Abstract
Background
Acute necrotising pancreatitis carries significant mortality, morbidity, and resource use. There is considerable uncertainty as to how people with necrotising pancreatitis should be treated.
Objectives
To assess the benefits and harms of different interventions in people with acute necrotising pancreatitis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 4), MEDLINE, EMBASE, Science Citation Index Expanded, and trials registers to April 2015 to identify randomised controlled trials (RCT). We also searched the references of included trials to identify further trials.
Selection criteria
We considered only RCTs performed in people with necrotising pancreatitis, irrespective of aetiology, presence of infection, language, blinding, or publication status for inclusion in the review.
Data collection and analysis
Two review authors independently identified trials and extracted data. We calculated the odds ratio (OR) and mean difference with 95% confidence intervals (CI) using Review Manager 5 based on an available‐case analysis using fixed‐effect and random‐effects models. We planned a network meta‐analysis using Bayesian methods, but due to sparse data and uncertainty about the transitivity assumption, performed only indirect comparisons and used Frequentist methods.
Main results
We included eight RCTs with 311 participants in this review. After exclusion of five participants, we included 306 participants in one or more outcomes. Five trials (240 participants) investigated the three main treatments: open necrosectomy (121 participants), minimally invasive step‐up approach (80 participants), and peritoneal lavage (39 participants) and were included in the network meta‐analysis. Three trials (66 participants) investigated the variations in the main treatments: early open necrosectomy (25 participants), delayed open necrosectomy (11 participants), video‐assisted minimally invasive step‐up approach (12 participants), endoscopic minimally invasive step‐up approach (10 participants), minimally invasive step‐up approach (planned surgery) (four participants), and minimally invasive step‐up approach (continued percutaneous drainage) (four participants). The trials included infected or sterile necrotising pancreatitis of varied aetiology.
All the trials were at unclear or high risk of bias and the overall quality of evidence was low or very low for all the outcomes. Overall, short‐term mortality was 30% and serious adverse events rate was 139 serious adverse events per 100 participants. The differences in short‐term mortality and proportion of people with serious adverse events were imprecise in all the comparisons. The number of serious adverse events and adverse events were fewer in the minimally invasive step‐up approach compared to open necrosectomy (serious adverse events: rate ratio 0.41, 95% CI 0.25 to 0.68; 88 participants; 1 study; adverse events: rate ratio 0.41, 95% CI 0.25 to 0.68; 88 participants; 1 study). The proportion of people with organ failure and the mean costs were lower in the minimally invasive step‐up approach compared to open necrosectomy (organ failure: OR 0.20, 95% CI 0.07 to 0.60; 88 participants; 1 study; mean difference in costs: USD ‐11,922; P value < 0.05; 88 participants; 1 studies). There were more adverse events with video‐assisted minimally invasive step‐up approach group compared to endoscopic‐assisted minimally invasive step‐up approach group (rate ratio 11.70, 95% CI 1.52 to 89.87; 22 participants; 1 study), but the number of interventions per participant was less with video‐assisted minimally invasive step‐up approach group compared to endoscopic minimally invasive step‐up approach group (difference in medians: 2 procedures; P value < 0.05; 20 participants; 1 study). The differences in any of the other comparisons for number of serious adverse events, proportion of people with organ failure, number of adverse events, length of hospital stay, and intensive therapy unit stay were either imprecise or were not consistent. None of the trials reported long‐term mortality, infected pancreatic necrosis (trials that included participants with sterile necrosis), health‐related quality of life at any time frame, proportion of people with adverse events, requirement for additional invasive intervention, time to return to normal activity, and time to return to work.
Authors' conclusions
Low to very low quality evidence suggested that the minimally invasive step‐up approach resulted in fewer adverse events, serious adverse events, less organ failure, and lower costs compared to open necrosectomy. Very low quality evidence suggested that the endoscopic minimally invasive step‐up approach resulted in fewer adverse events than the video‐assisted minimally invasive step‐up approach but increased the number of procedures required for treatment. There is currently no evidence to suggest that early open necrosectomy is superior or inferior to peritoneal lavage or delayed open necrosectomy. However, the CIs were wide and significant benefits or harms of different treatments cannot be ruled out. The TENSION trial currently underway in Netherlands is assessing the optimal way to perform the minimally invasive step‐up approach (endoscopic drainage followed by endoscopic necrosectomy if necessary versus percutaneous drainage followed by video‐assisted necrosectomy if necessary) and is assessing important clinical outcomes of interest for this review. Implications for further research on this topic will be determined after the results of this RCT are available.
Plain language summary
Treatment methods for people with necrotising pancreatitis (pancreatic destruction due to inflammation of pancreas)
Review question
How should people with necrotising pancreatitis be treated?
Background
The pancreas is an organ in the abdomen (tummy) that secretes several digestive enzymes (substances that enable and speed up chemical reactions in the body) into the pancreatic ductal system, which empties into the small bowel. It also contains the Islets of Langerhans, which secrete several hormones including insulin (helps regulate blood sugar). Acute pancreatitis is sudden inflammation of the pancreas and can lead to destruction of the pancreas (pancreatic necrosis). Pancreatic necrosis can be infected or non‐infected (sterile). Pancreatic necrosis can lead to failure of other organs, such as the lungs and kidneys, and is a life‐threatening illness. The main treatments for pancreatic necrosis include removal of the dead tissue (debridement or necrosectomy), peritoneal lavage (washing dead tissue out of the abdomen, drainage (inserting a tube or 'drain' to drain out the fluid collection around the pancreas), or initial drainage followed by necrosectomy if necessary (called the minimally invasive 'step‐up' approach). The minimally invasive step‐up approach can be performed in different ways. For example, in video‐assisted minimally invasive step‐up approach, necrosectomy is performed after a period of drainage through a key‐hole operation; in the endoscopic minimally invasive step‐up approach, necrosectomy is performed with the help of an endoscope (instrument used to look inside the abdomen).
The best way to treat people with necrotising pancreatitis is not clear. We sought to resolve this issue by searching for existing studies on the topic. We included all randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) whose results were reported to 7 April 2015.
Study characteristics
Eight trials including 311 participants met the inclusion criteria for the review, of whom 306 participants were included in various comparisons. The treatments compared in five trials included necrosectomy, peritoneal lavage, and the step‐up approach. Three other trials compared variations in timing of necrosectomy and methods of step‐up approach. The participants in the trials had infected or sterile pancreatic necrosis resulting from varying causes.
Key results
Overall, the short‐term death rate (mortality over a short time) was 30% and serious adverse events (side effects or complications) rate was 139 per 100 participants. The differences in short‐term mortality and percentage of people with serious adverse events were imprecise in all the comparisons. The number of serious adverse events and adverse events were fewer in the minimally invasive step‐up approach compared to open necrosectomy. The complications resulting from the disease and treatment included heart failure (heart does not pump enough blood around the body at the correct pressure), lung failure (lungs do not remove waste products from the blood), kidney failure (kidneys do not remove waste products from the blood), and blood poisoning (micro‐organisms and their poisons are in the blood). The percentage of people with organ failure and the average costs were lower in the minimally invasive step‐up approach compared to open necrosectomy. The number of adverse events were more with the video‐assisted minimally invasive step‐up approach compared to the endoscopic‐assisted minimally invasive step‐up approach but the total numbers of procedures performed were less with the video‐assisted minimally invasive step‐up approach compared to the endoscopic minimally invasive step‐up approach. The differences in any of the other comparisons for number of serious adverse events, percentage of people with organ failure, number of adverse events, length of hospital stay, and intensive therapy unit stay were either imprecise or were not consistent. None of the trials reported long‐term mortality, infected pancreatic necrosis (in trials that included participants with sterile necrosis), health‐related quality of life (which measures physical, mental, emotional, and social functioning), percentage of people with adverse events, requirement for additional invasive intervention, time to return to normal activity, and time to return to work.
Quality of the evidence
The overall quality of evidence was low or very low for all the measurement because the trials were at high risk of bias (e.g. prejudice of people who conducted the trial and trial participants who prefer one treatment over another) and were small trials. As a result, further studies are required on this topic.
Summary of findings
Summary of findings for the main comparison. Interventions for necrotising pancreatitis: mortality.
| Interventions for necrotising pancreatitis: mortality | |||||
| Patient or population: people with necrotising pancreatitis Settings: secondary or tertiary care Intervention: various interventions vs. control for necrotising pancreatitis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Intervention | ||||
| Short‐term mortality | |||||
| Peritoneal lavage vs. open necrosectomy | 329 per 1000 | 482 per 1000 (264 to 708) | OR 1.9 (0.73 to 4.94) | 80 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 |
| Minimally invasive step‐up approach vs. open necrosectomy | 329 per 1000 | 242 per 1000 (136 to 397) | OR 0.65 (0.32 to 1.34) | 160 (2 studies) | ⊕⊝⊝⊝ very low1,2,3,4 |
| Delayed open necrosectomy vs. early open necrosectomy | 329 per 1000 | 124 per 1000 (29 to 404) | OR 0.29 (0.06 to 1.38) | 36 (1 study) | ⊕⊝⊝⊝ very low1,2,3 |
| Minimally invasive step‐up approach: video‐assisted vs. endoscopic | 100 per 1000 | 333 per 1000 (44 to 845) | OR 4.5 (0.41 to 49.08) | 22 (1 study) | ⊕⊝⊝⊝ very low1,2,3 |
| Minimally invasive step‐up approach: planned surgery vs. continued percutaneous drainage | 225 per 1000 | 859 per 1000 (157 to 995) | OR 21 (0.64 to 689.99) | 8 (1 study) | ⊕⊝⊝⊝ very low1,2,3 |
| None of the trials reported long‐term mortality | |||||
| *The basis for the assumed risk was the mean control group proportion across all studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 The trial(s) was (were) at unclear or high risk of bias. 2 Sample size was small. 3 Confidence intervals overlapped clinically significant effect and no effect. 4 There was moderate heterogeneity as indicated by the I2 statistic.
Summary of findings 2. Interventions for necrotising pancreatitis: other primary outcomes.
| Interventions for necrotising pancreatitis: other primary outcomes | |||||
| Patient or population: people with necrotising pancreatitis Settings: secondary or tertiary care Intervention: interventions for necrotising pancreatitis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Intervention | ||||
| Serious adverse events (proportion) | |||||
| Minimally invasive step‐up approach vs. open necrosectomy | 714 per 1000 | 487 per 1000 (259 to 716) | OR 0.38 (0.14 to 1.01) | 72 (1 study) | ⊕⊝⊝⊝ very low1,2,3 |
| Serious adverse events (number) | |||||
| Peritoneal lavage vs. open necrosectomy | 1662 per 1000 | 2123 per 1000 (1527 to 2950) | Rate ratio 1.28 (0.92 to 1.78) | 56 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 |
| Minimally invasive step‐up approach vs. open necrosectomy | 1662 per 1000 | 689 per 1000 (422 to 1125) | Rate ratio 0.41 (0.25 to 0.68) | 88 (1 study) | ⊕⊝⊝⊝ very low1,2,3 |
| Minimally invasive step‐up approach: video‐assisted vs. endoscopic | 535 per 1000 | 6716 per 1000 (384 to 117455) | Rate ratio 12.55 (0.72 to 219.54) | 22 (1 study) | ⊕⊝⊝⊝ very low1,2,3 |
| Organ failure | |||||
| Minimally invasive step‐up approach vs. open necrosectomy | 400 per 1000 | 118 per 1000 (45 to 286) | OR 0.20 (0.07 to 0.60) | 88 (1 study) | ⊕⊕⊝⊝ low1,2 |
| Minimally invasive step‐up approach: video‐assisted vs. endoscopic | 116 per 1000 | 669 per 1000 (87 to 977) | OR 15.4 (0.73 to 322.88) | 22 (1 study) | ⊕⊝⊝⊝ very low1,2,3 |
| None of the trials that included participants with sterile necrosis reported the proportion of people with infected pancreatic necrosis None of the trials reported the health‐related quality of life at any time frame | |||||
| *The basis for the assumed risk is the control group proportions or rates across studies except for the comparison minimally invasive step‐up approach: video‐assisted vs. endoscopic; we used the mean rate of serious adverse events in the minimally invasive step‐up approach from other trials as the control event rate since there were no serious adverse events in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 The trial(s) was (were) at unclear or high risk of bias. 2 Sample size was small. 3 Confidence intervals overlapped clinically significant effect and no effect.
Summary of findings 3. Interventions for necrotising pancreatitis for necrotising pancreatitis: secondary outcomes.
| Interventions for necrotising pancreatitis: secondary outcomes | |||||
| Patient or population: people with necrotising pancreatitis Settings: secondary or tertiary care Intervention: various interventions vs. control for necrotising pancreatitis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Intervention | ||||
| Adverse events (number) | |||||
| Peritoneal lavage vs. open necrosectomy | 1696 per 1000 | 1713 per 1000 (1070 to 2742) | Rate ratio 1.01 (0.63 to 1.62) | 21 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4 |
| Minimally invasive step‐up approach vs. open necrosectomy | 1696 per 1000 | 703 per 1000 (431 to 1148) | Rate ratio 0.41 (0.25 to 0.68) | 88 (1 study) | ⊕⊕⊝⊝ low1,3 |
| Minimally invasive step‐up approach: video‐assisted vs. endoscopic | 100 per 1000 | 1170 per 1000 (152 to 8987) | Rate ratio 11.7 (1.52 to 89.87) | 22 (1 study) | ⊕⊝⊝⊝ very low1,3 |
| Length of hospital stay: 5 trials reported the length of hospital stay but this was not reported in a format that could be meta‐analysed. There were no statistically significant differences reported in the length of hospital stay in any of the 5 trials (3 comparisons: peritoneal lavage vs. open necrosectomy (2 trials; 58 participants); minimally invasive step‐up approach vs. open necrosectomy (2 trials; 160 participants); minimally invasive step‐up approach: video‐assisted vs. endoscopic (1 trial; 20 participants)) that provided information on the length of hospital stay | |||||
| Length of ITU stay: 3 trials reported the length of ITU stay but this was not reported in a format that could be meta‐analysed. There was major inconsistency between 2 trials (58 participants) that reported ITU stay in the comparison between peritoneal lavage and open necrosectomy. There was no statistically significant difference in the length of ITU stay between the minimally invasive step‐up approach and open necrosectomy in the only trial (88 participants) that reported this outcome in the comparison between minimally invasive step‐up approach and open necrosectomy | |||||
| Number of treatments: only 1 trial (20 participants) reported the number of treatments in each group, but this was not reported in a format that could be meta‐analysed. The number of treatments were statistically significantly fewer (2 fewer treatments) in the video‐assisted minimally invasive step‐up approach group (median: 1 treatment per participant) compared to endoscopic minimally invasive step‐up approach group (median: 3 treatments per participant) | |||||
| Costs: only 1 trial (88 participants) reported the costs in each group but this was not reported in a format that could be meta‐analysed without imputation of data. The costs were statistically significantly less (USD 11,922 cheaper) in the minimally invasive step‐up approach (mean costs per participant: USD 86,653) compared to open necrosectomy (mean costs per participant: USD 98,575) | |||||
| None of the trials reported the proportion of people with adverse events , requirement for additional invasive intervention , time to return to normal activity , or time to return to work | |||||
| *The basis for the assumed risk is the control event rates across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ITU: intensive therapy unit. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 The trial(s) was (were) at unclear or high risk of bias. 2 There was moderate heterogeneity as indicated by the I2 statistic. 3 Sample size was small. 4 Confidence intervals overlapped clinically significant effect and no effect.
Background
Description of the condition
The pancreas is an abdominal organ that secretes several digestive enzymes into the pancreatic ductal system that empties into the small bowel. It also contains the Islets of Langerhans, which secrete several hormones including insulin (NCBI 2014). Acute pancreatitis is a sudden inflammatory process in the pancreas, with variable involvement of adjacent (nearby) organs or other organ systems (Bradley 1993). The annual incidence of acute pancreatitis ranges from 5 to 30 per 100,000 population (Roberts 2013; Yadav 2006). There has been an increase in the incidence of acute pancreatitis since the late 2000s in the UK and US (Roberts 2013; Yang 2008). Acute pancreatitis is the most common gastrointestinal (digestive tract) cause of hospital admission in the US (Peery 2012). Gallstones and alcohol are the two main causes for acute pancreatitis. Approximately 50% to 70% of acute pancreatitis is caused by gallstones (Roberts 2013; Yadav 2006). Increasing age, male gender, and lower socioeconomic class are associated with higher incidence of acute pancreatitis (Roberts 2013).
The diagnosis of acute pancreatitis is made when at least two of the following three features are present (Banks 2013).
Acute onset of a persistent, severe, epigastric pain often radiating to the back.
Serum lipase activity (or amylase activity) at least three times greater than the upper limit of normal.
Characteristic findings of acute pancreatitis on contrast‐enhanced computed tomography (CECT) and less commonly magnetic resonance imaging (MRI) or transabdominal ultrasonography.
Depending upon the type of inflammation, acute pancreatitis can be classified into interstitial oedematous pancreatitis (diffuse (widespread) or occasionally localised enlargement of the pancreas due to inflammatory oedema as seen on CECT) or necrotising pancreatitis (necrosis involving either the pancreas or peripancreatic tissues, or both) (Banks 2013). Approximately 90% to 95% of people with acute pancreatitis have interstitial oedematous pancreatitis, while the remainder have necrotising pancreatitis (Banks 2013). Necrotising pancreatitis is diagnosed by impaired enhancement on CECT but the typical CECT features may take several days to develop (Banks 2013). Local complications of acute necrotising pancreatitis include acute necrotic collection (first four weeks of acute pancreatitis) and walled‐off necrosis (has a well‐defined inflammatory wall that usually develops at or beyond four weeks after the onset of acute pancreatitis) (Banks 2013). The systemic complications of acute pancreatitis include worsening of pre‐existing illnesses such as heart or chronic lung disease (Banks 2013). The mortality rates following an attack of acute pancreatitis are between 6% and 20% (Roberts 2013; Yadav 2006).
The clinical manifestation of acute pancreatitis is believed to be caused by activation of inflammatory pathways either directly by the pathological insult or indirectly by activation of trypsinogen (an enzyme that digests protein or a protease) resulting in formation of trypsin, a protease that can breakdown the pancreas (Sah 2013). This activation of inflammatory pathways manifests clinically as systemic inflammatory response syndrome (SIRS) (Banks 2013; Sah 2013; Tenner 2013). SIRS is characterised by two or more of the following criteria (Bone 1992).
Body temperature below 36 °C or above 38 °C.
Heart rate greater than 90 beats/minute.
Respiratory rate greater than 20 breaths/minute or partial pressure of carbon dioxide (pCO2) less than 32 mm Hg.
White blood cell count greater than 12,000/mm3, less than 4000/mm2, or greater than 10% immature (band) forms.
Depending upon the presence of transient organ failure involving one of more of lungs, kidneys, and cardiovascular system (heart and blood vessels) lasting up to 48 hours, or persistent organ failure of the lungs, kidneys, and cardiovascular system lasting beyond 48 hours, acute necrotising pancreatitis can be moderately severe (transient organ failure) or severe (persistent organ failure). Necrotising pancreatitis may be sterile or infected (Banks 2013). Various theories exist with regards to how pancreatic and peripancreatic tissues become infected. These include spread of infection from blood circulation, lymphatics, bile, small bowel (duodenum) through the pancreatic duct, and bacterial movement through the large bowel wall (translocation) (Schmid 1999). Infected necrotising pancreatitis carries a significantly worse prognosis than sterile necrotising pancreatitis with a mean in‐hospital mortality of more than 30% for people with infected necrotising pancreatitis; this increases to more than 40% in the subgroup of people with organ failure in addition to infection (Petrov 2010).
See Appendix 1 for a glossary of terms.
Description of the intervention
The main purpose of treatment is to decrease the mortality and morbidity associated with acute necrotising pancreatitis. The various treatment strategies in acute necrotising pancreatitis include early surgical debridement (surgical removal of damaged, dead, or infected tissue, or necrosectomy, which can be performed by open surgery or by minimally invasive retroperitoneal debridement), delayed necrosectomy (delaying the surgery by about four weeks), percutaneous drainage, endoscopic transluminal drainage, and a step‐up approach that consists of endoscopic or percutaneous drainage followed by laparoscopic necrosectomy if required (Bakker 2012; Mouli 2013; Tenner 2013; Van Brunschot 2014; Van Santvoort 2010a; Van Santvoort 2011). The complications related to the treatments include failure of adequate treatment of necrotising pancreatitis or performing a major procedure in an already unwell person leading to organ failure, sepsis, and death. All of these treatments are supported by appropriate fluid treatment and appropriate nutritional treatment. Some centres might also use antibiotics routinely or if the necrosis is infected as supportive treatment.
How the intervention might work
The interventions work by removal of the necrosis or infected necrosis, thereby eliminating the trigger factors of inflammation and infection.
Why it is important to do this review
The American College of Gastroenterology guidelines suggest that in clinically stable people with infected necrotising pancreatitis, delayed necrosectomy is the main treatment option, while in clinically unstable people with infected pancreatic necrosis, early necrosectomy should be considered (Tenner 2013). Studies have shown that less invasive approaches, such as percutaneous drainage followed by necrosectomy if required and endoscopic transluminal drainage, provide better results than surgical debridement (Bakker 2012; Van Santvoort 2010a). Thus, the optimal management of people with pancreatic necrosis is unclear. Multiple treatment comparison or network meta‐analysis allows comparison of several treatments simultaneously and provides information of the relative effect of one treatment versus another even when no direct comparison has been made. There is no Cochrane network meta‐analysis on this topic. This systematic review and network meta‐analysis will identify the relative effects of different treatments and identify any research gaps.
Objectives
To assess the benefits and harms of different interventions in people with acute necrotising pancreatitis.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs). We included studies reported as full text, those published as abstract only, and unpublished data.
Types of participants
We included adults with acute necrotising pancreatitis irrespective of the presence or absence of infection irrespective of aetiology. In the presence of an adequate number of trials, we planned to perform a separate network meta‐analysis for infected necrotising pancreatitis and uninfected necrotising pancreatitis.
Types of interventions
We planned to include trials comparing one or more of the following interventions.
Early surgical debridement (as soon as diagnosis is established) or early open necrosectomy.
Delayed surgical debridement (delayed by at least three days after diagnosis of necrotising pancreatitis) or delayed open necrosectomy.
Endoscopic drainage.
Percutaneous drainage.
Peritoneal lavage.
Step‐up approach (primary percutaneous or endoscopic drainage or video‐assisted followed by open surgical debridement if symptoms persist or worsen in three days or a similar period).
We anticipated that all the groups will receive conservative supportive treatment in terms of appropriate fluid treatment; appropriate nutritional treatment; and renal, ventilatory, or cardiovascular support depending upon the organ failure. We also anticipated that antibiotics may be used routinely or in people with infected pancreatic necrosis and considered this a part of conservative treatment. As such, we did not include trials that evaluate the role of antibiotics or nutrition since different forms of conservative treatments are not the focus of this review.
Types of outcome measures
Primary outcomes
-
Mortality.
Short‐term mortality (in‐hospital mortality or mortality within six months).
Long‐term mortality (at maximal follow‐up).
-
Serious adverse events (within six months). We accepted the following definitions of serious adverse events.
International Conference on Harmonisation ‐ Good Clinical Practice (ICH‐GCP) guideline (ICH‐GCP 1996): serious adverse events defined as any untoward medical occurrence that results in death, is life threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability/incapacity.
Other variations of ICH‐GCP classifications such as Food and Drug Administration (FDA) classification (FDA 2006), Medicines and Healthcare products Regulatory Agency (MHRA) classification (MHRA 2013).
Infected pancreatic necrosis (cytology or culture confirmed).
Organ failure (however defined by authors).
-
Health‐related quality of life (using any validated scale).
Short‐term (four weeks to three months).
Medium‐term (greater than three months to one year).
Long‐term (greater than one year).
Secondary outcomes
Adverse events (within six months). We accepted all adverse events reported by the study author irrespective of the severity of the adverse event.
-
Measures of decreased complications and earlier recovery (within six months).
Length of hospital stay (including the index admission for acute necrotising pancreatitis and any disease‐related or intervention‐related re‐admissions including those for recurrent episodes).
Length of intensive therapy unit (ITU) stay (including the index admission for acute necrotising pancreatitis and any disease‐ or intervention‐related re‐admissions).
Requirement for additional invasive intervention such as necrosectomy.
Total number of treatments (number of procedures to complete the treatment).
Time to return to normal activity (return to pre‐acute necrotising pancreatitis episode mobility without any additional carer support).
Time to return to work (in people who were employed previously).
Costs (within six months).
We based the choice of these clinical outcomes on the necessity to assess whether the pharmacological interventions were effective in decreasing the complications, thereby decreasing the length of ITU and hospital stay; decreasing any additional interventions; and resulting in earlier return to normal activity and work, and improvement in quality of life. The costs provide an indication of resource requirement.
Reporting of the outcomes listed here were not inclusion criteria for the review.
Search methods for identification of studies
Electronic searches
We conducted a literature search to identify all published and unpublished RCTs. The literature search identified potential studies in all languages. We translated any non‐English language papers and assessed them fully for potential inclusion in the review as necessary.
We searched the following electronic databases to identify potential studies:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2015 Issue 4) (Appendix 2);
MEDLINE (1966 to April 2015) (Appendix 3);
EMBASE (1988 to April 2015) (Appendix 4); and
Science Citation Index (1982 to April 2015) (Appendix 5).
We also conducted a search of ClinicalTrials.gov (Appendix 6) and the World Health Organization ‐ International Clinical Trials Registry Platform (WHO ICTRP) (Appendix 7) on 7 April 2015. Please note that these search strategies were the same as for another Cochrane protocol on pharmacological interventions for acute pancreatitis (Gurusamy 2014), and so may contain additional search terms that might result in additional results not relevant for this review but has allowed easier management of searches.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We contacted authors of identified trials and asked them to identify other published and unpublished studies.
We searched for errata or retractions from eligible trials on PubMed on 24 February 2016 (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Three review authors (KG, AB, and AH) independently screened titles and abstracts for inclusion of all the potential studies we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports and three review authors (KG, AB, and AH) independently screened the full text and identified studies for inclusion and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We planned to contact investigators of trials of unclear eligibility. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table.
Data extraction and management
We used a standard data collection form for study characteristics and outcome data that had been piloted on at least one study in the review. Three review authors (KG, AB, and AH) extracted study characteristics from included studies and detailed them in the 'Characteristics of included studies' table. We extracted the following study characteristics.
Methods: study design, total duration study and run in, number of study centres and location, study setting, withdrawals, date of study.
Participants: number, mean age, age range, gender, presence of infection, inclusion criteria, exclusion criteria.
Interventions: intervention, comparison, concomitant interventions, number of participants randomised in each group.
Outcomes: primary and secondary outcomes specified and collected, time points reported. For binary outcomes, we obtained the number of participants with events and number of participants included in the analysis in each group. For continuous outcomes, we obtained the unit or scale of measurement, mean, standard deviation, and the number of participants included in the analysis for each group. For count outcomes, we obtained the number of events and number of participants included in the analysis in each group. For time‐to‐event outcomes, we planned to obtain the number of people with events, the mean duration of follow‐up of participants in the trial, and the number of participants included in the analysis for each group.
Notes: funding for trial, notable conflicts of interest of trial authors.
Three review authors (KG, AB, and AH) independently extracted outcome data from included studies. If outcomes were reported at multiple time points, we extracted the data for all time points. We obtained the information on the number of participants with adverse events (or serious adverse events) and the number of adverse events (or serious adverse events) where applicable. We extracted all the costs using the currency reported by trial authors and converted to US dollars (USDs) on February 2016. We extracted data for every trial arm that was an included intervention. If outcome data were reported in an unusable way, we attempted to contact the study authors and try to obtain usable data. If we were unable to obtain usable data despite this, we summarised the unusable data in tables. We resolved disagreements by consensus. One review author (KG) copied across the data from the data collection form into Review Manager 5 (RevMan 2012). We double checked that the data were entered correctly by comparing the study reports with how we presented the data in the systematic review.
Assessment of risk of bias in included studies
Three review authors (KG, AB, and AH) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion. We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We graded each potential source of bias as high, low, or unclear risk of bias and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different from a participant‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table. We planned to present the risk of bias in each pair‐wise comparison in separate tables. However, the risk of bias was low or unclear in all the trials. Therefore, we did not present this information for each pair‐wise comparison but provided this in a table arranged according to the intervention and control.
When considering treatment effects, we planned to take into account the risk of bias for the studies that contributed to that outcome using a sensitivity analysis.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the Differences between protocol and review section.
Measures of treatment effect
For dichotomous variables (short‐term mortality, proportion of participants with adverse events, requirement for additional interventions), we calculated the odds ratio (OR) with 95% confidence interval (CI) or credible interval (CrI). For continuous variables, such as length of hospital stay, ITU stay, time to return to normal activity, time to return to work, and costs, we calculated the mean difference (MD) with 95% CI or CrI. We used the standardised mean difference (SMD) with 95% CrI for quality of life if studies used different scales. For count outcomes, such as the number of adverse events, we calculated the rate ratio (RaR) with 95% CI or CrI. For time‐to‐event data, such as long‐term mortality, we used the hazard ratio (HR) with 95% CI or CrI.
A common way that trialists indicate when they have skewed data is by reporting medians and interquartile ranges. When we encountered this, we have reported the median and interquartile range in a table.
Unit of analysis issues
The unit of analysis was the individual participant with acute necrotising pancreatitis. As anticipated, we did not find any cluster‐randomised trials for this comparison but if we had identified any cluster‐randomised trials, we planned to obtain the effect estimate adjusted for the clustering effect. If this was not available from the report or from the authors, we planned to exclude the trial from the meta‐analysis.
In multi‐arm trials, the models account for the correlation between trial‐specific treatment effects from the same trial.
Dealing with missing data
We attempted to contact investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). For binary, count, and time‐to‐event outcomes, we performed an intention‐to‐treat analysis whenever possible (Newell 1992). If this was not possible, we performed an available‐case analysis but assessed the impact of best‐best, best‐worst, worst‐best, and worst‐worst scenario analyses on the results for binary outcomes. For continuous outcomes, we performed an available‐case analysis. If we were unable to obtain the information from the investigators or study sponsors, we planned to impute mean from median (i.e. consider median as the mean) and standard deviation from standard error, interquartile range, or P values according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), but assess the impact of including such studies as indicated in a sensitivity analysis. If we were unable to calculate the standard deviation from standard error, interquartile range, or P values, we planned to impute the standard deviation as the highest standard deviation in the remaining trials included in the outcome fully aware that this method of imputation decreases the weight of the studies in the meta‐analysis of MD and shift the effect towards no effect for SMD. We planned to assess the impact of including such studies using a sensitivity analysis. However, we did not perform this imputation since the majority of the trials did not report the mean (i.e. they reported the median) or standard deviation, or both.
Assessment of heterogeneity
We assessed heterogeneity in each pair‐wise comparison by assessing the I2 statistic, Chi2 test with significance set at a P value less than 0.10, and visual inspection. We also used the Tau2 statistic to measure heterogeneity among the trials in each analysis. The Tau2 statistic provides a measure of the variability of the effect estimate across studies in a random‐effects model (Higgins 2011). If we identified substantial heterogeneity, we planned to explore it using meta‐regression.
Assessment of reporting biases
We attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results using a sensitivity analysis.
If we were able to pool more than 10 trials for a specific comparison, we planned to create and examine a funnel plot to explore possible publication biases. We planned to use Egger's test to determine the statistical significance of the reporting bias (Egger 1997). We planned to consider a P value of less than 0.05 statistically significant reporting bias.
Data synthesis
We undertook meta‐analyses only where this was meaningful (i.e. if the treatments, participants, and the underlying clinical questions were similar). In general, we favoured performing a meta‐analysis and have clearly highlighted the reason for not performing a meta‐analysis if such an analysis was not possible. We planned to conduct network meta‐analyses to compare multiple interventions simultaneously for each of the primary and secondary outcomes. Network meta‐analysis combines direct evidence within trials and indirect evidence across trials (Mills 2012).
We planned to obtain a network plot to ensure that the trials were connected by treatments using Stata/IC 12 (StataCorp LP) (see Appendix 8 for the Stata command that we planned to use). We planned to apply network meta‐analysis to each connected network. We planned to conduct a Bayesian network meta‐analysis using the Markov chain Monte Carlo method in WinBUGS 1.4. We planned to model the treatment contrast (e.g. log OR for binary outcomes, MD or SMD for continuous outcomes, RaR for count outcomes, HR for time‐to‐event outcomes) for any two interventions ('functional parameters') as a function of comparisons between each individual intervention and an arbitrarily selected reference group ('basic parameters') (Lu 2004). We planned to use open necrosectomy as the reference group. We planned to perform the network analysis as per the guidance from The National Institute for Health and Care Excellence Decision Support Unit (NICE DSU) documents (Dias 2014). Further details of the codes used and the technical details of how we planned to perform the analysis are shown in Appendix 9 and Appendix 10. In short, we planned to use non‐informative priors and three initial values, a burn‐in of 30,000 simulations to ensure convergence (we planned to use longer burn‐in if the models did not converge in 30,000 simulations), and obtained the posterior estimates after further 100,000 simulations. We planned to run the fixed‐effect and random‐effects models (assuming homogenous between‐trial variance across comparisons) for each outcome. We planned to choose the fixed‐effect model if it resulted in an equivalent fit (assessed by residual deviances, number of effective parameters, and deviance information criteria (DIC)) as the random‐effects model. A lower DIC indicates a better model fit. We planned to use the random‐effects model if it resulted in a better model fit as indicated by a DIC lower than that of fixed‐effect model by at least three. In addition, we planned to perform a treatment‐by‐design random‐effects inconsistency model (Higgins 2012; White 2012). We planned to consider that the inconsistency model had a better model than the random‐effects consistency model (standard random‐effects network meta‐analysis model) if the model fit of the inconsistency model (as indicated by DIC) was at least three lower than the random‐effects consistency model.
For multi‐arm trials, one can enter the data from all the arms in a trial. This is entered as the number of people with events and the number of people exposed to the event using the binomial likelihood and logit link for binary outcomes; the mean and standard error using the normal likelihood and identity link for continuous outcomes requiring calculation of the MD; the mean and standard error of the treatment differences using the normal likelihood and identity link for continuous outcomes requiring calculation of the SMD; number of events and the number of people exposed to the event using the Poisson likelihood and log link for count outcomes; and follow‐up time in the study, number of people with event, and the number of people exposed to the event using the binomial likelihood and cloglog link for time‐to‐event outcomes. We planned to report the treatment contrasts (e.g. log ORs for binary outcomes, MDs for continuous outcomes, etc.) of the different treatments in relation to the reference treatment (i.e. open necrosectomy), the residual deviances, number of effective parameters, and DIC for fixed‐effect model and random‐effects model for each outcome. We also planned to report the parameters used to assess the model fit (i.e. residual deviances, number of effective parameters, and DIC) for the inconsistency model for all the outcomes and the between‐trial variance for the random‐effects model (Dias 2012a; Dias 2012b; Higgins 2012; White 2012). If the inconsistency model results in a better model fit than the consistency models, the transitivity assumption is likely to be untrue and the effect estimates obtained may not be reliable. We planned to highlight such outcomes where the inconsistency model resulted in a better model fit than the consistency models. We then planned to perform a separate network meta‐analysis for interventions for infected versus sterile necrotising pancreatitis and assess the inconsistency again. If there was no evidence of inconsistency in the revised analysis, we planned to present the results of the analysis for infected and sterile necrotising pancreatitis separately. If there was persistent evidence of inconsistency, we presented the results from the direct comparison in the 'Summary of findings' table.
We planned to calculate the 95% CrIs of treatment effects (e.g. ORs for binary outcomes, MDs for continuous outcomes, etc.) in the Bayesian meta‐analysis, which is similar in use to the 95% CIs in the Frequentist meta‐analysis. These are the 2.5th percentile and 97.5th percentiles of the simulations. We planned to report the mean effect estimate and the 95% CrI for each pair‐wise comparison in a table. We planned to estimate the probability that each intervention ranks at one of the possible positions and present this information in graphs. It should be noted that a less than 90% probability that the treatment is the best treatment is unreliable (i.e. one should not conclude that the treatment is the best treatment for that outcome if the probability of being the best treatment is less than 90%) (Dias 2012a). We planned to present the cumulative probability of the treatment ranks (i.e. the probability that the treatment is within the top two, the probability that the treatment is within the top three, etc.) in graphs. We planned to plot the probability that each treatment is best for each of the different outcomes (rankograms), which are generally considered more informative (Dias 2012a; Salanti 2011). However, because of sparse data, lack of direct and indirect evidence for any comparisons, and concerns about the transitivity assumption, we performed indirect comparisons only using methods described by Bucher et al. (Bucher 1997), and have presented the indirect comparison in Appendix 12. Although we planned to perform the direct comparisons using the same codes, we used the Review Manager 5 statistical algorithm for direct comparisons (RevMan 2012), which allowed us to present information in the standard way of representing information in Cochrane reviews.
In the presence of adequate data where authors reported the outcomes of participants at multiple follow‐up time points, we planned to follow the methods suggested by Lu et al. to perform the meta‐analysis (Lu 2007).
'Summary of findings' table
We created 'Summary of findings' tables using all the outcomes. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data to the meta‐analyses for the pre‐specified outcomes. We planned to use methods and recommendations described in the GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis (Puhan 2014). However, since the network meta‐analysis was not performed and because of the concerns about the transitivity assumption, we presented only the results of direct comparisons. We have justified all decisions to downgrade or upgrade the quality of studies using footnotes and made comments to aid the reader's understanding of the review where necessary. We considered whether there was any additional outcome information that was not able to be incorporated into meta‐analyses and noted this in the comments and planned to state if it supported or contradicted the information from the meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We planned to assess the differences in the effect estimates between the following subgroups using meta‐regression with the help of the code shown in Appendix 6 when at least one trial was included in each subgroup.
Presence of infection (infected necrotising pancreatitis versus sterile necrotising pancreatitis).
Type of surgical intervention (open versus minimally invasive surgery).
Routine antibiotic prophylaxis versus none.
Early enteral nutrition versus parenteral nutrition.
We planned to calculate the interaction term (Dias 2012c). If the 95% CrI of the interaction term did not overlap zero, we planned to consider this statistically significant.
Sensitivity analysis
We planned to perform sensitivity analysis defined a priori to assess the robustness of our conclusions:
excluding trials at unclear or high risk of bias (one or more of the risk of bias domains classified as unclear or high);
excluding trials in which either mean or standard deviation, or both were imputed;
imputation of binary outcomes under best‐best, best‐worst, worst‐best, and worst‐worst scenarios.
Reaching conclusions
We have based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We have avoided making recommendations for practice and have provided clear implications for research.
Results
Description of studies
Results of the search
We identified 13,062 references through electronic searches of CENTRAL (1092 references), MEDLINE (OvidSP) (5049 references), EMBASE (OvidSP) (4386 references), Science Citation Index expanded (2328 references), ClinicalTrials.gov (35 references) and WHO ICTRP (172 references). We identified no references by searching reference lists. After removing duplicate references, there were 9957 references. We excluded 9916 clearly irrelevant references through reading titles and abstracts. We retrieved 41 references for further assessment in detail, from the full publication. We excluded 25 references for the reasons stated in Excluded studies and the Characteristics of excluded studies table. One trial (three references) is an ongoing trial without any interim data (van Brunschot 2013). In total, 13 references describing eight trials fulfilled the inclusion criteria (Characteristics of included studies table) (Bakker 2012; Kivilaakso 1984; Litvin 2010; Maroske 1981; Mier 1997; Schroder 1991; Shenvi 2014; Van Santvoort 2010b). Figure 1 shows the reference flow.
1.
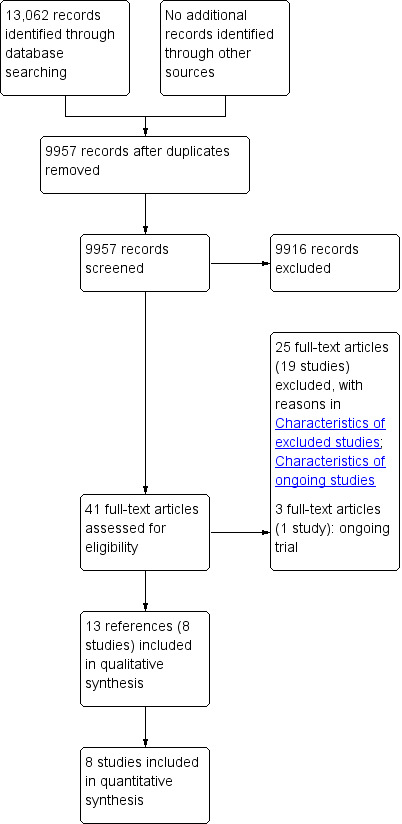
Study flow diagram.
Included studies
The review included eight RCTs (Bakker 2012; Kivilaakso 1984; Litvin 2010; Maroske 1981; Mier 1997; Schroder 1991; Shenvi 2014; Van Santvoort 2010b). All the eight trials were two‐armed trials. Three trials included only people with suspected or confirmed infected pancreatic necrosis (Bakker 2012; Shenvi 2014; Van Santvoort 2010b). The remaining five trials included infected or sterile necrotising pancreatitis (Kivilaakso 1984; Litvin 2010; Maroske 1981; Mier 1997; Schroder 1991). Only one trial restricted the participants based on aetiology (Schroder 1991). This trial included only people with necrotising pancreatitis due to alcohol (Schroder 1991). Two trials had no information on aetiology (Maroske 1981; Shenvi 2014). In the remaining trials, there was no restriction based on aetiology (Bakker 2012; Kivilaakso 1984; Litvin 2010; Mier 1997; Van Santvoort 2010b).
Of the three trials that included only participants with suspected or confirmed infected pancreatic necrosis, one trial used routine antibiotics but this was not for prophylaxis (Bakker 2012); the second trial used antibiotics routinely in majority of the participants prior to randomisation (Van Santvoort 2010b); and the third trial provided no information on antibiotic use (Shenvi 2014). In the remaining five trials that included participants with infected or sterile pancreatic necrosis, three trials used routine antibiotic treatment (Kivilaakso 1984; Mier 1997; Schroder 1991); and two trials provided no information on antibiotic use (Litvin 2010; Maroske 1981). None of the trials reported details on enteral versus parenteral nutrition.
The eight trials randomised 311 participants to intervention or control. After exclusion of five participants in one trial, 306 participants contributed to one or more outcomes in this review. Only two trials reported the follow‐up period (Bakker 2012; Van Santvoort 2010b). Both trials followed up participants for six months. The remaining trials did not report the follow‐up period (Kivilaakso 1984; Litvin 2010; Maroske 1981; Mier 1997; Schroder 1991; Shenvi 2014). However, it appeared that trials followed up participants only until discharge or for a short period of time following discharge (Kivilaakso 1984; Litvin 2010; Maroske 1981; Mier 1997; Schroder 1991; Shenvi 2014). We summarised the interventions and controls in the different trials and the timing of the intervention and control in the different studies below.
-
Peritoneal lavage versus open necrosectomy.
Kivilaakso 1984: peritoneal lavage (17 participants) versus open necrosectomy (18 participants). The timing of intervention after diagnosis was not clearly reported but it appeared that the intervention and control were performed as soon as possible.
Maroske 1981: peritoneal lavage (12 participants) versus open necrosectomy (12 participants). The timing of intervention and control after diagnosis was not clearly reported but it appeared that the intervention and control were performed as soon as possible.
Schroder 1991: peritoneal lavage (10 participants) versus open necrosectomy (11 participants). The intervention was performed as soon as possible.
-
Minimally invasive step‐up approach versus open necrosectomy.
Litvin 2010: minimally invasive step‐up approach (37 participants) versus open necrosectomy (35 participants). The timing of intervention and control after diagnosis was not reported clearly. The mean time to minimally‐invasive approach was 4.3 days from onset of acute pancreatitis.
Van Santvoort 2010b: minimally invasive step‐up approach (43 participants) versus open necrosectomy (45 participants). The intervention and control was postponed for at least four weeks after onset of pancreatitis if possible.
Minimally invasive step‐up approach involved percutaneous or endoscopic drainage in both trials followed by open necrosectomy in one trial (Litvin 2010) or video‐assisted necrosectomy in one trial (Van Santvoort 2010b).
-
Variations in open necrosectomy
Mier 1997: delayed open necrosectomy (11 participants) versus early open necrosectomy (25 participants). Timing: delayed open necrosectomy: at least 12 days after diagnosis; early open necrosectomy: within 48 to 72 hours of diagnosis.
-
Variations in the minimally invasive step‐up approach
Bakker 2012: video‐assisted minimally invasive step‐up approach (12 participants) versus endoscopic minimally invasive step‐up approach (10 participants). In the video‐assisted group, necrosectomy was performed after percutaneous drainage while endoscopic necrosectomy was performed after endoscopic drainage in the endoscopic group. The timing of intervention after confirmation of diagnosis was not reported.
Shenvi 2014: minimally invasive step‐up approach (planned surgery) (four participants) versus minimally invasive step‐up approach (continued percutaneous drainage) (four participants). The intervention and control were performed after failed percutaneous drainage, which was attempted for at least one week.
The Characteristics of included studies table lists the outcomes reported in individual trials. Table 4 shows the inclusion and exclusion criteria and the risk of bias according to the comparisons. The interval between diagnosis and treatment was not clear in many of the trials as shown above; so we were unable to assess the transitivity assumption (i.e. the assumption that similar participants were included in all trials).
1. Characteristics of studies (arranged according to comparisons).
| Study name | Inclusion and exclusion criteria | Number of people in intervention group | Number of people in control group | Risk of bias | ||||||
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | ||||
| Peritoneal lavage vs. open necrosectomy | ||||||||||
| Kivilaakso 1984 | Inclusion criteria People with acute fulminant (haemorrhagic) pancreatitis Exclusion criteria People with oedematous pancreatitis | 17 | 18 | Unclear | Low | Unclear | Unclear | Low | Low | Unclear |
| Maroske 1981 | Inclusion criteria People with acute haemorrhagic (necrotising) pancreatitis | 12 | 12 | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear |
| Schroder 1991 | Inclusion criteria People aged under 50 years with fulminant acute pancreatitis resulting from alcohol abuse | 10 | 11 | Unclear | Low | Unclear | Unclear | Low | Low | Unclear |
| Minimally invasive step‐up approach vs. open necrosectomy | ||||||||||
| Litvin 2010 | Inclusion criteria People with acute necrotising pancreatitis | 37 | 35 | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear |
| Van Santvoort 2010b |
Inclusion criteria
People with suspected or confirmed infected necrotising pancreatitis
Exclusion criteria
Flare‐up of chronic pancreatitis Previous exploratory laparotomy during the current episode of pancreatitis Previous drainage or surgery for confirmed or suspected infected necrosis Pancreatitis caused by abdominal surgery An acute intraabdominal event (e.g. perforation of a visceral organ, bleeding, or the abdominal compartment syndrome) |
43 | 45 | Unclear | Low | Unclear | Low | Low | Low | Low |
| Variations in open necrosectomy (delayed open necrosectomy vs. early open necrosectomy) | ||||||||||
| Mier 1997 | Inclusion criteria People with fulminant necrotising pancreatitis | 11 | 25 | Unclear | Unclear | Unclear | Unclear | High | High | Unclear |
| Variations in the minimally invasive step‐up approach (video‐assisted vs. endoscopic) | ||||||||||
| Bakker 2012 |
Inclusion criteria
Adults needing necrosectomy for suspected or confirmed infected necrotising pancreatitis who could undergo both endoscopic or surgical necrosectomy, based on computed tomographic imaging
Exclusion criteria
Previous surgical or endoscopic necrosectomy Previous exploratory laparotomy Pancreatitis as a consequence of abdominal surgery A flare‐up of chronic pancreatitis Abdominal compartment syndrome Perforation of a visceral organ Bleeding as indication for intervention |
12 | 10 | Unclear | Unclear | Unclear | Low | Low | Low | Low |
| Variations in the minimally invasive step‐up approach (planned surgery vs. continued percutaneous drainage) | ||||||||||
| Shenvi 2014 | Inclusion criteria People with diagnosis of infectious pancreatic necrosis managed with percutaneous catheter drainage for 10‐15 days and people who did not show significant improvement on percutaneous catheter drainage | 4 | 4 | Low | Low | High | High | Low | High | Low |
Excluded studies
We excluded 25 references of 19 studies because they were not conducted in people with necrotising pancreatitis (Ai 2010; Balldin 1983; Ihse 1986; Mayer 1985; Radenkovic 2010; Ranson 1976), or they were non‐randomised studies (Amorotti 1998; Connor 2005; Cooper 1982; Dronov 2009; Krautzberger 1985; Pascual 2013; Schroder 1990; Teerenhovi 1989; Van Santvoort 2011), quasi‐randomised studies (Ranson 1990), or were comments (Armbruster 1998; Brand 2010; Levi 2010).
Risk of bias in included studies
None of the included trials were at low risk of bias. Figure 2 and Figure 3 summarise the risk of bias in the individual domains.
2.
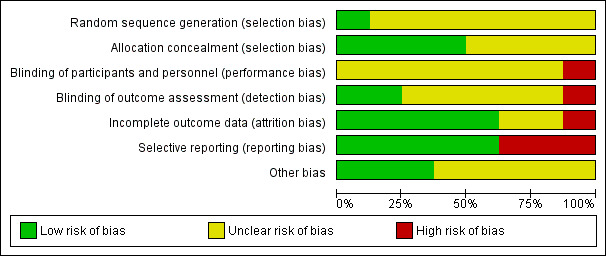
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
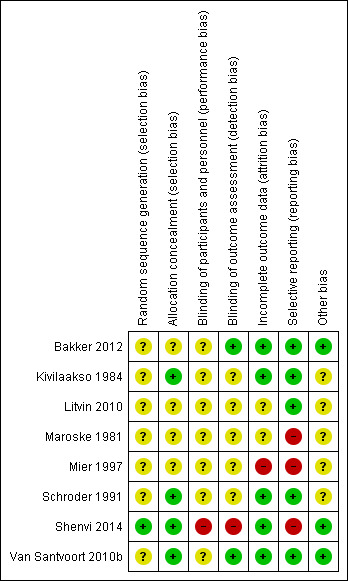
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only one trial was at low risk of bias for random sequence generation (Shenvi 2014). Four trials were at low risk of bias for allocation concealment (Kivilaakso 1984; Schroder 1991; Shenvi 2014; Van Santvoort 2010b). Thus, only one trial was of low risk of bias for both random sequence generation and allocation concealment and we considered it at low risk of selection bias.
Blinding
None of the trials reported blinding of participants and healthcare providers. This was impossible or unethical for most of the comparisons included in this review. Thus, none of the trials were at low risk of performance bias. Two trials achieved blinding of outcome assessors (Bakker 2012; Van Santvoort 2010b). We considered these two trials at low risk of detection bias.
Incomplete outcome data
Five trials included all participants for analysis of clinical outcomes and we considered them at low risk of attrition bias (Bakker 2012; Kivilaakso 1984; Schroder 1991; Shenvi 2014; Van Santvoort 2010b).
Selective reporting
Five trials reported mortality and morbidity and we considered them at low risk of selective reporting bias (Bakker 2012; Kivilaakso 1984; Litvin 2010; Schroder 1991; Van Santvoort 2010b).
Other potential sources of bias
Three trials reported source of funding and we considered them at low risk of bias (Bakker 2012; Shenvi 2014; Van Santvoort 2010b). There were no other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Table 1; Table 2; and Table 3 summarise the effects of interventions. None of the trials reported long‐term mortality, quality of life at any time frame, requirement for additional intervention, time to return to normal activity, and time to return to work.
Three trials could not be included for indirect comparison as there were variations of minimally invasive approach (Bakker 2012; Shenvi 2014) or open necrosectomy (Mier 1997).
Mortality
Short‐term mortality
All eight trials reported short‐term mortality (Bakker 2012; Kivilaakso 1984; Litvin 2010; Maroske 1981; Mier 1997; Schroder 1991; Shenvi 2014; Van Santvoort 2010b). As shown in Analysis 1.1, there were no statistically significant differences in any of the direct comparisons. The effect estimates for each of the comparisons were as follows.
1.1. Analysis.
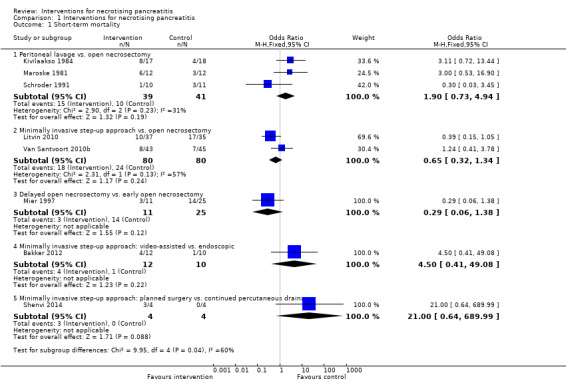
Comparison 1 Interventions for necrotising pancreatitis, Outcome 1 Short‐term mortality.
Peritoneal lavage versus open necrosectomy (OR 1.90, 95% CI 0.73 to 4.94; 80 participants; 3 studies; I2 = 31%).
Minimally invasive step‐up approach versus open necrosectomy (OR 0.65, 95% CI 0.32 to 1.34; 160 participants; 2 studies; I2 = 57%).
Delayed open necrosectomy versus early open necrosectomy (OR 0.29, 95% CI 0.06 to 1.38; 36 participants; 1 study).
-
Variations in the minimally invasive step‐up approach.
Minimally invasive step‐up approach: video‐assisted versus endoscopic (OR 4.50, 95% CI 0.41 to 49.08; 22 participants; 1 study).
Minimally invasive step‐up approach: planned surgery versus continued percutaneous drainage (OR 21.00, 95% CI 0.64 to 689.99; 8 participants; 1 study).
There was no evidence of heterogeneity in the comparison of peritoneal lavage with open necrosectomy (I2 = 31%; Chi2 test for heterogeneity = 0.23). There was moderate heterogeneity in the comparison of minimally invasive step‐up approach with open necrosectomy (I2 = 57%; Chi2 test for heterogeneity = 0.13). There was no difference in the interpretation of results using fixed‐effect versus random‐effects models for these two comparisons. The remaining comparisons had only one trial and the issues of heterogeneity and fixed‐effect versus random‐effects model did not arise.
The absolute unadjusted proportions of people with short‐term mortality in different interventions were as follows.
Open necrosectomy (irrespective of timing): 28.1% (34/121).
Early open necrosectomy: 56% (14/25).
Delayed open necrosectomy: 27.3% (3/11).
Minimally invasive step‐up approach (all): 22.5% (18/80).
Video‐assisted minimally invasive step‐up approach: 33.3% (4/12).
Endoscopic minimally invasive step‐up approach: 10% (1/10).
Minimally invasive step‐up approach (planned surgery): 75% (3/4).
Minimally invasive step‐up approach (percutaneous drainage): 0% (0/4).
Peritoneal lavage: 38.5% (15/39).
Long‐term mortality
None of the trials reported long‐term mortality.
Serious adverse events
Serious adverse events (proportion)
Only one trial reported the proportion of participants who developed serious adverse events such as organ failure and sepsis (Litvin 2010). There was no statistically significant difference in the proportion of participants who developed serious adverse events between the minimally invasive step‐up approach (18/37; 48.6%) and open necrosectomy (25/35; 71.4%) (OR 0.38, 95% CI 0.14 to 1.01; 72 participants; 1 study) (Analysis 1.2).
1.2. Analysis.
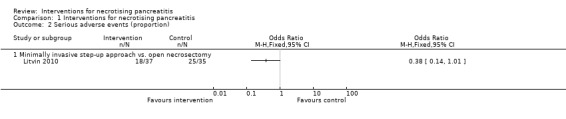
Comparison 1 Interventions for necrotising pancreatitis, Outcome 2 Serious adverse events (proportion).
Serious adverse events (number)
Four trials reported the number of serious adverse events such as sepsis, abscess, pulmonary insufficiency, renal insufficiency, and re‐operations (Bakker 2012; Kivilaakso 1984; Schroder 1991; Van Santvoort 2010b). As shown in Analysis 1.3, the number of serious adverse events were fewer in the minimally invasive step‐up approach compared to open necrosectomy (RaR 0.41, 95% CI 0.25 to 0.68; 88 participants; 1 study). There were no statistically significant differences in the comparisons of peritoneal lavage versus open necrosectomy (RaR 1.28, 95% CI 0.92 to 1.78; 56 participants; 2 studies; I2 = 60%) and minimally invasive step‐up approach: video‐assisted versus endoscopic (RaR 12.55, 95% CI 0.72 to 219.54; 22 participants; 1 study).
1.3. Analysis.
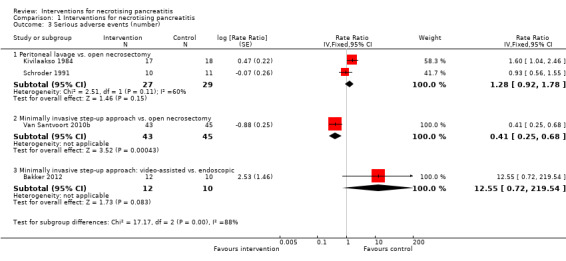
Comparison 1 Interventions for necrotising pancreatitis, Outcome 3 Serious adverse events (number).
There was moderate heterogeneity in the comparison of peritoneal lavage versus open necrosectomy (I2 = 60%; Chi2 test for heterogeneity = 0.11). There was no difference in the interpretation of results using fixed‐effect versus random‐effects models for this comparison. The remaining comparisons had only one trial and the issues of heterogeneity and fixed‐effect versus random‐effects model did not arise.
The absolute unadjusted number of serious adverse events per 100 participants in different interventions were as follows.
Open necrosectomy: 166.2 events per 100 participants (123/74).
Minimally invasive step‐up approach: 53.5 events per 100 participants (23/43).
Video‐assisted minimally invasive step‐up approach: 58.3 events per 100 participants (7/12).
Endoscopic minimally invasive step‐up approach: 0 events per 100 participants (0/10).
Peritoneal lavage: 285.2 events per 100 participants (77/27).
Infected pancreatic necrosis
None of the trials that included participants with sterile pancreatic necrosis reported the number of participants who developed infection during the course of treatment or during the follow‐up.
Organ failure
Two trials reported the proportion of people with organ failure (Bakker 2012; Van Santvoort 2010b). As shown in Analysis 1.4, the proportion of people with organ failure was lower in the minimally invasive step‐up approach (11.6%) compared to open necrosectomy group (40%) (OR 0.20, 95% CI 0.07 to 0.60; 88 participants; 1 study). There was no statistically significant difference in the minimally invasive step‐up approach: video‐assisted (41.7%) compared to endoscopic (0%) (OR 15.40, 95% CI 0.73 to 322.88; 22 participants; 1 study).
1.4. Analysis.
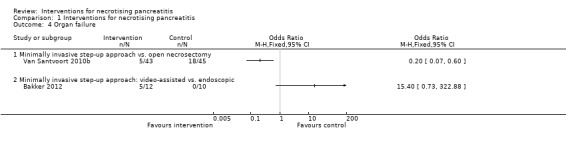
Comparison 1 Interventions for necrotising pancreatitis, Outcome 4 Organ failure.
Health‐related quality of life
None of the trials reported health‐related quality of life at any time frame.
Adverse events
Adverse events (proportion)
None of the trials reported the proportion of people with adverse events.
Adverse events (number)
Three trials reported the number of adverse events (Bakker 2012; Schroder 1991; Van Santvoort 2010b). As shown in Analysis 1.5, the number of adverse events were fewer in the minimally invasive step‐up approach compared to open necrosectomy (RaR 0.41, 95% CI 0.25 to 0.68; 88 participants; 1 study) and in the endoscopic minimally invasive step‐up approach compared to the video‐assisted minimally invasive step‐up approach (RaR minimally invasive step‐up approach: video‐assisted versus endoscopic: 11.70, 95% CI 1.52 to 89.87; 22 participants; 1 study). There was no statistically significant difference in the peritoneal lavage compared to open necrosectomy (RaR 1.01, 95% CI 0.63 to 1.62; 21 participants; 1 study).
1.5. Analysis.
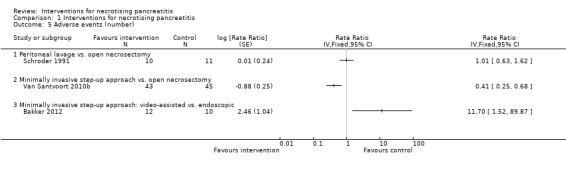
Comparison 1 Interventions for necrotising pancreatitis, Outcome 5 Adverse events (number).
The absolute unadjusted number of adverse events per 100 participants in different interventions were as follows.
Open necrosectomy: 169.6 events per 100 participants (95/56).
Minimally invasive step‐up approach: 53.5 events per 100 participants (23/43).
Peritoneal lavage: 340 events per 100 participants (34/10).
Video‐assisted minimally invasive step‐up approach: 116.7 events per 100 participants (14/12).
Endoscopic minimally invasive step‐up approach: 10 events per 100 participants (1/10).
Length of hospital stay
Five trials reported the length of hospital stay (Bakker 2012; Kivilaakso 1984; Litvin 2010; Schroder 1991; Van Santvoort 2010b). None of the trials reported the mean and standard deviation. One trial reported the mean and standard error of the mean (Kivilaakso 1984). Two trials reported median and P values (Bakker 2012; Van Santvoort 2010b). In the remaining two trials, it was unclear whether mean or median hospital stay was reported (Litvin 2010; Schroder 1991). These two trials did not report the standard deviation (Litvin 2010; Schroder 1991). Therefore, we could not perform a meta‐analysis. We have tabulated the mean length of hospital stay in each group and the differences in length of hospital stay between the intervention and control groups in Analysis 1.6. As shown in the Analysis 1.6, there was either no statistically significant difference or the statistical significance of the difference was not known in all the comparisons.
1.6. Analysis.
Comparison 1 Interventions for necrotising pancreatitis, Outcome 6 Length of hospital stay.
| Length of hospital stay | ||||||
|---|---|---|---|---|---|---|
| Study | Intervention: Median (days) | Intervention: Number of participants | Control: Median (days) | Control: Number of participants | Difference in median (days) | Statistical significance/ standard deviation |
| Peritoneal lavage vs. open necrosectomy | ||||||
| Kivilaakso 1984 | 41.7 (mean) | 17 | 41.1 (mean) | 18 | 0.6 | No statistically significant difference Standard deviation: not stated |
| Schroder 1991 | 44.3 (not clear whether this was mean or median) | 10 | 56.1 (not clear whether this was mean or median) | 11 | ‐11.8 | Statistical significance not reported nor could be calculated Standard deviation: not stated |
| Minimally invasive step‐up approach vs. open necrosectomy | ||||||
| Litvin 2010 | 19.7 (not clear whether this was mean or median) | 37 | 29.7 (not clear whether this was mean or median) | 35 | ‐10 | Statistical significance not reported nor could be calculated Standard deviation: not stated |
| Van Santvoort 2010b | 50 (median) | 43 | 60 (median) | 45 | ‐10 | P = 0.53 Standard deviation: not stated |
| Minimally invasive step‐up approach: video‐assisted vs. endoscopic | ||||||
| Bakker 2012 | 36 | 10 | 45 | 10 | ‐9 | P = 0.91 Standard deviation: not stated |
Length of intensive therapy unit stay
Three trials reported the length of ITU stay (Kivilaakso 1984; Schroder 1991; Van Santvoort 2010b). None of the trials reported the mean and standard deviation. One trial reported the mean and standard error of the mean (Kivilaakso 1984). One trial reported median and P value (Van Santvoort 2010b). In one trial, it was unclear whether mean or median ITU stay was reported (Schroder 1991). This trial did not report the standard deviation (Schroder 1991). Therefore, we could not perform a meta‐analysis. We have tabulated the mean length of ITU stay in each group and the differences in length of ITU stay between the intervention and control groups in Analysis 1.7. As shown in the Analysis 1.7, the length of ITU stay was statistically significantly longer (by eight days) in the peritoneal lavage group than in the open necrosectomy group in one trial (Kivilaakso 1984), but was shorter by 10 days in the other trial involving the same comparison (Schroder 1991). Although the second trial did not report the statistical significance of the difference in the length of ITU stay (Schroder 1991), we cannot be certain whether there was a difference between the peritoneal lavage group and the open necrosectomy group because of the major inconsistency in the two studies. There was no statistically significant difference in the length of ITU stay between the minimally invasive step‐up approach and open necrosectomy in the only trial that reported this outcome in this comparison (Van Santvoort 2010b).
1.7. Analysis.
Comparison 1 Interventions for necrotising pancreatitis, Outcome 7 Length of intensive therapy unit stay.
| Length of intensive therapy unit stay | ||||||
|---|---|---|---|---|---|---|
| Study | Intervention: Median (days) | Intervention: Number of participants | Control: Median (days) | Control: Number of participants | Difference in median (days) | Statistical significance/ standard deviation |
| Peritoneal lavage vs. open necrosectomy | ||||||
| Kivilaakso 1984 | 20.7 (mean) | 17 | 12.3 (mean) | 18 | 8.4 | Statistically significant Standard deviation: not stated |
| Schroder 1991 | 16.2 (not clear whether this was mean or median) | 10 | 25.9 (not clear whether this was mean or median) | 11 | ‐9.7 | Statistical significance not reported nor could be calculated Standard deviation: not stated |
| Minimally invasive step‐up approach vs. open necrosectomy | ||||||
| Van Santvoort 2010b | 9 | 43 | 11 | 45 | ‐2 | 0.26 Standard deviation: not stated |
Requirement for additional invasive intervention such as necrosectomy
None of the trials reported the requirement for additional invasive intervention.
Total number of treatments
Only one trial reported the total number of treatments (Bakker 2012). This trial reported the median and P value rather than the mean and standard deviation. Therefore, we tabulated the total number of treatments in Analysis 1.8. As shown in Analysis 1.8, the total number of treatments was statistically significantly fewer in the video‐assisted minimally invasive step‐up approach compared to the endoscopic minimally invasive step‐up approach.
1.8. Analysis.
Comparison 1 Interventions for necrotising pancreatitis, Outcome 8 Number of treatments.
| Number of treatments | ||||||
|---|---|---|---|---|---|---|
| Study | Intervention: Median | Intervention: Number of participants | Control: Median | Control: Number of participants | Difference in median | Statistical significance/ standard deviation |
| Minimally invasive step‐up approach: video‐assisted vs. endoscopic | ||||||
| Bakker 2012 | 1 | 10 | 3 | 10 | 2 | P = 0.007 (fewer treatments favouring video assisted necrosectomy group) Standard deviation: not stated |
Time to return to normal activity
None of the trials reported time to return to normal activity.
Time to return to work
None of the trials reported time to return to work.
Costs
Only one trial reported the costs (Van Santvoort 2010b). The costs included treatment costs and loss of productivity costs. This trial did not report the standard deviation. Therefore, we have tabulated the costs in Analysis 1.9. As shown in Analysis 1.9, the costs were statistically significantly less in the minimally invasive step‐up approach than open necrosectomy.
1.9. Analysis.
Comparison 1 Interventions for necrotising pancreatitis, Outcome 9 Costs.
| Costs | |||||||
|---|---|---|---|---|---|---|---|
| Study | Intervention: Mean (USD) | Intervention: Number of participants | Control: Mean (USD) | Control: Number of participants | Difference in means (USD) | Statistical significance/ standard deviation | Comment |
| Minimally invasive step‐up approach vs. open necrosectomy | |||||||
| Van Santvoort 2010b | 86,653 | 43 | 98,575 | 45 | ‐11,922 | Statistically significantly lower costs favouring step‐up approach Standard deviation: not stated |
Converted from euros to US dollars on 22 February 2016 (1 Euro = 1.1 USD) |
Subgroup analysis
We presented the direct comparisons for the primary outcomes for the following subgroups: infected or suspected necrotising pancreatitis only (none of the trials reported participants with sterile necrosis only) and for routine antibiotic use in trials that did not restrict participants to infected or suspected necrotising pancreatitis. As shown in the Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; and Analysis 2.5, there were no alterations in the results because of subgroup analysis. We did not perform the remaining subgroup analysis as all the trials in the step‐up approach used open necrosectomy as the final resort and was an integral part of the step‐up approach and none of the trials reported the use of early enteral nutrition versus parenteral nutrition.
2.1. Analysis.
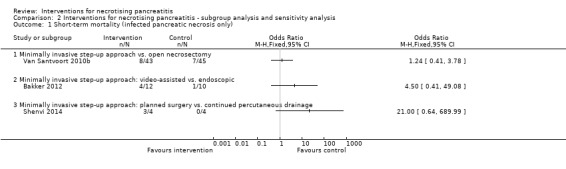
Comparison 2 Interventions for necrotising pancreatitis ‐ subgroup analysis and sensitivity analysis, Outcome 1 Short‐term mortality (infected pancreatic necrosis only).
2.2. Analysis.
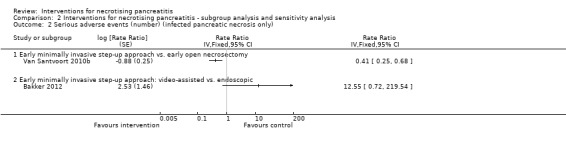
Comparison 2 Interventions for necrotising pancreatitis ‐ subgroup analysis and sensitivity analysis, Outcome 2 Serious adverse events (number) (infected pancreatic necrosis only).
2.3. Analysis.
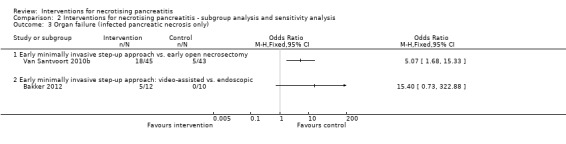
Comparison 2 Interventions for necrotising pancreatitis ‐ subgroup analysis and sensitivity analysis, Outcome 3 Organ failure (infected pancreatic necrosis only).
2.4. Analysis.
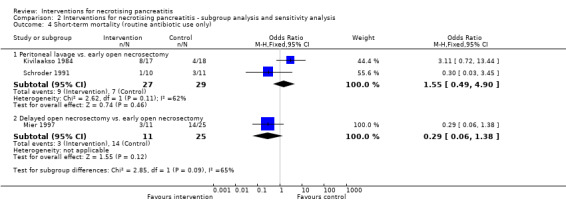
Comparison 2 Interventions for necrotising pancreatitis ‐ subgroup analysis and sensitivity analysis, Outcome 4 Short‐term mortality (routine antibiotic use only).
2.5. Analysis.
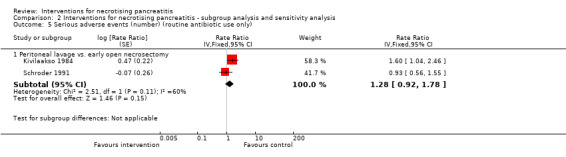
Comparison 2 Interventions for necrotising pancreatitis ‐ subgroup analysis and sensitivity analysis, Outcome 5 Serious adverse events (number) (routine antibiotic use only).
Sensitivity analysis
Short‐term mortality was the only outcome in the trial that had post‐randomisation exclusions (Mier 1997). There was a total of five post‐randomisation exclusions (one in the early open necrosectomy group and four in the delayed open necrosectomy group). The reasons for exclusions were: one exclusion from early necrosectomy group because the participant had received medical treatment initially by error; three exclusions from the delayed necrosectomy group who did not require surgery and resolved by conservative treatment; and one exclusion from the delayed necrosectomy group because of mesenteric ischaemia. Since it was reasonable to conclude that the three exclusions in the delayed necrosectomy group did not die, we performed an intention‐to‐treat analysis considering that these exclusions did not die and imputed the different scenarios for one participant in each group. As shown in Analysis 2.6, there was no change in the results because of imputation.
2.6. Analysis.
Comparison 2 Interventions for necrotising pancreatitis ‐ subgroup analysis and sensitivity analysis, Outcome 6 Short‐term mortality: sensitivity analysis.
We did not perform the remaining sensitivity analysis since all the trials were at unclear or high risk of bias and we did not impute either the mean or standard deviation.
Reporting bias
We did not use a funnel plot to explore reporting bias because of the presence of few trials.
Discussion
Summary of main results
This systematic review included eight RCTs. A total of 306 participants in these eight RCTs contributed to one or more outcomes for this review. Necrotising pancreatitis is a morbid disease with a short‐term mortality of approximately 30% of participants included in this review. The serious adverse event rate was 139 serious adverse events per 100 participants. The mean or median length of hospital stay varied between 20 and 60 days and the mean or median length of ITU stay varied between nine and 26 days in the trials that reported these outcomes. The mean costs related to treatment and loss of productivity in six months were more than USD 85,000 per participant in the only trial that assessed the costs.
In terms of comparisons between treatments, we presented only direct comparisons. This was because of sparse data and none of the comparisons had both direct and indirect evidence resulting in difficulty in assessing whether the transitivity assumption was true. We presented the analysis using the Frequentist methods since they follow the standard Cochrane format. We conducted indirect comparisons but presented the results in Appendix 12 because of the difficulty in assessing whether the transitivity assumption was true. There were no statistically significant differences in short‐term mortality and proportion of people with serious adverse events in any of the comparisons. The number of serious adverse events and adverse events were fewer in the minimally invasive step‐up approach compared to open necrosectomy. The proportion of people with organ failure and the mean costs were also lower in the minimally invasive step‐up approach compared to open necrosectomy. The number of adverse events were more with the video‐assisted minimally invasive step‐up approach compared to the endoscopic minimally invasive step‐up approach but the total number of interventions were fewer with the video‐assisted minimally invasive step‐up approach compared to the endoscopic minimally invasive step‐up approach. There were no statistically significant or consistent differences in any of the other comparisons for number of serious adverse events, proportion of people with organ failure, number of adverse events, length of hospital stay, and ITU stay. None of the trials reported long‐term mortality, infected pancreatic necrosis (trials that included participants with sterile necrosis), health‐related quality of life at any time frame, proportion of people with adverse events, requirement for additional invasive intervention, time to return to normal activity, and time to return to work.
Overall completeness and applicability of evidence
The trials included in this review included infected and sterile necrotising pancreatitis of varied aetiology. Therefore, the results of this review are applicable to people with sterile necrotising pancreatitis of varied aetiology. The timing of intervention differed between the studies and the information on the timing of the intervention from diagnosis of necrotising pancreatitis was unclear in many of the included studies. However, the interventions were likely to have been carried out immediately or within a few days after the diagnosis of necrotising pancreatitis unless the authors specifically delayed the treatment (e.g. delayed necrosectomy) or delayed the treatment as much possible.
Quality of the evidence
The overall quality of evidence was low or very low for the outcomes for which we could assess the quality of evidence. The major reason for this was the risk of bias in the trials. While blinding of healthcare providers or participants cannot be achieved ethically for many of the comparisons, blinding of outcome assessors can be achieved with appropriate study design. There were no difficulties in ensuring that the trial is low risk of bias in other domains. However, many of the trials were also of unclear or high risk of bias in other domains. These risks of bias can be avoided by appropriate planning and reporting. Another major issue for downgrading the quality of evidence was imprecision. The review included only 306 participants. The actual number of participants included was even less than this since there were many comparisons and not all studies reported all the outcomes. There was moderate heterogeneity in some of the comparisons (short‐term mortality: minimally invasive step‐up approach versus open necrosectomy and number of serious adverse events: peritoneal lavage versus open necrosectomy), but there was good overlap of CIs for these outcomes. Variations in the way that intervention and control were performed could possibly explain this and the heterogeneity does not alter the interpretation of evidence. Of the outcomes for which we did not assess the GRADE of evidence formally, there was significant heterogeneity for the length of ITU stay: the length of ITU stay was statistically significantly longer (by eight days) in peritoneal lavage group than open necrosectomy group in one trial (Kivilaakso 1984), but was shorter by 10 days in the other trial involving the same comparison (Schroder 1991). While variations in the way intervention and control could explain some difference in the effects estimated in the trial, these differences are unlikely to account for such a major difference in the estimated effects. This decreases the confidence in the differences in ITU stay calculated in these studies.
There was also inconsistency in the effect of the intervention across outcomes. Despite the fewer complications in the minimally invasive approach, there was no statistically significant difference in the length of hospital stay despite the large differences noted (the median hospital stay was 10 days less in the minimally invasive group compared to open necrosectomy group). This may be because of the multiple complications that develop in each person with differing severity resulting in significant within‐group variability in the length of hospital stay. However, the minimally invasive step‐up approach appears to be better than open necrosectomy for most outcomes.
Potential biases in the review process
We followed the Cochrane Handbook for Systematic Reviews of Interventions for the conduct of the direct comparison of the review and the guidance from the NICE DSU documents (Dias 2014). Two review authors selected studies and extracted data reducing the errors in data collection. We used formal search strategies to identify the trials. While the likelihood of missing trials from the identified references was low, the review included the time frame before the mandatory trial registration era and it was possible that some trials were not reported in journals because of their results. However, one has to be pragmatic and accept that this is the best level of evidence that is currently available.
While network meta‐analysis has its advantages in combining direct and indirect evidence (resulting in more precise evidence) and Bayesian network meta‐analysis allows calculation of probability of being best treatments, these advantages were limited in this review because of the sparse data, lack of direct and indirect evidence for a single comparison, and concerns about the transitivity assumption. Therefore, we used direct comparisons to arrive at conclusions and presented the indirect comparisons in Appendix 12.
Agreements and disagreements with other studies or reviews
This is the first systematic review of RCTs for the management of necrotising pancreatitis. We agree with Litvin et al. and Van Santvoort et al. that the minimally invasive step‐up approach is better than open necrosectomy (Litvin 2010; Van Santvoort 2010b). We disagreed with Kivilaakso et al. and Schroder et al., since we found no evidence to suggest that necrosectomy is better or worse than peritoneal lavage (Kivilaakso 1984; Schroder 1991). We also disagreed with Mier et al., since we found no evidence that delayed necrosectomy is better than early necrosectomy (Mier 1997).
Authors' conclusions
Implications for practice.
Low to very low quality evidence suggested that the minimally invasive step‐up approach resulted in fewer adverse events, fewer serious adverse events, less organ failure, and lower costs compared to open necrosectomy. Very low quality evidence suggested that the endoscopic minimally invasive step‐up approach resulted in fewer adverse events than the video‐assisted minimally invasive step‐up approach but increased the number of procedures required to treat the participant. There is currently no evidence to suggest that early open necrosectomy is superior or inferior to peritoneal lavage or delayed open necrosectomy. However, the confidence intervals were wide and significant benefits or harms of different treatments could not be ruled out.
Implications for research.
The TENSION trial that is currently underway in the Netherlands is assessing the optimal way to perform the minimally invasive step‐up approach (endoscopic drainage followed by endoscopic necrosectomy if necessary versus percutaneous drainage followed by video‐assisted necrosectomy if necessary) and is assessing important clinical outcomes of interest for this review. Implications for further research on this topic will be determined after the results of this randomised controlled trial are available.
Acknowledgements
We thank Karin Dearness, Managing Editor, Cochrane Upper Gastrointestinal and Pancreatic Diseases (UGPD) Group for providing administrative and logistical support for the preparation of the current review.
We thank the copy editors and Cochrane Editorial Unit for their comments.
Appendices
Appendix 1. Glossary of terms
Acute: sudden.
Aetiology: cause.
Amylase: an enzyme that breaks down carbohydrates.
Contrast‐enhanced computed tomography (CECT): computer tomography scan performed after injecting a dye in order to improve the scan's ability to distinguish between normal and abnormal tissues.
Enzyme: substances that enable and speed up chemical reactions that are necessary for the normal functioning of the body.
Endoscopic: with the help of an endoscope, a tube inserted into body (in this context, through the mouth and into the stomach).
Endoscopic transluminal drainage: endoscopic drainage inserted through an opening (in this context, through an opening in the stomach).
Epigastric pain: upper central abdominal pain.
Insulin: substance that helps regulate blood sugar.
Laparoscopic necrosectomy: removal of dead, damaged, or infected tissue by way of keyhole surgery.
Lipase: an enzyme that breaks down fat.
Lymphatics: vessels that convey lymph.
Morbidity: illness (in this context, it means complications).
Mortality: death.
Necrosis: death and decomposition of living tissue usually caused by lack of blood supply but can be caused by other pathological insult.
Oedema: swelling.
Pathological insult: substance or mechanism that causes the condition.
Percutaneous drainage: drainage carried out by insertion of drain from the external surface of the body, usually guided by an ultrasound or computed tomography scan.
Peripancreatic tissues: tissues surrounding the pancreas.
Pharmacological: drug related.
Prophylaxis: prevention.
Protease: an enzyme that digests protein.
Retroperitoneal: behind the abdominal cavity.
Sepsis: blood poisoning which activates the body’s defence mechanism excessively.
Serum: clear fluid that separates out when blood clots.
Transient: temporary.
Transabdominal ultrasonography: standard ultrasound in which the ultrasound probe is placed on the abdomen (tummy) to view structures inside the abdomen.
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Pancreatitis, Acute Necrotizing] this term only
#2 MeSH descriptor: [Pancreatitis] this term only and with qualifier(s): [Etiology ‐ ET]
#3 MeSH descriptor: [Pancreas] this term only and with qualifier(s): [Abnormalities ‐ AB, Pathology ‐ PA, Physiopathology ‐ PP]
#4 (acute near/3 pancrea*)
#5 (necro* near/3 pancrea*)
#6 (inflam* near/3 pancrea*)
#7 ((interstitial or edema* or oedema*) near/2 pancrea*)
#8 #1 or #2 or #3 or #4 or #5 or #6 or #7
Appendix 3. MEDLINE search strategy
1. Pancreatitis, Acute Necrotizing/
2. Pancreatitis/et
3. Pancreas/ab, pa, pp
4. (acute adj3 pancrea*).mp.
5. (necro* adj3 pancrea*).mp.
6. (inflam* adj3 pancrea$).mp.
7. ((interstitial or edema* or oedema*) adj2 pancrea*).mp.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. randomized controlled trial.pt.
10. controlled clinical trial.pt.
11. randomized.ab.
12. placebo.ab.
13. drug therapy.fs.
14. randomly.ab.
15. trial.ab.
16. groups.ab.
17. 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16
18. exp animals/ not humans.sh.
19. 17 not 18
20. 8 and 19
Appendix 4. EMBASE search strategy
1. acute hemorrhagic pancreatitis/
2. Pancreatitis/et
3. acute pancreatitis/
4. (acute adj3 pancrea*).mp.
5. (necro* adj3 pancrea*).mp.
6. (inflam* adj3 pancrea*).mp.
7. ((interstitial or edema* or oedema*) adj2 pancrea*).mp.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. Clinical trial/
10. Randomized controlled trial/
11. Randomization/
12. Single‐Blind Method/
13. Double‐Blind Method/
14. Cross‐Over Studies/
15. Random Allocation/
16. Placebo/
17. Randomi?ed controlled trial*.tw.
18. Rct.tw.
19. Random allocation.tw.
20. Randomly allocated.tw.
21. Allocated randomly.tw.
22. (allocated adj2 random).tw.
23. Single blind*.tw.
24. Double blind*.tw.
25. ((treble or triple) adj blind*).tw.
26. Placebo*.tw.
27. Prospective study/
28. or/9‐27
29. Case study/
30. Case report.tw.
31. Abstract report/ or letter/
32. or/29‐31
33. 28 not 32
34. 8 and 33
Appendix 5. Science Citation Index Expanded search strategy
# 1 TS=((acute or necro* or inflam* or interstitial or edema* or oedema*) near/3 pancrea*)
# 2 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*)
# 3 #2 AND #1
Appendix 6. ClinicalTrials.gov search strategy
"Interventional" [STUDY‐TYPES] AND acute pancreatitis [DISEASE] AND ( "Phase 2" OR "Phase 3" OR "Phase 4" ) [PHASE]
Appendix 7. WHO ICTRP search strategy
Distal pancreatectomy AND laparoscop*
Appendix 8. Stata code for network plot
networkplot t1 t2, labels(T1 T2 T3 ...)
Appendix 9. Winbugs code
Source of code:
Consistency models: Dias 2014
Inconsistency models: White 2012 (modifications were performed for continuous, count, and time‐to‐event outcomes)
Binary outcome
Binary outcome ‐ fixed‐effect model
# Binomial likelihood, logit link # Fixed effects model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH STUDIES mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dbin(p[i,k],n[i,k]) # binomial likelihood # model for linear predictor logit(p[i,k]) <‐ mu[i] + d[t[i,k]] ‐ d[t[i,1]] # expected value of the numerators rhat[i,k] <‐ p[i,k] * n[i,k] #Deviance contribution dev[i,k] <‐ 2 * (r[i,k] * (log(r[i,k])‐log(rhat[i,k])) + (n[i,k]‐r[i,k]) * (log(n[i,k]‐r[i,k]) ‐ log(n[i,k]‐rhat[i,k]))) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) } totresdev <‐ sum(resdev[]) # Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } # pairwise ORs and LORs for all possible pair‐wise comparisons, if nt>2 for (c in 1:(nt‐1)) { for (k in (c+1):nt) { or[c,k] <‐ exp(d[k] ‐ d[c]) lor[c,k] <‐ (d[k]‐d[c]) } } # ranking on relative scale for (k in 1:nt) { # rk[k] <‐ nt+1‐rank(d[],k) # assumes events are “good” rk[k] <‐ rank(d[],k) # assumes events are “bad” best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Binary outcome ‐ random‐effects model
# Binomial likelihood, logit link # Random effects model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH STUDIES w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm delta[i,1] <‐ 0 # treatment effect is zero for control arm mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dbin(p[i,k],n[i,k]) # binomial likelihood logit(p[i,k]) <‐ mu[i] + delta[i,k] # model for linear predictor rhat[i,k] <‐ p[i,k] * n[i,k] # expected value of the numerators #Deviance contribution dev[i,k] <‐ 2 * (r[i,k] * (log(r[i,k])‐log(rhat[i,k])) + (n[i,k]‐r[i,k]) * (log(n[i,k]‐r[i,k]) ‐ log(n[i,k]‐rhat[i,k]))) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific LOR distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of LOR distributions (with multi‐arm trial correction) md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # precision of LOR distributions (with multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k # adjustment for multi‐arm RCTs w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # cumulative adjustment for multi‐arm trials sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) } } totresdev <‐ sum(resdev[]) # Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) # pairwise ORs and LORs for all possible pair‐wise comparisons, if nt>2 for (c in 1:(nt‐1)) { for (k in (c+1):nt) { or[c,k] <‐ exp(d[k] ‐ d[c]) lor[c,k] <‐ (d[k]‐d[c]) } } # ranking on relative scale for (k in 1:nt) { # rk[k] <‐ nt+1‐rank(d[],k) # assumes events are “good” rk[k] <‐ rank(d[],k) # assumes events are “bad” best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Binary outcome ‐ inconsistency model (random‐effects)
# Binomial likelihood, logit link, inconsistency model # Random effects model # Treatment by design interactions # ns = number of studies, nt = number of treatments, A = total number of treatment arms in all trials, and D = the number of designs have to be stated. # The main data are arranged with one record per arm: d and study indicate which design and study that arm belongs to, t indicates its treatment, and b indicates the first treatment in that design. r and n are the numbers of events and individuals in the arm. The supplementary data offset and offset.design list the rows in which the first arm of each trial and of each design is found. model { for(i in 1:ns) { eff.study[i, b[offset[i]], b[offset[i]]] <‐0 for(k in (offset[i] + 1):(offset[i + 1]‐1)) { eff.study[i,t[k],b[k]] <‐eff.des[d[k],t[k]] + RE[i,t[k]] ‐ RE[i,b[k]] } } # Random effects for heterogeneity for(i in 1:ns) { RE[i,1] <‐0 RE[i,2:nt] ˜ dmnorm(zero[], Prec[,]) } # Prec is the inverse of the structured heterogeneity matrix for(i in 1:(nt‐1)) { for(j in 1:(nt‐1)){ Prec[i,j] <‐2*(equals(i,j)‐1/nt)/(tau*tau) } } for(i in 1:A) { logit(p[i]) <‐mu[study[i]] + eff.study[study[i],t[i],b[i]] r[i] ˜ dbin(p[i],n[i])} # For computing DIC for(i in 1:A) { rhat[i] <‐p[i] * n[i] dev[i] <‐2 * (r[i] * (log(r[i])‐log(rhat[i])) + (n[i]‐r[i]) * (log(n[i]‐r[i]) ‐ log(n[i]‐ rhat[i]))) } devs <‐sum(dev[]) # Priors for(i in 1:ns) { mu[i] ˜ dnorm(0,0.01) } tau ˜ dunif(0,2) } # *** PROGRAM ENDS
Continuous outcome (mean difference)
Continuous outcome (mean difference) ‐ fixed‐effect model
# Normal likelihood, identity link # Fixed effect model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH STUDIES mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS var[i,k] <‐ pow(se[i,k],2) # calculate variances prec[i,k] <‐ 1/var[i,k] # set precisions y[i,k] ˜ dnorm(theta[i,k],prec[i,k]) # model for linear predictor theta[i,k] <‐ mu[i] + d[t[i,k]] ‐ d[t[i,1]] #Deviance contribution dev[i,k] <‐ (y[i,k]‐theta[i,k])*(y[i,k]‐theta[i,k])*prec[i,k] } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero for control arm # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } # ranking on relative scale for (k in 1:nt) { rk[k] <‐ rank(d[],k) # assumes lower is better # rk[k] <‐ nt+1‐rank(d[],k) # assumes lower outcome is worse best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Continuous outcome (mean difference) ‐ random‐effects model
# Normal likelihood, identity link # Random effects model for multi‐arm trials model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH STUDIES w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm delta[i,1] <‐ 0 # treatment effect is zero for control arm mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS var[i,k] <‐ pow(se[i,k],2) # calculate variances prec[i,k] <‐ 1/var[i,k] # set precisions y[i,k] ˜ dnorm(theta[i,k],prec[i,k]) theta[i,k] <‐ mu[i] + delta[i,k] # model for linear predictor #Deviance contribution dev[i,k] <‐ (y[i,k]‐theta[i,k])*(y[i,k]‐theta[i,k])*prec[i,k] } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific MD distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of MD distributions, with multi‐arm trial correction md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # precision of MD distributions (with multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k # adjustment, multi‐arm RCTs w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # cumulative adjustment for multi‐arm trials sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) } } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero for control arm # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) # ranking on relative scale for (k in 1:nt) { rk[k] <‐ rank(d[],k) # assumes lower is better # rk[k] <‐ nt+1‐rank(d[],k) # assumes lower outcome is worse best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Continuous outcome (mean difference) ‐ inconsistency model (random‐effects)
# Normal likelihood, identity link, inconsistency model # Random effects model # Treatment by design interactions # ns = number of studies, nt = number of treatments, A = total number of treatment arms in all trials, and D = the number of designs have to be stated. # The main data are arranged with one record per arm: d and study indicate which design and study that arm belongs to, t indicates its treatment, and b indicates the first treatment in that design. y, se, and n are the mean, standard error, and number of individuals in the arm. The supplementary data offset and offset.design list the rows in which the first arm of each trial and of each design is found. model { for(i in 1:ns) { eff.study[i, b[offset[i]], b[offset[i]]] <‐0 for(k in (offset[i] + 1):(offset[i + 1]‐1)) { eff.study[i,t[k],b[k]] <‐eff.des[d[k],t[k]] + RE[i,t[k]] ‐ RE[i,b[k]] } } # Random effects for heterogeneity for(i in 1:ns) { RE[i,1] <‐0 RE[i,2:nt] ˜ dmnorm(zero[], Prec[,]) } # Prec is the inverse of the structured heterogeneity matrix for(i in 1:(nt‐1)) { for(j in 1:(nt‐1)){ Prec[i,j] <‐2*(equals(i,j)‐1/nt)/(tau*tau) } } for(i in 1:A) { var[i] <‐ pow(se[i],2) # calculate variances prec[i] <‐ 1/var[i] # set precisions y[i] ˜ dnorm(theta[i],prec[i]) # normal likelihood theta[i] <‐mu[study[i]] + eff.study[study[i],t[i],b[i]] # model for linear predictor } # For computing DIC for(i in 1:A) { dev[i] <‐ (y[i]‐theta[i])*(y[i]‐theta[i])*prec[i] } devs <‐sum(dev[]) # Priors for(i in 1:ns) { mu[i] ˜ dnorm(0,0.01) } tau ˜ dunif(0,2) for(i in 1:D) { for(k in (offset.design[i] + 1):(offset.design[i] + num.ests[i])) { eff.des[i,t[k]] ˜ dnorm(0,0.01) } } } # *** PROGRAM ENDS
Continuous outcome (standardised mean difference)
The standardised mean difference and its standard error for each treatment comparison will be calculated using the statistical algorithms used by Review Manager 5 (RevMan 2012).
Continuous outcome (standardised mean difference) ‐ fixed‐effect model
# Normal likelihood, identity link # Trial‐level data given as treatment differences # Fixed effects model model{ # *** PROGRAM STARTS for(i in 1:ns2) { # LOOP THROUGH 2‐ARM STUDIES y[i,2] ˜ dnorm(delta[i,2],prec[i,2]) # normal likelihood for 2‐arm trials #Deviance contribution for trial i resdev[i] <‐ (y[i,2]‐delta[i,2])*(y[i,2]‐delta[i,2])*prec[i,2] } for(i in (ns2+1):(ns2+ns3)) { # LOOP THROUGH THREE‐ARM STUDIES for (k in 1:(na[i]‐1)) { # set variance‐covariance matrix for (j in 1:(na[i]‐1)) { Sigma[i,j,k] <‐ V[i]*(1‐equals(j,k)) + var[i,k+1]*equals(j,k) } } Omega[i,1:(na[i]‐1),1:(na[i]‐1)] <‐ inverse(Sigma[i,,]) #Precision matrix # multivariate normal likelihood for 3‐arm trials y[i,2:na[i]] ˜ dmnorm(delta[i,2:na[i]],Omega[i,1:(na[i]‐1),1:(na[i]‐1)]) #Deviance contribution for trial i for (k in 1:(na[i]‐1)){ # multiply vector & matrix ydiff[i,k]<‐ y[i,(k+1)] ‐ delta[i,(k+1)] z[i,k]<‐ inprod2(Omega[i,k,1:(na[i]‐1)], ydiff[i,1:(na[i]‐1)]) } resdev[i]<‐ inprod2(ydiff[i,1:(na[i]‐1)], z[i,1:(na[i]‐1)]) } for(i in 1:(ns2+ns3)){ # LOOP THROUGH ALL STUDIES for (k in 2:na[i]) { # LOOP THROUGH ARMS var[i,k] <‐ pow(se[i,k],2) # calculate variances prec[i,k] <‐ 1/var[i,k] # set precisions delta[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] } } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } # ranking on relative scale for (k in 1:nt) { rk[k] <‐ nt+1‐rank(d[],k) # assumes higher HRQoL is “good” #rk[k] <‐ rank(d[],k) # assumes higher outcome is “bad” best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Continuous outcome (standardised mean difference) ‐ random‐effects model
# Normal likelihood, identity link # Trial‐level data given as treatment differences # Random effects model model{ # *** PROGRAM STARTS for(i in 1:ns2) { # LOOP THROUGH 2‐ARM STUDIES y[i,2] ˜ dnorm(delta[i,2],prec[i,2]) # normal likelihood for 2‐arm trials #Deviance contribution for trial i resdev[i] <‐ (y[i,2]‐delta[i,2])*(y[i,2]‐delta[i,2])*prec[i,2] } for(i in (ns2+1):(ns2+ns3)) { # LOOP THROUGH THREE‐ARM STUDIES for (k in 1:(na[i]‐1)) { # set variance‐covariance matrix for (j in 1:(na[i]‐1)) { Sigma[i,j,k] <‐ V[i]*(1‐equals(j,k)) + var[i,k+1]*equals(j,k) } } Omega[i,1:(na[i]‐1),1:(na[i]‐1)] <‐ inverse(Sigma[i,,]) #Precision matrix # multivariate normal likelihood for 3‐arm trials y[i,2:na[i]] ˜ dmnorm(delta[i,2:na[i]],Omega[i,1:(na[i]‐1),1:(na[i]‐1)]) #Deviance contribution for trial i for (k in 1:(na[i]‐1)){ # multiply vector & matrix ydiff[i,k]<‐ y[i,(k+1)] ‐ delta[i,(k+1)] z[i,k]<‐ inprod2(Omega[i,k,1:(na[i]‐1)], ydiff[i,1:(na[i]‐1)]) } resdev[i]<‐ inprod2(ydiff[i,1:(na[i]‐1)], z[i,1:(na[i]‐1)]) } for(i in 1:(ns2+ns3)){ # LOOP THROUGH ALL STUDIES w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm delta[i,1] <‐ 0 # treatment effect is zero for control arm for (k in 2:na[i]) { # LOOP THROUGH ARMS var[i,k] <‐ pow(se[i,k],2) # calculate variances prec[i,k] <‐ 1/var[i,k] # set precisions } for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific SMD distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of random effects distributions, with multi‐arm trial correction md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # precision of random effects distributions (with multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k # adjustment, multi‐arm RCTs w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # cumulative adjustment for multi‐arm trials sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) } } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) # ranking on relative scale for (k in 1:nt) { rk[k] <‐ nt+1‐rank(d[],k) # assumes higher HRQoL is “good” # rk[k] <‐ rank(d[],k) # assumes higher outcome is “bad” best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Continuous outcome (standardised mean difference) ‐ inconsistency model (random‐effects)
# Normal likelihood, identity link # Trial‐level data given as treatment differences # Random effects model model { for(i in 1:ns) { eff.study[i, t[i,1], t[i,1]] <‐0 for(k in 2:na[i]) { eff.study[i,t[i,k],t[i,1]] <‐eff.des[design[k],t[i,k]] + RE[i,t[i,k]] ‐ RE[i, t[i,1]] } } # Random effects for heterogeneity for(i in 1:ns) { RE[i,1] <‐0 RE[i,2:nt] ˜ dmnorm(zero[], Prec[,]) } # Prec is the inverse of the structured heterogeneity matrix for(i in 1:(nt‐1)) { for(j in 1:(nt‐1)){ Prec[i,j] <‐2*(equals(i,j)‐1/nt)/(tau*tau) } } for(i in 1:ns2) { # LOOP THROUGH 2‐ARM STUDIES y[i,2] ˜ dnorm(delta[i,2],prec[i,2]) # normal likelihood for 2‐arm trials #Deviance contribution for trial i resdev[i] <‐ (y[i,2]‐delta[i,2])*(y[i,2]‐delta[i,2])*prec[i,2] } for(i in (ns2+1):(ns2+ns3)) { # LOOP THROUGH THREE‐ARM STUDIES for (k in 1:(na[i]‐1)) { # set variance‐covariance matrix for (j in 1:(na[i]‐1)) { Sigma[i,j,k] <‐ V[i]*(1‐equals(j,k)) + var[i,k+1]*equals(j,k) } } Omega[i,1:(na[i]‐1),1:(na[i]‐1)] <‐ inverse(Sigma[i,,]) #Precision matrix # multivariate normal likelihood for 3‐arm trials y[i,2:na[i]] ˜ dmnorm(delta[i,2:na[i]],Omega[i,1:(na[i]‐1),1:(na[i]‐1)]) #Deviance contribution for trial i for (k in 1:(na[i]‐1)){ # multiply vector & matrix ydiff[i,k]<‐ y[i,(k+1)] ‐ delta[i,(k+1)] + eff.study[i,t[i,k],t[i,1]] z[i,k]<‐ inprod2(Omega[i,k,1:(na[i]‐1)], ydiff[i,1:(na[i]‐1)]) } resdev[i]<‐ inprod2(ydiff[i,1:(na[i]‐1)], z[i,1:(na[i]‐1)]) } for(i in 1:(ns2+ns3)){ # LOOP THROUGH ALL STUDIES w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm delta[i,1] <‐ 0 # treatment effect is zero for control arm for (k in 2:na[i]) { # LOOP THROUGH ARMS var[i,k] <‐ pow(se[i,k],2) # calculate variances prec[i,k] <‐ 1/var[i,k] # set precisions } for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific SMD distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of random effects distributions, with multi‐arm trial correction md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # precision of random effects distributions (with multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k # adjustment, multi‐arm RCTs w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # cumulative adjustment for multi‐arm trials sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) } } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) for(i in 1:D) { for(k in (offset.design[i] + 1):(offset.design[i] + num.ests[i])) { eff.des[i,t[i,k]] ˜ dnorm(0,0.01) } } } } # *** PROGRAM ENDS
Count outcome
Count outcome ‐ fixed‐effect model
# Poisson likelihood, log link # Fixed effects model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH STUDIES mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dpois(theta[i,k]) # Poisson likelihood theta[i,k] <‐ lambda[i,k]*E[i,k] # failure rate * exposure # model for linear predictor log(lambda[i,k]) <‐ mu[i] + d[t[i,k]] ‐ d[t[i,1]] #Deviance contribution dev[i,k] <‐ 2*((theta[i,k]‐r[i,k]) + r[i,k]*log(r[i,k]/theta[i,k])) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } # pairwise RRs and LRRs for all possible pair‐wise comparisons, if nt>2 for (c in 1:(nt‐1)) { for (k in (c+1):nt) { rater[c,k] <‐ exp(d[k] ‐ d[c]) lrater[c,k] <‐ (d[k]‐d[c]) } } # ranking on relative scale for (k in 1:nt) { # rk[k] <‐ nt+1‐rank(d[],k) # assumes events are “good” rk[k] <‐ rank(d[],k) # assumes events are “bad” best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Count outcome ‐ random‐effects model
# Poisson likelihood, log link # Random effects model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH STUDIES w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm delta[i,1] <‐ 0 # treatment effect is zero for control arm mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dpois(theta[i,k]) # Poisson likelihood theta[i,k] <‐ lambda[i,k]*E[i,k] # failure rate * exposure # model for linear predictor log(lambda[i,k]) <‐ mu[i] + d[t[i,k]] ‐ d[t[i,1]] #Deviance contribution dev[i,k] <‐ 2*((theta[i,k]‐r[i,k]) + r[i,k]*log(r[i,k]/theta[i,k])) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific LOR distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of LOR distributions (with multi‐arm trial correction) md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # precision of LOR distributions (with multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k # adjustment for multi‐arm RCTs w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # cumulative adjustment for multi‐arm trials sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) } } totresdev <‐ sum(resdev[]) # Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) # pairwise ORs and LORs for all possible pair‐wise comparisons, if nt>2 for (c in 1:(nt‐1)) { for (k in (c+1):nt) { or[c,k] <‐ exp(d[k] ‐ d[c]) lor[c,k] <‐ (d[k]‐d[c]) } } # ranking on relative scale for (k in 1:nt) { # rk[k] <‐ nt+1‐rank(d[],k) # assumes events are “good” rk[k] <‐ rank(d[],k) # assumes events are “bad” best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Count outcome ‐ inconsistency model (random‐effects)
# Poisson likelihood, log link, inconsistency model # Random effects model # Treatment by design interactions # ns = number of studies, nt = number of treatments, A = total number of treatment arms in all trials, and D = the number of designs have to be stated. # The main data are arranged with one record per arm: d and study indicate which design and study that arm belongs to, t indicates its treatment, and b indicates the first treatment in that design. r and E are the numbers of successes and exposures in the arm. The supplementary data offset and offset.design list the rows in which the first arm of each trial and of each design is found. model { for(i in 1:ns) { eff.study[i, b[offset[i]], b[offset[i]]] <‐0 for(k in (offset[i] + 1):(offset[i + 1]‐1)) { eff.study[i,t[k],b[k]] <‐eff.des[d[k],t[k]] + RE[i,t[k]] ‐ RE[i,b[k]] } } # Random effects for heterogeneity for(i in 1:ns) { RE[i,1] <‐0 RE[i,2:nt] ˜ dmnorm(zero[], Prec[,]) } # Prec is the inverse of the structured heterogeneity matrix for(i in 1:(nt‐1)) { for(j in 1:(nt‐1)){ Prec[i,j] <‐2*(equals(i,j)‐1/nt)/(tau*tau) } } for(i in 1:A) { r[i] ˜ dpois(theta[i]) # Poisson likelihood theta[i] <‐ lambda[i]*E[i] # failure rate * exposure log(lambda[i]) <‐mu[study[i]] + eff.study[study[i],t[i],b[i]] # model for linear predictor } # For computing DIC for(i in 1:A) { dev[i] <‐ 2*((theta[i]‐r[i]) + r[i]*log(r[i]/theta[i])) } devs <‐sum(dev[]) # Priors for(i in 1:ns) { mu[i] ˜ dnorm(0,0.01) } tau ˜ dunif(0,2) for(i in 1:D) { for(k in (offset.design[i] + 1):(offset.design[i] + num.ests[i])) { eff.des[i,t[k]] ˜ dnorm(0,0.01) } } } # *** PROGRAM ENDS
Time‐to‐event outcome
Time‐to‐event outcome ‐ fixed‐effect model
# Binomial likelihood, cloglog link # Fixed effects model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH STUDIES mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dbin(p[i,k],n[i,k]) # Binomial likelihood # model for linear predictor cloglog(p[i,k]) <‐ log(time[i]) + mu[i] + d[t[i,k]] ‐ d[t[i,1]] rhat[i,k] <‐ p[i,k] * n[i,k] # expected value of the numerators #Deviance contribution dev[i,k] <‐ 2 * (r[i,k] * (log(r[i,k])‐log(rhat[i,k])) + (n[i,k]‐r[i,k]) * (log(n[i,k]‐r[i,k]) ‐ log(n[i,k]‐rhat[i,k]))) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero for control arm # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } # ranking on relative scale for (k in 1:nt) { # rk[k] <‐ rank(d[],k) # assumes lower is better rk[k] <‐ nt+1‐rank(d[],k) # assumes lower outcome is worse best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Time‐to‐event outcome ‐ random‐effects model
# Binomial likelihood, cloglog link # Random effects model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH STUDIES w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm delta[i,1] <‐ 0 # treatment effect is zero for control arm mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dbin(p[i,k],n[i,k]) # Binomial likelihood # model for linear predictor cloglog(p[i,k]) <‐ log(time[i]) + mu[i] + delta[i,k] rhat[i,k] <‐ p[i,k] * n[i,k] # expected value of the numerators #Deviance contribution dev[i,k] <‐ 2 * (r[i,k] * (log(r[i,k])‐log(rhat[i,k])) + (n[i,k]‐r[i,k]) * (log(n[i,k]‐r[i,k]) ‐ log(n[i,k]‐rhat[i,k]))) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific LOR distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of LOR distributions, with multi‐arm trial correction md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # precision of LOR distributions (with multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k # adjustment, multi‐arm RCTs w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # cumulative adjustment for multi‐arm trials sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) } } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) # ranking on relative scale for (k in 1:nt) { # rk[k] <‐ rank(d[],k) # assumes lower is better rk[k] <‐ nt+1‐rank(d[],k) # assumes lower outcome is worse best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Time‐to‐event outcome ‐ inconsistency model (random‐effects)
# Binomial likelihood, cloglog link, inconsistency model # Random effects model # Treatment by design interactions # ns = number of studies, nt = number of treatments, A = total number of treatment arms in all trials, and D = the number of designs have to be stated. # The main data are arranged with one record per arm: d and study indicate which design and study that arm belongs to, t indicates its treatment, and b indicates the first treatment in that design. r ,n, and time are the numbers of events, individuals, and follow‐up time in the arm. The supplementary data offset and offset.design list the rows in which the first arm of each trial and of each design is found. model { for(i in 1:ns) { eff.study[i, b[offset[i]], b[offset[i]]] <‐0 for(k in (offset[i] + 1):(offset[i + 1]‐1)) { eff.study[i,t[k],b[k]] <‐eff.des[d[k],t[k]] + RE[i,t[k]] ‐ RE[i,b[k]] } } # Random effects for heterogeneity for(i in 1:ns) { RE[i,1] <‐0 RE[i,2:nt] ˜ dmnorm(zero[], Prec[,]) } # Prec is the inverse of the structured heterogeneity matrix for(i in 1:(nt‐1)) { for(j in 1:(nt‐1)){ Prec[i,j] <‐2*(equals(i,j)‐1/nt)/(tau*tau) } } for(i in 1:A) { r[i] ˜ dbin(p[i],n[i]) # Binomial likelihood cloglog(p[i]) <‐ log(time[i]) + mu[study[i]] + eff.study[study[i],t[i],b[i]] # model for linear predictor } # For computing DIC for(i in 1:A) { dev[i] <‐ 2 * (r[i] * (log(r[i])‐log(rhat[i]))+ (n[i]‐r[i]) * (log(n[i]‐r[i]) ‐ log(n[i]‐rhat[i]))) } devs <‐sum(dev[]) # Priors for(i in 1:ns) { mu[i] ˜ dnorm(0,0.01) } tau ˜ dunif(0,2) for(i in 1:D) { for(k in (offset.design[i] + 1):(offset.design[i] + num.ests[i])) { eff.des[i,t[k]] ˜ dnorm(0,0.01) } } } # *** PROGRAM ENDS
Appendix 10. Technical details of network meta‐analysis
The posterior probabilities (effect estimates or values) of the treatment contrast (i.e. log odds ratio, mean difference, standardised mean difference, rate ratio, or hazard ratio) may vary depending upon the initial values to start the simulations. In order to control the random error due to the choice of initial values, we performed the network analysis for three different initial values (starting values) as per the guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) documents (Dias 2014). If the results from three different initial values are similar and stable (convergence), then the results are reliable. It is important to discard the results of the initial simulations as they can be significantly affected by the choice of the priors and only include the results of the simulations obtained after the convergence. The discarding of the initial simulations is called 'burn in'. We planned to run the models for all outcomes for 30,000 simulations for 'burn in' for three different chains (a set of initial values). We planned to run the models for another 100,000 simulations to obtain the effect estimates. We planned to obtain the effect estimates from the results of all the three chains (different initial values). We planned to ensure that the results in the three different chains were similar in order to control for random error due to the choice of initial values. We planned to do this in addition to the visual inspection of convergence obtained after simulations in the burn in. In order to avoid the influence of the priors chosen in the model, we planned to use non‐informative priors.
We planned to run three different models for each outcome. Fixed‐effect model assumes that the treatment effect is the same across studies. The random‐effects consistency model assumes that the treatment effect is distributed normally across the studies but assumes that the transitivity assumption is satisfied (i.e. the population studied, the definition of outcomes, and the methods used were similar across studies and that there is consistency between the direct comparison and indirect comparison). A random‐effects inconsistency model does not assume transitivity assumption. If the inconsistency model resulted in a better model fit than the consistency model, the results of the network meta‐analysis can be unreliable and so should be interpreted with extreme caution. If there was evidence of inconsistency, we planned to identify areas in the network where substantial inconsistency might be present in terms of clinical and methodological diversities between trials and, when appropriate, limit network meta‐analysis to a more compatible subset of trials.
We planned to base the choice of the model between fixed‐effect model and random‐effects model on the model fit as per the guidelines of the NICE TSU (Dias 2014). We planned to assess the model fit by deviance residuals and deviance information criteria (DIC) according to NICE TSU guidelines (Dias 2014). A difference of three or five in the DIC is not generally considered important (Dias 2012c). We planned to use the simpler model (i.e. fixed‐effect model was used since the DIC was similar between the fixed‐effect model and random‐effects model). We planned to use the random‐effects model if it resulted in a better model fit as indicated by a DIC lower than that of fixed‐effect model by at least three.
We planned to calculate the effect estimates of the treatment and the 95% credible intervals using the following additional code. # pairwise ORs and MD for all possible pair‐wise comparisons, if nt>2 for (c in 1:(nt‐1)) { for (k in (c+1):nt) { OR[c,k] <‐ exp(d[k] ‐ d[c]) #MD[c,k] <‐ (d[k]‐d[c]) } }
where c indicates control group, k indicates intervention group, OR indicates odds ratio or other ratios, and MD indicates mean difference or other differences.
Appendix 11. Winbugs code for subgroup analysis
Source of code: Dias 2012c.
Categorical covariate
Only the code for random‐effects model for a binary outcome is shown. The differences in the code are underlined. We planned to make similar changes for other outcomes.
# Binomial likelihood, logit link, subgroup # Random effects model for multi‐arm trials model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH STUDIES w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm delta[i,1] <‐ 0 # treatment effect is zero for control arm mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dbin(p[i,k],n[i,k]) # binomial likelihood # model for linear predictor, covariate effect relative to treat in arm 1 logit(p[i,k]) <‐ mu[i] + delta[i,k] + (beta[t[i,k]]‐beta[t[i,1]]) * x[i] rhat[i,k] <‐ p[i,k] * n[i,k] # expected value of the numerators #Deviance contribution dev[i,k] <‐ 2 * (r[i,k] * (log(r[i,k])‐log(rhat[i,k])) + (n[i,k]‐r[i,k]) * (log(n[i,k]‐r[i,k]) ‐ log(n[i,k]‐rhat[i,k]))) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific LOR distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of LOR distributions (with multi‐arm trial correction) md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # precision of LOR distributions (with multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k # adjustment for multi‐arm RCTs w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # cumulative adjustment for multi‐arm trials sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) } } totresdev <‐ sum(resdev[]) # Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment beta[1] <‐ 0 # covariate effect is zero for reference treatment for (k in 2:nt){ # LOOP THROUGH TREATMENTS d[k] ˜ dnorm(0,.0001) # vague priors for treatment effects beta[k] <‐ B[k] # exchangeable covariate effect B[k] ˜ dnorm(0,.0001) # vague prior for covariate effect } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) # treatment effect when covariate = z[j] for (k in 1:nt){ # LOOP THROUGH TREATMENTS for (j in 1:nz) { dz[j,k] <‐ d[k] + (beta[k]‐beta[1])*z[j] } } # *** PROGRAM ENDS
Continuous covariate
# Binomial likelihood, logit link, continuous covariate # Random effects model for multi‐arm trials model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH STUDIES w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm delta[i,1] <‐ 0 # treatment effect is zero for control arm mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dbin(p[i,k],n[i,k]) # binomial likelihood # model for linear predictor, covariate effect relative to treat in arm 1 logit(p[i,k]) <‐ mu[i] + delta[i,k] + (beta[t[i,k]]‐beta[t[i,1]]) * (x[i]‐mx) rhat[i,k] <‐ p[i,k] * n[i,k] # expected value of the numerators #Deviance contribution dev[i,k] <‐ 2 * (r[i,k] * (log(r[i,k])‐log(rhat[i,k])) + (n[i,k]‐r[i,k]) * (log(n[i,k]‐r[i,k]) ‐ log(n[i,k]‐rhat[i,k]))) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific LOR distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of LOR distributions (with multi‐arm trial correction) md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # precision of LOR distributions (with multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k # adjustment for multi‐arm RCTs w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # cumulative adjustment for multi‐arm trials sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) } } totresdev <‐ sum(resdev[]) # Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment beta[1] <‐ 0 # covariate effect is zero for reference treatment for (k in 2:nt){ # LOOP THROUGH TREATMENTS d[k] ˜ dnorm(0,.0001) # vague priors for treatment effects beta[k] <‐ B[k] # exchangeable covariate effect B[k] ˜ dnorm(0,.0001) # vague prior for covariate effect } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) # treatment effect when covariate = z[j] (un‐centring treatment effects) for (k in 1:nt){ for (j in 1:nz) { dz[j,k] <‐ d[k] ‐ (beta[k]‐beta[1])*(mx‐z[j]) } } # pairwise ORs and LORs for all possible pair‐wise comparisons, if nt>2 for (c in 1:(nt‐1)) { for (k in (c+1):nt) { # at mean value of covariate or[c,k] <‐ exp(d[k] ‐ d[c]) lor[c,k] <‐ (d[k]‐d[c]) # at covariate=z[j] for (j in 1:nz) { orz[j,c,k] <‐ exp(dz[j,k] ‐ dz[j,c]) lorz[j,c,k] <‐ (dz[j,k]‐dz[j,c]) } } } } # *** PROGRAM ENDS
Appendix 12. Indirect comparisons
Indirect comparisons were possible for three outcomes: short‐term mortality, serious adverse events (number), and adverse events (number) for the comparison between the minimally invasive step‐up approach and peritoneal lavage (Figure 19). Indirect comparisons could not be performed for the remaining outcomes because of the lack of any trial reporting the outcome (long‐term mortality, infected pancreatic necrosis, health‐related quality of life, proportion of people with adverse events, requirement for additional invasive intervention, time to return to normal activity, and time to return to work), presence of only one trial for the outcome (proportion of people with serious adverse events, total number of treatments, and costs), absence of a common comparator in the trials allowing indirect comparison (organ failure), or lack of data in a format that could be meta‐analysed (length of hospital stay and length of intensive therapy unit stay).
4.
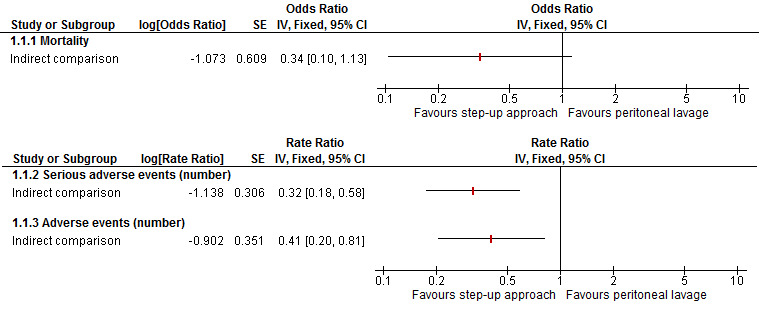
Indirect comparison of minimally invasive step‐up approach versus peritoneal lavage for the outcomes where indirect comparisons was possible.
There was no statistically significant difference in the short‐term mortality between the two groups (odds ratio 0.34, 95% confidence interval (CI) 0.10 to 1.13). The number of serious adverse events and adverse events were fewer in the minimally invasive step‐up approach than peritoneal lavage (serious adverse events: rate ratio 0.32, 95% CI 0.18 to 0.58 and adverse events: rate ratio 0.41, 95% CI 0.20 to 0.81).
Data and analyses
Comparison 1. Interventions for necrotising pancreatitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term mortality | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Peritoneal lavage vs. open necrosectomy | 3 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.73, 4.94] |
| 1.2 Minimally invasive step‐up approach vs. open necrosectomy | 2 | 160 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.32, 1.34] |
| 1.3 Delayed open necrosectomy vs. early open necrosectomy | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.38] |
| 1.4 Minimally invasive step‐up approach: video‐assisted vs. endoscopic | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.5 [0.41, 49.08] |
| 1.5 Minimally invasive step‐up approach: planned surgery vs. continued percutaneous drainage | 1 | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | 21.0 [0.64, 689.99] |
| 2 Serious adverse events (proportion) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Minimally invasive step‐up approach vs. open necrosectomy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serious adverse events (number) | 4 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Peritoneal lavage vs. open necrosectomy | 2 | 56 | Rate Ratio (Fixed, 95% CI) | 1.28 [0.92, 1.78] |
| 3.2 Minimally invasive step‐up approach vs. open necrosectomy | 1 | 88 | Rate Ratio (Fixed, 95% CI) | 0.41 [0.25, 0.68] |
| 3.3 Minimally invasive step‐up approach: video‐assisted vs. endoscopic | 1 | 22 | Rate Ratio (Fixed, 95% CI) | 12.55 [0.72, 219.54] |
| 4 Organ failure | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Minimally invasive step‐up approach vs. open necrosectomy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Minimally invasive step‐up approach: video‐assisted vs. endoscopic | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse events (number) | 3 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 5.1 Peritoneal lavage vs. open necrosectomy | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Minimally invasive step‐up approach vs. open necrosectomy | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Minimally invasive step‐up approach: video‐assisted vs. endoscopic | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Length of hospital stay | Other data | No numeric data | ||
| 6.1 Peritoneal lavage vs. open necrosectomy | Other data | No numeric data | ||
| 6.2 Minimally invasive step‐up approach vs. open necrosectomy | Other data | No numeric data | ||
| 6.3 Minimally invasive step‐up approach: video‐assisted vs. endoscopic | Other data | No numeric data | ||
| 7 Length of intensive therapy unit stay | Other data | No numeric data | ||
| 7.1 Peritoneal lavage vs. open necrosectomy | Other data | No numeric data | ||
| 7.2 Minimally invasive step‐up approach vs. open necrosectomy | Other data | No numeric data | ||
| 8 Number of treatments | Other data | No numeric data | ||
| 8.3 Minimally invasive step‐up approach: video‐assisted vs. endoscopic | Other data | No numeric data | ||
| 9 Costs | Other data | No numeric data | ||
| 9.1 Minimally invasive step‐up approach vs. open necrosectomy | Other data | No numeric data |
Comparison 2. Interventions for necrotising pancreatitis ‐ subgroup analysis and sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term mortality (infected pancreatic necrosis only) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Minimally invasive step‐up approach vs. open necrosectomy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Minimally invasive step‐up approach: video‐assisted vs. endoscopic | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Minimally invasive step‐up approach: planned surgery vs. continued percutaneous drainage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Serious adverse events (number) (infected pancreatic necrosis only) | 2 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Early minimally invasive step‐up approach vs. early open necrosectomy | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Early minimally invasive step‐up approach: video‐assisted vs. endoscopic | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Organ failure (infected pancreatic necrosis only) | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Early minimally invasive step‐up approach vs. early open necrosectomy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Early minimally invasive step‐up approach: video‐assisted vs. endoscopic | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term mortality (routine antibiotic use only) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Peritoneal lavage vs. early open necrosectomy | 2 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.49, 4.90] |
| 4.2 Delayed open necrosectomy vs. early open necrosectomy | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.38] |
| 5 Serious adverse events (number) (routine antibiotic use only) | 2 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 Peritoneal lavage vs. early open necrosectomy | 2 | Rate Ratio (Fixed, 95% CI) | 1.28 [0.92, 1.78] | |
| 6 Short‐term mortality: sensitivity analysis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Delayed open necrosectomy vs. early open necrosectomy (per‐protocol analysis) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Delayed open necrosectomy vs. early open necrosectomy (intention‐to‐treat: best‐best scenario) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Delayed open necrosectomy vs. early open necrosectomy (intention‐to‐treat: best‐worst scenario) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Delayed open necrosectomy vs. early open necrosectomy (intention‐to‐treat: worst‐best scenario) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Delayed open necrosectomy vs. early open necrosectomy (intention‐to‐treat: worst‐worst scenario) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bakker 2012.
| Methods | Randomised controlled trial | |
| Participants | Country: The Netherlands Number randomised: 22 Post‐randomisation exclusions: 0 (0%) Revised sample size: 22 Mean age: 63 years Females: 6 (27.3%) Mean duration of follow‐up: 12 months Inclusion criteria Adults needing necrosectomy for suspected or confirmed infected necrotising pancreatitis who could undergo both endoscopic or surgical necrosectomy, based on CT imaging Exclusion criteria Previous surgical or endoscopic necrosectomy Previous exploratory laparotomy Pancreatitis as a consequence of abdominal surgery A flare‐up of chronic pancreatitis Abdominal compartment syndrome Perforation of a visceral organ Bleeding as indication for intervention |
|
| Interventions | Participants were randomly assigned to 1 of 2 groups Group 1: minimally invasive step‐up approach (video‐assisted) (n = 12) Group 2: minimally invasive step‐up approach (endoscopic) (n = 10) Open necrosectomy was performed if minimal access necrosectomy was unsuccessful |
|
| Outcomes | Mortality, complications, hospital stay, number of necrosectomies | |
| Notes | Clinical endpoints were reported for 2 participants who were excluded from analysis of laboratory parameters and were included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An adjudication committee consisting of 5 gastrointestinal surgeons and 2 gastroenterologists independently reviewed all clinical end points and performed a blinded outcome assessment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were included for clinical outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported |
| Other bias | Low risk | Quote: "Dr Bakker is sponsored by grant number ZonMw 17099.2902 from the Netherlands Organization for Health Research and Development to perform clinical studies on necrotizing pancreatitis" Comment: no source of funding bias or any other bias |
Kivilaakso 1984.
| Methods | Randomised controlled trial | |
| Participants | Country: Finland. Number randomised: 35 Post‐randomisation exclusions: 0 (0%) Revised sample size: 35 Mean age: 37 years Females: 5 (14.3%) Mean duration of follow‐up: not stated Inclusion criteria People with acute fulminant (haemorrhagic) pancreatitis Exclusion criteria People with oedematous pancreatitis |
|
| Interventions | Participants were randomly assigned to 1 of 2 groups Group 1: open necrosectomy (n = 18) A subtotal pancreatectomy was performed in the open necrosectomy group Group 2: peritoneal lavage (n = 17) |
|
| Outcomes | Mortality, complications, hospital stay, intensive therapy unit stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "After establishment of the clinical diagnosis of acute fulminant pancreatitis, the patient was subjected to laparotomy where the clinical diagnosis was confirmed, whereafter the patient was allocated at random to either pancreatic resection or peritoneal lavation group by the method of supernumerally sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were included for analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported |
| Other bias | Unclear risk | Comment: source of funding was not available. There was no other bias |
Litvin 2010.
| Methods | Randomised controlled trial | |
| Participants | Country: Republic of Belarus Number randomised: 72 Post‐randomisation exclusions: not stated Revised sample size: 72 Mean age: not stated Females: not stated Mean duration of follow‐up: not stated Inclusion criteria People with acute necrotising pancreatitis |
|
| Interventions | Participants were randomly assigned to 1 of 2 groups Group 1: minimally invasive step‐up approach (n = 37) Group 2: open necrosectomy (n = 35) Minimally invasive step‐up approach: initially minimally invasive necrosectomy (further details not available) followed by open necrosectomy if necessary |
|
| Outcomes | Mortality, complications, hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported |
| Other bias | Unclear risk | Comment: source of funding was not available. There was no other bias |
Maroske 1981.
| Methods | Randomised controlled trial | |
| Participants | Country: Germany Number randomised: 24 Post‐randomisation exclusions: not stated Revised sample size: 24 Mean age: not stated Females: not stated Mean duration of follow‐up: not stated Inclusion criteria People with acute haemorrhagic (necrotising) pancreatitis |
|
| Interventions | Participants were randomly assigned to 1 of 2 groups Group 1: open necrosectomy (n = 12) Pancreatic resection was performed as necessary Group 2: peritoneal lavage (n = 12) Not clear whether the peritoneal lavage was performed percutaneously or by open surgery |
|
| Outcomes | Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | High risk | Comment: complications were not reported |
| Other bias | Unclear risk | Comment: source of funding was not available. There was no other bias |
Mier 1997.
| Methods | Randomised controlled trial | |
| Participants | Country: Mexico Number randomised: 41 Post‐randomisation exclusions: 5 (12.2%) Revised sample size: 36 Mean age: 40 years Females: 14 (38.9%) Mean duration of follow‐up: not stated Inclusion criteria People with fulminant necrotising pancreatitis |
|
| Interventions | Participants were randomly assigned to 1 of 2 groups Group 1: early open necrosectomy (n = 25) Group 2: delayed open necrosectomy (n = 11) Necrosectomy was delayed by at least 12 days In both groups, planned re‐laparotomies were performed |
|
| Outcomes | Mortality | |
| Notes | Reasons for post‐randomisation exclusions: 1 from early group because the participant had received medical treatment initially by error; 3 from delayed group who did not require surgery and resolved by conservative treatment; 1 from delayed group because of mesenteric ischaemia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation exclusions |
| Selective reporting (reporting bias) | High risk | Comment: complications were not reported |
| Other bias | Unclear risk | Comment: source of funding was not available. There was no other bias |
Schroder 1991.
| Methods | Randomised controlled trial | |
| Participants | Country: Finland Number randomised: 21 Post‐randomisation exclusions: not stated Revised sample size: 21 Mean age: 38 years Females: 2 (9.5%) Mean duration of follow‐up: not stated Inclusion criteria People under 50 years of age with fulminant acute pancreatitis resulting from alcohol abuse |
|
| Interventions | Participants were randomly assigned to 1 of 2 groups Group 1: open necrosectomy (n = 11) Pancreatic resection was performed as necessary Group 2: peritoneal lavage (n = 10) Peritoneal lavage was performed using a minilaparotomy |
|
| Outcomes | Mortality, complications, hospital stay, intensive therapy unit stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "After establishment of the clinical diagnosis of acute fulminant pancreatitis, the patient was subjected to laparotomy where the clinical diagnosis was confirmed, whereafter the patient was allocated at random to either pancreatic resection or peritoneal lavation group by the method of supernumerally sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all patients were included for analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported |
| Other bias | Unclear risk | Comment: source of funding was not available. There was no other bias |
Shenvi 2014.
| Methods | Randomised controlled trial | |
| Participants | Country: India Number randomised: 8 Post‐randomisation exclusions: not stated Revised sample size: 8 Mean age: not stated Females: not stated Mean duration of follow‐up: not stated Inclusion criteria People with diagnosis of infectious pancreatic necrosis managed with percutaneous catheter drainage for 10‐15 days and people who did not show significant improvement on percutaneous catheter drainage |
|
| Interventions | Participants were randomly assigned to 1 of 2 groups Group 1: minimally invasive step‐up approach (planned surgery) (n = 4) Group 2: minimally invasive step‐up approach (continued percutaneous drainage) (n = 4) |
|
| Outcomes | Mortality | |
| Notes | Authors provided reply to our request for further information in September 2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Based on table of random numbers at a block of 4 patients" (author reply) |
| Allocation concealment (selection bias) | Low risk | Quote: "Covered envelopes used" (author reply) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Since there was difference in the timing of intervention it was not possible to blind the patient and healthcare providers after allocation" (author reply) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Since there was difference in the timing of intervention it was not possible to blind the patient and healthcare providers after allocation" (author reply) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation exclusions |
| Selective reporting (reporting bias) | High risk | Quote: morbidity was not measured (author reply) |
| Other bias | Low risk | Quote: "This study was a part of MCh(Surgical Gastroenterology) curriculum and was funded by the institution" Comment: no source of funding bias or any other bias |
Van Santvoort 2010b.
| Methods | Randomised controlled trial | |
| Participants | Country: the Netherlands Number randomised: 88 Post‐randomisation exclusions: 0 (0%) Revised sample size: 88 Mean age: 57 years Females: 24 (27.3%) Mean duration of follow‐up: 12 months (13.6%) Inclusion criteria People with suspected or confirmed infected necrotising pancreatitis Exclusion criteria Flare‐up of chronic pancreatitis Previous exploratory laparotomy during the current episode of pancreatitis Previous drainage or surgery for confirmed or suspected infected necrosis Pancreatitis caused by abdominal surgery An acute intraabdominal event (e.g. perforation of a visceral organ, bleeding, or the abdominal compartment syndrome) |
|
| Interventions | Participants were randomly assigned to 1 of 2 groups Group 1: minimally invasive step‐up approach (n = 43) Group 2: open necrosectomy (n = 45) Minimally invasive step‐up: initially percutaneous or transgastric drainage was performed followed by video‐assisted necrosectomy after 2 attempts at percutaneous or transgastric drainage |
|
| Outcomes | Mortality, complications, hospital stay, intensive therapy unit stay, costs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed centrally by the study coordinator" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A blinded outcome assessment was performed by an adjudication committee consisting of eight experienced gastrointestinal surgeons who independently reviewed all data regarding complications" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were included for clinical outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported |
| Other bias | Low risk | Quote: "Supported by a grant (945‐06‐910) from the Dutch Organization for Health Research and Development" Comment: no source of funding bias or any other bias |
CT: computed tomography; n: number of participants.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ai 2010 | Not in acute necrotising pancreatitis |
| Amorotti 1998 | Not a randomised controlled trial |
| Armbruster 1998 | Comment on an included trial (Mier 1997) |
| Balldin 1983 | Not in acute necrotising pancreatitis |
| Brand 2010 | Comment on an included trial (Van Santvoort 2010b) |
| Connor 2005 | Not a randomised controlled trial |
| Cooper 1982 | Not a randomised controlled trial |
| Dronov 2009 | Not a randomised controlled trial |
| Ihse 1986 | Not in acute necrotising pancreatitis |
| Krautzberger 1985 | Not a randomised controlled trial |
| Levi 2010 | Comment on an included trial (Van Santvoort 2010b) |
| Mayer 1985 | Not in acute necrotising pancreatitis |
| Pascual 2013 | Not a randomised controlled trial |
| Radenkovic 2010 | Not in acute necrotising pancreatitis |
| Ranson 1976 | Not in acute necrotising pancreatitis |
| Ranson 1990 | Quasi‐randomised study (based on chart number) |
| Schroder 1990 | Not a randomised controlled trial |
| Teerenhovi 1989 | Not a randomised controlled trial |
| Van Santvoort 2011 | Not a randomised controlled trial |
Characteristics of ongoing studies [ordered by study ID]
van Brunschot 2013.
| Trial name or title | TENSION trial |
| Methods | Randomised controlled trial |
| Participants | People with infected pancreatic necrosis |
| Interventions | Intervention: minimally invasive step‐up approach (endoscopic): initial endoscopic transgastric drainage followed by endoscopic transgastric necrosectomy if necessary Control: minimally invasive step‐up approach (surgical): initial percutaneous drainage followed by video‐assisted surgical necrosectomy or open surgical necrosectomy |
| Outcomes | Mortality, major complications, health‐related quality of life, requirement for additional intervention, length of hospital stay, length of intensive therapy unit stay, costs |
| Starting date | 2011 |
| Contact information | s.vanbrunschot@pancreatitis.nl |
| Notes | Recruitment has been completed and currently follow‐up information is being collected |
Differences between protocol and review
We revised delayed necrosectomy to any period beyond three days of diagnosis. Our initial choice of definition was an arbitrary decision. In the included trial, delayed necrosectomy was defined as necrosectomy performed after a minimum of 12 days of onset.
We added peritoneal lavage as one of the treatment arms as this was one of the treatments used for the management of people with necrotising pancreatitis.
We included costs within six months since the costs were reported for six months rather than three months. We included this information since the costs within six months were related to the treatment costs and loss of productivity resulting from necrotising pancreatitis.
While network meta‐analysis has its advantages in combining direct and indirect evidence (resulting in more precise evidence) and Bayesian network meta‐analysis allows calculation of probability of being best treatments, these advantages were limited in this review because of the sparse data, lack of direct and indirect evidence for any comparisons, and concerns about the transitivity assumption. Therefore, we used Frequentist methods, which allowed presentation of information in the standard Cochrane format for direct comparisons, and we presented indirect comparisons in Appendix 12.
Contributions of authors
Conceiving the review: KG.
Designing the review: KG.
Co‐ordinating the review: KG.
Designing search strategies: KG.
Study selection: KG, AB, AH.
Data extraction: KG, AB, AH.
Writing the review: KG.
Providing general advice on the review: BRD, SP.
Securing funding for the review: KG.
Performing previous work that was the foundation of the current study: KG.
Sources of support
Internal sources
University College London, UK.
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure, Cochrane Programme Grant, or Cochrane Incentive funding to the Cochrane Hepato‐Biliary and Upper Gastrointestinal and Pancreatic Diseases Groups. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, the National Health Service, or the Department of Health. The funding covered salary, equipment, and other resources to complete this review.
Declarations of interest
This report was independently research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ Evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the review author(s) and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health.
New
References
References to studies included in this review
Bakker 2012 {published data only}
- Bakker J, Santvoort HC, Brunschot S. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. Zentralblatt für Chirurgie 2012;137(3):201‐2. [DOI] [PubMed] [Google Scholar]
- Bakker OJ, Santvoort HC, Brunschot S, Geskus RB, Besselink MG, Bollen TL, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012;307(10):1053‐61. [DOI] [PubMed] [Google Scholar]
Kivilaakso 1984 {published data only}
- Kivilaakso E, Lempinen M, Makelainen A. Pancreatic resection versus peritoneal lavation for acute fulminant pancreatitis. A randomized prospective study. Annals of Surgery 1984;199(4):426‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Litvin 2010 {published data only}
- Litvin A, Khokha V. Stepped approach in the treatment of severe acute pancreatitis. Pancreatology 2010;10 (2‐3):352. [Google Scholar]
Maroske 1981 {published data only}
- Maroske D. Acute hemorrhagic necrotizing pancreatitis ‐ laparotomy or peritoneal‐lavage ‐ results of a prospective controlled randomized clinical‐study. Medecine & Chirurgie Digestives 1981;10(5):402‐7. [Google Scholar]
Mier 1997 {published data only}
- Mier J, Leon EL, Castillo A, Robledo F, Blanco R. Early versus late necrosectomy in severe necrotizing pancreatitis. American Journal of Surgery 1997;173(2):71‐5. [DOI] [PubMed] [Google Scholar]
Schroder 1991 {published data only}
- Schroder T, Sainio V, Kivisaari L, Puolakkainen P, Kivilaakso E, Lempinen M. Pancreatic resection versus peritoneal lavage in acute necrotizing pancreatitis: a prospective randomized trial. Annals of Surgery 1991;214(6):663‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shenvi 2014 {published data only}
- Shenvi S, Gupta R, Singh R, Khullar M, Kang M, Bhasin D. Timing of surgical intervention after percutaneous catheter drainage (PCD) in step up approach of severe acute pancreatitis. HPB 2014;16(Suppl 2):82. [DOI] [PubMed] [Google Scholar]
- Shenvi SD, Gupta R, Singh R, Khullar M, Kang M, Rana SS, et al. Timing of surgical intervention after percutaneous catheter drainage (PCD) in step up approach of severe acute pancreatitis. Gastroenterology 2014;146(5 Suppl 1):S‐1019. [Google Scholar]
Van Santvoort 2010b {published data only}
- Besselink MG, Santvoort HC, Nieuwenhuijs VB, Boermeester MA, Bollen TL, Buskens E, et al. Minimally invasive 'step‐up approach' versus maximal necrosectomy in patients with acute necrotising pancreatitis (PANTER trial): design and rationale of a randomised controlled multicenter trial [isrctn13975868]. BMC Surgery 2006;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et al. A step‐up approach or open necrosectomy for necrotizing pancreatitis. New England Journal of Medicine 2010;362(16):1491‐502. [DOI] [PubMed] [Google Scholar]
- Santvoort HC, Besselink MG, Cirkel GA, Gooszen HG. A nationwide Dutch study into the optimal treatment of patients with infected necrotising pancreatitis: the PANTER trial [Landelijk onderzoek naar optimale behandeling van patienten met geinfecteerde necrotiserende pancreatitis: PANTER‐trial]. Nederlands Tijdschrift voor Geneeskunde 2006;150(33):1844‐6 (Dutch). [PubMed] [Google Scholar]
- Santvoort HC, Besselink MG, Gooszen HG. Obtaining medical ethical approval for a multicentre, randomised study: prospective evaluation of a ponderous process. Nederlands Tijdschrift voor Geneeskunde 2008;152(38):2077‐83. [PubMed] [Google Scholar]
References to studies excluded from this review
Ai 2010 {published data only}
- Ai X, Qian X, Pan W, Xu J, Hu W, Terai T, et al. Ultrasound‐guided percutaneous drainage may decrease the mortality of severe acute pancreatitis. Journal of Gastroenterology 2010;45(1):77‐85. [DOI] [PubMed] [Google Scholar]
Amorotti 1998 {published data only}
- Amorotti C, Mosca D, Palladino L, Rossi A, Cioni G. The role of surgery in severe acute pancreatitis. Indications and timing from a prospective study [Italian]. Chirurgia 1998;11(5):311‐7. [Google Scholar]
Armbruster 1998 {published data only}
- Armbruster C, Kriwanek S. Early versus late necrosectomy in severe necrotizing pancreatitis. American Journal of Surgery 1998;175(4):341. [PubMed] [Google Scholar]
Balldin 1983 {published data only}
- Balldin G, Borgström A, Genell S, Ohlsson K. The effect of peritoneal lavage and aprotinin in the treatment of severe acute pancreatitis. Research in Experimental Medicine. Zeitschrift fur die Gesamte Experimentelle Medizin Einschliesslich Experimenteller Chirurgie 1983;183(3):203‐13. [DOI] [PubMed] [Google Scholar]
Brand 2010 {published data only}
- Brand M, Maier SKG. Acute necrotizing pancreatitis: minimally invasive stage treatment or open necrosectomy? Results of a randomized, prospective multicenter study [German]. Medizinische Klinik 2010;105(7):506‐7. [Google Scholar]
Connor 2005 {published data only}
- Connor S, Raraty MGT, Howes N, Evans J, Ghaneh P, Sutton R, et al. Surgery in the treatment of acute pancreatitis ‐ minimal access pancreatic necrosectomy. Scandinavian Journal of Surgery 2005;94(2):135‐42. [DOI] [PubMed] [Google Scholar]
Cooper 1982 {published data only}
- Cooper MJ, Williamson RC, Pollock AV. The role of peritoneal lavage in the prediction and treatment of severe acute pancreatitis. Annals of the Royal College of Surgeons of England 1982;64(6):422‐7. [PMC free article] [PubMed] [Google Scholar]
Dronov 2009 {published data only}
- Dronov OI, Koval's'ka IO, Kovalenko AP. Dynamic laparoscopy and staged necrectomy of the retroperitoneal space in patients with acute necrotizing pancreatitis. Klinichna Khirurhiia 2009;7‐8:21‐2. [PubMed] [Google Scholar]
Ihse 1986 {published data only}
- Ihse I, Evander A, Gustafson I, Holmberg JT. Influence of peritoneal lavage on objective prognostic signs in acute pancreatitis. Annals of Surgery 1986;204(2):122‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krautzberger 1985 {published data only}
- Krautzberger W, Buchler M, Block S, Beger HG. Necrotizing pancreatitis: peritoneal lavage or lavage of the lesser sac?. Langenbecks Archiv für Chirurgie 1985;366(1):612. [Google Scholar]
Levi 2010 {published data only}
- Levi M. Step‐up less invasive approach in necrotizing pancreatitis is better than standard laparotomy. Nederlands Tijdschrift voor Geneeskunde 2010;154(21):1018. [Google Scholar]
Mayer 1985 {published data only}
- Mayer AD, McMahon MJ, Corfield AP, Cooper MJ, Williamson RC, Dickson AP, et al. Controlled clinical trial of peritoneal lavage for the treatment of severe acute pancreatitis. New England Journal of Medicine 1985;312(7):399‐404. [DOI] [PubMed] [Google Scholar]
- Mayer AD, McMahon MJ, Corfield AP, Cooper MJ, Williamson RCN, Dixon AP, et al. A randomized trial of peritoneal‐lavage for the treatment of severe acute‐pancreatitis. Gastroenterology 1984;86(5):1178. [Google Scholar]
- Mayer AD, McMahon MJ, Corfield AP, Cooper MJ, Williamson RCN, Dixon AP, et al. Therapeutic peritoneal lavage in patients with severe acute pancreatitis. Gut 1984;25(Suppl 2):A579. [Google Scholar]
Pascual 2013 {published data only}
- Pascual I, Sabater L, Anon R, Calvete J, Pacheco G, Munoz E, et al. Surgical versus nonsurgical treatment of infected pancreatic necrosis: more arguments to change the paradigm. Journal of Gastrointestinal Surgery 2013;17(9):1627‐33. [DOI] [PubMed] [Google Scholar]
Radenkovic 2010 {published data only}
- Radenkovic D, Bajec D, Gregoric P, Jeremic V, Ivancevic N, Bumbasirevic V, et al. Decompressive laparotomy versus percutaneous puncture in abdominal compartment syndrome in acute pancreatitis: background and design of randomised study. Pancreatology 2010;10 (2‐3):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radenkovic D, Bajec D, Ivancevic N, Gregoric P, Karamarkovi A, Djukic V, et al. Decompressive laparotomy with temporary abdominal closure versus percutaneous puncture with placement of abdominal catheter in patients with abdominal compartment syndrome during acute pancreatitis (Decompress study): background and design of multicenter, randomised, controlled study. Pancreatology 2009;9(4):494‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radenkovic D, Bajec D, Ivancevic N, Jeremic V, Djukic V, Stefanovic B, et al. Decompressive laparotomy with temporary abdominal closure versus percutaneous puncture with placement of abdominal catheter in patients with abdominal compartment syndrome during acute pancreatitis (Decompress study): background and design of multicenter, randomised, controlled study. Pancreas 2008;37(4):490‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radenkovic D, Bajec D, Ivancevic N, Jeremic V, Gregoric P, Karadzic B. Decompressive laparotomy with temporary abdominal closure versus percutaneous puncture with placement of abdominal catheter in patients with abdominal compartment syndrome during acute pancreatitis (Decompress study): background and design of multicenter, randomised, study. Pancreas 2009;38(8):1039‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radenkovic DV, Bajec D, Ivancevic N, Bumbasirevic V, Milic N, Jeremic V, et al. Decompressive laparotomy with temporary abdominal closure versus percutaneous puncture with placement of abdominal catheter in patients with abdominal compartment syndrome during acute pancreatitis: background and design of multicenter, randomised, controlled study. BMC Surgery 2010;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranson 1976 {published data only}
- Ranson JH, Rifkind KM, Turner JW. Prognostic signs and nonoperative peritoneal lavage in acute pancreatitis. Surgery, Gynecology and Obstetrics 1976;143(2):209‐19. [PubMed] [Google Scholar]
Ranson 1990 {published data only}
- Ranson JH, Berman RS. Long peritoneal lavage decreases pancreatic sepsis in acute pancreatitis. Annals of Surgery 1990;211(6):708‐16; discussion 16‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroder 1990 {published data only}
- Schroder T, Puolakkainen P, Ramo J, Nuutinen P, Kivilaakso E, Kiviluoto T, et al. Long term results after pancreatic resection and peritoneal lavage for acute hemorrhagic pancreatitis. Surgical Research Communications 1990;7(2‐3):145‐9. [Google Scholar]
Teerenhovi 1989 {published data only}
- Teerenhovi O, Nordback I, Eskola J. High volume lesser sac lavage in acute necrotizing pancreatitis. British Journal of Surgery 1989;76(4):370‐3. [DOI] [PubMed] [Google Scholar]
Van Santvoort 2011 {published data only}
- Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 2011;141(4):1254‐63. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
van Brunschot 2013 {published data only}
- Brunschot S, Santvoort H, Boermeester M, Dijkgraaf M, Timmer R, Bruno M, et al. Endoscopic transluminal step‐up approach versus surgical step‐up approach in patients with infected necrotizing pancreatitis (TENSION): design and rationale of a randomized controlled multicenter trial. Pancreatology 2012;12(6):574‐5. [Google Scholar]
- Brunschot S, Grinsven J, Voermans RP, Bakker OJ, Besselink MG, Boermeester MA, et al. Transluminal endoscopic step‐up approach versus minimally invasive surgical step‐up approach in patients with infected necrotising pancreatitis (TENSION trial): design and rationale of a randomised controlled multicenter trial [ISRCTN09186711]. BMC Gastroenterology 2013;13:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunschot S, Santvoort H, Boermeester M, Dijkgraaf M, Timmer R, Bruno M, et al. Endoscopic versus surgical step‐up approach in patients with infected necrotizing pancreatitis (TENSION): design and rationale of a randomized controlled multicenter trial. Pancreas 2012;41(8):1410. [Google Scholar]
Additional references
Banks 2013
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis ‐ 2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62(1):102‐11. [DOI] [PubMed] [Google Scholar]
Bone 1992
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101(6):1644‐55. [DOI] [PubMed] [Google Scholar]
Bradley 1993
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11‐13, 1992. Archives of Surgery 1993;128(5):586‐90. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Dias 2012a
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 1: introduction to evidence synthesis for decision making, 2012. www.nicedsu.org.uk/TSD1%20Introduction.final.08.05.12.pdf (accessed 13 November 2014).
Dias 2012b
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials, 2012. www.nicedsu.org.uk/TSD4%20Inconsistency.final.15April2014.pdf (accessed 13 November 2014). [PubMed]
Dias 2012c
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU Technical Support Document 3: heterogeneity: subgroups, meta‐regression, bias and bias‐adjustment, 2012. www.nicedsu.org.uk/TSD3%20Heterogeneity.final%20report.08.05.12.pdf (accessed 13 November 2014).
Dias 2014
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta‐analysis of randomised controlled trials, 2014. www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%2015April2014.pdf (accessed 13 November 2014). [PubMed]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2006
- Center for Biologics Evaluation and Research, U.S. Food, Drug Administration. Guidance for industry adverse reactions section of labeling for human prescription drug and biological products ‐ content and format, 2006. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075057.pdf (accessed 13 November 2014).
Gurusamy 2014
- Gurusamy K. Pharmacological interventions for acute pancreatitis: a network meta‐analysis. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD011384] [DOI] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2012
- Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH‐GCP 1996
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1996. [Google Scholar]
Lu 2004
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine 2004;23(20):3105‐24. [DOI] [PubMed] [Google Scholar]
Lu 2007
- Lu G, Ades AE, Sutton AJ, Cooper NJ, Briggs AH, Caldwell DM. Meta‐analysis of mixed treatment comparisons at multiple follow‐up times. Statistics in Medicine 2007;26(20):3681‐99. [DOI] [PubMed] [Google Scholar]
MHRA 2013
- Medicines and Healthcare products Regulatory Agency (MHRA). Clinical trials for medicines: safety reporting ‐ SUSARs and DSURs, 2013. www.mhra.gov.uk/Howweregulate/Medicines/Licensingofmedicines/Clinicaltrials/Safetyreporting‐SUSARsandASRs/ (accessed on 13 November 2014).
Mills 2012
- Mills EJ, Ioannidis JP, Thorlund K, Schunemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta‐analysis. JAMA 2012;308(12):1246‐53. [DOI] [PubMed] [Google Scholar]
Mouli 2013
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta‐analysis. Gastroenterology 2013;144(2):333‐40. [DOI] [PubMed] [Google Scholar]
NCBI 2014
- NCBI. MeSH. NLM Controlled Vocabulary. Pancreas. www.ncbi.nlm.nih.gov/mesh/68010179 (accessed 13 November 2014).
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Peery 2012
- Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143(5):1179‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrov 2010
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010;139(3):813‐20. [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ (Clinical Research Edition) 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roberts 2013
- Roberts SE, Akbari A, Thorne K, Atkinson M, Evans PA. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Alimentary Pharmacology and Therapeutics 2013;38(5):539‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sah 2013
- Sah RP, Dawra RK, Saluja AK. New insights into the pathogenesis of pancreatitis. Current Opinion in Gastroenterology 2013;29(5):523‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [DOI] [PubMed] [Google Scholar]
Schmid 1999
- Schmid SW, Uhl W, Friess H, Malfertheiner P, Buchler MW. The role of infection in acute pancreatitis. Gut 1999;45(2):311‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tenner 2013
- Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. American Journal of Gastroenterology 2013;108(9):1400‐15. [DOI] [PubMed] [Google Scholar]
Van Brunschot 2014
- Brunschot S, Fockens P, Bakker OJ, Besselink MG, Voermans RP, Poley JW, et al. Endoscopic transluminal necrosectomy in necrotising pancreatitis: a systematic review. Surgical Endoscopy 2014;28(5):1425‐38. [DOI] [PubMed] [Google Scholar]
Van Santvoort 2010a
- Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et al. A step‐up approach or open necrosectomy for necrotizing pancreatitis. New England Journal of Medicine 2010;362(16):1491‐502. [DOI] [PubMed] [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Research Synthesis Methods 2012;3(2):111‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
WinBUGS 1.4 [Computer program]
- Imperial College and MRC, UK. WinBUGS with DoodleBUGS. Version 1.4.3. Imperial College and MRC, UK, 2007.
Yadav 2006
- Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas 2006;33(4):323‐30. [DOI] [PubMed] [Google Scholar]
Yang 2008
- Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol‐related liver and pancreatic disease in the United States. Archives of Internal Medicine 2008;168(6):649‐56. [DOI] [PubMed] [Google Scholar]


