Abstract
Key Clinical Message
Three‐dimensional multiplanar analysis and real‐time three‐dimensional guidance using transesophageal echocardiography can help to identify and access the ideal position for transseptal puncture even in the presence of atrial septal occluders.
Abstract
Transseptal puncture (TSP) for the percutaneous mitral valve edge‐to‐edge repair (PMVR) after percutaneous atrial septal defect (ASD) closure is a rare and challenging issue. Here, we present a case illustrating the feasibility of real‐time three‐dimensional transesophageal echocardiographic guidance for TSP without ASD closure device injury.
Keywords: atrial septal defect closure, percutaneous mitral valve edge‐to‐edge repair, three‐dimensional transthoracic echocardiography, transseptal puncture
Three‐dimensional multiplanar analysis and real‐time three‐dimensional guidance using transesophageal echocardiography can help to identify and access the ideal position for transseptal puncture even in the presence of atrial septal occluders.
1. INTRODUCTION
Advances in transcatheter structural heart disease interventions have expanded the targeted transseptal approach to the left atrium (LA). Moreover, procedure‐oriented precise transseptal punctures are mandatory to optimize anatomical positioning. Transcatheter edge‐to‐edge repair using MitraClip requires a 4.0–4.5‐cm distance from the mitral annulus to the puncture line. However, the presence of a percutaneous ASD closure device restricts the puncture site, which makes the procedure difficult. Developing three‐dimensional analysis, including multiplanar reconstruction (MPR) and real‐time volume rendering (VR) using transesophageal echocardiography, enables both pre‐procedural planning and intraprocedural guidance. This report shared the usefulness of MPR analysis before the procedure and real‐time VR during transseptal puncture in patients with ASD closure devices.
2. CASE REPORT
2.1. History of presentation
A 69‐year‐old woman was referred to our institution for New York Heart Association class II heart failure symptoms. Her systolic blood pressure dropped from 130 mmHg to 90 mmHg during hemodialysis. In addition, transthoracic echocardiography (TTE) revealed moderate to severe mitral regurgitation (MR) with a dilated LA.
2.2. Past medical history
She had a history of hypertension, end‐stage renal failure requiring hemodialysis diagnosed 15 years ago, old cerebral infarction, permanent atrial fibrillation, right subclavian vein stenosis treated with stents, and ASD. At 65 years, ASD was percutaneously treated using a 32‐mm Amplatzer Septal Occluder (Abbott Vascular).
At 67 years, MR became moderate in severity without any symptoms, but the regurgitation progressed from moderate to severe during hospitalization.
2.3. Investigations
The baseline TTE showed a normal left ventricular ejection fraction of 65% with a mildly dilated end‐diastolic diameter of 50 mm and end‐systolic diameter of 32 mm. The left atrial diameter was dilated to 55 mm. Moreover, mitral valve leaflets showed non‐prolapsed, non‐tethering morphology. However, moderate to severe MR with the posteriorly directed jet was visualized with a proximal iso‐velocity surface area (PISA) of 0.39 cm2 and a regurgitant fraction of 64%. Therefore, MR etiology was diagnosed as atrial functional MR because she was complicated with permanent atrial fibrillation.
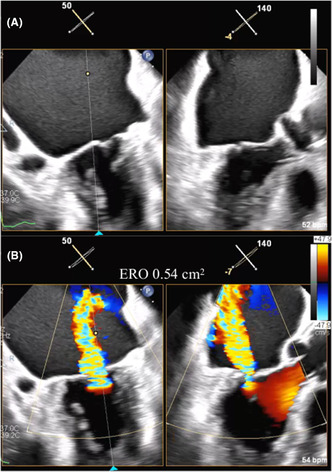
Repeated TTE revealed non‐prolapsed, non‐tethering mitral leaflets (flat valve) and severe MR with a PISA of 0.54 cm2 between A2 and P2 (Figure 1A). The mitral valve annulus was 33 mm in transverse dimensions and 28 mm in longitudinal dimensions on 3D multiplanar reconstruction (MPR). Moreover, the anterior and posterior mitral leaflet length was 24 mm and 8 mm, respectively, while the mitral valve opening area was 4.02 cm2.
FIGURE 1.
(A) Biplane view of bi‐commissure and left ventricular outflow tract showing a non‐prolapsed and non‐tethering leaflet morphology. (B) Color Doppler view showing wide regurgitant jet from central lesion.
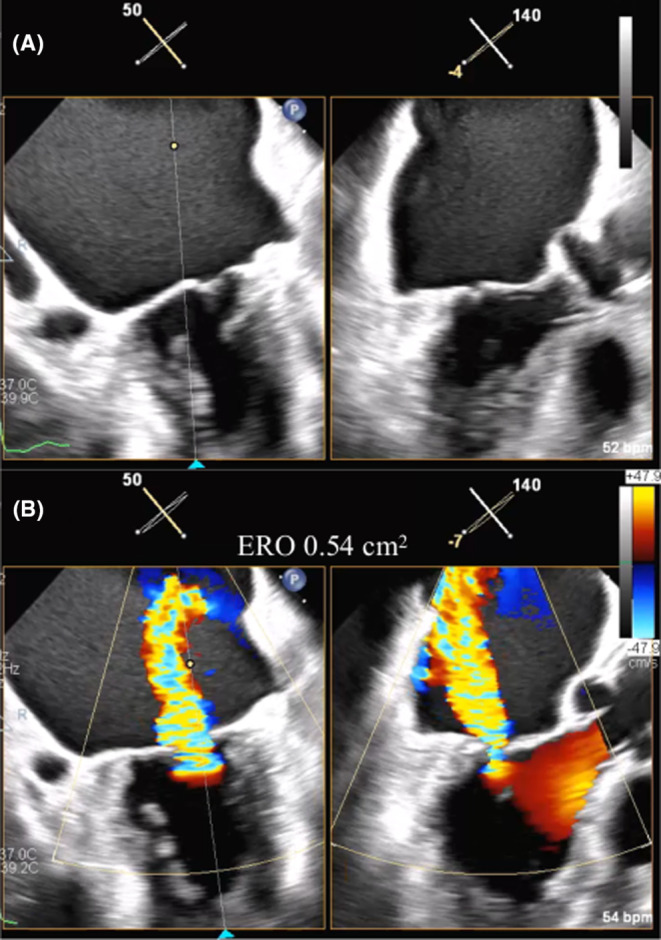
3D‐MPR analysis was used to estimate the best puncture point. Based on a four‐chamber view at 0°, the range of interest was arranged for the maximal thickness of the mitral valve and interatrial septum (IAS), and 3D data were obtained. The best transseptal puncture (TSP) point was estimated on the IAS, which was the cross point from the extended line at 40–45 mm from the mitral annulus (Figure 2A–C). This line should be 30° anteriorly from the transverse line of the A2P2‐closure (Figure 2D). A similar view was expressed in multidetector‐row computed tomography (Figure 2E). The demonstration device showed that the MitraClip was designed for a 30° anterior approach (Figure 2F). In this patient, the ideal puncture point was estimated to be 43 mm from the mitral annulus, within the fossa ovalis, and just out of the Amplatzer Septal Occluder. MitraClip (Abbott Vascular) was assumed to be safely applied.
FIGURE 2.
(A) Multiplanar reconstruction showing the best puncture point (red arrow) within the fossa ovalis on a four‐chamber view. (B) Bicaval view showing the best puncture point (red arrow) out of the ASO device. (C) Estimated best puncture point (red dot) on 3D volume rendering view. (D) Estimated puncture point showing a 30° anterior side from the A2 and P2 closing line. (E) CT enface view showing the puncture point (red dot) correlated with TEE volume rendering view. (F) Demo device showing a nearly 30° approach angle. ASO, Amplatzer Septal Occluder; CT, computed tomography; Inf, inferior; LA, left atrium; MV, mitral valve; RA, right atrium; Sup, superior; TEE, transoesophageal echocardiography.
The patient's general condition was frailty. Because of the impaired motor function of an old cerebral infarction, the 6‐min walking distance was only 60 m. Pulmonary function showed mixed disorder with a 68.4% (%VC) and 64.8% FEV1.0. The STS score for mitral valve replacement was 19.116%. The cardiovascular team decided to perform percutaneous mitral valve edge‐to‐edge repair (PMVR) because of the high surgical risk.
2.4. Management
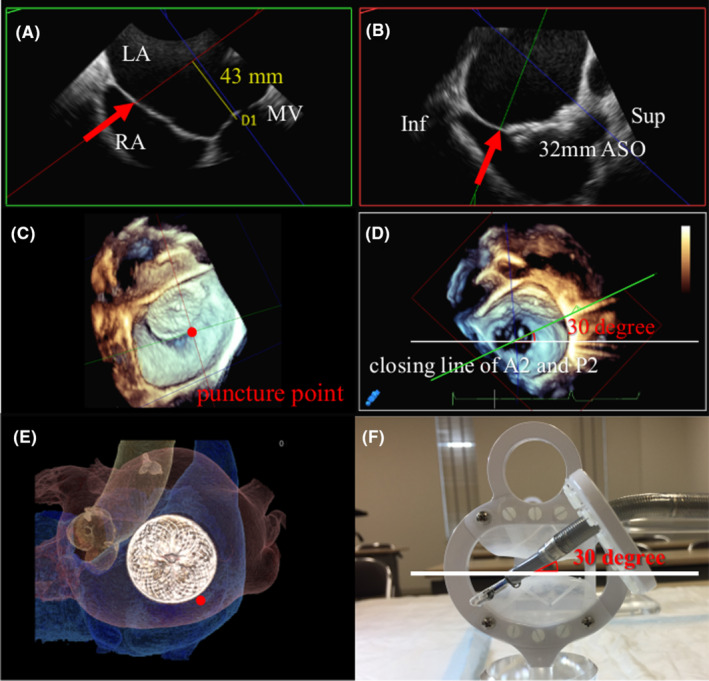
PMVR was performed under general anesthesia and TEE guidance. Real‐time 3D‐TEE was used for volume rendering (VR), and the puncture needle was guided to the estimated puncture point (Figure 3A). Real‐time VR imaging visualized the tenting point and guided the needle to move posteriorly to the estimated puncture point (Figure 3B). The point inferoposterior to the Amplatzer Septal Occluder was punctured under confirmation by TEE and fluoroscopy (Figure 3C). A steerable guide catheter approached the mitral valve at nearly 30° anteriorly, as estimated during the pre‐procedural TEE analysis (Figure 3D).
FIGURE 3.
(A) Intraprocedural real‐time 3D‐TEE volume rendering view showing puncture needle (red arrow) as tenting of the septum. (B) Puncture needle (red arrow) guided by real‐time 3D‐TEE to the posterior side. (C) Fluoroscopic view showing puncture needle (red arrow) at the inferoposterior side of the device. (D) Steerable guide catheter approaching the mitral valve at nearly 30° as estimated by the pre‐procedural TEE. ASO, Amplatzer Septal Occluder; SGC, steerable guide catheter; TEE, transoesophageal echocardiography.
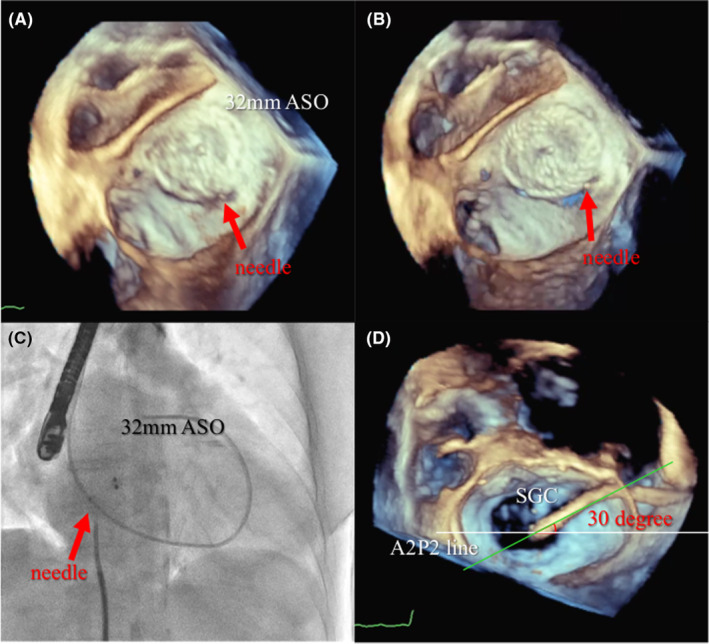
An NT clip was implanted because the mitral valve opening was borderline at 4.02 cm2, and leaflet degeneration due to hemodialysis was a concern (Figure 4A). After the clip was implanted at the A2 and P2, the MR decreased to mild (Figure 4B) with the mitral valve area of 1.80 cm2 and the transmitral mean pressure gradient (TMPG) of 2.7 mmHg (Figure 4C), avoiding mitral stenosis. The fluoroscopic view showed the guide catheter located inferoposterior to the Amplatzer device (Figure 4D).
FIGURE 4.
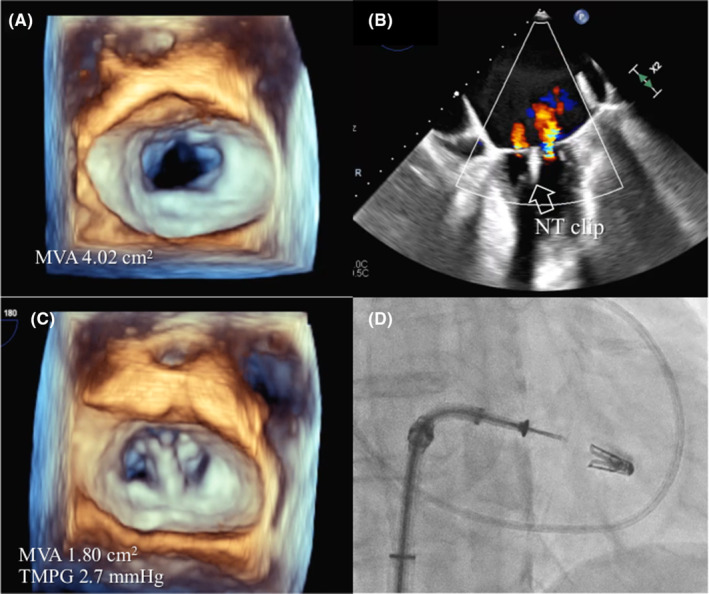
(A) 3D enface view of the mitral valve at baseline with an MVA of 4.02 cm2. (B) Mild residual regurgitant jet treated by one NT clip (white arrow). (C) Mitral valve resulting in MVA of 1.80 cm2 and TMPG of 2.7 mmHg. (D) Fluoroscopic view showing the inferoposterior relationship of the guide catheter. MVA, mitral valve area; TMPG, transmitral mean pressure gradient.
The procedure was typically performed to puncture the best point under TEE guidance without damage to the Amplatzer Septal Occluder.
3. DISCUSSION
TSP is the first step to a successful PMVR procedure. 1 The puncture site is usually posterior within the fossa ovalis and should be adjusted based on the clipping point on the mitral valve. 2 A three‐dimensional illustration is helpful for educational TSP imaging. However, TSP is performed under 2D‐TEE guidance. 3 The ideal 3D‐guided, case‐based TSP point has not been shown.
The ideal puncture point, determined by 3D analysis, was on the interatrial septum 30° anterior to A2P2's closure line at 40–45 mm from the estimated clipping point on the mitral leaflet.
TEE is one of the best interatrial septum imaging modalities. 4 Specifically, 3D‐MPR reconstruction is feasible to determine the best puncture point based on individual cases. Moreover, this point can be expressed with VR images.
This case of MR with ASD was treated with a large Amplatzer Septal Occluder. MPR analysis with 3D‐TEE helped determine the puncture point. Jonathan et al. reported the usefulness of computed tomography in PMVR procedures treated with Amplatzer Septal Occluders. 5 However, computed tomography images are not shown simultaneously on the monitor during the procedure. The 3D‐TEE movies can be displayed concurrently during the procedure to guide the septal puncture needle through VR images to the estimated best puncture point.
The mid‐posterior point on IAS is recommended to puncture in PMVR. 6 Conversely, the best puncture point varies according to cardiac morphology, such as an enlarged left atrium, or prior treatment, such as percutaneous ASD closure. The two‐dimensional guidance alone has limitations in such conditions. Despite computed tomography's ability to draw a clear image, they cannot visualize puncture needles during the procedure. MPR analysis and VR images using 3D‐TEE applied to the pre‐procedural analysis and intraprocedural guidance. Moreover, tissue tenting by the puncture needle is visualized on real‐time VR images to guide the puncture needle intuitively and safely.
4. CONCLUSIONS
This first case report demonstrated the usefulness of pre‐procedural analysis and real‐time 3D‐TEE‐guided TSP in a PMVR procedure after percutaneous ASD closure.
AUTHOR CONTRIBUTIONS
Hiroyuki Tabata: Writing – original draft. Akihiro Isotani: Writing – review and editing. Shinichi Shirai: Writing – review and editing. Kenji Ando: Writing – review and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors have no potential conflict of interest relevant to this article.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
None.
Tabata H, Isotani A, Shirai S, Ando K. Three‐dimensional transesophageal echocardiography‐guided transseptal puncture for percutaneous mitral valve edge‐to‐edge repair post‐percutaneous atrial septal defect closure. Clin Case Rep. 2023;11:e7794. doi: 10.1002/ccr3.7794
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
REFERENCES
- 1. Simard T, El Sabbagh A, Lane C, et al. Anatomic approach to transseptal puncture for structural heart interventions. JACC Cardiovasc Intv. 2021;14:1509‐1522. doi: 10.1016/j.jcin.2021.04.037 [DOI] [PubMed] [Google Scholar]
- 2. Alkhouli M, Rihal C, Holmes DR. Transseptal techniques for emerging structural heart interventions. JACC Cardiovasc Intv. 2016;9:2465‐2480. doi: 10.1016/j.jcin.2016.10.035 [DOI] [PubMed] [Google Scholar]
- 3. Radinovic A, Mazzone P, Landoni G, Agricola E, Regazzoli D, Della‐Bella P. Different transseptal puncture for different procedure: optimization of left atrial catheterization guided by transesophageal echocardiography. Ann Card Anaesth. 2016;19:589‐593. doi: 10.4103/0971-9784.191548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faletra FF, Nucifora G, Ho SY. Imaging the atrial septum using real‐time three‐dimensional transesophageal echocardiography: technical tups, normal anatomy, and its role in transseptal puncture. J Am Soc Echocardiogr. 2011;24:24593‐24599. doi: 10.1016/j.echo.2011.01.022 [DOI] [PubMed] [Google Scholar]
- 5. Yap J, Chen S, Stripe B, et al. Transseptal access for left heart structural interventions in the setting of prior atrial septal defect closure. Catheter Cardiovasc Interv. 2020;95:414‐419. doi: 10.1002/ccd.28548 [DOI] [PubMed] [Google Scholar]
- 6. Wunderlich NC, Siegel RJ. Peri‐interventional echo assessment for the MitraClip procedure. Eur Heart J Cardiovasc Imaging. 2013;14:935‐949. doi: 10.1093/ehjci/jet060 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.


