Abstract
Isolated hypogonadotropic hypogonadism (IHH) is a rare disease with hypogonadism and infertility caused by the defects in embryonic migration of hypothalamic gonadotropin-releasing hormone (GnRH) neurons, hypothalamic GnRH secretion or GnRH signal transduction. PROKR2 gene, encoding a G-protein coupled receptor PROKR2, is one of the most frequently mutated genes identified in IHH patients. However, the functional consequences of several PROKR2 mutants remain elusive. In this study, we systematically analyzed the Gαq, Gαs and ERK1/2 signaling of 23 IHH-associated PROKR2 mutations which are yet to be functionally characterized. We demonstrate that blockage of Gαq, instead of MAPK/ERK pathway, inhibited PROK2-induced migration of PROKR2-expressing cells, implying that PROKR2-related IHH results primarily due to Gαq signaling pathway disruption. Combined with previous reports, we categorized a total of 63 IHH-associated PROKR2 mutations into four distinct groups according Gαq pathway functionality: (i) neutral (N, >80% activity); (ii) low pathogenicity (L, 50–80% activity); (iii) medium pathogenicity (M, 20–50% activity) and (iv) high pathogenicity (H, <20% activity). We further compared the cell-based functional results with in silico mutational prediction programs. Our results indicated that while Sorting Intolerant from Tolerant predictions were accurate for transmembrane region mutations, mutations localized in the intracellular and extracellular domains were accurately predicted by the Combined Annotation Dependent Depletion prediction tool. Our results thus provide a functional database that can be used to guide diagnosis and appropriate genetic counseling in IHH patients with PROKR2 mutations.
Introduction
Isolated hypogonadotropic hypogonadism (IHH) is a rare reproductive endocrine disease that typically results in absent puberty, hypogonadism and infertility. IHH is biochemically characterized by abnormally low or undetectable concentrations of circulating sex steroids in the setting of inappropriately normal or low levels of pituitary gonadotropins (1,2). Approximately 50% of IHH patients have concomitant olfactory defects, an association that is categorized as Kallmann syndrome (KS), whereas IHH patients with normal olfaction are referred to as normosmic IHH (nIHH) subjects (3,4). IHH is primarily caused by the deficiency in hypothalamic gonadotropin-releasing hormone (GnRH), which stimulates the pituitary gonadotropins (luteinizing hormone and follicle stimulating hormone) (2,5). During early embryogenesis, GnRH neurons originate from the olfactory epithelium, migrate along the axons of developing olfactory sensory neurons (olfactory/vomeronasal/terminal nerve neurons), enter the forebrain traversing the developing olfactory bulb and eventually migrate to the medial preoptic area of the hypothalamus (6). Therefore, the normal development of the olfactory structures/terminal nerve is deemed as a prerequisite for normal GnRH neuron migration (3,7–9).
Thus far, more than 60 genes have been associated with IHH amongst which the PROKR2 gene, encoding a G-protein coupled receptor, PROKR2, is one of the most frequently mutated genes. The ligand for PROKR2, PROK2, has been shown to be critical chemotactic molecule that attracts neural stem cells expressing PROKR2 to migrate from the subventricular zone (SVZ) toward the olfactory bulb (10–12). Correspondingly, mice deficient in Prok2 or Prokr2 shows atrophy of the olfactory bulb, and the neural stem cells across the rostral migratory stream are halted on the way to migration without reaching the olfactory bulb (13). Furthermore, hypothalamic GnRH neurons are severely decreased in Prok2 or Prokr2-deficient mice, leading to IHH. Guided by the phenotypes of Prok2 or Prokr2 knockout mice, candidate gene analysis in IHH patients has implicated both PROKR2 and PROK2 mutations in IHH patients across different ethnic populations. Many IHH-associated PROKR2 mutations have been previously shown to disrupt prokineticin 2 signaling function as assayed with cell-based assays. Interestingly, PROKR2 can activate different G protein signaling pathways, including Gαq, Gαs and Gαi/o pathways (14). However, some IHH-associated PROKR2 mutations may only affect a certain signaling pathway sparing the other pathways, a phenomenon described as biased signaling (15). However, it is unclear if such biased signaling is pervasive across all PROKR2 mutations. Similarly, the primary G protein signaling pathway(s) involved in the pathogenesis of PROKR2-related IHH remains to be elucidated.
To address these outstanding questions, in this study, by using a novel cell migration assay, we demonstrate that disruption of Gαq signaling pathway may primarily underlie the pathogenesis of PROKR2 mutations identified in IHH patients. Blockage of Gαq but not of MAPK/ERK signaling pathway inhibited the migration of PROKR2-expressed cells induced by the ligand PROK2. We systematically compared cell-based functional assays for all IHH-related PROKR2 mutations with commonly used in silico analysis to identify the correlations between these functional characterization methods.
Results
PROKR2 mutations from the IHH cohorts
Chinese IHH cohort: PROKR2 was the most frequently mutated gene (11.94%, 37/310) among all reported IHH-associated genes after analysis of 310 Chinese IHH probands (198 KS and 112 nIHH) with whole exome sequencing. As shown in Supplementary Material, Table S1, a total of 13 different mutations were identified in PROKR2 gene. Among them three substitutions were recurrent: W178S, Y113H and R353H mutations occurred in 20, 8 and 2 IHH patients, respectively, while rest of the mutations only appeared once in this cohort. Within 11 IHH probands in whom parental DNA were available, all probands inherited their PROKR2 mutation from their parents. One proband (L1721) carried a homozygous W178S mutation and two probands (L1701 and L1747) carried compound heterozygous mutations. The remaining majority of probands only harbored heterozygous PROKR2 mutations. Furthermore, oligogenic mutations (i.e. one patient carries gene mutations in two or more IHH genes) were very common in this cohort of IHH patients. Among the 37 probands with PROKR2 mutations, 16 probands carried only PROKR2 mutation, whereas the remaining 21 probands exhibited oligogenic inheritance with additional mutations in other IHH-associated genes.
MGH IHH cohort: Eighty four out of 1555 IHH probands (5.4%) harbored rare 37 mutations (single nucleotide variants/copy number variations) affecting the PROKR2 gene in the MGH IHH cohort. The vast majority of the PROKR2 mutations in the IHH cohort have previously been functionally characterized and published previously (16). Seven PROKR2 mutations that have not been previously functionally validated were included in this study and are shown in Supplementary Material, Table S1. Notably, all PROKR2 variants occurred in an oligogenic state with additional mutations in other IHH genes. Two subjects harbored more than one PROKR2 variant, and segregation analyses showed that neither of these patients were compound heterozygotes.
Functional analysis of IHH-related PROKR2 mutations across all GPCR-signaling pathways
To fully understand the functional consequences of the IHH-associated PROKR2 mutations, we generated 23 PROKR2 mutants which have been identified in IHH patients yet have not been fully functionally characterized (17–23). These include 9 novel mutations identified in study (Chinese and MGH IHH cohort) and 14 mutations from the literature. We used an aequorin-based assay, luciferase-based cAMP assay and the levels of p-ERK1/2 to analyze Gαq, Gαs and MAPK/ERK signal transduction, respectively. As shown in Table 1 and Supplementary Material, Figure S1, all truncating mutations as well as missense mutations R135C, P302L and S324R lose their transduction ability in all three signaling pathways. In addition, the E319K and M239T mutations were severely damaging and impaired signaling of all three pathways. However, some mutations showed evidence of biased signaling deficits. Specifically, seven mutations (Y33H, R80H, L91F, A103V, A189S, V236M and H310Q) did not cause significant defects in MAPK/ERK signaling but had varying yet significantly impaired signaling of the Gαq and Gαs pathways. Finally, T11I mutation had normal Gαs signaling and V233M has normal Gαq signaling, but the other two pathways were decreased by these two mutations.
Table 1.
Functional analysis of 23 IHH-related PROKR2 mutations
| Mutations | Gαq | Gαs | ERK1/2 |
|---|---|---|---|
| Disrupted all three pathwaysa | |||
| R80fs*82 | × | × | × |
| Y140X | × | × | × |
| K162X | × | × | × |
| P256Lfs*51 | × | × | × |
| T330fs*5 | × | × | × |
| R135C | × | × | × |
| P302L | × | × | × |
| S324R | × | × | × |
| Affected all three pathwaysb | |||
| M239T | ↓↓ | × | ↓ |
| E319K | ↓↓ | ↓↓ | ↓ |
| Affected two pathwaysb | |||
| V233M | − | ↓ | ↓ |
| T11I | ↓ | − | ↓ |
| Y33H | ↓ | ↓↓ | − |
| R80H | ↓ | ↓↓ | − |
| L91F | ↓ | ↓ | − |
| A103V | ↓ | ↓↓ | − |
| A189S | ↓ | ↓↓ | − |
| V236M | ↓↓ | ↓ | − |
| H310Q | ↓ | ↓↓ | − |
| Affected one pathwayb | |||
| G57C | − | − | ↓ |
| R264H | − | ↓ | − |
| L176F | ↓ | − | − |
| V180M | ↓ | − | − |
−: neutral; ↓: low pathogenicity; ↓↓: medium pathogenicity; ×: high pathogenicity.
a‘Disrupted’ refers to the observation when signaling in all three pathways was totally disrupted with no activity.
b‘Affected’ refers to the observation when at least one signaling pathway is not totally disrupted with evidence of some preserved activity.
Gαq signaling is required for the PROK2-induced migration of PROKR2-expressing cells
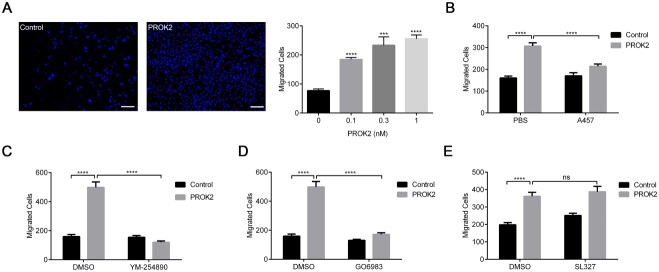
Although PROKR2 mutations variably disrupted Gαq, Gαs and MAPK/ERK signal transduction pathways in this study, we next wished to determine which of these pathways were relevant for cell migratory function. Previous reports have demonstrated that PROK2 functions as a chemotactic molecule and attracts neural stem cells located in the SVZ that highly expresses PROKR2 and guides their migration toward the olfactory bulb via the rostral migratory stream (10). This chemotactic function of PROK2 is also deemed critical for embryonic GnRH migration from the olfactory placode (12). To clarify which G protein signaling pathway(s) is involved in this function, we setup a novel transwell assay to analyze the chemotactic effect of PROK2-PROKR2 signaling. Chinese hamster ovary (CHO) cells stably expressing PROKR2 were loaded onto the upper chamber, and PROK2 was added into the lower chamber (24). As shown in Figure 1A and B and Supplementary Material, Figure S2A, PROK2 dose-dependently promoted the migration of PROKR2-bearing CHO cells to the lower chamber, which is inhibited by a PROKR2 antagonist A457 (25). Then, we added various inhibitors to explore the involvement of different G protein signaling pathways in cell migration (26,27). As shown in Figure 1C–E and Supplementary Material, Figure S2B–D, the Gαq signaling pathway inhibitor YM-254890 inhibited the PROK2-induced cell migration. Consistently, the PKC inhibitor Gö6983 also significantly inhibited PROK2-induced cell migration. However, the MAPK/ERK signaling pathway inhibitor SL327 failed to inhibit PROK2-induced cell migration. Our results thus suggest that Gαq is required for the PROK2-induced migration of PROKR2-bearing cells, implying that disrupted Gαq may underlie the chemotactic defects resulting from impaired prokineticin 2 signaling. Thus, our observations implicate the disruption of downstream Gαq signaling as the primary driver of the pathogenesis of IHH in humans with PROKR2 mutations.
Figure 1.
Gαq signaling is required for the PROK2-induced migration of PROKR2-expressing cells. (A) PROK2 dose-dependently induced the migration of CHO cells stably expressing PROKR2 as assayed with transwell experiments. Left, representative images of migrated cells without PROK2 treatment (control) and with 1 nM PROK2. Right, the statistical data of three independent experiments. (B–E) The effects of PROKR2 antagonist A457 (0.1 μM), Gαq signaling pathway inhibitor YM-254890 (1 μM), PKC inhibitor Gö6983 (10 μM) and MAPK/ERK signaling pathway inhibitor SL327 (10 μM) on the migration of PROKR2-expressing cells induced by PROK2 (1 nM). Each value was the mean ± SEM of three independent experiments, ns, not significant; *P ˂ 0.05; ***P ˂ 0.001; ****P ˂ 0.0001, unpaired t test.
Categorization of PROKR2 mutations according to their Gαq-signaling activity
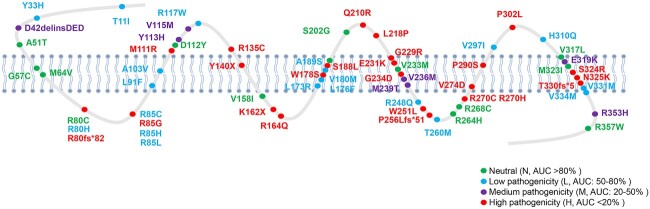
In the light of the above observations that strongly suggested that disrupted Gαq pathways underlie the pathogenesis of IHH induced by PROKR2 mutations, we aggregated the Gαq-signaling ability of PROKR2 mutations reported in this study and previously reported studies that characterized Gαq signaling in IHH subjects with PROKR2 mutations. In total, as shown in Figure 2 we categorized 63 PROKR2 mutations (15,25,28–32) according to their Gαq-signaling activity into four distinct functional categories: (i) neutral (N), with AUC (area under curve) > 80% of wild-type (WT) PROKR2; (ii) low pathogenicity (L), with AUC 50–80% of WT PROKR2; (iii) medium pathogenicity (M), with AUC 20–50% of WT PROKR2 and (iv) high pathogenicity (H), with AUC < 20% of WT PROKR2. As shown in Figure 2 and Supplementary Material, Tables S2 and S3, 13 mutations (A51T, G57C, M64V, R80C, D112Y, V158I, S202G, V233M, R264H, R268C, V317L, M323I and R357W) were considered as N; 19 mutations (T11I, Y33H, R80H, R85C, R85H, R85L, L91F, A103V, R117W, L173R, L176F, V180M, A189S, R248Q, T260M, V297I, H310Q, V331M and V334M) were considered as L; 7 mutations (D42delinsDED, Y113H, V115M, V236M, M239T, E319K and R353H) were considered as M and 24 mutations (R80fs*82, R85G, M111R, R135C, Y140X, K162X, R164Q, W178S, S188L, Q210R, L218P, G229R, E231K, G234D, W251L, P256Lfs *51, R270C, R270H, V274D, P290S, P302L, S324R, N325K and T330fs*5) were considered as H.
Figure 2.
Schematic diagram of all IHH-related PROKR2 mutations according the Gαq signaling pathway. The green dots represent neutral (N, AUC > 80% of WT PROKR2); the blue dots represent low pathogenicity (L, AUC 50–80% of WT PROKR2); the purple dots represent medium pathogenicity (M, AUC 20–50% of WT PROKR2) and the red dots represent high pathogenicity (H, AUC < 20% of WT PROKR2).
Comparison of cell-based functional data with in silico prediction
We then compared our category with five frequently used in silico function-prediction software (CADD, SIFT, Polyphen2, MutationTaster and MutationAssessor). Combined Annotation Dependent Depletion (CADD) and Sorting Intolerant from Tolerant (SIFT) classified variants into T (tolerable) and D (deleterious or damaging); Mutation Assessor classified variants into neutral (N), low (L), medium (M) and high(H); Polyphen2 classified variants into three categories B, benign, P, possibly damaging and D, probably damaging, and MutationTaster classified variants into four categories polymorphism_automatic (P), polymorphism(N), disease_causing(D) and disease_causing_automatic(A), where polymorphism _automatic and disease_causing_automatic indicate that the mutation has been recorded in known databases and the evidence is clear. We therefore matched our cell-based categories with these different prediction data, which is listed in Supplementary Material, Table S2. As shown in Supplementary Material, Table S3, the accuracy to predict mutations in the transmembrane region for CADD, SIFT, Polyphen2, MutationTaster and MutationAssessor is 14/24 (58.3%), 17/24 (70.8%), 13/24 (54.2%), 13/24 (54.2%) and 9/24 (37.5%), respectively. The accuracy to predict mutations in the extracellular region for CADD, SIFT, Polyphen2, MutationTaster and MutationAssessor is 13/16 (81.3%), 11/16 (68.8%), 10/16 (62.5%), 9/16 (56.3%) and 4/16 (25.0%), respectively. The accuracy to predict mutations in the intracellular region for CADD, SIFT, Polyphen2, MutationTaster and MutationAssessor is 10/18 (55.6%), 8/18 (44.4%), 5/18 (27.8%), 8/18 (44.4%) and 3/18 (16.7%), respectively. Taken together, our data support the notion that of the prediction programs, CADD was able to predict the mutations located in the intracellular and extracellular domains more accurately, whereas SIFT was able to predict the mutations in the transmembrane region more accurately.
Discussion
Cell migration is a fundamental biological process involved in human development, and the abnormal cellular migration is known to result in a variety of developmental disorders (33,34). In mammals, the ontogeny of GnRH neurons is intricately intertwined with olfactory neurogenesis. Embryonic GnRH neurons migrate from the medial olfactory placode to the hypothalamus along the olfactory vomeronasal neurons (3,7,8,10–12). While the olfactory sensory neurons project to the main olfactory bulb, the GnRH neurons migrate into the forebrain and eventually reach the medial preoptic area of the hypothalamus where they synchronize pulsatile GnRH secretion that in turn governs reproductive maturation. During development, neural stem cells located in the SVZ of the brain generate new interneurons that migrate along the rostral migratory stream to the olfactory bulb. G protein-coupled receptors (GPCRs), a family of seven transmembrane receptors, are engaged in a broad range of physiological activities, including cell migration. Amongst these GPCRs, PROKR2 and its ligand PROK2 have been implicated to facilitate cellular migration of both GnRH neurons and the olfactory interneurons emanating from the SVZ (10–12). Despite the critical role of PROK2 signaling in directing these migratory processes, the precise GPCR-related intracellular signaling pathways/mechanisms that govern these neuronal migration processes remain unclear. Evidence from this study and the literature have implicated that PROKR2 mutations are common contributor to IHH, with a significant majority of mutations linked to the KS form of IHH, a disorder characterized by impaired embryonic GnRH migration. Thus, functional analyses of IHH-associated PRORK2 mutations provide an opportunity to dissect the precise downstream intracellular signaling cascades that govern the neuronal migration of both GnRH and olfactory interneurons.
Accumulated evidence suggest that GPCRs may induce cell migration through coupling to various Gα-proteins, such as Gαi/o, Gαs, Gαq and Gα12/13 (35). In this scenario, Gα-specific inhibitors or RNAi are used to investigate the involvement of specific Gα proteins. For instance, by using pertussis toxin (PTX), which can irreversibly and specifically uncoupled Gαi from receptors, it is uncovered that Gαi participates in the cell migration induced by a variety of GPCRs, such as PAF receptor and S1P receptor (36–39). In addition to the classic G-protein coupled signaling, activated GPCRs also recruit arrestin proteins to initiate a broad variety of signaling events such as activation of the MAPK pathway (40–42). The arrestin-mediated GPCR signaling is also linked to physiological outputs such as cell migration. In the β-arrestin2-deficient mice, the lymphocytes are unable to migrate under CXCL12 stimulation (43). Furthermore, β-arrestin2-depleted T cells cannot migrate to inflammation sites in an animal model of asthma (44). GPCRs may regulate cell migration through transactivation of tyrosine kinase receptors, such as EGFR. For instance, the migration of vascular smooth muscle cells induced by thrombin is totally blocked by specific EGFR inhibitors (45). And the migration of keratinocytes and fibroblasts is inhibited by HB-EGF neutralizing antibody or an HB-EGF antagonist (CRM197) (46). Prior studies have shown that WT PROKR2 can activate different G-protein (Gαq, Gαs and Gαi/o) subtypes (15). Furthermore, a recent study showed that while PROKR2 interacts with Gαq and Gαi/o with comparable efficiencies, Gαs coupling may occur at a lower extent compared to the other G protein subtypes (47). Given these diverse intracellular pathways linked to PROKR2 function, in this study, we systematically examined the Gαq, Gαs and ERK1/2 signaling of 23 IHH-associated PROKR2 mutations. Our functional analyses of 23 PROKR2 mutations showed variable disruption across all these pathways.
For dissecting which of the studied intracellular signaling pathways was specifically responsible for the PROK2/PROKR2-related cell migration defects, we used a novel cell migration assay to examine PROK2 signaling. Our study results show that PROK2-PROKR2 signaling induced cellular migration is primarily mediated by the Gαq pathway, as this is blocked by YM-254890 instead of ERK1/2 inhibitor SL327. Although we cannot exclude the involvement of other signaling pathways, such as Gαs, Gαi/o and Gα12/13 in the cell migration induced by PROKR2 activation, our cell-based experiments suggest the importance of Gαq in the pathology of IHH induced by PROK2/PROKR2 mutations. Hence, to expand on these observations regarding the importance of Gαq signaling cascade in IHH, we aggregated functional studies (this study and all available reports) that have examined Gαq pathway signaling for IHH-associated PROKR2 mutations. At least 10 literatures that analyzed the function of mutant PROKR2s include Gαq pathway (15,16,28–32,48–50). In total, 63 PROKR2 mutations with Gαq signaling studies were examined and we then summarized the Gαq pathway defects into four distinct groups to reflect the relative pathogenicity. Our summarization of Gαq-related functional data shows that just over 50% of PROKR2 mutations (32/63) showed neutral or low pathogenicity, while 31/63 mutations showed medium/high pathogenicity. These findings raise the possibility that PROKR2 mutations with neutral or low pathogenicity categorization through Gαq signaling deficit may represent nonpathogenic alleles that may not necessarily causal for the IHH phenotype in mutation bearing subjects. Alternatively, it is possible that some PROKR2 mutations with preserved Gαq signaling may confer pathogenicity for normosmic forms of IHH. It is well recognized that PROKR2 variants are also implicated in normosmic forms of IHH (nIHH) and the pathogenic mechanisms of PROKR2 mutations in nIHH may differ from KS pathogenesis. It is to be emphasized that the cell migration assay results and the associated Gαq deficits identified in this study may specifically relate to GnRH migratory defects and hence may be more specific to IHH patients presenting with the KS form of IHH and may not be applicable for nIHH subjects. For example, the subject with V233M variant (Table 1) presented with a nIHH and while his Gαq signaling was intact, he had deficits in Gαs and ERK1/2 signaling. Thus, this observation may indicate that PRORK2-related nIHH may not be causally related to Gαq deficits but may relate to Gαs and/or ERK1/2 signaling deficits. Thus, in nIHH patients with variants that have preserved Gαq signaling, evaluation of other G-protein pathways should be considered. Additionally, for PROKR2 variants with such biased signaling deficits, further genetic inquiry to identify other causal variants in known or novel IHH genes must also be undertaken especially given the oligogenic basis of IHH.
Finally, since cell-based functional analyses may not be always feasible for all newly discovered PROKR2 mutations, we compared our Gαq-based categorization with five commonly used functional prediction software (CADD, SIFT, Polyphen2, MutationTaster and MutationAssessor). Typically, in silico prediction platforms utilize several criteria including evolutionary amino acid/nucleotide conservation, the location/context within the protein sequence and biochemical consequence of the amino acid substitution. Hence, in this study, we utilized multiple software programs for sequence variant interpretation given the differing strengths and weaknesses of each platform. We found the CADD platform was superior in predicting pathogenicity of mutations located in the intracellular and extracellular domains, whereas SIFT platform performed the best for mutations located in the transmembrane region. Thus, the predictive sensitivity for prediction platforms for PROKR2 mutations suggesting that the discriminatory capability of the programs was dependent on the location of the PROKR2 mutation. Our observations suggest the future efforts that incorporate in silico prediction must utilize an array of different programs given their differing capabilities. Finally, taken together with our cell-based migration assays, our study results suggest that for newly identified PROKR2 mutations in IHH patients (especially in those with KS form of IHH), variant pathogenicity should be assessed initially with Gαq functional studies. If access to such assays is not available, in silico prediction could be used based on location of mutations—CADD prediction assessment for intracellular and extracellular domains, whereas SIFT prediction assessment will be preferable for mutations in the transmembrane region.
Although this study represents the largest reported study to aggregate Gαq signaling deficits in IHH, there are some limitations. First, majority of the mutations in PROKR2 occurred in the heterozygous state and our cellular assays did not examine heterozygosity using WT co-transfection experiments as has been previously reported (16). Furthermore, while this study implicated Gαq-signaling as the critical determinant for chemotactic/migratory function of PROK2 signaling relating to GnRH/olfactory ontogeny, PROKR2 mutations are also implicated in patients with nIHH, with no discernible olfactory deficits. Hence, it is possible that other signaling cascades may also be relevant for PROK2-related deficits that extend beyond cell migratory defects. In this regard, it is notable that PROK2 signaling is implicated to other diverse physiologic processes such as intestinal contraction, circadian regulation and metabolic homeostasis (51–54), where other G-protein signaling pathways (Gαi/o, Gαs and/or Gα12/13) may be implicated. Finally, while we systematically characterized the functional deficits, concomitant genotype–phenotype correlations were not examined. Future studies will be needed to specifically correlate the Gαq deficits with olfactory, reproductive and other nonreproductive phenotypes seen in IHH subjects.
In summary, our data suggest that disrupted Gαq pathway underlies the pathogenesis of IHH induced by PROKR2 mutations. After comparing the data from the cell-based assays and the commonly used prediction software, our studies also suggest that the SIFT prediction tool was able to more accurately predict PROKR2 mutations localized in the transmembrane region, whereas CADD tool was superior to other in silico tools in predicting pathogenicity of PROKR2 mutations localized in the intracellular and extracellular domains.
Materials and Methods
Patients and clinical evaluation
Chinese IHH cohort: We recruited 310 Chinese IHH probands from Xiangya Hospital (Changsha, China) and the People’s Hospital of Henan Province (Zhengzhou, China). The diagnostic criteria of IHH are described previously (55). All patients or their guardians gave written informed consents for hormonal, anthropometric and genetic detection. The studies were approved by the Ethics Committee of School of Life Sciences, Central South University.
MGH IHH cohort: A total of 1555 IHH (n = 776 with KS and n = 779 nIHH) patients (1084 males and 471 females) were recruited at the Reproductive Endocrine Unit/Massachusetts General Hospital (MGH). IHH was defined by: (i) absent/incomplete puberty by 18 years of age; (ii) serum testosterone < 100 ng/dL (men) or estradiol < 20 pg/mL (women) with low/normal serum gonadotropins; (iii) otherwise normal anterior pituitary function; (iv) normal serum ferritin concentrations and (v) normal magnetic resonance imaging of the pituitary region. Both self-reported olfaction and University of Pennsylvania Smell Identification Test (UPSIT) scores were used to classify KS and nIHH (56). Clinical charts and patient questionnaires (300 questions), with regard to reproductive and nonreproductive history, were reviewed. Nonreproductive features involved bone, face/head, cardiovascular, hearing, neurodevelopmental, neurologic, skin and eye disorders. Family history was obtained and, whenever possible, family members were recruited by our study team.
Whole exome sequencing
Peripheral blood DNA extraction, the whole exome sequencing and PROKR2 mutation screening were performed as described previously (57). Genomic DNA was extracted from the peripheral blood lymphocytes (3 mL/patient) using the phenol/chloroform method. Then, we analyzed the genomic DNA of each patient by whole exome sequencing. The whole exome was captured by the SureSelect Human All Exon Kit (Agilent Technologies, Santa Clara, CA, USA) and sequenced on an Illumina HiSeq 2500 instrument (Illumina, San Diego, CA, USA). The sequenced reads were aligned to the human genome reference (University of California, Santa Cruz hg19; http://www.epigenomebrowser.org/index.html) using a Burrows-Wheeler Aligner (bwa-0.7.17). The single nucleotide variants and small insertions and deletions (Indels) were generated with Genome Analysis Toolkit (GATK-4.0.2.1; Broad Institute, Cambridge, MA, USA) Unifed Genotyper in parallel with the Samtools (samtools-1.7; www.htslib.org/doc/samtools.html) pipeline. We then screened for rare mutations (<1% in the dbSNP, ExAC, ESP6500 and 1000 Genomes database) of PROKR2. Deleterious variants were predicted by (i) CADD (https://cadd.gs.washington.edu/; a scaled CADD score of 20 means that a variant is among the top 1% of deleterious variants in the human genome; a scaled CADD score of 30 means that the variant is in the top 0.1%); (ii) SIFT (http://sift.bii.a-star.edu.sg/); (iii) polymorphism phenotyping v.2 (Polyphen2;http://genetics.bwh.harvard.edu/pph2/i); (iv) MutationTaster (http://www.mutationtaster.org/) and (v) MutationAssessor (http://mutationassessor.org/r3/).
PROKR2 mutant plasmid construction
The human PROKR2 with an N-terminal Flag tag was constructed as previously described (57). The QuikChange Primer Design (https://www.agilent.com/store/primerDesignProgram.jsp) was used to design mutant primers. PCR was performed in a total volume of 50 μL according to the manufacturer’s instructions of Thermo Scientific Phusion High-Fidelity DNA Polymerase (#F-530S; ThermoFisher Scientific, USA), and the amplified products were purified and recovered using HiPure Plasmid Micro Kit C (P1001-03C; Magen, China). The purified product was digested with DpnI enzyme and transformed into bacteria. All the constructs were verified by Sanger sequencing by Sangon Biotech (Shanghai, China). The sequencing results were confirmed to be correct by using SnapGene software (v.2.3.2; SnapGene, USA).
G protein signaling pathway detection
An aequorin-based luminescent assay was used to measure mobilization of intracellular Ca2+ as previously described (30). CHO cells stably expressing the photoprotein aequorin were transiently transfected with WT PROKR2 or their respective mutants. Two days after transfection, the cells were charged in Opti-MEM (Invitrogen, Carlsbad, CA, USA) containing 8 μM coelenterazine cp (Invitrogen) at 37°C for 2 h. Cells were detached by brief trypsinization and maintained in Hank’s balanced salt solution plus 10 mM HEPES, pH 7.5 and 0.1% bovine serum albumin at about 5 × 105 cells/mL. Luminescence measurements were made using a Sirius Single Tube Luminometer (Berthold Detection Systems GmbH, Pforzheim, Germany). PROK2 (PeproTech, Rocky Hill, NJ, USA) were serially diluted in Hank’s balanced salt solution plus 10 mM HEPES, pH 7.5 and 0.1% bovine serum albumin. Approximately 100 μL of cells were injected into the tubes with 10 μL of PROK2. The luminescence was monitored for 25 s (peak 1), then 90 μL of 1% Triton X-100 was injected to lyse the cells, and the luminescence was continuously monitored for another 20 s (peak 2). The AUC of peak 1 was divided by the total AUC of peaks 1 and 2, and the maximal responses from WT receptors were normalized to 100.
Analysis of ERK1/2 activation with western blot: HEK293 cells were transfected with plasmids expressing WT or mutant PROKR2. At 40 h after transfection, the cells were stimulated with 5 nM PROK2 for 5 min. Cells were then washed with Phosphate buffer saline (PBS) and lysed in SDS lysis buffer. The phosphorylated and total ERK1/2 were detected by immunoblotting with p-ERK1/2 (Cell Signaling Technology, Danvers, MA, USA) or total ERK1/2 (Cell Signaling Technology) antibodies, respectively (29).
The GloSensor cAMP Assay Kit (Promega, Madison, WI, USA) was used to measure the concentration of cytoplasmic cAMP according to the manufacturer’s protocol. In brief, HEK293 cells cultured in 96-well plates were transiently transfected with 100 ng pGloSensor-22F cAMP plasmid and 100 ng WT or mutant PROKR2-expressing plasmids. Twenty-four hours after transfection, the cells were pre-equilibrated with GloSensor cAMP Reagent at 37°C for 2 h. PROK2 was serially diluted and added to each well. The plate was quickly put to the luminometer, and then measurements were taken (29).
Transwell experiment
A stable CHO cell line expressing PROKR2 was resuspended by serum-free medium, counted and adjusted cell density to 250 cells/μL. Inhibitors of different G protein signaling pathways were added into cell resuspension solution and medium (YM-254890, FUJIFILM Wako Chemicals USA Corporation; SL-327, Selleck, China; Gö6983, Sigma Aldrich Inc, USA). A total of 5 × 105 cells suspension was taken into the upper chamber of transwell plates and 500 μL medium containing PROK2 or PBS in the lower chamber. After 24 h of cultivation in cell incubator, cells were discarded in the medium and fixed with methanol at room temperature for 20 min. The nonmigrated cells in the upper chamber were wiped and washed with PBS. Cells that migrate to the other side of the membrane were stained with DAPI for 30 min and washed with PBS. The plates were imaged using an inverted fluorescent microscope (DMI3000 B, Leica, Germany).
Data analysis
A repeated-measures ANOVA followed by an unpaired Student’s t test was used to analyze the data for differences. All statistical analyses were performed using Prism 9.2 (GraphPad Software, La Jolla, CA, USA).
Supplementary Material
Acknowledgement
We are grateful to all IHH patients and families for their participation in the research and support, and to all those involved whose involvement has advanced the understanding of the pathogenesis of IHH.
Conflict of interest statement. The authors have no relevant financial or non-financial interests to disclose.
Contributor Information
Xinying Wang, School of Life Sciences, Central South University, Changsha, Hunan 410078, China; Hunan Key Laboratory of Medical Genetics, Central South University, Changsha, Hunan 410078, China; Hunan Key Laboratory of Animal Models for Human Diseases, Central South University, Changsha, Hunan 410078, China.
Danna Chen, Department of Basic Medical Sciences, Changsha Medical University, Changsha, Hunan 410219, China.
Yaguang Zhao, School of Life Sciences, Central South University, Changsha, Hunan 410078, China; Hunan Key Laboratory of Medical Genetics, Central South University, Changsha, Hunan 410078, China; Hunan Key Laboratory of Animal Models for Human Diseases, Central South University, Changsha, Hunan 410078, China.
Meichao Men, Health Management Center, Xiangya Hospital, Central South University, Changsha, Hunan 410078, China.
Zhiheng Chen, School of Life Sciences, Central South University, Changsha, Hunan 410078, China; Department of Pediatrics, Third Xiangya Hospital, Central South University, Changsha, Hunan 410013, China.
Fang Jiang, School of Life Sciences, Central South University, Changsha, Hunan 410078, China; Hunan Key Laboratory of Medical Genetics, Central South University, Changsha, Hunan 410078, China; Hunan Key Laboratory of Animal Models for Human Diseases, Central South University, Changsha, Hunan 410078, China.
Ruizhi Zheng, Department of Endocrinology, The People's Hospital of Henan Province, Zhengzhou, Henan 450003, China.
Maria I Stamou, Reproductive Endocrine Unit, Massachusetts General Hospital and the Center for Reproductive Medicine, Boston, MA 02141, USA.
Lacey Plummer, Reproductive Endocrine Unit, Massachusetts General Hospital and the Center for Reproductive Medicine, Boston, MA 02141, USA.
Ravikumar Balasubramanian, Reproductive Endocrine Unit, Massachusetts General Hospital and the Center for Reproductive Medicine, Boston, MA 02141, USA.
Jia-Da Li, School of Life Sciences, Central South University, Changsha, Hunan 410078, China; Hunan Key Laboratory of Medical Genetics, Central South University, Changsha, Hunan 410078, China; Hunan Key Laboratory of Animal Models for Human Diseases, Central South University, Changsha, Hunan 410078, China; Hunan International Scientific and Technological Cooperation Base of Animal Models for Human Disease, Changsha, Hunan 410078, China.
Funding
National Natural Science Foundation of China (81900948, 82070815, 31972913 and 81770780); Key Research and Development Programs from Hunan Province (2021DK2001); Guangdong Key Project in ‘Development of New Tools for Diagnosis and Treatment of Autism’ (2018B030335001); Training Program for Excellent Young Innovators of Changsha (kq2106074); Postgraduate Scientific Research Innovation Project of Hunan Province (CX2018B054 and CX20190103); United States National Institutes of Health (P50 HD028138, R01 HD096324 and F32 HD108873).
Data availability statement
The authors confirm that any required links or identifiers for the data are present in the manuscript as described.
References
- 1. Beate, K., Joseph, N., Nicolas, D.R. and Wolfram, K. (2012) Genetics of isolated hypogonadotropic hypogonadism: role of GnRH receptor and other genes. Int. J. Endocrinol., 2012, 147893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balasubramanian, R. and Crowley, W.F., Jr. (2011) Isolated GnRH deficiency: a disease model serving as a unique prism into the systems biology of the GnRH neuronal network. Mol. Cell. Endocrinol., 346(1–2), 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim, S.-H. (2015) Congenital hypogonadotropic hypogonadism and Kallmann syndrome: past, present, and future. Endocrinol. Metab. (Seoul), 30(4), 456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boehm, U., Bouloux, P., Dattani, M., de Roux, N., Dodé, C., Dunkel, L., Dwyer, A., Giacobini, P., Hardelin, J., Juul, A. et al. (2015) Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism--pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol., 11(9), 547–564. [DOI] [PubMed] [Google Scholar]
- 5. Casoni, F., Malone, S., Belle, M., Luzzati, F., Collier, F., Allet, C., Hrabovszky, E., Rasika, S., Prevot, V., Chédotal, A. and Giacobini, P. (2016) Development of the neurons controlling fertility in humans: new insights from 3D imaging and transparent fetal brains. Development, 143(21), 3969–3981. [DOI] [PubMed] [Google Scholar]
- 6. Oleari, R., Caramello, A., Campinoti, S., Lettieri, A., Ioannou, E., Paganoni, A., Fantin, A., Cariboni, A. and Ruhrberg, C. (2019) PLXNA1 and PLXNA3 cooperate to pattern the nasal axons that guide gonadotropin-releasing hormone neurons. Development, 146(21), 1–8. [DOI] [PubMed] [Google Scholar]
- 7. Bonomi, M., Libri, D.V., Guizzardi, F., Guarducci, E., Maiolo, E., Pignatti, E., Asci, R., Persani, L. and Idiopathic Central Hypogonadism Study Group of the Italian Societies of Endocrinology and Pediatric Endocrinology Diabetes (2012) New understandings of the genetic basis of isolated idiopathic central hypogonadism. Asian J. Androl., 14, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bianco, S.D.C. and Kaiser, U.B. (2009) The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat. Rev. Endocrinol., 5(10), 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taroc, E.Z.M., Prasad, A., Lin, J.M. and Forni, P.E. (2017) The terminal nerve plays a prominent role in GnRH-1 neuronal migration independent from proper olfactory and vomeronasal connections to the olfactory bulbs. Biol. Open., 6(10), 1552–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ng, K.L., Li, J.-D., Cheng, M.Y., Leslie, F.M., Lee, A.G. and Zhou, Q.-Y. (2005) Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science, 308(5730), 1923–1927. [DOI] [PubMed] [Google Scholar]
- 11. Puverel, S., Nakatani, H., Parras, C. and Soussi-Yanicostas, N. (2009) Prokineticin receptor 2 expression identifies migrating neuroblasts and their subventricular zone transient-amplifying progenitors in adult mice. J. Comp. Neurol., 512(2), 232–242. [DOI] [PubMed] [Google Scholar]
- 12. Wen, Y., Zhang, Z., Li, Z., Liu, G., Tao, G., Song, X., Xu, Z., Shang, Z., Guo, T., Su, Z. et al. (2019) The PROK2/PROKR2 signaling pathway is required for the migration of most olfactory bulb interneurons. J. Comp. Neurol., 527(18), 2931–2947. [DOI] [PubMed] [Google Scholar]
- 13. Matsumoto, S.I., Yamazaki, C., Masumoto, K.H., Nagano, M., Naito, M., Soga, T., Hiyama, H., Matsumoto, M., Takasaki, J., Kamohara, M. et al. (2006) Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc. Natl. Acad. Sci. U. S. A., 103(11), 4140–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Casella, I. and Ambrosio, C. (2021) Prokineticin receptors interact unselectively with several G protein subtypes but bind selectively to β-arrestin 2. Cell. Signal., 83, 1–9. [DOI] [PubMed] [Google Scholar]
- 15. Sbai, O., Monnier, C., Dodé, C., Pin, J.P., Hardelin, J.P. and Rondard, P. (2014) Biased signaling through G-protein-coupled PROKR2 receptors harboring missense mutations. FASEB J., 28(8), 3734–3744. [DOI] [PubMed] [Google Scholar]
- 16. Cox, K., Oliveira, L., Plummer, L., Corbin, B., Gardella, T., Balasubramanian, R. and Crowley, W. (2018) Modeling mutant/wild-type interactions to ascertain pathogenicity of PROKR2 missense variants in patients with isolated GnRH deficiency. Hum. Mol. Genet., 27(2), 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miraoui, H., Dwyer, A.A., Sykiotis, G.P., Plummer, L., Chung, W., Feng, B., Beenken, A., Clarke, J., Pers, T.H., Dworzynski, P. et al. (2013) Mutations in FGF17, IL17RD, DUSP6, SPRY4, and FLRT3 are identified in individuals with congenital hypogonadotropic hypogonadism. Am. J. Hum. Genet., 92(5), 725–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Costa-Barbosa, F.A., Balasubramanian, R., Keefe, K.W., Shaw, N.D., Al-Tassan, N., Plummer, L., Dwyer, A.A., Buck, C.L., Choi, J.H., Seminara, S.B. et al. (2013) Prioritizing genetic testing in patients with Kallmann syndrome using clinical phenotypes. J. Clin. Endocrinol. Metab., 98, E943–E953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goncalves, C.I., Patriarca, F.M., Aragues, J.M., Carvalho, D., Fonseca, F., Martins, S., Marques, O., Pereira, B.D., Martinez-de-Oliveira, J. and Lemos, M.C. (2019) High frequency of CHD7 mutations in congenital hypogonadotropic hypogonadism. Sci. Rep., 9, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou, C., Niu, Y., Xu, H., Li, Z., Wang, T., Yang, W., Wang, S., Wang, D.W. and Liu, J. (2018) Mutation profiles and clinical characteristics of Chinese males with isolated hypogonadotropic hypogonadism. Fertil. Steril., 110, 486–495. [DOI] [PubMed] [Google Scholar]
- 21. Bergman, J.E., de Ronde, W., Jongmans, M.C., Wolffenbuttel, B.H., Drop, S.L., Hermus, A., Bocca, G., Hoefsloot, L.H. and van Ravenswaaij-Arts, C.M. (2012) The results of CHD7 analysis in clinically well-characterized patients with Kallmann syndrome. J. Clin. Endocrinol. Metab., 97, E858–E862. [DOI] [PubMed] [Google Scholar]
- 22. Sarfati, J., Guiochon-Mantel, A., Rondard, P., Arnulf, I., Garcia-Piñero, A., Wolczynski, S., Brailly-Tabard, S., Bidet, M., Ramos-Arroyo, M., Mathieu, M.l. et al. (2010) A comparative phenotypic study of Kallmann syndrome patients carrying monoallelic and biallelic mutations in the prokineticin 2 or prokineticin receptor 2 genes. J. Clin. Endocrinol. Metab., 95, 659–669. [DOI] [PubMed] [Google Scholar]
- 23. Abreu, A.P., Trarbach, E.B., de Castro, M., Frade Costa, E.M., Versiani, B., Matias Baptista, M.T., Garmes, H.M., Mendonca, B.B. and Latronico, A.C. (2008) Loss-of-function mutations in the genes encoding prokineticin-2 or prokineticin receptor-2 cause autosomal recessive Kallmann syndrome. J. Clin. Endocrinol. Metab., 93, 4113–4118. [DOI] [PubMed] [Google Scholar]
- 24. Bullock, C., Li, J. and Zhou, Q. (2004) Structural determinants required for the bioactivities of prokineticins and identification of prokineticin receptor antagonists. Mol. Pharmacol., 65, 582–588. [DOI] [PubMed] [Google Scholar]
- 25. Chen, D.N., Ma, Y.T., Liu, H., Zhou, Q.Y. and Li, J.D. (2014) Functional rescue of Kallmann syndrome-associated prokineticin receptor 2 (PKR2) mutants deficient in trafficking. J. Biol. Chem., 289, 15518–15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oh, J., Kim, E. and Nam, T. (2018) Phycoerythrin-derived tryptic peptide of a red alga Pyropia yezoensis attenuates glutamate-induced ER stress and neuronal senescence in primary rat hippocampal neurons. Mol. Nutr. Food Res., 62, e1700469. [DOI] [PubMed] [Google Scholar]
- 27. Yin, W., Liu, H., Peng, Z., Chen, D., Li, J. and Li, J.D. (2014) Mechanisms that underlie the internalization and extracellular signal regulated kinase 1/2 activation by PKR2 receptor. Cell. Signal., 26, 1118–1124. [DOI] [PubMed] [Google Scholar]
- 28. Cole, L.W., Sidis, Y., Zhang, C., Quinton, R., Plummer, L., Pignatelli, D., Hughes, V.A., Dwyer, A.A., Raivio, T., Hayes, F.J. et al. (2008) Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J. Clin. Endocrinol. Metab., 93, 3551–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao, Y., Wu, J., Wang, X., Jia, H., Chen, D.N. and Li, J.D. (2019) Prokineticins and their G protein-coupled receptors in health and disease. Prog. Mol. Biol. Transl. Sci., 161, 149–179. [DOI] [PubMed] [Google Scholar]
- 30. Peng, Z., Tang, Y., Luo, H., Jiang, F., Yang, J., Sun, L. and Li, J.D. (2011) Disease-causing mutation in PKR2 receptor reveals a critical role of positive charges in the second intracellular loop for G-protein coupling and receptor trafficking. J. Biol. Chem., 286, 16615–16622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Libri, D.V., Kleinau, G., Vezzoli, V., Busnelli, M., Guizzardi, F., Sinisi, A.A., Pincelli, A.I., Mancini, A., Russo, G., Beck-Peccoz, P. et al. (2014) Germline prokineticin receptor 2 (PROKR2) variants associated with central hypogonadism cause differental modulation of distinct intracellular pathways. J. Clin. Endocrinol. Metab., 99, E458–E463. [DOI] [PubMed] [Google Scholar]
- 32. Raivio, T., Avbelj, M., McCabe, M.J., Romero, C.J., Dwyer, A.A., Tommiska, J., Sykiotis, G.P., Gregory, L.C., Diaczok, D., Tziaferi, V. et al. (2012) Genetic overlap in Kallmann syndrome, combined pituitary hormone deficiency, and septo-optic dysplasia. J. Clin. Endocrinol. Metab., 97, E694–E699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parrini, E., Conti, V., Dobyns, W.B. and Guerrini, R. (2016) Genetic basis of brain malformations. Mol. Syndromol., 7, 220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pan, Y.H., Wu, N. and Yuan, X.B. (2019) Toward a better understanding of neuronal migration deficits in autism spectrum disorders. Front. Cell. Dev. Biol., 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilkie, T. and Yokoyama, S. (1994) Evolution of the G protein alpha subunit multigene family. Soc. Gen. Physiol. Ser., 49, 249–270. [PubMed] [Google Scholar]
- 36. Kaslow, H. and Burns, D. (1992) Pertussis toxin and target eukaryotic cells: binding, entry, and activation. FASEB J., 6, 2684–2690. [DOI] [PubMed] [Google Scholar]
- 37. Sunyer, T., Monastirsky, B., Codina, J. and Birnbaumer, L. (1989) Studies on nucleotide and receptor regulation of Gi proteins: effects of pertussis toxin. Mol. Endocrinol., 3, 1115–1124. [DOI] [PubMed] [Google Scholar]
- 38. Brown, S., Jala, V., Raghuwanshi, S., Nasser, M., Haribabu, B. and Richardson, R. (2006) Activation and regulation of platelet-activating factor receptor: role of G(i) and G(q) in receptor-mediated chemotactic, cytotoxic, and cross-regulatory signals. J. Immunol., 177, 3242–3249. [DOI] [PubMed] [Google Scholar]
- 39. Yoon, C., Hong, B., Moon, H., Lim, S., Suh, P., Kim, Y., Chae, C. and Gho, Y. (2008) Sphingosine-1-phosphate promotes lymphangiogenesis by stimulating S1P1/Gi/PLC/Ca2+ signaling pathways. Blood, 112, 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Claing, A., Laporte, S., Caron, M. and Lefkowitz, R. (2002) Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog. Neurobiol., 66, 61–79. [DOI] [PubMed] [Google Scholar]
- 41. McDonald, P., Chow, C., Miller, W., Laporte, S., Field, M., Lin, F., Davis, R. and Lefkowitz, R. (2000) Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science, 290, 1574–1577. [DOI] [PubMed] [Google Scholar]
- 42. Luttrell, L., Ferguson, S., Daaka, Y., Miller, W., Maudsley, S., Della Rocca, G., Lin, F., Kawakatsu, H., Owada, K., Luttrell, D. et al. (1999) Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science, 283, 655–661. [DOI] [PubMed] [Google Scholar]
- 43. Fong, A., Premont, R., Richardson, R., Yu, Y., Lefkowitz, R. and Patel, D. (2002) Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc. Natl. Acad. Sci. U. S. A., 99, 7478–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walker, J., Fong, A., Lawson, B., Savov, J., Patel, D., Schwartz, D. and Lefkowitz, R. (2003) Beta-arrestin-2 regulates the development of allergic asthma. J. Clin. Invest., 112, 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kalmes, A., Vesti, B., Daum, G., Abraham, J. and Clowes, A. (2000) Heparin blockade of thrombin-induced smooth muscle cell migration involves inhibition of epidermal growth factor (EGF) receptor transactivation by heparin-binding EGF-like growth factor. Circ. Res., 87, 92–98. [DOI] [PubMed] [Google Scholar]
- 46. Yahata, Y., Shirakata, Y., Tokumaru, S., Yang, L., Dai, X., Tohyama, M., Tsuda, T., Sayama, K., Iwai, M., Horiuchi, M. and Hashimoto, K. (2006) A novel function of angiotensin II in skin wound healing. Induction of fibroblast and keratinocyte migration by angiotensin II via heparin-binding epidermal growth factor (EGF)-like growth factor-mediated EGF receptor transactivation. J. Biol. Chem., 281, 13209–13216. [DOI] [PubMed] [Google Scholar]
- 47. Casella, I. and Ambrosio, C. (2021) Prokineticin receptors interact unselectively with several G protein subtypes but bind selectively to beta-arrestin 2. Cell. Signal., 83, 110000. [DOI] [PubMed] [Google Scholar]
- 48. Dodé, C. and Rondard, P. (2013) PROK2/PROKR2 signaling and Kallmann syndrome. Front. Endocrinol., 4, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Monnier, C., Dodé, C., Fabre, L., Teixeira, L., Labesse, G., Pin, J., Hardelin, J. and Rondard, P. (2009) PROKR2 missense mutations associated with Kallmann syndrome impair receptor signalling activity. Hum. Mol. Genet., 18, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abreu, A., Kaiser, U. and Latronico, A. (2010) The role of prokineticins in the pathogenesis of hypogonadotropic hypogonadism. Neuroendocrinology, 91, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheng, M.Y., Bullock, C.M., Li, C., Lee, A.G., Bermak, J.C., Belluzzi, J., Weaver, D.R., Leslie, F.M. and Zhou, Q.Y. (2002) Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature, 417, 405–410. [DOI] [PubMed] [Google Scholar]
- 52. Prosser, H.M., Bradley, A., Chesham, J.E., Ebling, F.J., Hastings, M.H. and Maywood, E.S. (2007) Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proc. Natl. Acad. Sci. U. S. A., 104, 648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li, M., Bullock, C.M., Knauer, D.J., Ehlert, F.J. and Zhou, Q.Y. (2001) Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol. Pharmacol., 59, 692–698. [DOI] [PubMed] [Google Scholar]
- 54. Fullone, M.R., Maftei, D., Vincenzi, M., Lattanzi, R. and Miele, R. (2022) Identification of regions involved in the physical interaction between melanocortin receptor accessory protein 2 and prokineticin receptor 2. Biomol. Ther., 12, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hou, Q., Wu, J., Zhao, Y., Wang, X., Jiang, F., Chen, D.N., Zheng, R., Men, M. and Li, J.D. (2020) Genotypic and phenotypic spectrum of CCDC141 variants in a Chinese cohort with congenital hypogonadotropic hypogonadism. Eur. J. Endocrinol., 183, 245–254. [DOI] [PubMed] [Google Scholar]
- 56. Kasemsuk, N., Thanaviratananich, S. and Piromchai, P. (2020) A study of 30 odors panel smell identification test, smell detection threshold and University of Pennsylvania Smell Identification Test (UPSIT) in Thailand. Auris Nasus Larynx, 47, 1003–1008. [DOI] [PubMed] [Google Scholar]
- 57. Zhao, Y., Wu, J., Jia, H., Wang, X., Zheng, R., Jiang, F., Chen, D.N., Chen, Z. and Li, J.D. (2019) PROKR2 mutations in idiopathic hypogonadotropic hypogonadism: selective disruption of the binding to a Galpha-protein leads to biased signaling. FASEB J., 33, 4538–4546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that any required links or identifiers for the data are present in the manuscript as described.