Abstract
In order to improve the yield of mung bean peel polysaccharide, on the basis of single-factor experiments, the ultrasonic assisted extraction conditions were optimized by response surface methodology (RSM). The results showed that under the conditions of material-liquid ratio of 1: 40, temperature 77 °C, ultrasonic power 216 W and extraction time 47 min, the extraction rate of mung bean peel polysaccharide was the best, which was 2.55 %. The extracted polysaccharide was phosphorylated and its antioxidant activity in vitro was studied. The results suggested that the modified polysaccharide had a significant scavenging effect on hydroxyl radicals and enhanced the ability of anti-lipid peroxidation, which offered ideas and methods for the development and application of mung bean peel polysaccharide.
Keywords: Phosphorylation modification, Mung bean peel polysaccharide, Antioxidation, Ultrasonic-assisted extraction
1. Introduction
Mung bean, as a traditional medicinal and edible plant, has a long planting history in China [1]. Because the development depth of mung bean is not enough, mung bean skin is often discarded as a waste, resulting in waste. Mung bean skin contains proteins, lipids, polyphenols, vitamins, flavonoids and other substances [2], [3]. In addition, mung bean skin also contains important bioactive components, such as polysaccharides. Polysaccharide is a kind of natural macromolecular compound connected by 10 or more glycosidic bonds. Because of its abundant physiological activities and low toxicity, it is widely used in food, medical, health care, cosmetics and other fields [4], [5], [6]. Research have found that mung bean peel polysaccharide (MBP) has antioxidant, anti-tumor, antibacterial, immune regulation and so on [7], [8]. The activity of polysaccharide is affected by molecular weight, structure, water solubility etc., which make the activity of polysaccharide difficult to reach the ideal level. Related studies have confirmed that chemical modification of polysaccharide can improve the functional activity of polysaccharide, and modified products with different activities can be obtained by different modifications. For instance, chitosan has improved its water solubility and antibacterial activity after chemical modification [9]. Sepia esculent Ink polysaccharide has stronger anticoagulant ability after phosphorylation modification [10]. Phosphorylated Dictyophora indusiata polysaccharide possesses better antioxidant and anti-tumor activity [11]. Common chemical modification methods include acetylation, carboxymethylation, sulfation, phosphorylation, selenization, etc. [12].
Now, the extraction ways of polysaccharide mainly include traditional water extraction, ultrasonic assisted extraction, acid extraction, alkali extraction, microwave assisted extraction, enzyme extraction, supercritical fluid extraction and other methods [13]. Among many auxiliary extraction ways, ultrasonic-assisted extraction has been applied to the extraction of natural products by a growing number of scholars because of its short extraction time and high extraction efficiency. The cavitation effect generated by ultrasound produces strong shear force in the cavitation region and increases the local temperature and pressure, forcing the cell wall to crack, so that the intracellular substances enter the solvent and promotes the release of intracellular active components [14], [15]. Herein, response surface methodology (RSM) [16] was utilized to optimize the ultrasonic extraction process of mung bean peel polysaccharide, and the gained polysaccharide were phosphorylated and explored for its antioxidant activity, which provided reference value for the research and application of mung bean peel polysaccharide.
2. Materials and methods
2.1. Materials and reagents
Mung beans were bought from local supermarket. The purity of related reagents was analytically pure.
2.2. Ultrasonic assisted extraction of mung bean peel polysaccharide (MBP)
First, 80 % ethanol (1: 10 g / mL) was added to the dried mung bean peel powder and soaked for 24 h to remove lipid and pigment molecules, after soaking, the powder was filtered and dried naturally [8]. Secondly, distilled water with a certain solid–liquid ratio was added to 5 g treated mung bean peel powder to extract mung bean peel polysaccharide under certain ultrasonic conditions. Then the filtrate was centrifuged and concentrated under reduced pressure, deproteinized, and the supernatant was collected and dialyzed in tap water and distilled water for 24 h. After that, added 95% ethanol to the solution to let the final ethanol concentration 80% [17]. Finally, the precipitate was collected by centrifugation and freeze-dried to obtain mung bean peel polysaccharide. The standard curve of glucose was drawn, and the concentration of polysaccharide extract was obtained by phenol–sulfuric acid method. The extraction rate of polysaccharides was calculated using the following formula:
Yield(%) = [(C·V·B)/m]·100
Where C was polysaccharide concentration; V was volume; B was dilution multiple; m was sample mass.
2.3. Single factor experiments
The ratio of material to solvent (1: 10,1: 20,1: 30,1: 40,1: 50 g / mL), time (30 min, 40 min, 50 min, 60 min, 70 min), power (144 W, 180 W, 216 W, 252 W, 288 W), temperature (40 °C, 50 °C, 60 °C, 70 °C, 80 °C) were chosen as four factors. The yield of polysaccharide was used as the index, and the single-factor experiments were implemented sequentially according to the method of 2.2. The optimal conditions got in each experiment were one of the conditions for the next single-factor test.
2.4. Response surface experimental design
On the basis of single factor experiment results and literature review [18], time (A), ultrasonic power (B) and temperature (C) were taken as three experimental factors, and the yield of polysaccharide was used as the response value. The optimal range of each factor was selected to design a three-factor and three-level response surface experiment (Table 1). The Design-expert 12 software was used to perform regression fitting on various factors so as to optimize the extraction conditions of mung bean peel polysaccharide.
Table 1.
Test table of three factors and three levels.
| Levels | Factors | ||
|---|---|---|---|
| A. Time(min) | B. Ultrasonic power(W) | C. Temperature(°C) | |
| 1 | 30 | 180 | 60 |
| 2 | 40 | 216 | 70 |
| 3 | 50 | 252 | 80 |
2.5. Preparation of P-MBP
Phosphorylated polysaccharides were prepared according to Chen et al. [19]. 0.5 g MBP was dissolved in 25 mL DMF, sealed and stirred for 2 h. In ice bath condition, slowly dropwise added 5 mL of POCl3 to 15 mL of pyridine solution. When a great number of light-yellow solids appeared in the solution, the DMF solution of MBP was slowly added to it. Then the mixed solution was moved to an oil bath at 55 °C for 3 h. After the reaction was complete, the solution was cooled to indoor temperature, and the pH of the solution was adjusted to neutral with NaOH(1 mol /L) solution. Eventually, P-MBP was acquired by alcohol precipitation, centrifugation, precipitation re-dissolution, dialysis and freeze-drying.
2.6. Determination of degree of substitution
2.6.1. Drawing of phosphate standard curve
According to the method of Ye et al. [20], the standard curve was drawn, and measured the absorbance at 580 nm. The phosphate concentration was the abscissa and the absorbance was the ordinate.
2.6.2. Determination of phosphate content
Phosphate content was measured by the way of Zhang et al. [21]. In short, put 0.05 g sample in a beaker, slowly added 0.5 mL concentrated sulfuric acid and 0.5 mL concentrated nitric acid,and then the beaker was heated in a 45 °C water bath until smoke was generated. After cooling, 30 % hydrogen peroxide solution (0.5 mL) was added and heated again until no smoke was generated. Repeated the above steps once until the solution was pale yellow and no smoke was generated. After cooling, 6 mol/L HCl (0.5 mL) was added and heated in a water bath to completely decompose the acid. Lastly, the mixture was diluted to 50 mL with distilled water, and 5 mL solution was taken to determine the phosphate content. The degree of substitution was expressed as [22]:
DS = 5.23P / (100–3.32P)
Where P was the percentage of phosphate (%).
2.7. Infrared analysis
The appropriate amount of MBP and P-MBP were pressed with KBr, respectively, and determined by FT-IR in the wavelength range of 4000–400 cm−1.
2.8. Oxidation resistance test
2.8.1. Determination of antioxidant activity of hydroxyl radical
Hydroxyl radical determination method on the basis of the previous slightly modified [8]. 1 mL of FeSO4 (6 mmol/L), 1 mL of salicylic acid–ethanol solution (6 mmol/L), and 1 mL of hydrogen peroxide (6 mmol/L) were added to 1 mL polysaccharide solution (4 mg/mL, 3.5 mg/mL, 3 mg/mL~0.5 mg/mL). Then, mixed well and reacted in a water bath at 37 °C for 30 min. With distilled water as blank control and VC as positive control, the scavenging activity of hydroxyl radical was calculated by measuring the absorbance at 510 nm.
Hydroxyl scavenging activity (%) = [1–(Ab –Ac) ⁄ (Ao)] × 100%
Where Ab was sample’ absorbance; Ac was the background absorbance of the sample; Ao was blank’ absorbance.
2.8.2. Determination of anti-lipid peroxidation ability
The resistance to lipid peroxidation was determined by Li et al. [15]. In brief, added 1 mL soybean lecithin solution (PBS with pH = 7 as solvent) and 0.2 mL FeSO4 solution (10 mmol/L) to 1 mL polysaccharide solution (0.5 mg/L, 1 mg/L~4.0 mg/L). It is then reacted in 37 °C water bath for 40 min. The mixture was cooled down to indoor temperature, 0.5 mL TCA (20%, w/v) and 0.5 mL thiobaric acid (0.8%, w/v) were added, heated in a 90 °C water bath for 15 min, and then cooled again to room temperature. Measured absorbance at 535 nm. Vc was used as a positive control and PBS solution as a blank control to calculate lipid peroxidation capacity.
Inhibition rate (%)=(Ai-Aj)/Ai
In the formula, Ai was the absorbance of the blank, and Aj was the absorbance of the sample.
3. Results and discussion
3.1. Glucose standard curve
The obtained glucose standard curve was y = 15.194x + 0.0744, R2 = 0.9991.
3.2. The influence of various factors on the extraction rate
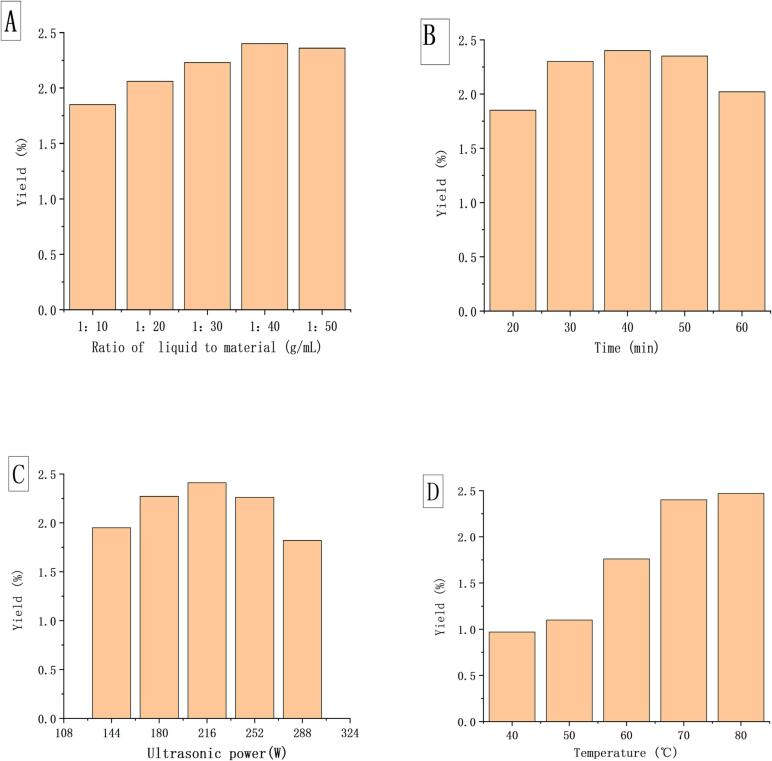
As the Fig. 1A showed, the yield of MBP increased with the increase of material-water ratio, and reached the peak when the solid–liquid ratio was 1: 40. When the solid to liquid ratio was low, the sample was not mixed with water sufficiently, and the extraction was not complete, which was not conducive to the release of intracellular polysaccharide, resulting in low extraction rate. When the ratio of material to solvent was high, the extraction rate decreased, which might be because the increase of the solvent made the distance of internal diffusion longer, resulting in the decrease of hydration and affecting the mass transfer [16], [23]. Therefore, 1: 40 was selected as the best solid–liquid ratio.
Fig. 1.
Single factor condition analysis: A: Influence of solid–liquid ratio on MBP yield; B: Influence of time on MBP yield; C: Influence of ultrasonic power on MBP yield; D: Influence of temperature on MBP yield.
As illustrated in Fig. 1B, with the increase of time, the production first increased and then decreased, and reached the maximum at 40 min. This might be due to the relatively long time, ultrasonic cavitation was conducive to the diffusion of polysaccharide from the cell to the solvent. However, when the time was too long, the thermal effect and mechanical action of ultrasound were heightened, resulting in the degradation of polysaccharide molecules, thereby reducing the yield [15], [24].
As shown in 1C, the yield of MBP increased with the increase of ultrasonic power. This might be because the mechanical effect increased with the increase of ultrasonic power, which accelerated the dissolution and release of intracellular polysaccharide. When the ultrasonic power was greater than 216 W, the polysaccharide was degraded by the severe destruction of ultrasound, resulting in a decrease in yield [25], [26].
As shown in Fig. 1D, when the temperature increased, the yield of polysaccharide increased significantly. This might be due to the higher temperature, the more intense thermal motion of polysaccharide molecules, which accelerated the diffusion and dissolution of polysaccharide [3]. When the temperature was 70–80 °C, the yield of polysaccharide increased more gently. Excessive temperature would cause the breakage of glycosidic bonds to degrade polysaccharide, thereby reducing the thermal stability and activity of polysaccharide [27]. Thus, 70 °C was chosen as the best condition.
3.3. Response surface optimization of ultrasonic-assisted extraction conditions of polysaccharide
3.3.1. Response surface test design and results
Taking the polysaccharide yield as the response value, a three-factor three-level response surface experiment was designed on the basis of the single factor experiment.The experimental design and results were exhibited in Table 2.
Table 2.
RSM experimental design and results.
| Run | Factors | Yield (%) | ||
|---|---|---|---|---|
| A:Time(min) | B:Ultrasonic power(W) | C:Temperature(°C) | ||
| 1 | 30 | 252 | 70 | 2 |
| 2 | 40 | 216 | 70 | 2.44 |
| 3 | 50 | 252 | 70 | 2.37 |
| 4 | 30 | 180 | 70 | 2.23 |
| 5 | 40 | 252 | 80 | 2.43 |
| 6 | 50 | 180 | 70 | 1.99 |
| 7 | 50 | 216 | 80 | 2.48 |
| 8 | 40 | 252 | 60 | 1.45 |
| 9 | 40 | 216 | 70 | 2.4 |
| 10 | 40 | 180 | 60 | 1.4 |
| 11 | 30 | 216 | 80 | 2.46 |
| 12 | 40 | 180 | 80 | 2.31 |
| 13 | 40 | 216 | 70 | 2.41 |
| 14 | 50 | 216 | 60 | 1.69 |
| 15 | 30 | 216 | 60 | 1.6 |
Multiple regression analysis was carried out by Design-Expert 12 software, and the following regression equation was attained:
Y(%) = 2.42 + 0.0300*A + 0.0400*B + 0.4425*C + 0.1525*AB-0.0175*AC + 0.0175*BC-0.0546*A2-0.2146*B2-0.3046*C2
3.3.2. Model analysis of variance results
The consequences of variance analysis of the model were shown in Table 3.
Table 3.
Response surface model analysis of variance of MBP yield.
| Source | Sum of Squares | df | Mean Square | F-value | p-value | significant |
|---|---|---|---|---|---|---|
| Model | 2.16 | 9 | 0.2401 | 147.03 | < 0.0001 | significant |
| A (Time) | 0.0072 | 1 | 0.0072 | 4.41 | 0.0898 | |
| B(Ultrasonic power) | 0.0128 | 1 | 0.0128 | 7.84 | 0.0380 | |
| C(Temperature) | 1.57 | 1 | 1.57 | 959.05 | < 0.0001 | |
| AB | 0.0930 | 1 | 0.0930 | 56.95 | 0.0006 | |
| AC | 0.0012 | 1 | 0.0012 | 0.7500 | 0.4261 | |
| BC | 0.0012 | 1 | 0.0012 | 0.7500 | 0.4621 | |
| A2 | 0.0110 | 1 | 0.0110 | 6.74 | 0.0485 | |
| B2 | 0.1700 | 1 | 0.1700 | 104.09 | 0.0002 | |
| C2 | 0.3425 | 1 | 0.3425 | 209.72 | < 0.0001 | |
| Residual | 0.0082 | 5 | 0.0016 | |||
| Lack of Fit | 0.0073 | 3 | 0.0024 | 5.62 | 0.1549 | Not significant |
| Pure Error | 0.0009 | 2 | 0.0004 | |||
| Cor Total | 2.17 | 14 | ||||
| R2 = 0.9962 | R2adj = 0.9895 | CV = 1.91 |
F-value was used to reflect whether the model is significant. The larger the value, the more significant the model is. In this experiment, the F-value was 147.03, indicating that the model was significant. The p-value was used to reflect the significance of the influence of various factors in the model. Generally speaking, p-value < 0.05 indicated that the model term was significant; when p-value < 0.001, the model term is extremely significant. In this experiment, B, C, AB, A2, B2 and C2 were significant model items. According to Table 3, the factors affecting the yield of polysaccharides were temperature > ultrasonic power > time. R2 = 0.9962, R2adj = 0.9895, and the lack of fit term was not significant (p-value greater than 0.05), indicating that the model had a good fitting degree and could better analyze and predict the conditions of ultrasonic-assisted extraction of MBP. It also showed that the experimental method was reliable.
3.3.3. Confirmation and verification of the optimal conditions of response surface
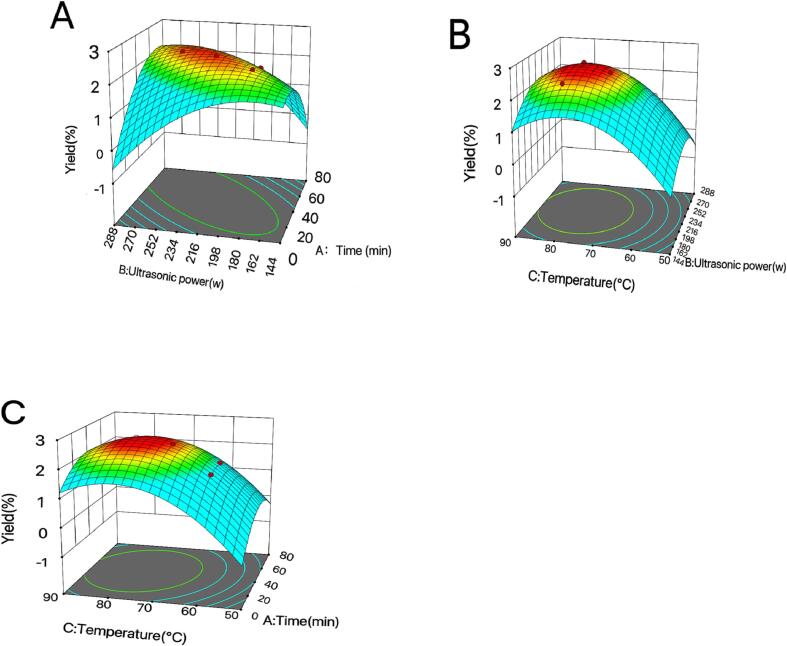
The 3D diagram of any two-factor interaction could be obtained through the model. As shown in Fig. 2A, the response surface graph was steep, indicating that there was a certain interaction between ultrasonic time and ultrasonic power. Based on the analysis of the results of the response surface experiment, the optimum conditions for the extraction of MBP were gained: the ultrasonic time was 46.5694 min, the ultrasonic power was 228.83 W, and the temperature was 77.1819 °C. Under this condition, the predicted value of polysaccharide extraction rate was 2.59 %. Referred to the best experiment designed by the model for verification. For the convenience of operation, the ultrasonic time was 47 min, the power was 216 W, the temperature was 77 °C, and the three-time average extraction rate of polysaccharide is 2.55%. There was not much difference between the predicted value and the predicted value, demonstrating that the optimal conditions optimized by the model were more accurate.
Fig. 2.
Response surface diagram of factor interaction.
3.4. Phosphorylation analysis of MBP
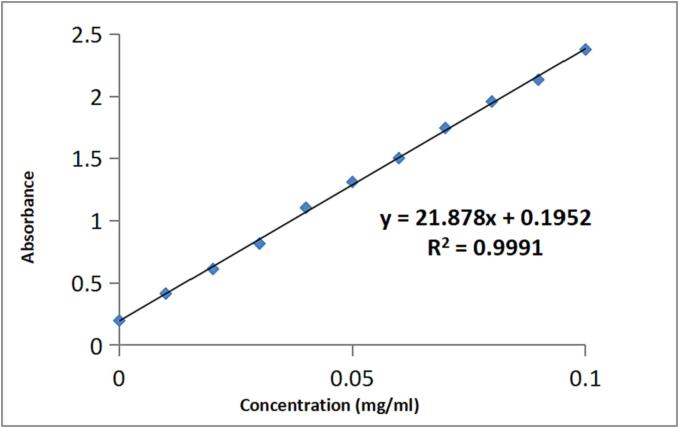
PMBP was acquired by POCl3-pyridine method with a yield of 57.78 %. Studies had shown that phosphorus oxychloride was a severe reactive phosphorylation reagent, and a higher degree of phosphorylated substitution products could be got under mild reaction conditions [28], [29]. It was generally believed that the degree of substitution was an important parameter affecting the activity of the modified product. High degree of substitution could improve the activity of the product. The standard curve of phosphate was shown in Fig. 3. According to the standard curve and formula, the degree of phosphorylation DS was 0.64. This indicated that it was feasible to modify MBP by this way. Liu et al. [10]prepared PSIP by sodium trimetaphosphate-sodium tripolyphosphate method, and the substitution degree of PSIP was 0.0828. This manifested that the degree of substitution of phosphorylated polysaccharide was closely related to the modification method, and also proved that POCl3-pyridine method was an effective method to obtain highly substituted phosphorylated polysaccharide. However, in the process of phosphorylation modification, pH has a great influence on the modified product [30]. Therefore, it is necessary to control the reaction in weak acid or weak base to obtain the desired product.
Fig. 3.
Phosphate standard curve.
3.5. Infrared spectrum analysis
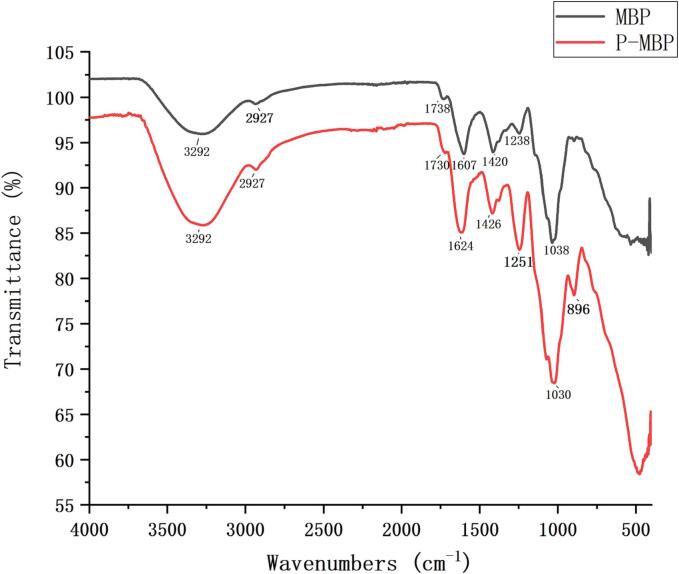
The detection results of the functional groups of the two polysaccharides by infrared spectroscopy were shown in Fig. 4. Among them, the broad peak of 3292 cm−1 was owing to the stretching vibration of O-H; the absorption peaks at 2927 cm−1 and 1420 cm−1 were due to C-H stretching vibration and variable angle vibration [31]. The absorption peak around 1040 cm−1 indicated the presence of a pyran ring structure in the polysaccharide [32]. There was a weak absorption peak owing to C = O stretching vibration at about 1740 cm−1 and a absorption peak caused by COOH at about 1620 cm−1, indicating that there was a small amount of uronic acid in the polysaccharide before and after modification [33], [34]. Compared with MBP, P-MBP increased two absorption peaks at 1251 cm−1 and 896 cm−1, which were by the reason of the stretching vibration of P = O and the characteristic absorption of P-O-C, respectively [35], [36]. This suggested that MBP successfully introduced phosphate group. The overall peak shape of the two polysaccharides was similar, illustrating that the main structure of polysaccharide did not change although it was chemically modified.
Fig. 4.
Infrared spectra of MBP and PMBP.
3.6. Analysis of antioxidant activity of polysaccharides in vitro
3.6.1. Hydroxyl radical scavenging activity
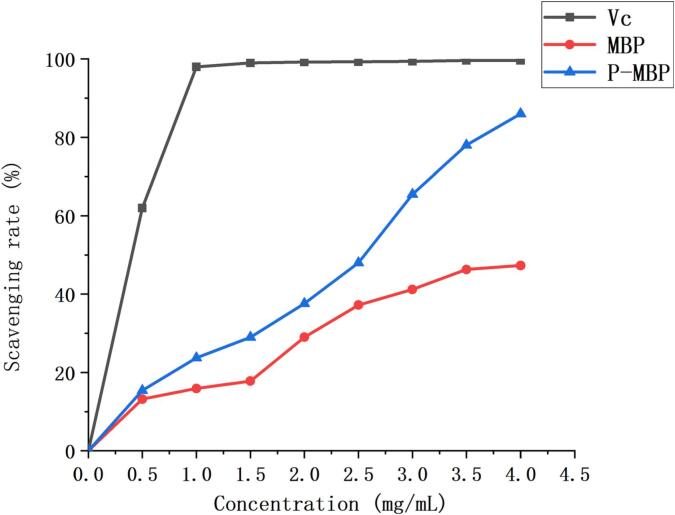
Hydroxyl radical was considered to be a harmful free radical, which could cause damage to biological macromolecules in cells and cause various diseases. Hence, scavenging hydroxyl radicals is important for the body. As illustrated (Fig. 5), there was a certain positive correlation between the concentration of polysaccharide and the clearance rate of hydroxyl radicals. It was similar to the results of Lai and Zhong et al. [37], [38], when the concentration of MBP reached 4 mg/mL, the clearance rate was 47.3 %. Compared with MBP, the scavenging activity of P-MBP on hydroxyl radicals was significantly improved. At a concentration of 4 mg/mL, the clearance rate was 86 %, and the scavenging rate may continue to increase. This might be because on the one hand, the solubility of modified polysaccharide in water was improved; on the other hand, after phosphorylation modification, the structure of polysaccharide molecules changed partially. PO43-was introduced into the sugar ring, which enhanced the chelating ability with Fe2+, thus inhibiting the production of free radicals and improving the clearance rate of hydroxyl radicals [39].
Fig. 5.
Hydroxyl radical scavenging rate.
3.6.2. Anti-lipid peroxidation ability
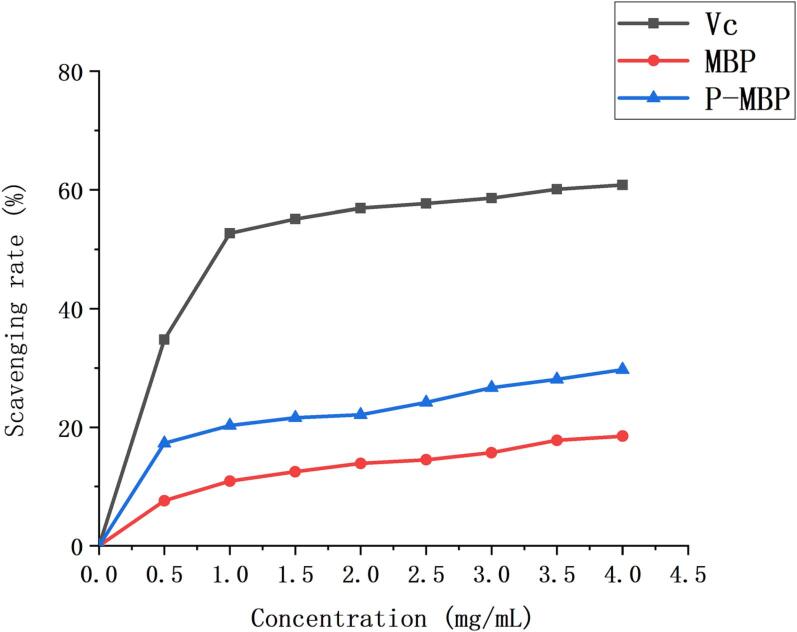
A large number of studies had shown that lipid peroxidation had certain toxicity to cells and was an important cause of various diseases, such as brain tumors and neurological diseases [40], [41], [42]. Therefore, it was necessary to find a way to resist lipid peroxidation damage, and natural antioxidant polysaccharides had attracted much attention because of their good antioxidant activity and low cytotoxicity. As shown in Fig. 6, when the concentration was 4 mg /mL, the scavenging rate of PMBP was 29.7%, and its anti-lipid peroxidation ability was higher than that of unmodified MBP, but lower than that of VC. Compared with the antioxidant capacity of hydroxyl radicals, phosphorylated mung bean peel polysaccharide did not significantly improve the anti-lipid peroxidation ability, which may be due to the different physical and chemical properties of polysaccharides and the different antioxidant mechanisms for different free radicals [43]. In short, the modification of polysaccharide could enhance the antioxidant capacity to a certain extent, but the antioxidant mechanism of polysaccharides had not been fully elucidated. It was expected that more experimental data would be available to support the antioxidant mechanism in the future.
Fig. 6.
Anti-lipid scavenging ability.
4. Conclusion
In this study, the conditions of ultrasonic-assisted extraction of mung bean peel polysaccharide were optimized by RSM. When the conditions of solid–liquid ratio 1:40, temperature 77 °C, ultrasonic power 216 W, and time 47 min, the extraction rate of mung bean peel polysaccharide was 2.55 %. The phosphorylated polysaccharide with a degree of substitution of 0.64 was obtained by POCl3 / pyridine modification. The successful modification was proved by infrared spectroscopy. In vitro antioxidant experiments proved that phosphorylation modification could enhance the antioxidant capacity of polysaccharide. This offered a method for the development and utilization of mung bean peel polysaccharide.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Gangliang Huang, Email: huangdoctor226@163.com.
Hualiang Huang, Email: hlhuang@wit.edu.cn.
References
- 1.Hou D., Yousaf L., Xue Y., Hu J., Wu J., Hu X., Feng N., Shen Q. Mung Bean (Vigna radiata L.): Bioactive Polyphenols, Polysaccharides, Peptides, and Health Benefits. Nutrients. 2019;11(6):1238. doi: 10.3390/nu11061238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei Y., Wang X., Shao X., Xu F., Wang H. Sucrose treatment of mung bean seeds results in increased vitamin C, total phenolics, and antioxidant activity in mung bean sprouts. Food Science & Nutrition. 2019;7(12):4037–4044. doi: 10.1002/fsn3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y., Zheng J., Gan R.-Y., Zhou T., Xu D.-P., Li H.-B. Optimization of Ultrasound-Assisted Extraction of Antioxidants from the Mung Bean Coat. Molecules. 2017;22(4):638. doi: 10.3390/molecules22040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirzadeh M., Arianejad M.R., Khedmat L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydrate Polymers. 2020;229:115421. doi: 10.1016/j.carbpol.2019.115421. [DOI] [PubMed] [Google Scholar]
- 5.Wang B., Xu Y., Chen L., Zhao G., Mi Z., Lv D., Niu J. Optimizing the Extraction of Polysaccharides from Bletilla ochracea Schltr. Using Response Surface Methodology (RSM) and Evaluating their Antioxidant Activity. Processes. 2020;8(3):341. [Google Scholar]
- 6.Shi L. isolation and purification methods of polysaccharides from natural products: A review. International Journal of Biological Macromolecules. 2016;92:37–48. doi: 10.1016/j.ijbiomac.2016.06.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a. Qin L, Chen S, Xie L, et al. Mechanisms of RAW264.7 macrophages immunomodulation mediated by polysaccharide from mung bean skin based on RNA-seq analysis. Food Research International, 2022, 154: 111017. b. Jiang L, Wang W, Wen P, et al. Two water-soluble polysaccharides from mung bean skin: Physicochemical characterization, antioxidant and antibacterial activities. Food Hydrocolloids, 2020, 100: 105412. [DOI] [PubMed]
- 8.a. Song ZY, Xiong X, Huang GL. Ultrasound-assisted extraction and characteristics of maize polysaccharides from different sites. Ultrasonics Sonochemistry, 2023, 95: 106416. b. Xiong X, Yang W, Huang GL, Huang HL. Ultrasonic-assisted extraction, characteristics and activity of Ipomoea batatas polysaccharide. Ultrasonics Sonochemistry, 2023, 96: 106420. c. Wang Y, Xiong X, Huang GL. Ultrasound-assisted extraction and analysis of maidenhairtree polysaccharides. Ultrasonics Sonochemistry, 2023, 95: 106395. d. Zhou S, Huang G. Extraction, structure characterization and biological activity of polysaccharide from coconut peel. Chemical and Biological Technologies in Agriculture, 2023, 10(1): 15. e. Zhou S, Huang G. Extraction, structural analysis and antioxidant activity of aloe polysaccharide. Journal of Molecular Structure, 2023, 1273: 134379.
- 9.Madera-Santana T.J., Herrera-Méndez C.H., Rodríguez-Núñez J.R. An overview of the chemical modifications of chitosan and their advantages. Green Materials. 2018;6(4):131–142. [Google Scholar]
- 10.Liu, Li, Luo. Effect of Carboxymethylation and Phosphorylation on the Properties of Polysaccharides from Sepia esculenta Ink: Antioxidation and Anticoagulation in Vitro. Marine Drugs, 2019, 17(11): 626. [DOI] [PMC free article] [PubMed]
- 11.Deng C., Fu H., Xu J., Shang J., Cheng Y. Physiochemical and biological properties of phosphorylated polysaccharides from Dictyophora indusiata. International Journal of Biological Macromolecules. 2015;72:894–899. doi: 10.1016/j.ijbiomac.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 12.a. Liu Y, Huang G. Extraction and derivatisation of active polysaccharides. Journal of Enzyme Inhibition and Medicinal Chemistry, 2019, 34(1): 1690-1696. b. Fan, Y., Huang, G. Preparation, structural analysis and antioxidant activity of polysaccharides and their derivatives from Pueraria lobata. Chemistry and Biodiversity, 2023, 20, e202201253. c. Mei, X., Tang, Q., Huang, G., Long, R., & Huang, H. (2020). Preparation, structural analysis and antioxidant activities of phosphorylated (1→3)-β-D-glucan. Food Chem., 309, 125791. d. Chen F, Huang G, Huang H. Preparation, analysis, antioxidant activities in vivo of phosphorylated polysaccharide from Momordica charantia. Carbohydrate Polymers, 2021, 252: 117179. e. Yang W, Huang GL. Chemical modification and structural analysis of polysaccharide from Solanum tuberdsm. Journal of Molecular Structure, 2023, 1285, 135480. f. Zhang, W., Huang, G. Preparation, structural characteristics, and application of taro polysaccharides in food. Journal of the Science of Food and Agriculture, 2022, 102, 6193-6201. g. Li, J., Fan, Y., Huang, G., Huang, H. Extraction, structural characteristics and activities of Zizylphus vulgaris polysaccharides. Industrial Crops and Products, 2022, 178,114675. h. Lin, B., Huang, G. An important polysaccharide from fermentum. Food Chemistry: X, 2022, 15,100388. i. Tang, Z., Huang, G. Extraction, structure, and activity of polysaccharide from Radix astragali. Biomedicine and Pharmacotherapy, 2022, 150,113015. j. Lin, B., Huang, G. Extraction, isolation, purification, derivatization, bioactivity, structure-activity relationship, and application of polysaccharides from White jellyfungus. Biotechnology and Bioengineering, 2022, 119(6), 1359-1379. k. Zhou, S., Huang, G., Huang, H. Extraction, derivatization and antioxidant activities of onion polysaccharide. Food Chemistry, 2022, 388,133000.
- 13.a. Yang, W., Huang, G. Extraction methods and activities of natural glucans. Trends in Food Science and Technology, 2021, 112, 50-57. b. Huang, G., Chen, F., Yang, W., Huang, H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol., 2021, 109, 564-568. c. Yang, W., Huang, G., Chen, F., Huang, H. Extraction/synthesis and biological activities of selenopolysaccharide. Trends Food Sci. Technol., 2021, 109, 211-218. d. Benchamas, G., Huang, G., Huang, S., Huang, H. Preparation and biological activities of chitosan oligosaccharides. Trends in Food Science and Technology, 2021, 107, 38-44. e. Tang, Q., Huang, G. Improving method, properties and application of polysaccharide as emulsifier. Food Chemistry, 2022, 376,131937. f. Li, B., Huang, G. Preparation, structure-function relationship and application of Grifola umbellate polysaccharides. Industrial Crops and Products, 2022, 186,115282.
- 14.Cui R., Zhu F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends in Food Science & Technology. 2021;107:491–508. [Google Scholar]
- 15.a. Li J, Chen Z, Shi H, et al. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrasonics Sonochemistry, 2023, 93: 106295. b. Ebringerová A, Hromádková Z. An overview on the application of ultrasound in extraction, separation and purification of plant polysaccharides. Open Chemistry, 2010, 8(2): 243-57. [DOI] [PMC free article] [PubMed]
- 16.Fan Y., Huang G. Preparation and analysis of Pueraria lobata polysaccharides. ACS Biomaterials Science & Engineering. 2023;9(5):2329–2334. doi: 10.1021/acsbiomaterials.2c01479. [DOI] [PubMed] [Google Scholar]
- 17.Song Q., Jiang L., Yang X., Huang L., Yu Y., Yu Q., Chen Y.i., Xie J. Physicochemical and functional properties of a water-soluble polysaccharide extracted from Mung bean (Vigna radiate L.) and its antioxidant activity. International Journal of Biological Macromolecules. 2019;138:874–880. doi: 10.1016/j.ijbiomac.2019.07.167. [DOI] [PubMed] [Google Scholar]
- 18.Huang J.W., Cheng Y.L. Comparaison on extraction of polysaccharides from mung bean hull. Food Science and Technology. 2013 [Google Scholar]
- 19.Chen F., Huang G., Yang Z., Hou Y. Antioxidant activity of Momordica charantia polysaccharide and its derivatives. International Journal of Biological Macromolecules. 2019;138:673–680. doi: 10.1016/j.ijbiomac.2019.07.129. [DOI] [PubMed] [Google Scholar]
- 20.Ye M., Yuan R.-Y., He Y.-L., Du Z.-Z., Ma X.-J. Phosphorylation and anti-tumor activity of exopolysaccharide from Lachnum YM120. Carbohydrate Polymers. 2013;97(2):690–694. doi: 10.1016/j.carbpol.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M., Su N., Huang Q., Zhang Q., Wang Y., Li J., Ye M. Phosphorylation and antiaging activity of polysaccharide from Trichosanthes peel. Journal of Food and Drug Analysis. 2017;25(4):976–983. doi: 10.1016/j.jfda.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen F., Huang G., Huang H. Preparation, analysis, antioxidant activities in vivo of phosphorylated polysaccharide from Momordica charantia. Carbohydrate Polymers. 2021;252:117179. doi: 10.1016/j.carbpol.2020.117179. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Q., Ren D., Yang N., Yang X. Optimization for ultrasound-assisted extraction of polysaccharides with chemical composition and antioxidant activity from the Artemisia sphaerocephala Krasch seeds. International Journal of Biological Macromolecules. 2016;91:856–866. doi: 10.1016/j.ijbiomac.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 24.Hou X., Huang X., Li J., Jiang G., Shen G., Li S., Luo Q., Wu H., Li M., Liu X., Chen A., Ye M., Zhang Z. Extraction Optimization and Evaluation of the Antioxidant and α-Glucosidase Inhibitory Activity of Polysaccharides from Chrysanthemum morifolium cv. Hangju. Antioxidants. 2020;9(1):59. doi: 10.3390/antiox9010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying Z., Han X., Li J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chemistry. 2011;127(3):1273–1279. doi: 10.1016/j.foodchem.2011.01.083. [DOI] [PubMed] [Google Scholar]
- 26.Zhu C.-P., Zhai X.-C., Li L.-Q., Wu X.-X., Li B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chemistry. 2015;177:139–146. doi: 10.1016/j.foodchem.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Qu Y., Li C., Zhang C., Zeng R., Fu C. Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohydrate Polymers. 2016;148:345–353. doi: 10.1016/j.carbpol.2016.04.081. [DOI] [PubMed] [Google Scholar]
- 28.Wang J., Wang Y., Xu L.u., Wu Q., Wang Q.i., Kong W., Liang J., Yao J., Zhang J.i. Synthesis and structural features of phosphorylated Artemisia sphaerocephala polysaccharide. Carbohydrate Polymers. 2018;181:19–26. doi: 10.1016/j.carbpol.2017.10.049. [DOI] [PubMed] [Google Scholar]
- 29.Li J., Shi H., Yu J., Lei Y., Huang G., Huang H. Extraction and properties of Ginkgo biloba leaf polysaccharide and its phosphorylated derivative. Industrial Crops and Products. 2022;189:115822. [Google Scholar]
- 30.Huang G., Huang H. The derivatization and antitumor mechanisms of polysaccharides. Future Med. Chem. 2017;9(16):1931–1938. doi: 10.4155/fmc-2017-0132. [DOI] [PubMed] [Google Scholar]
- 31.Babamoradi N., Yousefi S., Ziarati P. Optimization of ultrasound-assisted extraction of functional polysaccharides from common mullein (Verbascum thapsus L.) flowers. Journal of Food Process Engineering. 2018;41(7):e12851. [Google Scholar]
- 32.Yang W., Zhang Y., Tang A.o., Ruan Q., Huang G. Preparation and antioxidant activity of phosphorylated polysaccharide from purple sweet potato. Chemical Biology & Drug Design. 2021;98(5):828–834. doi: 10.1111/cbdd.13936. [DOI] [PubMed] [Google Scholar]
- 33.Xie L., Shen M., Wen P., Hong Y., Liu X., Xie J. Preparation, characterization, antioxidant activity and protective effect against cellular oxidative stress of phosphorylated polysaccharide from Cyclocarya paliurus. Food and Chemical Toxicology. 2020;145:111754. doi: 10.1016/j.fct.2020.111754. [DOI] [PubMed] [Google Scholar]
- 34.Duan S., Zhao M., Wu B., Wang S., Yang Y.u., Xu Y., Wang L. Preparation, characteristics, and antioxidant activities of carboxymethylated polysaccharides from blackcurrant fruits. International Journal of Biological Macromolecules. 2020;155:1114–1122. doi: 10.1016/j.ijbiomac.2019.11.078. [DOI] [PubMed] [Google Scholar]
- 35.Song Y.i., Ni Y., Hu X., Li Q. Effect of phosphorylation on antioxidant activities of pumpkin (Cucurbita pepo, Lady godiva) polysaccharide. International Journal of Biological Macromolecules. 2015;81:41–48. doi: 10.1016/j.ijbiomac.2015.07.055. [DOI] [PubMed] [Google Scholar]
- 36.Wang X., Zhang Z., Yao Q., Zhao M., Qi H. Phosphorylation of low-molecular-weight polysaccharide from Enteromorpha linza with antioxidant activity. Carbohydrate Polymers. 2013;96(2):371–375. doi: 10.1016/j.carbpol.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 37.Lai F., Wen Q., Li L., Wu H., Li X. Antioxidant activities of water-soluble polysaccharide extracted from mung bean (Vigna radiata L.) hull with ultrasonic assisted treatment. Carbohydrate Polymers. 2010;81(2):323–329. [Google Scholar]
- 38.Zhong K., Lin W., Wang Q., Zhou S. Extraction and radicals scavenging activity of polysaccharides with microwave extraction from mung bean hulls. International Journal of Biological Macromolecules. 2012;51(4):612–617. doi: 10.1016/j.ijbiomac.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 39.Zhou S., Huang G. Preparation, structure and activity of polysaccharide phosphate esters. Biomedicine & Pharmacotherapy. 2021;144:112332. doi: 10.1016/j.biopha.2021.112332. [DOI] [PubMed] [Google Scholar]
- 40.Gaschler M.M., Stockwell B.R. Lipid peroxidation in cell death [J] Biochemical and Biophysical Research Communications. 2017;482(3):419–425. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peña-Bautista C., Vento M., Baquero M., Cháfer-Pericás C. Lipid peroxidation in neurodegeneration. Clinica Chimica Acta. 2019;497:178–188. doi: 10.1016/j.cca.2019.07.037. [DOI] [PubMed] [Google Scholar]
- 42.Jaganjac M., Cindrić M., Jakovčević A., Žarković K., Žarković N. Lipid peroxidation in brain tumors. Neurochemistry International. 2021;149:105118. doi: 10.1016/j.neuint.2021.105118. [DOI] [PubMed] [Google Scholar]
- 43.Lin Y., Pi J., Jin P., Liu Y., Mai X., Li P., Fan H. Enzyme and microwave co-assisted extraction, structural characterization and antioxidant activity of polysaccharides from Purple-heart Radish. Food Chemistry. 2022;372:131274. doi: 10.1016/j.foodchem.2021.131274. [DOI] [PubMed] [Google Scholar]