Abstract
Background
Despite autism spectrum disorder (ASD) and mentalization being two words often associated in the literature, the assessment of this ability in individuals with ASD in the clinical setting is still limited. Indeed, there are no standardized Theory of Mind (ToM) tests that are adaptable to different cognitive profiles, such as individuals with language poverty, and intellectual or memory impairments. This study proposes a non-verbal test (Intentions Attribution-Comic Strip Test; IA-CST) to evaluate the ability to infer the intentions of others, a basic component of ToM, in the clinical setting.
Method
In Study 1, the test was administered to 261 healthy individuals and we performed structural validation using Exploratory Graph Analysis. In Study 2, the final version of the test was administered to 32 individuals with ASD to assess the known group validity of the measure by comparing their scores with a sample of IQ-matched controls. Moreover, we performed logistic regression and ROC curve to preliminarily assess the diagnostic performance of the IA-CST.
Results
The IA-CST resulted in a 3-dimension measure with good structural stability. Group comparison indicated that the ASD group shows significantly lower performance in intention attribution but not in inferring causal consequences. The test demonstrated known group validity and that, preliminarily, it is suitable for implementation within the clinical practice.
Conclusions
The results support the IA-CST as a valid non-verbal task for evaluating intentions attribution in the clinical setting. Difficulties in ToM are early and relevant in ASD, so assessing these aspects is valuable for structuring individualized and evidence-based interventions.
Keywords: Autism spectrum disorders, Intention attribution, Psychometric proprieties, Theory of Mind, Clinical utility
Introduction
Theory of Mind (ToM) is the ability to naturally infer the intentions, beliefs, thoughts and feelings of others, which is useful for predicting their behavior [1, 2] and is one of the main components of social cognition, i.e., a multidimensional construct that refers to the ability to process the social world [3]. ToM is a skill that develops along a continuum and follows defined stages [4, 5], ranging from basic skills (i.e., joint attention) to more complex forms of mentalization (i.e., attributing different mental states to several people). The ability to understand the behavior of others requires an awareness that there is an intention behind an action, a capacity that seems to develop right back in early childhood [6]. Understanding an action first requires identifying what has been done; actions can be identified at a lower level, through details indicating how the action was performed, and at a higher level, through details indicating why and with what effect the action was performed and the effects it had [7]. Recognizing one's actions at the highest level is usually an indication of being aware of one's mind as the cause of the behavior [7, 8]. Action identification makes it possible to keep track of the inference of mental states and this principle can be applied to one's own mental state as well as that of others [7].
Literature suggests that a deficit in planning an action in a specific situation explains the difficulty of mentally representing the intention of that action [9, 10]. This model of functioning has been studied mainly in schizophrenia [11–14]. The ability to understand the intentions of others has also been shown to be impaired in individuals with autism spectrum disorder (ASD) [15–17]. Several studies have suggested that people with ASD have a different way of action processing [16, 18, 19] and would have difficulty in anticipating the actions of others and representing goal-orientated behaviors [19, 20]. This difficulty was evident in the task characterized by illustrated story, known as the “comic strip task”, which required the sequencing of a goal-directed action [19]. The inability to identify and predict actions could be one of the causes of the difficulties in social interaction and the perception of social information in individuals with ASD [21, 22].
Despite the strong association between ASD and deficit in ToM abilities, the literature reports conflicting results [23–25]. Some studies suggest that adults with ASD have significant difficulty in inferring the mental and emotional states of others [26–29]. On the other hand, other studies report similar performances in both individuals with ASD and healthy controls in ToM's tasks [30, 31]. Furthermore, there is a scarcity of data available to demonstrate, at group level, how individuals with ASD, especially adolescents and adults, compare with IQ-matched healthy controls [24]. Several instruments have been developed to evaluate mentalizing ability, such as the Reading the Mind in the Eyes test [26, 32], the Theory of Mind Assessment Scale [33], and the Edinburgh Social Cognition Test [23], whose psychometric properties have been evaluated. However, the use of ToM measures remains mainly confined to the field of research, and their application in clinical practice is still a challenge. In addition, most measures for assessing ToM, such as the Strange Stories [27], require well-developed expressive and receptive language (including, for example, long verbal descriptions and instructions), or involve a huge memory load making it difficult to administer to individuals with ASD that have impaired verbal and cognitive abilities [29, 34, 35].
Several strategies have been suggested to simplify the ToM tasks and facilitate comprehension of the instructions: in some cases, situations similar to the subjects' daily lives were presented; in others, repetition of the story was made available when the patient seemed not to understand; and in yet others, visual aids such as drawings or vignettes were provided [36–39]. The use of vignettes seems to have proved useful in the assessment of ToM in clinical populations, particularly in schizophrenia [40].
We could not find studies in which there is a corresponding task of understanding the intentions used in young adults and adults with ASD. In two of our previous studies [5, 34], we used a comic strip task in children with ASD. In these studies, children were presented with three pictures that told a social story; they were then given two images representing alternative endings and asked to choose the appropriate one [41, 42]. We wondered whether the construction of a similar task using comic strips in which the linguistic and memory component was minimized might be useful in demonstrating that a deficit in intention attribution does not depend on other cognitive deficits. In accordance with Baron-Cohen and collaborators [43], we compared the abilities of the ToM with the understanding of physical causality. Based on these premises, we propose the Intentions Attribution-Comic Strip Test (IA-CST), a test aimed at evaluating the ability to infer characters’ intentions and understand their behaviors. This test can be interpreted as a measure of basic ToM ability and as a precursor of higher-order mentalization skills. Thus, the aim of our study is twofold: (1) to validate a new non-verbal test for the evaluating the attribution of intentions on a large sample, including adolescents and adults (Study 1); (2) to compare the performance of individuals with ASD with IQ-matched controls (Study 2). In a broader perspective, our study aims to provide a standardized tool for the evaluation of a ToM ability that is practical and language-free, to be integrated within the clinical setting to support diagnostic evaluation and intervention planning in ASD.
Study 1: construction and validation of the IA-CST
Methods
Procedure
Scale validation was performed following three phases [44]. In the first phase, items were constructed and reviewed by experts (2 psychometricians, 1 neuropsychologist, and 1 biostatistician) with extensive knowledge and experience in the field of autism and social cognition. Successively, a convenience sample was used to remove possible confounding items (i.e., items that might have been unclear or difficult to interpret). In the second phase, we performed a structural validation of the scale performing an Exploratory Graph Analysis and a Confirmatory Factor Analysis. Moreover, the relation between performance on the IA-CST and the Advanced Theory of Mind [45, 46], a verbal test of cognitive ToM (concurrent validity), was analyzed.
Lastly, in the third phase reported in Study 2, we preliminarily assess external validation of the new measure assessing known group validity.
The Ethics Committee approved the protocol prior to the recruitment of participants, according to the principles established by the Declaration of Helsinki. Written informed consent and socio-demographic information were provided by all participants, as well as their parents when underage, prior to test administration. Each participant was assessed individually in a quiet room without any distractions. Their responses were registered using paper and pencil. A psychologist was present in the room during the administration to provide any further information if necessary.
Measures
Raven’s Standard Progressive Matrices
Raven’s Standard Progressive Matrices (SPM) [47] were used to assess non-verbal intelligence and IQ level was calculated following conversion tables. The SPM consists of 60 items, each of which requires the completion of a set of figures with the missing one, from a presented pattern. Each item becomes progressively more difficult, requiring analysis, coding and interpretation skills. We chose to use SPM as its administration is shorter, less demanding and less stressful than typical IQ tests (e.g., Wechsler scales).
Advanced Theory of Mind (A-ToM)
A-ToM [45, 46] is an Italian adaptation of a cognitive ToM task (i.e., Strange Stories) that Blair and Cipollotti [45] used and was first proposed by Happé [27]. Happé [27] defined the Strange Stories task as an “advanced” ToM task and proposed that it would be useful for individuals with high-functioning forms of ASD who might otherwise succeed at (first-order) ToM tests. This task includes a two-level investigation of the story protagonist’s mental states, because the stories contain an understanding question and a key question to explain the cause of his/her behavior [48]. In our study, we used the Italian adaptation [46], which consists of a shorter version of 13 stories that describe real events; for correct interpretation, the task requires the subject to go beyond the literal meaning of the text and to draw an inference about the story protagonist’s mental state. A score of 1 is assigned for each item if the comprehension and the justification questions are answered correctly, and 0 otherwise. For more details, please refer to Happé [27] and Mazza et al. [29].
Intention attribution-comic strip test (IA-CST)
IA-CST is a comic strip test that evaluates the ability to infer characters’ intentions and understand their behaviors. It consists of six cartoon-like vignettes illustrating a sequence of purposeful actions performed by a character in a daily life scenario. The test also includes a series of items assessing physical causality. For each item, participants are shown three vignettes describing the action or causal relation, after which they are presented with three vignettes each containing an alternative conclusion to the scenario. The participant is asked to choose the correct ending from those proposed. Specifically, the three possible endings represent: (a) the correct ending, which is understandable if the participant can understand the protagonist's intention (or causal relation); (b) a wrong ending very similar to the last picture of the sequence; c) a wrong ending describing an everyday action not associated with the sequence. Correct conclusions indicated by participants represent a score of 1 point, and 0 otherwise.
Construction of IA-CST
A total of 38 stimuli were originally constructed by one member of the research team. Stimuli were designed to elicit the participant’s ability to deduce the character’s intention or physical causal inference. Specifically, intention stimuli were designed to try to elicit first- or second-order intentions. After constructing the items, the research team met in order to review each of the proposed stimuli following a qualitative content validity approach. During this meeting, items considered unclear or possible confounders or not an adequate measure of target dimensions were reviewed or discarded. A total of 12 stimuli were removed from the pool, while the remaining were considered adequate by the whole research team. Then, the 26 stimuli were administered to the healthy sample (see Participant section). The percentage of correct responses for each item was calculated. This preliminary analysis led to the discarding of three other items because they presented an extremely high percentage of errors (> 70%); the subsequent review of these items indicated that their correct answers could be difficult to interpret and they were discarded. At the end of this phase, the number of items considered adequate for the subsequent step was 23, henceforth coded from I1 to I23.
Participants
A total of 261 healthy individuals (age range in years 14–48, mean chronological age 20.09 ± 4.71, 122 males and 139 females, IQ mean 94.57 ± 4.22), participated in the study during the second phase of the study. They were Italian native speakers and recruited by opportunity from local structures and organizations. If a participant reported a current or past history of substance abuse, neurological and/or psychiatric disorders were excluded from the study. Details of the participants' characteristics are given in Table 1.
Table 1.
Socio-demographic data and social cognition measures results of the healthy sample
| Group age | Full sample | Statistics | p | ||
|---|---|---|---|---|---|
| 14–18 | 19–48 | ||||
| N | 82 | 179 | 261 | ||
| Gender | |||||
| Male (N) | 52 | 70 | 122 | < 0.01 | |
| Female (N) | 30 | 109 | 139 | ||
| Mean age (SD) | 15.18 (1.08) | 22.34 (3.97) | 20.09 (4.71) | ||
| Mean years of education (SD) | 10.16 (1.02) | 14.97 (1.14) | 13.46 (2.49) | ||
| Mean QI (SD) | 94.44 (4.46) | 94.64 (4.11) | 94.57 (4.22) | F1,259 = 0.123 | 0.73 |
| Social cognition measures | |||||
| A-ToM | 9.30 (1.49) | 11.26 (1.92) | 10.65 (2.01) | F1,259 = 62.93 | < 0.001 |
| Total IA-CST | 8.45 (0.71) | 8.33 (0.68) | 8.36 (0.69) | F1,259 = 1.85 | 0.17 |
Significant comparisons (p < 0.05) are reported in bold
Statistical analysis
Exploratory Graph Analysis (EGA) was performed to investigate items latent factors of the scale. This method was originally proposed by Golino and Epskamp [49]. Within this framework items of the scale are considered nodes of the network and edges between nodes partial correlation coefficients [50]. In this approach, latent constructs are characterized in terms of subnetworks within a wider network described by nodes (item) and edges (partial correlations). The main goal of EGA is to detect clusters of highly connected nodes, according to a separation function, known as modularity [51], and a corresponding optimal separation configuration. This goal is achieved by performing separated random walk explorations which should stochastically converge to the optimal cluster separation if the pattern exists. This approach should enable us to set out the underlying subnetwork structure. Some points of strength of this approach are that its results are comparable, or even better, to other traditional techniques used to detect latent dimensions [49, 50, 52], it can overcome problems related to the choice of a rotation method [53, 54], and it reduces the risk of researcher-related error or bias [50, 53].
A Gaussian Graphical Model (GGM) [55], was estimated through a variant of the least absolute shrinkage and selection operator (LASSO) [56], namely graphical LASSO [57]. This is a regularization technique used to estimate the model and parameters of the GGM [58].
A tuning parameter is set by minimizing an Extended Bayesian Criteria (EBIC) [59], which is an in-index to estimate optimal model fitting [60], which, in turn, is regulated by a parameter γ. EGA implements and algorithm which sets γ based on network resulting connections. For a detailed description please refer to Golino et al. [50].
LASSO reduces at zero edges with little values [60, 61] allowing to limit false-positive edges and returning conservative and replicable results [62]. Number of dimensions were detected using the Walktrap algorithm [63] as proposed by Golino and Epskamp [49]. Then stability of dimensions and items were evaluated through a bootstrap approach to assess the generalizability of results as proposed by Christensen and Golino [64]. A non-parametric bootstrap was performed with 1000 iterations. Descriptive statistics such as median, CI 95% and frequency of numbers of factors were obtained through all the bootstraps. Moreover, structural consistency, i.e., how often the empirical EGA dimension is exactly replicated, and item stability, i.e., each times each item is placed in each dimension, as indicated by Christensen and Golino [64], item stability values ≥ 0.70 were considered as acceptable. Unstable items were then removed, and another model was constructed without those items.
The final model was then further evaluated by performing confirmatory factor analysis (CFA) and goodness of fit was assessed by calculating comparative fit index (CFI), root mean square of error approximation (RMSEA), goodness of fit index (GFI) and Chi-square/degrees of freedom (Chisq/df) where a good fitting was considered by CFI > 0.90; RMSEA < 0.08, GFI > 0.90 and Chisq/df < 3. The internal consistency and reliability were assessed using Cronbach’s α. In addition, we performed Pearson correlation to investigate the relationship between the IA-CST and a verbal test of social cognition (A-ToM).
Results
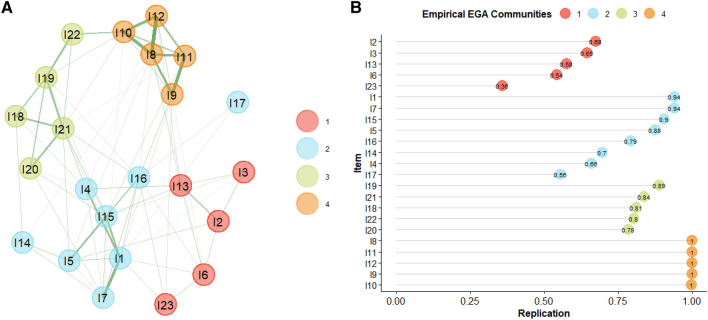
The first network resulting from EGA is reported in Fig. 1A, the model indicates a 4-dimension solution. The median number of dimensions showed by the bootstrapped networks was 4 (CI 95% [2.54, 5.45]). This analysis indicated that 48% of the networks were characterized by 4 dimensions. In terms of structural consistency, namely how frequent is the occurrence of a dimension, we got the following distribution: dimension 1 was observed 19% times, dimension 2 was 22%, dimension 3 was 60% and dimension 4 was 99%, indicating low structural consistency for all dimensions, except for dimension number 4.
Fig. 1.
Exploratory graph analysis and item stability results from the first item selection
Taken together, these results were interpreted as indicating low structural stability of the networks.
This conclusion was further confirmed by item stability analysis (Fig. 1B) where all of Dimension 1’s items were not stable (< 0.70). These results lead to remove all the items of Dimension 1. Moreover, Dimension 2 showed two unstable items (item stability < 0.70) and one item with stability near the cut-off (I14, item stability = 0.71), we choose to comprehend this item within unstable items, accordingly these three items were removed. Items were removed to find a better structural solution for the network. Then, items from the three Dimensions (excluding Dimension 1) were reviewed to assess the construct represented by each of them. The revision indicated that items of Dimension 2 were evaluating First Order Intention Attribution except for one item which was considered a causal effect item by the research team, thus it was removed from further analysis. Revision of Dimension 3 items indicated that they measure Causal Inference while revision of items of Dimension 4 indicated that they measure Second Order Intention Attribution. Thus, the remaining items were used to conduct a second EGA.
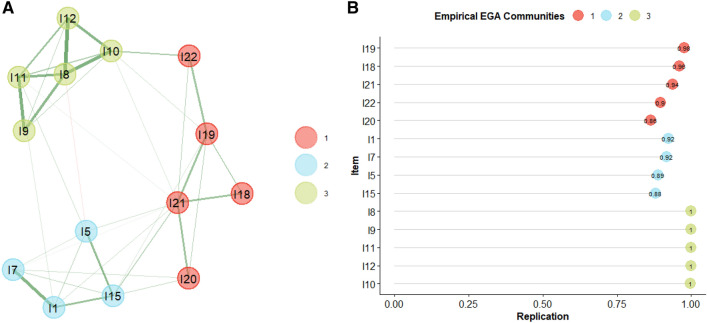
The results of the second EGA are reported in Fig. 2A. The model indicates a 3-dimension solution, where Dimension 1 represents Causal Inference, Dimension 2 represents Second Order Intention Attribution and Dimension 3 represents First Order Intention Attribution, according to the previous review. A description of each dimension is reported in Table 2.
Fig. 2.
Exploratory graph analysis and item stability results after unstable items removal
Table 2.
Dimensions’ description of the final network model
| Dimension | Description | Vignette example | Item |
|---|---|---|---|
| Causal Inference (Control Condition) | A correct answer implies an understanding of the cause–effect relationship of the objects or people in the scene. Inference of intention is not required to pass these items | The cartoon shows an object X going against an object Y. The correct answer shows Y being moved by object X | I18, I19, I20, I21, I22 |
| First Order Intention Attribution | A correct answer implies an understanding of the character’s intentions. The correct answer, to be indicated by the participant, shows the very moment of the achievement of the goal by the protagonist of the scene | The cartoon shows a person who wants to reach an object beyond his reach. Correct answer depicts the man with the object in his hand | I8, I9, I10, I11, I12 |
| Second Order Intention Attribution | A correct answer implies an understanding of the character’s intentions. The correct answer, to be indicated by the participant, shows an action necessary to achieve the goal, which is not yet reached by the protagonist | The cartoon shows a person who wants to reach an object beyond his reach. Correct answer depicts the man while performing an action that will enable him to achieve the objective | I1, I5, I7, I15 |
Bootstrap results indicated a median of 3 dimensions with a relative narrow CI 95% [2.34, 3.65], this number of dimensions was obtained in 88% of the simulated networks. Structural consistency results indicated good stability for Dimension 1 (78%), Dimension 2 (85%), and Dimension 3 (99%). Taken together these results were interpreted as indicating good structural stability of the networks. This conclusion was also confirmed by item stability results where all items showed high item stability values (all > 0.80, please refer to Fig. 2B). Thus, a 3-dimension structure for the items considered in the analysis could be considered as a stable and replicable solution.
Finally, as further confirmation, the three-dimension model represented in Fig. 2, was further inspected through CFA. The CFA model fitting of data resulted in CFI = 0.86; RMSEA = 0.07, GFI = 0.91 and Chisq/df = 2.55. Considering that RMSEA, GFI and Chisq/df indicated a good fitting, while CFI reached a close value to 0.90, we interpreted this result as an acceptable fitting of the data. Thus, based on EGA results and CFA results we established as final structure of the IA-CST the three-dimension model, shown in Fig. 2.
In summary, the network analysis performed two structural stability analyses, one referred to the number of dimensions representing our data, and another one to the item stability, letting us understand how much chance influenced the latent items structures.
Final scale: the IA-CST
The final scale consists of 14 stimuli divided into three subscales: Causal Inference (C IA-CST), First Order Intention (1st IA-CST) and Second Order Intention (2nd IA-CST). The total scores for each subscale that can be obtained range from 0 to 5 for C IA-CST, 0–5 for 1st IA-CST and 0–4 for 2nd IA-CST. For the Causal Inference, we calculated the 5th percentile as a threshold; the results indicated that to proceed to the other two conditions, the subject should correctly answer at least 4 out of 5 control items.
Moreover, a total score of Intention Attribution abilities was obtained summing the dimensions of First and Second order Intention attribution scores (Total IA-CST = 1st IA-CST + 2nd IA-CST, range 0–9). Reliability for the Total IA-CST was good (α = 0.7) and very good for 1st IA-CST subscale (α = 0.84); an acceptable reliability was found for the 2nd IA-CST (α = 0.6) and C IA-CST (α = 0.6) subscales. Correlation results show that subscales of First and Second order Intentions significantly correlated with A-ToM total score (r = 0.324, p = 0.017 – r = 0.390, p = 0.004, respectively). In addition, the total score of Intention Attribution abilities correlated with the total score of the A-ToM (r = 0.492, p < 0.001).
Study 2: clinical validity
Methods
Participant
Thirty-two Level 1 ASD (mean chronological age 18.53 ± 2.53, mean IQ 94.07 ± 10.58, mean years of education 10.84 ± 1.61) were recruited by the Reference Regional Centre for Autism (CRRA) [65]. The diagnosis was made according to Autism Diagnostic Observation Schedule-2 [66] and the Diagnostic and Statistical Manual of Mental Disorders-5th [67] by experienced clinicians. ASD participants were excluded from the study if they presented concurrent psychiatric or medical conditions and cognitive impairment. Moreover, 32 typically developing (TD) participants (mean chronological age 19.19 ± 3.01, mean IQ 95.70 ± 4.44, mean years of education 12.63 ± 2.83) were matched with ASD by IQ, age, and gender.
Statistical analysis
To preliminary assess the IA-CST potential clinical use and known group validity, the resulting IA-CST task from the previous analysis was then assessed to a group of 32 TD and 32 ASD matched by IQ, gender, and chronological age. IA-CST scores were compared through the Mann–Whitney test, and group differences were assessed with a non-parametric test as, given sample sizes, we performed a Shapiro–Wilk test which indicated that the IA-CST measures for both groups did not follow a normal distribution. We performed Spearman correlations between IQ, age and years of education with IA-CST scores for both groups to explore dimensions associated with the measure. A binomial logistic model was implemented to understand if scores on the IA-CST could be used to predict ASD diagnosis, then diagnostic performance was evaluated by Receiver Operating Characteristic curve (ROC), the optimal cut-off was estimated by Youden Index, AUC accuracy values between 0.90–1.00 were considered as excellent, 0.80–0.90 as good accuracy, 0.70–0.80 as fair accuracy and 0.70–0.60 as poor accuracy [68]. Then an EGA approach was also performed with the ASD sample to assess IA-CST dimensions within the clinical group, however, the best fitting model was an empty network, thus results are not reported.
Analysis was performed using R [69], the EGAnet package version 0.9.9 [70], and the lavaan package, version 0.5–12 BETA [71]. ROC curve analysis was performed with SPSS 25.0 [72].
Results
Thirty-two young adults with ASD (28 males and 4 females) were compared with 32 TD participants (25 males and 7 females). Groups were matched by IQ (t(62) = 0.80; p = 0.42) and did not show differences regarding chronological age (t(62) = 0.94, p = 0.34) and gender (χ2 (1, N = 64) = 0.98, p = 0.32). The two groups show a difference in years of education (t(62) = 3.09, p < 0.01). Demographical and clinical information are reported in Table 3. Then, according to item clusters reported in Fig. 2 and in Table 1, groups were then compared on the total score of Causal Inference (C IA-CST), First Order Intention (1st IA-CST), and Second Order Intention (2nd IA-CST) of the IA-CST.
Table 3.
Demographic data of the samples and clinical information regarding ASD
| ASD (N = 32) |
TD (N = 32) |
Test statistic | p | |
|---|---|---|---|---|
| Gender (M; F) | 28; 4 | 25; 7 | χ2 (1, N = 64) = 0.98 | 0.32 |
| Mean chronological age (SD) | 18.53 (2.53) | 19.19 (3.01) | t(62) = 0.94 | 0.34 |
| Mean IQ (SD) | 94.07 (10.58) | 95.70 (4.44) | t(62) = 0.80 | 0.42 |
| Mean years of education (SD) | 10.84 (1.61) | 12.63 (2.83) | t(62) = 3.09 | < 0.01 |
| Clinical information | ||||
| Mean year of first diagnosis (SD) | 10.68 (5.34) | – | – | – |
| ADOS-2 (Module 4) | – | – | – | |
| Communication | 4.33 (1.63) | – | – | – |
| Social interaction | 8.66 (3.35) | – | – | – |
| Communication + social interaction | 13.00 (4.62) | – | – | – |
| Stereotyped behaviors and restricted interests | 0.40 (0.51) | – | – | – |
Significant comparisons (p < 0.05) are reported in bold
IA-CST scores for both groups are presented in Table 4. Results indicated a significant difference in 1st IA-CST (U = 318.00, z = − 2.95, p = 0.003) where the ASD group showed lower scores (Mdn = 4.50) compared to the TD group (Mdn = 5.00) and, a significant difference in 2nd IA-CST (U = 292, z = − 3.40, p < 0.001) where the ASD group showed lower scores (Mdn = 3.50) compared to the TD group (Mdn = 4.00); a significant difference in Total IA-CST (U = 251.50, z = − 3.66, p < 0.001) where the ASD group showed lower scores (Mdn = 8.00) compared to the TD group (Mdn = 9.00). Results did not indicate a difference regarding C IA-CST (U = 386.00. z = − 1.82, p = 0.068) within the comparison between ASD (Mdn = 5.00) and TD (Mdn = 5.00).
Table 4.
IA-CST scores comparison between ASD and TD groups, and descriptive statistics according to gender
| ASD group | TD group | Z | p | |||
|---|---|---|---|---|---|---|
| Total sample (N = 32) | Total sample (N = 32) | |||||
| Mean (SD) | Median (1st–3rd quartile) | Mean (SD) | Median (1st–3rd quartile) | |||
| C IA-CST | 4.54 (0.76) | 5 (4–5) | 4.83 (0.45) | 5 (5–5) | − 1.82 | 0.068 |
| 1st IA-CST | 4.28 (0.92) | 4.50 (4–5) | 4.83 (0.37) | 5 (5–5) | − 2.95 | 0.003 |
| 2nd IA-CST | 2.96 (1.25) | 3.50 (2–4) | 3.87 (0.34) | 4 (4–4) | − 3.40 | < 0.001 |
| Total IA-CST | 7.25 (1.84) | 8 (6–9) | 8.70 (0.46) | 9 (8–9) | − 3.66 | < 0.001 |
| IA-CST score by gender | ||||||
|---|---|---|---|---|---|---|
| ASD males (N = 28) | ASD females (N = 4) | |||||
| Mean (SD) | Median (1st–3rd quartile) | Mean (SD) | Median (1st–3rd quartile) | |||
| C IA-CST | 4.48 (0.80) | 5 (4–5) | 5.00 (0.00) | 5 (5–5) | ||
| 1st IA-CST | 4.25 (0.96) | 4.5 (4–5) | 4.50 (0.57) | 4.5 (4–5) | ||
| 2nd IA-CST | 2.96 (1.26) | 3.5 (2–4) | 3.00 (1.41) | 3.5 (1.5–4) | ||
| Total IA-CST | 7.21 (1.87) | 8 (6–9) | 7.50 (1.91) | 8 (5.5–9) | ||
| TD males (N = 25) | TD females (N = 7) | |||||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (1st–3rd quartile) | Mean (SD) | Median (1st–3rd quartile) | |||
| C IA-CST | 4.86 (0.35) | 5 (5–5) | 4.81 (0.54) | 5 (5–5) | ||
| 1st IA-CST | 4.86 (0.35) | 5 (5–5) | 4.81 (0.40) | 5 (5–5) | ||
| 2nd IA-CST | 3.86 (0.35) | 4 (4–4) | 3.87 (0.34) | 4 (4–4) | ||
| Total IA-CST | 8.73 (0.45) | 9 (8–9) | 8.68 (0.47) | 9 (8–9) | ||
Z statistics are obtained from Mann–Whitney test; significant differences (p < 0.05) are reported in bold
Spearman correlations are reported in Table 5, results indicated that only IQ for the ASD group was significantly correlated with C IA-CST (r = 0.42; p = 0.02).
Table 5.
Spearman correlations for both groups between IA and age with IA-CST scores
| C IA-CST | 1st IA-CST | 2nd IA-CST | Total IA-CST | |
|---|---|---|---|---|
| TD group | ||||
| Chronological age | 0.20 | − 0.01 | 0.26 | 0.17 |
| IQ | 0.02 | − 0.10 | − 0.05 | − 0.12 |
| Years of education | 0.13 | − 0.02 | 0.25 | 0.17 |
| ASD group | ||||
| Chronological age | − 0.06 | 0.03 | 0.12 | 0.05 |
| IQ | 0.42 | 0.23 | 0.23 | 0.32 |
| Years of education | 0.14 | 0.17 | 0.13 | 0.14 |
Significant correlations (p < 0.05) are reported in bold
Finally, logistic regression was carried out to evaluate the effect of Total IA-CST on the likelihood on receive an ASD diagnosis or not. The model was statistically significant if compared to a null model (χ2(1) = 19.60, p < 0.001), furthermore, it explained 36% of the variation in group membership (according to Nagelkerke R2) and it correctly predicted 68% of the sample. Total IA-CST was a significant predictor (β = − 1.22, SE = 0.40, Wald’s χ2(1) = 9.24, p = 0.002) indicating that this score could help in differentiating between ASD and TD groups. Based on this outcome, we constructed a ROC curve with Total IA-CST score to address its diagnostic performance. ROC curve of Total IA-CST results indicated a fair classification accuracy (AUC = 0.75, SE = 0.06, p = 0.001, CI 95% [0.63, 0.87]), where the best cut-off for an ASD diagnosis was a score < 9 (sensitivity = 71%, specificity = 66%, overall correct classification = 68%).
Discussion
Theory of Mind is widely studied in ASD research, and several instruments have been developed to evaluate this ability [29, 34, 73]. Moreover, most standardized tests of ToM require well-developed expressive and receptive language skills and cannot be used among individuals with little or no verbal ability [29, 34, 35]. This leads to the exclusion of a sub-group of individuals with ASD (those with moderate-or-severe language impairments and/or individuals with intellectual disabilities) for the assessment of ToM abilities whose deficit is considered one of the main characteristics of this clinical condition [3, 26, 35, 74]. Furthermore, many ToM tasks are long or complex, so they could be difficult to apply in clinical settings [75]. The present study was conducted to contribute to evaluating the reliability and validity of a non-verbal test, the IA-CST, that could be introduced in clinical practice to support diagnostic evaluation. The IA-CST consists of a series of cartoon-type stimuli to assess intentionality and causal inference (control condition). To the best of our knowledge, currently, there are no standardized batteries or single tests that allow an assessment of non-verbal attribution of intentions.
Results from structural analysis of the IA-CST indicated unstable items, which were removed, and a three-dimension model structure as optimal; CFA further confirmed this solution, indicating that it was suitable for our sample. Thus, we obtained three subscores, namely First Order Intention Attribution, Second Order Intention Attribution and Causal Inference, as the control score, then a Total Score of Intention Attribution could be calculated by summing the two intention attribution scores. Specifically, for items investigating First Order Intention Attribution, the story is constructed so that the correct ending shows the moment when the character achieves the goal (e.g., the character has the object he wanted in his hand); in Second Order Intention Attribution, the correct ending shows the character performing the action necessary to achieve the goal. The Causal Inference condition does not involve an inference of intentions but an understanding of the cause–effect relationship of objects or people in the scene (e.g., an object as it falls). We suggest using the score obtained in the Causal Inference condition as a control score, which allows access to the intention attribution series if the subject is able to identify at least 4 of the 5 items. Furthermore, our results show that the IA-CST shows good concurrent validity. Although we found significant but weak correlations between the two subscales of First and Second Order Intentions with the A-ToM, the Total Score of the Intentions Attribution showed a moderate correlation with the A-ToM. These results are consistent with the hypothesis that the ability to understand the intentions of others by observing their actions is a prerequisite for the ability to produce reasoning about the mental state, and explain the thoughts and feelings of others, as measured by the A-ToM [1, 76]. In fact, according to Happé and Frith [4], the construct of social cognition can be understood as a complex network that includes distinct components, such as agent identification, self-processing and mental state attribution. All these components are interconnected and influence the development of appropriate social behaviors [4, 77–80]. Our results demonstrate how the different components of social cognition, as measured by the tests used, represent different but related skills and are involved in understanding social agents and social interactions. In particular, we supported the hypothesis that one component, such as action recognition and intention attribution assessed by the IA-CST, may be a (sub)component of another, such as mental state attribution and empathy [79]. It is known that in ASD individuals there is an atypical development of the different (sub)components of social cognition [80], so having specific measures assessing single abilities represents an added value in both the research and clinical field. In Study 2, we preliminarily assessed the clinical validity of the IA-CST and compared the performance of the clinical group, ASD individuals, with IQ-matched controls. The group comparison indicated that ASD individuals show no difficulty in the control condition compared to the IQ-matched group. In contrast, the ASD group shows a significantly lower performance in the two series investigating intention attribution. This confirms that the IA-CST is a useful test with which to identify subtle impairments in intention attribution, which is independent of the comprehension of physical events but is related only to mentalizing abilities. In fact, our results showed that individuals with ASD are significantly impaired in the intention attribution conditions, highlighting an impairment in the mechanisms necessary to understand the intentions of others. Since individuals with ASD are known to show difficulties in social cognition processes [27, 43, 77, 79], one would expect that even in the IA-CST, individuals with ASD would experience difficulties, so this finding indicates the known group validity of the task. Regression analysis confirms this evidence, demonstrating that the ability to attribute intentions is a significant predictor in differentiating between ASD and TD individuals. Further confirmation is provided by ROC analysis, which supports the effectiveness of the IA-CST in discriminating, with a fair level of accuracy, between the ASD and the TD group. The best sensitivity and specificity of the IA-CST, and therefore a higher probability of identifying individuals with ASD, were obtained with a score < 9 (best cut-off). Taken together, these results suggest the potential for using the test in clinical practice. The IA-CST would provide an additional tool during the diagnostic process, incorporating, and placed alongside measures considered to be the gold-standard for the diagnosis and assessment of autism. Standard neuropsychological assessments and diagnostic procedures often lack information on social cognition abilities and, as a result, do not provide appropriate indications for the treatment of these deficits [81]. Impairments in ToM abilities are early and relevant in ASD, so assessing these aspects, along with symptomatic, cognitive, functional, and adaptive features, is also valuable for structuring interventions.
We propose an instrument that is easy to administer and allows for the assessment of a basic ToM ability necessary for the development of higher-level skills. The assessment of the different components of social cognition in autism is important for the delineation of a functioning profile of the individual, highlighting strengths and weaknesses. Currently, the interventions for ASD adolescents and adults most frequently reported in the literature [82] are mainly aimed at improving communicative-relational abilities. However, whether the treatment does not consider the potential impairment of more basic abilities, the risk is that interventions on higher-level abilities will not be effective. The construction of interventions must therefore be individualized, evidence-based, and targeted to the specific impaired skill. The use of specific tests, such as the one we have proposed, also provides valuable information for the structuring and follow-up of interventions for low and medium functioning individuals.
We are aware that our study has some limitations. One of these is the relatively small ASD sample sizes; future studies should explore differences and the diagnostic performance of the IA-CST with larger sample sizes. Moreover, our preliminary results indicated a fair level of accuracy in terms of classification, suggesting its potential for use in clinical-diagnostic assessments. There are gold-standard instruments that clearly the IA-CST cannot replace, but it can be used as an integrative tool to assess another dimension of individual functioning. From this perspective, we would like to underline that a strength of the IA-CST is its short length (14 items in total) and the possibility of administering it to people with poor verbal skills. Our test involves visual-perceptual processing skills. The literature often reports that individuals with ASD have high levels of visual discrimination and perceptual functioning [83, 84], however, this aspect remains controversial [85, 86]. In fact, some studies have shown that individuals with ASD have a deficit in visual processing [85–87]. For example, it has been suggested that atypical gaze patterns in ASD individuals may affect the ability to understand the observed actions, primarily due to abnormalities in visual attention [86, 88]. The population with ASD is largely heterogeneous, so it is likely that, for some individuals with ASD, the use of pictures may facilitate performance, while for others their use may be a disadvantage [86]; when interpreting results, it is important to bear this in mind. One of the fundamental tasks of the clinician is to choose the most appropriate tests based on the characteristics of the individual concerned. During the evaluation process, the clinician should consider any additional relevant investigations in order to fully understand and properly interpret the results.
Among the limitations, we should report that the validation process could be further expanded. The significant correlations that emerged between the IA-CST and the A-ToM were weak/moderate, so this should be further investigated. Moreover, in our framework, items were reviewed by experts; then, through EGA we assessed the internal structure, and we evaluated its ability to discriminate a sample that is known to be impaired in the construct that the instrument is intended to measure.
Furthermore, although the absolute (RMSEA, GFI) and parsimonious (Chisq/df) fit indices reach the commonly accepted criteria, the incremental fit index (CFI) is just below the proposed cut-off (> 90) [89] and, from a broader perspective, we aim to improve it, although these indices should not be interpreted as binary judgements, but rather as an approximation of the data to the model that is more realistic than a perfect fit [90]. Even if our results provide evidence in favor of the validity of the proposed measure, further studies should evaluate additional aspects, for instance, assessing test–retest reliability, predictive validity, and other types of external validity. Indeed, the process of validating a measure consists in obtaining a lot of evidence in favor of its validity. Future studies should overcome these limitations and extend the application of the test also to individuals with ASD at lower functioning.
Acknowledgements
Not applicable.
Author contributions
ILD and MA contributed equally to collecting data, writing the original draft preparation, and data interpretation. AB, RV, and FM contributed to the analysis and interpretation of the data. MM conceived designed and supervised the work and data analysis. MV contributed to the critical revision and supervision of the work. All authors read and edited the manuscript and approved the final version.
Funding
The authors received no financial support for the research.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with Declaration of Helsinki and the rules of good clinical practice. The ethics committee of the NHS Local Health Unit (Azienda Sanitaria Locale 1) approved the experimental protocol (n. 0052505/21). Informed consent to participate in the study was obtained from all individual participants, as well as their parents when underage.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ilenia Le Donne and Margherita Attanasio contributed equally to this work and share first authorship.
References
- 1.Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978;1(4):515–526. doi: 10.1017/S0140525X00076512. [DOI] [Google Scholar]
- 2.Leslie AM. Pretense and representation: the origins of "theory of mind". Psychol Rev. 1987;94(4):412. doi: 10.1037/0033-295X.94.4.412. [DOI] [Google Scholar]
- 3.Mazza M, Mariano M, Peretti S, Masedu F, Pino MC, Valenti M. The role of theory of mind on social information processing in children with autism spectrum disorders: a mediation analysis. J Autism Dev Disord. 2017;47(5):1369–1379. doi: 10.1007/s10803-017-3069-5. [DOI] [PubMed] [Google Scholar]
- 4.Happé F, Frith U. Annual research review: towards a developmental neuroscience of atypical social cognition. J Child Psychol Psychiatry. 2014;55(6):553–557. doi: 10.1111/jcpp.12162. [DOI] [PubMed] [Google Scholar]
- 5.Pino MC, Mazza M, Mariano M, et al. Simple mindreading abilities predict complex theory of mind: developmental delay in autism spectrum disorders. J Autism Dev Disord. 2017;47(9):2743–2756. doi: 10.1007/s10803-017-3194-1. [DOI] [PubMed] [Google Scholar]
- 6.Aschersleben G, Hofer T, Jovanovic B. The link between infant attention to goal-directed action and later theory of mind abilities. Dev Sci. 2008;11(6):862–868. doi: 10.1111/j.1467-7687.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 7.Kozak MN, Marsh AA, Wegner DM. What do I think you're doing? Action identification and mind attribution. J Pers Soc Psychol. 2006;90(4):543–555. doi: 10.1037/0022-3514.90.4.543. [DOI] [PubMed] [Google Scholar]
- 8.Levy SR, Freitas AL, Salovey P. Construing action abstractly and blurring social distinctions: implications for perceiving homogeneity among, but also empathizing with and helping, others. J Pers Soc Psychol. 2002;83(5):1224–1238. doi: 10.1037//0022-3514.83.5.1224. [DOI] [PubMed] [Google Scholar]
- 9.Frith CD, Done DJ. Experiences of alien control in schizophrenia reflect a disorder in the central monitoring of action. Psychol Med. 1989;19(2):359–363. doi: 10.1017/s003329170001240x. [DOI] [PubMed] [Google Scholar]
- 10.Harvey PD. Reality monitoring in mania and schizophrenia. The association of thought disorder and performance. J Nerv Ment Dis. 1985;173(2):67–73. doi: 10.1097/00005053-198502000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Brüne M. "Theory of mind" in schizophrenia: a review of the literature. Schizophr Bull. 2005;31(1):21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- 12.Frith CD. Theory of mind in schizophrenia. In: David AS, Cutting JC, editors. The neuropsychology of schizophrenia. Hove: Lawrence Erlbaum Associates; 1994. [Google Scholar]
- 13.Hardy-Baylé MC. Organisation de l'action, phénomènes de conscience et représentation mentale de l'action chez des schizophrènes. Actual Psychiatr. 1994;24(1):9–18. [Google Scholar]
- 14.Sarfati Y, Hardy-Baylé MC. How do people with schizophrenia explain the behaviour of others? A study of theory of mind and its relationship to thought and speech disorganization in schizophrenia. Psychol Med. 1999;29(3):613–620. doi: 10.1017/s0033291799008326. [DOI] [PubMed] [Google Scholar]
- 15.Schütz M, Ciaramidaro A, Martinelli A, Öller R, Hartmann D, Hein G, et al. Communicative intentions in autism spectrum disorder. Res Autism Spectr Disord. 2020;79:101666. doi: 10.1016/j.rasd.2020.101666. [DOI] [Google Scholar]
- 16.Vivanti G, McCormick C, Young GS, et al. Intact and impaired mechanisms of action understanding in autism. Dev Psychol. 2011;47(3):841–856. doi: 10.1037/a0023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams D, Happé F. Representing intentions in self and other: studies of autism and typical development. Dev Sci. 2010;13(2):307–319. doi: 10.1111/j.1467-7687.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser MD, Pelphrey KA. Disrupted action perception in autism: behavioral evidence, neuroendophenotypes, and diagnostic utility. Dev Cogn Neurosci. 2012;2(1):25–35. doi: 10.1016/j.dcn.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zalla T, Labruyere N, Georgieff N. Goal-directed action representation in autism. J Autism Dev Disord. 2006;36(4):527–540. doi: 10.1007/s10803-006-0092-3. [DOI] [PubMed] [Google Scholar]
- 20.Chambon V, Farrer C, Pacherie E, Jacquet PO, Leboyer M, Zalla T. Reduced sensitivity to social priors during action prediction in adults with autism spectrum disorders. Cognition. 2017;160:17–26. doi: 10.1016/j.cognition.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Sinha P, Kjelgaard MM, Gandhi TK, et al. Autism as a disorder of prediction. Proc Natl Acad Sci U S A. 2014;111(42):15220–15225. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with autism. Psychol Sci. 2003;14(2):151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- 23.Baksh RA, Abrahams S, Bertlich M, et al. Social cognition in adults with autism spectrum disorders: validation of the Edinburgh Social Cognition Test (ESCoT) Clin Neuropsychol. 2021;35(7):1275–1293. doi: 10.1080/13854046.2020.1737236. [DOI] [PubMed] [Google Scholar]
- 24.Brewer N, Young RL, Barnett E. Measuring theory of mind in adults with autism spectrum disorder [published correction appears in J Autism Dev Disord. 2017 Jul;47(7):1942-1943] J Autism Dev Disord. 2017;47(7):1927–1941. doi: 10.1007/s10803-017-3080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole EJ, Slocombe KE, Barraclough NE. Abilities to explicitly and implicitly infer intentions from actions in adults with autism spectrum disorder. J Autism Dev Disord. 2018;48(5):1712–1726. doi: 10.1007/s10803-017-3425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The, "Reading the Mind in the Eyes" Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42(2):241–251. doi: 10.1111/1469-7610.00715. [DOI] [PubMed] [Google Scholar]
- 27.Happé FG. An advanced test of theory of mind: understanding of story characters' thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord. 1994;24(2):129–154. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- 28.Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(Pt 8):1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 29.Mazza M, Pino MC, Keller R, et al. Qualitative differences in attribution of mental states to other people in autism and schizophrenia: what are the tools for differential diagnosis? J Autism Dev Disord. 2022;52(3):1283–1298. doi: 10.1007/s10803-021-05035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkovski M, Enticott PG, Hughes ME, Rossell SL, Fitzgerald PB. Atypical neural activity in males but not females with autism spectrum disorder. J Autism Dev Disord. 2016;46(3):954–963. doi: 10.1007/s10803-015-2639-7. [DOI] [PubMed] [Google Scholar]
- 31.Roeyers H, Buysse A, Ponnet K, Pichal B. Advancing advanced mind-reading tests: empathic accuracy in adults with a pervasive developmental disorder. J Child Psychol Psychiatry. 2001;42(2):271–278. doi: 10.1111/1469-7610.00718. [DOI] [PubMed] [Google Scholar]
- 32.Olderbak S, Wilhelm O, Olaru G, Geiger M, Brenneman MW, Roberts RD. A psychometric analysis of the reading the mind in the eyes test: toward a brief form for research and applied settings. Front Psychol. 2015;6:1503. doi: 10.3389/fpsyg.2015.01503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosco FM, Gabbatore I, Tirassa M, Testa S. Psychometric properties of the theory of mind assessment scale in a sample of adolescents and adults. Front Psychol. 2016;7:566. doi: 10.3389/fpsyg.2016.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pino MC, Masedu F, Vagnetti R, et al. Validity of social cognition measures in the clinical services for autism spectrum disorder. Front Psychol. 2020;11:4. doi: 10.3389/fpsyg.2020.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colle L, Baron-Cohen S, Hill J. Do children with autism have a theory of mind? A non-verbal test of autism vs. specific language impairment. J Autism Dev Disord. 2007;37(4):716–723. doi: 10.1007/s10803-006-0198-7. [DOI] [PubMed] [Google Scholar]
- 36.Brunet E, Sarfati Y, Hardy-Baylé MC, Decety J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia. 2003;41(12):1574–1582. doi: 10.1016/s0028-3932(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 37.Drury VM, Robinson EJ, Birchwood M. 'Theory of mind' skills during an acute episode of psychosis and following recovery. Psychol Med. 1998;28(5):1101–1112. doi: 10.1017/s0033291798006850. [DOI] [PubMed] [Google Scholar]
- 38.Frith CD, Corcoran R. Exploring 'theory of mind' in people with schizophrenia. Psychol Med. 1996;26(3):521–530. doi: 10.1017/s0033291700035601. [DOI] [PubMed] [Google Scholar]
- 39.Pickup GJ, Frith CD. Theory of mind impairments in schizophrenia: symptomatology, severity and specificity. Psychol Med. 2001;31(2):207–220. doi: 10.1017/s0033291701003385. [DOI] [PubMed] [Google Scholar]
- 40.Sarfati Y, Hardy-Baylé MC, Besche C, Widlöcher D. Attribution of intentions to others in people with schizophrenia: a non-verbal exploration with comic strips. Schizophr Res. 1997;25(3):199–209. doi: 10.1016/s0920-9964(97)00025-x. [DOI] [PubMed] [Google Scholar]
- 41.Cornish K, Rinehart N, Gray K, Howlin P. Comic strip task. Melbourne: Monash University Developmental Neuroscience and Genetic Disorders Laboratory and Monash University Centre for Developmental Psychiatry and Psychology. 2010.
- 42.Sivaratnam CS, Cornish K, Gray KM, Howlin P, Rinehart NJ. Brief report: assessment of the social-emotional profile in children with autism spectrum disorders using a novel comic strip task. J Autism Dev Disord. 2012;42(11):2505–2512. doi: 10.1007/s10803-012-1498-8. [DOI] [PubMed] [Google Scholar]
- 43.Baron-Cohen S, Leslie AM, Frith U. Mechanical, behavioural and Intentional understanding of picture stories in autistic children. Br J Dev Psychol. 1986;4(2):113–125. doi: 10.1111/j.2044-835X.1986.tb01003.x. [DOI] [Google Scholar]
- 44.Flake JK, Pek J, Hehman E. Construct validation in social and personality research: current practice and recommendations. Soc Psychol Personal Sci. 2017;8(4):370–378. doi: 10.1177/1948550617693063. [DOI] [Google Scholar]
- 45.Blair RJ, Cipolotti L. Impaired social response reversal A case of 'acquired sociopathy'. Brain. 2000;123(Pt 6):1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- 46.Prior M, Sartori G, Marchi S. Cognizione sociale e comportamento: uno strumento per la misurazione. Padova. 2003.
- 47.Raven J. Human assessment and cultural factors. Boston: Springer; 1983. The progressive matrices and mill hill vocabulary scale in western societies; pp. 107–114. [Google Scholar]
- 48.Pino MC, Mazza M. The use of "Literary Fiction" to promote mentalizing ability. PLoS ONE. 2016;11(8):e0160254. doi: 10.1371/journal.pone.0160254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golino HF, Epskamp S. Exploratory graph analysis: a new approach for estimating the number of dimensions in psychological research. PLoS ONE. 2017;12(6):e0174035. doi: 10.1371/journal.pone.0174035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golino H, Shi D, Christensen AP, et al. Investigating the performance of exploratory graph analysis and traditional techniques to identify the number of latent factors: a simulation and tutorial. Psychol Methods. 2020;25(3):292–320. doi: 10.1037/met0000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newman ME. Modularity and community structure in networks. Proc Natl Acad Sci U S A. 2006;103(23):8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Golino H, Demetriou A. Estimating the dimensionality of intelligence like data using Exploratory Graph Analysis. Intelligence. 2017;62:54–70. doi: 10.1016/j.intell.2017.02.007. [DOI] [Google Scholar]
- 53.Browne MW. An overview of analytic rotation in exploratory factor analysis. Multivar Behav Res. 2001;36:111–150. doi: 10.1207/S15327906MBR3601_05. [DOI] [Google Scholar]
- 54.Christensen AP, Golino H, Silvia PJ. A psychometric network perspective on the validity and validation of personality trait questionnaires. Eur J Pers. 2020;34(6):1095–1108. doi: 10.1002/per.2265. [DOI] [Google Scholar]
- 55.Lauritzen SL. Graphical models. Oxfordshire: Clarendon Press; 1996. [Google Scholar]
- 56.Tibshirani R. Regression shrinkage and selection via the lasso. J Roy Stat Soc: Ser B (Methodol) 1996;58(1):267–288. [Google Scholar]
- 57.Friedman J, Hastie T, Tibshirani R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics. 2008;9(3):432–441. doi: 10.1093/biostatistics/kxm045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Epskamp S, Waldorp LJ, Mõttus R, Borsboom D. The Gaussian graphical model in cross-sectional and time-series data. Multivariate Behav Res. 2018;53(4):453–480. doi: 10.1080/00273171.2018.1454823. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, Chen Z. Extended Bayesian information criteria for model selection with large model spaces. Biometrika. 2008;95(3):759–771. doi: 10.1093/biomet/asn034. [DOI] [Google Scholar]
- 60.Epskamp S, Fried EI. A tutorial on regularized partial correlation networks. Psychol Methods. 2018;23(4):617–634. doi: 10.1037/met0000167. [DOI] [PubMed] [Google Scholar]
- 61.McNeish DM. Using Lasso for predictor selection and to assuage overfitting: a method long overlooked in behavioral sciences. Multivariate Behav Res. 2015;50(5):471–484. doi: 10.1080/00273171.2015.1036965. [DOI] [PubMed] [Google Scholar]
- 62.Costantini G, Epskamp S, Borsboom D, Perugini M, Mõttus R, Waldorp LJ, Cramer AO. State of the aRt personality research: a tutorial on network analysis of personality data in R. J Res Pers. 2015;54:13–29. doi: 10.1016/j.jrp.2014.07.003. [DOI] [Google Scholar]
- 63.Pons P, Latapy M. Computing communities in large networks using random walks. J Graph Algorithms Appl. 2006;10(2):191–218. doi: 10.7155/jgaa.00124. [DOI] [Google Scholar]
- 64.Christensen AP, Golino H. Estimating the stability of psychological dimensions via bootstrap exploratory graph analysis: a Monte Carlo simulation and tutorial. Psych. 2021;3(3):479–500. doi: 10.3390/psych3030032. [DOI] [Google Scholar]
- 65.Valenti M, Vagnetti R, Masedu F, Pino MC, Rossi A, Scattoni ML, et al. Register-based cumulative prevalence of Autism Spectrum Disorders during childhood and adolescence in Central Italy. Epidemiol Biostat Public Health. 2019;16(4):e13226. doi: 10.2427/13226. [DOI] [Google Scholar]
- 66.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule (ADOS- 2): manual (2nd edn). Los Angeles, CA: Western Psychological Services. 2012
- 67.American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DSM-5®) Washington, DC: American Psychiatric Pub; 2013. [Google Scholar]
- 68.Metz CE. Current problems in ROC analysis. In Proceedings of the chest imaging Conference 1987. Department of Medical Physics, University of Wisconsin-Madison; 1988.
- 69.R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. https://www.R-project.org/.
- 70.Golino H, Christensen AP. EGAnet: Exploratory Graph Analysis – A framework for estimating the number of dimensions in multivariate data using network psychometrics. R package version 0.9.9. 2021.
- 71.Rosseel Y. Lavaan: an R package for structural equation modeling and more. Version 0.5–12 (BETA) J Stat Softw. 2012;48(2):1–36. doi: 10.18637/jss.v048.i02. [DOI] [Google Scholar]
- 72.IBM Corp. IBM SPSS Statistics for Windows. Armonk, NY: IBM Corp. 2017; https://hadoop.apache.org
- 73.Morrison KE, Pinkham AE, Kelsven S, Ludwig K, Penn DL, Sasson NJ. Psychometric evaluation of social cognitive measures for adults with autism. Autism Res. 2019;12(5):766–778. doi: 10.1002/aur.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baron-Cohen S. Mindblindness: an essay on autism and theory of mind. Cambridge: MIT press; 1995. [Google Scholar]
- 75.Dodich A, Cerami C, Canessa N, Crespi C, Iannaccone S, Marcone A, Realmuto S, Lettieri G, Perani D, Cappa SF. A novel task assessing intention and emotion attribution: Italian standardization and normative data of the Story-based Empathy Task. Neurol Sci. 2015;36(10):1907–1912. doi: 10.1007/s10072-015-2281-3. [DOI] [PubMed] [Google Scholar]
- 76.Leslie AM. Pretending and believing: issues in the theory of ToMM. Cognition. 1994;50(1–3):211–238. doi: 10.1016/0010-0277(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 77.Pino MC, Vagnetti R, Masedu F, et al. Mapping the network of social cognition domains in children with autism spectrum disorder through graph analysis. Front Psychiatry. 2020;11:579339. doi: 10.3389/fpsyt.2020.579339.Published2020Oct30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vagnetti R, Pino MC, Masedu F, et al. Exploring the social cognition network in young adults with autism spectrum disorder using graph analysis. Brain Behav. 2020;10(3):e01524. doi: 10.1002/brb3.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Happé F, Cook JL, Bird G. The structure of social cognition: In(ter)dependence of sociocognitive processes. Annu Rev Psychol. 2017;3(68):243–267. doi: 10.1146/annurev-psych-010416-044046. [DOI] [PubMed] [Google Scholar]
- 80.Pino MC, Mariano M, Peretti S, D’Amico S, Masedu F, Valenti M, Mazza M. When do children with autism develop adequate social behaviour? Cross-sectional analysis of developmental trajectories. Eur J Dev Psychol. 2020;17(1):71–87. doi: 10.1080/17405629.2018.1537876. [DOI] [Google Scholar]
- 81.Henry JD, von Hippel W, Molenberghs P, Lee T, Sachdev PS. Clinical assessment of social cognitive function in neurological disorders. Nat Rev Neurol. 2016;12(1):28–39. doi: 10.1038/nrneurol.2015.229. [DOI] [PubMed] [Google Scholar]
- 82.Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383:896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 83.Baron-Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos Trans R Soc Lond B Biol Sci. 2009;364(1522):1377–1383. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- 85.Funabiki Y, Shiwa T. Weakness of visual working memory in autism. Autism Res. 2018;11(9):1245–1252. doi: 10.1002/aur.1981. [DOI] [PubMed] [Google Scholar]
- 86.Trembath D, Vivanti G, Iacono T, Dissanayake C. Accurate or assumed: visual learning in children with ASD. J Autism Dev Disord. 2015;45(10):3276–3287. doi: 10.1007/s10803-015-2488-4. [DOI] [PubMed] [Google Scholar]
- 87.Williams DL, Goldstein G, Carpenter PA, Minshew NJ. Verbal and spatial working memory in autism. J Autism Dev Disord. 2005;35(6):747–756. doi: 10.1007/s10803-005-0021-x. [DOI] [PubMed] [Google Scholar]
- 88.Masedu F, Vagnetti R, Pino MC, Valenti M, Mazza M. Comparison of visual fixation trajectories in toddlers with autism spectrum disorder and typical development: a Markov Chain Model. Brain Sci. 2021;12(1):10. doi: 10.3390/brainsci12010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hopwood CJ, Donnellan MB. How should the internal structure of personality inventories be evaluated? Pers Soc Psychol Rev. 2010;14(3):332–346. doi: 10.1177/1088868310361240. [DOI] [PubMed] [Google Scholar]
- 90.Knekta E, Runyon C, Eddy S. One size doesn't fit all: using factor analysis to gather validity evidence when using surveys in your research. CBE Life Sci Educ. 2019;18(1):rm1. doi: 10.1187/cbe.18-04-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.