Abstract
The cornea serves as an important barrier structure to the eyeball and is vulnerable to injuries, which may lead to scarring and blindness if not treated promptly. To explore an effective treatment that could achieve multi-dimensional repair of the injured cornea, the study herein innovatively combined modified mRNA (modRNA) technologies with adipose-derived mesenchymal stem cells (ADSCs) therapy, and applied IGF-1 modRNA (modIGF1)-engineered ADSCs (ADSCmodIGF1) to alkali-burned corneas in mice. The therapeutic results showed that ADSCmodIGF1 treatment could achieve the most extensive recovery of corneal morphology and function when compared not only with simple ADSCs but also IGF-1 protein eyedrops, which was reflected by the healing of corneal epithelium and limbus, the inhibition of corneal stromal fibrosis, angiogenesis and lymphangiogenesis, and also the repair of corneal nerves. In vitro experiments further proved that ADSCmodIGF1 could more significantly promote the activity of trigeminal ganglion cells and maintain the stemness of limbal stem cells than simple ADSCs, which were also essential for reconstructing corneal homeostasis. Through a combinatorial treatment regimen of cell-based therapy with mRNA technology, this study highlighted comprehensive repair in the damaged cornea and showed the outstanding application prospect in the treatment of corneal injury.
Keywords: adipose-derived mesenchymal stem cells, IGF1-modified mRNA, corneal alkali burn, corneal wound healing, limbal stem cells, corneal nerves, corneal neovascularization
Graphical abstract

Fu and colleagues describe a combinatorial treatment regimen of modified mRNA-engineered stem cells to promote corneal wound healing. This therapeutic scheme achieves comprehensive recovery of alkali-burned corneas, and may further provide the bright prospect for the application of modified mRNA technology in the treatment of corneal diseases.
Introduction
The cornea is the most important refractive medium of the eye, which is of great significance for the formation of good vision.1,2 Due to the particularity of its location, the cornea is vulnerable to a variety of injuries, such as chemical injuries or thermal burns.3,4 Corneal alkali burn injuries resulting from alkaline chemicals cause a protracted course of corneal damage and remain a leading cause of blindness worldwide.5,6 Current clinical treatments for corneal alkali injury aim to ameliorate excessive inflammation or aim to promote corneal epithelial repair. However, these methods often fail to achieve a comprehensive effect. For example, corticosteroid eye drops play an important role in inflammatory inhibition, but their long-term use may induce ocular complications, compromise corneal integrity, or even lead to corneal melting.7,8 Alternative and novel approaches for corneal repair have generated contradictory results. In particular, amniotic membrane transplantation has been widely applied in the clinical setting, but the quick dissolution and detachment of amniotic membrane limits the repair to some extent.9 In the later stages of corneal injuries, allograft transplantation has shown encouraging results to partially restore the structure and function of cornea.10,11 However, corneal donor resources are limited, and postoperative rejection often results in poor long-term outcomes. Thus, the multi-dimensional and complex repair process of corneal injury requires effective and comprehensive treatments.
In recent years, mesenchymal stem/stromal cells (MSCs) have been widely applied to treat ocular disorders due to their unique biological properties and potential value for clinical application.12,13 Among MSCs, adipose-derived mesenchymal stem cells (ADSCs) are favored due to their ease of acquisition and wide range of tissue sources as well as low immunogenicity profile.14 ADSCs have important immunomodulatory and anti-inflammatory properties, and subconjunctival injection of ADSCs in corneal alkali-burned animals was previously deemed beneficial.15,16 The cell therapy promoted corneal wound healing through the inhibition of stromal inflammation and the prevention of neovascularization and fibrosis. However, the overall recovery of injured cornea requires not only restoring corneal tissue structure but also reconstructing the corneal homeostasis, including nerves and limbus. The effects of ADSCs on corneal nerves and limbal stem cells (LSCs) have been rarely reported, because it might be difficult to be achieved simply via ADSC therapy.16,17 One avenue worth investigating is the modification of ADSCs that would further improve their efficacy and minimize injury progression in the acute phases of injury more efficiently.
To improve therapeutic effectiveness, ADSCs are often genetically modified to enhance their specific functions through exogenous genetic transfection according to the disease characteristics.18 Compared with DNA/viral-based technologies, transiently expressed modified mRNAs (modRNAs), as a new and efficient gene delivery vector and nucleic acid therapy approach, overcome the potential safety risks and have been employed clinically for prophylactic use.19,20,21,22 Applications for mRNA in applied and regenerative medicine are still emerging.23,24 Recently, our team documented the benefits of a cell-based mRNA delivery platform for improved therapeutic indications. More specifically, we have employed synthetic chemically modRNAs in combination with cell-based therapies as an advanced regenerative strategy for treating limb ischemia, myocardial infarction, bone defects, or improving fat grafting.20,25,26,27 To the best of our knowledge, a cell-mediated modRNA delivery system that repairs corneal injury has not been documented.
Therapeutic proteins to treat corneal defects and injuries are currently being investigated and are reviewed extensively elsewhere.10 Insulin-like growth factor-1 (IGF-1), a member of the insulin gene family, is a multifunctional cytokine with a wide range of biological activities, and it plays an important role in maintaining and regulating cell growth, proliferation, differentiation, maturation, and regeneration.28,29,30 Furthermore, IGF-1 is an essential neurotrophic factor, which can promote nerve regeneration and repair after peripheral nerve injury.31,32 In ophthalmology, IGF-1 promotes IGF receptor expression in limbal cells and induces the differentiation of LSCs.33 Several clinical studies have demonstrated the use of IGF-1-containing eyedrops to promote epithelial repair and nerve regeneration of injured corneas, while the short half-life and low ocular surface availability of recombinant proteins are bottleneck-restricting effects.34,35 Given these reports and based on the above functions and properties of IGF-1, we hypothesized that the enhancement of ADSCs with IGF1-modRNA (ADSCmodIGF1) could more robustly result in the regeneration of corneal nerves and repopulation of LSCs, and thus could support an efficient therapeutic scheme for comprehensively treating injured corneas.
Results
modIGF1 transfection promotes proliferation and migration of ADSCs
To assess the feasibility of enhancing human ADSCs properties with modRNAs, we first analyzed the transfection efficiency and expression kinetics of a GFP modRNA reporter construct (modGFP). The initial findings were consistent to those of our previous study, which concluded that Lipofectamine-mediated modRNA transfections were well tolerated in human ADSCs.20 We found that the GFP protein expression peaked within the first 24 h and the signal was sustained for at least 6 days following transfection (Figure 1A). Furthermore, we noted that the modRNA transfection efficiency reached 90%+ within the first 24 h post-transfection when transfecting a ratio of 1 μg modRNA to every 1 × 105 ADSCs (Figure 1B). This transfection ratio coincided with a mean fluorescence intensity value of 1.8 × 106 at 24 h post-transfection (Figure 1C).
Figure 1.
Properties of mRNA-enhanced human ADSCs
(A–C) The expression kinetics and transfection efficiency of modGFP in ADSCs. (A) Representative fluorescence images showing daily GFP signal kinetics in ADSCs from D1 to D6 post-transfection. Scale bar, 50 μm. (B) Flow cytometry analysis of transfection efficiency at 24 h post-transfection. (C) Flow cytometry analysis of mean fluorescence intensity at 24 h post-transfection. (D) Transcript analysis of intracellular IGF-1 mRNA at 24 h post-transfection. (E) Secretion kinetics of newly produced IGF-1 proteins in culture medium following modIGF1 transfection into ADSCs. (F) Cell surface marker expression of ADSCs analyzed at 24 h post-transfection. (G) Induction of adipogenic, osteogenic, and chondrogenic differentiation revealed the multipotent capacity of modRNA-transfected ADSCs. Successful differentiations of the lineages were detected and analyzed using oil red O, alizarin red S, and Alcian blue staining, respectively. Scale bar, 20 μm. (H) The proliferation capacity of the mRNA transfected ADSCs was measured and compared with control ADSCs (CTR). (I) The migration potential of ADSCs in each group was evaluated using transwell assay at 24 h post-seeding. Scale bar, 100 μm. (J) The number of migrated ADSCs was quantified and analyzed. Data are presented as the mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001; n = 3 (B–F) and 4 (H and J).
To determine the secretory capabilities of ADSCs following modRNA transfer, we next explored the transfection and expression characteristics of IGF1-modRNAs (modIGF1) at the same ratio of modRNA to cells as the GFP experiments above. Through RT-PCR, we found an approximately 8,000-fold increase in IGF-1 transcripts in the modIGF1-transfected ADSC group at 24 h post-transfection compared with modGFP-transfected and naïve (ADSCs lacking mRNA transfection) control cells (Figure 1D). Considering that IGF-1 is a secreted factor, we detected the newly produced IGF-1 protein concentration within the culture medium for six consecutive days with an enzyme-linked immunosorbent assay (ELISA) kit. The results revealed that the levels of IGF-1 protein concentration in cell culture supernatants were significantly higher in the modIGF1-transfected group compared with control groups for the first 48 h post-transfection (Figure 1E).
To evaluate the effects that IGF-1 modRNA transfections had on ADSCs, we characterized cell surface gene expression and differentiation capacity, as well as proliferation and migratory capabilities. First, a panel of known antibodies identifying MSC-like cells were selected, which included CD73, CD90, CD105, CD14, CD34, and CD45.36 We compared cell surface marker gene expression on modRNA-transfected ADSCs 24 h post-transfection and compared those profiles to both an inert modRNA reporter sequence encoding firefly luciferase (modLuc) and untransfected ADSCs (CTR). The results showed that modRNA transfections had little effect on driving phenotypic changes to the ADSC cell surface profile (Figure 1F). In addition, we collected conditioned medium at 24 h post-transfection and quantified the concentration of key soluble mediators secreted by ADSCs. The results showed that the secretion of TGF-β1, IL-6, SDF-1, and HGF, which related to the growth and immune regulation, anti-fibrosis, or chemotactic functions of ADSCs, would not be affected by modRNA transfections (Figure S1).37,38 Of further interest, we found that the modRNA-enhanced ADSCs maintain their trilineage stemness, and that overexpression of mRNAs did not negatively alter the cells’ adipogenic, osteogenic, or chondrogenic differentiation capabilities (Figure 1G).
IGF-1 has been shown previously to enhance the proliferation and migratory capacity of mesenchymal stromal cells.39,40 We therefore sought to determine the proliferative and migratory capacity of ADSCs following modIGF1 transfection. The CCK8 assay concluded that ADSCs transfected with modIGF1 resulted in superior cell proliferation capabilities when compared with ADSCs modified with the modLuc reporter or naïve controls (Figure 1H). In addition, the migration capacity of each group was measured by transwell assay, the findings of which revealed that modIGF1 transfection significantly improved the migration potential of ADSCs (Figures 1I and 1J).
ADSCs enriched with modIGF1 promote corneal wound healing after acute alkali burn
Next, to evaluate the maintenance period of ADSCs in vivo, we loaded naïve and modIGF1-enhanced ADSCs with red fluorescence dye 1,1′ -dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD) and traced the cells following subconjunctival injections in BALB/c mice. Consecutive image tracking was performed using an in vivo imaging system (IVIS) every 2 days for a period of 16 days. The relative fluorescence at each time point was normalized to D0 and was measured and analyzed. The linear chart revealed no significant differences in the relative fluorescence between the two groups and thus concluded that modIGF1 transfection did not affect the maintenance period of ADSCs in vivo (Figure S2). Of importance, these data indicated that the ADSCs could be maintained in vivo for more than 1 week, covering the acute phase of corneal alkali burn, which was previously reported to be conducive to continuous repair of the damaged cornea.41
To examine the therapeutic efficacy of IGF-1-modified ADSCs to heal cornea, we employed an alkali burn injury model. Mice were randomly assigned into four groups, where they received: IGF-1 eyedrops treatment, or subconjunctival injections containing either phosphate-buffered saline (PBS), ADSCs transfected with modLuc (ADSCmodLuc), or ADSCs transfected with modIGF1 (ADSCmodIGF1) (schematic diagram of study plan shown in Figure 2A). An additional control group not receiving any injury or treatment was included as a benchmark. Another schematic diagram that highlights the timeline, study time points, and specific treatments is presented in Figure S3. After consecutive examinations, bright-field corneal images taken by a slit-lamp microscope showed that subconjunctival injection of ADSCmodIGF1 could significantly alleviate the edema and allow the transparency of the injured corneas to quickly return to normal levels (Figures 2B and 2C). In addition, the fluorescein-staining images revealed that IGF-1 eyedrops treatment, the subconjunctival injection of ADSCmodLuc or ADSCmodIGF1 could effectively accelerate the rate of wound healing, but only the degree of corneal epithelial defects in the ADSCmodIGF1 group was significantly lower than that of the CTR group (Figures 2D and 2E). Moreover, corneal thickness is an important parameter in terms of measuring the degree of corneal damage and repair.42 The results of continuous anterior segment optical coherence tomography (OCT) follow-up examinations revealed that ADSCmodLuc, IGF-1 eyedrops, and ADSCmodIGF1 treatments all aided in restorative tissue repair and the latter two groups both had significant effects on the elimination of corneal edema (Figures 2F and 2G). Among treatment groups, only ADSCmodIGF1 therapy could promote corneal thickness to normal (dotted line) during the study period (Figure 2G). Of interest, the results stemming from the ADSCmodLuc treatment group provided some evidence that simple ADSCs could promote limited recovery to the acute alkali-burned corneas, while the consecutive IGF-1 protein eyedrops also promoted the recovery of injured corneas to some extent as reported. Nevertheless, modIGF1-enriched ADSCs, which combined the therapeutic advantages of ADSCs and IGF-1, significantly accelerated the comprehensive recovery of transparency, thickness, and epithelial defects of the injured corneas.
Figure 2.
ADSCs engineered with modIGF1 promoted corneal wound healing after alkali burn
(A) Schematic diagram depicting the construction of corneal alkali burn model and treatments including the subconjunctival injection of either PBS or modRNA-enhanced ADSCs, or eyedrops containing rhIGF1 protein. (B) Representative bright-field corneal images revealed transparency of normal and injured corneas at D0, D4, D8, and D16 after alkali burn. (C) Analysis of clinical scores of corneal transparencies at various time points. (D) Representative fluorescein staining images showed normal corneas and the degree of corneal epithelial deficiency in injured corneas at D0, D4, D8, and D16 after alkali burn. (E) Analysis and comparison of the corneal fluorescein grading from each group. (F) Representative anterior segment OCT images showed normal corneas and corneal edema degree of injured corneas at D0, D1, D2, D4, D8, and D16 after alkali burn. (G) Central corneal thicknesses of five groups were analyzed. Data are presented as the mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; n = 8.
modIGF1-engineered ADSCs enhance morphological and functional recovery of alkali-burned corneas
To gain further insights into the cellular and molecular recovery of the injured corneas following treatment with modRNA-enhanced ADSCs, we performed immunostainings and morphological analyses of relevant indicators of corneal functionality on the corneal slides of each group. Clinical evaluations demonstrated that the alkali-burned corneas in the PBS control group (CTR) displayed a modest innate wound healing ability. However, hematoxylin and eosin (H&E) and Masson’s trichrome staining results showed that the histomorphology of the CTR group remained poor 16 days after the injury, which was reflected in the abnormal cell morphology and arrangement of corneal epithelium as well as the loose and disordered arrangement of the corneal stromal fibers (Figure 3A). In comparison, corneas treated with ADSCmodLuc or IGF-1 eyedrops gave rise to relatively thicker epithelium and more compact stroma, but appeared inferior to the corneal morphology found in the ADSCmodIGF1 treatment group (Figure 3A). From the histological staining results, we measured and analyzed the corneal epithelial thickness and collagen volume ratios of each group. We found that corneas of ADSCmodIGF1 treatment group had significantly thicker epithelial layers and larger relative collagen volume, indicting closest to normal corneas (Figures 3B and 3C).
Figure 3.
Morphological and cellular recovery of alkali-burned corneas following modIGF1-enhanced ADSC treatment
(A) Representative H&E staining, Masson’s trichrome staining, and αSMA staining images of normal corneas and corneas at D16 after alkali burn in each group are presented. Scale bars, 20 μm. (B) Ratios of corneal epithelial thickness in each group were compared. (C) Percentages of collagen volume in corneas from each group were analyzed and shown. (D) The comparison of percentages of αSMA-positive corneal area in each group was shown. (E) Representative immunofluorescence staining images of whole corneas revealed the expression and distribution of PAX6 in each group. Scale bar, 200 μm. The lower images at larger magnifications highlighted the expression of PAX6 at central or peripheral corneas, respectively. Scale bars, 50 μm. Nuclei were counterstained with DAPI. (F) Representative immunofluorescence staining images of p63 in whole corneas from each group. Scale bar, 200 μm. Corresponding magnified images of central and peripheral areas. Scale bar, 50 μm. Nuclei were counterstained with DAPI. (G and H) The numbers of PAX6-positive cells in central and peripheral corneas from each group were calculated and analyzed. (I and J) Analysis and comparison of p63-positive cells in central and peripheral corneal areas from each group. Data are presented as the mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; n = 9 (B) and 4 (C, D, and G–J).
We next sought to evaluate the levels of fibrosis following injury and treatment. Results stemming from αSMA staining and analysis revealed a severe degree of fibrosis in the CTR group. However, the degree of fibrosis among the alternative treatment groups were much less, where in particular the ADSCmodIGF1 treatment group appeared to effectively alleviate the occurrence of scarring in the injured corneas (Figures 3A and 3D). Furthermore, we performed immunofluorescence staining to detect functional indicators related to the corneal epithelium. We first analyzed the expression and distribution of PAX6, an essential transcription factor related to corneal homeostasis.43 Based on the staining results of the whole corneas from the different groups undergoing alkali injury and treatment, we discovered a significant increase in the number of PAX6-expressing cells in the corneas in the ADSCmodIGF1 treatment group (Figure 3E). Most of the corneal epithelium in the ADSCmodIGF1 treatment group expressed PAX6. In comparison, the fluorescence intensity of PAX6 in the corneal epithelium of the control group was much weaker, indicating that the cornea was in poor condition. The magnified images (lower panel Figure 3E) provide clear nuclear localization signals of PAX6 in the central and peripheral corneas, respectively, and the number of PAX6+ epithelial cells found in the ADSCmodIGF1 treatment group was significantly higher than those of the other treatment groups (Figures 3G and 3H). The detection of the corneal-specific epithelial keratin marker CK12 further demonstrated the essential role of ADSCmodIGF1 in corneal epithelial regeneration (Figures S4A and S4D).
The stemness characteristic of corneal epithelial cells was also detected and the immunofluorescence staining of stemness marker p63 showed that the ADSCmodLuc and IGF-1 eyedrops treatment groups could promote the accumulation of p63-positive cells to a certain extent (Figures 3F, 3I, and 3J).44 Moreover, the numbers of corneal epithelial cells positive for p63 both in the central and peripheral areas were significantly higher in the ADSCmodIGF1 group compared with the other three injured groups (Figures 3F, 3I, and 3J). To determine the proliferation capacity of the corneal epithelial cells within the different treatment groups following injury and recovery, we performed immunofluorescence staining using antibodies directed against PCNA. A strong proliferation response was detected in the corneal epithelium of mice treated with ADSCmodIGF1, which was reflected in the significantly greater number of PCNA-positive cells both in the central and peripheral epithelial areas compared with those in the control groups (Figures S4B, S4E, and S4F). Subsequently, we also detected the epithelial barrier using the known functional marker, E-cadherin.45 The results importantly indicated that ADSCmodIGF1 treatment also substantially improved corneal epithelial barrier recovery (Figure S4C and S4G).
modIGF1-engineered ADSCs prevent corneal neovascularization and lymphangiogenesis but promote corneal nerve regeneration in alkali-burned mice
Given that severe inflammation resulting from acute alkali burn in corneas could promote neovascularization and lymphangiogenesis, we identified the expression patterns of CD31 and LYVE1, respectively, in the corneal stroma of each treatment group using an immunohistochemistry approach. The growth of new capillaries and lymphatic vessels appeared diminished or completely inhibited following ADSCmodIGF1 treatment in the injured corneas compared with the control treatment groups (Figures 4A–4D). Following detailed molecular analysis, the number of newly formed capillaries in the injured corneas of mice in the control group averaged 10.00 ± 3.54/field. The number of capillaries found in the ADSCmodIGF1 treatment group was significantly lower, with an average of approximately 1.25 ± 0.83/field (Figures 4A and 4C). Analysis from lymphangiogenesis presented a similar statistical trend, as the number of lymphatic vessels in the ADSCmodIGF1 group was 0.50 ± 0.50/field, which was significantly less than the 6.25 ± 0.83/field reported in the CTR group. Besides, the ADSCmodLuc treatment and IGF-1 eyedrops also definitely played a positive role in the inhibition of angiogenesis and lymphangiogenesis (Figures 4A–4D).
Figure 4.
modIGF1-engineered ADSCs suppressed corneal angiogenesis and lymphangiogenesis and promoted corneal nerve repair
(A and B) Representative immunohistochemistry staining images of blood vessel marker CD31 and lymphatic marker LYVE1, respectively. Scale bars, 50 μm. Red arrows indicated capillaries and lymphatic vessels representatively. (C and D) Numbers of capillaries and lymphatic vessels per field in corneas of each group were counted and compared. (E) Representative images of whole corneas in each group staining for tubulin β-III and the respective images of central and peripheral corneal nerve fibers are presented. Scale bars, 50 μm. (F) The occupancy of nerves in the central corneas were quantified as percent/area and compared between groups. (G) The occupancy of nerves in the peripheral corneas were quantified as percent/area and compared between groups. Data are presented as the mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; n = 4 (C and D) and 5 (F and G).
Next, to reveal the morphological distribution of nerve fibers in the corneas of each group following injury and treatment, the whole corneas were stained for tubulin β-III. The respective representative images of the central and peripheral corneal fibers demonstrated that ADSCmodIGF1 treatment undoubtedly played an important role in promoting the regeneration and repair of nerve fibers in the alkali-burned corneas (Figure 4E). The nerve fibers found within the central and peripheral areas of the corneas were quantified. The fiber density in the ADSCmodIGF1 treatment group were significantly the densest among all treatment groups when compared with the density of nerve fibers found in the normal group, i.e., mice not receiving alkali injury or treatment (Figures 4F and 4G).
modIGF1-engineered ADSCs have a direct effect on nerve growth in vitro
To further investigate the effects of modIGF1-engineered ADSCs on the growth and proliferation of nerves, primary trigeminal ganglion (TG) cells were cultivated in vitro with and without the co-culture of mRNA-enhanced or control ADSCs for 24 h. Tubulin β-III staining and statistical analysis revealed that the percent area occupied by nerves increased in the ADSC co-culture groups, but the ADSCmodIGF1 group significantly stimulated the proliferation and growth of TG cells compared with controls (Figures 5A and 5B). Further analyses were then performed to compare the neurite length and intersection number of the neurons in each group by tracing the neurites of representative neurons. As indicated from the results, neurites receiving ADSCmodIGF1 treatment were more complex and the mean neurite length was significantly longer compared with the control groups. Of interest, we found that co-culture with ADSCs alone could promote some neuronal growth, as shown with the ADSCmodLuc group (Figures 5C and 5D). Subsequently, the intersection number of neurites from each group was calculated and analyzed based on the neurite tracings by Sholl analysis. The generated pseudo-color images of the representative neurons by Sholl analysis were called intersection masks, and the color intensity represented the number of intersections or branches (Figure 5C). The line chart presented in Figure 5E depicts the intersection number with the change of distance from the soma. As shown, the highest intersection number for neurites in the ADSCmodIGF1 group was between 40 and 75 μm, which reached more than 25/neuron. As for the neurons in the ADSCmodLuc group, the intersection number reached a peak of approximately 24.37 ± 2.57/neuron at 35 μm from the soma. Furthermore, the largest intersection number of the control neurons was only 15.47 ± 1.22/neuron at 40 μm from the soma, which was significantly less compared with the other groups. To better elucidate these findings, the area under curve of each group was calculated, together with the maximum/mean intersection number, and the results verified the pro-growth effects stemming from the modIGF1-enhanced ADSCs (Figures 5F–5H).
Figure 5.
modIGF1-engineered ADSCs stimulated the proliferation and growth of trigeminal neurons in vitro
(A) Representative immunofluorescence staining images of tubulin β-III for primary trigeminal neurons after 24 h co-culture with modRNA-engineered ADSCs. Nuclei were counterstained with DAPI. Scale bar, 10 μm. (B) The percent areas occupied by nerves in each group were compared. (C) Representative images of neurons in each group were picked and the tracings of the corresponding neurites were tracked by ImageJ. The tracings were then analyzed by Sholl analysis to figure out the intersection numbers of the neurons in different groups. Scale bars, 50 μm. (D) Mean length of neurites in each group was analyzed according to the tracings. (E) Line chart depicting the intersection number with the change of distance from soma was presented. (F–H) The area under curve (AUC), maximum intersection number and mean intersection number for each group was analyzed respectively. Data are presented as the mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; n = 8 (B) and 3 (D–H).
Characteristics of LSCs co-cultured with modRNA-engineered ADSCs
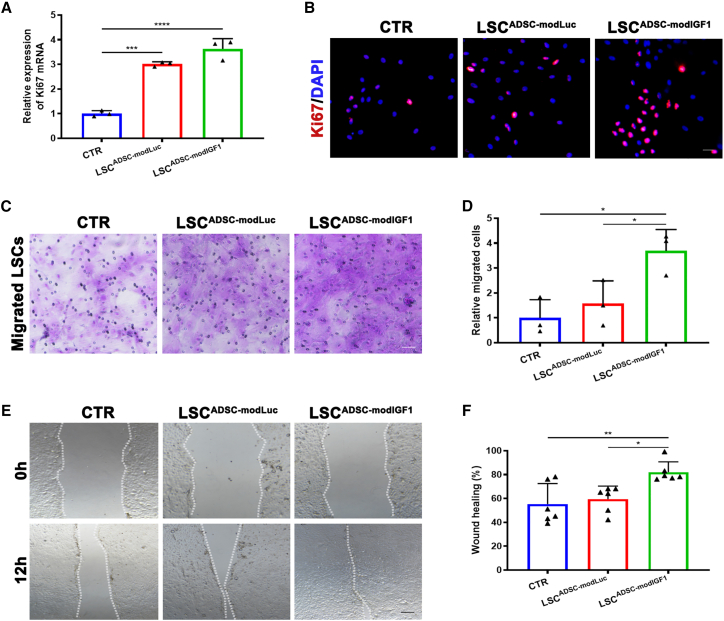
To elucidate the underlying therapeutic mechanism that the ADSCmodIGF1 treatment group had on the alkali-burned corneas, we again cultured primary LSCs either in the absence of ADSCs or in co-culture with mRNA-enhanced ADSCs. A series of experiments were performed to characterize cell proliferation rates and the migratory capacity of LSCs following co-culture with ADSCs. Notably, we found that LSC proliferation was stimulated by co-culture with ADSCs. Furthermore, LSC co-culture with modIGF1-engineered ADSCs (LSCADSC-modIGF1 group) most significantly stimulated proliferation events over the control groups, as indicated by levels of Ki67 gene expression (Figure 6A). Immunocytochemistry staining results of Ki67 were consistent with the RT-PCR findings, suggesting that ADSCmodIGF1 treatment was most beneficial to the promotion of LSC proliferation (Figure 6B). Subsequently, we employed transwell and scratch-wound assays to investigate the migratory effects of LSCs in co-culture with the ADSC treatment groups. These results obtained from these studies indicated that both ADSCmodLuc and ADSCmodIGF1 could promote the migration of LSCs, but a more significant effect was seen on the LSCs in co-culture with ADSCmodIGF1 (Figures 6C–6F). We also transfected LSCs directly with modRNA to explore potential changes in the proliferation and migration characteristics by CCK8 and transwell assay. We found that the direct transfection of modIGF1 into LSCs could also significantly promote their proliferation and migration capacity (Figure S5).
Figure 6.
Proliferation and migration capacity of LSCs co-cultured with modRNA-engineered ADSCs
(A) Expression levels of Ki67 mRNA in LSCs after 24 h co-culture with modRNA-engineered ADSCs. (B) Representative immunofluorescence staining images of Ki67 for LSCs at 24 h after co-culture. Nuclei were counterstained with DAPI. Scale bar, 20 μm. (C) Representative images of migrated LSCs by transwell migration assay after co-culture with different groups of ADSCs for 24 h. Scale bar, 50 μm. (D) Relative number of migrated cells dyed with crystal violet was compared. (E) Representative images of scratch assay from the three experimental LSCs groups captured at time 12 h. The dotted line represented the interfaces/cell fronts. Scale bar, 100 μm. (F) The percentage of wound healing was analyzed and compared. Data are presented as the mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; n = 3 (A and D) and 6 (F).
We next sought to evaluate to what extent the stemness and differentiation properties of LSCs could be altered following co-culture with modRNA-engineered ADSCs. At acute time intervals following transfection, RT-PCR revealed that ADSCmodIGF1 co-culture did not alter the stemness of LSCs while inhibiting their differentiation into corneal epithelial cells. This was made evident by the increased mRNA expression levels of stemness markers p75NTR and CK15, as well as the decreased mRNA expression levels of differentiation markers PAX6 and CK12 (Figures 7A–7D). The results of immunofluorescence staining were consistent with the trend of RT-PCR results. Although ADSCs transfected with modLuc could also maintain stemness and inhibit the differentiation characteristics to a certain extent, those effects stemming from the ADSCmodIGF1 group was more obvious (Figures 7E–7L). In addition, the barrier function of cells was also an important indicator of measuring the cell state, and the immunofluorescence staining of E-cadherin indicated that modIGF1-engineered ADSCs could effectively promote the junction formation between cells (Figure 7M).
Figure 7.
Characterization of LSCs in co-culture with modRNA-enhanced ADSCs
(A–D) The relative mRNA expression levels of stemness markers p75NTR and CK15, and differentiation markers PAX6 and CK12, in LSCs co-cultured with modRNA-engineered ADSCs for 24 h. (E–H) Representative immunofluorescence staining images of p75NTR, CK15, PAX6, and CK12 for LSCs at 24 h after co-culture. Nuclei were counterstained with DAPI. Scale bars, 20 μm. (I–L) Relative fluorescence intensity of each group was analyzed according to the results of immunofluorescence staining. (M) Representative immunofluorescence staining images of barrier function marker E-cadherin for LSCs at 24 h after co-culture. Nuclei were counterstained with DAPI. Scale bar, 50 μm. Data are presented as the mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001; n = 3.
Discussion
Herein, we innovatively applied cell-based delivery of modRNA to an ocular surface disorder using a murine model of corneal alkali burn injury. The enhancement of ADSCs with IGF1-modRNA effectively promoted the rapid recovery of corneal morphology and function, the repair and regeneration of corneal nerves, and the proliferation and migration of LSCs. We showed that the combination of ADSC cell therapy and mRNA technologies could be readily exploited to accelerate the comprehensive repair of the injured cornea in a multi-dimensional manner.
As the most essential structural barrier to the eye, cornea functions to protect the intraocular tissue from environmental factors. Destruction of the corneal structure, transparency, and function can easily lead to severe vision loss. Clinically, despite their different etiologies, a variety of corneal injuries or diseases, including chemical injury, thermal burn, exposure keratopathy, and neurotrophic keratitis, have similar pathological manifestations, such as corneal epithelial defects, neovascularization, corneal nerve injury, and LSC reduction.46,47 Repair of the injured cornea involves regeneration of various tissue structures including corneal epithelium, stroma, nerves, and limbus, which necessitates the synthesis of a complex cascade of biological reactions.48,49 Thus, it is of great value to promote the corneal repair both efficiently and comprehensively.
Among current therapeutic methods, stem cell therapy appears to be a considerably promising treatment for the interplay between tissue regeneration and wound healing of damaged tissue. In particular, the application of ADSCs in the treatment of ocular surface injury caused by LSC deficiency has been extensively studied, and it has been shown previously that ADSCs effectively reduce corneal opacity, inflammation, and neovascularization, while promoting corneal epithelium recovery.50,51 However, there are relatively few studies of ocular injuries that employ ADSCs with a focus to repair corneal nerves or regulate LSCs. Furthermore, corneal chemical injuries can be characterized into four stages, namely immediate, acute, early reparative, and late reparative. The management of care during the acute phase is accepted as crucial for the prognosis of the injured cornea.15,52 Considering that the therapeutic role of ADSCs has been suggested via secreting paracrine factors, it is of great significance to explore the means with which we can accelerate their rate of action and promote their efficacy to prevent the progression of corneal injury during the acute phase.37,38
To improve the therapeutic ability of ADSCs and achieve a better prognosis of acute alkali-burned cornea, we used modRNA technology to genetically modify ADSCs with the multifunctional cytokine IGF-1. IGF-1 shows a promising application prospect in repairing corneal injury and can promote healing of persistent corneal epithelial deficiency by inducing epithelial cell migration and proliferation.53 Previous studies have shown that ocular surface application of IGF-1 promoted the recovery of corneal epithelial ultrastructure and corneal nerve regeneration after laser in situ keratomileusis.54 Furthermore, several clinical studies have confirmed the constructive effect of a local application treatment with IGF-1, which was shown to improve healing rates of corneal epithelial defects and promote corneal nerve regeneration.34,35 However, IGF-1 protein applied in the form of eyedrops in previous studies often had the certain disadvantages of short action time, high loss rate, and repeated administration, which led to the weakening of drug efficacy. Thus, it was of great research significance to explore new drug delivery methods to the ocular surface.
In our research, we combined mRNA technology with stem cell therapy to achieve better recovery effects. Firstly, we validated modRNA expression in human ADSCs demonstrated overexpression of mRNA target genes were well tolerated. This was especially denoted through the evaluation of the cellular properties on ADSCs with the forced mRNA expression, which included phenotypic evaluation, differentiation capabilities, and an unaltered ADSC secretome (Figures 1 and S1). Furthermore, the sudden burst of transiently overexpressed IGF-1 protein resulted in enhanced proliferation and migration characteristics of the ADSCs (Figure 1).
Through the construction of an alkali burn model and continuous clinical examinations, ADSCmodIGF1 treatment efficiently promoted the transparency and thickened repair of injured corneas to normal levels and accelerated the healing rate of corneal epithelial defects (Figure 2). These results indicated that IGF-1 overexpression could indeed improve the in vivo efficacy of ADSCs. Further histochemical staining results were consistent with previous studies and showed that ADSCs could indeed promote corneal wound healing and retain their critical anti-inflammation, anti-neovascularization, and anti-fibrosis functions. It is worth mentioning that the IGF-1 recombinant protein eyedrops also had a positive yet more subtle effect on corneal repair in our research, especially as reported in the recovery of corneal epithelia and nerves.33,34,35 However, treatment with modIGF1-overexpressing ADSCs exhibited a superior capability to repair injured cornea, as the corneas in this group had more complete epithelia, but also more compact and orderly stroma, fewer neovascularization and lymphatic vessels, and denser nerve endings (Figures 3 and 4). The component structures of the cornea, along with limbus, are an integral whole. It is worth noting that the corneal nerves, which are important for corneal sensation and nutrition,55,56 and LSCs, which are essential for maintaining the dynamic balance of the corneal epithelium, are of great significance for the restoration of the complete morphology and function of the cornea.
To gain more extensive insights on the mechanisms that facilitate the multi-dimensional repair of the damaged cornea, we evaluated the effects of mRNA-enhanced ADSCs on corneal nerves and LSCs. The in vitro co-culture of modRNA enriched-ADSCs and primary TG cells revealed that ADSCmodIGF1 could significantly promote the proliferation and growth of TG neurons, not only in the density of nerve fibers, but also in the length of neurites and the number of intersections, which had a significantly better effect compared with ADSCs transfected with control modRNA (Figure 5). We therefore concluded that modIGF1-enhanced ADSCs could better promote the overall recovery of the injured cornea, in part through corneal nerve repair.
The dynamic properties and regenerative ability of corneal epithelium can help maintain its ultrastructure and function.57,58,59 Therefore, rapid re-epithelialization of the damaged corneal site is of great significance to maintain the normal state and function of the cornea.60,61 With the in-depth study of stem cells, the epithelial repair hypothesis based on LSCs has been widely accepted. LSCs, located at the basal layer of the limbal epithelium in the corneal and scleral transition area, are usually quiescent when the corneal epithelium is at steady state, but can divide symmetrically or asymmetrically in response to different environmental stimuli.62,63,64 The centripetal differentiation and migration of LSCs can renew and replenish corneal epithelial cells continuously, thus ensuring the dynamic balance of corneal epithelium. In our study, it was found that the corneal epithelia treated with ADSCmodIGF1 accumulated the greatest number of cells with stemness and proliferation characteristics among all treated groups, suggesting the most significantly improved reparative and regenerative capacity of the corneal epithelium (Figures 3F and S4A). The results also showed that control ADSCs and IGF-1 eyedrop treatments also had a certain degree of promoting effects on corneal injury repair, including the recovery of epithelial and stromal reconstruction, and simple ADSCs played a better role in improving limbal cells activity (Figures 3G–3J). Consequently, primary human LSCs in co-culture with ADSCmodIGF1 revealed significantly increased proliferation and migration properties (Figure 6). In addition, ADSCmodIGF1 could maintain the stemness of LSCs while inhibiting their differentiation into terminally mature corneal epithelial cells (Figure 7). The number of LSCs in vivo is limited, and the gradual differentiation and migration from stem cells to terminal corneal epithelial cells until shedding suggests the continuous loss of stem cells. As reflected by our in vitro experimental results, ADSCmodIGF1 could help increase the number of stem/progenitor cells and inhibit their rapid differentiation into corneal epithelium to some extent. This undoubtedly could enhance the in vivo limbal and corneal stem/progenitor cell reserve, which had an important therapeutic significance with respect to the rapid healing of the damaged cornea. As indicated in Figure 8, we believe that the subconjunctival injection of modIGF1-engineered ADSCs offers an optimal alternative for the treatment of corneal injury and has the potential to achieve comprehensive efficacy.
Figure 8.
Schematic diagram modeling the cellular engineering and subconjunctival delivery of modRNA-enhanced ADSCs as a novel therapy for in vivo corneal injury repair
In conclusion, we demonstrated the therapeutic potential of ADSCs overexpressing IGF-1 in a murine model of acute corneal alkali burn. In addition, we provided further evidence on the underlying molecular mechanisms responsible for enhancing properties of corneal neurons and LSCs. This study demonstrated a combinatorial approach utilizing stem cell therapy and mRNA technologies to promote the multi-dimensional repair of damaged cornea. The platform described herein could lead to more widespread applications for treating genetic- or trauma-related ocular injuries.
Materials and methods
Isolation and culture of ADSCs
The study was approved by the Medical Ethics Committee of the Ninth People’s Hospital affiliated to Shanghai Jiao Tong University, School of Medicine. Adipose tissues were collected from healthy female patients (aged 20–30 years) who underwent full-incision blepharoplasty surgeries for cosmetic purposes. Excised segments of adipose tissues were cut into small pieces and digested with 0.1% collagenase type A (Roche, Manheim, Germany) at 37°C for 8 h, followed by centrifugation at 1,500 rpm for 10 min. The precipitated cells were resuspended and cultured in DMEM (HyClone, GE Healthcare, Little Chalfont, UK) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, CA) and 1% penicillin-streptomycin antibiotic (Gibco, Carlsbad, CA) at 37°C.20,65 The medium was changed every 2 days and ADSCs were subcultured using TrypLE (Gibco) when they reached 80%–90% confluence. ADSCs between passages 3 and 5 were used for all studies described.
Isolation and culture of LSCs
Human limbal rings from donors were collected after central corneal button removed for corneal transplantation under the approval of the Eye Bank and Medical Ethics Committee of the Ninth People’s Hospital affiliated to Shanghai Jiao Tong University, School of Medicine. The obtained limbal ring tissue was incubated with 10 mg/mL Dispase II (Sigma-Aldrich, St. Louis, MO) at 4°C for 8 h. Subsequently, the epithelial cell sheets were separated from the underlying stroma and digested by TrypLE at 37°C for 5 min. The obtained single-cell suspensions were seeded and cultured in DMEM/F12 medium (Invitrogen) containing 10% FBS, 1% insulin-transferrin-selenium (Life Technologies, Carlsbad, CA), 1% non-essential amino acids (Life Technologies), 0.4 μg/mL hydrocortisone (Wako, Osaka, Japan), 2 mM L-glutamine (Life Technologies), 10 ng/mL epidermal growth factor (Life Technologies), and 1% penicillin-streptomycin antibiotic.66 The medium was changed every 2 days. LSCs were subcultured using TrypLE when the cells reached 80%–90% confluence. The first to third passages of LSCs were used for further experiments.
modRNA synthesis and formulation
modRNA was synthesized according to the methods described previously.67,68 In brief, T7 RNA polymerase-mediated transcription was performed from a linearized DNA template, which incorporated generic 5′ and 3′ UTRs and a poly(A) tail. RNA was purified using Ambion MEGAclear spin columns and residual 5′-phosphates were removed by treating the purified RNA with Antarctic Phosphatase (New England Biolabs) at 37°C for 30 min. The purity and concentration of RNA were assessed using NanoDrop spectrophotometers (Thermo Fisher Scientific, Waltham, MA) and the modRNA was resuspended at 1 μg/μL in 10 mM Tris HCl and 1 mM EDTA for further use. During the in vitro transcription synthesis, uridine was fully replaced with N1-methylpseudouridine. The open reading frame sequence for IGF-1, GFP, and luciferase modRNAs are provided in the Table S1.
modRNA transfection
ADSCs were transfected using Lipofectamine MessengerMAX Reagent (Invitrogen) as described previously.20,25 In brief, 2 μL Lipofectamine MessengerMAX Reagent were used per 1 μg modRNA to transfect 1 × 105 ADSCs. modRNA and transfection reagents were first diluted in Opti-MEM (Invitrogen) separately and incubated for 5 min at room temperature (RT). Subsequently, the two mixes were pooled together and left to incubate for 15 min to generate modRNA-lipid complexes. The complexes were exposed to cells for 4 h, after which the medium was completely replaced with standard medium for further culture until analysis or removed to collect cells for further experiments.
GFP modRNA expression in ADSCs was confirmed by photographing at 1, 2, 3, 4, 5, and 6 days after transfection. The transfection efficiency and mean fluorescence intensities of GFP modRNA were measured by C6 flow cytometry (Beckman Coulter, CA) 24 h after transfection.
Real-time PCR
Total RNA was extracted from different subsets of ADSCs or LSCs using an EZ-press RNA Purification Kit (EZBioscience, MN) and the reverse transcription was employed using PrimeScript RT reagent kit (Takara Bio, Otsu, Japan) according to the manufacturer’s instructions. PCR detection was carried out on a 7500 Real-Time PCR Detection System (Thermo Fisher Scientific) using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster, CA) and relative expression was determined using the 2-ΔΔCT method. Primers specific for human IGF-1 gene and the other primers sequences used in the research are shown in Table S2.
ELISA
To quantify the expression kinetics of IGF-1 following ADSC transfection, the supernatant was collected at various time points (at 1, 2, 3, 4, 5, and 6 days) after transfection and levels of IGF-1 protein were determined using an ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The optical density values of absorbance were measured on a microplate reader (ELX800, BioTek), and the protein content was determined using a standard curve. In addition, conditioned medium was collected at 24 h post-transfection for quantifying soluble mediators secreted by ADSCs of different groups. The protein concentrations of TGF-β1, IL-6, SDF-1, and HGF were detected using ELISA kits (R&D Systems) and then analyzed.
Transwell assay
To detect the migratory ability, 24-well polycarbonate membrane cell culture plate inserts with 8-μm pore size (SPL Life Sciences, Korea) were used. A number of 2 × 103 cells/well of LSCs were seeded in the upper chamber of the transwell chamber and the respective modRNA-engineered ADSCs were seeded to the lower chamber to explore the impact of modRNA-transfected ADSCs on the migratory ability of LSCs. After 24 h of co-culture incubation, liquid was discarded, and the cells were fixed with 4% paraformaldehyde (PFA) at RT for 15 min. After staining with 0.1% crystal violet (Biyuntian Biotechnology, China) at RT for 20 min, cells were photographed and counted from three randomly selected areas using a microscope. To explore the direct impact of modRNA transfection on the migratory ability of ADSCs or LSCs, 2 × 103 cells/well, which had been transfected for 24 h, were seeded in the upper chamber containing 200 μL serum-free medium, and 600 μL of normal culture medium containing 10% FBS was added to the lower chamber. After 24 h of incubation, follow-up steps were the same as those described above.
Wound healing assay
LSCs (5 × 105) that had been co-cultured with modRNA-engineered ADSCs for 24 h were seeded in a six-well plate with 100% confluence. Linear scratch wounds were created using a 200-μL sterile pipette tip. Next, the plate was washed with PBS (Gibco) three times to remove the suspended cells, and the cells were cultured in serum-free medium. After 0 and 12 h, the cells were cultured with 10 μg/mL mitomycin C to inhibit cell proliferation. The wounded area was imaged at the same position under the microscope and the distance between the wound sides was calculated by the ImageJ software.
Proliferation assay
modRNA-engineered ADSCs or LSCs were seeded in a 96-well plate at a concentration of 1 × 103 cells/well. Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) was used to conduct a cell proliferation assay according to the manufacturer’s instructions before and after modRNA transfection. Optical densities were recorded at 450 nm on a microplate reader.
Phenotypic profile of ADSCs
The phenotypic profiles of ADSCs were detected for the cell identification. In brief, 5 × 104 ADSCs were collected and incubated with fluorescence-conjugated CD73, CD90, CD105, CD14, CD34, and CD45 antibodies (BD Biosciences, San Jose, CA) at RT for 30 min at 24 h post-transfection. Quantitative analysis was performed using C6 flow cytometry (Beckman Coulter) and results of triplicate experiments were calculated with the FlowJo software (Tree Star, Ashland, OR).
Trilineage differentiation of ADSCs
The trilineage differentiation potentials of ADSCs were tested as described previously.69 All induction procedures were performed according to the manufacturer’s instructions (OriCell, Cyagen Biosciences). At 24 h post-transfection with modRNA, the ADSCs were incubated with adipogenic, osteogenic, or chondrogenic differentiation induction medium for 21 days. The ADSCs were then fixed in 4% PFA, followed by oil red O, alizarin red S, or Alcian blue staining. The images of differentiated ADSCs were captured and compared.
Immunocytochemical staining
The LSCs or TG neurons were seeded into 24-well plates after co-culture for 24 h with modRNA-engineered ADSCs. After 12 h of culture, the cells were rinsed three times with PBS, followed by fixation with 4% PFA for 15 min and permeabilization with 0.3% Triton X-100 (Sigma-Aldrich) in PBS for 15 min. Then the cells were blocked with 5% donkey serum (Sigma-Aldrich) for 1 h at RT. Subsequently, cells were incubated with Ki67 (Cell Signaling Technology, 3 Trask Lane Danvers, MA), p75NTR (Cell Signaling Technology), CK15 (Abcam, San Francisco, CA), PAX6 (Abcam), CK12 (Abcam), or E-cadherin (Abcam) overnight at 4°C. Cells were then incubated with the 1:400 dilution of Alexa Fluor-conjugated secondary antibodies (BD Biosciences) at RT for 1 h. Cell nuclei were stained with DAPI (Invitrogen) for 10 min. Subsequently, the stained cells were examined and photographed using a fluorescence microscope (Nikon Eclipse 80i; Nikon Instruments, Tokyo, Japan) and then ImageJ was used to analyze the fluorescence intensity.
Primary TG cells culture and staining
Primary TG cells were cultured using previously described methods.70,71 In brief, the whole TG tissues were obtained from healthy BALB/c mice and digested with papain and collagenase/Dispase to isolate the TG cells. The cells were then separated in Percoll gradients and seeded onto poly-D-lysine- and laminin-coated slides (Thermo Fisher Scientific). Neurobasal medium (Gibco) supplemented with B27 (Life Technologies) was used for culture and was changed every other day. After incubation for 1 week, the TG cells were co-cultured with modRNA-engineered ADSCs for 24 h, and then the cells were stained with an anti-tubulin β-III antibody (Abcam). Afterward, the density of neurite outgrowth, the neurite length, and the intersection number were calculated and analyzed by ImageJ with the NeuronJ and Sholl Analysis plug-ins.
Corneal alkali burn model
All experimental procedures with mice in this study were approved by the Medical Ethics Committee of Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, as well as carried out in accordance with the guidelines of the Chinese Animal Administration and the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Healthy 8-week-old female BALB/c mice were used in this study, and the corneal alkali burn model was established as described previously.72 In brief, mice were anesthetized and received ocular administration of oxybuprocaine hydrochloride eye drops (Santen Pharmaceutical, Kita-ku, Osaka, Japan), then a round Whatman III filter paper (2 mm in diameter) presoaked with 1 N NaOH (Sigma-Aldrich) was placed on the central cornea of the right eye for 40 s, followed by flushing the wound surface with PBS for 1 min.
Animal grouping and treatment
There were five animal groups, apart from the uninjured group receiving no treatment; the alkali-burned mice were randomly divided into the control (CTR), ADSCmodLuc, IGF-1 Eyedrop and ADSCmodIGF1 groups. The CTR group received subconjunctival injection of 10 μL PBS. Mice in the ADSCmodLuc group and ADSCmodIGF1 group were subconjunctivally injected with 1 × 105 modLuc-engineered or modIGF1-engineered ADSCs, respectively, which were suspended in 10 μL of PBS, using a 50-μL syringe and a 33-gauge metal needle (Hamilton, Reno, NV). As for the IGF-1 Eyedrop group, the mice received 1 μg/mL recombinant human IGF-1 (rhIGF1) protein (PeproTech, Rocky Hill, NJ) treatment one drop per eye 4 times a day for 16 consecutive days.
To detect the disassembly of ADSCs in vivo, mice injected subconjunctivally with DiD (5 μL, 100 μg DiD/mL; Invitrogen)-loaded simple or modIGF1-transfected ADSCs were imaged by IVIS every other day for 16 days.
Clinical evaluations
Clinical examinations were conducted for each group at the indicated times and the investigators were all blinded to the group assignment during scoring and data analysis. Slit-lamp microscope (SL-D7, Topcon, Tokyo, Japan) was used to evaluate the corneal opacity at days 0, 4, 8, and 16 after the alkali burn, and the corneal edema scores were recorded according to a scale from 0 to 4; 0 = completely clear; 1 = slightly hazy, iris, pupils easily visible; 2 = slightly opaque, iris, pupils still detectable; 3 = severely opaque iris, pupils hardly visible; and 4 = completely opaque, with no view of the iris and/or pupils.73 Afterward, corneal fluorescein staining was conducted using 2% fluorescein sodium (Sigma-Aldrich) and the corneal epithelial defects were evaluated and photographed under cobalt blue light at days 0, 4, 8, and 16 after the alkali burn. Corneal epithelial defect scores were evaluated and analyzed in accordance with the National Eye Institute/Industry grading scale. Moreover, the central corneal thickness of each group was examined and measured by OCT (Heidelberg Engineering, Germany) at days 0, 1, 2, 4, 8, and 16, and the average of three reading was recorded.
Histology and immunohistochemical staining
The mice were ethically sacrificed at day 16 and the enucleated eyes were paraffin-embedded and cut into 6-μm sections. Subsequently, the sections were stained with H&E or Masson’s trichrome (Sigma, St. Louis, MO) according to the manufacturer’s instructions. As for the immunohistochemical staining, the sections were incubated with 5% donkey serum for 1 h at RT, followed by staining with the primary antibody of αSMA (Abcam), CD31 (Abcam), or LYVE1 (Abcam) at 4°C overnight and then with the corresponding secondary antibody (1:2,000, Abcam) at RT for 1 h. The stained slides were viewed and photographed using the fluorescence microscope, and then ImageJ was used to analyze the corneal epithelial thickness, the percentage of stromal collagen volume, and αSMA-positive areas.
Immunofluorescence staining
For immunofluorescence staining, the eyeball sections were permeabilized by 0.3% Triton X-100 in PBS for 15 min and then blocked with 5% donkey serum for 1 h at RT. Afterward, primary antibodies against PAX6 (Abcam), PCNA (Cell Signaling Technology), p63 (Abcam), CK12 (Abcam), and E-cadherin (Abcam) were used and, after incubation overnight at 4°C, the sections were further incubated with the Alexa Fluor-conjugated secondary antibodies at RT for 1 h. Subsequently, nuclei were counterstained with DAPI for 10 min. ImageJ was used for analyzing the fluorescence intensity of each group.
As for the corneal nerve staining, the procedure was performed as described previously.74 The whole-mount corneas of each group were fixed in Zamboni fixative solution (Solarbio, Beijing, China) for 1 h, and then blocked by 0.1% Triton X-100, 2% goat serum, and 2% bovine serum albumin in PBS for 2 h. Afterward, the corneas were incubated with tubulin β-III at 4°C overnight and then within the Alexa Fluor 488-conjugated secondary antibody at RT for 1 h. The corneas were cut into petal shapes, and the nerve morphology was examined and captured using a fluorescence microscope. ImageJ was used to calculate the percentage area occupied by corneal sub-basal nerve fibers.
Statistical analysis
All data are presented as means ± standard deviation (SD). Depending on the grouping, p values were analyzed respectively using Student’s t test, one-way analysis of variance (ANOVA), followed by post hoc analysis Tukey test or two-way ANOVA followed by Bonferroni post hoc test (GraphPad Software, San Diego, CA). A value of p < 0.05 was considered statistically significant.
Data availability
All data and materials supporting the findings of this manuscript are presented in the paper and/or the supplemental information. Additional data are available from the corresponding authors upon reasonable request.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31800809 to Y.L., 82271041 to Y.F., and 82272178 to W.F.), the Biomaterials and Regenerative Medicine Institute Cooperative Research Project, Shanghai Jiao Tong University School of Medicine (2022LHA06 to Y.F.), the Program of Shanghai Academic Research Leader (22XD1401800 to Y.F.), Shanghai Natural Science Foundation (20ZR1434500 to W.F.), Biomedical Engineering fund of Shanghai Jiao Tong University (YG2021GD04 to W.F.), and Science and Technology Development Foundation of Shanghai Pudong (PKJ2020-Y06 to W.F.).
Author contributions
Study design, F.Y., D.G., Y.F., W.F., and Y.L.; modRNA preparation, W.F. and H.W.; experiments and data acquisition, F.Y., D.G., and D.Y.; manuscript preparation, F.Y. and D.G.; manuscript revision and editing, N.W., Y.F., W.F., and Y.L.; funding acquisition, Y.F., W.F., and Y.L.
Declaration of interests
The authors declared no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.05.002.
Contributor Information
Yang Lu, Email: luyang8311@hotmail.com.
Wei Fu, Email: fuweizhulu@163.com.
Yao Fu, Email: drfuyao@126.com.
Supplemental information
References
- 1.Chan E., Chong E.W., Lingham G., Stevenson L.J., Sanfilippo P.G., Hewitt A.W., Mackey D.A., Yazar S. Prevalence of keratoconus based on scheimpflug imaging: the raine study. Ophthalmology. 2021;128:515–521. doi: 10.1016/j.ophtha.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Long K., Liu Y., Li W., Wang L., Liu S., Wang Y., Wang Z., Ren L. Improving the mechanical properties of collagen-based membranes using silk fibroin for corneal tissue engineering. J. Biomed. Mater. Res. A. 2015;103:1159–1168. doi: 10.1002/jbm.a.35268. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto Y., Funamoto S., Sasaki S., Honda T., Hattori S., Nam K., Kimura T., Mochizuki M., Fujisato T., Kobayashi H., Kishida A. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials. 2010;31:3941–3948. doi: 10.1016/j.biomaterials.2010.01.122. [DOI] [PubMed] [Google Scholar]
- 4.Tan D.T.H., Dart J.K.G., Holland E.J., Kinoshita S. Corneal transplantation. Lancet. 2012;379:1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 5.Bourne R.R.A., Flaxman S.R., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J.H., Leasher J., Limburg H., et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob. Health. 2017;5:e888–e897. doi: 10.1016/S2214-109X(17)30293-0. [DOI] [PubMed] [Google Scholar]
- 6.Ahmmed A.A., Ting D.S.J., Figueiredo F.C. Epidemiology, economic and humanistic burdens of Ocular Surface Chemical Injury: a narrative review. Ocul. Surf. 2021;20:199–211. doi: 10.1016/j.jtos.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Tallab R.T., Stone D.U. Corticosteroids as a therapy for bacterial keratitis: an evidence-based review of 'who, when and why'. Br. J. Ophthalmol. 2016;100:731–735. doi: 10.1136/bjophthalmol-2015-307955. [DOI] [PubMed] [Google Scholar]
- 8.Jinagal J., Gupta P.C., Pilania R.K., Ram J. Systemic toxicity of topical corticosteroids. Indian J. Ophthalmol. 2019;67:559–561. doi: 10.4103/ijo.IJO_1091_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C., Du T., Mu G., Wang J., Gao X., Long F., Wang Q. Evaluation and ultrastructural changes of amniotic membrane fragility after UVA/riboflavin cross-linking and its effects on biodegradation. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ljubimov A.V., Saghizadeh M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanbhag S.S., Chanda S., Donthineni P.R., Basu S. Surgical management of unilateral partial limbal stem cell deficiency: conjunctival autografts versus simple limbal epithelial transplantation. Clin. Ophthalmol. 2021;15:4389–4397. doi: 10.2147/OPTH.S338894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zakirova E.Y., Valeeva A.N., Aimaletdinov A.M., Nefedovskaya L.V., Akhmetshin R.F., Rutland C.S., Rizvanov A.A. Potential therapeutic application of mesenchymal stem cells in ophthalmology. Exp. Eye Res. 2019;189 doi: 10.1016/j.exer.2019.107863. [DOI] [PubMed] [Google Scholar]
- 13.Galindo S., de la Mata A., López-Paniagua M., Herreras J.M., Pérez I., Calonge M., Nieto-Miguel T. Subconjunctival injection of mesenchymal stem cells for corneal failure due to limbal stem cell deficiency: state of the art. Stem Cel Res. Ther. 2021;12:60. doi: 10.1186/s13287-020-02129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertheuil N., Chaput B., Ménard C., Varin A., Laloze J., Watier E., Tarte K. Adipose mesenchymal stromal cells: definition, immunomodulatory properties, mechanical isolation and interest for plastic surgery. Ann. Chir. Plast. Esthet. 2019;64:1–10. doi: 10.1016/j.anplas.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Dinç E., Dursun Ö., Yilmaz G., Kurt A.H., Ayaz L., Vatansever M., Özer Ö., Yilmaz Ş.N. Evaluation of anti-Inflammatory and antiapoptotic effects of bone marrow and adipose-derived mesenchymal stem cells in acute alkaline corneal burn. J. Ocul. Pharmacol. Ther. 2021;37:24–34. doi: 10.1089/jop.2020.0103. [DOI] [PubMed] [Google Scholar]
- 16.Almaliotis D., Thomas A., Komnenou A., Gounari E., Almpanidou S., Siempis T., Papaioannou N., Koliakos G., Papakonstantinou E., Sotiropulos K., Karampatakis V. Evaluation of clinical and histological outcomes of adipose-derived mesenchymal stem cells in a rabbit corneal alkali burn model. Stem Cell Int. 2021;2021 doi: 10.1155/2021/6610023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sikora B., Skubis-Sikora A., Prusek A., Gola J. Paracrine activity of adipose derived stem cells on limbal epithelial stem cells. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-99435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu X., Ru Y., Chu C., Lv Y., Gao Y., Jia Z., Huang Y., Zhang Y., Zhao S. Lentivirus-mediated IL- 10-expressing Bone Marrow Mesenchymal Stem Cells promote corneal allograft survival via upregulating lncRNA 003946 in a rat model of corneal allograft rejection. Theranostics. 2020;10:8446–8467. doi: 10.7150/thno.31711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur K., Zangi L. Modified mRNA as a therapeutic tool for the heart. Cardiovasc. Drugs Ther. 2020;34:871–880. doi: 10.1007/s10557-020-07051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu F., Witman N., Yan D., Zhang S., Zhou M., Yan Y., Yao Q., Ding F., Yan B., Wang H., et al. Human adipose-derived stem cells enriched with VEGF-modified mRNA promote angiogenesis and long-term graft survival in a fat graft transplantation model. Stem Cel Res. Ther. 2020;11:490. doi: 10.1186/s13287-020-02008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadas Y., Katz M.G., Bridges C.R., Zangi L. Modified mRNA as a therapeutic tool to induce cardiac regeneration in ischemic heart disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017 Jan;;9 doi: 10.1002/wsbm.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamb Y.N. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81:495–501. doi: 10.1007/s40265-021-01480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillmore J.D., Gane E., Taubel J., Kao J., Fontana M., Maitland M.L., Seitzer J., O'Connell D., Walsh K.R., Wood K., et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 2021;385:493–502. doi: 10.1056/NEJMoa2107454. [DOI] [PubMed] [Google Scholar]
- 24.Trepotec Z., Lichtenegger E., Plank C., Aneja M.K., Rudolph C. Delivery of mRNA therapeutics for the treatment of hepatic diseases. Mol. Ther. 2019;27:794–802. doi: 10.1016/j.ymthe.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Z., Witman N., Wang W., Li D., Yan B., Deng M., Wang X., Wang H., Zhou G., Liu W., et al. Cell-mediated delivery of VEGF modified mRNA enhances blood vessel regeneration and ameliorates murine critical limb ischemia. J. Control Release. 2019;310:103–114. doi: 10.1016/j.jconrel.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Geng Y., Duan H., Xu L., Witman N., Yan B., Yu Z., Wang H., Tan Y., Lin L., Li D., et al. BMP-2 and VEGF-A modRNAs in collagen scaffold synergistically drive bone repair through osteogenic and angiogenic pathways. Commun. Biol. 2021;4:82. doi: 10.1038/s42003-020-01606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ai X., Yan B., Witman N., Gong Y., Yang L., Tan Y., Chen Y., Liu M., Lu T., Luo R., et al. Transient secretion of VEGF protein from transplanted hiPSC-CMs enhances engraftment and improves rat heart function post MI. Mol. Ther. 2023;31:211–229. doi: 10.1016/j.ymthe.2022.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee P.D., Giudice L.C., Conover C.A., Powell D.R. Insulin-like growth factor binding protein-1: recent findings and new directions. Proc. Soc. Exp. Biol. Med. 1997;216:319–357. doi: 10.3181/00379727-216-44182. [DOI] [PubMed] [Google Scholar]
- 29.Doorn J., Roberts S.J., Hilderink J., Groen N., van Apeldoorn A., van Blitterswijk C., Schrooten J., de Boer J. Insulin-like growth factor-I enhances proliferation and differentiation of human mesenchymal stromal cells in vitro. Tissue Eng. Part A. 2013;19:1817–1828. doi: 10.1089/ten.TEA.2012.0522. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadi R., Esmaeil-Sani Z., Amini K. Effect of local administration of insulin-like growth factor I combined with inside-out artery graft on peripheral nerve regeneration. Injury. 2013;44:1295–1301. doi: 10.1016/j.injury.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Kostereva N.V., Wang Y., Fletcher D.R., Unadkat J.V., Schnider J.T., Komatsu C., Yang Y., Stolz D.B., Davis M.R., Plock J.A., Gorantla V.S. IGF-1 and chondroitinase ABC augment nerve regeneration after vascularized composite limb allotransplantation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slavin B.R., Sarhane K.A., von Guionneau N., Hanwright P.J., Qiu C., Mao H.Q., Höke A., Tuffaha S.H. Insulin-Like growth factor-1: a promising therapeutic target for peripheral nerve injury. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.695850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trosan P., Svobodova E., Chudickova M., Krulova M., Zajicova A., Holan V. The key role of insulin-like growth factor I in limbal stem cell differentiation and the corneal wound-healing process. Stem Cell Dev. 2012;21:3341–3350. doi: 10.1089/scd.2012.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishida T., Chikama T.I., Morishige N., Yanai R., Yamada N., Saito J. Persistent epithelial defects due to neurotrophic keratopathy treated with a substance p-derived peptide and insulin-like growth factor 1. Jpn. J. Ophthalmol. 2007;51:442–447. doi: 10.1007/s10384-007-0480-z. [DOI] [PubMed] [Google Scholar]
- 35.Yamada N., Matsuda R., Morishige N., Yanai R., Chikama T.I., Nishida T., Ishimitsu T., Kamiya A. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br. J. Ophthalmol. 2008;92:896–900. doi: 10.1136/bjo.2007.130013. [DOI] [PubMed] [Google Scholar]
- 36.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y., Wang Y., Li Q., Liu K., Hou J., Shao C., Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 38.Bunnell B.A. Adipose tissue-derived mesenchymal stem cells. Cells. 2021;10:3433. doi: 10.3390/cells10123433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huat T.J., Khan A.A., Pati S., Mustafa Z., Abdullah J.M., Jaafar H. IGF-1 enhances cell proliferation and survival during early differentiation of mesenchymal stem cells to neural progenitor-like cells. BMC Neurosci. 2014;15:91. doi: 10.1186/1471-2202-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y., Yu X., Lin S., Li X., Zhang S., Song Y.H. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007;356:780–784. doi: 10.1016/j.bbrc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 41.Bizrah M., Yusuf A., Ahmad S. An update on chemical eye burns. Eye (Lond) 2019;33:1362–1377. doi: 10.1038/s41433-019-0456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binotti W.W., Bayraktutar B., Ozmen M.C., Cox S.M., Hamrah P. A review of imaging biomarkers of the ocular surface. Eye Contact Lens. 2020;46(Suppl. 2) doi: 10.1097/ICL.0000000000000684. S84-s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouyang H., Xue Y., Lin Y., Zhang X., Xi L., Patel S., Cai H., Luo J., Zhang M., Zhang M., et al. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature. 2014;511:358–361. doi: 10.1038/nature13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pellegrini G., Dellambra E., Golisano O., Martinelli E., Fantozzi I., Bondanza S., Ponzin D., McKeon F., De Luca M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L., Hartley R., Reiss B., Sun Y., Pu J., Wu D., Lin F., Hoang T., Yamada S., Jiang J., Zhao M. E-cadherin plays an essential role in collective directional migration of large epithelial sheets. Cell. Mol. Life Sci. 2012;69:2779–2789. doi: 10.1007/s00018-012-0951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kousha O., Kousha Z., Paddle J. Exposure keratopathy: incidence, risk factors and impact of protocolised care on exposure keratopathy in critically ill adults. J. Crit. Care. 2018;44:413–418. doi: 10.1016/j.jcrc.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 47.Jabbour S., Ashton C., Balal S., Kaye A., Ahmad S. The management of neurotrophic keratitis. Curr. Opin. Ophthalmol. 2021;32:362–368. doi: 10.1097/ICU.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 48.Raghunathan V.K., Thomasy S.M., Strøm P., Yañez-Soto B., Garland S.P., Sermeno J., Reilly C.M., Murphy C.J. Tissue and cellular biomechanics during corneal wound injury and repair. Acta Biomater. 2017;58:291–301. doi: 10.1016/j.actbio.2017.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Li C., Zhu Q., Liang R., Xie C., Zhang S., Hong Y., Ouyang H. A long-term retaining molecular coating for corneal regeneration. Bioact. Mater. 2021;6:4447–4454. doi: 10.1016/j.bioactmat.2021.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin H.F., Lai Y.C., Tai C.F., Tsai J.L., Hsu H.C., Hsu R.F., Lu S.N., Feng N.H., Chai C.Y., Lee C.H. Effects of cultured human adipose-derived stem cells transplantation on rabbit cornea regeneration after alkaline chemical burn. Kaohsiung J. Med. Sci. 2013;29:14–18. doi: 10.1016/j.kjms.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shang Q., Chu Y., Li Y., Han Y., Yu D., Liu R., Zheng Z., Song L., Fang J., Li X., et al. Adipose-derived mesenchymal stromal cells promote corneal wound healing by accelerating the clearance of neutrophils in cornea. Cell Death Dis. 2020;11:707. doi: 10.1038/s41419-020-02914-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao L., Li Z.R., Su W.R., Li Y.P., Lin M.L., Zhang W.X., Liu Y., Wan Q., Liang D. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaidyanathan U., Hopping G.C., Liu H.Y., Somani A.N., Ronquillo Y.C., Hoopes P.C., Moshirfar M. Persistent corneal epithelial defects: a review article. Med. Hypothesis Discov. Innov. Ophthalmol. 2019;8:163–176. [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C., Peng Y., Pan S., Li L. Effect of insulin-like growth factor-1 on corneal surface ultrastructure and nerve regeneration of rabbit eyes after laser in situ keratomileusis. Neurosci. Lett. 2014;558:169–174. doi: 10.1016/j.neulet.2013.10.063. [DOI] [PubMed] [Google Scholar]
- 55.Rózsa A.J., Beuerman R.W. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain. 1982;14:105–120. doi: 10.1016/0304-3959(82)90092-6. [DOI] [PubMed] [Google Scholar]
- 56.Müller L.J., Marfurt C.F., Kruse F., Tervo T.M.T. Corneal nerves: structure, contents and function. Exp. Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 57.Lavker R.M., Kaplan N., Wang J., Peng H. Corneal epithelial biology: lessons stemming from old to new. Exp. Eye Res. 2020;198 doi: 10.1016/j.exer.2020.108094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amitai-Lange A., Altshuler A., Bubley J., Dbayat N., Tiosano B., Shalom-Feuerstein R. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells. 2015;33:230–239. doi: 10.1002/stem.1840. [DOI] [PubMed] [Google Scholar]
- 59.Richardson A., Wakefield D., Di Girolamo N. Fate mapping mammalian corneal epithelia. Ocul. Surf. 2016;14:82–99. doi: 10.1016/j.jtos.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Lu L., Reinach P.S., Kao W.W. Corneal epithelial wound healing. Exp. Biol. Med. (Maywood) 2001;226:653–664. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- 61.Zieske J.D. Extracellular matrix and wound healing. Curr. Opin. Ophthalmol. 2001;12:237–241. doi: 10.1097/00055735-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Castro-Muñozledo F., Gómez-Flores E. Challenges to the study of asymmetric cell division in corneal and limbal epithelia. Exp. Eye Res. 2011;92:4–9. doi: 10.1016/j.exer.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Bonnet C., González S., Roberts J.S., Robertson S.Y.T., Ruiz M., Zheng J., Deng S.X. Human limbal epithelial stem cell regulation, bioengineering and function. Prog. Retin. Eye Res. 2021;85 doi: 10.1016/j.preteyeres.2021.100956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dziasko M.A., Daniels J.T. Anatomical features and cell-cell interactions in the human limbal epithelial stem cell niche. Ocul. Surf. 2016;14:322–330. doi: 10.1016/j.jtos.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Yan D., Yan C., Yu F., Zhang S., Chen L., Wu N., Shao C., Yao Q., Sun H., Fu Y. Exploitation of human mesenchymal stromal cell derived matrix towards the structural and functional restoration of the ocular surface. Biomater. Sci. 2020;8:4712–4727. doi: 10.1039/d0bm00787k. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Q., Chen P., Di G., Zhang Y., Wang Y., Qi X., Duan H., Xie L. Ciliary neurotrophic factor promotes the activation of corneal epithelial stem/progenitor cells and accelerates corneal epithelial wound healing. Stem Cells. 2015;33:1566–1576. doi: 10.1002/stem.1942. [DOI] [PubMed] [Google Scholar]
- 67.Warren L., Manos P.D., Ahfeldt T., Loh Y.H., Li H., Lau F., Ebina W., Mandal P.K., Smith Z.D., Meissner A., et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Richner J.M., Himansu S., Dowd K.A., Butler S.L., Salazar V., Fox J.M., Julander J.G., Tang W.W., Shresta S., Pierson T.C., et al. Modified mRNA vaccines protect against Zika Virus infection. Cell. 2017;169:176. doi: 10.1016/j.cell.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Zheng H., Yu Z., Deng M., Cai Y., Wang X., Xu Y., Zhang L., Zhang W., Li W. Fat extract improves fat graft survival via proangiogenic, anti-apoptotic and pro-proliferative activities. Stem Cel Res. Ther. 2019;10:174. doi: 10.1186/s13287-019-1290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malin S.A., Davis B.M., Molliver D.C. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat. Protoc. 2007;2:152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- 71.Yang S., Zhang Y., Zhang Z., Dan J., Zhou Q., Wang X., Li W., Zhou L., Yang L., Xie L. Insulin promotes corneal nerve repair and wound healing in Type 1 Diabetic mice by enhancing Wnt/β-Catenin signaling. Am. J. Pathol. 2020;190:2237–2250. doi: 10.1016/j.ajpath.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 72.Yan D., Yu F., Chen L., Yao Q., Yan C., Zhang S., Wu N., Gong D., Sun H., Fu Y., Shao C. Subconjunctival injection of regulatory T cells potentiates corneal healing via orchestrating inflammation and tissue repair after acute alkali burn. Invest. Ophthalmol. Vis. Sci. 2020;61:22. doi: 10.1167/iovs.61.14.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoeruek E., Ziemssen F., Henke-Fahle S., Tatar O., Tura A., Grisanti S., Bartz-Schmidt K.U., Szurman P., Tübingen Bevacizumab Study Group Safety, penetration and efficacy of topically applied bevacizumab: evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol. 2008;86:322–328. doi: 10.1111/j.1600-0420.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J., Dai Y., Wei C., Zhao X., Zhou Q., Xie L. DNase I improves corneal epithelial and nerve regeneration in diabetic mice. J. Cell. Mol. Med. 2020;24:4547–4556. doi: 10.1111/jcmm.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials supporting the findings of this manuscript are presented in the paper and/or the supplemental information. Additional data are available from the corresponding authors upon reasonable request.