-segment elevation in V1-V3 and frequent premature ventricular contractions (b). Brugada-like ECG change, manifested by the typical ECG pattern with ST-segment elevations in V1–V2, was gradually apparent (c, d). Twelve days later, the ECG was normalized (e)
Entrectinib, at a dose of 600 mg orally once per day, was administered as third-line chemotherapy after 4.5 years of treatment with crizotinib, owing to the development of metastases in the lung, brain, and adrenal gland. Laboratory tests showed no remarkable abnormalities before treatment with entrectinib (Table 1). Three days after starting entrectinib, the patient complained of nausea and presented with low blood pressure (55/42 mmHg). ECG showed ST-segment elevation in V1-V3 and frequent premature ventricular contractions (Fig. 1B). Laboratory data revealed no severe electrolyte abnormalities (Table 2). The patient was transferred to the intensive care unit (ICU) due to hypotension and hemodynamic instability.
Table 1.
Laboratory results before treatment with entrectinib
| Laboratory parameters | Values |
|---|---|
| White blood cell (109/L) | 3.6 |
| Hemoglobin (g/L) | 127* |
| Platelet (109/L) | 132* |
| Urea nitrogen (mg/dL) | 15.9 |
| Creatinine (mg/dL) | 0.77 |
| Sodium (mEq/L) | 140 |
| Potassium (mEq/L) | 4.5 |
| Bilirubin (mg/dL) | 0.5 |
| Alanine aminotransferase (U/L) | 24 |
| Aspartate aminotransferase (U/L) | 20 |
| Albumin (g/dL) | 3.5* |
| C reactive protein (mg/dL) | 0.31* |
*Indicates an out-of-normal range
Table 2.
Laboratory results at admission to ICU
| Laboratory parameters | Values |
|---|---|
| White blood cell (109/L) | 5.5 |
| Hemoglobin (g/L) | 121* |
| Platelet (109/L) | 143* |
| Urea nitrogen (mg/dL) | 24* |
| Creatinine (mg/dL) | 1.61* |
| Sodium (mEq/L) | 132* |
| Potassium (mEq/L) | 4.5 |
| Magnesium (mg/dL) | 2.2 |
| Bilirubin (mg/dL) | 0.3 |
| Alanine aminotransferase (U/L) | 107* |
| Aspartate aminotransferase (U/L) | 129* |
| Albumin (g/dL) | 3.3* |
| C reactive protein (mg/dL) | 0.09 |
| Troponin T (ng/mL) | 0.065* |
*Indicates an out-of-normal range
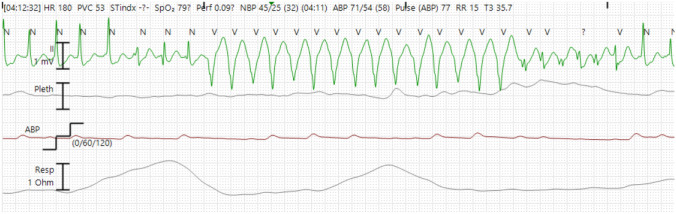
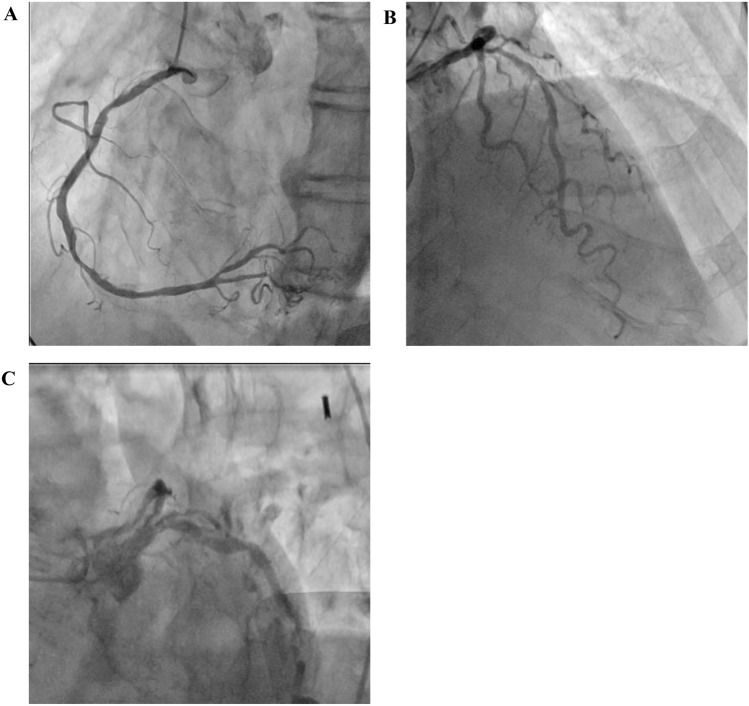
Ventricular tachycardia (VT) was observed; therefore, antiarrhythmic agents (150 mg of amiodarone and 50 mg of lidocaine) were administered intravenously (Fig. 2). The echocardiogram of the patient, performed by a cardiologist, showed no regional wall motion abnormality and the left ventricular ejection fraction (LVEF) was within the normal range. However, an emergency coronary angiography was performed due to suspicion of an acute coronary syndrome, showing no severe stenosis in the coronary arteries (Fig. 3). During treatment, a Brugada-like ECG change, manifested by the typical ECG pattern with ST-segment elevations in V1–V2, was observed (Fig. 1C, D). Therefore, it was assumed that the lethal arrhythmia was caused by entrectinib, and the treatment was terminated. The patient recovered from multiorgan failure, and the acidemia stabilized. Hence, the use of vasopressors was terminated approximately 30 h after admission to the ICU. The ECG also gradually normalized, and the patient no longer had recurrent VT within 12 days (Fig. 1E). Cytotoxic chemotherapy was planned as next-line treatment.
Fig. 2.
Electrocardiogram recorded using remote electrocardiographic monitoring at admission to the intensive care unit. A rhythm strip showed ventricular tachycardia
Fig. 3.
Coronary artery angiography. Right a and left b, c coronary angiogram
Discussion
Entrectinib is a multikinase inhibitor of ROS1 and TRK that was approved by the Pharmaceuticals and Medical Devices Agency in Japan in 2019. In phase I/II studies (ALKA-372–001, STARTRK-1, and STARTRK-2), this agent was active with durable disease control in patients with ROS1 fusion-positive NSCLC [2]. In these studies, most treatment-related adverse events were graded as either 1 or 2. Although there were no deaths related to entrectinib, serious cardiac disorders occurred in three patients (2%). Myocarditis is one of these disorders and was previously described in a case report [3] manifesting as impaired LVEF. In this case, the echocardiogram revealed a maintained LVEF, indicating no evidence of myocarditis or congestive heart failure. Corrected QT (QTc) prolongation can lead to life-threatening complications such as torsade de Pointes, VT, and sudden cardiac death. In clinical trials, QTc prolongation was noted in two patients (1.5%); however, no Torsade de Pointes has been reported with entrectinib [2, 4]. In this case, QTc prolongation, a potential cause of VT, was observed only at the onset of the symptoms and was not sustained (Fig. 1B). Hypomagnesemia and hypokalemia may cause QTc prolongation; however, severe electrolyte abnormalities were not observed in this patient (Table 2). These results indicate the presence of an additional cause of VT.
Drug-induced BrS is defined as the presence of a normal pretreatment ECG with the development of the Brugada pattern after exposure to certain drugs and the risk of ventricular arrhythmias [5]. This syndrome is often asymptomatic; however, syncope, VT, and ventricular fibrillation can potentially occur, typically 72 h or later after drug administration [6]. An association between tyrosine kinase inhibitors and drug-induced BrS has been described in oncology [7–9]. In this case, no apparent abnormality was observed on ECG at baseline. However, three days after starting entrectinib, symptomatic arrhythmias occurred, and a typical Brugada ECG pattern was observed the day after the onset of arrhythmia. This abnormal change normalized with the discontinuation of entrectinib, and these results are consistent with those of the previous report [6]. Another possible mechanism is the drug-induced unmasking of the underlying genetic predisposition to BrS. To our knowledge, this is the first report of a potential association between entrectinib and BrS. More frequent ECG recordings should be considered after initiating entrectinib treatment to detect an abnormality earlier.
The underlying mechanism of drug-induced BrS has not been fully elucidated; however, several reports suggest that drug-induced BrS occurs through the blockage of depolarizing (inward) sodium or calcium channels. These are the same cardiac ion channels that malfunction in the common genetic type of BrS [6, 10]. This implies that treatment of drug-induced BrS requires discontinuation of the causative drug, and VT associated with drug-induced BrS should be treated with defibrillation or amiodarone [5]. In this case, although the administration of antiarrhythmic agents and vasopressors was needed, the patient recovered from lethal arrhythmia only after the termination of entrectinib.
The results of a literature search with the terms “tyrosine kinase inhibitors” and “Brugada syndrome” in Pubmed resulted in two case reports [7, 8]. The available clinical information is summarized in Table 3. Data was available on three males, including the male in our case. Excluding our case, one patient had chronic myeloid leukemia (CML) and the other had melanoma. The patient with CML whose ECG showed right bundle branch block at baseline was treated with dasatinib. After one year, he presented with syncope with a typical ECG of Brugada pattern. The other patient with melanoma whose ECG also showed right bundle branch block at baseline was treated with dabrafenib and trametinib. After five months, his ECG also showed a typical ECG of Brugada pattern, although the patient was asymptomatic. The patient with CML continued treatment with dasatinib after implantable cardioverter defibrillator insertion. The patient with melanoma withdrew the treatment with dabrafenib and trametinib.
Table 3.
Summary of previous case reports on tyrosine kinase inhibitors and BrS
| Reference | Age | Sex | Primary tumor | TKI | Time to onset | Baseline ECG | Symptom | TKI-Treatment |
|---|---|---|---|---|---|---|---|---|
| [7] | 69 | Male | Chronic myeloid leukemia | Dasatinib | One year | Right bundle branch block | Syncope | Continued treatment with dasatinib (after ICD insertion) |
| [8] | 50 | Male | Melanoma | Dabrafenib and trametinib | Five months | Right bundle branch block | Asymptomatic | Withdrawal |
| Our case | 81 | Male | ROS1-positive NSCLC | Entrectinib | Three days | No apparent abnormality | Nausea | Discontinued permanently |
ECG electrocardiogram, ICD implantable cardioverter defibrillator, NSCLC non-small cell lung cancer, TKI tyrosine kinase inhibitor
A literature search with the terms “cardiac” and “entrectinib” in Pubmed revealed two case reports, both on females [3, 11]. The available clinical information is summarized in Table 4. Both females and the male patient in our case report had ROS1-positive NSCLC. Two patients were treated with 600 mg of entrectinib once daily, and one patient was treated with reduced dose of 400 mg once daily owing to oral dysesthesia and creatinine increased at a dose of 600 mg. Time to onset of cardiac-related events with entrectinib ranged from three to 19 days. Symptoms at onset of these events included chest pain, dyspnea, shortness of breath, edema, and nausea. Diagnosis of these events included myocarditis, heart failure, and Brugada syndrome. All patients recovered after supportive care and interruption of entrectinib. Entrectinib was discontinued permanently in two cases. In one case, it was re-started with a once daily reduced dose of 400 mg. Entrectinib-induced cardiac events were found to have occurred early in the treatment period (Table 4); therefore, treatment with entrectinib should be initiated in a hospital, and continuous or repeated ECG monitoring for entrectinib-induced BrS should be considered. Further analyses with a greater number of cases are needed to elucidate the relationship between entrectinib and BrS.
Table 4.
Summary of previous case reports of cardiac-related events with entrectinib
| Reference | Age | Sex | Primary tumor | Dose (mg/day) | Time to onset | Symptom | Diagnosis | Outcome | Entrectinib |
|---|---|---|---|---|---|---|---|---|---|
| [3] | 51 | Female | ROS1-positive NSCLC | 600 | Two weeks | Chest pain and dyspnea | Myocarditis | Recover | Temporarily discontinued and re-started with reduced dose |
| [11] | 74 | Female | ROS1-positive NSCLC | 400 | 19 days | Shortness of breath and edema | Heart failure | Recover | Discontinued permanently |
| Our case | 81 | Male | ROS1-positive NSCLC | 600 | Three days | Nausea | Brugada syndrome (Ventricular tachycardia) | Recover | Discontinued permanently |
NSCLC non-small cell lung cancer
Conclusion
We encountered a case of fatal arrhythmia due to drug-induced BrS caused by entrectinib. Cardiac toxicity induced by entrectinib was reported in only 2% of cases; however, it is necessary to initiate this agent in a hospital, monitor ECG changes with special attention, and consider early therapeutic interventions and discontinuation of the drug in the case of drug-induced BrS.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
All authors contributed to the conception and design of the study. KF and TH wrote the first draft of the manuscript.
Funding
This case report was not supported by any funds, grants, or other means of support.
Data availability
All data included in this report are available upon reasonable request from the corresponding author.
Declarations
Conflict of interest
T. Hase received personal fees and research funding from Chugai Pharmaceutical Co. outside the submitted work. All remaining authors have no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Inform consent
The patient gave written informed consent as a part of an observational study approved by the Ethics Review Committee of Nagoya University Graduate School of Medicine (No. 2018–0386).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:261–270. doi: 10.1016/s1470-2045(19)30690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonseca M, Chen DH, Walker JM, et al. Entrectinib-related myocarditis in a young female patient with metastatic non-small cell lung cancer. BMJ Case Reports. 2021;14:e243946. doi: 10.1136/bcr-2021-243946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martineau C, Turcotte M-K, Otis N, et al. Management of adverse events related to first-generation tyrosine receptor kinase inhibitors in adults: a narrative review. Support Care Cancer. 2022;30:10471–10482. doi: 10.1007/s00520-022-07401-y. [DOI] [PubMed] [Google Scholar]
- 5.Tisdale JE, Chung MK, Campbell KB, et al. Drug-induced arrhythmias: a scientific statement from the american heart association. Circulation. 2020;142:e214–e233. doi: 10.1161/cir.0000000000000905. [DOI] [PubMed] [Google Scholar]
- 6.Konigstein M, Rosso R, Topaz G, et al. Drug-induced brugada syndrome: clinical characteristics and risk factors. Heart Rhythm. 2016;13:1083–1087. doi: 10.1016/j.hrthm.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Sgherza N, Rossi AVR, Colonna P, et al. Use of tyrosine kinase inhibitors in a patient with Brugada syndrome and chronic myeloid leukemia. Int J Hematol. 2013;98:483–486. doi: 10.1007/s12185-013-1395-8. [DOI] [PubMed] [Google Scholar]
- 8.Nardin C, Colas M, Badoz M, et al. Brugada syndrome induced by BRAF and MEK inhibitors in a melanoma patient. Eur Heart J. 2017;38:2151–2151. doi: 10.1093/eurheartj/ehx133. [DOI] [PubMed] [Google Scholar]
- 9.Tang CPS, Lip GYH, McCormack T, et al. Management of cardiovascular complications of bruton tyrosine kinase inhibitors. Brit J Haematol. 2022;196:70–78. doi: 10.1111/bjh.17788. [DOI] [PubMed] [Google Scholar]
- 10.Turker I, Ai T, Itoh H, et al. Drug-induced fatal arrhythmias: acquired long QT and Brugada syndromes. Pharmacol Therapeut. 2017;176:48–59. doi: 10.1016/j.pharmthera.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Otsu Y, Kata Y, Takayasu H, et al. Entrectinib-induced heart failure in a patient with metastatic lung adenocarcinoma a case report. Cureus. 2022;14:e32174. doi: 10.7759/cureus.32174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this report are available upon reasonable request from the corresponding author.