Abstract
Aims
Cardiac implantable electronic devices (CIED) are important tools for managing arrhythmias, improving hemodynamics, and preventing sudden cardiac death. Device-related infections (DRI) remain a significant complication of CIED and are associated with major adverse outcomes. We aimed to assess the trend in CIED implantations, and the burden and morbidity associated with DRI.
Methods and results
The 2011–2018 National Inpatient Sample database was searched for admissions for CIED implantation and DRI. A total of 1 604 173 admissions for CIED implantations and 71 007 (4.4%) admissions for DRI were reported. There was no significant change in annual admission rates for DRI (3.96–4.59%, P value for trend = 0.98). Those with DRI were more likely to be male (69.3 vs. 57%, P < 0.001) and have a Charlson comorbidity index score ≥3 (46.6 vs. 36.8%, P < 0.001). The prevalence of congestive heart failure (CHF) increased in those admitted with DRI over the observation period. Pulmonary embolism, deep vein thrombosis, and post-procedural hematoma were the most common complications in those with DRI (4.1, 3.6, and 2.90%, respectively). Annual in-hospital mortality for those with DRI ranged from 3.9 to 5.8% (mean 4.4%, P value for trend = 0.07). Multivariate analysis identified CHF [odds ratio (OR) = 1.67; 95% confidence interval (CI) = 1.35–2.07], end-stage renal disease (OR = 1.90; 95% CI = 1.46–2.48), coagulopathy (OR = 2.94; 95% CI = 2.40–3.61), and malnutrition (OR = 2.50; 95% CI = 1.99–3.15) as the predictors of in-hospital mortality for patients admitted with DRI.
Conclusion
Device-related infection is relatively common and continues to be associated with high morbidity and mortality. The prevalence of DRI has not changed significantly despite technical and technological advances in cardiac devices and their implantation.
Keywords: Cardiac implantable electronic device, Device-related infection, Prognosis, Mortality, Cost, Length of stay
Graphical Abstract
Graphical Abstract.
CHF, congestive heart failure; CIED, cardiac implantable electronic device; DRI, device-related infections; ESRD, end-stage renal disease; NIS, national inpatient sample.
What’s new?
The number of cardiac implantable electronic device (CIED) implantations steadily declined from 2011 to 2014 (P < 0.001) and remained stable during 2014–2018. The decline was seen in all categories of CIED.
Years 2011–2018 witnessed the highest reported incidence of device-related infection (DRI) between 3.9 and 4.8% despite significant technological and technical advances in CIED design and implantation. As evident in our study, the persistently high annual incidence of DRI might be explained by CIED implantation in patients with an increasing number of comorbid conditions.
Compared to patients admitted for device implantation, those with DRI were more likely to be men, of nonwhite race, and from a low-income status.
The in-hospital mortality rate in patients admitted with DRI was 4.3% in this study, which is lower than the 5–8% reported incidence from previous reports in the Medicare fee-for-service database.
From 2011 to 2018, the inflation-adjusted mean total hospital charges in patients admitted with DRI increased by 36% to $239 232.
Introduction
Cardiac implantable electronic devices (CIED) include a wide variety of tools used for long-term chronotropic and hemodynamic support, as well as the prevention of sudden cardiac death in patients with heart disease. These devices include increasingly sophisticated permanent pacemakers (PPM), implantable cardioverter defibrillators (ICD), and cardiac resynchronization therapy (CRT) devices with or without antitachycardia pacing and defibrillator capabilities. Prior studies have shown an upward trend in annual CIED implantation. From 1993 to 2006, 2.4 million patients underwent PPM implantation and 0.8 million received an ICD while 369 000 PPM and 74 000 ICD units were replaced.1 In the following 3 years (2007–2009), an additional 0.6 million PPM alone were implanted in the USA, with a progressive rise in the number of dual compared to single-chamber units implanted in patients with higher prevalence of comorbid conditions.2 In an international survey of 61 countries, the USA had the highest number of CIED implanted in 2009, which included 225 567 PPM, 133 262 ICD, and 49 255 CRT units.3 The increase in the number of CIED implants is likely driven by the improved survival of patients with heart disease and the expanded indications for their use.4,5
Device-related infection (DRI) is a major complication of CIED implantation and is associated with significant morbidity, mortality, and financial healthcare burden.6–9 The incidence of DRI averaged 1.61% between 1993 and 2008 and to a rate of 2.41% in 2008.7 In a 12-year study (2000–2012) using the National Inpatient Sample (NIS), including 4 144 683 device-related procedures, the rate of DRI was 2.06%.8 In another study of 97 750 patients from a Danish registry, the rate of DRI ranged from 1.19% for PPM to 3.35% for CRT-defibrillator between 1982 and 2018.10 The increasing rate of DRI, despite significant technical and technological advances in devices and their implantation, may be partially explained by the increasing use of more complex devices in older patients with higher comorbidities.10–12 Several procedural, device-, or patient-related risk factors have been identified for DRI.13,14 Trends of CIED implantation and DRI in the USA in the years following 2012 have not been previously studied. The present study aimed to assess these trends along with morbidity associated with DRI.
Methods
Data source
Data were obtained from the NIS database, a part of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality, for calendar years 2011–2018.15 The database contains discharge-level data for ∼8 million hospital stays from ∼1000 hospitals annually. It is designed to approximate a 20% stratified sample of community hospitals. A total of 46 states, representing ∼96% of the US population, participate in NIS. Hospital ownership, patient volume, teaching status, urban or rural location, and geographic region are used for stratified sampling, and discharge weights provided by the sponsor are used to obtain national estimates. The database is publicly available and contains deidentified information; therefore, the study was deemed exempt from institutional research board review.
Study population
The NIS data were queried to identify baseline characteristics (age, sex, race, and comorbidities), hospital-level characteristics (hospital bed size, region, teaching status, primary expected payer, and median household income for patient’s ZIP code), and outcome variables (in-hospital mortality, mean length of stay, and mean total hospital charge) for adults (age ≥ 18-year-old) that underwent CIED implantation or had DRI between 2011 and 2018 using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. Cardiac implantable electronic device implantations included PPM, ICD, and CRT devices and were identified using appropriate ICD codes for implantation as shown in Supplementary material online, Table S1. Device-related infections were identified using previously known methods in one of two ways: (i) an ICD-9-CM or ICD-10-CM diagnostic code for DRI along with any procedure codes for CIED implantation or CIED/lead removal, revision, or replacement and (ii) any CIED removal code along with signs of system infection.7,16
Study outcome measures
The primary outcomes of interest were in-hospital mortality, mean length of stay, and mean total inflation-adjusted hospital charge. The secondary outcomes of interest were temporal trends in CIED implantation and DRI, their associated adverse events, and predictors of DRI-related mortality.
Statistical analysis
Weighted data were used for all statistical analyses. Results were expressed as numbers (%) for categorical variables and mean ± standard deviation for continuous variables. Differences between groups were analyzed with the use of Student’s t-test for continuous variables and the χ2 test for categorical variables, respectively. Stepwise, forward selection, logistic regression was used to identify predictors of in-hospital mortality in patients admitted with DRI. The regression model was adjusted for demographics, hospital characteristics, comorbidities, and complications as deemed important. Adjusted odds ratio (OR) and 95% confidence intervals (CI) were used to report the results of logistic regression. A two-tailed P < 0.05 was considered statistically significant. Statistical analyses were performed using statistical software STATA IC version 16.1 (StataCorp, College Station, TX). Comparisons between those with CIED implantation alone and patients with DRI were made using the independent samples Mann–Whitney U test.
Results
Patient characteristics
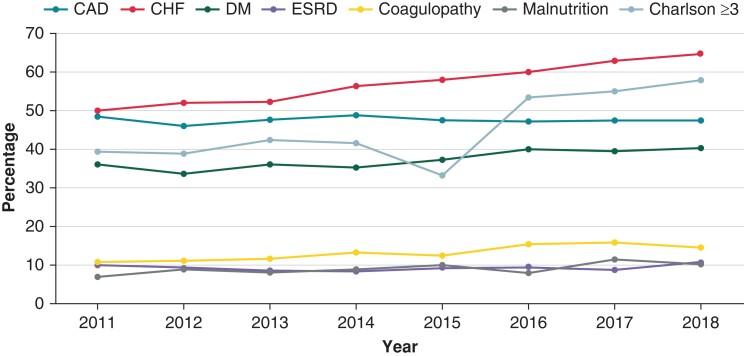
The baseline demographic, clinical, and hospital characteristics of all patients admitted for CIED implantation and DRI between 2011 and 2018 are summarized in Table 1. A total of 1 604 173 admissions for CIED implantations and 71 007 (4.4%) admissions for DRI were reported. Compared to those admitted for CIED implantation, patients with DRI were younger (67 vs. 73 years) and more often male (69.3 vs. 57%). The distribution of body mass index (BMI) was different in CIED implantation and DRI cohorts. Patients undergoing CIED implantation were more likely to have a BMI of 30–39 while patients with DRI were more likely to be morbidly obese with a BMI ≥40 kg/m2. In addition, patients admitted with DRI were less often of the white race (69.5 vs. 72.5%) and more likely to have lower household income (31 vs. 27%), Medicaid as the primary payer (9.5 vs. 5.4%), and a higher number of comorbid conditions as assessed by a Charlson comorbidity index ≥3 (46.6 vs. 36.8%). Some of the specific comorbid conditions more commonly noted in those with DRI included congestive heart failure (CHF) (58.2%), diabetes (38%), end-stage renal disease (ESRD) (9.1%), coagulopathy (13.1%), and malnutrition (8.9%). Among these comorbidities, the prevalence of CHF increased during the study period (Figure 1, 50% in 2011 to 64.4% in 2018, P for trend <0.001).
Table 1.
Baseline demographic, clinical, and hospital characteristics of patients admitted for cardiac implantable electronic device implantation or device-related infection
| CIED implantation | DRI | P value | |
|---|---|---|---|
| (n = 1 604 173) | (n = 71 007) | ||
| Mean age, years | 73 | 67 | <0.001 |
| Male, number (%) | 914 378 (57) | 49 207 (69.3) | <0.001 |
| White race, number (%) | 1 163 025 (72.5) | 49 350 (69.5) | <0.001 |
| Primary payer, number (%) | <0.001 | ||
| Medicare | 1 203 130 (75) | 48 995 (69) | |
| Medicaid | 86 625 (5.4) | 6746 (9.5) | |
| Private insurance | 253 459 (15.8) | 12 284 (17.3) | |
| Others | 60 959 (3.8) | 2982 (4.2) | |
| Body mass index, number (%) | <0.001 | ||
| <19 | 96 250 (6%) | 7101 (10%) | |
| 19–29 | 224 584 (14%) | 11 360 (16%) | |
| 30–39 | 721 878 (45%) | 25 563 (36%) | |
| ≥40 | 561 461 (35%) | 26 983 (38%) | |
| Median household income percentile | <0.001 | ||
| 0–25th | 433 127 (27) | 22 012 (31) | |
| 26–50th | 418 689 (26.1) | 18 959 (26.7) | |
| 51–75th | 399 439 (24.9) | 16 687 (23.5) | |
| 76–100th | 352 918 (22) | 13 349 (18.8) | |
| Charlson comorbidity index score | <0.001 | ||
| 0 | 352 918 (22) | 11 361 (16) | |
| 1 | 360 940 (22.5) | 13 633 (19.2) | |
| 2 | 299 980 (18.7) | 12 923 (18.2) | |
| ≥ 3 | 590 335 (36.8) | 33 090 (46.6) | |
| Comorbidities, number (%) | |||
| Coronary artery disease | 757 170 (47.2) | 34 510 (48.6) | 0.0004 |
| Hypertension | 755 566 (47.1) | 24 640 (34.7) | <0.001 |
| Diabetes mellitus | 556 648 (34.7) | 26 983 (38) | <0.001 |
| Congestive heart failure | 717 065 (44.7) | 41 326 (58.2) | <0.001 |
| Peripheral vascular disease | 97 855 (6.1) | 4402 (6.2) | 0.76 |
| Chronic kidney disease (stage ≥3) | 259 876 (16.2) | 12 426 (17.5) | <0.001 |
| End-stage renal disease | 54 542 (3.4) | 6462 (9.1) | <0.001 |
| Chronic obstructive pulmonary disease | 251 855 (15.7) | 12 568 (17.7) | <0.001 |
| Hyperlipidemia | 816 524 (50.9) | 31 740 (44.7) | <0.001 |
| Atrial fibrillation | 672 149 (41.9) | 29 965 (42.2) | 0.56 |
| Atrial flutter | 139 563 (8.7) | 4758 (6.7) | <0.001 |
| Atrioventricular block | 598 357 (37.3) | 14 060 (19.8) | <0.001 |
| Sick sinus syndrome | 571 086 (35.6) | 9515 (13.4) | <0.001 |
| Thyroid disease | 283 939 (17.7) | 11 006 (15.5) | <0.001 |
| Malignancy | 52 938 (3.3) | 2343 (3.3) | 0.12 |
| Coagulopathy | 104 271 (6.5) | 9302 (13.1) | <0.001 |
| Cirrhosis | 14 438 (0.9) | 1136 (1.6) | <0.001 |
| Malnutrition | 48 125 (3) | 6320 (8.9) | <0.001 |
CIED, cardiac implantable electronic device; DRI, device-related infection.
Figure 1.
Trends of comorbidities and Charlson index in patients with device-related infection (DRI) from 2011 to 2018. Trends for coronary artery disease (P = 0.94) and end-stage renal disease (P = 0.88) were statistically insignificant while all other comorbidities showed significant interval increase (P < 0.001 for all others). CAD, coronary artery disease; Charlson, Charlson comorbidity index; CHF, congestive heart failure; DM, diabetes mellitus; ESRD, end-stage renal disease.
Trends in cardiac implantable electronic device implantation and device-related infection
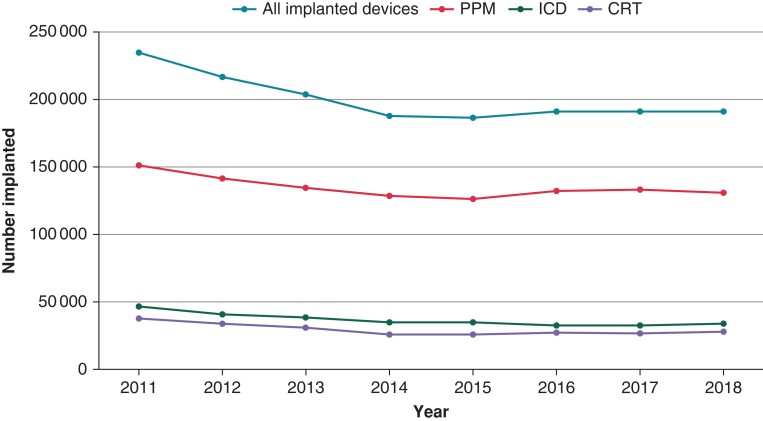
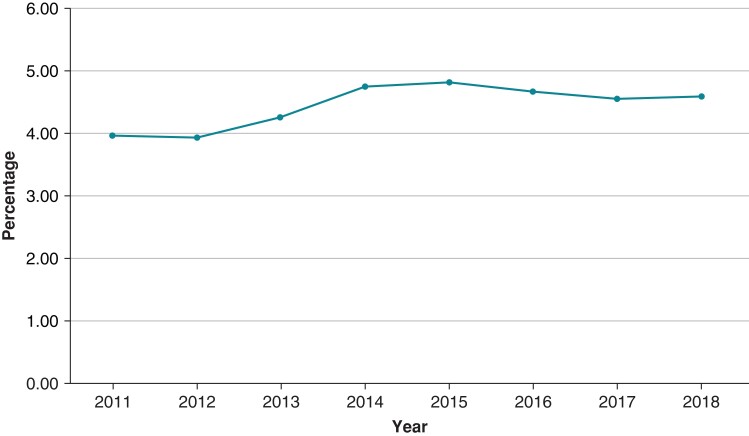
The annual number of CIED implantations and DRI from 2011 to 2018 is shown in Figures 2 and 3 and Tables 2 and 3. The number of CIED implantations steadily declined from 234 543 in 2011 by 20% to 187 630 in 2014 (P < 0.001) and remained stable during 2014–2018. The decline was seen in all categories of CIED (Figure 2). The percentage of DRI remained stable around a mean of 4.4% throughout the study period (P = 0.98) (Table 3).
Figure 2.
Trend of cardiac implantable electronic device (CIED) implantation from 2011 to 2018 by device type. All trends were statistically significant at P < 0.001 level. CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; PPM, permanent pacemaker.
Figure 3.
Trend of device-related infection (DRI) between 2011 and 2018 (P trend = 0.98).
Table 2.
Number of cardiac implanted electronic devices inserted annually from 2011 to 2018
| Year | Total number of implanted devices | Permanent pacemakers | Implantable cardioverter defibrillators | Cardiac resynchronization therapy devices |
|---|---|---|---|---|
| 2011 | 234 543 | 150 897 | 46 376 | 37 270 |
| 2012 | 216 920 | 141 830 | 41 205 | 33 885 |
| 2013 | 203 595 | 134 910 | 38 455 | 30 230 |
| 2014 | 187 630 | 127 825 | 34 910 | 24 895 |
| 2015 | 186 035 | 126 055 | 34 415 | 25 565 |
| 2016 | 192 145 | 132 625 | 32 640 | 26 880 |
| 2017 | 191 630 | 133 140 | 32 330 | 26 160 |
| 2018 | 191 675 | 130 940 | 33 260 | 27 475 |
| Total | 1 604 173 | 1 078 222 | 293 591 | 232 360 |
Table 3.
Numbers and percentages of device-related infections reported annually between 2011 and 2018
| Year | Number | Percentage (infections/total device implanted) |
|---|---|---|
| 2011 | 9307 | 3.96% |
| 2012 | 8550 | 3.94% |
| 2013 | 8690 | 4.26% |
| 2014 | 8925 | 4.75% |
| 2015 | 8990 | 4.83% |
| 2016 | 8985 | 4.67% |
| 2017 | 8750 | 4.56% |
| 2018 | 8810 | 4.59% |
| Total = 71 007 | Average = 4.44% |
Trends in primary outcomes
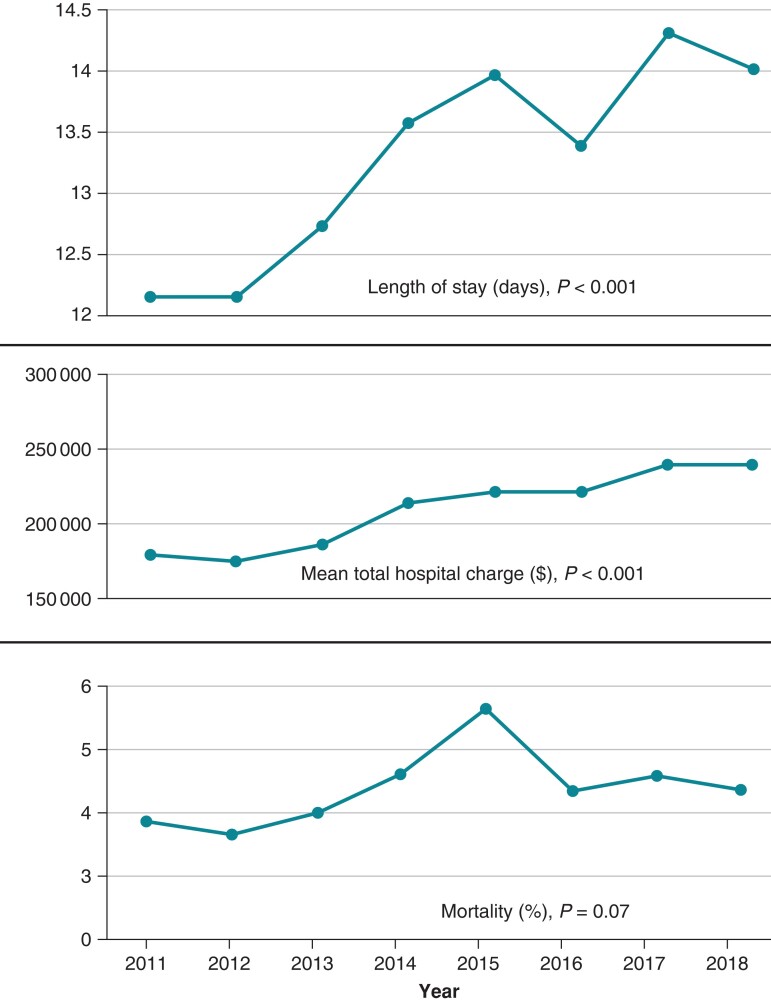
Hospitalizations for DRI had a significantly higher mean length of stay (13.2 vs. 6.1 days), mean inflation-adjusted total hospital charge (207 514 vs. 140 409 US dollars), and in-hospital mortality (4.3 vs. 0.98%) compared to hospitalizations with CIED implantations (Table 4). From years 2011 to 2018, the mean length of stay of DRI hospitalizations increased from ∼12 days in 2011 to ∼14 days in 2018 (P < 0.001), mean inflation-adjusted total hospital charge increased by 36%, while in-hospital mortality increased from 3.9% in 2011 to 4.6% in 2018 with a peak in 2015 (Figure 4).
Table 4.
Outcomes and healthcare resource utilization associated with cardiac implantable electronic device implantation and device-related infection
| CIED implantation | DRI | P value | |
|---|---|---|---|
| (n = 1 604 173) | (n = 71 007) | ||
| Mortality, n (%) | 15 844 (0.98) | 3109 (4.3) | <0.001 |
| Mean inflation-adjusted total hospital charges ($) | 140 409 | 207 514 | <0.001 |
| Mean length of stay (days) | 6.1 | 13.2 | <0.001 |
CIED, cardiac implantable electronic device; DRI, device-related infection.
Figure 4.
Top panel: the mean hospital length of stay of patients with device-related infection (DRI) increased from ∼12 days in 2011 to ∼14 days in 2018 (P < 0.001). Middle panel: the mean inflation-adjusted total hospital charges increased by 36% during this time (P < 0.001). Bottom panel: in-hospital mortality increased from 3.9% in 2011 to 4.6% in 2018 with a peak in 2015 (P = 0.07).
Complications in cardiac implantable electronic device implantation and infection
Compared to patients admitted for CIED implantation, patients admitted with DRI had a significantly higher number of total adverse events (Table 5, 16.1 vs. 4.6%, P < 0.001). Pulmonary embolism was the most common (4.13%) adverse event suffered by those with DRI.
Table 5.
Comparison of adverse events associated with cardiac implantable electronic device insertion and device-related infection
| Types of adverse events | CIED | DRI | P value |
|---|---|---|---|
| (n = 1 604 173) | (n = 71 007) | ||
| Pericardial effusion | 18 127 (1.13%) | 1669 (2.35%) | <0.001 |
| Cardiac tamponade | 5614 (0.35%) | 575 (0.81%) | <0.001 |
| Cardiac perforation | 3690 (0.23%) | 454 (0.64%) | <0.001 |
| Arteriovenous fistula | 289 (0.018%) | 26 (0.036%) | 0.31 |
| Hematoma—post procedure | 14 117 (0.88%) | 2059 (2.90%) | <0.001 |
| Hemorrhage—post procedure | 7700 (0.48%) | 1207 (1.70%) | <0.001 |
| Pulmonary embolism | 10 106 (0.63%) | 2933 (4.13%) | <0.001 |
| Deep vein thrombosis | 14 438 (0.90%) | 2556 (3.60%) | <0.001 |
CIED, cardiac implantable electronic device; DRI, device-related infection.
Predictors of mortality in patients with device-related infection
Predictors of in-hospital mortality in patients admitted with DRI are shown in Table 6. Predictors of increased mortality include small hospital bed size (OR 1.40, CI 1.08–1.81, P = 0.01), CHF (OR 1.67, CI 1.35–2.07, P < 0.001), ESRD (OR 1.90, CI 1.46–2.48, P < 0.001), coagulopathy (OR 2.94, CI 2.40–3.61, P < 0.001), and malnutrition (OR 2.50, CI 1.99–3.15, P < 0.001).
Table 6.
Predictors of mortality in patients admitted with device-related infection
| Predictors | Mortality (n = 3109) | |||
|---|---|---|---|---|
| Univariate odds ratio (95% confidence interval) | P value | Multivariate odds ratio (95% confidence interval) | P value | |
| Age group | ||||
| 18–45 | 0.47 (0.32–0.69) | <0.001 | 0.34 (0.21–0.54) | <0.001 |
| 46–60 | 0.95 (0.78–1.16) | 0.63 | ||
| 61–75 | 1.24 (1.05–1.46) | 0.008 | ||
| >75 | 1.00 (0.84–1.18) | 0.99 | ||
| Body mass index, number (%) | ||||
| <19 | 1.69 (0.97–2.93) | 0.06 | ||
| 19–29 | 1.28 (0.79–2.08) | 0.30 | ||
| 30–39 | 0.50 (0.30–0.81) | 0.006 | ||
| ≥40 | 1.18 (0.84–1.66) | 0.32 | ||
| Hospital bed size | 0.01 | |||
| Small | 0.76 (0.53–1.09) | 0.14 | 1.40 (1.08–1.81) | |
| Medium | 0.85 (0.68–1.06) | 0.15 | ||
| Large | 1.23 (1.01–1.50) | 0.033 | ||
| Comorbidities | ||||
| CAD | 0.66 (0.56–0.78) | <0.001 | 0.78 (0.64–0.94) | 0.01 |
| Hypertension | 0.32 (0.26–0.41) | <0.001 | 0.56 (0.42–0.73) | <0.001 |
| Diabetes | 0.87 (0.74–1.04) | 0.13 | ||
| CHF | 2.05 (1.72–2.45) | <0.001 | 1.67 (1.35–2.07) | <0.001 |
| PVD | 1.06 (0.77–1.45) | 0.70 | ||
| CKD ≥ 3 | 1.37 (1.13–1.65) | 0.001 | ||
| ESRD | 2.88 (2.37–3.50) | <0.001 | 1.90 (1.46–2.48) | <0.001 |
| COPD | 0.96 (0.78–1.19) | 0.74 | ||
| Hyperlipidemia | 0.50 (0.42–0.60) | <0.001 | 0.62 (0.50–0.76) | <0.001 |
| Atrial fibrillation | 0.97 (0.82–1.15) | 0.76 | ||
| Atrial flutter | 1.52 (1.16–2) | 0.002 | ||
| Atrioventricular block | 1.01 (0.83–1.23) | 0.91 | ||
| Sick sinus syndrome | 0.32 (0.22–0.46) | <0.001 | 0.43 (0.29–0.64) | <0.001 |
| Thyroid disease | 0.74 (0.58–0.95) | 0.021 | ||
| Malignancy | 1.14 (0.75–1.74) | 0.51 | ||
| Coagulopathy | 3.72 (3.12–4.44) | <0.001 | 2.94 (2.40–3.61) | <0.001 |
| Cirrhosis | 1.36 (0.77–2.39) | 0.27 | ||
| Malnutrition | 3.92 (3.25–4.73) | <0.001 | 2.50 (1.99–3.15) | <0.001 |
Significance of bold values represents Multivariate odds ratio (95% Confidence Interval). CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; PVD, peripheral vascular disease.
Discussion
Key findings
The main findings of the study were as follows: (i) the number of CIED implants declined from 2011 to 2014, followed by a stable annual number of implants from 2015 to 2018; (ii) the annual incidence of DRI remained stable between 2011 and 2018; (iii) compared to patients admitted for CIED implantation, those with DRI were more likely to be men, of nonwhite race, and from a low-income household; (iv) overall burden of comorbidities increased during the study period in patients admitted for CIED implantation as assessed by a Charlson comorbidity index ≥3; (v) the mean length of stay and inflation-adjusted total hospital charge significantly increased for DRI admissions over the study period; and (vi) for the patients admitted with DRI, the in-hospital mortality was relatively high (4.3%) with CHF, ESRD, malnutrition, and coagulopathy identified as being the strongest predictors of mortality.
Trends in cardiac implantable electronic device implantation
This study elucidates the most recent data on national trends in CIED implantations and DRI in the USA. From 1993 to 2008, pacemaker implantations increased by 45%, while ICD implantations increased exponentially by 504%.7 The current study shows that CIED implantations declined from 2011 to 2014 and remained stable from 2015 to 2018. The decline was seen in all categories of CIED. This is likely explained, at least partly, by adopting new clinical guidelines and reimbursement policies for device therapies in clinical practice.11,12,17 A recently published study from Olmsted County, MN, has shown local trends in CIED implantation from 1998 to 2018, similar to the above national trends from our study.15
Trends in device-related infection
Using a national database, Voigt et al.16 showed that through 1996–2003, incidence of DRI increased at a rate (overall 3.1-fold increase) faster than CIED implants (1.49-fold rise). The annual incidence of DRI markedly increased from 2004 to 2008, peaking at 2.4%.7 Another study showed that the annual incidence of DRI increased from 1.45% in 2008 to 3.41% in 2012.8 The incidence of DRI in Olmsted County, MN, was also shown to increase from 1988 to 2015 in 7-year periods.17 Our study indicates that between 2011 and 2018, the annual incidence of DRI remained stable at 3.9–4.8%. A study covering only the year 2016 has reported a comparable annual incidence of DRI at 4.2%.13 Recent years have witnessed the highest incidence of DRI despite significant technological and technical advances in CIED design and implantation. As evident in our study, the persistently high annual incidence of DRI might be explained by CIED implantation in patients with an increasing number of comorbid conditions. Another study has also suggested that the rise in DRI in the past might be due to implantation in patients with higher comorbid conditions and performance of more complex procedures.18
Comparison of admission for cardiac implantable electronic device implantation and device-related infection
Compared to those admitted for CIED implantation, those with DRI were more often men, of nonwhite race, from lower-income households, and had Medicaid as their primary payer. In addition, those with DRI also had a higher burden of comorbidities including diabetes, CHF, chronic kidney disease, ESRD, coagulopathy, cirrhosis, and malnutrition. A pooled analysis has identified many of the similar comorbidities listed above as risk factors for DRI with ESRD being the strongest risk factor.9 A recent survey conducted at 234 centers across 62 countries revealed significantly low compliance with current guidelines and recommendations for preventing DRI.19 The study also found substantial global variability in the prevention and management of CIED infections.
Adverse events and outcomes
In this study, adverse events were significantly higher among patients admitted for DRI compared to CIED implantation (16.1 vs. 4.6%). All adverse events, including pulmonary embolism, deep vein thrombosis, hematoma, cardiac tamponade, and cardiac perforation, were more common among patients admitted with DRI. The incidence of pulmonary embolism was higher (4.13 vs. 0.1%) in our study compared to a prior multicenter study looking at in-hospital complications of 1684 patients undergoing lead extraction for DRI.20 Pulmonary embolism can occur from the migration of emboli from an infected device lead, either spontaneously or during device extraction. The in-hospital mortality rate in patients admitted with DRI was 4.3% in this study, which is lower than the 5–8% reported incidence from previous reports in the Medicare fee-for-service database.21,22 In our study, CHF, ESRD, malnutrition, and coagulopathy were associated with higher mortality among DRI patients. Others have shown an association between CHF, frailty, corticosteroid use, and infective endocarditis-related infection and in-hospital mortality among patients with DRI.23,24 Like our study, advanced age was not found to be a predictor of in-hospital mortality by others.25
Resource utilization
From 2011 to 2018, the mean length of stay for patients admitted with DRI increased by an average of 2 days. Our study’s mean length of stay of 14 days in 2018 was similar to the mean length of stay of 13.7 days in 2016.13 From 2011 to 2018, the inflation-adjusted mean total hospital charge in patients admitted with DRI increased by 36% to $239 232 in 2018. This was a continuation of a rising trend with a 26% increase in inflation-adjusted total hospital charge from 2003 to 2011 in a prior study.26 Extending the duration of hospitalization by even a single day incurs significant healthcare spending for patients with DRI, and the rapidly rising cost of DRI hospitalizations reflects a growing financial burden on the healthcare system.9 In addition, increasing costs could partly be attributed to an increasingly complex population of patients admitted with DRI.
Conclusions
Annual CIED implantations have declined in recent years but are associated with a remarkably high incidence of DRI. These infections cause significant morbidity and mortality among patients and incur a substantial financial burden on the healthcare system.
Limitations
Data in NIS are retrospective. The accuracy of patient selection based on ICD codes depends on the precision with which ICD codes were entered into patients’ records at the time of discharge. The transition from ICD-9-CM to ICD-10-CM on 1 October 2015 adds a potential source of error in trend analysis. Patients admitted for CIED implantation and DRI represent two independent cohorts. Due to the way DRI is defined in our study, we have predominantly included patients with systemic infection that underwent device/lead extraction. Our study likely excluded many patients with local pocket infections and patients that did not undergo device/lead extraction. Although a limitation, DRI was defined as such to maintain comparability with previously published trends that defined DRI in the same manner. Device-specific ICD codes for lead removal, revision, or replacement were not available. For this reason, the association of specific device type with infection or mortality could not be determined. The NIS database uses ICD codes documented at the time of discharge to maintain a record of patient diagnoses and procedures. Unfortunately, the ICD code for the use of an antibiotic envelope has infrequently made it to the discharge-level documentation and thus we would not be able to make a definitive comment on that without risking the introduction of unsupported conclusions. Although very helpful, a breakdown of cause of death could not be included as it is not reported in the database. Despite these limitations, the overall congruence of our observations with previous reports adds validity to our conclusions.
Supplementary Material
Contributor Information
Vivek Modi, Department of Cardiology, St. Luke’s University Health Network, Heart and Vascular Center, 801 Ostrum Street, Bethlehem, PA 18015, USA.
Kashyap Shah, Department of Cardiology, St. Luke’s University Health Network, Heart and Vascular Center, 801 Ostrum Street, Bethlehem, PA 18015, USA.
Bruce Ferraro, Department of Cardiology, St. Luke’s University Health Network, Heart and Vascular Center, 801 Ostrum Street, Bethlehem, PA 18015, USA.
Leyla Gasimli-Gamache, Department of Cardiology, St. Luke’s University Health Network, Heart and Vascular Center, 801 Ostrum Street, Bethlehem, PA 18015, USA.
Sudip Nanda, Department of Cardiology, St. Luke’s University Health Network, Heart and Vascular Center, 801 Ostrum Street, Bethlehem, PA 18015, USA.
Steven Stevens, Department of Cardiology, St. Luke’s University Health Network, Heart and Vascular Center, 801 Ostrum Street, Bethlehem, PA 18015, USA.
Jamshid Shirani, Department of Cardiology, St. Luke’s University Health Network, Heart and Vascular Center, 801 Ostrum Street, Bethlehem, PA 18015, USA.
Supplementary material
Supplementary material is available at Europace online.
Data availability
All relevant data are within the manuscript except National Inpatient Database files, which can be accessed at https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp.
References
- 1. Kurtz SM, Ochoa JA, Lau E, Shkolnikov Y, Pavri BB, Frisch Det al. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993–2006: implantation trends and patient profiles for pacemakers and ICDs. Pacing Clin Electrophysiol 2010;33:705–11. [DOI] [PubMed] [Google Scholar]
- 2. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RTet al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009. J Am Coll Cardiol 2012;60:1540–5. [DOI] [PubMed] [Google Scholar]
- 3. Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009—a World Society of Arrhythmia’s Project: 2009 survey cardiac pacemakers and ICDS. Pacing Clin Electrophysiol 2011;34:1013–27. [DOI] [PubMed] [Google Scholar]
- 4. Goldberger Z, Lampert R. Implantable cardioverter-defibrillators: expanding indications and technologies. JAMA 2006;295:809. [DOI] [PubMed] [Google Scholar]
- 5. Boriani G, Ziacchi M, Nesti M, Battista A, Placentino F, Malavasi VLet al. Cardiac resynchronization therapy: how did consensus guidelines from Europe and the United States evolve in the last 15 years? Int J Cardiol 2018;261:119–29. [DOI] [PubMed] [Google Scholar]
- 6. Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo Ret al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017;14:e503–51. [DOI] [PubMed] [Google Scholar]
- 7. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RTet al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States. J Am Coll Cardiol 2011;58:1001–6. [DOI] [PubMed] [Google Scholar]
- 8. Joy PS, Kumar G, Poole JE, London B, Olshansky B. Cardiac implantable electronic device infections: who is at greatest risk? Heart Rhythm 2017;14:839–45. [DOI] [PubMed] [Google Scholar]
- 9. Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MGet al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections—endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace 2020;22:515–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olsen T, Jørgensen OD, Nielsen JC, Thøgersen AM, Philbert BT, Johansen JB. Incidence of device-related infection in 97 750 patients: clinical data from the complete Danish device-cohort (1982–2018). Eur Heart J 2019;40:1862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM, Freedman RA, Gettes LSet al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol 2008;51:e1–62.18498951 [Google Scholar]
- 12. National coverage determination (NCD) for cardiac pacemakers: single chamber and dual chamber permanent cardiac pacemakers (20.8.3).
- 13. Rennert-May E, Chew D, Lu S, Chu A, Kuriachan V, Somayaji R. Epidemiology of cardiac implantable electronic device infections in the United States: a population-based cohort study. Heart Rhythm 2020;17:1125–31. [DOI] [PubMed] [Google Scholar]
- 14. Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang Set al. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016;374:533–41. [DOI] [PubMed] [Google Scholar]
- 15. Vaidya VR, Asirvatham R, Kowlgi GN, Dai M-Y, Cochuyt JJ, Hodge DOet al. Trends in cardiovascular implantable electronic device insertion between 1988 and 2018 in Olmsted County. JACC Clin Electrophysiol 2022;8:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol 2006;48:590–1. [DOI] [PubMed] [Google Scholar]
- 17. Dai M, Cai C, Vaibhav V, Sohail MR, Hayes DL, Hodge DOet al. Trends of cardiovascular implantable electronic device infection in 3 decades. JACC Clin Electrophysiol 2019;5:1071–80. [DOI] [PubMed] [Google Scholar]
- 18. Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol 2010;33:414–9. [DOI] [PubMed] [Google Scholar]
- 19. Traykov V, Bongiorni MG, Boriani G, Burri H, Costa R, Dagres Net al. Clinical practice and implementation of guidelines for the prevention, diagnosis and management of cardiac implantable electronic device infections: results of a worldwide survey under the auspices of the European Heart Rhythm Association. Europace 2019;21:1270–9. [DOI] [PubMed] [Google Scholar]
- 20. Byrd CL, Wilkoff BL, Love CJ, Sellers TD, Reiser C. Clinical study of the laser sheath for lead extraction: the total experience in the United States. Pacing Clin Electrophysiol 2002;25:804–8. [DOI] [PubMed] [Google Scholar]
- 21. Sohail MR. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med 2011;171:1821. [DOI] [PubMed] [Google Scholar]
- 22. Rizwan Sohail M, Henrikson CA, Jo Braid-Forbes M, Forbes KF, Lerner DJ. Increased long-term mortality in patients with cardiovascular implantable electronic device infections: long-term mortality with CIED infections. Pacing Clin Electrophysiol 2015;38:231–9. [DOI] [PubMed] [Google Scholar]
- 23. Viganego F, O’Donoghue S, Eldadah Z, Shah MH, Rastogi M, Mazel JAet al. Effect of early diagnosis and treatment with percutaneous lead extraction on survival in patients with cardiac device infections. Am J Cardiol 2012;109:1466–71. [DOI] [PubMed] [Google Scholar]
- 24. Habib A, Le KY, Baddour LM, Friedman PA, Hayes DL, Lohse CMet al. Predictors of mortality in patients with cardiovascular implantable electronic device infections. Am J Cardiol 2013;111:874–9. [DOI] [PubMed] [Google Scholar]
- 25. Sridhar ARM, Lavu M, Yarlagadda V, Reddy M, Gunda S, Afzal Ret al. Cardiac implantable electronic device-related infection and extraction trends in the U.S.: lead infection and extraction trends in the U.S. Pacing Clin Electrophysiol 2017;40:286–93. [DOI] [PubMed] [Google Scholar]
- 26. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IMet al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427–520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript except National Inpatient Database files, which can be accessed at https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp.