Abstract
Acute kidney injury (AKI) survivors have a dynamic posthospital course which warrants close monitoring. Remote patient monitoring (RPM) could be used to improve quality and efficiency of AKI survivor care.
Objective:
The objective of this report was to describe the development and preliminary feasibility of an AKI RPM program launched in October 2021.
Setting:
Academic medical center.
Patients:
Patients enrolled in the AKI RPM program were those who experienced AKI during a hospitalization and underwent nephrology consultation.
Measurements/Methods:
At enrollment, patients were provided with home monitoring technology and underwent weekly laboratory assessments. Nurses evaluated the data daily and adhered to prespecified protocols for management and escalation of care if needed.
Results:
Twenty patients were enrolled in AKI RPM in the first 5 months. Median duration of program participation was 36 (31, 40) days. Eight patients (40%) experienced an unplanned readmission, or an emergency department visit, half (N = 4) of which were attributed to AKI and related circumstances. Of the 9 postgraduation survey respondents, all were satisfied with the RPM program and 89% would recommend RPM to other patients with similar health conditions.
Limitations:
Acute kidney injury RPM was made possible by the existing infrastructure in our integrated health system and the robust resources available in the Mayo Clinic Center for Digital Health. Such infrastructure may not be universally available which could limit scale and generalizability of such a program.
Conclusions:
Remote patient monitoring can offer a unique opportunity to bridge the care transition from hospital to home and increase access to quality care for the AKI survivors.
Keywords: remote patient monitoring, acute kidney injury, telehealth, health technology, digital health
Abrégé
Les survivants d’un épisode d’insuffisance rénale aiguë (IRA) ont un parcours post-hospitalier dynamique qui justifie une surveillance étroite. La télésurveillance des patients (TSP) pourrait être employée pour améliorer la qualité et l’efficacité des soins pour les survivants de l’IRA.
Objectif:
L’objectif de ce rapport était de décrire le développement et la faisabilité préliminaire d’un programme de TSP-IRA (télésurveillance des patients atteints d’IRA) en octobre 2021.
Cadre:
Centre médical universitaire
Sujets:
Les patients inscrits au programme de TSP-IRA étaient des patients qui avaient vécu un épisode d’IRA lors d’une hospitalisation et obtenu une consultation en néphrologie.
Mesures et méthodologie:
Au moment de l’inclusion, les patients ont reçu un dispositif de surveillance à domicile et se sont soumis à des évaluations de laboratoire hebdomadaires. Les infirmières ont évalué les données quotidiennement et ont respecté des protocoles prédéfinis pour la gestion et l’escalade des soins si nécessaire.
Résultats:
Vingt patients ont été inclus dans le programme de TSP-IRA au cours des cinq premiers mois. La durée médiane de participation au programme était de 36 (31, 40) jours. Huit patients (40%) ont dû être réadmis de façon non planifiée ou ont dû faire une visite aux urgences; pour la moitié d’entre eux (N = 4) en raison de l’IRA et de circonstances connexes. Parmi les neuf répondants qui ont répondu au sondage à la complétion du programme, tous se sont dits satisfaits du programme de TSP et 89% le recommanderaient à d’autres patients ayant des problèmes de santé similaires.
Limites:
Le programme de TSP-IRA a été rendu possible grâce à l’infrastructure existante dans notre système de santé intégré et aux ressources robustes disponibles au Mayo Clinic Center for Digital Health. Une telle infrastructure n’est peut-être pas universellement disponible, ce qui pourrait limiter l’ampleur et la généralisabilité d’un tel programme.
Conclusion:
La TSP peut offrir une occasion unique de faciliter la transition des soins entre l’hôpital et le domicile et d’accroître l’accès à des soins de qualité pour les survivants d’un épisode d’IRA.
Introduction
Acute kidney injury (AKI) affects approximately 20% of hospitalized patients 1 and is associated with worse clinical and patient-centered outcomes.1,2 One strategy to reduce the burden of long-term adverse outcomes is to enhance follow-up care.3,4 Recent evidence suggested that almost 30% of AKI patients lacked basic kidney health follow-up after hospital discharge, 5 and few received follow-up in nephrology clinics.3,4 The dynamic posthospital course and inadequate and delayed follow-up of AKI survivors exposes patients to poor outcomes during a critical time.
Digital health solutions like remote patient monitoring (RPM) could be used to improve quality and efficiency of AKI survivor care. 6 Remote monitoring has been successfully used in other medical conditions, 7 but has not been well characterized among AKI survivors. This article aimed to describe the development and implementation of a novel AKI RPM pilot program launched at Mayo Clinic.
Materials and Methods
Stakeholder Engagement
The AKI RPM program was planned and launched in collaboration with the Mayo Clinic Center for Digital Health at the Mayo Clinic Rochester, MN, campus. The study was approved by the Mayo Clinic Institutional Review Board (#22-000931).
Beginning April 2021, key stakeholders were identified and engaged including nephrologists, administrators from the hospital practice and the Center for Digital Health, inpatient and outpatient nurse managers and clinical nurse specialists, health care informaticists, scheduling support staff, and an implementation coordinator. Through the human-centered design process, we interacted directly with patients to create journey maps that were used to frame the program and implementation process. Care processes, digital infrastructure, and practice management guidelines were developed during biweekly meetings over 6 months.
Patient Identification
Candidates for AKI RPM met prespecified eligibility criteria and agreed to participate in the program. For feasibility reasons, potential AKI RPM candidates were limited to those who experienced stage 2 or 3 AKI 8 and underwent nephrology consultation while hospitalized. It was reasoned that patients with a nephrology consultation were likely to have a documented cause of AKI and a complete diagnostic work-up before discharge to assist in the postdismissal survivor care plans. Patients who required temporary acute dialysis, but were liberated by discharge, were eligible for participation. Patients treated with immunosuppression for kidney disease or transplantation were excluded due to pre-established care guidelines (Supplementary Table S1). Patients with established chronic kidney disease (CKD) and kidney care were eligible with the agreement of their primary nephrologist. Patients unable to participate in follow-up at Mayo Clinic in Rochester, MN, or in the Mayo Clinic Health System were excluded from the pilot. The Mayo Clinic Health System is a series of 44 community and rural hospitals, clinics, and care facilities across Minnesota, Iowa, and Wisconsin. Initially, patients were identified for potential participation by the hospital nephrology consulting team.
Patient Referral and Enrollment
During the discharge planning phase, patients were assessed to determine their eligibility for enrollment in the AKI RPM program. Those deemed suitable candidates were subsequently enrolled at the time of discharge. As part of the program, patients received education prior to discharge, and typically, a welcome call was made to them the day after they left the hospital. Acute kidney injury RPM technology including a scale, blood pressure cuff, pulse oximeter, and tablet for symptom assessment was then mailed directly to the patient. An introductory phone call to the program to provide education and instructions on the equipment was coordinated by the Mayo Clinic Center for Digital Health.
Monitoring and Escalation Pathways
Remote monitoring occurred for at least 4 weeks and up to 90 days after discharge. Remote patient monitoring patients were asked to monitor blood pressure, heart rate, and weight daily and report symptoms on standardized questionnaires that probed shortness of breath, edema, and bladder and bowel function. Weekly in-center laboratory assessments for serum creatinine and electrolytes were scheduled for the duration of the AKI RPM program. A urinalysis with microscopy and microalbumin were scheduled for 4 weeks after hospital dismissal.
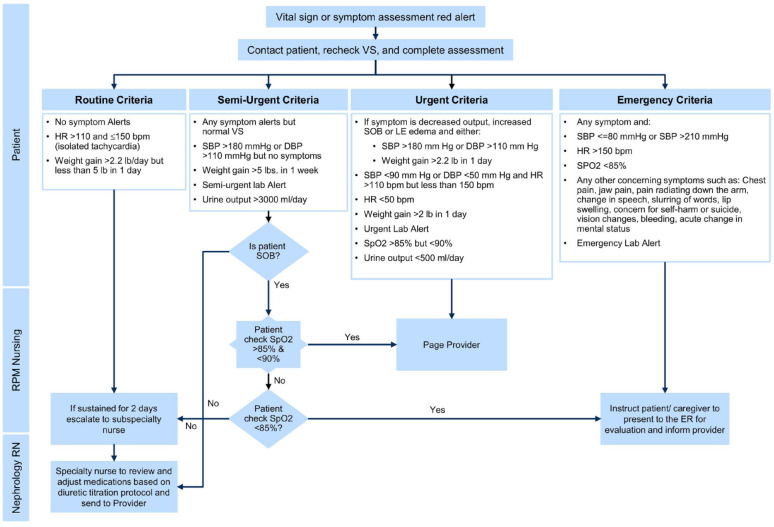
After discharge, the patients’ vital signs, self-reported symptoms, and weekly laboratory findings were reviewed remotely daily by an RPM nurse. They called the patients once weekly to review laboratory values and as needed for vital sign and symptom assessment alerts. Remote patient monitoring nurses were available 7 days per week, from 07:00 to 19:00. Values for the objective or subjective data were categorized as routine, semi-urgent, urgent, or emergent (Figure 1). Routine and semi-urgent results identified by RPM nurses were first escalated to a nurse with specialty training in nephrology. The clinical scenario was reviewed by the nephrology nurse, recommendations provided to the patient, repeat testing ordered if needed, and the supervising nephrology specialist informed as appropriate. Urgent results were escalated from the RPM nurse directly to the nephrology specialist. Emergent results prompted a direct referral to the local emergency department. All decisions were made by RPM nurses or providers, and there were no automated recommendations provided. Communication among providers occurred via electronic health record (EHR) messaging (routine or semi-urgent results) or via a dedicated pager (urgent or emergent results).
Figure 1.
The figure shows the algorithm of escalation of care based on vital signs and laboratory abnormalities. RPM nurses review data daily and call patients at a minimum once weekly. Vital signs, symptoms, and laboratory data are classified into 4 groups: routine, semi-urgent, urgent, and emergent. If a patient has a parameter that meets emergent criteria, RPM nurses direct the patient to the emergency department for further evaluation. Urgent criteria are referred directly to the nephrology provider via a dedicated pager. Patients who meet routine or semi-urgent criteria are monitored by RPM nurses in collaboration with specialty nurses. They review medications, titrate diuretics, and schedule follow-up based on a prespecified protocol.
bpm = beats per minute; DBP = diastolic blood pressure; ER = emergency room; HR = heart rate; LE = lower extremity; SBP = systolic blood pressure; SOB = shortness of breath; VS = Vital sign.
Graduation
Acute kidney injury RPM participants were eligible for graduation if they remained off dialysis, with a stable serum creatinine (defined as no increase of creatinine by more than 0.2 mg/dL) for 2 consecutive weeks and had no urgent or emergent results in the preceding 1-week interval. While the final disposition was at the discretion of the nephrologist, in general, individuals with an estimated glomerular filtration rate (eGFR) > 45 mL/min/1.73 m2 and no evidence of albuminuria were triaged to primary care. An eGFR <45 mL/min/1.73 m2 or albuminuria > 300 mg/g prompted triage to nephrology follow-up. After graduation, all participants were sent a survey inquiring about their RPM experience (Supplementary Table S2).
Iterative Prototyping
The program was launched in October 2021 and iterative refinement occurred throughout the pilot phase as part of continuous quality improvement. One major modification was the creation of an EHR-embedded list of potential AKI RPM candidates. Patient identification transitioned from a manual process to routine screening of this EHR list by nurses within the Center for Digital Health. AKI was identified with the KDIGO serum creatinine criteria 8 and listed in an EHR-registry. Admission eGFRs were calculated with the CKD EPI eGFR creatinine equation (mL/min/1.73 m2). 9 The preadmission baseline creatinine concentration for AKI staging and eGFR calculation was the median of all outpatient creatinine values in the 6-months to −7 days before the hospitalization. 10 If unavailable, this value was estimated using the MDRD equation with an estimated glomerular filtration rate (eGFR) of 60 mL/min/1.73 m2. 11 Currently, approximately 35 to 40 patients can be screened daily within about 30 minutes, although only a few patients are eligible at any given time if imminent discharge to home within our program geographical radius is expected. Another improvement was modification of escalation criteria for calcium concentrations which were frequently abnormal, but rarely of clinical consequence. For patients who were dismissed to a primary care provider, a telephone visit with a nephrology nurse was added within 1 week after graduation to confirm stability of clinical status and the forthgoing follow-up plan.
Preliminary Results
Twenty patients were enrolled in AKI RPM in the first 5 months with a median (interquartile range [IQR]) age of 67 years (61, 72), 13 (65%) were men, and all identified as non-Hispanic whites. Three patients (15%) had stage 1 AKI, 1 patient (5%) had stage 2, and 16 (80%) patients had stage 3 AKI. Five patients (25%) required an intensive care unit (ICU) stay, and 8 patients (40%) required dialysis during the index hospitalization. Median creatinine concentrations were 1.5 mg/dL (1.2, 1.7) at baseline, 3.2 mg/dL (1.8, 3.8) at admission, and 4.7 mg/dL (3.9, 5.4) at peak. Fourteen patients (70%) were discharged from the hospital on diuretics. Median duration of AKI RPM participation was 36 (31, 40) days. Eight patients (40%) experienced an unplanned readmission, or an emergency department visit, half (N = 4) of which were attributed to AKI and related circumstances.
To highlight the potential benefits of an AKI RPM program, 2 illustrative cases from our experience are summarized in Supplementary Figure S1.
Patient Satisfaction
Overall, 9 (45%) patients completed part or all of the postparticipation survey (Supplementary Table S2). The majority agreed that the RPM program helped them feel comfortable managing their health care, that the equipment was easy to use, that the team explained things in a manner that was easy to understand and would recommend RPM to other patients with similar health conditions.
Discussion
In this article, we described the development and implementation of an AKI RPM program. To the best of our knowledge, the use of RPM for AKI survivors has not been characterized. As highlighted by the illustrative cases, our experience suggests that AKI RPM may facilitate earlier hospital discharge, allow for close monitoring of electrolytes and recovering kidney function, facilitate rapid titration of kidney active medications (renally eliminated, nephrotoxic, or nephroprotective) that may have been manipulated due to the hospitalization, and enable direct handoff to the long-term care provider (nephrology specialist or primary care). Overall, preliminary results showed that patients were satisfied with the AKI RPM program, they felt comfortable using RPM equipment, and enjoyed the interaction with the team.
While AKI RPM appears promising, limitations exist. Although the program was intended to reach patients with stages 2 and 3 AKI only, in rare cases patients with stage 1 AKI were enrolled. It appeared after chart review that these were patients with severe CKD at baseline. We infer that nephrologists elected to enroll patients in AKI RPM to promote close kidney follow-up even though the degree of AKI was less severe. Acute kidney injury RPM was made possible by the existing infrastructure in our integrated health system, the robust resources available in the Mayo Clinic Center for Digital Health, and patients and caregivers demonstrating digital readiness. Such infrastructure may not be universally available which could limit scale and spread of such a program. The AKI RPM program was launched in the Midwest United States with a predominately non-Hispanic Caucasian population. Future AKI survivor efforts should explore feasibility and effectiveness in more diverse patient populations with regard to geography, degree of rurality, and race/ethnicity. This report aimed to characterize program development and key features and not the effectiveness of AKI RPM. Future research will explore relevant process metrics including alert frequency, intensity, and interventions, and outcome considerations including unplanned hospital readmissions or emergency department visits, kidney function, and patient and provider satisfaction. Key counterbalances will be monitored including the risk for excessive and sometimes unnecessary interventions that emerge with augmented clinical monitoring. Individualization of the frequency of clinical and laboratory monitoring is expected with iterative improvements to the program. The priority at the outset of program development was patient safety. Future expansion is expected to include patients not previously seen by nephrologists during their hospitalization.
Conclusions
In conclusion, the AKI RPM workflow developed was feasible and addressed the important gap for AKI care after discharging from the hospital. Digital health solutions such as RPM offer a unique opportunity to bridge the care transition from hospital to home, increase access to quality care for the most vulnerable AKI survivors, and direct the attention of nephrologists to patients most likely to benefit from specialty consultation.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581231192746 for Development and Implementation of an Acute Kidney Injury Remote Patient Monitoring Program: Research Letter by Mariam Charkviani, Erin F. Barreto, Kristina K. Pearson, Brigid M. Amberg, Rachel H. Amundson, Sarah J. Bell, Eric J. Cleveland, Craig E. Daniels, Christopher M. Kohler, Angela M. Leuenberger, Lindsey M. Philpot, David A. Ramirez, Karen J. Reinschmidt, Ziad Zoghby and Andrea G. Kattah in Canadian Journal of Kidney Health and Disease
Acknowledgments
The authors express their gratitude to Drs Sandhya Manohar, Kianoush Kashani, and Ms Vicki Hines for their contributions to the AKI RPM program.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Z.Z.—ChroniSense Medical, Ltd: consultant, Kadmon Corporation: research funding (site PI for a phase 2 clinical trial), EPIC Corporation: advisory or leadership role (unpaid), and member of Nephrology Steering Board Committee. The rest of the authors have nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the National Institute of Allergy and Infectious Diseases under award number K23AI143882 (PI; E.F.B.) and the Agency for Healthcare Research and Quality HS028060-01 (PI; E.F.B.). The funding sources had no role in study design, data collection, analysis, or interpretation, writing the report or the decision to submit the report for publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
ORCID iDs: Mariam Charkviani  https://orcid.org/0000-0002-8854-8796
https://orcid.org/0000-0002-8854-8796
Erin F. Barreto  https://orcid.org/0000-0002-0996-1487
https://orcid.org/0000-0002-0996-1487
Andrea G. Kattah  https://orcid.org/0000-0001-7228-9876
https://orcid.org/0000-0001-7228-9876
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kashani K, Rosner MH, Haase M, et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol. 2019;14(6):941-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Odutayo A, Wong CX, Farkouh M, et al. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol. 2017;28(1):377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thanapongsatorn P, Chaikomon K, Lumlertgul N, et al. Comprehensive versus standard care in post-severe acute kidney injury survivors, a randomized controlled trial. Critical Care. 2021;25(1):322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silver SA, Adhikari NK, Bell CM, et al. Nephrologist follow-up versus usual care after an acute kidney injury hospitalization (FUSION): a randomized controlled trial. Clin J Am Soc Nephrol. 2021;16(7):1005-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barreto EF, Schreier DJ, May HP, et al. Incidence of serum creatinine monitoring and outpatient visit follow-up among acute kidney injury survivors after discharge: a population-based cohort study. Am J Nephrol. 2021;52(10-11):817-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vegesna A, Tran M, Angelaccio M, Arcona S. Remote patient monitoring via non-invasive digital technologies: a systematic review. Telemed J E Health. 2017;23(1):3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scarpioni R, Manini A, Chiappini P. Remote patient monitoring in peritoneal dialysis helps reduce risk of hospitalization during Covid-19 pandemic. J Nephrol. 2020;33(6):1123-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179-84. [DOI] [PubMed] [Google Scholar]
- 9. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thongprayoon C, Cheungpasitporn W, Kittanamongkolchai W, et al. Optimum methodology for estimating baseline serum creatinine for the acute kidney injury classification. Nephrology (Carlton). 2015;20(12):881-886. [DOI] [PubMed] [Google Scholar]
- 11. Ghosh E, Eshelman L, Lanius S, et al. Estimation of baseline serum creatinine with machine learning. Am J Nephrol. 2021;52(9):753-762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581231192746 for Development and Implementation of an Acute Kidney Injury Remote Patient Monitoring Program: Research Letter by Mariam Charkviani, Erin F. Barreto, Kristina K. Pearson, Brigid M. Amberg, Rachel H. Amundson, Sarah J. Bell, Eric J. Cleveland, Craig E. Daniels, Christopher M. Kohler, Angela M. Leuenberger, Lindsey M. Philpot, David A. Ramirez, Karen J. Reinschmidt, Ziad Zoghby and Andrea G. Kattah in Canadian Journal of Kidney Health and Disease