Abstract
Induced pluripotent stem cells (iPSCs) express a broad spectrum of tumor-associated antigens and exert prophylactic effects on various tumors. However, some problems remain, such as potential tumorigenicity, challenges in transport to the lymph nodes and spleen, and limited antitumor effects. Thus, designing a safe and effective iPSC-based tumor vaccine is necessary. We prepared iPSC-derived exosomes and incubated them with DCs (dendritic cells) for pulsing to explore their antitumor effects in murine melanoma models. The antitumor immune response induced by the DC vaccine pulsed with iPSC exosomes (DC + EXO) was assessed in vitro and in vivo. After DC + EXO vaccination, extracted spleen T cells effectively killed a variety of tumor cells (melanoma, lung cancer, breast cancer, and colorectal cancer) in vitro. In addition, DC + EXO vaccination significantly inhibited melanoma growth and lung metastasis in mouse models. Furthermore, DC + EXO vaccination induced long-term T cell responses and prevented melanoma rechallenge. Finally, biocompatibility studies showed that the DC vaccine did not significantly alter the viability of normal cells and mouse viscera. Hence, our research may provide a prospective strategy of a safe and effective iPSC-based tumor vaccine for clinical use.
Keywords: induced pluripotent stem cells, exosomes, DC vaccine, melanoma
Graphical abstract

Huang and colleagues found that iPSC exosomes carry tumor-associated antigens. They prepared iPSC-derived exosomes and combined them with dendritic cells to explore their antitumor effects in melanoma models. This research offers a prospective strategy for a safe and effective iPSC-based tumor vaccine.
Introduction
Tumor vaccine refers to presenting tumor-associated antigen to provoke specific antitumor immunity and kill tumor cells.1,2,3 Over the past few decades, tumor vaccines have achieved remarkable clinical results with multiple clinical products in an increasing number of clinical trials.4,5,6 However, many obstacles still exist, such as efficient mobilization of the immune system, available vaccine sources, and wide-spectrum effects. These obstacles prompted researchers to seek feasible solutions.7,8
In recent years, induced pluripotent stem cells (iPSCs) have been increasingly applied to the field of tumor vaccines.9,10,11 Kooreman et al.10,12,13 found that iPSCs share similar gene expression patterns with tumor cells, and that iPSCs express a broad spectrum of tumor-associated antigens; these results indicated that iPSCs can produce various tumor antigens that activate antitumor responses and induce tumor regression.10,12,13 However, some problems remain. First, iPSC vaccines need to be irradiated before administration to inhibit tumorigenicity, so intact iPSCs carry potential safety risks.10 Second, as a cellular vaccine, iPSCs are large and easy to intercept, and it is difficult for them to be transported to the lymph nodes and spleen.14 Moreover, current iPSC vaccines exert limited antitumor effects, probably due to the improper presentation of iPSC-derived antigens and the immunosuppressive microenvironment of established tumors.11,13 Therefore, there is a need to develop safer and more effective iPSC-based vaccines as an alternative strategy.
Exosomes are extracellular vesicles that are secreted by cells and are rich in proteins, nucleic acids, and lipids.15,16,17 In the exploration of various cancer immunotherapies, exosome vaccines have shown outstanding performance.18,19,20,21,22 Wolfers et al. first discovered that the delivery of exosomes from solid tumors to dendritic cells (DCs) results in the activation of T cell-mediated immune responses that ultimately lead to tumor rejection.22 Furthermore, bone marrow-derived dendritic cells (BMDCs) that are loaded with exosomes in vitro exert an excellent therapeutic effect on established tumors.22 Subsequently, Andre et al. reported that exosomes collected from patients’ malignant ascites constitute a source of tumor antigens that can be presented to cytotoxic T lymphocytes by DCs, which then lead to an antitumor response.15 The superior properties of exosomes over conventional antigens include two aspects. First, exosomes contain molecules essential for antigen presentation, such as MHC class I and II, co-stimulatory molecules, heat shock proteins (HSPs), and intercellular adhesion molecules.18,21,22,23,24,25 Second, exosomes are small in size, easy to be taken up, and safe; indeed, these cell-free products pose no risk of tumorigenicity.26,27 These results suggest that iPSC-derived exosomes may be a better source of tumor vaccines compared with intact iPSCs.
However, to achieve ideal antitumor effects based on iPSC-derived exosomes, it is important to identify a proper strategy for the uptake and presentation of iPSC-derived antigens despite the immunosuppressive microenvironment of tumors. DCs were discovered by Nobel Laureate Ralph Steinman in 1973,28 and this discovery revolutionized the field of vaccination.25 Many years of studies have focused on the use of DC vaccines against cancer, which have resulted in satisfactory clinical prognosis.29,30,31 Compared with conventional vaccines, DC vaccines exert both tumor therapeutic and prophylaxis effects32; the advantage of these vaccines is that adequate maturation of DC in vitro avoids the endogenous immunosuppressed microenvironment in tumors,33,34,35 suggesting that this is an ideal strategy for iPSC tumor vaccines.
In this study, we prepared exosomes derived from human iPSCs (hiPSCs) and mouse iPSCs (miPSCs) and incubated them with DCs for pulsing to explore their antitumor effects in melanoma models. DC + EXO induced a robust antitumor response and inhibited melanoma progression in prophylactic, therapeutic, lung metastasis, and recurrence models, and they caused no significant adverse effects. Overall, DC + EXO could be a safe and effective tumor vaccine, holding the prospect of clinical application in cancer therapy.
Results
Characterization of iPSC exosomes and uptake by DCs
To characterize iPSC-derived exosomes, we followed similar schedules (Figures S1A–S1E) and established mouse (Figures S2A–S2E) and hiPSC cell lines (Figures S3A–S3E) with morphology and pluripotent identification. Then we prepared iPSC-derived exosomes by ultracentrifugation as described previously.36 The expression of iPSC exosome markers (Calnexin, HSP70, TSG101, CD63, and CD81) was measured by western blotting. Consistent with the standard stipulated by the International Association of Extracellular Vesicles,37 both hiPSC and miPSC exosomes expressed exosomal proteins such as HSP70, TSG101 (cytoplasmic protein), and CD63 (transmembrane protein) with no expression of Calnexin (endoplasmic reticulum protein) (Figure 1A). We detected several major tumor-associated antigens in the iPSC exosomes, such as epidermal growth factor receptor (EGFR), melanoma antigen recognized by T cells 1 (MART-1), glycoprotein 100 (gp100), carcinoembryonic antigen (CEA), and mucin 1 (MUC1) (Figures 1B and 1C). EGFR is a tyrosine kinase receptor that mediates cell growth, differentiation, and survival signals, which is mutated or overexpressed in many solid tumors, such as lung cancer, breast cancer, colorectal cancer, head and neck cancer, and glioblastoma38; MART-1 and gp100 are melanocyte differentiation antigens that are frequently expressed in melanoma cells;39,40,41 CEA is a glycoprotein that is overexpressed in various cancers, especially colorectal cancer42; MUC1 is a mucin that is aberrantly expressed and glycosylated in many epithelial cancers, such as breast, ovarian, and pancreatic cancer.43 These results indicate that the iPSC exosome-pulsed DC vaccine may induce antitumor immunity by presenting a broad spectrum of tumor-associated antigens to the immune system, which may indicate their promising application as a tumor vaccine. In addition, exosomes isolated from both cell lines exhibited typical exosome morphology (cup- or disc shaped) with diameters ranging from 30 to 150 nm as verified by transmission electron microscopy (Figure 1D). Nanoparticle tracking analysis (NTA) showed that miPSC and hiPSC exosomes had similar particle sizes between 30 and 150 nm (Figure 1E). Finally, by BCA protein quantification, we detected that with the same cell number and preparation process (isolate exosomes from the supernatant of 1 × 107 cells after 24 h culture by ultracentrifugation and resuspended by 100 μL PBS), the yield of hiPSC-derived exosomes was approximately 667 times higher than that of DLD-1-derived exosomes, and the yield of miPSC exosomes was approximately 197 times higher than the yield of B16-derived exosomes (Figure 1F), which indicates promising large-scale production for clinical use.
Figure 1.
Characterization of iPSC-derived exosomes
(A) Western blotting was performed to measure the expression of exosome markers. (B) Western blot assay was performed to detect the expression of EGFR, MART-1, gp100, CEA, and MUC1 in hEXO and mEXO. Each lane was loaded with 20 μg of exosomal protein. Positive control (PC) was established using 10 μg of standard proteins, allocating 2 μg for each protein. (C) The statistical result of relative gray values. (D) Morphology of exosomes observed by transmission electron microscopy. Scale bars, 50 nm. (E) NTA of the particle size distribution of exosomes. (F) Comparison of the exosome yields from miPSCs to B16F10 and hiPSCs to DLD-1 (n = 5, data are expressed as mean ± SEM, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
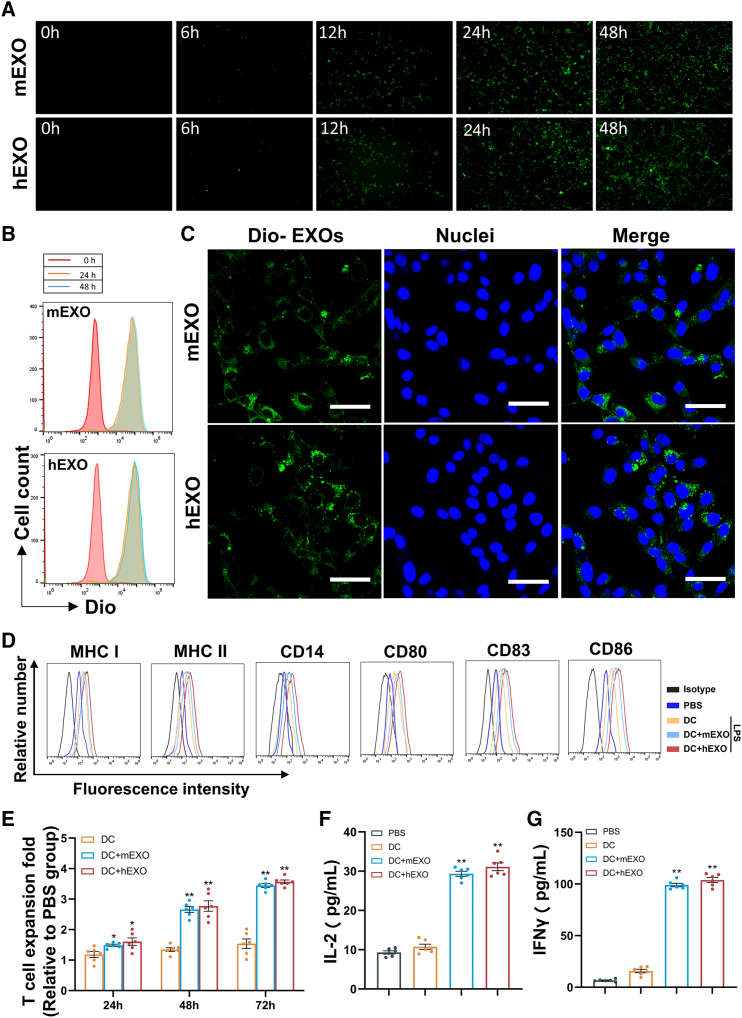
DC uptake of iPSC exosomes and antitumor effect in vitro
To investigate whether iPSC exosomes can be effectively taken up by DCs, DiO-labeled iPSC exosomes (DiO-EXOs) were coincubated with DCs for 48 h, and images of exosome uptake were obtained by fluorescence microscopy. The fluorescence values were detected by flow cytometry. From 0 to 48 h, a gradual increase in exosome uptake was observed, approaching a peak at 24 h (Figures 2A and 2B). Furthermore, DiO-labeled iPSC exosomes appeared perinuclear after taken up into the cell (Figure 2C). These results indicate that both miPSC and hiPSC exosomes can be efficiently taken up by DCs. Notably, iPSC exosomes uptake promoted DC maturation and activation as demonstrated by elevated levels of MHC class I and II, and expression of costimulatory factors CD80, CD83, and CD86, and CD14, a differentiation and activation marker on DC + mEXO surface compared with untreated DCs (Figure 2D). As a complementary approach, we observed increased levels of MHC class I and II, and expression of CD80, CD83, CD86, and CD14 on the surface of DC2.4 cells after iPSC exosome uptake (Figure S4), indicating that iPSC exosomes can also induce maturation and activation of DC2.4 cells. These results suggest that iPSC exosomes are capable of activating DCs.
Figure 2.
DC uptake of iPSC exosomes and antitumor effect in vitro
(A) DC uptake of DIO-labeled iPSC exosomes detected by fluorescence microscopy at different time points. (B) DC uptake of iPSC exosomes was quantitatively measured by flow cytometry at 24 and 48 h, respectively. (C) Confocal fluorescence microscopy images showing the localization of iPSC exosomes in DCs. Green represents DIO-labeled exosomes; blue represents nuclei stained with Hoechst 33342. Scale bars, 50 μm. Naive T cells were stimulated with treatment in each group for 24–72 h. BMDC were incubated with EXO in the presence of 5 μg/mL LPS. (D) The expression of DC mature markers was detected by flow cytometry including CD14, CD80, CD83, CD86, MHC class I and II on BMDC. (E) CCK8 was used to measure T cell proliferation. (F and G) ELISA was used to measure IFN-γ and IL-2 levels in the culture medium at 24 h (n = 6). Comparison was performed between DC + EXO and control or blank DC-immunized groups. All data are expressed as mean ± SEM, ∗p < 0.05, ∗∗p < 0.01.
To examine the in vitro activation of T cells by iPSC exosome-pulsed DCs, we performed a T lymphocyte stimulation assay. T cells were randomly assigned to coincubate with a PBS control, blank DCs, DCs pulsed with hiPSC-derived exosomes (DC + hEXO), or DCs pulsed with murine iPSC-derived exosomes (DC + mEXO) for 72 h, respectively. As shown in Figure 2E, compared with the phosphate-buffered saline (PBS) group, T cells in the DC + hEXO and DC + mEXO groups expanded by 3.3- and 3.5-fold, respectively, within 72 h. The T cells in the DC group did not expand significantly. In addition, compared with the PBS group or DC group, the DC + hEXO, and DC + mEXO groups significantly promoted the secretion of IL-2 and IFN-γ from the corresponding T cells (Figures 2F and 2G). No significant difference was found between the DC + hEXO and DC + mEXO groups. These results indicate that DC + hEXO and DC + mEXO can activate T cells in vitro.
Lymphocytotoxic effect induced by DC + EXO
To confirm whether DC + EXOs has a broad spectrum of anticancer activities, we pulsed DCs by coincubating mEXOs with different concentrations to assess the killing efficiency of splenic CD8+ T cells after DC + mEXO immunization. As shown in Figure S5, as the mEXO concentration increased, the efficiency of splenic CD8+ T cells for killing B16F10 cells peaked at 80 μg/mL. Then, we coincubated 80 μg/mL mEXOs with DCs for loading to explore the killing efficiency of splenic T cells on four tumor cell lines at different effector-to-target cell ratio conditions. Spleen T cells have potent killing activity against B16-F10, LLC, 4T1, and MC38 cells (Figures 3A–3D). At the effect cell to target cell ratio of 20:1, DC + mEXO induced lymphotoxicity responses against B16-F10 cells (53.0% ± 0.7% specific killing), LLC cells (40.0% ± 0.9% specific killing), 4T1 cells (30.1% ± 1.8% specific killing), and MC38 cells (23.5% ± 0.6% specific killing), and DC + hEXO induced lymphotoxicity against B16F10 cells (49.3% ± 0.6% specific killing), LLC cells (38.9% ± 0.4% specific killing), 4T1 cells (23.6% ± 1.1% specific killing), and MC38 cells 21.5% ± 0.4% specific killing). The response was more effective than that of the DC group or the PBS group. In addition, as the effector-to-target cell ratio escalates from 5:1 to 20:1, cytotoxic effect grows correspondingly. A continued increase could potentially lead to near 100% efficiency of T cell-induced tumor cell elimination, eventually plateauing at this level.44,45 To test whether the killing effect was produced based on the shared epitopes between iPSCs and cancer cells, we prepared a DC vaccine pulsed with exosomes from B16-F10 (DC + BEXO) and incubated them with spleen T cells. Compared with the PBS group or DC group, the DC + BEXO groups significantly promoted the secretion of IFN-γ and IL-2 from the corresponding T cells (Figure S6A and S6B). Then we inoculated mice with DC + BEXO with the same schedule, DC + BEXOs also induced lymphotoxicity responses against miPSCs (54.4% ± 1.0%) (Figure 3E) and hiPSCs (45.0% ± 0.9%) (Figure 3F). These results indicate that both DC + hEXO and DC + mEXO have broad-spectrum antitumor activities based on shared antigen epitopes, especially against melanoma.
Figure 3.
Lymphocytotoxicity effect induced by DC + EXO and DC + BEXO
After vaccinating the mouse with DCs pulsed with iPSC exosomes, the killing efficiency of splenic T cells against B16F-10 (A), LLC (B), 4T1 (C), and MC38 (D) at different effector-target ratio conditions was determined. Comparison was performed between DC + EXO and PBS or blank DC-immunized groups (n = 6). Data are expressed as mean ± SEM, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. After vaccinating the mouse with DCs pulsed with B16-F10 exosomes, the killing efficiency of splenic T cells against (E) miPSC and (F) hiPSC (n = 6). Comparison was performed between DC + BEXO and control or blank DC-immunized groups. All data are expressed as mean ± SEM, ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
The prophylactic effect of DC + EXO on melanoma
To explore the efficacy of our iPSC exosome-pulsed DC vaccine in preventing tumor progression in vivo, mice were pre-vaccinated with DC + EXO (Figure 4A). Mice injected with PBS served as a negative control group; Mice injected with DC pulsed with bone mesenchymal stem cell-derived exosomes (DC + BMEXO) served as control to represent exosomes from standard cells; mice injected with DC pulsed with B16F10-derived exosomes (DC + BEXO) served as positive control. The PBS, DC, and DC + BMEXO groups showed apparent tumor growth after B16F10 cell inoculation. Notably, significant tumor inhibition was found in mice inoculated with DC + mEXO, DC + hEXO, and DC + BEXO (Figures 4B, 4C, and S7A–S7C), and survival rates over 40 days increased to 70%, 50%, and 70% (Figure 4D), respectively. In addition, the DC + mEXO and DC + BEXO groups showed similar tumor growth and survival. These results indicate that DC + hEXO and DC + mEXO vaccines effectively prevent melanoma progression in mice.
Figure 4.
DC + EXO-mediated in vivo prophylaxis experiment
(A) Timeline of vaccination versus tumor. s.c., subcutaneous injection; i.v., intravenous injection). (B and C) Tumor sizes among the six groups treated (n = 6). (D) Cumulative survival of B16-F10 inoculated mice after DC + EXO pre-vaccination (n = 10). After the last vaccination, (E) the percentage of CD8+ T cells and CD4+ T cells, (F) the ratio of CD8+ T cells to CD4+ T cells, and (G) serum IFN-γ levels were determined (n = 6). All data are expressed as mean ± SEM, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant.
To examine the in vivo immune response induced by DC + EXO, we analyzed splenic T cell subsets by flow cytometry. Seven days after the last immunization, the T lymphocytes among the spleen cells from immunized mice were examined. As shown in Figures 4E and 4F, the number of CD8+ T lymphocytes in DC + mEXO, DC + hEXO, and DC + BEXO groups significantly increased compared with that in the PBS, DC, and DC + BMEXO groups, although no difference was observed in the number of CD4+ T lymphocytes. In addition, the ratio of CD8+ to CD4+ T lymphocytes in the DC + mEXO, DC + hEXO, and DC + BEXO groups also significantly increased compared with that in the PBS, DC, and DC + BMEXO groups. We also detected the level of IFN-γ in the serum of the immunized mice. The results showed that the levels of IFN-γ in the serum of mice in the DC + mEXO, DC + hEXO, and DC + BEXO groups were significantly higher than those in the PBS and DC groups (Figure 4G). There was no significant difference between the DC + mEXO and DC + BEXO groups. These results suggest that DC + hEXO and DC + mEXO could effectively activate CD8+ T lymphocytes in vivo.
The therapeutic effect of DC + EXO on melanoma
To explore the therapeutic effect of the iPSC exosome-pulsed DC vaccine on established tumors in vivo, mice were preinoculated with 1 × 106 B16-F10 cells and injected with the DC vaccine 5 days later (Figure 5A). Mice injected with PBS served as the control group. Mice injected with DC pulsed with DC + BMEXO served as control to represent exosomes from normal cells; mice injected with DC pulsed with B16F10-derived exosomes served as positive control. As shown in Figures 5B, 5C, and S8A–S8C, significant tumor growth occurred in the PBS-, blank DC-, and DC + BMEXO-immunized groups. Tumor growth was significantly inhibited in mice treated with DC + mEXO, DC + hEXO, and DC + BEXO, and the survival rates over 40 days increased to 60%, 40%, and 60%, respectively (Figure 5D). These results suggest that DC + hEXO and DC + mEXO vaccines effectively inhibited the growth of established melanoma and increased survival rate. To analyze the changes in the tumor microenvironment of mice treated with DC + EXO, we digested tumor tissue and extracted lymphocytes, and we analyzed the changes in their lymphocyte phenotypes by flow cytometry. The numbers of CD8+ T lymphocytes in the tumor tissues of the DC + hEXO-, DC + mEXO-, and DC + BEXO-treated mice were significantly increased compared with those in the PBS-, blank DC-, and DC + BMEXO-treated mice (Figures 5E and 5F), although no difference was observed in the number of CD4+ T lymphocytes, indicating that DC + hEXO and DC + mEXO mainly activated cytotoxic T lymphocytes. Notably, the numbers of CD25+FoxP3+CD4+ regulatory T cells (Tregs) in the tumor tissues of mice treated with DC + hEXO, DC + mEXO, and DC + BEXO were significantly reduced compared with those in PBS-, DC-, and DC + BMEXO-treated mice (Figures 5G and 5H). In addition, no significant differences were observed between the DC + mEXO and DC + BEXO groups. Overall, DC + hEXO and DC + mEXO treatment improved the immunosuppressive tumor microenvironment of melanoma in mice.
Figure 5.
DC + EXO-mediated in vivo therapeutic assay
(A) Timeline of tumor cell and DC vaccine injection schedule. s.c., subcutaneous injection; i.v., intravenous injection. (B and C) Tumor sizes among the six groups after treatment (n = 6). (D) Cumulative survival of melanoma-bearing mice (n = 10). After receiving DC + EXO treatment, the proportion of (E and F) CD8+ T, CD4+ T (n = 6) and (G and H) CD4+CD25+Foxp3+ Treg cells (n = 6) in mouse melanoma tissues changed between DC + EXO and control or blank DC-immunized groups. All data are expressed as mean ± SEM, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant.
To investigate the underlying mechanisms of the enhanced tumor rejection triggered by exposure to DC + mEXO, we inoculated mice with B16F10 cells (1 × 106) and divided them randomly into six groups. Depleting antibodies against cell surface markers were administered intraperitoneally (i.p.) starting 1 day prior to DC + mEXO therapy initiation to deplete macrophage (colony-stimulating factor 1 receptor [CSF1R]), NK cells (asialo ganglio-N-tetraosylceramide [ASGM1]), CD8+ T cells (CD8), or CD4+ T cells (CD4),46,47,48 with confirmation of specific cell type depletion by flow cytometry of peripheral blood mononuclear cells (Figure S9A). The mice were then vaccinated with DC + mEXO or PBS (as control) four times at days 5, 8, 11, and 14 after B16F10 inoculation. As Figures S9B–S9D demonstrates, mice vaccinated with DC + mEXO displayed significant tumor regression with an intact immune system, while tumors progressed and survival rate declined in CD8-depleted mice despite DC + mEXO exposure. Macrophages and CD4+ T cell blockade did not affect the efficacy of DC + mEXO vaccination, while the limitation of NK cells led to some degree of tumor progression in DC + mEXO-vaccinated mice. Overall, the antibody depletion studies showed that CD8+ T cells play a critical role in tumor rejection, with other immune cell subsets such as NK cells playing some role in tumor regression.
The inhibiting effect of DC + EXO on melanoma lung metastasis and tumor recurrence
To explore the efficacy of the iPSC exosome-pulsed DC vaccine in inhibiting melanoma lung metastasis in vivo, we inoculated 2 × 105 B16F10 cells via the tail vein and administered the DC + EXO 5 days later (Figure 6A). Mice injected with PBS served as the control. As shown in Figures 6B and 6C, the numbers of lung metastatic nodules and lung weight were significantly reduced in the DC + mEXO, DC + hEXO, and DC + BEXO immunized groups compared with the control, blank DC, and DC + BMEXO-immunized groups. No significant differences were observed between the DC + mEXO and DC + BEXO groups. These results suggest that the DC + EXO vaccine effectively prevents melanoma lung metastases in mice.
Figure 6.
DC + EXO-mediated inhibition of melanoma lung metastasis and recurrence
(A) Timeline of the tumor cell inoculation and DC vaccination. i.v., intravenous injection. (B and C) Comparison of the number of metastatic nodules in the lungs of mice with melanoma (n = 6). (D) Comparison of the lung weight between different groups (n = 6). (E–G) DC + EXO-vaccinated mice (complete tumor regression up to 110 days) were reinoculated with B16-F10-luciferase cells subcutaneously, and tumor growth was monitored over time (n = 6). (E) Fluorescence intensity of melanoma at day 20 post inoculation. (F) Tumor weight at day 20 post inoculation. (G) Tumor growth curve after B16-F10-luciferase rechallenge. (H and I) PBMCs were isolated from tumor-eradicated mice (up to 110 days post inoculation) and stimulated with B16-F10-luciferase lysates or PBS as control, and the proportion of effector memory T cells was analyzed by flow cytometry (n = 6). All comparison was performed between DC + EXO and naive mice groups. All data are expressed as mean ± SEM, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant.
To investigate the growth of melanoma more clearly, we established the luciferase-expressed B16-F10 cell line (B16-F10-luciferase) by infecting B16-F10 with lentivirus carrying the luciferase-mCherry-puromycin-expressing gene (Figures S10A and S10B). After DC + EXO vaccination, we inoculated mice with 2 × 105 B16-F10-luciferase, the tumor-eradicated mice were subcutaneously rechallenged with 2 × 105 B16-F10 cells on day 110 after primary tumor inoculation. Notably, rejection of re-inoculated B16-F10 cells was observed in 83% DC + mEXO vaccinated mice and 50% DC + hEXO vaccinated mice, while melanoma gradually formed over time in naive mice (Figures 6E–6G and S11A–S11E). We restimulated tumor-eradicated mice with B16-F10-luciferase cell lysates and collected PBMCs from them to investigate the memory T cells. The ratio of CD44+CD62L−-activated effector T cells significantly increases in comparison with the naive mice group after the B16-F10-luciferase lysate treatment (Figure 6I). These results indicate that the DC + EXO vaccine can generate durable immune memory against B16-F10 and prevent melanoma recurrence.
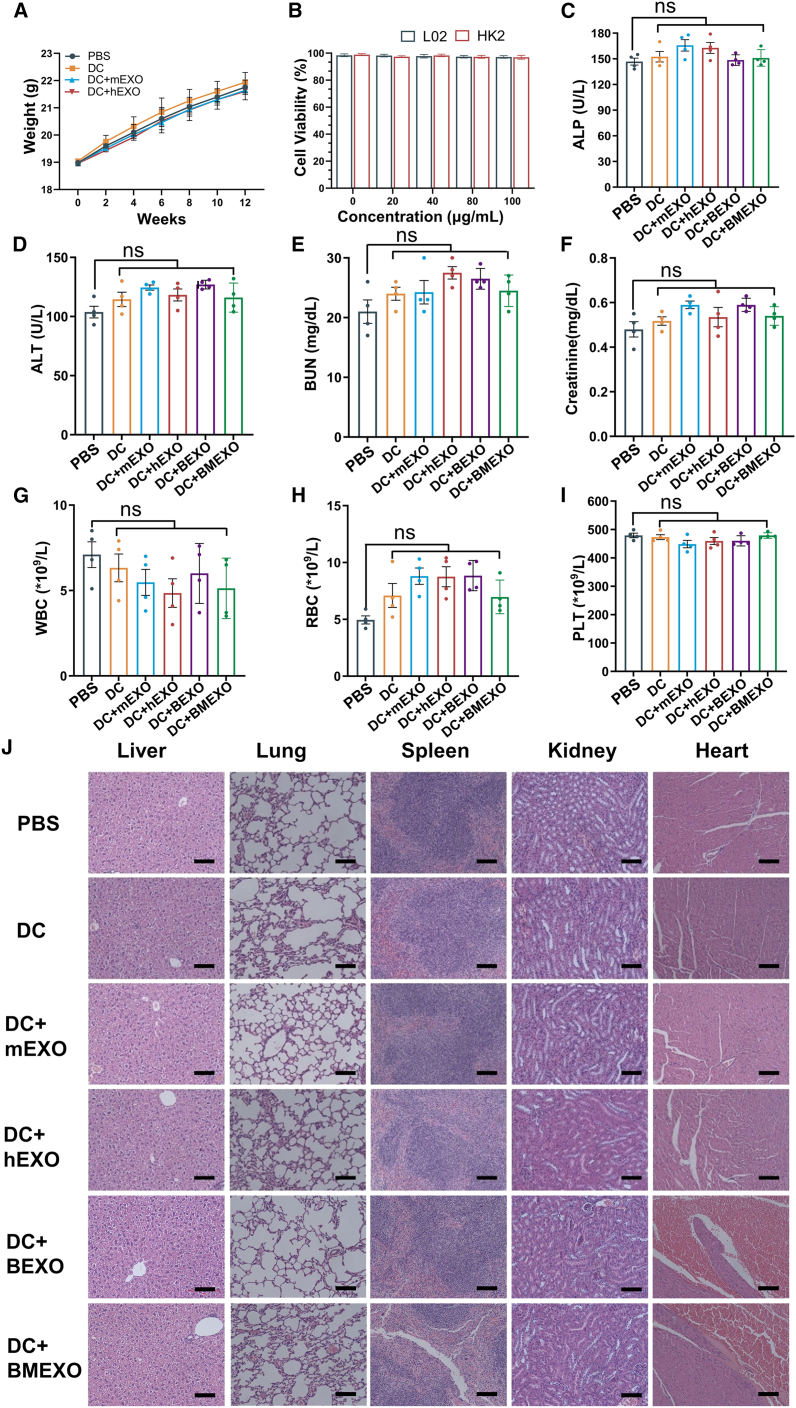
Biocompatibility experiments of DC + EXO vaccine
To verify the biosafety of DC + EXO, we continuously monitored the body weights of the mice. No significant difference was found in the body weight between different groups after 12 weeks of observation (Figure 7A). In addition, we detected the viability of the human L02 normal liver cell line and the HK2 normal kidney proximal convoluted tubule epithelial cell line by CCK8 assay after coincubation with DC + EXO. No significant difference was found between the different concentration groups, indicating that DC + EXO does not alter the viability of these human normal liver and kidney proximal convoluted tubule epithelial cell lines (Figure 7B). In addition, we measured the levels of alkaline phosphatase and alanine transaminase, which are indicators of liver damage (Figures 7C and 7D). We also measured creatinine and blood urea nitrogen levels, which are indicators of liver damage (Figures 7E and 7F). The platelets, red blood cells, and white blood cells were also monitored. Compared with the control group, there were no significant differences in the DC + EXO-treated groups. Finally, we made H&E-stained sections of vaccinated and control mice for analysis. The results showed that the DC vaccine caused no obvious toxicity to the mouse viscera (Figure 7G). To sum up, the DC + EXO vaccine displays good biosafety in vivo.
Figure 7.
Analyses of DC-EXO safety
(A) Changes in the body weight of mice after vaccination. (B) The effect of DC vaccines pulsed with iPSC exosomes on the activity of the human L02 normal liver cell line and the HK2 normal kidney proximal convoluted tubule epithelial cell line. (C) Alkaline phosphatase (ALP), (D) alanine transaminase (ALT), (E) blood urea nitrogen (BUN), and (F) creatinine (Cre) levels in mouse serum, and (G) white blood cell (WBC), (H) red blood cell (RBC), (I) platelet (PLT) levels in peripheral blood were detected after DC-EXO vaccination (n = 4). (J) Effects of iPSC exosome-pulsed DC vaccine on mice viscera: H&E-stained sections of heart, liver, spleen, lung, and kidney. Scale bars, 100 μm. Statistically significant differences were observed compared with the PBS group. All data are expressed as mean ± SEM; ns, not significant.
Discussion
iPSCs have been shown to express tumor-associated antigens in previous studies and to exert a prophylactic effect on various tumors.10,49 However, some problems remain, such as potential safety issues due to teratoma development,10 challenges in transport to the lymph nodes and spleen,14 and limited antitumor effects.11,13 Here, iPSC exosomes serve as broad-spectrum antigen carriers and exert robust and long-term antitumor effects when combined with DC immunotherapy. Significantly, DC-EXO treatment improved the immunosuppressive tumor microenvironment in mouse melanoma models, as manifested by increased levels of immunostimulatory cytokines, increased infiltration of CD8+ T cells, and decreased numbers of immunosuppressive Treg cells. Based on these results, iPSC-derived exosomes have the potential to be an effective antigen source for enhancing DC antitumor immunity, and DC pulsed with iPSC exosomes can serve as a promising tumor vaccine strategy. To the best of our knowledge, this study is the first to report the efficacy of iPSC exosomes in the field of tumor vaccines.
DC vaccines are a type of cancer immunotherapy that use DCs to present antigen and stimulate the immune system to attack cancer cells. DC exosome vaccines are created by taking exosomes from DCs, loading them with tumor antigens, and then injecting them back into the patient to stimulate an immune response against the cancer.50 In 1998, Zitvogel et al. first demonstrated that DC-derived exosomes (dexosomes) expressing MHC class I and II as well as T cell costimulatory molecules can facilitate immune cell-dependent tumor rejection.20 The DC exosome vaccines and DC vaccines are both based on DCs but are two different immunotherapy strategies. The DC vaccine involves the isolation, ex vivo loading, and reinfusion of DCs into patients, while the DC exosome vaccine involves the isolation, purification, and administration of dexosomes into patients.50 The advantages of the DC exosome vaccine over the DC vaccine include the following: (1) dexosomes are more stable and easier to store and transport than DCs, (2) dexosomes can cross the blood-brain barrier and target tumors in the central nervous system, and (3) dexosomes can avoid the tumor-mediated immunosuppression and the functional limitation of the commonly used monocyte-derived DCs.32,51 However, the DC exosome vaccine also faces some challenges, such as the low yield of dexosomes, the heterogeneity of dexosome composition, and the potential immunogenicity of dexosome components.50 Therefore, more research is needed to optimize the production, characterization, and delivery of dexosomes for cancer immunotherapy. In summary, the DC exosome vaccine is a promising immunotherapy strategy that has some advantages over the DC vaccine in terms of stability, targeting, and immunosuppression resistance. However, it also faces some technical and biological challenges that need to be addressed in future studies.
In recent years, exosomes have emerged as promising agents for cancer diagnosis and immunotherapy, especially in the field of tumor vaccines.19,30,52,53,54 A recent study unveiled an innovative tumor vaccine that utilizes cis-spliced chimeric RNA-loaded extracellular vesicles derived from DCs. It is noteworthy that, apart from the conventional antigen sources found in traditional tumor vaccines, this study demonstrated that cis-spliced chimeric RNA can also generate tumor antigens.52 In addition, Wang and co-workers reported engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer.30 Tracing back to 2001, Wolfers et al. first proposed that tumor exosomes are rich in tumor antigens and exert good antitumor effects.22 Andre et al. first verified that exosomes isolated from abdominal ascites contained tumor-rejection antigens and activated tumor-specific cytotoxic T cells in different tumor types.15 In this study, we propose the feasibility of the use of iPSC exosomes as tumor vaccines for the first time and show that these exosomes exert excellent therapeutic effects and inhibit lung metastases through in vitro pulsing of activated DCs. This study provides a solid theoretical basis for further research on iPSC exosome-based vaccines.
The choice of the antigen source is crucial for the use of DC vaccines in immunotherapy as the heterogeneous expression of tumor antigens.55 Previous studies have reported that a single antigen carried a risk of immune escape, in which immune response was not elicited by HLA-restricted peptides.56,57 Herein, we applied iPSC exosomes as multiple antigen sources in DC immunotherapy. This method takes advantage of the unique characteristics of iPSC-EXOs carrying broad-spectrum antigens, which can activate tumor-specific CD8+ cytotoxic T cells, thereby amplifying the immunotherapy effect of DCs, as indicated by the inhibitory effect on various tumors in vitro and melanoma in vivo. Moreover, DC + mEXO and DC + hEXO both induced effective antitumor reaction. This indicates that the hiPSC exosome may elicit cross-reactive immunity. Thus, iPSC exosomes represent a novel and effective antigen source for DC vaccines and solve the issue of limited tumor antigen sources for patients in current research.
According to previous studies, the main explanation for the limited antitumor effect of the iPSC-based tumor vaccine is improper antigen presentation and the immunosuppressive microenvironment of established tumors.11,13 This study combined the iPSC-based tumor vaccine with DC-based immunotherapy, which exerted excellent antitumor effects. The advantage of these vaccines is that adequate maturation of DCs in vitro avoids the endogenous immunosuppressed microenvironment in tumors.32,58,59 Overall, it represents an ideal strategy for the development of effective iPSC-based tumor vaccines. In further research, the combination with the heat shock method holds the potential to enhance the yield of exosomes and increase the presence of HSP70 within these exosomes.60,61 HSP70 plays a crucial role in the process of antigen presentation. By improving these factors, the efficacy of iPSC exosome-based tumor vaccines can be ultimately enhanced. In addition, combination with a PD-1 immune checkpoint inhibitor or an immunologic adjuvant such as CPG, which can be loaded into iPSC exosomes, is also a promising immunotherapy strategy.
In this study, we generated miPSCs by extracting embryonic fibroblasts from pregnant mice and infecting them with a CytoTune-iPS Sendai reprogramming kit 2.0. By preparing autologous iPSCs to provide a more precise tumor antigen group,10 the best antitumor effect of the DC-EXO vaccine was achieved. In addition, we collected peripheral blood from healthy people and infected their PBMCs with the CytoTune-iPS Sendai reprogramming kit 2.0 to generate hiPSCs. Our results showed that hiPSC exosomes also exert an antitumor effect and serve as a vaccine source to inhibit melanoma progression in vivo. On the one hand, this study helps validate the feasibility of the use of iPSC exosomes as vaccines. On the other hand, DC + hEXO and DC + mEXO vaccines both effectively prevent tumor progression, which could be attributed to two main reasons. First, hiPSC- and miPSC-derived exosomes (iPSC-EXOs) express similar antigens such as EGFR, MART-1, gp100, CEA, and MUC1 (Figure 1B). These antigens can be recognized by both human and mouse immune systems and elicit specific antitumor responses. Second, these antigens have 85.5%,62 86%,63 71%,64 53%,65 and 48%65 similarity of protein sequences between human and mouse sources, respectively, which means that they can bind to MHC molecules of both species and stimulate T cells.66,67 This results in their similar antitumor effect. Therefore, both hiPSC- and miPSC-EXOs can serve as potent adjuvants for DC vaccines and induce robust antitumor immunity. This phenomenon is consistent with previous reports of tumor vaccines based on iPSCs,14 ESCs,68,69 and other tumor antigens.69,70 These results provide a solid theoretical basis for further clinical trials with hiPSC exosome vaccines.
In summary, we demonstrated for the first time that iPSC-derived exosomes could serve as an antigen source of tumor vaccines and combined this with DC-based immunotherapy. The DC vaccine pulsed with iPSC-derived exosomes can induce a broad-spectrum antitumor response and inhibit melanoma progression in prophylactic, therapeutic, and lung metastasis models with no significant adverse effects. Therefore, our research proposes a safe and effective iPSC-based tumor vaccine. Given the systemic extensive antitumor effects and available antigen source, our strategy is emerging as a promising approach for cancer prevention and immunotherapy.
Materials and methods
Mice and cell lines
C57B/L6 mice (H-2b; 6–8 weeks) were purchased from the Guangdong Animal Experiment Center. All experimental procedures involving animals were approved by the Fifth Affiliated Hospital of Sun Yat-sen University and Animal Care and Use Occasion (00172). Mouse cell lines (H-2b), including LLC, MC38 colon cancer cells, 4T1 breast cancer cells, and B16-F10 melanoma cells, were purchased from Shanghai Cell Bank, Chinese Academy of Sciences. Complete DMEM medium (supplemented with 10% fetal bovine serum [FBS]) was used for the culture of B16-F10 and LLC cells. Complete RPMI medium was used for the culture of MC38 and 4T1 cells (supplemented with 10% FBS). MiPSCs were cultured in Oricell mESC serum-free culture medium (Cyagen) on well plates previously coated with gelatin, and hiPSCs were cultured in serum-free Stemflex medium (Gibco) on well plates previously coated with Matrigel.
Preparation of miPSCs
MEFs were isolated with trypsin and cultured in RPMI medium supplemented with 10% FBS at 37°C, 20% O2, and 5% CO2. A total of 1 × 105 embryonic fibroblasts were seeded on 0.2% gelatin-coated plates and infected with a CytoTune-iPS Sendai reprogramming kit 2.0 (Thermo Fisher Scientific). When ESC-like clones appeared, Oricell mESC serum-free medium (Cyagen) was used for culture. Once clones were grown, miPSCs were passaged and purified by flow sorting with an SSEA-1 antibody.
Preparation of hiPSCs
PBMCs were isolated by Ficoll density gradient centrifugation from a healthy volunteer in our laboratory. (Written informed consent was obtained from healthy donors enrolled. This study was approved by the Ethics Review Committee in the Fifth Affiliated Hospital of Sun Yat-sen University.) Then, 1 × 105 PBMCs were seeded onto Matrigel-coated well plates. On the fourth day of culture, the CytoTune-iPS Sendai reprogramming kit 2.0 was used to infect PBMCs. The cells were transferred to Stemfex medium on day 7 of induction. ESC-like clones appeared in approximately 14 days and, when the clones were grown, hiPSCs were passaged and purified by fluorescence-activated cell sorting with an SSEA-4 antibody.
Preparation of Bone Marrow-derived Mesenchymal Stem Cells (BM-MSCs)
Mouse BM-MSCs were isolated and cultured according to a previous protocol.71 In brief, bone marrow cells were flushed out from the bone marrow cavity of C57B/L6 mice and then incubated with the following antibodies at 4°C for 20 min: Sca-1, CD29, CD45, and CD11b, all purchased from BioLegend. Next, the Sca-1+CD29+CD45−CD11b−BM-MSCs were sorted by flow cytometry (BD Biosciences) and cultured at 37°C with 5% CO2 supplement. The culture medium for BM-MSCs consisted of DMEM, streptomycin/penicillin, and 10% exosome-free FBS.
Preparation of exosomes
As described previously,72 the cell culture supernatant was pre-centrifuged at 800 × g for 5 min to remove large cell debris, followed by centrifugation at 2,000 × g for 10 min to remove small cell debris as well as large cell vesicles. Then a 0.22 μm membrane was used to filter the supernatant and the exosomes were isolated by ultracentrifugation at 100,000 × g for 90 min. PBS was then added and the exosomes were centrifuged at 100,000 × g for 90 min and repeated twice to wash away contaminating proteins. Finally, the protein and nanoparticle concentrations were quantitatively measured by NTA and a BCA protein assay kit, respectively.
Western blot assay of tumor-associated antigens
The exosomes were collected and washed twice with PBS. Subsequently, they were lysed in lysis buffer and incubated on ice for 15 min. Afterward, the samples were centrifuged at 12,000 × g for 15 min at 4°C, and the supernatant was collected. The protein concentration of the supernatant was measured using a BCA protein assay kit. The exosome loading was standardized at 20 μg for each sample. Positive control was established using 10 μg of standard proteins, allocating 2 μg for each protein, including EGFR, gp100, CEA, MUCI, and MART-1. To separate the proteins, a 12% SDS-polyacrylamide gel was utilized, and the proteins were electro-transferred onto a polyvinylidene fluoride membrane (Millipore, Bedford, MA). The membranes were then blocked with 5% non-fat milk in TBST for 1 h at room temperature. Subsequently, the membranes were incubated with primary antibodies overnight at 4°C. The primary antibodies used in this study were as follows: anti-EGFR (1:2,000, ab52894), anti-gp100 (1:1,000, ab137078), anti-CEA (1:1,000, aa35-428), anti-MUCI (1:1,000, ab109185), and anti-MART-1 (1:2,000, ab210546). After incubation with the primary antibodies, the membranes were incubated with the respective secondary antibodies for 1.5 h. The second antibodies used in this study were anti-rabbit IgG (1:5,000, ab6721). The protein signal was visualized using the enhanced chemiluminescence kit (ECL-plus; Thermo Fisher Scientific). After the western blotting procedure, ImageJ software was used to quantify the gray value of western blot bands.
Induction and culture of mouse BMDCs
As described previously,73 tibia and femurs were dissected to isolate myeloid progenitor cells in C57BL/6 mice. The cells were cultured in complete RPMI medium supplemented with 10% exosome-depleted FBS (centrifugation at 100,000 × g for 16 h) for 4 h to allow adherence followed by discarding the supernatant and switching to a complete RPMI medium supplemented with GM-CSF (20 ng/mL, PeproTech, Rocky Hill, NJ) and IL-4 (20 ng/mL, PeproTech). On day 8 of induction, immature DCs were collected.
Preparation of DC + EXO
To generate DCs pulsed with iPSC exosomes, 1 × 106 immature DCs were incubated with a culture medium supplemented with 80 μg/mL exosomes and 5 μg/ml LPS for 48 h at 37°C to promote DC maturation.8 As a control group, another batch of immature DCs were incubated with a culture medium containing only 5 μg/mL LPS for the same duration and temperature. After 48 h, the supernatant was discarded, and the DCs were collected.
T cell proliferation assay
MHC-matched C57BL/6 mouse T lymphocytes (1 × 107) were cultured with DCs or DC + EXO for 4 days at varying DC:T lymphocyte ratios (20:1, 10:1, and 5:1). CCK8 was added into three-well plates at 24, 48, and 72 h, respectively, Finally, T cell proliferation was reflected using absorbance values at 450 nm.
ELISA and lymphocyte toxicity assay
All the mice in the different groups had blood drawn 7 days after the final vaccination. Serum levels of IFN-γ and IL-2 were evaluated using a commercial ELISA kit (Bender Medsystems, San Diego, CA). Mouse CD8+ T cells from splenocytes were used as effector cells to measure lymphocyte toxicity. Target cells (1 × 105) were mixed with effector cells at different effector/target cell ratios (5:1, 10:1, and 20:1). After 10 h of incubation, we discarded suspended T cells and detected the viability of the target cells using an LDH kit (Thermo Fisher Scientific). To assess the lymphocyte toxicity, the lysis ratio of target cell was set using the formula: 100 × (experimental signal reading × spontaneous background signal value)/(maximum signal value of target cells × spontaneous background signal value of target cells).
Tumor prophylaxis assay
In the tumor prophylaxis assay, the C57BL/6 mice were randomly assigned to receive either a PBS control, DCs, DC + hEXO, or DC + mEXO inoculation. The mice in experimental groups were intravenously inoculated with DC + EXO (1 × 106/mouse) or DCs four times (days 1, 5, 8, and 11). The control mice were injected with PBS. Tumors were induced by injecting 1 × 106 B16-F10 cells subcutaneously into the left groin of each mouse 7 days following the last immunization. Mouse tumor growth was monitored every other day and, when it reached a volume of 2,000 mm3, the mice were euthanized. Tumor volume was set as (0.5 × w2 x h), where w is the shortest diameter and h is the longest diameter.
Tumor therapeutic assay
In the tumor therapeutic assay, C57BL/6 mice were subcutaneously inoculated with B16-F10 cells (1 × 106) in the left groin. After 4 days, mice were randomly assigned to receive PBS control, DCs, DC + hEXO, DC + mEXO, DC + BEXO, or DC + BMEXO inoculation. The mice in the experimental groups were intravenously inoculated with DC + EXO (1 × 106/mouse) four times (days 5, 8, 11, and 14). The control mice were injected with PBS or DCs. Mouse tumor growth was monitored every other day and, when it reached a volume of 2,000 mm3, the mice were euthanized. Tumor volume was set as (0.5 × w2 × h), where w is the shortest diameter and h is the longest diameter.
Antigen depletion assay
Mice were inoculated with B16F10 cells (1 × 106) and randomly assigned to six groups. Four groups received DC + mEXO therapy and depleting antibodies against CSF1R (300 μg/mouse, every other day, BioXcell),46,47,48 ASGM1 (50 μL/mouse, twice weekly, BioLegend),46,48 CD8 (400 μg/mouse, twice weekly, BioXcell),46,47,48 or CD4 (200 μg/mouse, once a week, BioXcell)47,48 to eliminate macrophages, NK cells, CD8+ T cells, or CD4+ T cells, respectively.46,47,48 One group received DC + mEXO therapy without antibody depletion. The control group received PBS only. Depleting antibodies were administered intraperitoneally 1 day before and during the DC + mEXO therapy, which was given four times at days 5, 8, 11, and 14 after B16F10 inoculation. Mouse tumor growth was monitored every other day until it reached a volume of 2,000 mm3 when the mice were euthanized.
Analysis of lung metastasis of melanoma
In the experimental metastasis study, B16-F10 cells (2 × 105) were intravenously inoculated into C57BL/6 mice through the tail vein. After 3 days, mice were randomly assigned to receive either a PBS control, DCs, DC + hEXO, DC + mEXO, DC + BEXO, or DC + BMEXO inoculation. The mice in the experimental groups were intravenously inoculated with DC + EXO (1 × 106/mouse) four times (days 5, 8, 11, and 14) through the tail vein. The control mice were injected with PBS or DCs. Twenty days later, the mice were euthanized and dissected to observe the lung metastasis of melanoma.
Tumor rechallenge assay
In the tumor rechallenge assay, C57BL/6 mice were subcutaneously inoculated with B16-F10-luciferase cells (1 × 106) in the left groin. Then the mice were intravenously inoculated with DC + EXO (1 × 106/mouse) four times (days 5, 8, 11, and 14). Mice with complete tumor regression were rechallenged with 2 × 105 B16-F10-luciferase cells at day 110, naive mice receiving 2 × 105 B16-F10-luciferase cells served as control. Tumor growth was monitored every other day. At day 130, tumor burden was detected by the IVIS spectrum system after mice received 10 mg/kg introperitoneal injection of D-luciferin. Then the mice were euthanized and dissected to detect the tumor volume and weight.
Biocompatibility experiment of DC + EXO
For the in vivo biosafety evaluation of DC + EXO vaccine, C57BL/6J mice were randomly divided into six groups (n = 4 per group) and injected via the tail vein with PBS, DC, DC + mEXO, DC + hEXO, DC + BEXO, or DC + BMEXO, respectively. The injection was repeated four times once every 3 days. The body weight of the mice was monitored every 2 days. At the end of the experiment, the mice were euthanized and blood samples were collected for biochemical analysis. The major organs (heart, liver, spleen, lung, and kidney) were also harvested for histopathological examination.
For the cytotoxicity assessment of DC + EXO on human normal cell lines, the human L02 normal liver cell line and the HK2 normal kidney proximal convoluted tubule epithelial cell line were seeded in 96-well plates at a density of 5 × 103 cells/well and incubated overnight. Then, the cells were treated with different concentrations of DC + EXO (0, 20, 40, 80, and 100 μg/mL) for 24 h. The cell viability was measured by CCK8 assay according to the manufacturer’s instructions. The absorbance was read at 450 nm using a microplate reader. The cell viability was calculated as the percentage of the control group.
Statistical analysis
All the results are presented as the means ± SEM. Data from multiple groups were analyzed using single-factor analysis of variance (ANOVA). Student's t test and Tukey's post hoc test in a normal test were used to determine significance. GraphPad Prism was used for all statistical analysis, and the following levels of statistical significance were applied: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005, and ∗∗∗∗p < 0.001.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and the Supplemental information. The data that support the study and other findings within this research are available from the corresponding authors upon reasonable request.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (82072062), the Development Project of Foshan Fourth People’s Hospital (FSSYKF-2020003and FSSYKF-2020017), and the Department of Science and Technology of Guangdong Province to the Guangdong Provincial Key Laboratory of Biomedical Imaging (2018B030322006).
Author contributions
Conception and design, X.H. and B.H.; development of methodology, R.W. and T.Z.; acquisition of data, R.W. and T.Z.; analysis and interpretation of data, R.W. and T.Z.; writing, review, and/or revision of the manuscript, R.W. and T.Z.; supervision, X.H. and B.H.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.06.005.
Contributor Information
Bingzong Hou, Email: houbz@mail.sysu.edu.cn.
Xi Huang, Email: huangxi6@mail.sysu.edu.cn.
Supplemental information
References
- 1.Saxena M., van der Burg S.H., Melief C.J.M., Bhardwaj N. Therapeutic cancer vaccines. Nat. Rev. Cancer. 2021;21:360–378. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 2.Zheng X., Xu H., Yi X., Zhang T., Wei Q., Li H., Ai J. Tumor-antigens and immune landscapes identification for prostate adenocarcinoma mRNA vaccine. Mol. Cancer. 2021;20:160. doi: 10.1186/s12943-021-01452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo J., De May H., Franco S., Noureddine A., Tang L., Brinker C.J., Kusewitt D.F., Adams S.F., Serda R.E. Cancer vaccines from cryogenically silicified tumour cells functionalized with pathogen-associated molecular patterns. Nat. Biomed. Eng. 2022;6:19–31. doi: 10.1038/s41551-021-00795-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger M.F., Mardis E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018;15:353–365. doi: 10.1038/s41571-018-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin U., Türeci Ö. Personalized vaccines for cancer immunotherapy. Science (New York, N.Y.) 2018;359:1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 6.Hu Z., Ott P.A., Wu C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018;18:168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang F., Schrörs B., Löwer M., Türeci Ö., Sahin U. Identification of neoantigens for individualized therapeutic cancer vaccines. Nature reviews. Drug Discov. 2022;21:261–282. doi: 10.1038/s41573-021-00387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasso M.S., Mitrousis N., Wang Y., Briquez P.S., Hauert S., Ishihara J., Hubbell J.A., Swartz M.A. Lymphangiogenesis-inducing vaccines elicit potent and long-lasting T cell immunity against melanomas. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abe4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen P.K., Riegler J., Wu J.C. Stem cell imaging: from bench to bedside. Cell stem cell. 2014;14:431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kooreman N.G., Kim Y., de Almeida P.E., Termglinchan V., Diecke S., Shao N.Y., Wei T.T., Yi H., Dey D., Nelakanti R., et al. Autologous iPSC-Based Vaccines Elicit Anti-tumor Responses In Vivo. Cell stem cell. 2018;22:501–513.e7. doi: 10.1016/j.stem.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyvaerts C., Breckpot K. Towards a personalized iPSC-based vaccine. Nat. Biomed. Eng. 2018;2:277–278. doi: 10.1038/s41551-018-0237-7. [DOI] [PubMed] [Google Scholar]
- 12.Hailemichael Y., Singh M., Overwijk W. Vaccinating with Stem Cells to Stop Cancer. Trends Mol. Med. 2018;24:524–526. doi: 10.1016/j.molmed.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Bernardes de Jesus B., Neves B.M., Ferreira M., Nóbrega-Pereira S. Strategies for Cancer Immunotherapy Using Induced Pluripotency Stem Cells-Based Vaccines. Cancers. 2020;12 doi: 10.3390/cancers12123581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhai Y., He X., Li Y., Han R., Ma Y., Gao P., Qian Z., Gu Y., Li S. A splenic-targeted versatile antigen courier: iPSC wrapped in coalescent erythrocyte-liposome as tumor nanovaccine. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abi6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andre F., Schartz N.E.C., Movassagh M., Flament C., Pautier P., Morice P., Pomel C., Lhomme C., Escudier B., Le Chevalier T., et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet (London, England) 2002;360:295–305. doi: 10.1016/s0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 16.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 18.Xu Z., Zeng S., Gong Z., Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol. Cancer. 2020;19:160. doi: 10.1186/s12943-020-01278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Z., Zuo B., Jing R., Gao X., Rao Q., Liu Z., Qi H., Guo H., Yin H. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J. Hepatol. 2017;67:739–748. doi: 10.1016/j.jhep.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 21.Rao Q., Zuo B., Lu Z., Gao X., You A., Wu C., Du Z., Yin H. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64:456–472. doi: 10.1002/hep.28549. [DOI] [PubMed] [Google Scholar]
- 22.Wolfers J., Lozier A., Raposo G., Regnault A., Théry C., Masurier C., Flament C., Pouzieux S., Faure F., Tursz T., et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W., Zhong W., Wang B., Yang J., Yang J., Yu Z., Qin Z., Shi A., Xu W., Zheng C., et al. ICAM-1-mediated adhesion is a prerequisite for exosome-induced T cell suppression. Dev. Cell. 2022;57:329–343.e7. doi: 10.1016/j.devcel.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morishita M., Takahashi Y., Matsumoto A., Nishikawa M., Takakura Y. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016;111:55–65. doi: 10.1016/j.biomaterials.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 25.Linette G.P., Carreno B.M. On the Twentieth Anniversary of Dendritic Cell Vaccines - Riding the Next Wave. Cancer Res. 2022;82:966–968. doi: 10.1158/0008-5472.Can-21-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamalkhah M., Asaadi Y., Azangou-Khyavy M., Khanali J., Soleimani M., Kiani J., Arefian E. MSC-derived exosomes carrying a cocktail of exogenous interfering RNAs an unprecedented therapy in era of COVID-19 outbreak. J. Transl. Med. 2021;19:164. doi: 10.1186/s12967-021-02840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalaj K., Figueira R.L., Antounians L., Lauriti G., Zani A. Systematic review of extracellular vesicle-based treatments for lung injury: are EVs a potential therapy for COVID-19? J. Extracell. Vesicles. 2020;9 doi: 10.1080/20013078.2020.1795365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinman R.M., Cohn Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palucka K., Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang L., Rong Y., Tang X., Yi K., Qi P., Hou J., Liu W., He Y., Gao X., Yuan C., Wang F. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer. 2022;21:45. doi: 10.1186/s12943-022-01515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wculek S.K., Cueto F.J., Mujal A.M., Melero I., Krummel M.F., Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 32.Fu C., Zhou L., Mi Q.S., Jiang A. DC-based vaccines for cancer immunotherapy. Vaccines. 2020;8:e40706. doi: 10.3390/vaccines8040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun K., Wang L., Zhang Y. Dendritic cell as therapeutic vaccines against tumors and its role in therapy for hepatocellular carcinoma. Cell. Mol. Immunol. 2006;3:197–203. [PubMed] [Google Scholar]
- 34.Gilboa E. DC-based cancer vaccines. J. Clin. Invest. 2007;117:1195–1203. doi: 10.1172/jci31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chacon J.A., Sarnaik A.A., Chen J.Q., Creasy C., Kale C., Robinson J., Weber J., Hwu P., Pilon-Thomas S., Radvanyi L. Manipulating the tumor microenvironment ex vivo for enhanced expansion of tumor-infiltrating lymphocytes for adoptive cell therapy. Clin. Cancer Res. 2015;21:611–621. doi: 10.1158/1078-0432.Ccr-14-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melo S.A., Luecke L.B., Kahlert C., Fernandez A.F., Gammon S.T., Kaye J., LeBleu V.S., Mittendorf E.A., Weitz J., Rahbari N., et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witwer K.W., Soekmadji C., Hill A.F., Wauben M.H., Buzás E.I., Di Vizio D., Falcon-Perez J.M., Gardiner C., Hochberg F., Kurochkin I.V., et al. Updating the MISEV minimal requirements for extracellular vesicle studies: building bridges to reproducibility. J. Extracell. Vesicles. 2017;6 doi: 10.1080/20013078.2017.1396823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholson R.I., Gee J.M., Harper M.E. EGFR and cancer prognosis. Eur. J. Cancer. 2001;37:S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 39.Tran E., Turcotte S., Gros A., Robbins P.F., Lu Y.C., Dudley M.E., Wunderlich J.R., Somerville R.P., Hogan K., Hinrichs C.S., et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ott P.A., Hu Z., Keskin D.B., Shukla S.A., Sun J., Bozym D.J., Zhang W., Luoma A., Giobbie-Hurder A., Peter L., et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahin U., Oehm P., Derhovanessian E., Jabulowsky R.A., Vormehr M., Gold M., Maurus D., Schwarck-Kokarakis D., Kuhn A.N., Omokoko T., et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585:107–112. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 42.Grunnet M., Sorensen J.B. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung cancer. 2012;76:138–143. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Kufe D.W. Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weigelin B., den Boer A.T., Wagena E., Broen K., Dolstra H., de Boer R.J., Figdor C.G., Textor J., Friedl P. Cytotoxic T cells are able to efficiently eliminate cancer cells by additive cytotoxicity. Nat. Commun. 2021;12:5217. doi: 10.1038/s41467-021-25282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiesgen S., Messinger J.C., Chintala N.K., Tano Z., Adusumilli P.S. Comparative analysis of assays to measure CAR T-cell-mediated cytotoxicity. Nat. Protoc. 2021;16:1331–1342. doi: 10.1038/s41596-020-00467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen L., Qin H., Zhao R., Zhao X., Lin L., Chen Y., Lin Y., Li Y., Qin Y., Li Y., et al. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abc2816. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y., Liu C., Peng W., Lizée G., Overwijk W.W., Liu Y., Woodman S.E., Hwu P. Antitumor T-cell responses contribute to the effects of dasatinib on c-KIT mutant murine mastocytoma and are potentiated by anti-OX40. Blood. 2012;120:4533–4543. doi: 10.1182/blood-2012-02-407163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moynihan K.D., Opel C.F., Szeto G.L., Tzeng A., Zhu E.F., Engreitz J.M., Williams R.T., Rakhra K., Zhang M.H., Rothschilds A.M., et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat. Med. 2016;22:1402–1410. doi: 10.1038/nm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ouyang X., Liu Y., Zhou Y., Guo J., Wei T.T., Liu C., Lee B., Chen B., Zhang A., Casey K.M., et al. Antitumor effects of iPSC-based cancer vaccine in pancreatic cancer. Stem Cel. Rep. 2021;16:1468–1477. doi: 10.1016/j.stemcr.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santos P., Almeida F. Exosome-Based Vaccines: History, Current State, and Clinical Trials. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.711565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nikfarjam S., Rezaie J., Kashanchi F., Jafari R. Dexosomes as a cell-free vaccine for cancer immunotherapy. J. Exp. Clin. Cancer Res. 2020;39:258. doi: 10.1186/s13046-020-01781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong X., Ke X., Wang L., Lin Y., Wang S., Yao Z., Li K., Luo Y., Liu F., Pan Y., et al. Neoantigen-based cancer vaccination using chimeric RNA-loaded dendritic cell-derived extracellular vesicles. J. Extracell. Vesicles. 2022;11 doi: 10.1002/jev2.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J., Li J., Peng Y., Du Y., Yang Z., Qi X. Dendritic cell derived exosomes loaded neoantigens for personalized cancer immunotherapies. J. Control Release. 2023;353:423–433. doi: 10.1016/j.jconrel.2022.11.053. [DOI] [PubMed] [Google Scholar]
- 54.Li K., Lin Y., Luo Y., Xiong X., Wang L., Durante K., Li J., Zhou F., Guo Y., Chen S., et al. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: a multicenter prospective study. Mol. Cancer. 2022;21:21. doi: 10.1186/s12943-022-01499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saxena M., Balan S., Roudko V., Bhardwaj N. Towards superior dendritic-cell vaccines for cancer therapy. Nat. Biomed. Eng. 2018;2:341–346. doi: 10.1038/s41551-018-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makarova-Rusher O.V., Medina-Echeverz J., Duffy A.G., Greten T.F. The yin and yang of evasion and immune activation in HCC. J. Hepatol. 2015;62:1420–1429. doi: 10.1016/j.jhep.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 57.Butterfield L.H., Ribas A., Dissette V.B., Lee Y., Yang J.Q., De la Rocha P., Duran S.D., Hernandez J., Seja E., Potter D.M., et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin. Cancer Res. 2006;12:2817–2825. doi: 10.1158/1078-0432.Ccr-05-2856. [DOI] [PubMed] [Google Scholar]
- 58.Gao S., Yang D., Fang Y., Lin X., Jin X., Wang Q., Wang X., Ke L., Shi K. Engineering Nanoparticles for Targeted Remodeling of the Tumor Microenvironment to Improve Cancer Immunotherapy. Theranostics. 2019;9:126–151. doi: 10.7150/thno.29431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mullins D.W., Sheasley S.L., Ream R.M., Bullock T.N.J., Fu Y.X., Engelhard V.H. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J. Exp. Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huber C.C., Callegari E.A., Paez M.D., Romanova S., Wang H. Heat Shock-Induced Extracellular Vesicles Derived from Neural Stem Cells Confer Marked Neuroprotection Against Oxidative Stress and Amyloid-β-Caused Neurotoxicity. Mol. Neurobiol. 2022;59:7404–7412. doi: 10.1007/s12035-022-03055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho J.A., Lee Y.S., Kim S.H., Ko J.K., Kim C.W. MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009;275:256–265. doi: 10.1016/j.canlet.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 62.Reiter J.L., Threadgill D.W., Eley G.D., Strunk K.E., Danielsen A.J., Sinclair C.S., Pearsall R.S., Green P.J., Yee D., Lampland A.L., et al. Comparative genomic sequence analysis and isolation of human and mouse alternative EGFR transcripts encoding truncated receptor isoforms. Genomics. 2001;71:1–20. doi: 10.1006/geno.2000.6341. [DOI] [PubMed] [Google Scholar]
- 63.Aris M., Zubieta M.R., Colombo M., Arriaga J.M., Bianchini M., Alperovich M., Bravo A.I., Barrio M.M., Mordoh J. MART-1- and gp100-expressing and -non-expressing melanoma cells are equally proliferative in tumors and clonogenic in vitro. J. Invest. Dermatol. 2012;132:365–374. doi: 10.1038/jid.2011.312. [DOI] [PubMed] [Google Scholar]
- 64.Kawakami Y., Eliyahu S., Sakaguchi K., Robbins P.F., Rivoltini L., Yannelli J.R., Appella E., Rosenberg S.A. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J. Exp. Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gatti-Mays M.E., Redman J.M., Donahue R.N., Palena C., Madan R.A., Karzai F., Bilusic M., Sater H.A., Marté J.L., Cordes L.M., et al. A Phase I Trial Using a Multitargeted Recombinant Adenovirus 5 (CEA/MUC1/Brachyury)-Based Immunotherapy Vaccine Regimen in Patients with Advanced Cancer. Oncologist. 2020;25:479–e899. doi: 10.1634/theoncologist.2019-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zitvogel L., Kroemer G. Cross-reactivity between cancer and microbial antigens. Oncoimmunology. 2021;10 doi: 10.1080/2162402x.2021.1877416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zitvogel L., Kroemer G. Cross-reactivity between microbial and tumor antigens. Curr. Opin. Immunol. 2022;75 doi: 10.1016/j.coi.2022.102171. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Z., Chen X., Chang X., Ye X., Li Y., Cui H. Vaccination with embryonic stem cells generates effective antitumor immunity against ovarian cancer. Int. J. Mol. Med. 2013;31:147–153. doi: 10.3892/ijmm.2012.1195. [DOI] [PubMed] [Google Scholar]
- 69.Ruan Z., Yang Z., Wang Y., Wang H., Chen Y., Shang X., Yang C., Guo S., Han J., Liang H., Wu Y. DNA vaccine against tumor endothelial marker 8 inhibits tumor angiogenesis and growth. J. Immunother. 2009;32:486–491. doi: 10.1097/CJI.0b013e3181a1d134. [DOI] [PubMed] [Google Scholar]
- 70.Adotévi O., Mollier K., Neuveut C., Dosset M., Ravel P., Fridman W.H., Tartour E., Charneau P., Wain-Hobson S., Langlade-Demoyen P. Targeting human telomerase reverse transcriptase with recombinant lentivector is highly effective to stimulate antitumor CD8 T-cell immunity in vivo. Blood. 2010;115:3025–3032. doi: 10.1182/blood-2009-11-253641. [DOI] [PubMed] [Google Scholar]
- 71.Li C.J., Xiao Y., Yang M., Su T., Sun X., Guo Q., Huang Y., Luo X.H. Long noncoding RNA Bmncr regulates mesenchymal stem cell fate during skeletal aging. J. Clin. Invest. 2018;128:5251–5266. doi: 10.1172/jci99044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., Lee J.J., Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mahaweni N.M., Kaijen-Lambers M.E., Dekkers J., Aerts J.G., Hegmans J.P. Tumour-derived exosomes as antigen delivery carriers in dendritic cell-based immunotherapy for malignant mesothelioma. J. Extracell. Vesicles. 2013;2:22492. doi: 10.3402/jev.v2i0.22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and the Supplemental information. The data that support the study and other findings within this research are available from the corresponding authors upon reasonable request.