Abstract
Background
Patients with cancer may be at increased risk of osteoporosis and fracture; however, gaps exist in the existing literature and the association between cancer and fracture requires further examination.
Methods
We conducted a population-based cohort study of Ontario patients with cancer (breast, prostate, lung, gastrointestinal, haematologic) diagnosed between January 2007 to December 2018 and 1:1 matched non-cancer controls. The primary outcome was incident fracture (end of follow-up December 2019). Multivariable Cox regression analysis was used to estimate the relative fracture risk with sensitivity analysis accounting for competing risk of death.
Results
Among 172,963 cancer patients with non-cancer controls, 70.6% of patients with cancer were <65 years old, 58% were female, and 9375 and 8141 fracture events were observed in the cancer and non-cancer group, respectively (median follow-up 6.5 years). Compared to non-cancer controls, patients with cancer had higher risk of fracture (adjusted HR [aHR] 1.10, 95% CI 1.07–1.14, p < 0.0001), which was also observed for both solid (aHR 1.09, 95% CI 1.05–1.13, p < 0.0001) and haematologic cancers (aHR 1.20, 95% CI 1.10–1.31, p < 0.0001). Sensitivity analysis accounting for competing risk of death did not change these findings.
Conclusions
Our study indicates that patients with cancer are at modest risk of fractures compared to non-cancer controls.
Subject terms: Quality of life, Cancer epidemiology
Background
As cancer outcomes have improved over time, survivorship has become increasingly important [1, 2]. The prevalence of osteoporosis and fractures in the general population is high, affecting 40% of postmenopausal women and 15–30% of men [3, 4]. Fractures result in morbidity and mortality, owing to pain, hospitalisation, reduced mobility and quality of life [5–7]. Moreover, prior studies suggest that 1 in 5 individuals with an osteoporosis-related fracture will die within 12 months [8, 9]. An increasing number of cancer survivors combined with high prevalence of fractures forms the rationale for addressing fracture risk in cancer survivors.
Both cancer and its treatment can compromise skeletal integrity and contribute to cancer-associated bone loss, as reflected by lower bone mineral density (BMD) in cancer patients than the general population [10]. Although prior studies have reported an increased risk of osteoporotic fractures in the cancer population, there are limitations to the existing literature. For instance, previous studies are limited by modest size and restricted to certain cancer subtypes with more data for solid cancers than haematologic cancers [11–21], or focus on older cancer survivors [22]. Furthermore, although evidence supports the use of dual-energy x-ray absorptiometry (DEXA) scans for BMD testing and antiresorptive therapy, studies in the general population report <20% of patients receive osteoporosis treatments, with this discrepancy between guidelines and real-world practice termed the ‘osteoporosis care gap’ [23]. While lower BMD has been demonstrated in patients with cancer compared to the general population [10], data on the usage of DEXA scans and appropriate osteoporosis-directed treatments in the cancer population is sparse. The purpose of this study was to examine fracture risk in patients with cancer, and in particular, the risk of fracture in subtypes of solid and haematologic cancers. Secondarily, we aimed to understand quality of fracture care and whether the ‘osteoporosis care gap’ exists in routine clinical practice.
Methods
Study design
We conducted a population-based retrospective cohort study using linked administrative databases, held at ICES (formerly the Institute for Clinical Evaluative Sciences), which captures individual-level records for all 14 million residents of Ontario, Canada. This study complied with ICES data confidentiality and privacy guidelines.
Data sources
Patients with cancer were identified using the Ontario Cancer Registry (OCR), a population-based provincial-wide cancer registry that captures all incident cancer diagnoses and all cancer deaths in Ontario residents since 1964 (except for non-melanoma skin cancers) [24, 25], using the International Classification of Diseases Oncology 3rd revision (ICD-O-3) codes (Appendix Table S1–S3). Comorbidity burden was measured using The Johns Hopkins’ ACG® System Version 1016, whereby patients are assigned up to 32 ACG® System Aggregated Disease groups (ADGs) to characterise patients’ comorbidity burden (cancer and comorbidities known to affect fracture/osteoporosis risk were excluded from the ADGs). Details on data sources can be found in Appendix methods.
Study population, exposure, and definitions
The study population consisted of Ontario residents ≥18 years old. The exposure cohort comprised of adults diagnosed with a common solid (breast, gastrointestinal, lung, and prostate) or haematologic cancer between January 1, 2007 and December 31, 2018. The exclusion criteria were as follows: invalid health card number; invalid date of death; invalid age or sex. Eligible cancer patients were 1:1 matched to eligible adults without cancer (non-cancer controls) based on age (by 5-year stratum) and sex. Individuals were followed until the first occurrence of an outcome of interest, death, or the end of the study period (December 31, 2019), whichever occurred first.
Outcomes
Index date was the date of cancer diagnosis for patients with cancer and matched non-cancer controls were assigned the same index date. For the primary analysis, both patients with cancer and non-cancer controls who had a fracture within two years of index date were excluded. A two-year look back period was chosen as it is generally accepted for identification of prevalent cases [26]. Furthermore, the re-fracture risk has been reported to be highest within 2 years of an initial fracture event [27]. Accordingly, the primary outcome was any incident fracture, defined as an emergency department (ED) visit, admission to hospital, or physician claim for any fracture of the wrist or forearm, shoulder or upper arm, thoracic spine, lumbar spine and pelvis, hip or femur, or lower leg or ankle (ICD 10th revision codes in Appendix Table S4). The ICD codes for fractures have been previously validated with high positive predictive value [28–30].
We excluded fractures secondary to trauma, seizure, motor vehicle collision, fall from a height, or primary bone malignancy. This algorithm used herein to identify incident fractures was as previously described [31], and ICD codes for fractures have been previously validated with high positive predictive value [28–30].
For exploratory analysis, the proportion who had a DEXA scan and antiresorptive therapy use with bisphosphonate or denosumab (≥65 years old for medication use) after a fracture in the subgroup of patients who developed an incident fracture were compared between cancer and non-cancer. Patients with cancer who developed an incident fracture were 1:1 matched to non-cancer controls who developed an incident fracture based on age and sex. These exploratory outcomes were determined in the following intervals among those who remained alive during these time intervals: 0–1 years, 1–2 years, 2–3 years, and 3–5 years.
Statistical analysis
Baseline data were analysed using descriptive statistics and reported using means and standard deviation, medians, and interquartile ranges (IQRs), or frequency and percentages, where appropriate. Distribution of baseline characteristics were compared between cancer and non-cancer using standardised differences to assess for balance (difference of <10% indicates balance) [32].
For the primary outcome analysis, we used cumulative incidence functions (CIFs) and multivariable Cox regression model to compare the risk of fracture between patients with cancer and non-cancer controls and estimated the hazard ratios (HRs) and 95% confidence intervals (CIs) based on a comparison of the time-to-event rates. We adjusted for the following covariates: rural residence status (categorised as residing in postal code areas with <10,000 residents), income quintile (assigned from Census data at the neighbourhood level based on postal code), comorbid disease burden, as well as comorbidities known to influence osteoporosis and fracture risk including chronic kidney disease (CKD), arthritis, Parkinson’s disease, dementia, chronic lung disease (asthma or chronic obstructive pulmonary disease), cerebrovascular disease, falls, and diabetes (ICD codes in Appendix Table S5). To account for the competing risk of death in patients with cancer, sensitivity analysis was conducted using the Fine and Grey model of subdistribution for the primary outcome. Sensitivity analysis was also conducted including eligible individuals with history of fracture two years prior to index date. Finally, sensitivity analysis stratified by sex were conducted.
Stratified analyses were conducted as above for patients with solid cancer and haematologic cancer, as well as its subtypes, while maintaining respective 1:1 matched non-cancer controls. The association between glucocorticoid use, particularly at high doses and prolonged courses has been well documented [33]. Given that high dose corticosteroids are part of standard of care for lymphoma treatment, we conducted analysis for indolent vs. aggressive lymphoma, as well as for the most common aggressive lymphoma, diffuse large B-cell lymphoma (DLBCL). Patients with DLBCL were defined as those with newly diagnosed DLBCL receiving first-line curative intent rituximab-containing chemoimmunotherapy.
For exploratory analysis, we examined: (1) the association between subtypes of cancer and fracture risk by anatomic site (spine and pelvis, hip and femur, lower leg and ankle, wrist and shoulder), (2) the association of patients aged <65 or ≥65 years with fracture risk in subtypes of cancer. Additionally, for exploratory analysis, the likelihood of having at least 1 DEXA scan and antiresorptive therapy use following incident fracture events was compared between patients with cancer and non-cancer controls. We calculated crude event rates with 95% CI of BMD testing with numerator being number of persons who had BMD assessment and denominator being time alive during the pre-specified time intervals post fracture, expressed as 100 person-years.
For all analyses, a two-tailed p value <0.05 was considered statistically significant. Analyses were conducted using SAS version 9.4 (Cary, NC, USA).
Results
Patient characteristics
Baseline characteristics are summarised in Table 1. The study cohort comprised 172,963 cancer patients (143,609 solid cancer patients and 29,354 haematologic cancer patients) as well as their 1:1 matched non-cancer controls. Within solid cancer, there were 56,424, 29,775, 27,973, and 29,437 patients with breast, colorectal, lung, and prostate cancer, respectively (Appendix Table S6 for characteristics). Within haematologic cancer, there were 21,363 patients with lymphoma (9709 with indolent lymphoma, 11,654 with aggressive lymphoma, and 3803 with DLBCL), 3973 and 4018 patients with leukaemia and multiple myeloma, respectively (Table S7 for characteristics). The majority of the cohort were aged <65 years (70.6%), and 57.9% were female.
Table 1.
Baseline characteristics of 1:1 age- and sex-matched cancer patients and matched non-cancer controls.
| Characteristic | Cancer (N = 172,963)a | Non-cancer control (N = 172,963) | Standardised difference (%) |
|---|---|---|---|
| Age ≥65 years, % | 29.4 | 29.4 | 0 |
| Sex (female), % | 57.9 | 57.9 | 0 |
| Rural residence, N (%) | 21,743 (12.6%) | 19,086 (11.0%) | 4.8 |
| Income quintile | |||
| 1 (lowest) | 32,492 (18.8%) | 34,406 (19.9%) | 2.8 |
| 5—n (%) | 36,397 (21.0%) | 35,630 (20.6%) | 1.1 |
| ADG score | |||
| Mean (SD) | 6.3 (3.4) | 4.2 (3.6) | 59.0 |
| Median (IQR) | 6 (4–8) | 4 (1–7) | |
| ADG category | |||
| 0-4, N (%) | 56,366 (32.6%) | 97,946 (56.6%) | 49.8 |
| 5-7, N (%) | 58,894 (34.1%) | 42,194 (24.4%) | 21.4 |
| 8-10, N (%) | 37,690 (21.8%) | 22,375 (12.9%) | 23.5 |
| 11+, N (%) | 20,013 (11.6%) | 10,448 (6.0%) | 19.6 |
| Comorbidities, N (%) | |||
| Dementia | 4684 (2.7%) | 4036 (2.3%) | 2.0 |
| CKD | 5286 (3.1%) | 2958 (1.7%) | 8.8 |
| Arthritis | 51,905 (30.0%) | 40,497 (23.4%) | 15.0 |
| Parkinson’s | 202 (0.1%) | 174 (0.1%) | 0.5 |
| Asthma or COPD | 43,251 (25.0%) | 30,162 (17.4%) | 18.6 |
| Cerebrovascular disease | 2056 (1.2%) | 1224 (0.7%) | 5.0 |
| Falls | 9858 (5.7%) | 7227 (4.2%) | 7.0 |
| Diabetes | 28,423 (16.4%) | 24,372 (14.1%) | 6.5 |
| CAD | 4657 (2.7%) | 3650 (2.1%) | 3.8 |
| Thyroid | 45,539 (26.3%) | 528 (0.3%) | 82.9 |
ADG aggregated diagnosis groups, CAD coronary artery disease, CKD chronic kidney disease, COPD chronic obstructive lung disease, IQR interquartile range, SD standard deviation.
*n = 143,609 patients with solid cancer; n = 29,354 patients with haematologic cancer.
As expected, cancer patients had greater comorbid disease burden than non-cancer controls (median ADG score 6 [IQR, 4–8)] vs 4 [IQR 1–7]). Cancer patients were more likely to have a diagnosis of chronic lung disease and arthritis, with standardised differences of 15%, and 19%, respectively, while other comorbidity covariates were balanced with standardised differences less than 10%.
Association between cancer and fracture
During a median follow-up of 6.5 years (IQR 3.7–9.5) for both cancer patients and non-cancer controls, there were 9375 patients with cancer who developed an incident fracture (unadjusted rate of 1.11 per 100 person years, 95% CI 1.09–1.14) while 8141 (0.74 per 100-person years, 95% CI 0.73–0.76) individuals without cancer developed an incident fracture (Appendix Table S8).
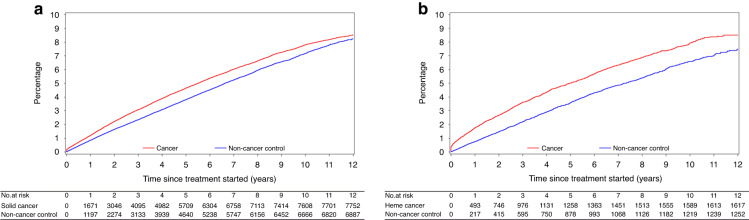
Cancer was associated with a significantly higher risk of an incident fracture in univariate (HR 1.10, 95% CI 1.06–1.13, p < 0.0001; Fig. 1 shows unadjusted CIF curve) and multivariable Cox regression analysis (adjusted HR [aHR] 1.10, 95% CI 1.07–1.14, p < 0.0001; Table 2). When examined separately in stratified analysis (while maintaining matched non-cancer controls), an increased fracture risk was observed for both solid (aHR 1.09, 95% CI 1.05–1.13, p < 0.0001; Fig. 2a shows unadjusted CIF curve) and haematologic cancers (aHR 1.20, 95% CI 1.10–1.31, p < 0.0001; Fig. 2b shows unadjusted CIF curve). Sensitivity analysis accounting for competing risk of death did not change these findings (Appendix Table S9). Similarly, sensitivity analysis including patients with a fracture two years prior to index date did not change these findings (Appendix Table S10).
Fig. 1. Cumulative incidence curves of incident fracture in a retrospectively identified population cohort.
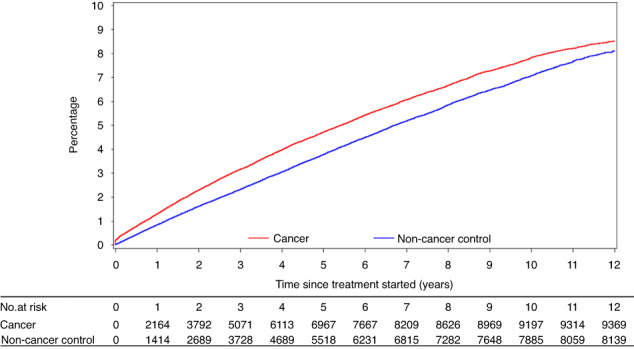
Curve compares patients with cancer to age- and sex-matched non-cancer controls.
Table 2.
Adjusted hazard ratios for incident osteoporotic fractures in cancer patients compared to non-cancer controls.
| Adjusted HR (95% CI)a | p value | |
|---|---|---|
| All patients with cancer vs. controls | ||
| Cancer cases | 1.10 (1.07–1.14) | <0.0001 |
| Solid vs. controls | ||
| Cancer cases | 1.09 (1.05–1.13) | <0.0001 |
| Haematologic vs. controls | ||
| Cancer cases | 1.20 (1.10–1.31) | <0.0001 |
| Lymphoma vs. controls | ||
| Cancer cases | 1.18 (1.06–1.31) | 0.0018 |
| DLBCL vs. controls | ||
| Cancer cases | 1.38 (1.07–1.77) | 0.012 |
| Indolent lymphoma vs. controls | ||
| Cancer cases | 1.36 (1.17–1.58) | <0.0001 |
| Aggressive lymphoma vs. controls | ||
| Cancer cases | 1.05 (0.90–1.22) | 0.53 |
CI confidence interval, CKD chronic kidney disease, COPD chronic obstructive lung disease, DLBCL diffuse large B cell lymphoma, HR hazard ratio.
aAdjusted for demographic income quintile and rural status, comorbidity burden measured by aggregated clinical group score, CKD, arthritis, dementia, asthma/COPD, cerebrovascular disease, falls, diabetes.
Fig. 2. Cumulative incidence curves of incident fracture.
Curves shown separately for patients with solid cancer (a) and haematologic cancer (b) compared to age- and sex-matched non-cancer controls.
The increase fracture risk was observed for both female and male patients with cancer (male aHR 1.15, 95% CI 1.08–1.22, p < 0.0001; female 1.09, 95% CI 1.05–1.13, p < 0.0001), solid cancer (male aHR 1.07, 95% CI 1.00–1.15, p = 0.05; female aHR 1.15, 95% CI 1.09–1.14, p < 0.0001), and male patients with haematologic cancer (male aHR 1.47, 95% CI 1.28–1.69, p < 0.0001) but not female patients (aHR 1.05, 95% CI 0.94–1.18, p = 0.38). Unadjusted CIF curves stratified by sex are shown in Appendix Fig. S1.
Association between subtypes of solid cancer and fracture
When stratifying by types of solid cancer, breast cancer (aHR 1.30, 95% CI 1.23–1.37, p < 0.0001) and prostate cancer (1.20, 95% 1.09–1.32, p = 0.0002) were associated with increased fracture risk, while no associated was observed for GI cancer (aHR 0.96, 95% CI 0.89–1.04, p = 0.29), and lower risk was observed for lung cancer (aHR 0.75, 95% CI 0.68–0.82, p < 0.0001). These results were not changed when accounting for competing risk of death (data not shown).
Association between subtypes of haematologic cancer and fracture
When stratifying by types of haematologic cancer, multiple myeloma was associated with higher risk of fracture (aHR 1.96, 95% CI 1.57–2.45, p < 0.0001), while lower risk was observed for leukaemia cancer (aHR 0.66, 95% CI 0.50–0.86, p = 0.0021). These results were not changed when accounting for competing risk of death (data not shown).
For patients with lymphoma, a higher incident fracture risk was observed (aHR 1.18, 95% CI 1.06–1.31, p = 0.0018; Table 2). When lymphoma was further classified as indolent and aggressive, respectively, patients with indolent lymphoma had higher fracture risk (aHR 1.36, 95% CI 1.17–1.58, p < 0.0001) compared to controls, while higher fracture risk was not observed in patients with aggressive lymphoma when compared to controls (aHR 1.05, 95% CI 0.90–1.22, p = 0.53) (Table 2). In a well-defined group of patients with DLBCL, higher fracture risk was observed (aHR 1.38, 95% CI 1.07–1.77, p = 0.012). These results were unchanged when accounting for competing risk of death (data not shown).
Fracture risk exploratory analysis
Our exploratory analyses suggests that the strength of association varied by fracture location, suggesting that spine, pelvis, hip, and femur fractures likely drove the association between cancer and fracture risk (Appendix Fig. S2). Moreover, the association between fracture risk by anatomical site varied by subtype of cancer (Appendix Fig. S3). For instance, breast cancer was associated with fracture risk in all fracture sites. However, colorectal cancer was associated with hip/femur fractures but not spine/pelvis fractures while prostate cancer was associated with higher risk of spine/pelvis fractures.
Adjusted models by subtype of cancer stratified by age <65 or age ≥65 years were carried out (Appendix Fig. S4). These findings suggest that cancer subtypes likely have differing effects on fracture risk in younger vs. older patients, whereby among patients older than 65 years, fracture risk was not associated with breast cancer, colorectal cancer, or lymphoma.
DEXA scan following incident fracture
Among those who developed a fracture, 7745 matched patients with cancer and non-cancer controls pairs were identified. In unadjusted matched comparison, patients with cancer had a higher likelihood of a DEXA scan than non-cancer controls within 1-year post fracture (cancer: 3.04 100-person years, 95% CI 2.90–3.19; non-cancer: 2.66 100-person years, 95% CI 2.54–2.79), and similarly between 1–2 years, 2–3 years and 3–5 years among matched pairs that remained alive during these intervals (Table 3 and Appendix Fig. S5A). When evaluating DEXA scan events by cancer subtype, patients with solid cancer had higher rate of BMD testing than non-cancer controls, while patients with haematologic cancer had a similar likelihood of a DEXA scan (Table 3 and Appendix Fig. S5A).
Table 3.
Incidence rate per 100 person-years with 95% confidence intervals of bone mineral density testing in subgroup of patients with cancer and non-cancer controls who developed an incident fracture.
| 0–1 year post fracture | 1–2 years post fracture | 2–3 years post fracture | 3–5 years post fracture | |
|---|---|---|---|---|
| All cancer | ||||
| Cancer | 3.04 (2.90, 3.19) n = 7745 | 1.61 (1.50, 1.71) n = 6819 | 1.23 (1.13, 1.32) n = 6513 | 1.47 (1.37, 1.58) n = 5914 |
| Non-cancer control | 2.66 (2.54, 2.79) n = 7745 | 1.07 (0.99, 1.15) n = 7583 | 0.95 (0.88, 1.03) n = 7472 | 1.07 (0.97, 1.13) n = 7192 |
| Solid cancer | ||||
| Solid cancer | 3.11 (3.08, 3.39) n = 6558 | 1.65 (1.53, 1.77) n = 5770 | 1.25 (1.15, 1.36) n = 5513 | 1.52 (1.40, 1.64) n = 5016 |
| Non-cancer control | 2.69 (2.53, 2.81) n = 6558 | 1.10 (1.01, 1.19) n = 6431 | 0.95 (0.87, 1.04) n = 6341 | 1.09 (1.00, 1.18) n = 6107 |
| Haematologic cancer | ||||
| Haematologic cancer | 2.68 (2.31, 3.04) n = 1170 | 1.23 (0.98, 1.47) n = 1036 | 0.96 (0.74, 1.18) n = 989 | 1.15 (0.90, 1.40) n = 895 |
| Non-cancer control | 2.47 (2.15, 2.79) n = 1170 | 0.84 (0.65, 1.03) n = 1144 | 0.90 (0.71, 1.09) n = 1121 | 0.88 (0.69, 1.08) n = 1069 |
Antiresorptive therapy following incident fracture
Among those who developed a fracture ≥65 years old, 3410 matched patients with cancer and non-cancer controls pairs were identified. In unadjusted matched comparison, patients with cancer were more likely to be treated with antiresorptive therapy than non-cancer controls within 1-year post fracture (cancer: 5.64 100-person years, 95% CI 5.31–5.96; non-cancer: 4.87 100-person years, 95% CI 4.60–5.15), which was not different beyond 1 year (Table 4 and Appendix Fig. S5B).
Table 4.
Incidence rate per 100 person-years with 95% confidence intervals for antiresorptive therapy (bisphosphonate or denosumab) use after incident fracture for cancer and non-cancer controls.
| 0–1 year post fracture | 1–2 years post fracture | 2–3 years post fracture | 3–5 years post fracture | |
|---|---|---|---|---|
| All cancer | ||||
| Cancer | 5.64 (5.31, 5.96) n = 3410 | 4.16 (3.87, 4.44) n = 2829 | 3.07 (2.82, 3.31) n = 2627 | 2.66 (2.42, 2.90) n = 2246 |
| Non-cancer control | 4.87 (4.60, 5.15) n = 3410 | 3.99 (3.74, 4.24) n = 3274 | 3.06 (2.82, 3.31) n = 3164 | 2.56 (2.35, 2.77) n = 2910 |
| Solid cancer | ||||
| Cancer | 5.66 (5.31, 6.01) n = 2900 | 4.13 (3.83, 4.44) n = 2423 | 3.03 (2.77, 3.30) n = 2257 | 2.61 (2.35, 2.86) n = 1940 |
| Non-cancer control | 4.77 (4.48, 5.06) n = 2900 | 3.97 (3.70, 4.23) n = 2787 | 3.02 (2.79, 3.26) n = 2701 | 2.50 (2.28, 2.72) n = 2500 |
| Haematologic cancer | ||||
| Cancer | 5.51 (4.65, 6.38) n = 499 | 4.31 (3.53, 5.09) n = 402 | 3.27 (2.57, 3.96) n = 367 | 3.00 (2.30, 3.70) n = 305 |
| Non-cancer control | 5.00 (4.27, 5.71) n = 499 | 3.67 (3.04, 4.29) n = 477 | 3.06 (2.48, 3.64) n = 452 | 2.51 (1.96, 3.05) n = 402 |
Discussion
In this population-based cohort study, a diagnosis of cancer was associated with a modest increased risk of an incident fracture. Our study is unique in that it provides contemporary comparative analysis of fracture risk in a large cohort of patients with various cancer subtypes, comprised of common solid cancers (including breast, prostate, lung, and gastrointestinal) and haematologic cancers, against an age- and sex-matched control population without cancer.
Findings of our study are concordant with prior studies demonstrating an increased fracture risk in patients with solid cancers [15, 16, 22]. For instance, results from the Women’s Health Initiative showed up to a twofold higher risk of fracture after a diagnosis of cancer, which was observed for breast, lung, skin, endometrial, and lymphoma cancers [15]. A recent report by Rees-Punia et al. showed that older cancer survivors had greater risk of fractures when compared to older individuals without cancer [22]. Our study adds to the existing body of literature in several ways. Existing literature examining the association between cancer and fracture risk has been predominantly limited to specific cancer subtypes, particularly hormone-sensitive solid cancers such as breast and prostate [11–14, 17, 18, 34–36]. Here, we included both patients with solid and haematologic cancers combined followed by a stratified association analysis by cancer subtype. In addition, our cohort comprised of younger patients with cancer, with 70% of included patients aged <65 years. Given that age is a well-known risk factor for osteoporosis and fractures, our study results provide evidence that fracture risk is an important health outcome even in younger cancer survivors. Notably, in exploratory analysis of fracture risk stratified by age, we found no increased fracture risk in patients older than 65 years with certain types of cancers including breast, colorectal, and lymphoma. As our multivariable models adjusted for important comorbidities associated with fracture risk which were not included in prior studies, this finding may be related to a stronger effect of other risk factors in the model compared to that of cancer. Nevertheless, future studies are required to delineate the relative contribution of age on fracture risk for various cancer subtypes.
Indeed, our results corroborate with existing literature that breast and prostate cancer are associated with greater fracture risk [11–14, 17, 18, 35, 36]. Lung cancer was associated with lower fracture risk compared to controls. Although lung cancer is known to be associated with skeletal-related adverse events (SREs) in the form of pathological fractures [16, 19], less data is known regarding osteoporotic fracture risk. The explanation for lower fracture risk observed for lung cancer here is unclear and requires future studies for clarification. For GI cancer, we did not observe an association with fracture, similar to prior reports [37, 38].
Our study comprises the largest cohort of patients with haematologic cancers to examine the association with fracture risk. Haematologic malignancies are a heterogenous group of cancers characterised by uncontrolled growth of haematopoietic or lymphoid tissues, which can exhibit an indolent to aggressive clinical course [39]. Herein, we report an increased risk of 18% for incident fractures in patients with haematologic cancer. For multiple myeloma, it is well known that SREs are common due to osteolytic lesions [40], and indeed, we observed the highest relative fracture risk for patients with multiple myeloma compared to controls. In acute leukaemias, although bone loss and increased fracture risk are common complications following haematopoietic transplant [20, 21], less is known regarding fracture risk in all patients with leukaemia including those without transplant. Here, we report lower fracture risk compared to controls, and prompts further study in patients with leukaemia.
Lymphoma is the most common type of haematologic malignancy, whereby the most common lymphoma subtypes include aggressive non-Hodgkin lymphoma such as DLBCL and indolent lymphomas such as follicular lymphoma (FL). With advances in chemoimmunotherapies, DLBCL has high cure rates, while FL has long survival, leading to substantial percentage of patients with lymphoma becoming long-term survivors. Glucocorticoids are included in many lymphoma regimens. For example, six cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) results in 3000 mg cumulatively over 18 weeks, with average of 24 mg/day, raising concern for glucocorticoid induced osteoporosis (GIO) [41, 42]. Here, we observed an estimated increased fracture risk of 18% in patients with lymphoma compared to persons without cancer. This corroborates with prior studies demonstrating BMD reduction and fracture risk in patients treated for non-Hodgkin lymphoma [43–48]. For instance, in the Danish study of 2589 lymphoma patients (DLBCL or FL), osteoporosis treatment initiation or low-impact fracture was increased compared to matched controls (61% relative risk), particularly within first 2 years after treatment [44]. In a study of 729 older DLBCL patients treated with R-CHOP, the cumulative fracture incidence was 11.4%, albeit without comparative analysis with matched controls [45]. These studies did not adjust for comprehensive list of comorbidities known to influence fracture risk as we did here and only few studies’ primary outcome was fracture, while others were focused on bone density. A caveat is the inability to adjust for important lifestyle variables and disease characteristics in our models.
When further stratifying lymphoma analysis for indolent and aggressive lymphoma, we found a 36% increased fracture risk for patients with indolent lymphoma. Although many patients with indolent lymphoma do not require treatment, osteoporosis was observed even in untreated patients with non-Hodgkin’s lymphoma [49]. Our study did not find higher fracture risk in aggressive lymphomas overall, but does for a more defined cohort of DLBCL patients treated with R-CHOP. This is congruent with prior studies demonstrating higher fracture risk in DLBCL patients who underwent R-CHOP treatment [44, 45, 50]. The precise reason for lack of significant fracture risk in all aggressive lymphomas is unclear and is hypothesis generating, requiring future studies to delineate and characterise the association between various lymphoma subtypes and fracture risk.
Our study has several limitations. As in all observational studies, there is a risk of misclassification and selection bias. Our primary outcome was non-traumatic fractures defined as exclusion of trauma using ICD codes; however, trauma ICD codes have not been validated, which may bias the interpretation of our results. Moreover, we could not capture minor or asymptomatic fractures, such as vertebral fractures that did not require hospital-based care. This may underestimate the association between cancer and fracture risk. We aimed to minimise misclassification bias with validated database definitions for cancer and defining the primary outcome as fracture, rather than osteoporosis which is likely under-reported in administrative databases. Furthermore, the potential for misclassification is reduced by using hospital-based fractures as our outcome. Although outside the scope of this study, longer follow-up in future studies is required to adequately evaluate fracture risk during different phases of cancer survivorship. We were unable to measure important factors that may affect the association between cancer and fracture risk, such as body mass index, family history, smoking status, menopausal status in women, caffeine or alcohol intake, and dietary and lifestyle factors. Cancer-related factors such as cancer stage, remission status, recurrence, and treatment regimens received were also not available and was not a primary aim of our study. As such, we could not adjust for these potential confounding factors in our multivariable models and residual confounding may be possible. Due to limitations of Ontario medication database (available for those >65 years old), we are unable to ascertain medications that predispose to fractures or osteoporosis treatment medications. Although we found more BMD testing and antiresorptive use following incident fracture, our sample size for these exploratory analysis limits a robust analysis and requires to be examined in future studies. We did not have access to BMD scan results, which usually guide frequency of follow-up BMD scans. Fractures are later stage of osteoporosis and underestimate the effect of cancer on BMD since not all patients with clinical osteoporosis experience fractures. Lastly, our observational study sought to evaluate the association of cancer with fracture risk, rather than establishing causality.
Despite these limitations, the large sample size of matched patients with cancer and stratified analysis for haematologic and solid cancer, sensitivity analyses accounting for patient death and previous fractures, as well as exploratory descriptive analysis of BMD testing and antiresorptive therapy adds to the growing body of literature on fracture risk in the cancer population.
In conclusion, patients with cancer are at modest risk of fractures compared to non-cancer controls, particularly for breast, prostate, lymphoma, and multiple myeloma. Our study results highlight that fracture prevention is likely important in optimising health outcomes in the growing number of cancer patients and survivors.
Supplementary information
Acknowledgements
This document used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under license from Canada Post Corporation and Statistics Canada. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI), Ontario Health (OH), Ontario Ministry of Health (MOH), and the Ontario Registrar General (ORG) information on deaths, the original source of which is ServiceOntario. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. We thank IQVIA Solutions Canada Inc. for use of their Drug Information File.
Author contributions
Conceptualisation: IYG, LM, MCC, KKWC. Data curation: LM, MCC, KKWC. Formal analysis: IYG, LM, MCC, KKWC. Funding acquisition: LM. Investigation: IYG, LM, MCC, KKWC. Methodology: all authors. Project administration, resources, software, supervision, validation, visualisation: LM, MCC. Writing—original draft: IYG. Writing—review and editing: all authors.
Funding
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This work was also funded by the Canadian Centre for Applied Research in Cancer Control (ARCC). ARCC receives core funding from the Canadian Cancer Society (Grant #2020-706936).
Data availability
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and providers (e.g. healthcare organisations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Research Ethics Board at Sunnybrook Health Sciences Center.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02353-4.
References
- 1.Lustberg MB, Reinbolt RE, Shapiro CL. Bone health in adult cancer survivorship. J Clin Oncol. 2012;30:3665–74. doi: 10.1200/JCO.2012.42.2097. [DOI] [PubMed] [Google Scholar]
- 2.Thanarajasingam G, Minasian LM, Baron F, Cavalli F, De Claro RA, Dueck AC, et al. Beyond maximum grade: modernising the assessment and reporting of adverse events in haematological malignancies. Lancet Haematol. 2018;5:e563–e598. doi: 10.1016/S2352-3026(18)30051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watts NB, Bilezikian JP, Camacho PM, Greenspan SL, Harris ST, Hodgson SF, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16:1–37. doi: 10.4158/EP.16.S3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Min Res. 2014;29:2520–6. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioannidis G, Papaioannou A, Hopman WM, Akhtar-Danesh N, Anastassiades T, Pickard L, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181:265–71. doi: 10.1503/cmaj.081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papaioannou A, Kennedy CC, Ioannidis G, Sawka A, Hopman WM, Pickard L, et al. The impact of incident fractures on health-related quality of life: 5 years of data from the Canadian Multicentre Osteoporosis Study. Osteoporos Int. 2009;20:703–14. doi: 10.1007/s00198-008-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Min Res. 2000;15:1384–92. doi: 10.1359/jbmr.2000.15.7.1384. [DOI] [PubMed] [Google Scholar]
- 8.Haentjens P, Magaziner J, Colon-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152:380–90. doi: 10.7326/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haleem S, Lutchman L, Mayahi R, Grice JE, Parker MJ. Mortality following hip fracture: trends and geographical variations over the last 40 years. Injury. 2008;39:1157–63. doi: 10.1016/j.injury.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Reuss-Borst M, Hartmann U, Scheede C, Weiss J. Prevalence of osteoporosis among cancer patients in Germany: prospective data from an oncological rehabilitation clinic. Osteoporos Int. 2012;23:1437–44. doi: 10.1007/s00198-011-1724-9. [DOI] [PubMed] [Google Scholar]
- 11.Chang CH, Chen SJ, Liu CY. Fracture risk and adjuvant therapies in young breast cancer patients: a population-based study. PLoS ONE. 2015;10:e0130725. doi: 10.1371/journal.pone.0130725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards BJ, Gradishar WJ, Smith ME, Pacheco JA, Holbrook J, McKoy JM, et al. Elevated incidence of fractures in women with invasive breast cancer. Osteoporos Int. 2016;27:499–507. doi: 10.1007/s00198-015-3246-3. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ, 3rd, Hartmann LC, Achenbach SJ, Atkinson EJ, Therneau TM, Khosla S. Fracture risk in women with breast cancer: a population-based study. J Bone Min Res. 2012;27:1196–205. doi: 10.1002/jbmr.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt N, Jacob L, Coleman R, Kostev K, Hadji P. The impact of treatment compliance on fracture risk in women with breast cancer treated with aromatase inhibitors in the United Kingdom. Breast Cancer Res Treat. 2016;155:151–7. doi: 10.1007/s10549-015-3661-3. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Maricic M, Aragaki AK, Mouton C, Arendell L, Lopez AM, et al. Fracture risk increases after diagnosis of breast or other cancers in postmenopausal women: results from the Women’s Health Initiative. Osteoporos Int. 2009;20:527–36. doi: 10.1007/s00198-008-0721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in patients with different types of cancer. Acta Oncol. 2009;48:105–15. doi: 10.1080/02841860802167490. [DOI] [PubMed] [Google Scholar]
- 17.Allain TJ. Prostate cancer, osteoporosis and fracture risk. Gerontology. 2006;52:107–10. doi: 10.1159/000090956. [DOI] [PubMed] [Google Scholar]
- 18.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 19.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–94. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1588::AID-CNCR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Ganguly S, Divine CL, Aljitawi OS, Abhyankar S, McGuirk JP, Graves L. Prophylactic use of zoledronic acid to prevent early bone loss is safe and feasible in patients with acute myeloid leukemia undergoing allogeneic stem cell transplantation. Clin Transplant. 2012;26:447–53. doi: 10.1111/j.1399-0012.2011.01527.x. [DOI] [PubMed] [Google Scholar]
- 21.Massenkeil G, Fiene C, Rosen O, Michael R, Reisinger W, Arnold R. Loss of bone mass and vitamin D deficiency after hematopoietic stem cell transplantation: standard prophylactic measures fail to prevent osteoporosis. Leukemia. 2001;15:1701–5. doi: 10.1038/sj.leu.2402264. [DOI] [PubMed] [Google Scholar]
- 22.Rees-Punia, E, Newton, CC, Parsons, HM, Leach, CR, Diver, WR, Grant, AC, et al. Fracture risk among older cancer survivors compared with older adults without a history of cancer. JAMA Oncol. 10.1001/jamaoncol.2022.5153 (2022). [DOI] [PMC free article] [PubMed]
- 23.Kanis JA, Svedbom A, Harvey N, McCloskey EV. The osteoporosis treatment gap. J Bone Min Res. 2014;29:1926–8. doi: 10.1002/jbmr.2301. [DOI] [PubMed] [Google Scholar]
- 24.Holowaty EJ, Norwood TA, Wanigaratne S, Abellan JJ, Beale L. Feasibility and utility of mapping disease risk at the neighbourhood level within a Canadian public health unit: an ecological study. Int J Health Geogr. 2010;9:21. doi: 10.1186/1476-072X-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin JR, Kreiger N, Marrett LD, Holowaty EJ. Cancer incidence registration and trends in Ontario. Eur J Cancer. 1991;27:1520–4. doi: 10.1016/0277-5379(91)90041-B. [DOI] [PubMed] [Google Scholar]
- 26.Austin PC, van Walraven C, Wodchis WP, Newman A, Anderson GM. Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49:932–9. doi: 10.1097/MLR.0b013e318215d5e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leslie WD, Yan L, Lix LM, Morin SN. Time dependency in early major osteoporotic and hip re-fractures in women and men aged 50 years and older: a population-based observational study. Osteoporos Int. 2022;33:39–46. doi: 10.1007/s00198-021-06166-0. [DOI] [PubMed] [Google Scholar]
- 28.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45:703–14. doi: 10.1016/0895-4356(92)90047-Q. [DOI] [PubMed] [Google Scholar]
- 29.Warriner AH, Patkar NM, Curtis JR, Delzell E, Gary L, Kilgore M, et al. Which fractures are most attributable to osteoporosis? J Clin Epidemiol. 2011;64:46–53. doi: 10.1016/j.jclinepi.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright NC, Daigle SG, Melton ME, Delzell ES, Balasubramanian A, Curtis JR. The design and validation of a new algorithm to identify incident fractures in administrative claims data. J Bone Min Res. 2019;34:1798–807. doi: 10.1002/jbmr.3807. [DOI] [PubMed] [Google Scholar]
- 31.Turner MR, Camacho X, Fischer HD, Austin PC, Anderson GM, Rochon PA, et al. Levothyroxine dose and risk of fractures in older adults: nested case-control study. BMJ. 2011;342:d2238. doi: 10.1136/bmj.d2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–34. doi: 10.1080/03610910902859574. [DOI] [Google Scholar]
- 33.Majumdar SR, Morin SN, Lix LM, Leslie WD. Influence of recency and duration of glucocorticoid use on bone mineral density and risk of fractures: population-based cohort study. Osteoporos Int. 2013;24:2493–8. doi: 10.1007/s00198-013-2352-3. [DOI] [PubMed] [Google Scholar]
- 34.Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013;9:699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadji P, Aapro MS, Body JJ, Gnant M, Brandi ML, Reginster JY, et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017;7:1–12. doi: 10.1016/j.jbo.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morote J, Morin JP, Orsola A, Abascal JM, Salvador C, Trilla E, et al. Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology. 2007;69:500–4. doi: 10.1016/j.urology.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Barzi A, Hershman DL, Till C, Barlow WE, Ramsey S, Lenz HJ, et al. Osteoporosis in colorectal cancer survivors: analysis of the linkage between SWOG trial enrollees and Medicare claims. Arch Osteoporos. 2019;14:83. doi: 10.1007/s11657-019-0629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan NF, Mant D, Carpenter L, Forman D, Rose PW. Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: a database study. Br J Cancer. 2011;105:S29–37. doi: 10.1038/bjc.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor J, Xiao W, Abdel-Wahab O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood. 2017;130:410–23. doi: 10.1182/blood-2017-02-734541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim C, Bhatta S, Cyprien L, Fonseca R, Hernandez RK. Incidence of skeletal-related events among multiple myeloma patients in the United States at oncology clinics: observations from real-world data. J Bone Oncol. 2019;14:100215. doi: 10.1016/j.jbo.2018.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann NY Acad Sci. 2002;966:73–81. doi: 10.1111/j.1749-6632.2002.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 42.van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology. 2000;39:1383–9. doi: 10.1093/rheumatology/39.12.1383. [DOI] [PubMed] [Google Scholar]
- 43.Anargyrou K, Fotiou D, Vassilakopoulos TP, Christoulas D, Makras P, Dimou M, et al. Low bone mineral density and high bone turnover in patients with non-Hodgkin’s lymphoma (NHL) who receive frontline therapy: results of a multicenter prospective study. Hemasphere. 2019;3:e303. doi: 10.1097/HS9.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baech J, Hansen SM, Jakobsen LH, Ovlisen AK, Severinsen MT, Brown PN, et al. Increased risk of osteoporosis following commonly used first-line treatments for lymphoma: a Danish nationwide cohort study. Leuk Lymphoma. 2020;61:1345–54. doi: 10.1080/10428194.2020.1723015. [DOI] [PubMed] [Google Scholar]
- 45.Booth S, Plaschkes H, Kirkwood AA, Gibb A, Horgan P, Higham C, et al. Fractures are common within 18 months following first-line R-CHOP in older patients with diffuse large B-cell lymphoma. Blood Adv. 2020;4:4337–46. doi: 10.1182/bloodadvances.2020002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cabanillas ME, Lu H, Fang S, Du XL. Elderly patients with non-Hodgkin lymphoma who receive chemotherapy are at higher risk for osteoporosis and fractures. Leuk Lymphoma. 2007;48:1514–21. doi: 10.1080/10428190701471973. [DOI] [PubMed] [Google Scholar]
- 47.Paccou J, Merlusca L, Henry-Desailly I, Parcelier A, Gruson B, Royer B, et al. Alterations in bone mineral density and bone turnover markers in newly diagnosed adults with lymphoma receiving chemotherapy: a 1-year prospective pilot study. Ann Oncol. 2014;25:481–6. doi: 10.1093/annonc/mdt560. [DOI] [PubMed] [Google Scholar]
- 48.Svendsen P, Shekhrajka N, Nielsen KL, Vestergaard P, Poulsen MO, Vistisen AK, et al. R-CHOP(-like) treatment of diffuse large B-cell lymphoma significantly reduces CT-assessed vertebral bone density: a single center study of 111 patients. Leuk Lymphoma. 2017;58:1105–13. doi: 10.1080/10428194.2016.1233543. [DOI] [PubMed] [Google Scholar]
- 49.Thompson MA, Huen A, Toth BB, Vassilopoulou-Sellin R, Hoff AO, Murphy WA, et al. Osteopenia and osteoporosis in untreated non-Hodgkin’s lymphoma patients: an important and potentially treatable survivorship issue in lymphoma. J Clin Oncol. 2007;25:9055. doi: 10.1200/jco.2007.25.18_suppl.9055. [DOI] [Google Scholar]
- 50.Huang LW, Sun D, Link TM, Lang T, Ai W, Kaplan LD, et al. High incidence of fractures after R-CHOP-like chemotherapy for aggressive B-cell non-Hodgkin lymphomas. Support Care Cancer. 2021;29:5399–408. doi: 10.1007/s00520-021-06120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and providers (e.g. healthcare organisations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.