Abstract
Background
Optical coherence tomography (OCT) is reported to be a feasible and safe imaging modality for the guidance of percutaneous coronary intervention (PCI) of complex lesions.
Methods
This multicenter, prospective registry assessed the minimum stent area (MSA) achieved under OCT guidance. A performance goal of 24% improvement in MSA over and above the recommendation set by the European Association of Percutaneous Cardiovascular Interventions Consensus 2018 (4.5 mm2 MSA for non-left main and 3.5 mm2 for small vessels). The incidence of contrast-induced nephropathy was also assessed. Core lab analysis was conducted.
Results
Five hundred patients (average age: 59.4 ± 10.1 years; 83% males) with unstable angina (36.8%), NSTEMI (26.4%), and STEMI (22%) were enrolled. The primary endpoint was achieved in 93% of lesions with stent diameter ≥2.75 mm (average MSA: 6.44 mm2) and 87% of lesions with stent diameter ≤2.5 mm (average MSA: 4.56 mm2). The average MSA (with expansion ≥80% cutoff) was 6.63 mm2 and 4.74 mm2 with a stent diameter ≥2.75 mm and ≤2.5 mm, respectively. According to the core lab analysis, the average MSA achieved with a stent diameter ≥2.75 mm and ≤2.5 mm was 6.23 mm2 and 3.95 mm2, respectively (with expansion ≥80% cutoff). Clinically significant serum creatinine was noted in two patients (0.45%). Major adverse cardiac events at 1 year were noted in 1.2% (n = 6) of the patients; all were cardiac deaths.
Conclusion
PCI under OCT guidance improves procedural and long-term clinical outcomes in patients with complex lesions not just in a controlled trial environment but also in routine clinical practice.
Keywords: Optical coherence tomography, Percutaneous coronary intervention, Complex lesions, Acute kidney injury, Minimum stent area, Clinical outcomes
1. Introduction
Optical coherence tomography (OCT) is considered a safe and feasible imaging modality for PCI guidance of coronary lesions, including complex lesions in calcific and tortuous vessels.1 Preprocedural evaluation of vessel and lumen dimensions along with the characterization of the lesions, can help facilitate accurate stent sizing and guide the stenting strategy. Postprocedural imaging enables strut-level evaluation of the stent result as well as guides PCI optimization.2
The role of OCT guidance in improving clinical outcomes has been reported in several clinical studies.3, 4, 5 The CLI-OPCI study, one of the first studies to evaluate OCT-guided PCI, suggested that OCT-guided PCI significantly reduced the rate of cardiac death, major adverse cardiac events (MACE), and the composite of myocardial infarction, cardiac death, or repeat revascularization when compared to angiography-guided PCI.6 A large cohort study involving the Pan-London (United Kingdom) PCI registry, concluded that OCI-guided PCI improved in-hospital events, procedural outcomes, and long-term survival.7
However, the utilization of intracoronary OCT is quite limited in several countries, including India. Some of the factors attributed to this include complexity in image interpretation, lack of standardized PCI guidance algorithm, transition difficulty (from intravascular ultrasound [IVUS] to OCT), and paucity of data.8 According to a 2018 report by the National Interventional Council, India), a considerable number of stents were deployed and PCIs were performed. However, adjunctive imaging modalities such as IVUS or OCT were used in only about 4% of these cases.9 Although OCT has been available in India for the last few years, literature on the use and benefits of OCT for guiding PCI is limited to single-center studies and case report series. There is also a paucity of data on the safety and effectiveness of OCT-guided PCI with DES implantation in complex lesions in Indian patients in the real-world setting. The current study was hence conducted to assess the effectiveness and safety of OCT-guided PCI in Indian patients with complex lesions.
2. Methods
2.1. Study design and patient population
This was a real-world, multicenter, prospective registry that enrolled 500 patients with complex lesions scheduled to undergo OCT-guided PCI between April 2019 and February 2020 across 16 sites in India with a follow-up period of 1 year.
The study enrolled patients aged ≥18 years with the following criteria: patients scheduled for a clinically indicated PCI procedure with medically treated diabetes mellitus and/or angiographically detected high-risk lesions, with at least one target lesion (i.e., culprit lesion responsible for either NSTEMI or STEMI >24 h from the onset of ischemic symptoms) in the planned target vessel; long or multiple lesions, bifurcation lesion with a side branch ≥2.5 mm by visual estimation; angiographic severe calcification; or in-stent restenosis. Patients with bypass graft stenoses, renal insufficiency (estimated glomerular filtration rate <50 mL/min/m2) or any other severe medical condition interfering with patients’ safety, contraindications to dual-antiplatelet therapy up to 1 year, and other comorbidities that may limit life expectancy to less than 1 year were excluded.
2.2. Procedure
The study was initiated after obtaining ethical committee approvals from respective institutions (CTRI Reg No. CTRI/2019/03/018301). All patients scheduled for a clinically indicated PCI procedure underwent diagnostic angiography to assess their suitability for inclusion in the study. OCT-guided PCI was performed on all patients; both before and after PCI, by experienced clinicians as per the standard methodology (EAPCI consensus guidelines I and II).2,10 OCT was performed using Dragonfly™ OPTIS™ imaging catheter (St. Jude Medical, CA). A contrast agent was used to clear the blood.
2.3. Core lab analysis
A designated core lab (Indian Cardiology Research Foundation, Chennai, India) was identified to independently review the OCT images. The core lab analyzed the data of 60% of patients, which were randomly selected from the enrolled patient population. Of this 60% of cases, ∼4% of cases were not analyzable as per the specifications/requirements of the core lab.
2.4. Enrolment and follow-up
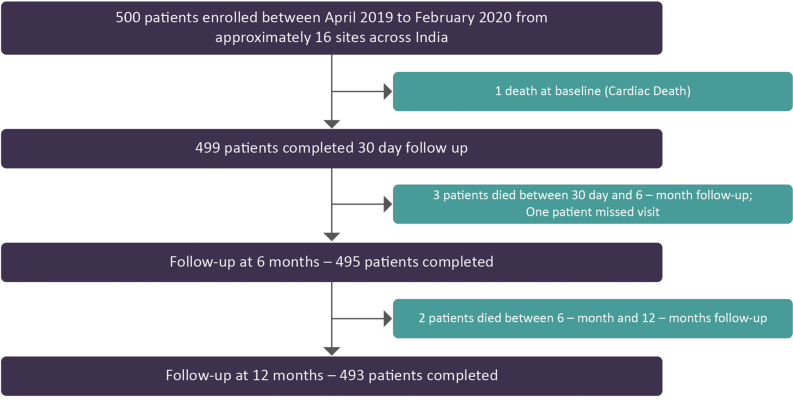
All the enrolled patients were followed up either telephonically or clinically at 30 days, 6 months and 1 year. Data related to study enrollment and follow-up are summarized in Supplementary Fig. 1.
2.5. Study hypothesis/design
According to the criteria for assessing optimal stent results in the consensus document of the European Association of Percutaneous Cardiovascular Interventions (EAPCI), a minimum stent area (MSA) of >5.5 mm2 by IVUS and >4.5 mm2 by OCT should be achieved in non-left main (LM) lesions. Further, a relative stent expansion of >80% (MSA divided by average reference lumen area) should be obtained in routine clinical practice.2 Additionally, according to a retrospective analysis of OCT MSA in small coronary arteries, the cutoff values of postintervention OCT MSA should be 3.5 mm2.11
Hence, it was considered that an improvement of acute performance in an OCT-guided PCI in complex lesions could be evaluated based on a performance goal set as per the ILUMIEN III study outcomes and EAPCI consensus document recommendations. Accordingly, a performance goal of 24% improvement in MSA 4.5 mm2 MSA for non-LM and 3.5 mm2 for small vessels was set.
2.6. Endpoints/objectives
The primary objectives were to assess the minimum stent area after OCT-guided PCI and the incidence of acute kidney injury (AKI) after OCT-guided PCI due to contrast-induced nephropathy (CIN). The secondary objectives were to assess the rate of major adverse cardiac events (MACE), cardiac death, and myocardial infarction (MI) at 30 days, 6 months, and 1 year.
| Definitions |
| Ostial lesion is defined as “a lesion involving within 3 mm of the origin of the vessel.”12 |
| Long or multiple lesions are defined as “intended total stent length in any single target vessel ≥28 mm”13 |
| Bifurcation lesion is defined as “a lesion with side branch ≥2.5 mm by visual estimation, intended to be treated with planned two-stent strategy (stenting in both the main branch and the side branch).”13 |
| Chronic total occlusion is defined as “a total occlusion with either a known duration of more than 3 months or the presence of bridging collaterals.”14 |
| Angiographic severe calcification is defined as “angiographically visible calcification on both sides of the vessel wall in the absence of cardiac motion.”13 |
| In-stent restenosis is defined as “a stenosis within the stented segment or its edge (5-mm segments adjacent to the stent) of >50% of the vessel diameter as determined by coronary angiography.”15 |
| Target vessel revascularization is defined as “any repeat percutaneous intervention or surgical bypass of any segment of the target vessel including the target lesion.”16 |
2.7. Data collection
Patient demographics, medical history, clinical presentation, and lesion and vessel characteristics were recorded at baseline. The following preprocedural variables were collected during the procedure as assessed by angiography and OCT: need for predilatation and intended need for lesion debulking (by means of scoring/cutting balloons and rotablation). Before PCI, diameter stenosis, proximal and distal vessel reference diameter, lesion length, intended stent length and diameter, and minimal lumen diameter were captured, while stent underexpansion, malapposition, lesion coverage, edge dissection, and tissue prolapse were assessed by OCT post-PCI, and documented. The need for post-PCI optimization was decided based on OCT findings by the treating physician. In this study, the participating sites also recorded the procedure time, radiation time, and contrast volume. At the time of follow-up (30 days, 6 months and 1 year), ongoing patient status including any repeat procedures was recorded.
2.8. Statistical analysis
No formal sample size was calculated as it was a single-arm observational registry. All the categorical variables were presented as numbers and percentages, and continuous data as mean and standard deviation. For primary endpoint analysis, data of 500 patients (involving 539 lesions) reported by sites were considered.
3. Results
3.1. Baseline characteristics
A total of 500 patients (average age: 59.4 ± 10.1 years; 83% males) with primarily unstable angina (36.8%), non-ST-segment elevation myocardial infarction (NSTEMI; 26.4%), and ST-segment elevation myocardial infarction (STEMI; 22%) were enrolled (Table 1). More than 50% of patients had diabetes and hypertension at baseline.
Table 1.
Baseline and lesion characteristics.
| Baseline demographics (N = 500) | N% |
|---|---|
| Female | 84 (17%) |
| Male | 416 (83%) |
| Average age (years) | 59.4 ± 10.1 |
| Medical History | |
| History of diabetes mellitus | 269 (53.8%) |
| Hypertension | 265 (53%) |
| Dyslipidemia | 77 (15%) |
| Previous PCI | 71 (14.2%) |
| Family history of CAD | 56 (11.2%) |
| Previous myocardial infarction | 45 (9%) |
| Previous CABG | 10 (2%) |
| Renal disease/dysfunction | 5 (0.8%) |
| Peripheral artery disease | 4 (0.8%) |
| Stroke | 3 (0.6%) |
| Bronchial asthma | 3 (0.6%) |
| COPD | 3 (0.6%) |
| Clinical Presentation (N = 500) | |
| Unstable angina | 184 (36.8%) |
| NSTEMI | 132 (26.4%) |
| Recent STEMI | 110 (22%) |
| Stable angina | 33 (6.6%) |
| Asymptomatic positive stress test | 12 (2.4%) |
| Silent ischemia | 4 (0.8%) |
| Others | 25 (5%) |
| Lesion characteristics | |
| Ostial lesion | 94 (17.4%) |
| Long lesion (>28 mm) | 444 (82.4%) |
| Average lesion length, mm | 34.2 ± 14.1 |
| In-stent restenosis | 62 (11.5%) |
| Thrombus present | 60 (11.1%) |
| Chronic total occlusion | 12 (2.2%) |
| Bifurcation lesion | 69 (12.8%) |
| Diffuse disease | 163 (30.2%) |
| Other lesion complexity (if any) | 33 (6.1%) |
| Calcification status of lesion | 299 (55.5%) |
| Mild | 178 (33.0%) |
| Moderate | 68 (12.6%) |
| Severe | 53 (9.8%) |
| Lesion complexity as per ACC/AHA | |
| A | 69 (12.8%) |
| B1 | 180 (33.4%) |
| B2 | 130 (24.1%) |
| C | 160 (29.7%) |
| TIMI flow | |
| I | 42 (7.8%) |
| II | 174 (32.3%) |
| III | 323 (60.0%) |
| Target lesion distribution (n = 554) | |
| Left anterior descending artery | 366 (66.1%) |
| Right coronary artery | 103 (19.1%) |
| Left circumflex artery | 58 (10.5%) |
| Left main artery | 23 (4.2%) |
| Ramus intermedius | 4 (0.7%) |
CABG: Coronary artery bypass grafting; CAD: Coronary artery disease; COPD: Chronic obstructive pulmonary disease; NSTEMI: Non-ST-segment elevation myocardial infarction; PCI: Percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction. ACC/AHA: American College of Cardiology and the American Heart Association; TIMI: Thrombolysis in myocardial infarction.
3.2. Lesion characteristics
Long lesions (>28 mm) were noted in 82% of the cases with an average length of 34.2 mm (Table 1) Diffuse disease was observed in 30% of the cases and bifurcation lesions in about 13% of the cases. Target lesions mainly involved the left anterior descending artery (66%) and right coronary artery (19.1%). About 55% of lesions were calcified, with mild calcification noted in 33% of cases. Thrombolysis in myocardial infarction (TIMI) flow grade III was observed in 60% of the cases.
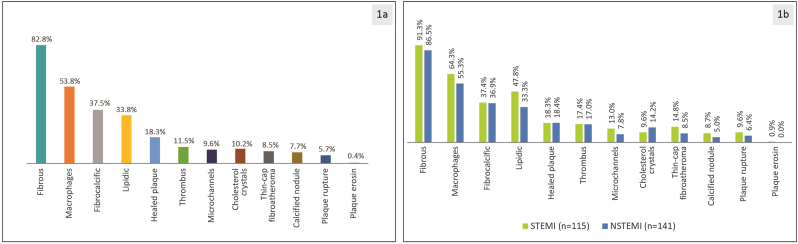
Further analysis of the lesions revealed fibrous plaque in 82.8% of the cases with the presence of macrophages in 53.8% of the overall lesions (Fig. 1a). Other major findings included fibrocalcification (37.5%), lipidic plaque (33.8%), healed plaque (18.3%), thrombus (11.5%), and cholesterol crystals (10.2%). Similarly, fibrous plaque was the common characteristic in 91.3% and 86.5% of the lesions among patients with STEMI and NSTEMI, respectively (Fig. 1b).
Fig. 1.
Lesion morphology (n = 312 lesions) a) overall b) in recent STEMI and NSTEMI as assessed by the core lab.
3.3. Primary endpoint
There were 127 lesions involving small vessels (≤2.5 mm) and 412 lesions involving large vessels (≥2.75 mm) (Table 2). The primary endpoint was achieved in 93% of lesions with a stent diameter ≥2.75 mm (average MSA: 6.44 mm2; n = 412) and in 87% of lesions with a stent diameter ≤2.5 mm (average MSA: 4.56 mm2; n = 127), with an average expansion of 101.53% and 97.10%, respectively (Table 2). The average MSA (with expansion ≥80% cutoff) was 6.62 mm2 and 4.74 mm2 with a stent diameter ≥2.75 mm and ≤2.5 mm, respectively.
Table 2.
Procedural characteristics, contrast volume, procedure time, and radiation exposure during the procedure.
| MSA Characteristics | Site reported | Analyzed by Core lab |
|---|---|---|
| Total lesions | 539 | 312 |
| Stent implanted ≥2.75 mm | ||
| Total lesions | 412 | 237 |
| Average MSA (mm2) | 6.44 | 6.04 |
| Average expansion (%) | 101.46 | 88.23 |
| Average MSA (with average expansion ≥80%) | 6.63 | 6.23 |
| Stent implanted ≤2.5 mm | ||
| Total lesions | 127 | 75 |
| Average MSA (mm2) | 4.56 | 4.00 |
| Average expansion (%) | 97.10 | 84.88 |
| Average MSA (with average expansion ≥80%) |
4.74 |
3.95 |
|
Other Characteristics |
Patients 1–250 |
Patients 251–500 |
| Contrast (mL); mean ± SD | ||
| Contrast used for angioplasty | 137.2 ± 69 | 154.3 ± 78.5 |
| Contrast used for OCT | 43.9 ± 21.6 | 49.5 ± 36.1 |
| Total contrast used | 181.1 ± 76.4 | 203.2 ± 82.4 |
| Radiation exposure; mean ± SD | ||
| CAK (mGy) | 3678.2 ± 5885.0 | 4498.0 ± 7675.2 |
| DAP (mGy-cm2) | 18825.9 ± 49585.5 | 52382.9 ± 112046.8 |
| Procedure time (min), mean ± SD | 74.4 ± 39 | 68.8 ± 37.5 |
CAK: Cumulative air kerma; DAP: Dose area product; OCT: Optical coherence tomography; SD: standard deviation. Note—Data for one lesion missing; MSA: Minimum stent area.
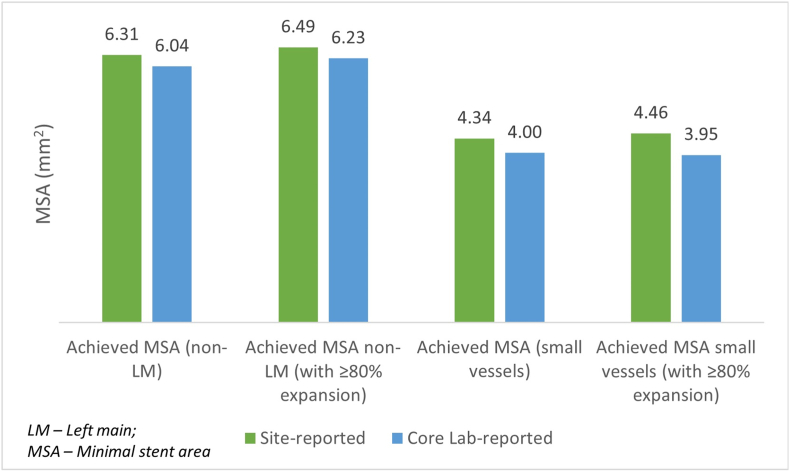
According to the core lab analysis, the average MSA achieved with a stent diameter ≥2.75 mm and ≤2.5 mm was 6.23 mm2 and 3.95 mm2, respectively (with expansion ≥80% cutoff). Fig. 2 provides a comparison of the site- and core lab-reported MSA of the same set of lesions (n = 312).
Fig. 2.
Comparison of MSA for site-reported and core lab-reported data (n = 312 lesions).
3.4. Acute kidney injury
Clinically significant serum creatinine level was noted only in 2 patients (0.45%), with 99% of patients free from CIN.
3.5. Secondary endpoints
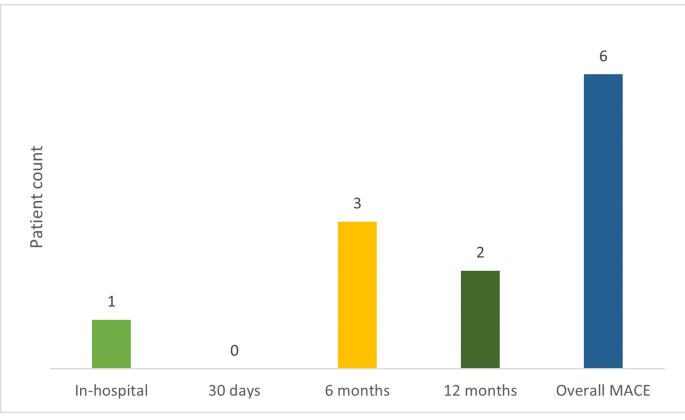
The incidence of MACE at 1 year (aggregate) was 1.2% (n = 6); all were cardiac deaths (Supplementary Fig. 2). The mean (±standard deviation [SD]) total contrast used in groups 1 (patients 1–250) and 2 (patients 251–500) was 181.1 (±76.4) mL and 203.2 (±82.4) mL, respectively (Table 2). The mean (±SD) radiation exposure in terms of cumulative air kerma was 3678.2 (±5885.0) mGy and 4498.0 (±7675.2) mGy, respectively. The mean (±SD) procedure time in groups 1 and 2 was 74.4 (±39) minutes and 68.8 (±37.5) minutes, respectively.
3.6. Impact of OCT
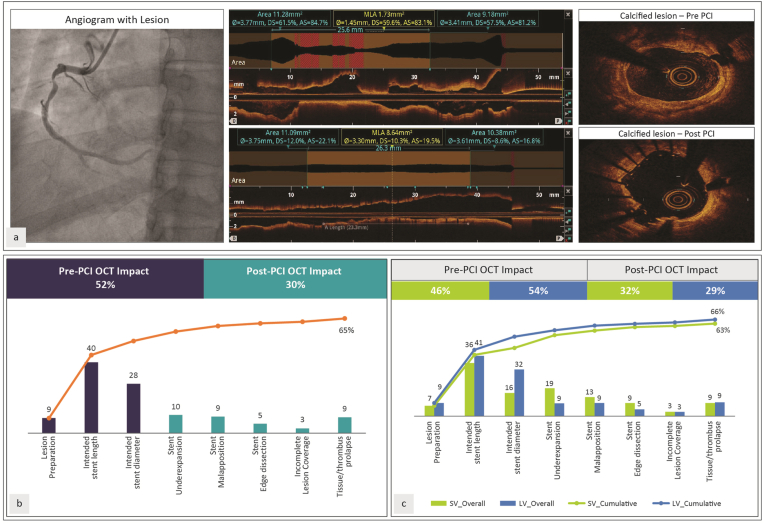
Overall strategy change following OCT was noted in 65% of the lesions (including preprocedure change in 52% and postprocedure change in 30%) (Fig. 3b). Strategy change following OCT was noted in 63% of the small vessel lesions and 66% of the lesions involving large vessels (Fig. 3c). Postprocedure optimization was noted in 32% of lesions in small vessels compared to 29% in large vessels.
Fig. 3.
(a) An illustrative case of Complex Lesion. (b) Impact of OCT in all vessels [Overall] (c) Impact of OCT in small (≤2.5 mm) and large (≥2.75 mm) vessels.
4. Discussion
Although available in India, the use of OCT for guiding PCI is limited. The existing evidence base for Indian settings includes single-center studies and isolated case reports. There is a lack of real-world insights on the effectiveness and safety of OCT-guided PCI for the management of complex lesions. In order to bridge the evidence gap contributing to the low adoption rate, this multi-site study was undertaken. This was the first Indian study that assesses the use of OCT in busy catheterization laboratories to improve procedural outcomes and establish that OCT-guided PCI is safe and effective in Indian settings for the management of complex lesions.
The primary objective of the current study was to assess the MSA after OCT-guided PCI and the incidence of CIN. Additionally, the incidence of MACE, cardiac death, and MI at 30 days, 6 months, and 1 year were also evaluated.
OCT guidance in complex PCI cases can help optimize stent expansion and detect edge dissections better than CA guidance.17,18 Furthermore, there is strong evidence supporting the use of intravascular imaging for stent placements in complex lesions and patients with ACS.2
Numerous intravascular ultrasound-based studies have consistently suggested that a stent cross-sectional area of 5.5 mm2 best discriminates subsequent events in non-left main lesions.19,20 Recent IVUS trials have reported a very low adverse event rate (1.5% within 1 year) in cases where MSA was greater than the distal reference lumen area.21 Based on the literature available and expert consensus, the EAPCI considered a cut-off of >80% for MSA (relative to average reference lumen area) to be a reasonable target to be achieved in clinical practice.2 In the current study, the criteria set by EAPCI guidelines for assessing optimal stent results with OCT in non-LM lesions was achieved in 93% of lesions with a stent diameter ≥2.75 mm (average MSA: 6.44 mm2) and in 87% of lesions with a stent diameter ≤2.5 mm (average MSA: 4.56 mm2), with an average expansion of 101.53% and 97.10%, respectively. Further, a relative stent expansion of >80% was also achieved in the current study. Stent expansion >80% is vital for optimizing PCI to decrease the risk of post-stent complications. OCT guidance in the current study was accordingly useful in optimizing treatment and led to an overall strategy change in 65% of the lesions involving small and large vessels. One of the major factors influenced by OCT guidance was the intended stent length for the pre-PCI treatment strategy and underexpansion for the post-PCI treatment strategy. Post-PCI OCT is beneficial in assessing this aspect owing to higher-resolution imaging capacity and semi-automated imaging analysis.22 The potential benefits of OCT-guided PCI have been proven in several clinical studies. In the ILUMIEN I trial, OCT was successfully used before PCI to guide clinical decision-making and modify the treatment strategy; and after PCI for the detection of stent under-expansion and malapposition to help guide additional postdilatation and stent implantation.23
According to the ULTIMATE trial which evaluated the 3-year outcome of IVUS-guided DES implantation, significantly lower rates of TVF and stent thrombosis were noted with IVUS-guided DES implantation compared to angiographic guidance.24 A strong recommendation for optimizing stenting was based on the outcomes noted in the IVUS EXCEL trial. Small final MSA (as evaluated by IVUS) after LM PCI was associated with an increased risk of adverse events (death, MI, and stent thrombosis).25 These studies highlight the beneficial role of intravascular imaging in guiding PCI procedures, especially those involving complex lesions.
Local interpretation of imaging modalities reflects the actual clinical practice but may be influenced by the expertise of the reader, risk averseness, and ability to read through calcification and other artifacts. Additionally, the knowledge of clinical history may lead to interpretation bias. Central core lab analysis is hence used to achieve a standardized assessment that is blinded to clinical information.26 In the current study, there was no major difference in the MSA values as evaluated by site evaluation and core lab analysis. It can, therefore, be suggested that the site's findings are a reliable indicator of the outcomes observed in the current study.
CIN is a common concern with OCT-guided PCI as the volume of contrast required during OCT-guided PCI can be high. The total volume of contrast agent used in the current study was as high as 203.2 ± 82.4 mL. However, a significant serum creatinine level was noted only in 0.45% of patients, indicating the safety of the procedure in patients with complex lesions. In a study that compared the incidence of decline in kidney function (DKI) in OCT-guided or IVUS-guided PCI, the incidence of acute and sustained DKI was comparable between the two groups, although the use of contrast agents was higher in the OCT group among patients with ACS.27 The results from this study show that the OCT-guided PCI in complex lesions was associated with very low rates of MACE (1.2%) at 1 year. This substantiates the safety of OCT in complex lesions. A meta-analysis that evaluated the role of imaging in PCI involving bare metal stents and DES showed that imaging guidance significantly lowered the incidence of death from all causes (odds ratio: 0.727; 95% confidence interval: 0.540–0.980; p < 0.01), along with the risk of MI and stent thrombosis.28 Another meta-analysis that compared the clinical outcomes following OCT-guided PCI with CA-guided PCI and IVUS-guided PCI reported that OCT-guided PCI was associated with reduced adverse events in terms of a composite of cardiac deaths, myocardial infarction, and repeat revascularizations, compared to CA-guided PCI. The outcomes were comparable with IVUS guidance.29
4.1. Limitations
The study is limited by its observational, nonrandomized design with no prospective head-to-head comparison of the data. Further, the sites had different levels of experience using OCT in daily practice which may have influenced patient selection, this being a real-world study. Although the operators were asked to follow standard procedure/guidelines during OCT and PCI, individual variations may have occurred, which may have influenced the outcomes. Additionally, the core lab data may not entirely reflect the findings that were recorded on-site. The cost-effectiveness of this approach was also not determined, which is an important aspect in developing countries like India.
5. Conclusion
OCT-guided PCI facilitates the achievement of guideline-recommended procedural outcomes and long-term clinical outcomes in patients with complex lesions. It is safe and effective not only in a controlled trial environment but also in routine clinical practice. The incidence of AKI was very low.
5.1. What is already known?
The use of OCT to improve procedural outcomes in clinical trial settings has been established; however, the replication of guidelines prescribed in real-world/clinical settings remains unknown.
5.2. What does this study add?
The current study evaluates the effectiveness and safety of OCT-guided PCI in Indian patients with complex lesions and concluded that OCT-guided PCI improves procedural outcomes among these patients and can be safely used in routine clinical practice in India. The guidelines prescribed criteria can be successfully incorporated in the real world. 99% of patients in this study were free from CIN.
Funding
The study has been funded by St. Jude Medical India Pvt. Ltd (now Abbott). The funding agency has no role in the study design, execution, analysis, and interpretation of results.
Declaration of competing interest
Authors MN and NEJW are employees of Abbott. The rest of the authors declare that they do not have any conflict of interest.
Acknowledgments
The authors would like to thank all study staff (co-investigators and research coordinators) for their support in executing the study and St. Jude Medical India Pvt. Ltd. (now Abbott) for funding the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2023.05.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
figs2.
References
- 1.Patted S., Porwal S., Ambar S., et al. One-year cardiovascular outcomes in patients treated with OCT-guided coronary angioplasty with stenting – a single centre study. J Cardiovasc Dis Diagn. 2000;8 [Google Scholar]
- 2.Räber L., Mintz G.S., Koskinas K.C., et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2018;14:656–677. doi: 10.4244/EIJY18M06_01. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto M.H., Kondo S., Mizukami T., et al. TACTICS investigators. Rationale and design of the TACTICS registry: optical coherence tomography guided primary percutaneous coronary intervention for patients with acute coronary syndrome. J Cardiol. 2022;80:505–510. doi: 10.1016/j.jjcc.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Meneveau N., Souteyrand G., Motreff P., et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS study (does optical coherence tomography optimize results of stenting) Circulation. 2016;134:906–917. doi: 10.1161/CIRCULATIONAHA.116.024393. [DOI] [PubMed] [Google Scholar]
- 5.Lee J.M., Choi K.H., Song Y.B., et al. On behalf of the RENOVATE-COMPLEX-PCI investigators. Intravascular imaging–guided or angiography-guided complex PCI. N Engl J Med. 2023;388:1668–1679. doi: 10.1056/NEJMoa2216607. [DOI] [PubMed] [Google Scholar]
- 6.Prati F., Di Vito L., Biondi-Zoccai G., et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention. 2012;8:823–829. doi: 10.4244/EIJV8I7A125. [DOI] [PubMed] [Google Scholar]
- 7.Jones D.A., Rathod K.S., Koganti S., et al. Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention: outcomes from the pan-london PCI cohort. JACC Cardiovasc Interv. 2018;11:1313–1321. doi: 10.1016/j.jcin.2018.01.274. [DOI] [PubMed] [Google Scholar]
- 8.Ali Z.A., Karimi Galougahi K., Mintz G.S., Maehara A., Shlofmitz R.A., Mattesini A. Intracoronary optical coherence tomography: state of the art and future directions. EuroIntervention. 2021;17:e105–e123. doi: 10.4244/EIJ-D-21-00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arramraju S.K., Janapati R.K., Sanjeeva Kumar E., et al. National interventional council data for the year 2018-India. Indian Heart J. 2020;72:351–355. doi: 10.1016/j.ihj.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson T.W., Räber L., di Mario C., et al. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2019;40:2566–2584. doi: 10.1093/eurheartj/ehz332. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo Y., Kubo T., Aoki H., et al. Optimal threshold of postintervention minimum stent area to predict in-stent restenosis in small coronary arteries: an optical coherence tomography analysis. Cathet Cardiovasc Interv. 2016;87:E9–E14. doi: 10.1002/ccd.26143. [DOI] [PubMed] [Google Scholar]
- 12.Lakhani M., Rajamanickam A., Kini A. In: Practical Manual of Interventional Cardiology. Kini A., Sharma S., Narula J., editors. Springer; London: 2014. Ostial lesion interventions; pp. 161–165. [Google Scholar]
- 13.Ali Z., Landmesser U., Karimi Galougahi K., et al. Optical coherence tomography-guided coronary stent implantation compared to angiography: a multicentre randomised trial in PCI - design and rationale of ILUMIEN IV: optimal PCI. EuroIntervention. 2021;16:1092–1099. doi: 10.4244/EIJ-D-20-00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senguttuvan N.B., Rao R.S., Kini A. In: Practical Manual of Interventional Cardiology. Kini A., Sharma S., Narula J., editors. Springer; London: 2014. Chronic total occlusions; pp. 177–186. [Google Scholar]
- 15.Her A.Y., Shin E.S. Current management of in-stent restenosis. Korean Circ J. 2018;48:337–349. doi: 10.4070/kcj.2018.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Garcia H.M., McFadden E.P., Farb A., et al. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Eur Heart J. 2018;39:2192–2207. doi: 10.1093/eurheartj/ehy223. [DOI] [PubMed] [Google Scholar]
- 17.Meneveau N., Souteyrand G., Motreff P., et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non–ST-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS study (Does Optical Coherence Tomography Optimize Results of Stenting) Circulation. 2016;134:906–917. doi: 10.1161/CIRCULATIONAHA.116.024393. [DOI] [PubMed] [Google Scholar]
- 18.Chamié D., Bezerra H.G., Attizzani G.F., et al. Incidence, predictors, morphological characteristics, and clinical outcomes of stent edge dissections detected by optical coherence tomography. JACC Cardiovasc Interv. 2013;6:800–813. doi: 10.1016/j.jcin.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Hong M.-K., Mintz G.S., Lee C.W., et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305–1310. doi: 10.1093/eurheartj/ehi882. [DOI] [PubMed] [Google Scholar]
- 20.Doi H., Maehara A., Mintz G.S., et al. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: an integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. J Am Coll Cardiol Intv. 2009;2:1269–1275. doi: 10.1016/j.jcin.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Mintz G.S., Guagliumi G. Intravascular imaging in coronary artery disease. Lancet. 2017;390:793–809. doi: 10.1016/S0140-6736(17)31957-8. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura D., Wijns W., Price M.J., et al. New volumetric analysis method for stent expansion and its correlation with final fractional flow reserve and clinical outcome: an ILUMIEN I substudy. JACC Cardiovasc Interv. 2018;11:1467–1478. doi: 10.1016/j.jcin.2018.06.049. [DOI] [PubMed] [Google Scholar]
- 23.Wijns W., Shite J., Jones M.R., et al. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J. 2015;36:3346–3355. doi: 10.1093/eurheartj/ehv367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao X.F., Ge Z., Kong X.Q., et al. 3-Year outcomes of the ULTIMATE trial comparing intravascular ultrasound versus angiography-guided drug-eluting stent implantation. JACC Cardiovasc Interv. 2021;14:247–257. doi: 10.1016/j.jcin.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Hunter G.W., Sharma V., Varma C., et al. The EXCEL trial: the interventionalists' perspective. Eur Cardiol. 2021;16:e01. doi: 10.15420/ecr.2020.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu M.T., Meyersohn N.M., Mayrhofer T., et al. Central core laboratory versus site interpretation of coronary CT angiography: agreement and association with cardiovascular events in the PROMISE trial. Radiology. 2018;287:87–95. doi: 10.1148/radiol.2017172181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemoto T., Minami Y., Sato T., et al. Contrast volume and decline in kidney function in optical coherence tomography-guided percutaneous coronary intervention. Int Heart J. 2019;60:1022–1029. doi: 10.1536/ihj.18-565. [DOI] [PubMed] [Google Scholar]
- 28.Alsidawi S., Effat M., Rahman S., Abdallah M., Leesar M. The role of vascular imaging in guiding routine percutaneous coronary interventions: a meta-analysis of bare metal stent and drug-eluting stent trials. Cardiovasc Ther. 2015;33:360–366. doi: 10.1111/1755-5922.12160. [DOI] [PubMed] [Google Scholar]
- 29.Kuku K.O., Ekanem E., Azizi V., et al. Optical coherence tomography-guided percutaneous coronary intervention compared with other imaging guidance: a meta-analysis. Int J Cardiovasc Imag. 2018;34:503–513. doi: 10.1007/s10554-017-1272-2. [DOI] [PubMed] [Google Scholar]