Abstract
Multiple clinical studies have treated mesothelin (MSLN)-positive solid tumors by administering MSLN-directed chimeric antigen receptor (CAR) T cells. Although these products are generally safe, efficacy is limited. Therefore, we generated and characterized a potent, fully human anti-MSLN CAR. In a phase 1 dose-escalation study of patients with solid tumors, we observed two cases of severe pulmonary toxicity following intravenous infusion of this product in the high-dose cohort (1–3 × 108 T cells per m2). Both patients demonstrated progressive hypoxemia within 48 h of infusion with clinical and laboratory findings consistent with cytokine release syndrome. One patient ultimately progressed to grade 5 respiratory failure. An autopsy revealed acute lung injury, extensive T cell infiltration, and accumulation of CAR T cells in the lungs. RNA and protein detection techniques confirmed low levels of MSLN expression by benign pulmonary epithelial cells in affected lung and lung samples obtained from other inflammatory or fibrotic conditions, indicating that pulmonary pneumocyte and not pleural expression of mesothelin may lead to dose-limiting toxicity. We suggest patient enrollment criteria and dosing regimens of MSLN-directed therapies consider the possibility of dynamic expression of mesothelin in benign lung with a special concern for patients with underlying inflammatory or fibrotic conditions.
Keywords: MSLN, chimeric antigen receptor (CAR) T cells, immunotherapy, cancer, cell transfer therapy
Graphical abstract

Haas, Albelda, Tanyi, and colleagues observe severe pulmonary toxicity in patients following treatment with high doses of potent CAR T cells engineered to recognize mesothelin. This appears to be due to dynamic upregulation of mesothelin in benign lung, suggesting that lung injury be included as an exclusion criterion.
Introduction
Over the past decade, chimeric antigen receptor (CAR) T cell therapy has achieved dramatic clinical success in patients with hematologic malignancies.1,2 However, challenges remain in demonstrating meaningful clinical activity in solid tumors.3,4
To develop effective cancer therapies targeting solid cancers, many groups have focused on targeting tumors that overexpress mesothelin.5 Mesothelin (MSLN) is a GPI-anchored surface protein that has been implicated in resistance to apoptosis, cancer invasion, and metastasis in tumors.6,7 Early immunohistochemical studies primarily identified mesothelial cells as a major source of mesothelin in tissues.8,9 However, it is highly overexpressed in many human cancers, including mesothelioma and pancreatic, ovarian, and lung cancers,5 supporting the selection of MSLN as a suitable target tumor antigen. This differential expression on tumors has led to the development of several types of cancer therapies targeting MSLN, including the antibody-drug conjugates (e.g., anetumab ravtansine),10 the chimeric anti-MSLN antibody amatuximab,11 the anti-MSLN vaccine CRS-207,12 MSLN-targeted CAR T cells,13,14 and mesothelin-targeted T cell receptors.15 Recent studies indicate that targeting of membrane-proximal epitopes of mesothelin may be more effective.16,17
For more than a decade, our group has focused on developing CAR T cells targeting MSLN. After conducting promising preclinical studies using a CAR that coupled a murine-derived anti-human mesothelin scFv called SS1 to cytoplasmic domains that included CD3-ζ and 4-1BB,13,18 we began a series of phase 1 clinical trials. To maximize safety, our first trial used mRNA electroporation to temporarily express the SS1-mesothelin CAR in activated autologous T cells (RNA CART-meso cells).19,20 Up to six doses of RNA CART-meso cells, given every other day, were infused intravenously into seven subjects with pancreatic cancer or malignant pleural mesothelioma (MPM) without off-target or on-target toxicities (e.g., pleuritis, pericarditis, or peritonitis) observed. Two patients showed some evidence of response by computed tomography (CT) or positron emission tomography scans. Only one severe adverse event was noted. In this event, one subject with MPM developed an anaphylactic reaction during a second set of infusions of RNA CART-meso cells after prolonged treatment interruption, which was likely the result of an immune response against the murine scFv CAR component.21
Given the lack of on-target off-tumor toxicity with RNA CART-meso cells, we subsequently conducted a second phase 1 trial (in patients with pancreatic cancer, MPM, and ovarian cancer) using the same CAR construct that was stably transduced into autologous T cells using a lentiviral vector.22 In this study, we escalated the dose of the CAR T cells with and without pre-treatment cyclophosphamide as a lymphoreduction strategy to improve CAR T cell persistence and efficacy. Encouragingly, the transduced CART-meso cells were well tolerated and CART-meso cells were detected in 7 of 10 tumor biopsies. The best overall response was stable disease (11 of 15 patients). However, CART-meso cells expanded modestly in the blood and persisted only transiently (approximately 28 days). Human anti-chimeric antibodies were detected in the blood of 8 of 14 patients, which may have played a role in the transient persistence of the CAR T cells.
Based on these biocorrelative and clinical results, we postulated that a fully human and more potent CAR would demonstrate enhanced persistence and antitumor activity. Using a phage display library, we identified such a fully human, MSLN-targeting single-chain variable antibody fragment (scFv) that was incorporated in a CAR (called M5). In preclinical studies, this M5 CAR had more potent in vitro and in vivo antitumor activity than the SS1-based CAR. We then initiated a phase 1 dose-escalation clinical trial that is currently ongoing (NCT03054298). The CAR was well tolerated at the initial dose of 107 CART/m2 with or without lymphodepletion (LD). However, when we increased the dose to 108 CART/m2 without LD, the first two patients in this cohort developed severe pulmonary toxicity, with one patient dying of respiratory failure while the other recovered.
In this report, we present these two cases of severe pulmonary toxicity observed in patients treated with high doses of potent T cells engineered to target MSLN. Our investigations into the cause of the toxicity revealed that low levels of MSLN expression can be upregulated on benign lung epithelial cells in the setting of lung injury, and that this pattern of expression can be recognized by MSLN CAR T cells, leading to diffuse lung injury. Our findings emphasize the importance of considering this potential toxicity in the design of future trials involving MSLN-targeted CARs, T cell receptors, or antibody conjugates, which are currently being developed by multiple academic and industry groups.
Results
Generation and characterization of highly active M5 huCART-meso T cells
To minimize the clinical risk of anti-CAR immunity that could result in anaphylaxis21 and immune-mediated loss of CAR T cells,2,23 we generated fully human CARs targeting MSLN. A human scFv library derived from B cells was screened using phage display technology24 to obtain human MSLN-binding scFv clones that were subsequently incorporated into CAR constructs. Twenty-two unique scFv sequences were inserted into a CAR construct that was based on the previously described design of a second-generation CAR25 that contained a CD8α hinge and transmembrane domains fused to 4-1BB and CD3-ζ cytoplasmic signaling domains. The resulting CAR plasmids were cloned into the pELPS lentiviral vector.13,26 The new CAR constructs were benchmarked against the MSLN-specific murine scFv-derived SS1 CAR, which had already been tested in clinical trials.19,21
Based on Jurkat T cell reporter cell line results and after reviewing the scFv sequence diversity, we chose eight CARs and expressed them in primary T cells. The resultant CAR T cells were exposed to the OVCAR8 and Panc 02.03 tumor cell lines. When co-cultured with the high MSLN-expressing cell line, OVCAR8 (Figure 1A), the M5, M11, M14, and M17 CARTs, as well as the SS1 CARTs, showed high levels of activation, as measured by interferon-γ (IFNγ) release (Figure 1B), and tumor cell killing (Figure 1C). However, when exposed to low MSLN-expressing Panc 02.03 cells (Figure 1A), only the M5 and M11 CARTs, but not M14, M17, or SS1 CARTs, released IFNγ and killed the target cells (Figures 1B and 1D). None of the meso-CAR constructs reacted with the NALM-6 leukemia line, a negative control, that does not express MSLN (Figure 1B).
Figure 1.
Characterization of novel anti-MSLN CAR constructs in vitro and in vivo
(A) Cancer cell lines with varying MSLN expression levels were selected to test CAR T cell reactivity. The ovarian cancer cell line OVCAR8 and the pancreatic cancer cell line Panc02.03 were stained with soluble SS1 scFv to quantify MSLN surface expression (numbers indicate MFI of entire cell population). (B) Cytokine secretion was assessed by co-culturing 25,000 CAR T cells with either OVCAR8, Panc02.03, or Nalm6 cells for 18 h at E:T = 1. Error bars reflect standard deviation. Supernatant was harvested, and the IFNγ concentration measured. (C and D) Impedance-based killing assays were performed by culturing CAR T cells with OVCAR8 (C) and Panc02.03 (D) for 16 h at defined E:T ratios. (E and F) Xenograft studies were performed using representative gastric cancer (E) and lung cancer (F) cells to assess in vivo CAR T cell reactivity. Inserts show MSLN expression by flow cytometry (red histogram: K1 α-MSLN Ab, blue: secondary Ab only). (E) A total of 2 × 106 cells of the intermediate MSLN-expressing gastric cancer line N87 were injected subcutaneously. On day 7 post cancer cell inoculation, 5 × 106 CAR-positive transduced T cells were injected i.v. (see arrow). Tumor growth was monitored by caliper measurements and plotted as tumor volume over time. Activated, but untransduced (UTD) cells served as negative control. (F) A total of 2.5 × 106 cells of the low MSLN-expressing lung cancer line A549 were injected i.v. Five days later, 5 × 106 CAR-positive T cells were injected i.v. (see arrow). Bioluminescence imaging (BLI) was conducted at the points indicated and plotted over time. UTD cells and no treatment served as negative controls. (G) Human plasma membrane protein binding cell array was used to assess MSLN binder specificity. SS1, MSLN-5, and MSLN-11 scFvs with AVI-HIS were applied to cell microarrays expressing 3,559 unique transcripts. Binding was detected using a secondary antibody conjugated to Alexa Fluor 647 and detection of ZsGreen served as transfection/expression control. The array contained three isoforms of MSLN, which were detected as medium to strong binders with all three scFvs. TAOK3, TAO kinase 3; PPAP2B, lipid phosphate phosphohydrolase; P2Ry1, purinergic receptor P2Y1.
We next tested the ability of M5, M11, and SS1 CARs to treat two human cancer xenograft models. The N87 cell line is derived from a gastric carcinoma and expresses intermediate levels of MSLN (Figure 1E, inset). Subcutaneous tumors were generated and then treated with a single dose of 5 × 106 CAR+ T cells on day 8 post cancer cell injection. Tumor regression was induced by all three CAR T cells (M5, M11, and SS1), but not by activated, but untransduced T (UTD) cells (Figure 1E). A549 cells are derived from a type II pneumocyte lung adenocarcinoma and express only very low levels of MSLN (Figure 1F, inset). A549 cells were injected intravenously (i.v.) where they home to the lung and establish an orthotopic lung cancer model. Tumor-bearing mice were treated with a single i.v. dose of 5 × 106 CAR+ T cells on day 5 post cancer cell injection. M5 and M11 CAR T cell treatment led to regression of the tumors, while SS1 CAR T cells had a marginal effect only slowing tumor growth (Figure 1F).
Biochemical studies were performed to determine the affinity and specificity of the lead scFv sequences for MSLN. Antibody affinity studies were performed using surface plasmon resonance (SPR) with MSLN immobilized on the chip. Despite the lower activity of the SS1 CAR in T cells, the Kd of the SS1 scFv was much lower (Kd = 0.1 nM), i.e., the affinity higher than that of the M5 (Kd = 26.9 nM) or M11 (Kd = 64.7) scFV (Figure S1). To examine the binding sites for the scFvs on MSLN, we performed epitope binning by testing the ability of scFvs to compete with SS1 for binding to MSLN. M5 and M11 were able to bind to the MSLN-SS1 complex, indicating that they bind a different epitope than SS1 (data not shown). To confirm this finding, binding of M5 and SS1 to MSLN was compared using hydrogen-deuterium exchange mass spectrometry (HDx-MS). In this technique, reduced deuterium exchange may occur when a protein binding partner directly blocks access to the solvent or allosterically regulates the structure of the bound protein. Negative values in “difference in deuterium uptake” (Figure S2) indicate that the MSLN-scFv complex undergoes less deuterium uptake relative to MSLN alone in the respective peptide regions. Figure S3 provides a summary of the protected regions for both the M5- and SS1-MSLN complexes. These data indicate that M5 exclusively protects the C-terminal region of MSLN; amino acid residues 485–490, 498–507, 532–537, and 545–572, and thus that the M5 epitope is C-terminal. In contrast, SS1 exclusively protects the N-terminal part of MSLN27 close in proximity to the MUC16 binding site.28 There was no observed overlap in the regions protected by SS1 and those protected by M5. The observation of two distinct protection patterns for SS1 and M5 indicate that M5 likely binds a distinct epitope from that bound by SS1, in accordance with the results obtained for SPR-based epitope binning. The region bound by SS1 is membrane distal; the region bound by M5 is membrane proximal (Figure S3). M11 binds to the same region of MSLN as M5 (data not shown).
In summary, these data show that the fully human M5 and M11 CARs are more active than the SS1 CAR in vitro and in vivo against tumors expressing low MSLN levels. This may result from the M5 and M11 CARs binding to a membrane-proximal portion of MSLN, unlike the SS1 CAR, which binds to a membrane distal portion of MSLN. A human plasma membrane protein binding cell array containing approximately 3,500 proteins was used to assess target selectivity using AVI-HIS-tagged MSLN scFvs. All three scFvs exhibited medium or strong binding to three distinct isoforms of human mesothelin protein expressed in the cell array (Figure 1G). No off-target medium or strong protein interactions were identified. The SS1 scFv produced the strongest signal to the MSLN isoforms and the MSLN-11 scFv showed higher levels of nonspecific background. The SS1 and MSLN-5 scFvs demonstrated very weak to weak reactivity to TAO kinase 3; however, this was considered nonspecific as (1) it was observed with other non-MSLN scFvs as well as control antibodies (data not shown) and (2) it is protein bound to the cellular membrane without an extracellular domain. Based on assays showing lower nonspecific binding, the M5 CAR was ultimately chosen to move forward into our clinical trial.
Clinical trial design
An institutional review board (IRB) and Food and Drug administration (FDA)-approved clinical trial entitled “Phase I Study of Human CAR Modified T Cells in Patients with Mesothelin Expressing Cancers” (IRB# 826085; UPCC# 02916; ClinicalTrials.gov identifier NCT03054298) was initiated on May 30, 2017. The trial was a standard 3 + 3 dose-escalation trial that included cohorts without and with lymphodepleting chemotherapy. At the time of the observed complications, the trial had enrolled 17 subjects and infused eight subjects.
In cohort 1 (1–3 × 107 M5 CART/m2), three subjects were infused, one with MPM and two with ovarian cancer. In cohort 2 (1–3 × 107 M5 CART/m2+Cytoxan [1 g/m2]), three subjects were infused, one with ovarian cancer and two with MPM. The subjects in these cohorts had a favorable safety profile without dose-limiting toxicities, cytokine release syndrome (CRS), or high-grade or unexpected toxicities. However, the first two subjects intravenously infused with a 10-fold higher dose without lymphodepletion in cohort 3 (1–3 × 108 CART/m2) developed severe pulmonary toxicity.
Clinical course of the first patient in cohort 3
Subject 02916–13 was a 61-year-old female with stage 2C, BRCA 1 positive (187delAG mutation), high-grade serous ovarian cancer who underwent a suboptimal cytoreduction. Nine years following this, she received six different chemotherapies, three secondary cytoreductions, and 5,000 cGy left pelvic sidewall radiation. She was the first patient to be infused with huCART-meso in cohort 3 (no lymphodepletion; 1–3 × 108 CART/m2). On day 0, the subject underwent her huCART-meso T cell infusion (3 × 108 CART/m2). She tolerated the infusion well and was discharged home following 3 h of monitoring after infusion, per protocol. Approximately 5 h post-infusion, she developed chills and fever to 102°F with no other symptoms. The chills resolved after taking acetaminophen, but her fever worsened to 103.6°F.
On day 1, she was instructed to come to the emergency department where she was febrile, tachycardic, and had developed hypotension (blood pressure approximately 80/40 mm Hg). She was transferred to the intensive care unit (ICU) where she received i.v. fluids, a norepinephrine i.v. infusion, and empiric broad-spectrum antibiotics. Her initial chest X-ray showed clear lung fields (Figure 2A). The subject remained alert, but she was confused to time and place, had stuttering with word-finding difficulty, and was unable to respond to commands. Both a non-contrast head magnetic resonance image and CT were within normal limits and these mental status changes improved within an hour without treatment. Oxygen therapy was provided at 2 L per minute by nasal cannula for tachypnea (26–30 breaths/min), a low oxygen saturation (SpO2 92%), and residual mental status changes.
Figure 2.
Serial chest radiologic studies captured the evolution of pulmonary toxicity in subjects 09 and 13
Both patients received serial chest radiologic studies following admission for complications arising in the setting of huCART-meso infusion. (A) Serial chest X-ray images document the evolution of pulmonary alveolar opacities consistent with non-cardiogenic pulmonary edema seen in subject 13. (B) Subject 09 received a chest CT approximately 2 weeks prior to huCART-meso infusion. After being admitted 2 days following huCART-meso infusion, she received multiple chest X-ray studies that documented new pulmonary alveolar opacities with notable air bronchograms (arrow) seen 2 days into her admission.
On day 2, the subject remained febrile to 102.9oF and required an increase in nasal oxygen flow to 4 L per minute. Due to persistent fevers, increasing oxygen requirement, hypotension, and evolving coagulopathy, the subject was started on a course of tocilizumab (8 mg/kg) and restarted on low-dose norepinephrine for hypotension. Her ferritin level was mildly elevated at 516. Her respiratory status continued to decline with increasing oxygen requirement, which correlated with worsening opacities on chest X-ray (Figure 2A) and the need for escalation to 100% oxygen by bilevel positive airway pressure (BIPAP) non-invasive ventilation support.
On day 3, she remained febrile to 102.3°F, tachypneic and hypoxic requiring continued BIPAP support, tachycardic with a labile blood pressure, and mildly coagulopathic. She was started on methylprednisolone (1 mg/kg) and received another dose of tocilizumab.
On day 4, despite becoming afebrile, she remained on BIPAP with 60% oxygen and had ongoing laboratory evidence of coagulopathy. Her chest X-ray showed worsening bilateral infiltrates (Figure 2A) concerning for non-cardiogenic pulmonary edema consistent with adult respiratory distress syndrome from CRS. Methylprednisolone, tocilizumab, and antibiotics were administered, but all bacterial cultures remained negative.
By day 5, the subject began to demonstrate clinical improvement. She remained afebrile, showed no confusion, did not require vasopressor support, and was tolerating high-flow nasal canula oxygen therapy. Her fibrinogen and prothrombin time normalized. On day 6, she remained afebrile with ongoing oxygen requirement improvement. On day 7, she was transferred out of the ICU. Over the next 2 days, her lab abnormalities resolved, she was weaned off oxygen, antibiotics were stopped, and she was discharged on day 9. RECIST measurements on day 28 showed an 8.4% increase in target tumor volume (stable disease). However, at 3 months, her tumor volume increased by 25% (progressive disease).
Clinical course of the second patient in cohort 3
Subject 02916–09 was a 74-year-old female with progressive MPM status post radical pleurectomy and multiple prior chemotherapy regimens. She also had a history of deep vein thrombosis (2013) and pulmonary embolism (2015) on chronic enoxaparin therapy. She received a baseline chest CT scan approximately 2 weeks prior to huCART-meso infusion that revealed progressive disease characterized by increased right pleural thickening and nodularity, increased mediastinal and right axillary lymphadenopathy, and three new left lung nodules smaller than 5 mm (Figure 2B).
On day 0, the subject received her huCART-meso T cell infusion (3 × 108 CART/m2) and was discharged home following 3 h of monitoring post-infusion per protocol.
On day 1, the subject was seen as an outpatient per protocol. She reported nausea and mild dyspnea above baseline without hypoxia but was otherwise stable. In a telephone encounter later that evening, she revealed worsening dyspnea but despite encouragement for the patient to present to the emergency department (ED) for evaluation, she declined this recommendation.
On day 2, the subject was again seen as an outpatient per protocol. On exam, she was weak and dyspneic at rest. Her oxygen saturation on room air was 84% and improved to 92% with supplemental oxygen at 2 L per minute by nasal cannula. Although afebrile, she was tachycardic and hypertensive. She was transferred to the ED for acute management where she rapidly progressed to hypoxemic respiratory failure unresponsive to 100% non-rebreather mask and requiring intubation. She was transferred to the medical ICU. Her chest X-ray showed new bilateral lower lobe pulmonary infiltrates (Figure 2B). She also became febrile and hypotensive, requiring fluid boluses and blood pressure support. She was given 10 mg dexamethasone and a dose of tocilizumab.
On day 3, she showed no improvement in her oxygenation or blood pressure and was administered a second dose of tocilizumab. Throughout the course of the day, her oxygenation continued to slowly worsen, and she developed more extensive alveolar infiltrates (Figure 2B).
Over the next 2 days, her hypoxemic respiratory failure remained refractory to maximal medical management that included paralysis, prone ventilation, methylprednisolone, cyclophosphamide, and inhaled prostacyclin. Given her advanced MPM stage and refractory hypoxemic respiratory failure, extensive discussions with the family concluded ongoing aggressive efforts would not have been within her goals of care, and the decision was made to remove life-sustaining therapies. The subject expired on day 5.
Biomarker analyses
For subjects 13 and 09, in vivo CAR T cell expansion kinetics in the peripheral blood were ascertained by quantitative PCR studies directed against the CAR vector sequence (Figure 3A). Results were expressed as vector copies per μg DNA. Subject 13 exhibited a small post-infusion increase in detectable CAR T cells on day 1 (∼200 copies/μg DNA) and then a larger increase at 7–10 days, peaking at ∼1,200 copies/μg of DNA. This pattern was similar in timing and magnitude to our previous subjects receiving mesothelin CARTs in solid tumor patients22 and was like that seen in patients in cohorts 1 and 2 (data not shown). Subject 09 showed an expected small increase in detectable CAR T cells on day 1 (∼400 copies/μg DNA) that then disappeared on day 2 and day 3. Planned serial measurements in subject 09 were limited by the subject’s worsening clinical course and death. Large numbers of circulating CAR T cells were thus not seen at the time when either of the subjects developed respiratory distress.
Figure 3.
Engraftment of huCART-meso cells and systemic cytokine analysis
(A) qPCR studies targeting the lentiviral DNA sequence of the huCART-meso cells were used to quantify the expansion kinetics of the mesoCART cells in peripheral blood specimens of subjects 13 and 09. (B) Non-cardiac C-reactive protein levels were assessed in pre- and post-infusion serum specimen. Serum cytokine levels were determined by multiplex Luminex assays on IFNγ (C), GM-CSF (D), TNFα (E), IL-6 (F), IL-10 (G), and IL-1RA (H). For specimens with cytokine values falling below the lowest control in the standard curve, a dashed line is included to denote the lowest concentration that can be reliably quantitated. Values below this line are plotted as reported, except for values reported as “out of range” that are plotted at zero. For both huCART-meso engraftment data and systemic cytokine measurements, pre-infusion specimens were designated day 0, and post-infusion specimens on the same day were designated day 0.5.
Cytokine profiling can be used to assist in identifying mechanisms driving adverse events, such as CRS, in CAR T cell therapy. Per study protocol, serum samples were collected at predetermined intervals before and after huCART-meso infusion. Figure 3 shows the patterns of non-cardiac C-reactive protein (CRP) (Figure 3B) and key cytokines including IFNγ (Figure 3C), granulocyte-macrophage colony stimulating factor (GM-CSF) (Figure 3D), tumor necrosis factor alpha (TNFα) (Figure 3E), interleukin (IL)-6 (Figure 3F), IL-10 (Figure 3G), and IL-1 receptor antagonist (IL-1RA) (Figure 3H) that have been implicated in different types of cytokine release syndromes and/or systemic sepsis. Table S1 shows the data for a larger panel of cytokines. In contrast to reports of CRS from CD19-directed CAR T cells where symptoms and increases in cytokines such as IL-6, IFNγ, IL-1RA, and GM-CSF typically appear 7–10 days after infusion (at the time of maximal T cell expansion),29 both subjects showed marked elevations at much earlier time points, although with slightly different kinetics. Subject 13 had large cytokine peaks on day 1 (24 h after CART infusion) with rapid resolution; the exceptions being that CRP peaked on day 2 and IL-6 on day 6. In contrast, subject 09 showed increases of most cytokines on day 1, but these continued to rise on day 2 and day 3. These changes were noted despite multiple doses of tocilizumab and methylprednisolone.
Because subject 09 had been previously treated with pembrolizumab, serial measurements of pembrolizumab levels in the serum were performed (Figure S4). ELISA results confirmed circulating levels of pembrolizumab to be present at 36 μg/mL 12 days prior to infusion with a reduction to 19 μg/mL on the day of huCART-meso infusion. Despite the subject’s last dose being over 50 days prior to infusion, pembrolizumab levels were present at levels well above the half maximal inhibitory concentration for PD-1.30 However, it is noteworthy that subject 13 was not treated with pembrolizumab in the months prior to huCART-meso infusion yet also developed profound pulmonary toxicity after treatment.
Pathology and correlative molecular studies
To better understand the mechanisms of pulmonary decompensation in subject 09, the family agreed to an autopsy. Multiple organ and tumor specimens were collected for both histological and molecular analyses. Gross pathology findings were notable for numerous tumor masses within the parenchyma and along visceral pleural surfaces of both the right and left lungs. Metastatic tumor deposits were also seen in the right infra-hilar lymph nodes, liver, omentum, and small bowel serosa. The lungs were diffusely boggy, and the cut surfaces were notable for diffuse parenchymal congestion. Despite abundant MSLN expression on benign pleural and pericardial mesothelial cells, these surfaces did not demonstrate gross signs of inflammation.
Microscopic examination of the lungs revealed a background of bilateral acute lung injury consisting of pulmonary edema, fibrin leakage, and focal hyaline membrane formation (Figure 4A, left and middle). In contrast to classic diffuse alveolar damage, there was an extensive polymorphous infiltrate within both the interstitium and alveolar spaces. The inflammatory infiltrate was composed of neutrophils, eosinophils, lymphocytes, and macrophages. Microscopic tumor masses were observed and found to be surrounded by numerous lymphocytes at the periphery (Figure 4A, right). Scattered lymphocytes in sparse numbers were seen within sites of tumor.
Figure 4.
Autopsy findings within the lungs for subject 09 are notable for acute lung injury, extensive T cell infiltrates, and abundant CAR T cells
(A) H&E-stained sections of lung tissue demonstrate acute lung injury including pulmonary edema, fibrin leakage, and focal hyaline membrane formation associated with an extensive polymorphous inflammatory infiltrate (left, middle). Scattered foci morphologically compatible with MPM are seen surrounded by lymphocytes that poorly infiltrate the tumor (right). Scale bars represent 250 μm (left), 50 μm (middle), and 500 μm (right). (B) Immunohistochemical studies reveal extensive T cell infiltrates in benign lung, with accumulation around vessels (arrow) and at the border of tumor deposits (arrowhead, left image). CD4+ T cells greatly outnumber CD8+ T cells (middle and right, respectively). Scale bars of the low-magnification images represent 500 μm. High-magnification images (30x) include scale bars corresponding to 30 μm. (C) qPCR studies targeting the WPRE sequence of the CAR T cells identify substantial enrichment of CAR T cells in lung and spleen at autopsy.
Immunohistochemical staining for CD3 was performed on sections of lung and demonstrated abundant T cell infiltrates. T cells were observed accumulating around larger vessels at the edge of, but not strongly within, tumor deposits (Figure 4B, left). Staining for CD4 and CD8 revealed a predominance of CD4+ T cells with both types displaying a similar spatial distribution as the CD3 stain (Figure 4B, middle and right). Neither CD4 nor CD8 stains identified regions of tumor that were heavily infiltrated by T cells, despite the presence of many surrounding T cells.
Quantitative PCR studies were performed on DNA extracted from autopsy specimens to capture the abundance and distribution of M5 huCART-meso cells in subject 09 (Figure 4C). Striking accumulation of detectable CAR signal was seen in bilateral benign lung specimens, with only spleen having comparable levels of CAR T cell involvement. Of several tumor nodules that were sampled, a single tumor specimen from the right lung demonstrated appreciable detection of CAR T cells. Samples of other solid organs did not have CAR T cell measurements near the levels observed in the lungs.
Mesothelin gene expression in human lung specimens
To identify the scope of cellular targets of M5 huCART-meso cells, immunohistochemical studies were performed on autopsy specimens of lung from subject 09 to identify sources of MSLN (Figure S5). As expected, deposits of MPM stained intensely for MSLN protein expression. Staining of the surrounding regions of benign pulmonary epithelial cells were nearly indistinguishable from the high background staining, so a low level of protein expression in the cells could not be formally excluded. Therefore, RNAScope studies were performed to examine the expression level of MSLN RNA across sections of lung in subject 09 (Figure S6). A subset of cells in regions of benign lung clearly stained positively for MSLN expression, suggesting pneumocytes may be sources of low levels of MSLN expression. This finding is consistent with single-cell RNA sequencing studies that identify a low level of MSLN RNA expression in pneumocytes (Figure S7).31
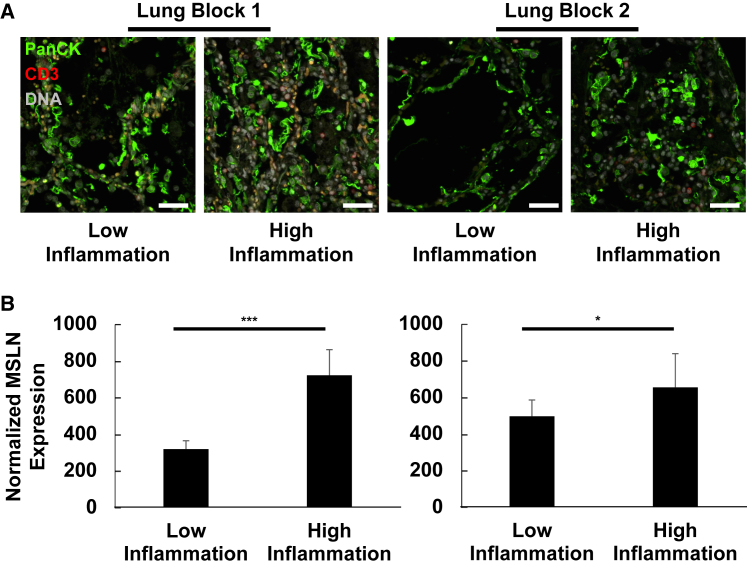
To map MSLN protein expression directly to pulmonary epithelial cells, digital spatial profiling was performed using the Nanostring GeoMx instrument.32 Using autopsy specimens from subject 09, sections of lung were cut from two independent blocks and stained with an antibody panel, including a validated antibody against MSLN, to be used for protein expression quantification. Tissues were then stained with a panel of morphology markers including fluorescently labeled antibodies against pan-cytokeratin and CD3, along with a chemical dye for DNA (Figure 5A). MSLN protein expression was then assessed by capturing signal only from cells positive for pan-cytokeratin, therefore selectively assaying relative MSLN protein expression in benign pulmonary epithelial cells. This technique demonstrated MSLN protein expression in this cell population, and it revealed a correlation between MSLN protein expression and the presence of surrounding inflammatory cells (Figure 5B). Specifically, areas with relatively high levels of inflammation had significantly higher levels of MSLN detected in the benign pulmonary epithelial cells. This finding raised the possibility that MSLN expression in epithelial cells may be dynamic and prone to upregulation in the setting of inflammation and/or injury.
Figure 5.
Inflammation correlates with enhanced MSLN protein expression in pulmonary epithelial cells from subject 09
(A) Representative immunofluorescence micrographs of independent autopsy specimens of lung collected from subject 09 are depicted. Heterogeneous levels of inflammatory infiltrates allow for direct comparison of MSLN protein expression from pulmonary epithelial cells (PanCK+ cells) from regions of relatively high vs. relatively low inflammation. Antibodies: PanCK (1:40, Cy3, green), CD3 (1:75, Alex Fluor 594, red). DNA was labeled using SYTO13 (gray). Scale bars represent approximately 50 μm. (B) MSLN protein expression was measured by digital counts from segments of PanCK+ cells. Normalized counts were generated by scaling data to the surface area of the illuminated segments. n = 10 segments per condition for block 1 (left) and n = 8 segments per condition for block 2 (right). ∗p < 0.05; ∗∗∗p < 0.001, two-tailed Student’s t test.
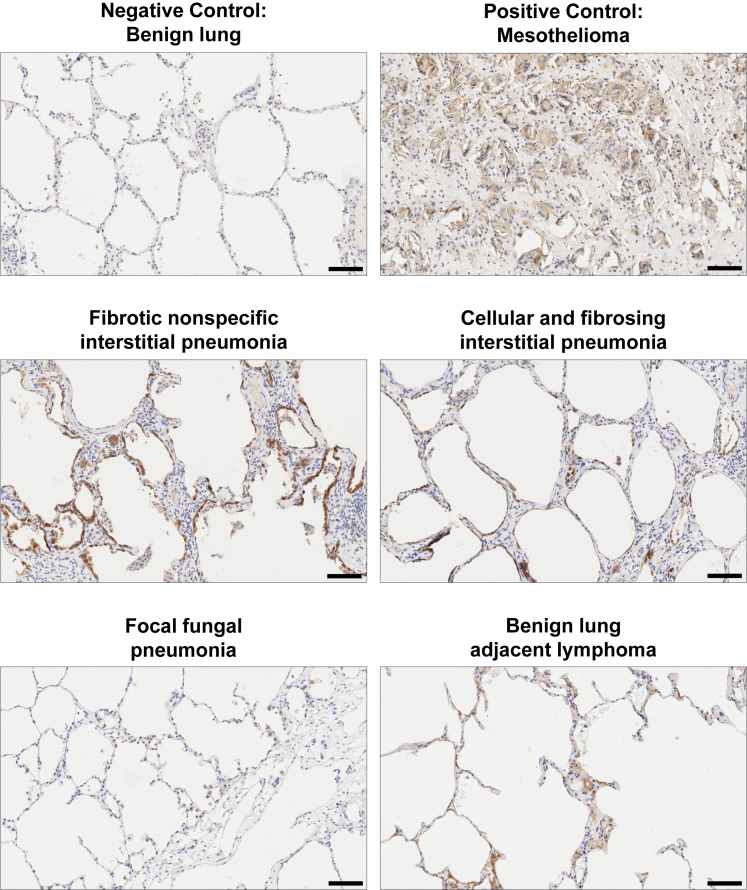
To determine if lung damage was associated with increased MSLN expression, we assembled a case series of human lung biopsy and autopsy specimens representing a wide spectrum of lung pathology (Table S2). Immunohistochemical staining for MSLN protein expression revealed multiple specimens to be positive for relatively strong staining of the pulmonary epithelium (Figure 6). Staining patterns ranged from linear stretches of intensely staining epithelial cells to scattered dim-positive alveolar cells. Collectively, this case series confirmed a pattern of dynamic expression of MSLN in the setting of lung injury.
Figure 6.
Immunohistochemical studies of human lung from a spectrum of inflammatory and/or fibrosing disease processes reveal variable expression of MSLN protein in alveolar cells
Immunohistochemical staining for MSLN was performed on either human biopsies or autopsy specimens across a spectrum of lung diseases. Mesothelioma was included as a positive control for the study. Images were obtained at ×10 magnification, and scale bars represent 100 μm.
Discussion
After safely treating dozens of patients with SS1 CARBBz T cells targeting MSLN in prior clinical trials, in this most recent trial with a more potent CAR construct that targets a membrane-proximal epitope, our first two subjects in the next log dose escalation developed severe pulmonary toxicity that was characterized by (1) onset of hypoxemia, pulmonary infiltrates, and hypotension approximately 1–2 days after infusion of the CAR T cells; (2) increases in CRP and inflammatory cytokines with a similar time course; and (3) minimal CAR T proliferation in the blood at the time of these adverse events. In the case where autopsy tissue was available, we observed an intense multicellular lung infiltrate with many endogenous T cells (primarily CD4+ lymphocytes) and high levels of CAR T cell accumulation as assessed by molecular testing. In subject 09, we also observed detectable expression of MSLN on non-malignant native lung epithelial cells. Subsequent studies of lung pathology specimens revealed that although epithelial cell expression of MSLN was generally low, we could observe marked increases in expression in inflammatory lung conditions, especially lung disease associated with fibrosis (Table S2).
Pulmonary toxicity has been previously observed after injection of adoptively transferred T cells. The most common scenario has been seen in CD19 CART and BCMA CART therapy in association with what has been defined as the cytokine release syndrome (CRS).33,34,35 This syndrome usually occurs 7–10 days after CART-19 infusion and is associated with hypoxia, fever, hypotension, and markedly elevated cytokine levels. The pathophysiology is thought to be due to proliferation of CAR T cells at the tumor site, in situ cytokine production by both activated CAR T cells and cellular components of the tumor microenvironment (especially macrophages), activation of “bystander” endogenous immune cells, and direct and indirect tumor cell killing. This results in a systemic inflammatory response that ultimately leads to endothelial injury and microvascular leakage in multiple tissues and organs, and their associated effects including hypoxia, hypotension, and/or organ damage.35
A second scenario was described by Morgan et al. after infusion of ERBB2-targeted CARTs.36 In this case, the subject began developing severe hypoxemia, pulmonary edema, and hypotension within 15 min of infusion of ERBB2-CARTs. There was an immediate high burst of cytokines (within 4 h) at levels similar to that seen in CRS, but with a more rapid onset. The authors proposed that this event was likely triggered by ERBB2-directed CAR T cells recognizing the target antigen on normal pulmonary cells during first-pass clearance in the lung with the resulting cytokine storm initiating an inflammatory cascade leading to pulmonary edema, and eventually multiorgan failure leading to death. Another potential example of CAR T cell-mediated on-target off-tumor respiratory toxicity was observed after treatment with a first-generation anti-CEACAM5 CAR (MFEζ) in a CAR T cell dose-related manner following preconditioning and IL-2 treatment.37 The observed transient acute respiratory toxicity was hypothesized to be caused by MFEζ CAR T cell recognition of CEACAM antigen expression, which was observed in normal lung epithelium by immunohistochemistry.
Our two subjects shared some of the same features as described in “classic CRS,” that is, severe hypoxemia, pulmonary infiltrates, and hypotension along with elevation of many inflammatory cytokines; however, the levels of cytokines observed were much lower and the kinetics of these changes were different in that they occurred earlier. This syndrome has not been reported frequently in the treatment of solid tumors, but has been observed in instances where significant CAR T cell expansion in the peripheral blood has occurred.38 Since the timing of symptom onset differs from the delayed onset often seen in CAR T cell trials and the number of circulating CARTs was quite low, classic CRS seems a less straightforward explanation for the clinical events that we observed.
Instead, we believe that the onset of pulmonary dysfunction in our cases was driven by local reactivity of highly active infused huCART-meso cells against lung epithelial cells with low levels of MSLN. This reactivity resulted in activation of local endogenous inflammatory cells accompanied by release of damaging cytokines and further recruitment of inflammatory cells. For subject 13, who did not have any known pre-existing lung condition, this activation appeared to be limited and reversible. Unfortunately, in subject 09, we observed progressive inflammation, organ dysfunction, and increased levels of circulating cytokines. The reason for these two outcomes is not clear but could relate to differences in the amount of MSLN expressed by benign pulmonary epithelial cells and detected by the infused huCART-meso cells. Subject 09 had MPM with diffuse pulmonary metastases and an unknown baseline level of pulmonary inflammation secondary to these tumor deposits and prior treatments that could have enhanced MSLN expression. In addition, it is also possible that the remaining pembrolizumab circulating in subject 09 could have enhanced the response of the huCART-meso cells or sensitized the subject by stimulating a subclinical level of pulmonary inflammation prior to infusion. The later possibility is supported by evidence that pneumonitis is a known complication of pembrolizumab therapy.39
Given our findings, we reassessed the conventional opinion that MSLN is not expressed on lung epithelial cells.8,9 MSLN has been a widely used target for drug development in cancer therapy,11,12,13,14,40 but pulmonary toxicity has not been commonly reported in these trials and many histologic surveys have not reported MSLN expression on epithelial cells of the lower airway.9,40 However, data from publicly available single-cell RNA sequencing studies of human lung demonstrate detectable MSLN RNA expression in pneumocytes (Figure S7).31 Importantly, we have uncovered a correlation between inflammatory lung injury and increased MSLN expression on benign pulmonary epithelial cells. Further, a series of human lung specimens encompassing a spectrum of inflammatory and/or fibrotic processes further substantiate a connection between lung inflammation or injury and increased MSLN expression (Table S2, Figure 6). It is unclear how epithelial cell injury is connected to the upregulation of MSLN expression, but associations with TLR5 and TLR7 activation41 and TGF-beta signaling have been proposed.42 Reports by other groups have shown that MSLN is dynamically expressed in fibroblasts41 and can be upregulated in mesothelial cells after induction of peritoneal adhesions in mice.43
It is possible that the low level of MSLN expression on pulmonary epithelial cells has likely remained underappreciated because of the relatively lower potency of previously tested MSLN-directed CAR T cell therapies. This appears to be the case with CAR T cells where our previous clinical trials with the SS1-based CARTs showed no evidence of pulmonary toxicity.19,20,22 In preclinical studies, we found that the SS1 CART-meso cells were not triggered by A549 cells (which express low levels of mesothelin [Figure 1]) and exerted no antitumor activity in vitro or in animal models. In contrast, the M5 huCART-meso cells were strongly activated by A549 cells and had good antitumor activity in vitro and in animal models. The relative contribution of the membrane-proximal location of the mesothelin-directed CAR T cell epitope,16 and/or avidity of the M5 scFv to increased potency and recognition of the low-density target remains unclear.
To our knowledge, this is the first clinical report detailing cases of direct pulmonary toxicity caused by MSLN-directed CAR T cells. However, on-target toxicity has been observed with SS1 ADC tested by the Pastan group at the National Cancer Institute. They found the dose-limiting toxicity was pleuropericarditis with the SS1-based ADC.44,45 Further, a close review of the literature reveals other groups have reported signs and/or symptoms consistent with pulmonary toxicity using other MSLN-directed therapies. In a phase I/II clinical trial of MSLN-directed TCR fusion construct T cells (gavocabtagene autoleucel), one of the seven treated patients experienced grade 3 “CRS” and grade 3 pneumonitis after infusion.15 In a phase I study of the antibody-drug conjugate anetumab ravtansine,46 this MSLN-directed therapy was associated with dyspnea in approximately 20% of the treated patients across three groups, with 6% of the total patients experiencing at least grade 3 dyspnea. Clearly, our data and these other experiences show that pulmonary toxicity is a possibility for highly active anti-MSLN therapeutics.
In addition to this specific warning, our experience and that of other groups highlight at least three important, more general considerations when designing and conducting future CAR T cell trials. Since most tumor antigens are usually not completely tumor-specific, the ability of a CAR to recognize even low levels of antigen expression should be considered carefully when choosing the optimal CAR for use in clinical trials. Our data suggest that studies of antibody affinity, in isolation, may not adequately predict reactivity of the scFv in cellular assays reliant on activity of the entire CAR construct. The SS1 scFv had a stronger binding affinity for MSLN (10-fold higher) compared with the M5 scFv; however, the CARs that contained the M5 scFv demonstrated a better ability to recognize low levels of the antigen on target cells. The reason for this discrepancy is unclear and may be due to the binding epitope of the scFv, the ease of immune synapse formation, or structural features of the entire CAR. It might be advisable to screen CARTs to find those that react with cells expressing antigen at levels compatible with malignant tissues without activation at lower expression levels seen in benign tissues.
A second lesson to be learned is that toxicity testing should consider more than just purely baseline conditions that might be seen in a young healthy person. Examining aged, inflamed, or appropriately diseased tissues for expression of target antigen is important since most cancer patients are older, often have concomitant diseases, and may have received tissue-altering treatments, such as chemotherapy and radiation. For example, a recent study showed upregulation of the acute myeloid leukemia CAR target CD93 when endothelial cells were exposed to inflammatory cytokines.47 Given that the lungs are a major early trafficking location for T cell products delivered by i.v. infusion in patients,48 the presence of pulmonary abnormalities with upregulation of the target antigen may be especially important, particularly in patients with thoracic malignancies who often have underlying inflammatory or fibrotic lung disease. An additional consideration could be the extent of metastatic disease. Widespread tumor burden may enhance the chance of an initial adverse reaction by increasing target upregulation due to target/tumor inflammatory responses, which propagates and augments normal tissue injury. Finally, concomitant therapies may worsen the situation. There is a possibility that the presence of pembrolizumab in the serum of subject 09 may have amplified or caused uncontrolled activation of the huCART-meso cells, although this has not been confirmed.
Last, these data confirm the importance of CAR T cell dose in the development of toxicities and stress the importance of careful dose titration. Although CARTs are “living drugs” that can proliferate to high numbers no matter what the initial starting dose, it appears that off-tumor effects are more likely when large numbers of CAR T cells are intravenously delivered and encounter their potential target antigen on the first pass through the lung. We saw no detectable pulmonary toxicity with the M5 CAR at doses lower than 3 × 108 cells in our initial dose escalation (including cohorts with lymphodepletion). In our subsequent cohorts after protocol modification, where we reduced the dose back to 3 × 107 cells, no pulmonary injury has been observed. The route of administration may also be important. In a trial of another mesothelin-specific CAR T cell therapy based on the m912 scFv and dosed by intrapleural administration alone or combined with pembrolizumab in patients with MPM, only one of 27 recipients showed grade 3 dyspnea.49
Finally, regarding our clinical trial, the significant toxicities observed in cohort 3 led to an immediate clinical trial hold. After considerable investigation, the trial was restarted with institutional and FDA approval, but with the following amendments to enhance safety, including: (1) the high-dose i.v. cohorts (108 cells/m2) were closed, (2) subjects with radiographic evidence of chronic lung disease (especially pulmonary fibrosis) or greater than lobar malignancy were excluded, (3) subjects were required to have at least 4 months of wash-out from their last dose of any immune checkpoint inhibitor, and (4) subjects were admitted for a minimum of 48 h following cell delivery for adverse event assessment. Instead of the high-dose cohorts, we designed two new cohorts. In one, subjects with malignant effusions from an MSLN-expressing cancer received a single intrapleural infusion of 3 × 107/m2 huCART-meso cells (without lymphodepletion). In a second cohort, a multidose strategy was adopted. Subjects received a dose of 1–3 × 107/m2 huCART-meso cells via intraperitoneal (i.p.) delivery on day 0, following a single dose of 1 g/m2 of cyclophosphamide administered 2–4 days prior to the cell infusion. This treatment was then followed by up to two additional i.v. infusions of huCART-meso cells at the same dose level, given at days 21 and 42 (without further lymphodepletion) assuming no toxicity from i.p. delivery. To date, no pulmonary toxicity, or other serious adverse events have occurred in more than 10 patients treated. The results of the trial will be shared in a separate report once enrollment is complete.
In summary, targeting tumor-enriched antigens remains a viable strategy in the clinical development of CAR T cell therapies for solid tumors. Thoughtful translational medicine studies aimed at predicting conditions bearing an elevated risk of toxicity must be combined with careful dose-escalation protocols and patient monitoring strategies. Dose-limiting toxicity can result in serious adverse events; however, for some products, repeated events can be prevented by modifications of dose, dosing route, and/or the selected patient population.
Materials and methods
CAR constructs
A human B cell-derived scFv library was screened using phage display technology to obtain human MSLN-binding scFvs to be incorporated into CAR constructs.24 After three rounds of panning using biotinylated human MSLN, positive clones were confirmed by ELISA. For creation of CAR constructs, scFv sequences were synthesized with a 5′ BamHI site directly followed by the ATG start codon and a 3′ silent BspEI site within the CD8α hinge region. BamHI and BspEI sites were used to clone the synthesized fragment into the pELPS-based CAR lentiviral vector13,26 containing CD8α hinge and transmembrane domains as well as cytoplasmic 4-1BB and CD3ζ domains.25
Cell lines
All cell culture media and additives were purchased from Gibco, ThemoFisher Scientific if not stated otherwise. The JNL reporter cell line was engineered from Jurkat E6-1 cells (ATCC TIB-152) and contains the firefly luciferase gene under the control of a minimal CMV promoter and tandem repeats of the NFAT transcriptional response element. Cells were cultured in RPMI with 10% heat-inactivated fetal bovine serum (HI-FBS) and 0.5 μg/mL puromycin. The ovarian adenocarcinoma OVCAR8 [(Fox Chase Cancer Center, Philadelphia, PA50) RPMI 1640, 10% HI-FBS] and Panc02.03 [(ATCC CRL-2553) RPMI, 15% HI-FBS, 0.1 U/mL Insulin], served as parental lines for OVCAR8-Luc and Panc02.03-Luc, which was generated by transduction with EF1α-Luciferase lentiviral particles (GenTarget). The gastric carcinoma cell line N87 (ATCC CRL-5822) and the lung carcinoma line A549 (ATCC CCL-185) were obtained from ATCC. The latter two were modified to express click beetle green luciferase (N87-CBG and A549-CBG, respectively).
Lentivirus production and titer determination
Lentivirus was produced by standard transfection methods of CAR plasmid mixed with three packaging plasmids pVSVg, pMDL.g/p, and pRSV.rev in LTX-293T cells (Takara Bio) using Lipofectamine2000 (ThermoFisher Scientific). The resulting viral preparations were stored in aliquots at −80°C. Lentiviral titer was determined by transduction of Sup-T1 cells and flow cytometric assessment of the percentage of CAR positivity (see the flow cytometry section). The average titer (four virus productions) for M5 CAR was 2.3 × 108 (±0.8 × 108) TU/mL.
CAR T cell generation for preclinical testing
T cells from healthy donors were obtained from the Research Donor Program at Novartis or the Human Immunology Core, University of Pennsylvania, under an IRB-approved protocol, or purchased from HemaCare. Bulk T cells (CD4 and CD8) were generated by negative selection using pan T cell isolation kit (Miltenyi Biotec) or obtained from the Human Immunology Core at University of Pennsylvania directly (RNA experiments, N87 and A549 studies in vivo). T cells were cultured in T cell medium (TCM: RPMI, 10% HI-FBS, 2 mM L-glutamine, 100 U/mL Pen/Strep, 1x NEAA, 1 mM sodium pyruvate, 10 mM HEPES, and 55 μM 2-mercaptoethanol) and were stimulated with anti-CD3/CD28 beads (Human T-Expander, ThermoFisher Scientific) at a cell:bead ratio of 1:3. Transductions with lentiviral particles were performed on day 1 post-stimulation using an MOI of 5–10. T cells were allowed to expand in a 37°C/5% CO2 incubator and were fed and split every 2 days starting day 3 post-stimulation. T cells were de-beaded and frozen in CryoStor CS10 (BioLife Solutions) on day 9–11 when their volume fell below 400 fL.
Flow cytometry
CAR expression was determined by staining with recombinant Fc-tagged MSLN protein followed by detection with a phycoerythrin (PE)-conjugated donkey anti-human immunoglobulin (Ig)G secondary antibody (Jackson ImmunoResearch), or by using Biotin-Protein L (GenScript) followed by detection with R-PE Streptavidin (Jackson ImmunoResearch). To analyze MSLN expression levels, cancer cell lines were incubated with Fc-block (1:10 in FACS buffer), washed once, then stained with His-tagged SS1 scFv (100 nM) followed by anti-His-PE secondary (Miltenyi Biotec). The anti-MSLN K1 monoclonal Ab (Biolegend) and PE Goat Anti-Mouse Ig (BD Biosciences) were used for N87 and A549 cell lines. Cells were analyzed on BD LSR Fortessa, and data were analyzed by FlowJo software.
Functional T cell assays
UTD cells were used to normalize the percentage of CAR-positive T cells in each sample. This guaranteed the use of the same number of CAR-expressing cells as well as the same total cell number in each sample.
Luciferase-based killing assay
Target cells expressing the luciferase reporter gene were washed and resuspended at 2.5 × 105 cells/mL in TCM; 100 μL of each target cell type were added in duplicate to a 96-well clear bottom black plate (Corning). Effector T cells were washed and resuspended at 2.5 × 106 cells/mL in TCM. Serial 2-fold dilution of effector T cells was prepared in TCM. One hundred microliters of T cells was added to the respective wells containing target cells. In addition, wells containing target cells alone were prepared. Plates were incubated at 37°C for 18 to 20 h. After that, 100 μL medium was removed from each well and replaced with 100 μL of Bright-Glo Substrate reagent (Promega). After incubation for 6 min, luminescent signal was detected using EnVision Multilabel Plate Reader (PerkinElmer). Percent killing was calculated as follows: killing (%) = (luminescence sample/mean luminescence target cells alone) ∗ 100.
Cytokine secretion assay
Target cells were washed and resuspended at 2.5 × 105 cells/mL in TCM (1 × 106 cells/mL in RPMI 1640, 10% HI-FBS medium [R10] for electroporated K562 study). One hundred microliters of each target cell type was added in duplicate to a 96-well round bottom plate (Corning). Effector T cells were washed, resuspended, and added to the respective wells at an E:T ratio of 1:1. In addition, wells containing T cells alone were prepared. After incubation at 37°C for 18 to 20 h, supernatant was harvested and subjected to an immunoassay (V-PLEX Human IFN-γ Kit).
Mouse studies
Male and female 6- to 8-week-old NOD-SCID-IL2rγ−/− (NSG) mice were purchased from Jackson Laboratories or bred in-house in the vivarium at the University of Pennsylvania and maintained in pathogen-free conditions. All the animals were handled in accordance with procedures approved by the University of Pennsylvania Institutional Animal Care and Use Committee regulations and guidelines. Studies and procedures were carried out in accordance with the guidelines for the ethical treatment of animals in research. For all CAR T cell transfers, UTD cells were used to normalize the percentage of CAR-positive T cells. This allowed the use of the same total number of T cells as well as CAR-expressing cells in each sample. Mice were randomized based on tumor burden (volume or bioluminescence imaging [BLI]) 1 day prior to CAR T cell transfer. Tumor volumes were determined by caliper measurement (two times per week) and calculated as follows:
N87 tumor model
A total of 2 × 106 N87-CBG cells were injected subcutaneously. Seven days later, 5 × 106 CAR+ T cells were injected i.v. to tumor-bearing mice.
A549 tumor model
A total of 2.5 × 106 A549-CBG cells were injected into mice (i.v.), and 5 days later, 5 × 106 CAR+ T cells were injected i.v. BLI was conducted at the different time points indicated using the IVIS Spectrum In Vivo Imaging System (PerkinElmer).
CAR T cell production for clinical testing
Engineered anti-MSLN CAR T cells were manufactured at the Cell and Vaccine Production Facility at the University of Pennsylvania (Philadelphia, PA) using leukapheresis products collected at the UPENN Apheresis Center. The apheresis products were processed at the Clinical Cell and Vaccine Production Facility to obtain a T cell population for manufacturing. T cells were activated using anti-CD3/28 conjugated paramagnetic microbeads (Life Technologies) and transduced with the specific lentiviral vector expressing the anti-MSLN transgene. The transgene was designed to encode the M5 scFv version coupled with the hinge, CD8 transmembrane domain, and the human 4-1BB and CD3ζ intracellular signaling domains. The GMP production of the lentiviral vector was performed at the UPENN Center for Advanced Retinal and Ocular Therapeutics. The transduced manufacturing cultures were maintained in static culture for the first days, followed by transfer to a Wave bioreactor up to day 9. At harvest, the cultures were washed, the magnetic beads removed, samples were collected for release testing, and the product was formulated in cryostorage bags. The target dose (1 × 107 up to 3 × 108 M5 CART/m2) was successfully manufactured in all runs. The product was released for infusion upon passing the Investigational New Drug-specified release testing for sterility, purity, and identity.
Quantification of CAR T cells in blood and tissues using quantitative PCR
Total genomic DNA was isolated directly from whole blood and tumor tissue, and huCART-meso levels were measured by qPCR using transgene-specific primers recognizing the 4-1BB-CD3ζ junctional fragment in the signaling domain of the CAR, as previously described.22 Transgene copies per microgram of genomic DNA are reported to quantify huCART-meso levels.
Quantification of cytokines and CRP
Cryopreserved serum samples obtained before and after huCART-meso infusion were thawed and analyzed for cytokine measurements using either a human cytokine magnetic 30-plex panel (Life Technologies) or a 31-plex panel (Millipore Sigma) as previously described.34
CRP was quantified in the clinical laboratory of the Hospital of the University of Pennsylvania, which is College of American Pathologists (CAP) accredited and maintains a Clinical Laboratory Improvement Amendments (CLIA) certificate.
Histopathology studies of human specimens
Formalin-fixed, paraffin-embedded autopsy specimens from subject 09 were sectioned, processed, and stained with hematoxylin and eosin in the clinical histopathology laboratory of the Hospital of the University of Pennsylvania, which is CAP accredited and maintains a CLIA certificate. Immunohistochemical studies for CD3, CD4, CD8, and mesothelin were also performed by the laboratory.
For the case series examining mesothelin expression in lung specimens, a diverse selection of autopsy and biopsy specimens were selected to encompass a range of pathologic processes including infection, fibrosis, inflammation, and diseases thought to cause only limited injury to the alveolar epithelial cells. Fresh sections were cut from all formalin-fixed paraffin-embedded blocks within 2 working days to avoid any decrement in staining intensity. The unstained sections were batched together and dewaxed. Heat-induced epitope retrieval was done for 20 min using ER2 solution (Leica Biosystems). Immunohistochemical staining was performed on a Leica Bond-III instrument using the Bond Polymer Refine Detection System (Leica Microsystems AR9800). Mesothelin antibody, Clone 5B2 (ThermoFisher MS-1320-R7) was used for antigen detection. Slide reading was performed by a board-certified anatomic pathologist.
In situ hybridization was performed using Advanced Cell Diagnostics (ACDBio/Biotechne) (Hayward, CA) RNAscope probes against mesothelin (NM_013404.4, catalog #413109). Peptidylprolyl Isomerase B (PPIB NM_000942.4, catalog #313909) and Dihydrodipicolinate reductase (DapB, EF191515, catalog #312039) were ordered from ACD/Biotechne. The in situ hybridization method followed protocols established by ACD Bio and Ventana Medical Systems using a 3,3′-Diaminobenzidine (DAB) chromogen. Experimental conditions were optimized first and negative (DapB) and positive (PPIB) control probes were included in each run. Briefly, 5-μm sections from formalin-fixed paraffin-embedded blocks were subjected to optimized preconditioning of 25 min and protease digestion for 16 min utilizing a Ventana Medical Systems Ultra autostainer followed by probe hybridization and signal amplification. Detection with horseradish peroxidase was then performed and slides were finished with a hematoxylin counter stain. For photomicrographs, slides were scanned at ×40 magnification using a Leica Aperio AT2 slide scanner (Buffalo Grove, IL).
Digital spatial profiling
Formalin-fixed, paraffin-embedded lung specimens from subject 09 were sectioned, baked, deparaffinized, and rehydrated before antigen retrieval according to the “GeoMx—nCounter Protein Manual Slide Preparation” protocol by the manufacturer. Slides were blocked and subjected to an overnight incubation with antibodies from the Nanostring Immuno-Oncology and Immunology core protein panel, a Geomx-compatible anti-MSLN antibody purchased from Nanostring (derived from Abcam clone EPR19025-42), an anti-PanCK antibody labeled with Cy3 (Nanostring), and an anti-CD3 antibody labeled in-house with Alexa Fluor 594 (Origene; Clone ID: UMAB54). The following day, slides were fixed and stained with a nuclear dye (SYTO13 purchased from Nanostring). Slides were imaged on the GeoMx DSP platform. Regions of relatively high vs. low inflammation were designated based on comparison with H&E-stained slides from the same blocks. To capture adequate signal from the epithelial cell population, large regions of interest encompassing 300 μm × 300 μm were designated and segments of cells positive for the anti-PanCK (Cy3) signal were selected for UV illumination and oligonucleotide capture by the instrument. The oligonucleotides were prepared for counting using the Nanostring nCounter prep station and subsequently counted on the Nanostring nCounter. Data were exported to the GeoMx DSP and analyzed following manufacturer-recommended quality control. Counts for each region of interest were normalized against the area of PanCK-positive cells sampled.
Single-cell RNA sequencing analysis
To assess the expression of MSLN mRNA in normal and fibrotic lungs, we queried the “Idiopathic Pulmonary Fibrosis Cell Atlas” (http://www.ipfcellatlas.com/).31 The site was developed by Nir Neumark and is currently being maintained by Carlos Cosme Jr. at Yale University. The platform allows investigators to plot single-cell RNA sequencing data for a selected gene from five independent studies examining normal and fibrotic human lung samples.
Human plasma membrane protein binding cell array
AVI-HIS ScFvs of SS1, MSLN-5, and MSLN-11 binders were generated and assessed in a human plasma membrane protein biding cell array (Retrogenix, Charles River Laboratories). Briefly, 3,559 expression vectors each encoding a full-length human plasma membrane protein were arrayed across 10 micorarray slides. An expression vector (plRES-hEGFR-IRES-ZsGreen1) was used to ensure that a minimal threshold of transfection efficiency had been achieved. Human HEK293 cells were seeded and used for reverse transfection/expression. ScFVs were added to each slide after fixation. Detection of binding was performed using an Alexa Fluor 647-labeled secondary antibody and images were scanned and analyzed using ImageQuant software. Hits were classified as strong, medium, weak, or very weak binders depending on the intensity of the duplicate spots.
Data availability
All non-HIPAA-protected data presented in this work are available from the authors upon request.
Acknowledgments
We would like to acknowledge the CCI Clinical Trials Unit for clinical research assistance and patient care. We thank the members of the CCI Translational Science and Correlative Studies Laboratory for analysis and interpretation. We appreciate the CCI Monitoring, Pharmacovigilance and Clinical Operations teams for their contributions to safety oversight and management of the clinical study. We thank Dr. Bruce Giantonio for contributions to this study as a medical director. We also would like to thank Novartis member May Liu and former members, Taylor Hickman, Benjamin Primack, Shawn Cogan, Tucker Ezell, Neeraja Idamakanti, Artur Veloso, Hui Gao, Qilong Wu, and Reshma Singh for their technical and scientific contribution to the lead CAR selection. The CAR design in the graphical abstract was created with BioRender.com. This study was supported by a sponsored research agreement between the University of Pennsylvania and Novartis. This work was also conducted with the support of a grant from the National Cancer Institute (P01-CA217805), which is applicable to S.M.A., A.R.H., C.H.J., L.A.L., and S.F.L.
Author contributions
A.R.H., R.J.G., L.A.L., J.J., A.R., A.C., E.D., K.S., X.L., M.M.D., N.C.S., Y.Z., J.A.F., S.F.L., G.P., R.M.Y., J.L.T., S.M.A., C.H.J., B.E., L.Z., F.X., J.A.T., M.R., B.G., K.M., and J.L.B. participated in the design, execution, and/or interpretation of the reported experiments or results. V.G., F.C., W.C., C.F., and S.J. participated in the acquisition or analysis of data. A.R.H., R.J.G., R.M.Y., S.M.A., C.H.J., J.L.T., B.E., L.Z., K.M., and J.L.B. wrote the paper, with all authors contributing to writing and providing feedback. C.H.J., A.R.H., J.L.T., and S.M.A supervised all aspects of the research.
Declaration of interests
C.H.J., R.M.Y., and M.M.D. are inventors on patents and/or patent applications licensed to Novartis Institutes of Biomedical Research and receive license revenue from such licenses. J.L.B., B.E., K.M., and L.Z. are holders of stock options and patents with Novartis Institutes for Biomedical Research. R.M.Y. and M.M.D. are inventors on patents and/or patent applications licensed to Tmunity Therapeutics and receive license revenue from such licenses. C.H.J. and A.C. are scientific cofounders of Tmunity Therapeutics. C.H.J. is a scientific cofounder of Capstan Therapeutics and is a member of the scientific advisory boards of AC Immune, Alaunos, BluesphereBio, Cabaletta, Carisma, Cartography, Cellares, Celldex, Decheng, Poseida, Verismo, and WIRB-Copernicus. B.E. and R.J.G. completed work on this study before becoming employees at Miltenyi Biotec and AstraZeneca, respectively. R.J.G. holds or may hold AstraZeneca stock. N.C.S. holds equity in Fate Therapeutics and Pfizer. J.A.F. has received grants and personal fees from Cartography Bio., grants from Tmunity Therapeutics, and personal fees from Retro Bio and Shennon Bio outside the submitted work. Additionally, J.A.F. holds patents related to CAR T cells for cancer that are licensed and associated with royalties. S.F.L. is an inventor on patents in the areas of CAR T and biomarkers at Penn that were assigned to Novartis; received research funding from Novartis, Tmunity, and Cabaletta; and consults for Kite/Gilead. M.M.D. is a consultant for Tmunity Therapeutics and is a Consultant and Member on the Scientific Advisory Board of Cellares Corporation.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.06.006.
Contributor Information
Andrew R. Haas, Email: andrew.haas2@pennmedicine.upenn.edu.
Steven M. Albelda, Email: albelda@pennmedicine.upenn.edu.
Janos L. Tanyi, Email: janos.tanyi@pennmedicine.upenn.edu.
Supplemental information
References
- 1.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F., et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turtle C.J., Hanafi L.A., Berger C., Hudecek M., Pender B., Robinson E., Hawkins R., Chaney C., Cherian S., Chen X., et al. Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson L.A., June C.H. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 2017;27:38–58. doi: 10.1038/cr.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yong C.S.M., Dardalhon V., Devaud C., Taylor N., Darcy P.K., Kershaw M.H. CAR T-cell therapy of solid tumors. Immunol. Cell Biol. 2017;95:356–363. doi: 10.1038/icb.2016.128. [DOI] [PubMed] [Google Scholar]
- 5.Hassan R., Thomas A., Alewine C., Le D.T., Jaffee E.M., Pastan I. Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J. Clin. Oncol. 2016;34:4171–4179. doi: 10.1200/JCO.2016.68.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morello A., Sadelain M., Adusumilli P.S. Mesothelin-Targeted CARs: Driving T Cells to Solid Tumors. Cancer Discov. 2016;6:133–146. doi: 10.1158/2159-8290.CD-15-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubbels J.A.A., Belisle J., Onda M., Rancourt C., Migneault M., Ho M., Bera T.K., Connor J., Sathyanarayana B.K., Lee B., et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kachala S.S., Bograd A.J., Villena-Vargas J., Suzuki K., Servais E.L., Kadota K., Chou J., Sima C.S., Vertes E., Rusch V.W., et al. Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clin. Cancer Res. 2014;20:1020–1028. doi: 10.1158/1078-0432.CCR-13-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ordóñez N.G. Application of mesothelin immunostaining in tumor diagnosis. Am. J. Surg. Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Golfier S., Kopitz C., Kahnert A., Heisler I., Schatz C.A., Stelte-Ludwig B., Mayer-Bartschmid A., Unterschemmann K., Bruder S., Linden L., et al. Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol. Cancer Ther. 2014;13:1537–1548. doi: 10.1158/1535-7163.MCT-13-0926. [DOI] [PubMed] [Google Scholar]
- 11.Hassan R., Ebel W., Routhier E.L., Patel R., Kline J.B., Zhang J., Chao Q., Jacob S., Turchin H., Gibbs L., et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan R., Ho M. Mesothelin targeted cancer immunotherapy. Eur. J. Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenito C., Milone M.C., Hassan R., Simonet J.C., Lakhal M., Suhoski M.M., Varela-Rohena A., Haines K.M., Heitjan D.F., Albelda S.M., et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl. Acad. Sci. USA. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adusumilli P.S., Cherkassky L., Villena-Vargas J., Colovos C., Servais E., Plotkin J., Jones D.R., Sadelain M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci. Transl. Med. 2014;6:261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong D.S., Johnson M., Tanyi J.L., MacMullen L., Tighe R., Jalbert L., Muzithras V.P., Zikaras K., Cardama A.Q., Hassan R. Abstract CT105: Preliminary safety and efficacy of gavocabtagene autoleucel (gavo-cel, TC-210), a T cell receptor fusion construct (TRuC™), in patients with treatment refractory mesothelin overexpressing solid tumors. Cancer Res. 2021;81 doi: 10.1158/1538-7445.AM2021-CT105. [DOI] [Google Scholar]
- 16.Liu X., Onda M., Watson N., Hassan R., Ho M., Bera T.K., Wei J., Chakraborty A., Beers R., Zhou Q., et al. Highly active CAR T cells that bind to a juxtamembrane region of mesothelin and are not blocked by shed mesothelin. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2202439119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomar S., Zhang J., Khanal M., Hong J., Venugopalan A., Jiang Q., Sengupta M., Miettinen M., Li N., Pastan I., et al. Development of Highly Effective Anti-Mesothelin hYP218 Chimeric Antigen Receptor T Cells With Increased Tumor Infiltration and Persistence for Treating Solid Tumors. Mol. Cancer Ther. 2022;21:1195–1206. doi: 10.1158/1535-7163.MCT-22-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y., Moon E., Carpenito C., Paulos C.M., Liu X., Brennan A.L., Chew A., Carroll R.G., Scholler J., Levine B.L., et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beatty G.L., Haas A.R., Maus M.V., Torigian D.A., Soulen M.C., Plesa G., Chew A., Zhao Y., Levine B.L., Albelda S.M., et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beatty G.L., O'Hara M.H., Lacey S.F., Torigian D.A., Nazimuddin F., Chen F., Kulikovskaya I.M., Soulen M.C., McGarvey M., Nelson A.M., et al. Activity of Mesothelin-Specific Chimeric Antigen Receptor T Cells Against Pancreatic Carcinoma Metastases in a Phase 1 Trial. Gastroenterology. 2018;155:29–32. doi: 10.1053/j.gastro.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maus M.V., Haas A.R., Beatty G.L., Albelda S.M., Levine B.L., Liu X., Zhao Y., Kalos M., June C.H. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 2013;1:26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas A.R., Tanyi J.L., O'Hara M.H., Gladney W.L., Lacey S.F., Torigian D.A., Soulen M.C., Tian L., McGarvey M., Nelson A.M., et al. Phase I Study of Lentiviral-Transduced Chimeric Antigen Receptor-Modified T Cells Recognizing Mesothelin in Advanced Solid Cancers. Mol. Ther. 2019;27:1919–1929. doi: 10.1016/j.ymthe.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamers C.H.J., Willemsen R., van Elzakker P., van Steenbergen-Langeveld S., Broertjes M., Oosterwijk-Wakka J., Oosterwijk E., Sleijfer S., Debets R., Gratama J.W. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117:72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 24.Hoogenboom H.R., de Bruïne A.P., Hufton S.E., Hoet R.M., Arends J.W., Roovers R.C. Antibody phage display technology and its applications. Immunotechnology. 1998;4:1–20. doi: 10.1016/s1380-2933(98)00007-4. [DOI] [PubMed] [Google Scholar]
- 25.Imai C., Mihara K., Andreansky M., Nicholson I.C., Pui C.H., Geiger T.L., Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 26.Milone M.C., Fish J.D., Carpenito C., Carroll R.G., Binder G.K., Teachey D., Samanta M., Lakhal M., Gloss B., Danet-Desnoyers G., et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma J., Tang W.K., Esser L., Pastan I., Xia D. Recognition of mesothelin by the therapeutic antibody MORAb-009: structural and mechanistic insights. J. Biol. Chem. 2012;287:33123–33131. doi: 10.1074/jbc.M112.381756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaneko O., Gong L., Zhang J., Hansen J.K., Hassan R., Lee B., Ho M. A binding domain on mesothelin for CA125/MUC16. J. Biol. Chem. 2009;284:3739–3749. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hay K.A., Hanafi L.A., Li D., Gust J., Liles W.C., Wurfel M.M., López J.A., Chen J., Chung D., Harju-Baker S., et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee M., Turner D.C., Felip E., Lena H., Cappuzzo F., Horn L., Garon E.B., Hui R., Arkenau H.T., Gubens M.A., et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann. Oncol. 2016;27:1291–1298. doi: 10.1093/annonc/mdw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumark N., Cosme C., Jr., Rose K.A., Kaminski N. The Idiopathic Pulmonary Fibrosis Cell Atlas. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020;319:L887–L893. doi: 10.1152/ajplung.00451.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merritt C.R., Ong G.T., Church S.E., Barker K., Danaher P., Geiss G., Hoang M., Jung J., Liang Y., McKay-Fleisch J., et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat. Biotechnol. 2020;38:586–599. doi: 10.1038/s41587-020-0472-9. [DOI] [PubMed] [Google Scholar]
- 33.Morris E.C., Neelapu S.S., Giavridis T., Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022;22:85–96. doi: 10.1038/s41577-021-00547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teachey D.T., Lacey S.F., Shaw P.A., Melenhorst J.J., Maude S.L., Frey N., Pequignot E., Gonzalez V.E., Chen F., Finklestein J., et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016;6:664–679. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzgerald J.C., Weiss S.L., Maude S.L., Barrett D.M., Lacey S.F., Melenhorst J.J., Shaw P., Berg R.A., June C.H., Porter D.L., et al. Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy for Acute Lymphoblastic Leukemia. Crit. Care Med. 2017;45:e124–e131. doi: 10.1097/CCM.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thistlethwaite F.C., Gilham D.E., Guest R.D., Rothwell D.G., Pillai M., Burt D.J., Byatte A.J., Kirillova N., Valle J.W., Sharma S.K., et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol. Immunother. 2017;66:1425–1436. doi: 10.1007/s00262-017-2034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narayan V., Barber-Rotenberg J.S., Jung I.Y., Lacey S.F., Rech A.J., Davis M.M., Hwang W.T., Lal P., Carpenter E.L., Maude S.L., et al. PSMA-targeting TGFbeta-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 2022;28:724–734. doi: 10.1038/s41591-022-01726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garon E.B., Rizvi N.A., Hui R., Leighl N., Balmanoukian A.S., Eder J.P., Patnaik A., Aggarwal C., Gubens M., Horn L., et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 40.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347 doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 41.Nishio T., Koyama Y., Liu X., Rosenthal S.B., Yamamoto G., Fuji H., Baglieri J., Li N., Brenner L.N., Iwaisako K., et al. Immunotherapy-based targeting of MSLN(+) activated portal fibroblasts is a strategy for treatment of cholestatic liver fibrosis. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2101270118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koyama Y., Wang P., Liang S., Iwaisako K., Liu X., Xu J., Zhang M., Sun M., Cong M., Karin D., et al. Mesothelin/mucin 16 signaling in activated portal fibroblasts regulates cholestatic liver fibrosis. J. Clin. Invest. 2017;127:1254–1270. doi: 10.1172/JCI88845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai J.M., Sinha R., Seita J., Fernhoff N., Christ S., Koopmans T., Krampitz G.W., McKenna K.M., Xing L., Sandholzer M., et al. Surgical adhesions in mice are derived from mesothelial cells and can be targeted by antibodies against mesothelial markers. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aan6735. [DOI] [PubMed] [Google Scholar]
- 44.Hassan R., Bullock S., Premkumar A., Kreitman R.J., Kindler H., Willingham M.C., Pastan I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin. Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 45.Hassan R., Kindler H.L., Jahan T., Bazhenova L., Reck M., Thomas A., Pastan I., Parno J., O'Shannessy D.J., Fatato P., et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin. Cancer Res. 2014;20:5927–5936. doi: 10.1158/1078-0432.CCR-14-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassan R., Blumenschein G.R., Jr., Moore K.N., Santin A.D., Kindler H.L., Nemunaitis J.J., Seward S.M., Thomas A., Kim S.K., Rajagopalan P., et al. First-in-Human, Multicenter, Phase I Dose-Escalation and Expansion Study of Anti-Mesothelin Antibody-Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors. J. Clin. Oncol. 2020;38:1824–1835. doi: 10.1200/JCO.19.02085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richards R.M., Zhao F., Freitas K.A., Parker K.R., Xu P., Fan A., Sotillo E., Daugaard M., Oo H.Z., Liu J., et al. NOT-Gated CD93 CAR T Cells Effectively Target AML with Minimized Endothelial Cross-Reactivity. Blood Cancer Discov. 2021;2:648–665. doi: 10.1158/2643-3230.BCD-20-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher B., Packard B.S., Read E.J., Carrasquillo J.A., Carter C.S., Topalian S.L., Yang J.C., Yolles P., Larson S.M., Rosenberg S.A. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J. Clin. Oncol. 1989;7:250–261. doi: 10.1200/JCO.1989.7.2.250. [DOI] [PubMed] [Google Scholar]
- 49.Adusumilli P.S., Zauderer M.G., Rivière I., Solomon S.B., Rusch V.W., O'Cearbhaill R.E., Zhu A., Cheema W., Chintala N.K., Halton E., et al. A Phase I Trial of Regional Mesothelin-Targeted CAR T-cell Therapy in Patients with Malignant Pleural Disease, in Combination with the Anti-PD-1 Agent Pembrolizumab. Cancer Discov. 2021;11:2748–2763. doi: 10.1158/2159-8290.CD-21-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson S.W., Laub P.B., Beesley J.S., Ozols R.F., Hamilton T.C. Increased platinum-DNA damage tolerance is associated with cisplatin resistance and cross-resistance to various chemotherapeutic agents in unrelated human ovarian cancer cell lines. Cancer Res. 1997;57:850–856. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All non-HIPAA-protected data presented in this work are available from the authors upon request.