Abstract
As the association of denitrification with global warming and nitrogen removal from ecosystems has gained attention in recent decades, numerous studies have examined denitrification rates and the distribution of denitrifiers across different environments. In this minireview, reported studies focused on coastal saline environments, including estuaries, mangroves, and hypersaline ecosystems, have been analysed to identify the relationship between denitrification and saline gradients. The analyses of the literature and databases stated the direct effect of salinity on the distribution patterns of denitrifiers. However, few works do not support this hypothesis thus making this topic controversial. The specific mechanisms by which salinity influences denitrifier distribution are not fully understood. Nevertheless, several physical and chemical environmental parameters, in addition to salinity, have been shown to play a role in structuring the denitrifying microbial communities. The prevalence of nirS or nirK denitrifiers in ecosystems is a subject of debate in this work. In general terms, in mesohaline environments, the predominant nitrite reductase is NirS type and, NirK is found predominantly in hypersaline environments. Moreover, the approaches used by different researchers are quite different, resulting in a huge amount of unrelated information, making it difficult to establish comparative analysis. The main techniques used to analyse the distribution of denitrifying populations along salt gradients have been also discussed.
Keywords: denitrification, saline ecosystem, halophilic microorganisms, nitrite reductase, denitrifiers distribution, coastal ecosystem
Importance of denitrification in saline and hypersaline environments in terms of denitrification rates and expression and abundance of denitrification genes in salinity gradients.
Introduction
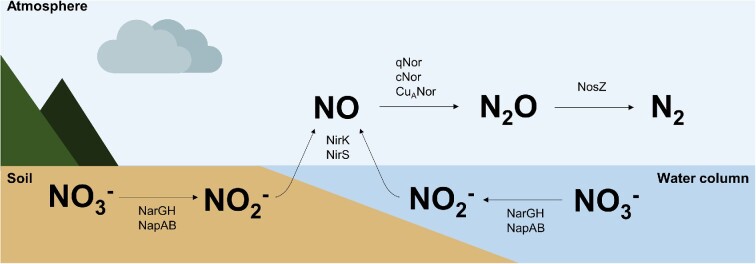
Denitrification is the most energetically favourable respiratory pathway in the absence of oxygen, where nitrate (NO3−) is sequentially reduced to nitrite (NO2−), nitric oxide (NO), nitrous oxide (N2O), and dinitrogen (N2), through a sequence of electrochemical gradient and a series of oxidoreductases (Fig. 1) (Richardson 2000, Philippot et al. 2007, Bakken et al. 2012, Xie et al. 2020). Numerous environmental factors modulate denitrification in different ecosystems, such as the availability of NO3− and NO2−, O2 solubility, temperature, or pH (Kaplan et al. 1979, Albina et al. 2019, Raboni et al. 2020). One of the most important factors is salinity, which has been shown to affect denitrification, among other N-cycle processes (Ardón et al. 2018).
Figure 1.
Summary of reactions and enzymes involved in the denitrification process. The complete reduction of nitrate to dinitrogen is driven by metalloenzymes nitrate reductase (NarGH: membrane-bound nitrate reductase; NapAB: periplasmic nitrate reductase), nitrite reductase (NirK: copper-containing nitrite reductases; NirS: cytochrome-cd1-dependent nitrite reductases), nitric oxide reductase (qNor: quinol dependent nitric oxide reductase; cNor: short-chain respiratory nitric oxide reductase; CuANor: copper-containing nitric oxide reductase), and nitrous oxide reductase (NosZ).
Saline and hypersaline environments are found worldwide. Among them, are oceans, salty lakes and lagoons, saline estuaries, and salty ponds (Oren 2002, Andrei et al. 2012, Fu et al. 2019). Moreover, sea level rise has already caused marine salt increases in coastal wetlands in many regions of the world (Herbert et al. 2015). This trend is expected to become more widespread as rates of sea level rise will increase from current rates of 2.2–3.6 mm year−1 up to 15.6 mm year–1 by 2100 (Stocker et al. 2013). Furthermore, salinization together with desertification are a global problem that affects soil parameters and structure. Moreover, these ecosystems are increasing in size and prevalence (Feng and Fu 2013, Torregrosa-Crespo et al. 2018).
Denitrification per se is an essential metabolic pathway driven by microorganisms in these ecosystems as it allows cellular respiration in the absence of oxygen even though implies the loss of N-fixed. However, in the last decades, this metabolic pathway has become even more relevant because anthropogenic activities currently lead to the contamination of saline systems by NO3− and NO2− (Martinez-Espinosa et al. 2007, Martínez-Espinosa et al. 2011, Ochoa-Hueso et al. 2014, Torregrosa-Crespo et al. 2018). Denitrification is with nitrification, the main biological sources of nitrous oxide emission. N2O is an intermediate of the denitrification process and can be the final product in partial denitrification carried out by bacteria and archaea. It is worth noting that a large percentage of haloarchaea, considered the most representative group of microorganisms in hypersaline environments, carry out partial denitrification (releasing NO or N2O into the atmosphere). This characteristic has led to the identification of hypersaline ecosystems as a potential source of nitrogenous gases (Miralles-Robledillo et al. 2021). This, together with the expansion and prevalence of saline environments, means that denitrification in this kind of ecosystem deserves to be studied in depth.
The research studies that relate denitrification and salinity are numerous, but the approach used to analyse this relationship is quite different, resulting in a huge amount of unrelated information: on the one hand, there are studies focused on denitrification rates, which quantitatively measure the loss of nitrogen from the habitat by denitrification, using, e.g. the isotope 15N (Hou et al. 2013); on the other hand, there are molecular studies to quantify the abundance and diversity of denitrification related genes or denitrifying microorganisms under salinity conditions (Lay et al. 2013, Lee and Francis 2017).
In this sense, because the metabolic potential for denitrification is widespread among many phylogenetically unrelated groups, a 16S rRNA-based approach or 16S rRNA analysis methods have never been appropriate to study denitrifying communities (Francis et al. 2013). Instead, the functional genes encoding key metalloenzymes in the denitrification pathway have proven to be useful molecular markers for denitrifying organisms; particularly, nitrite reductase (NiR), which catalyses the first committed step to a gaseous product (Zumft 1997). The choice for nirS or nirK as molecular markers is strengthened by the fact that the main difference between true denitrifiers and other microorganisms with NO3−-reducing activity is that the first group has two different types of enzymes, a cytochrome cd1-containing nitrite reductase encoded by nirS (cdNir) or a Cu-dependent nitrite reductase (CuNir) encoded by nirK (Zhou et al. 2016). For this reason, genes nirK and nirS have been proven useful molecular markers to resolve the structure of denitrifying communities in most environments (Zhou et al. 2016).
Considering the rising relevance of arid and saline habitats in the world and the role that denitrification plays in the nitrogen biogeochemical cycle globally and in particular in saline ecosystems, this review aims to summarize and update the information available on this metabolic pathway in different coastal ecosystems where the presence of salts is relevant: from coastal environments with marine influences, such as estuaries, bays, or mangroves, to coastal environments with extreme concentrations of salts: the so-called hypersaline environments.
Freshwater, slightly saline, and marine coastal environments
During the last decades, rapid industrialization, massive fertilization of crops and urbanization have produced huge amounts of NO3−, NO2−, and NH4+ reaching coastal regions, which has already exerted a serious threat to the environmental quality of estuarine and coastal environments, intensifying eutrophication (Cheung et al. 2003, Seitzinger 2008). Although denitrification involves a loss of available nitrogen in the ecosystem, it also works as a sink for the excessive N load that these habitats could accumulate due to anthropogenic activities. In estuarine sediments, denitrification can remove more than 50% of the NO3− from the water column, hence the relevance of denitrification in coastal environments, playing a key role in ameliorating the degree of eutrophication (Seitzinger et al. 2006, Caffrey et al. 2007, Gruber and Galloway 2008, Francis et al. 2013).
Estuaries
Estuaries are ecosystems where a freshwater river or stream meets the ocean. When freshwater and seawater combine, the water becomes brackish, or slightly salty. In these ecosystems, it is estimated that denitrification contributes up to 93.4% to the total nitrogen loss while anammox was much less quantitatively significant (Zheng et al. 2015). In fact, the rate of denitrification in sediments has been significantly higher than those in other kinds of natural environments (Devol 2015), making estuarine sediments significant players in the removal of reactive nitrogen (Hong et al. 2019). For this reason, it is critical to understand the community dynamics and distribution of the underlying denitrifiers in estuarine sediments.
One of the most studied ecosystems as an estuary model for denitrification is the Yangtze estuary, belonging to the Yangtze River, the longest in China and the whole of Asia. This river has been receiving an increasing load of anthropogenic nitrogen from fish farming, agricultural activities, and both industrial and domestic wastewater discharge, which has resulted in severely eutrophic status in the estuarine and adjacent coastal areas (Chai et al. 2006). Furthermore, due to these activities, it has been estimated that denitrification contributed 87.1% to 93.4% to the total N loss from intertidal sediments of the estuary based on previous experiments (Hou et al. 2013).
In the study of Zheng et al. (2015), surface sediments samples were collected from seven representative sites from the intertidal flats along the Yangtze Estuary to investigate the diversity, composition, and abundance of denitrifiers based on the nirS gene and to explore potential links of these communities to estuarine environmental variables, especially the salinity (concentrations between 0 and 20 ppt). The results showed that the highest nirS biodiversity (based on nucleotide sequence) was observed at the lower salinity concentration (0–1.5 ppt), while the lowest value occurred at the higher salinity site (10–20 ppt). In terms of quantification, the abundance of nirS-harbouring denitrifiers was significantly higher at the lower salinity sites (6.37 × 106–9.00 × 107 copies g−1 sediment) than at the higher salinity sites (1.01 × 106–7.50 × 106 copies g−1 sediment; p < .05). Overall, the results indicated that nirS-harbouring bacterial community structures in the sediments of the Yangtze Estuary (in terms of nirS biodiversity) correlated significantly with salinity (p = .002). In fact, of all the environmental parameters investigated (temperature, NH4+, NO3−, and NO2−, total phosphorous, organic carbon, and sediment mean size), only salinity showed a significant correlation with the nirS gene diversity (r = −0.549; p = .042; n = 14) (Zheng et al. 2015). This trend was consistent with the previous results in estuarine sediments, confirming that salinity plays a vital role in the N-cycle of the estuarine ecosystems (Francis et al. 2013). Nevertheless, the abundance of denitrifiers may also be reflected by denitrification rates and in this study, any significant correlations between nirS gene abundance and denitrification rates (p > .05) were found.
Another recent study performed along the Yangtze estuary performed by Wang et al. (2019a) confirmed the results obtained by Zheng et al. (2015), but in this case, the authors analysed the community composition and distribution of the nosZ gene by high throughput sequencing and q-PCR (Wang et al. 2019a). This study also claimed that salinity is important in structuring denitrifying bacteria and pointed out that the abundance and diversity of denitrifiers were observed to decrease with the increase in salinity level (Wang et al. 2019a).
As highlighted before, surface sediments are known to be hot spots where N-fixed get lost, reducing the anthropogenic nitrogen input. However, the community structure of denitrifiers and their role in nitrogen loss remain poorly understood in the subsurface estuarine sediments. In this sense, one of the newest studies was carried out by Xie et al. (2020). In this case, the area of study belonged to the Pearl River, located in the south of China, and salinity was shown to be significant in driving the shift of community structure of nirS-denitrifiers [Shannon index was positively correlated with it (p < .01, R = 0.507)], although pH was the most significant environmental parameter influencing community structuring of the denitrifiers in the sediments (F = 8.4, p < .05). These results conflict with the study mentioned before carried out by Wang et al. (2019a), where the diversity of denitrifiers has a negative correlation with salinity. Probably, this is because the salinity gradient explored in the Xie et al. (2020) study was higher (0–294.5 psu), whereas in the study of Wang et al. (2019a) was narrower (24.1–34.5 psu).
The abundance of nirS-type bacteria in the sediments showed significant spatial variations: vertically in each sediment core, the abundance of the nirS gene decreased gradually from the surface to the bottom layer. The abundance of the nirS gene ranged from 1.70 × 105 to 3.63 × 108 copies g−1 in the sediments, similar to those observed in a subtropical estuary in Mexico (Bahia del Tobari), which had 2.72 × 106–8.82 × 107 copies g−1 (Beman 2014). The average values of abundance were 1.11 × 108 copies g−1 and 2.14 × 107 copies g−1 in surface and bottom sediment, respectively.
However, despite these differences, the potential rates of denitrification were not significantly different and there was no correlation between microbial activities and the abundance of the nirS gene (p > .05). The mismatching of potential rates and the nirS abundance was also observed in Zheng et al. (2015), and it could be explained by the fact that researchers did not target the nirK encoding gene in the study (Zheng et al. 2015). This trend has been followed in many denitrification studies in estuaries.
In the same year, Fozia et al. (2020) published a study in the Indus River estuary, in Pakistan. This river has a total length of 3200 km, and human activities, such as excessive use of fertilizers in agriculture and improper disposal of wastewater contribute > 3 × 106 tons of nitrogen to the Indus River basin each year, causing different environmental problems in the area, such as coastal eutrophication (Wang et al. 2019b). In response to this question, this study aimed to investigate microbial reactive nitrogen loss by analysing the biodiversity, abundance, and distribution patterns of nirS-harbouring denitrifiers across the salinity gradient of the Indus estuary. For this purpose, 12 samples from different areas of the estuary were collected and analysed. The physicochemical properties of the samples, as well as denitrification rates and nirS abundance, were measured in each sample (Fozia et al. 2020). The results showed that the biodiversity of nirS-harbouring denitrifiers obtained in the Indus River estuary was similar to that obtained in other environments (such as the Chesapeake Bay estuary) (Francis et al. 2013), and that the nirS gene diversity showed nonsignificant-spatial-differences between freshwater, low salinity and high salinity sampling sites (one-way ANOVA, p > .05), compared to previous studies. The analyses also displayed that the denitrifier communities from the Indus River were divided into two phylogenetic groups: group I was retrieved in the high-salinity sediments (16–36 ppt) and group II was recovered in the low-salinity and freshwater sediments (0–15 ppt). This distribution suggests that, as in the Yangtze estuary, salinity is an important factor in the distribution of denitrifier communities (Zheng et al. 2015). Furthermore, the abundance of the nirS gene was reported to range from 5.3 × 106 to 2.5 × 108 copies g−1 dry sediment, which is also very similar to the quantified abundance in the Yangtze River estuary, and the Bahia del Tobari estuary, but significantly higher than the found in the Laizhou bay sediments (Beman 2014, Zheng et al. 2015, Fozia et al. 2020). Finally, denitrification rates in the Indus River estuary were estimated in the range of 0.01–6.27 μmol N kg−1 h−1, stating that denitrification contributes to 78.1% of the total nitrogen loss in the marsh sediments of this estuary (Fozia et al. 2020).
Some studies have attempted to identify differences in the ecological niches occupied by nirS and nirK-harbouring bacteria in estuarine ecosystems, resulting in a more precise description of the denitrification capacity as both versions are usually present: this is the case of the work conducted by Lee and Francis (2017). The location studied was the San Francisco Bay estuary, which is the largest on the west coast of the USA. It has long been an ecosystem subject to anthropogenic change: the greatest sources of nitrogen are agricultural return flow drains and municipal wastewater treatment facilities (Hager and Schemel 1992).
The study showed that nirS was consistently more abundant than nirK in all samples: nirS abundances ranged from 1.2 × 107 to 2.9 × 108 copies g−1 while nirK abundances ranged between 5.9 × 105 and 1.8 × 106 copies g−1 sediment. These measurements agreed with results from studies in other estuaries, such as Chesapeake Bay (Bulow et al. 2008), Colne Estuary (Smith et al. 2007), and Elkhorn Slough (Smith et al. 2014) and also in previous studies in San Francisco Bay (Mosier and Francis 2010). Reports of nirK abundance in estuaries are scarce, but the few studies focused on it yielded abundance results between 103 and 107 copies g−1 sediment (Abell et al. 2010, Smith et al. 2014). In general, when both genes have been quantified, nirK is at least one order of magnitude less abundant than nirS or it is not even detectable (Nogales et al. 2002, Lee and Francis 2017). Furthermore, studies that consider nirK quantification, have been restricted by the specificity of the PCR primers to detect only cluster I-type nirK, hence the abundance of nirK communities would be higher (Mosier and Francis 2010, Wei et al. 2015, Helen et al. 2016).
Also, to understand how environmental factors might affect the variations in gene abundances, nirK and nirS relative abundances were studied in connection with NO3− concentrations, temperature, and salinity, among others. In the case of salinity, nirK showed a significant negative effect (p = .004). However, one of the most remarkable findings of this study is the identification of an abundant group of nirK-harbouring microorganisms that seems to be preferentially abundant in high-salinity regions of the estuary (around 30 psu). Many studies have found nirK genes and transcripts to be in low abundance or entirely undetectable in saline environments (Mosier and Francis 2010, Francis et al. 2013, Smith et al. 2014).
In terms of diversity, nirK communities were markedly site-specific: several clades appeared to be endemic to a few sites of the study and most of them showed high similarity to those found in estuarine and marine sediments such as Chesapeake Bay (Fortunato et al. 2009) or South China Sea subseafloor sediment (Li et al. 2013). The most closely related sequences from cultured denitrifiers isolates were all Alpha-, Beta-, or Gamma-proteobacteria. Of great interest is that 215 of nirK sequences fell into a single group of ‘high salinity’ clades, sharing 85% nucleotide identity on average, and branched deeply from the rest of the tree. The closest matches were other sequences from San Francisco Bay sediment, from high salinity sites in San Francisco Bay and the South Bay (Mosier and Francis 2010). However, no published sequences from cultured representatives fell within this group. Despite their high divergence from other nirK sequences, the sequences in the high-salinity clade all shared the conserved region surrounding the catalytic histidine typically associated with type I Cu-Nir.
In the case of nirS, most sequences fell into a large clade that includes sequences from cultured Beta- and Gamma-proteobacteria. However, in contrast to nirK phylogeny, site specificity was less apparent in nirS phylogeny. Most of the clades were closely related to uncultured sequences from coastal and estuarine sediments such as the Jiazhou Bay (Dang et al. 2009) and the Arabian Sea (Yoshida et al. 2009).
Another interesting study, i.e. worth mentioning was carried out along the Columbia River, where five sampling points were analysed by metagenomics and metatranscriptomics techniques, including samples from the river, the estuary, the plume, and the ocean (Fortunato and Crump 2015). A dramatic change in microbial community composition from the river to the ocean was observed from the taxonomic profiles of 16S sequences identified in the metagenomes. Actinobacteria and Betaproteobacteria decreased across the salinity gradient. Gammaproteobacteria, especially the family Oceanospirillales, increased from the river to the ocean. Overall, the metagenomes from the different samples were highly similar across the salinity gradient, with an average Bray–Curtis similarity of 82% based on the normalized abundance of COG functions. However, the metatranscriptomes were less similar. The average similarity was only 31%. Metatranscriptomic data revealed that denitrification gene expression (napA, narG, nirK, norB, and nosZ) was increased in the samples from the estuary and that the narG gene was also highly expressed in the river and coastal ocean (Fortunato and Crump 2015). In the end, despite the taxonomic differences between samples, no relationship between denitrification and salinity was found, probably due to the similarity in the functionality of the communities found. The authors of this study suggested that these similarities are due to a combination of factors such as the rapid movement of the river and the similarity of conditions between samples in terms of oxygen and dissolved organic carbon (Fortunato and Crump 2015).
Mangroves
Mangroves grow in the coastal sediment habitats at transition zones between terrestrial, freshwater, and oceanic environments in tropic and subtropic regions (Bai et al. 2013).
They play an important role as hotspots, transforming and removing nutrient compounds (Bouillon et al. 2008, Pennings 2012). One of the most important characteristics of these ecosystems is that they are subjected to tidal action, which causes large changes in different parameters such as salinity, water temperature, and oxygen level, increasing the complexity of their study. The study of the mangroves is particularly important, as the total area of these habitats is declining by 1%–2% by year and is predicted to disappear in the next century if the actual rate of decline continues (Duke et al. 2007, Giri et al. 2011).
It is believed that nitrogen removal in mangrove wetlands is primarily dependent on aerobic nitrification and anaerobic denitrification by microorganisms (Fu et al. 2019). Many efforts have focused on how denitrifying communities respond to the salinity elevation in coastal, yet the answer remains ambiguous (Marton et al. 2012, Xie et al. 2014, Sheng et al. 2015).
Xiao et al. (2018) investigated seawater–groundwater exchange rates and inorganic nitrogen concentrations along a shore-perpendicular intertidal transect in a subtropical mangrove swamp. Swamps are areas where water is collected and one of their characteristics is that oxygen concentration is usually low; in this study, three hydrologic subzones were sampled (tidal creek, mangrove, and bare mudflat zones) in Daya Bay, China. Results showed that denitrification accounted for 90% of the total nitrogen loss, and anammox accounted for the remaining 10%. Specifically, the highest potential denitrification rates were measured at 9.16 nmol N g−1 h−1 in the surface sediment of the core (SCS) obtained in the mangrove zone of the transects, which was about eight times higher than that at the surface sediments taken from the mudflat zone. Also, SCS had the highest abundance of nirS gene at 2.65 × 107 copies g−1, much higher compared to the bottom part of the sediment core (SCB; 5.95 × 106 copies g−1). Although denitrification, generally, is an anaerobic process, the highest rates were measured on the surface of the sediments, where the oxygen concentration was higher than in the lower part. Thus, denitrifiers exhibited higher activities on the surface where the substrate (e.g. organic matter and NO3−) was sufficient (Xiao et al. 2018). This study suggests that nitrification may be coupled with denitrification in the sediments. The need for a minimum oxygen concentration to carry out this metabolic pathway cannot be ruled out as has previously been proposed (Torregrosa-Crespo et al. 2020a). Although denitrification was initially described as an anoxic metabolic pathway, aerobic denitrification is also suitable for several bacterial species as it has been extensively reported during this century (Kim et al. 2008, Hao et al. 2022).
Another type of test, i.e. carried out for the study of environmental factors on the N-cycle, especially salinity, is those that use small-scale experimental vertical flow constructed wetland systems. Fu et al. (2019) constructed mangrove wetlands by planting the salt-tolerant mangrove species Kandelia candel to investigate the influence of salinity fluctuations on the denitrification performance and denitrifying microbial community (Fu et al. 2019). On the one hand, a significant negative correlation between salinity and NO3−-N removal was observed (r = −0.983; p = .019): at salinity levels below 0.9%, the constructed wetlands could remove NO3−-N with efficiencies of 59 ± 22%, while at a salinity of 1.8%, the NO3−-N concentration of the effluent was even higher than that of the influent (1.42 mg l−1 vs. 1.27 mg l−1) (Fu et al. 2019); on the other hand, when salinity increased (from 0% to 1.8%), the abundance of the nirS gene decreased from 2.82 ± 1.63 × 107 copies g−1 to 1.52 ± 1.23 × 107, showing a negative correlation between the two data (r = −0.743; p = .016). Furthermore, there was also found a significant negative correlation between salinity and the total nitrogen removal rate (r = −0.957; p = .011), an indication that salinity would have a certain inhibitory effect on the growth of denitrifying microorganisms (Fu et al. 2019).
Other recent studies have focused in greater depth on the effect of salinity on denitrification in mangrove habitats, mainly through laboratory incubation experiments. Wang et al. (2018) investigated the response of mangrove surface sediments nitrifying and denitrifying communities to different salinities (0, 10, 20, and 30 ppt) during 28-day incubation. The activity of denitrification was calculated as the average N2O emission per day during a 12-day incubation after sampling. The emission rate decreased as salinity increased, with the minimum value detected in vials with salinities of 30 ppt after 28 days of incubation. Likewise, salinity affected the abundances of denitrifying genes: the nirK and nosZ abundances were significantly lower in 30-ppt samples compared to the other samples on day 28, while there was no significant change in nirS abundance. Also, on day 28, the nirK/nirS ratio was significantly greater in 0-ppt samples compared to the others.
A recent study in the Qi’ao Mangrove Wetland Park (China) reported that the nitrogen fixation rate (NFR) and clusters for nitrogen-fixing increased with the depth of mangrove sediments (Luo et al. 2021). By contrast, the abundance of functional enzymes that carried out denitrification (nirS and nirK) decreased with depth (Luo et al. 2021). Also, the salt concentration increased with the depth. When the cluster of denitrification-related genes was compared between surficial and deep sediments, the abundance decreased between 13.8% and 49.7% depending on the denitrification-related enzyme (Luo et al. 2021).
Li et al. (2020) determined the nosZ-denitrifier communities in surface sediments of nationwide distribution mangrove wetlands in China and their relationships with the physicochemical parameters of sediments, including salinity and total nitrogen. The analysis was performed in the Avicennia marina mangrove forest and mudflat. In this case, the salinity, sediment nitrogen, and carbon had a positive correlation with the denitrifier community in the A. marina forest indicating that sediment denitrifier density depended on mangrove habitat specificity (Li et al. 2020).
As well, Franklin et al. (2017) reported a positive relationship between salinity and denitrifier abundance in tidal wetlands of Virginia (USA) showing a greater abundance of nirS denitrifiers than nrfA DNRA microorganisms.
Several papers reported that salinity negatively affected denitrification activity, the abundance of denitrifiers, and the community composition, as mentioned above. These data are in concordance with the idea that denitrification is negatively affected by salinization across coastal environments (Ikenaga et al. 2010, Wang et al. 2011, Zhou et al. 2017, 2018, Fu et al. 2019, Luo et al. 2021). Many mechanisms may contribute to this phenomenon: the increase of salinity might affect the growth of the microorganisms and their metabolism and then, reduce the soil respiration (Wong et al. 2008), decreasing the oxygen consumption and thus inhibiting the anaerobic denitrifying process (Wong et al. 2008). Also, reducing nitrification by salinity elevation can decrease nitrate availability, limiting denitrification (Giblin et al. 2010). However, some research reflects the contrary, and in some locations, denitrification has a significant positive correlation with salinity and depth (Franklin et al. 2017, Li et al. 2020). The mechanism behind this could be the lower solubility of oxygen in salt water, which forces microorganisms to perform alternative respiration, such as denitrification (Miralles-Robledillo et al. 2021).
Coastal hypersaline environments
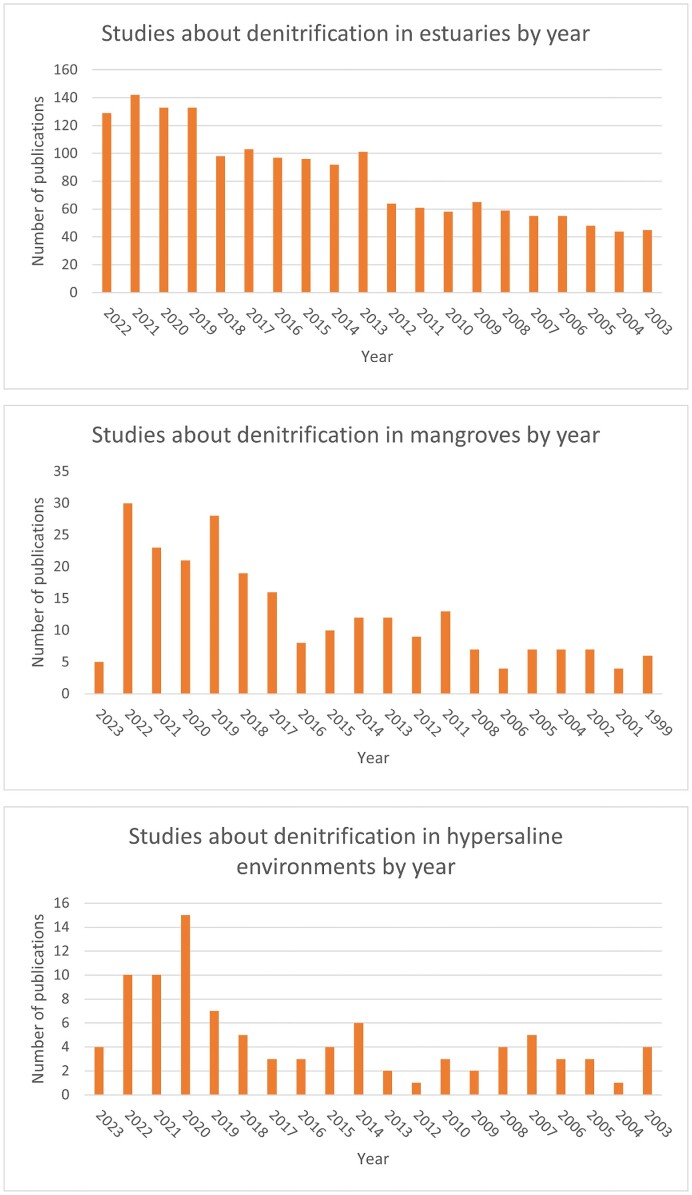
Hypersaline environments are in general worst described in terms of microbial biodiversity and denitrification than other geographically closed related ecosystems. A quick comparison using the database Web of Science demonstrates the differences in the number of publications by year in the three habitats analysed in this minireview (Fig. 2). The highest number of publications in the last 20 years corresponds to estuaries being the best-characterized ecosystems, followed by mangroves. However, the number of publications for hypersaline (not only coastal) environments is the lowest. Among the best-described hypersaline environments, the following examples could be highlighted.
Figure 2.
Bar charts comparing the number of publications available in the Web of Science database on denitrification over the last 20 years in hypersaline, mangroves, and estuarine ecosystems. (A) Number of publications by year about denitrification in mangroves. The search equation utilized to obtain the data was: estuar* AND denitrification (Topic). (B) Number of publications by year about denitrification in estuaries. The search equation utilized to obtain the data was: mangrove* AND denitrification (Topic). (C) Number of publications by year in hypersaline habitats. The search equation utilized to obtain the data was: hypersalin* AND denitrification (Topic).
The lost Hammer Spring comprises a cryoenvironment located on Alex Heiberg Island (Canada) and it is characterized by sub-zero temperatures, high salt concentration (25%), and oligotrophic (6.87 mg kg−1 of ammonia), microoxic (0.1–1 ppm), and reducing (−165 mV) conditions (Lay et al. 2013). Metagenomic and pyrosequencing analyses of the cDNA of its 16S rRNA genes were performed to determine the genetic and functional microbial components. Some genes involved in denitrification were detected, including narG, nirS, and nosZ. Surprisingly, any nitric oxide reductase read was found in the study (Lay et al. 2013). The taxonomic profile for each denitrification-related enzyme was highly different. Sequences related to Psychrobacter spp. and Nostoc spp. were the most important genera detected for narG, and Flavobacterium spp. and Kangiella spp. were the predominant genera containing nirS, whereas the nosZ gene was very restricted to deltaproteobacteria, especially Campylobacter spp. Although denitrification rates were not measured in the study, denitrification reads from metagenomic data were higher than nitrification and ammonification (Lay et al. 2013).
Desnues et al. (2007) performed a seasonal study to analyse the distribution of denitrifying and bacterial communities in a preconcentration pond of the Salin-de-Girauds salterns in Camargue (France) (Fourçans et al. 2004, Desnues et al. 2007). In the study, mat samples were collected in May 2000, January 2001, and June 2001. The salinity of the overlying water in each sample was 130 psu, 95 psu, and 120 psu, respectively (Fourçans et al. 2004, Desnues et al. 2007). Denitrification rates according to N2O production rates were similar in the three seasonal samples reaching values of 0.63 ± 17 mmol N m−2 d−1, 0.67 ± 0.09 mmol N m−2 d−1, and 0.88 ± 0.3 mmol N m−2 d−1, respectively. Additionally, in each sample, denitrifying populations were analysed, comparing DGGE patterns of 16S and nirS, and nirK genes, according to the depth of microbial mat and fluctuating parameters. The DGGE nirS pattern reported spatial and temporal changes according to depth and season and the nirS denitrifying community showed a significant change (R = 0.4749, p = .0028) comparing oxic and anoxic zones of the mat. On the one hand, the nirS community structure was significantly affected by environmental parameters (oxygen, pH, and sulfur) and preferentially deeply located in the permanent anoxic zone of the mat. On the other hand, nirK populations reported a differential change between oxic and anoxic zones, predominating in the upper layer of the mat with a large variation of physicochemical parameters. From an ecological point of view, the nirK microbial community seems to be greater adaptable (Desnues et al. 2007).
Another studied hypersaline ecosystem is the Ria Lagartos lagoon, which is an estuary within a protected environmental reserve on the northern coast of Yucatan (Mexico). This lagoon is under the effect of low precipitations and high evaporation, which produces eutrophication and an increase in the concentration of nitrogen (N) and phosphorus (P) in the area, as well as variations in salinity levels during the year (Valdes and Real 2004, Perry et al. 2010). Regarding these variations and the pressure of human activities, the aim of this study carried out by Valdes and Real (2004), was to determine the nitrogen and phosphorus balance and fluxes between the water column and the sediments of the lagoon.
For this study, 30 stations were sampled along the lagoon, and they were characterized by physicochemical analyses. Denitrification was detectable in all samples with values around 50 μmol m−2 h−1 and two stations above 100 μmol m−2 h−1 (47.4 μmol m−2 h−1 on average) and salinity was measured ranging from 60.05 to 147.52 psu (Valdes and Real 2004). The correlation between denitrification and salinity was not mentioned in the study, but it is stated that the total N in the sediments showed lower concentrations in the zone near the sea, and higher concentrations in the inner zone (Valdes and Real 2004). Furthermore, the estimated denitrification rates (47.4 μmol m−2 h−1 on average) were three times lower than the nitrification rates (150.5 μmol m−2 h−1 on average) and the average ammonium release (250 μmol m−2 h−1 on average) was almost twice the average nitrification (Valdes and Real 2004). The large differences between the three processes suggest that nitrogen recycling was very high and the yield low in this ecosystem. Moreover, the measured concentrations of organic matter (3.73% ± 1.65%), total nitrogen (99.14 ± 62.73 μmol g−1), and phosphorus (4.42 ± 1.82 μmol g−1) in lagoon sediments indicate that they may be sinks for these elements (Valdes and Real 2004).
Apart from these articles, there is not too much information about how denitrification is working in coastal hypersaline environments although denitrification driven by halophilic archaea and bacteria has been extensively described in vitro to characterize the enzymes involved and the pathway (Torregrosa-Crespo et al. 2020b, Lledó et al. 2004, 2020a, Miralles-Robledillo et al. 2023). This lack of knowledge should be considered to encourage new research focused on the nitrogen fluxes in these extremophilic ecosystems. Recent bioinformatic research about the most prevalent microorganisms in hypersaline habitats, the haloarchaea (Halobacteria class), analysed the denitrification capacity of these microorganisms by looking for denitrification genes in all the available haloarchaeal genomes in the NCBI Genome database, aiming to understand if the hypersaline habitats could be classified as sources or sinks of nitrogenous gases (Pruitt et al. 2007, Torregrosa-Crespo et al. 2018, Miralles-Robledillo et al. 2021). Regarding this, it was studied if these microorganisms carried a partial or a complete denitrification apparatus. The results showed that 70.5% of the reviewed species have the potential capability for denitrification, being 58.2% partial denitrifiers, and 12.3% of them complete denitrifiers (Miralles-Robledillo et al. 2021). The fact that most species encode at least one and more than half at least two key enzymes involved in denitrification indicates the importance of this process in ecosystems with low oxygen availability, such as hypersaline coastal environments. Moreover, it was also found that in this class of microorganisms, the denitrifiers whose theoretical end product is nitric oxide represent 12.8% of the total potential denitrifiers, whereas species in which denitrification end product would be nitrous oxide represent 46.5% (Miralles-Robledillo et al. 2021). Thus, 59.3% of the potential haloarchaeal denitrifiers can emit greenhouse gases into the atmosphere (Miralles-Robledillo et al. 2021). It is important to note that all archaea inhabiting hypersaline environments are the prevalent microorganisms and contain a clade II copper–nitrite reductase (NirK). This could explain the abundance of nirK in hypersaline ecosystems. Regarding the studies carried out in coastal hypersaline environments it can be deduced that the scientific community maybe is underestimating these habitats in terms of nitrogenous gas emissions.
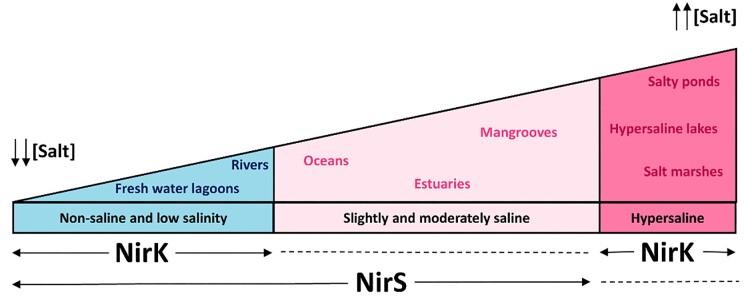
Finally, considering all the data about the NiR distribution collected in this minireview, it can be summarized that NirS and NirK showed a particular distribution depending on salinity. This distribution is shown in Fig. 3, that describes in which habitat each type of NiR is most abundant.
Figure 3.
Overview of the NiR-type distribution along aquatic environments regarding salinity based on the analysis of the literature done in this review. The dotted lines indicate the environments where the type of NiR is less abundant. Arrows indicate the higher abundance of that NiR type.
Considering all the mentioned studies carried out in coastal hypersaline environments it can be deduced that the scientific community maybe is underestimating these habitats in terms of nitrogenous gas emissions.
Conclusions
While salinity has a direct, although not fully understood, effect on the distribution patterns of denitrifiers, and more specifically nirS-harbouring bacterial communities, it is worth noting that a variety of physical/chemical environmental parameters might also be important in structuring estuarine microbial communities of denitrifiers with complex interactions (Dang et al. 2009, Francis et al. 2013, Zheng et al. 2015).
The prevalence of nirS or nirK denitrifiers in ecosystems is still a matter of debate. In general terms, there is a correlation between salt concentration and type of Nir abundance (Santoro et al. 2006), which indicates some kind of specialization of these enzymes. Studies in coastal ecosystems along salinity gradient conclude that with the increase of NaCl concentration (concentrations around 34.5 g/Kg), the predominant NiR in the microbial community is NirS (Santoro et al. 2006, Jones and Hallin 2010). However, in hypersaline environments (concentrations around or above 300 g l−1), the predominant NiR is NirK, found essentially in archaea species like Haloarcula marismortui, Haloarcula hispanica, Haloferax mediterranei, or Haloferax volcanii (Miralles-Robledillo et al. 2021).
This apparent specialization may be explained due to the more widespread nature of nirS sequences compared to nirK sequences along salinity gradients. The diversity of genes encoding NirS is founded in places with low and moderate NaCl concentrations while nirK-encoding genes are restricted to low salinity points or hypersaline environments, where the oxygen concentrations are low (Santoro et al. 2006, Miralles-Robledillo et al. 2021). This is summarized in Fig. 3. However, some studies reported nirK populations in the oxic layer with salt concentrations around 95 and 130 psu (Cole et al. 2004, Desnues et al. 2007). Although the prevalence of nirK in oxic layers is questionable, there is evidence of denitrification rate measurements in oxygenated microniches (Kim et al. 2008, Marchant et al. 2017, Hao et al. 2022).
The supposed advantage of NirK communities to the NirS at the highest salt concentrations might reside because of the different chemical structures of both enzymes, which determines the redox potential of the reactions catalysed in both cases. The first has copper centres with higher reducing power than the heme group of the cytochromes of NirS. This feature could explain the prevalence of NirK in environments whit low oxygen solubility and high reducing power like hypersaline habitats. Nevertheless, at the time of writing this work, there is no scientific evidence that verifies this hypothesis.
In this minireview, different research articles with different approaches have been analysed for all ecosystems. However, there are limitations in some of them that result in missing information that could be valuable for a better understanding of N fluxes and the importance of denitrification in these coastal ecosystems. Some studies only are focused on the measurements of physicochemical parameters (denitrification rates, total nitrogen…) and others only on the distribution or expression pattern of a specific type of enzyme (NirK/NirS/NosZ). Especially, a great part of the studies are focused only on the distribution of the nirS gene. Only analysing the distribution of a gene lead to an important loss of information because the presence of a gene does not necessarily mean that denitrification is happening in that ecosystem. This only confirms that are microorganisms with denitrification capacity. However, other articles integrate all these data using metagenomics, providing more details on what is happening with the microbial community in that specific sampling point. The different methods used in these articles are listed in Table 1. In summary, to obtain the most valuable data for the study of a specific environment, metagenomics and physicochemical measurements should be used together to overcome all the limitations that a study with a single approach and the use of less efficient techniques such as q-RTPCR can produce.
Table 1.
Summary of the advantages and disadvantages of three main techniques used in microbial population studies.
| Techniques | Advantages | Disadvantages | References |
|---|---|---|---|
| Physicochemical measurements | -Fast measurements. It does not need a meticulous handling | -Radiolabelling could be hazardous -Specific kits for radiolabelling It does not give molecular information |
Valdes and Real (2004), Wong et al. (2008), Giblin et al. (2010), Marton et al. (2012), Hong et al. (2019) |
| q-RTPCR and qPCR | - Amplification of specific gene -It can detect the expression (RNA) of the different genes |
-Weak or non-hybridization for divergent sequences -Requires optimization of the reaction (time-consuming) |
Desnues et al. (2007), Kim et al. (2008), Ikenaga et al. (2010), Wang et al. (2011), Bai et al. (2013), Hou et al. (2013), Xie et al. (2014), Zheng et al. (2015), Franklin et al. (2017), Lee and Francis (2017, 2018), Xiao et al. (2018), Fu et al. (2019), Fozia et al. (2020), Li et al. (2020), Luo et al. (2021) |
| Next-generation sequencing | -Whole metagenome or metatranscriptome sequencing -It can detect genes with divergent consensus sequences -More information about other bioprocesses |
-Cost of the service/reagents |
Giri et al. (2011), Lay et al. (2013), Fortunato and Crump (2015), Wang et al. (2019a), Luo et al. (2021) |
Finally, it is relevant to highlight that although denitrification has been studied in coastal environments like estuaries or mangroves, there is a lack of information about how denitrification works in hypersaline environments. There are almost no studies about denitrification rates and gene abundances in these areas, most of the research is focused on the description of the microbial diversity of these ecosystems (including the denitrifying community) (Han et al. 2017, Kimbrel et al. 2018, Pavloudi and Zafeiropoulos 2022). However, only by investigating the community we are missing relevant and sensitive information regarding the increase of hypersaline environments and their relationship with NO and N2O emissions (Miralles-Robledillo et al. 2021). Therefore, looking at the information available, more efforts should be made to understand denitrification in these extreme environments and how salinity gradient changes microbial population and denitrification capacity. Moreover, considering that some halophilic microorganisms have been proposed as good bioremediation agents for nitrate and nitrite removal in saline waters (Martínez-Espinosa et al. 2007).
Contributor Information
Javier Torregrosa-Crespo, Biochemistry and Molecular Biology, and Edaphology and Agricultural Chemistry Department, Faculty of Sciences, University of Alicante, Carretera San Vicente del Raspeig s/n, 03690 San Vicente del Raspeig, Alicante, Spain.
Jose María Miralles-Robledillo, Biochemistry and Molecular Biology, and Edaphology and Agricultural Chemistry Department, Faculty of Sciences, University of Alicante, Carretera San Vicente del Raspeig s/n, 03690 San Vicente del Raspeig, Alicante, Spain.
Eric Bernabeu, Biochemistry and Molecular Biology, and Edaphology and Agricultural Chemistry Department, Faculty of Sciences, University of Alicante, Carretera San Vicente del Raspeig s/n, 03690 San Vicente del Raspeig, Alicante, Spain.
Carmen Pire, Biochemistry and Molecular Biology, and Edaphology and Agricultural Chemistry Department, Faculty of Sciences, University of Alicante, Carretera San Vicente del Raspeig s/n, 03690 San Vicente del Raspeig, Alicante, Spain; Multidisciplinary Institute for Environmental Studies “Ramón Margalef” (IMEM), University of Alicante, Carretera San Vicente del Raspeig s/n, 03690 San Vicente del Raspeig, Alicante, Spain.
Rosa María Martínez-Espinosa, Biochemistry and Molecular Biology, and Edaphology and Agricultural Chemistry Department, Faculty of Sciences, University of Alicante, Carretera San Vicente del Raspeig s/n, 03690 San Vicente del Raspeig, Alicante, Spain; Multidisciplinary Institute for Environmental Studies “Ramón Margalef” (IMEM), University of Alicante, Carretera San Vicente del Raspeig s/n, 03690 San Vicente del Raspeig, Alicante, Spain.
Conflict of interest
None declared.
References
- Abell GCJ, Revill AT, Smith Cet al. Archaeal ammonia oxidizers and nirS-type denitrifiers dominate sediment nitrifying and denitrifying populations in a subtropical macrotidal estuary. ISME J. 2010;4. 10.1038/ismej.2009.105. [DOI] [PubMed] [Google Scholar]
- Albina P, Durban N, Bertron Aet al. Influence of hydrogen electron donor, alkaline pH, and high nitrate concentrations on microbial denitrification: a review. Int J Mol Sci. 2019;20. 10.3390/ijms20205163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei AŞ, Banciu HL, Oren A. Living with salt: metabolic and phylogenetic diversity of Archaea inhabiting saline ecosystems. FEMS Microbiol Lett. 2012;330:1–9. [DOI] [PubMed] [Google Scholar]
- Ardón M, Helton AM, Bernhardt ES. Salinity effects on greenhouse gas emissions from wetland soils are contingent upon hydrologic setting: a microcosm experiment. Biogeochemistry. 2018;140. 10.1007/s10533-018-0486-2. [DOI] [Google Scholar]
- Bai S, Li J, He Zet al. GeoChip-based analysis of the functional gene diversity and metabolic potential of soil microbial communities of mangroves. Appl Microbiol Biotechnol. 2013;97. 10.1007/s00253-012-4496-z. [DOI] [PubMed] [Google Scholar]
- Bakken LR, Bergaust L, Liu Bet al. Regulation of denitrification at the cellular level: a clue to the understanding of N2O emissions from soils. Phil Trans R Soc B. 2012;367:1226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beman JM. Activity, abundance, and diversity of nitrifying archaea and denitrifying bacteria in sediments of a subtropical estuary: bahía del Tóbari, Mexico. Estuar Coasts. 2014;37:1343–52. [Google Scholar]
- Bulow SE, Francis CA, Jackson GAet al. Sediment denitrifier community composition and nirS gene expression investigated with functional gene microarrays. Environ Microbiol. 2008;10. 10.1111/j.1462-2920.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- Caffrey JM, Bano N, Kalanetra Ket al. Ammonia oxidation and ammonia-oxidizing bacteria and archaea from estuaries with differing histories of hypoxia. ISME J. 2007;1. 10.1038/ismej.2007.79. [DOI] [PubMed] [Google Scholar]
- Chai C, Yu Z, Song Xet al. The status and characteristics of eutrophication in the Yangtze River (Changjiang) estuary and the adjacent East China Sea, China. Hydrobiologia. 2006;563. 10.1007/s10750-006-0021-7. [DOI] [Google Scholar]
- Cheung KC, Poon BHT, Lan CYet al. Assessment of metal and nutrient concentrations in river water and sediment collected from the cities in the Pearl River Delta, South China. Chemosphere. 2003;52. 10.1016/S0045-6535(03)00479-X. [DOI] [PubMed] [Google Scholar]
- Cole AC, Semmens MJ, LaPara TM. Stratification of activity and bacterial community structure in biofilms grown on membranes transferring oxygen. Appl Environ Microbiol. 2004;70:1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang H, Wang C, Li Jet al. Diversity and distribution of sediment nirS-encoding bacterial assemblages in response to environmental gradients in the eutrophied Jiaozhou Bay, China. Microb Ecol. 2009;58. 10.1007/s00248-008-9469-5. [DOI] [PubMed] [Google Scholar]
- Desnues C, Michotey VD, Wieland Aet al. Seasonal and diel distributions of denitrifying and bacterial communities in a hypersaline microbial mat (Camargue, France). Water Res. 2007;41:3407–19. [DOI] [PubMed] [Google Scholar]
- Devol AH. Denitrification, anammox, and N2 production in marine sediments. Ann Rev Mar Sci. 2015;7. 10.1146/annurev-marine-010213-135040. [DOI] [PubMed] [Google Scholar]
- Duke NC, Meynecke J-O, Dittmann Set al. A world without mangroves?. Science. 2007;317. 10.1126/science.317.5834.41b. [DOI] [PubMed] [Google Scholar]
- Feng S, Fu Q. Expansion of global drylands under a warming climate. Atmos Chem Phys. 2013;13:10081–94. [Google Scholar]
- Fortunato CS, Carlini DB, Ewers Eet al. Nitrifier and denitrifier molecular operational taxonomic unit compositions from sites of a freshwater estuary of Chesapeake Bay. Can J Microbiol. 2009;55. 10.1139/W08-124. [DOI] [PubMed] [Google Scholar]
- Fortunato CS, Crump BC. Microbial gene abundance and expression patterns across a river to ocean salinity gradient. PLoS ONE. 2015;10. 10.1371/JOURNAL.PONE.0140578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourçans A, De Oteyza TG, Wieland Aet al. Characterization of functional bacterial groups in a hypersaline microbial mat community (Salins-de-Giraud, Camargue, France). FEMS Microbiol Ecol. 2004;51. 10.1016/j.femsec.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Fozia, Zheng Y, Hou Let al. Community dynamics and activity of nirS-harboring denitrifiers in sediments of the Indus River Estuary. Mar Pollut Bull. 2020;153. 10.1016/j.marpolbul.2020.110971. [DOI] [PubMed] [Google Scholar]
- Francis CA, O'Mullan GD, Cornwell JCet al. Transitions in nirS-type denitrifier diversity, community composition, and biogeochemical activity along the Chesapeake Bay estuary. Front Microbiol. 2013;4. 10.3389/fmicb.2013.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RB, Morrissey EM, Morina JC. Changes in abundance and community structure of nitrate-reducing bacteria along a salinity gradient in tidal wetlands. Pedobiologia. 2017;60. 10.1016/j.pedobi.2016.12.002. [DOI] [Google Scholar]
- Fu G, Han J, Yu Tet al. The structure of denitrifying microbial communities in constructed mangrove wetlands in response to fluctuating salinities. J Environ Manage. 2019;238. 10.1016/j.jenvman.2019.02.029. [DOI] [PubMed] [Google Scholar]
- Giblin AE, Weston NB, Banta GTet al. The effects of salinity on nitrogen losses from an oligohaline estuarine sediment. Estuar Coasts. 2010;33. 10.1007/s12237-010-9280-7. [DOI] [Google Scholar]
- Giri C, Ochieng E, Tieszen LLet al. Status and distribution of mangrove forests of the world using Earth observation satellite data. Global Ecol Biogeogr. 2011;20. 10.1111/j.1466-8238.2010.00584.x. [DOI] [Google Scholar]
- Gruber N, Galloway JN. An earth-system perspective of the global nitrogen cycle. Nature. 2008;451. 10.1038/nature06592. [DOI] [PubMed] [Google Scholar]
- Hager SW, Schemel LE. Sources of nitrogen and phosphorus to Northern San Francisco Bay. Estuaries. 1992;15:40–52. [Google Scholar]
- Han R, Zhang X, Liu Jet al. Microbial community structure and diversity within hypersaline Keke Salt Lake environments. Can J Microbiol. 2017;63:895–908. [DOI] [PubMed] [Google Scholar]
- Helen D, Kim H, Tytgat Bet al. Highly diverse nirK genes comprise two major clades that harbour ammonium-producing denitrifiers. BMC Genomics. 2016;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert ER, Boon P, Burgin AJet al. A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere. 2015;6. 10.1890/ES14-00534.1. [DOI] [Google Scholar]
- Hong Y, Wu J, Guan Fet al. Nitrogen removal in the sediments of the Pearl River Estuary, China: evidence from the distribution and forms of nitrogen in the sediment cores. Mar Pollut Bull. 2019;138. 10.1016/j.marpolbul.2018.11.040. [DOI] [PubMed] [Google Scholar]
- Hou L, Zheng Y, Liu Met al. Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in marsh sediments of the Yangtze Estuary. J Geophys Res Biogeosci. 2013;118. 10.1002/jgrg.20108. [DOI] [Google Scholar]
- Ikenaga M, Guevara R, Dean ALet al. Changes in community structure of sediment bacteria along the Florida coastal everglades marsh-mangrove-seagrass salinity gradient. Microb Ecol. 2010;59. 10.1007/s00248-009-9572-2. [DOI] [PubMed] [Google Scholar]
- Jones CM, Hallin S. Ecological and evolutionary factors underlying global and local assembly of denitrifier communities. ISME J. 2010;4:633–41. [DOI] [PubMed] [Google Scholar]
- Kaplan W, Valiela I, Teal JM. Denitrification in a salt marsh ecosystem. Limnol Oceanogr. 1979;24. 10.4319/lo.1979.24.4.0726. [DOI] [Google Scholar]
- Kim M, Jeong SY, Yoon SJet al. Aerobic denitrification of Pseudomonas putida AD-21 at different C/N ratios. J Biosci Bioeng. 2008;106:498–502. [DOI] [PubMed] [Google Scholar]
- Kimbrel JA, Ballor N, Wu YWet al. Microbial community structure and functional potential along a hypersaline gradient. Front Microbiol. 2018;9:1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay CY, Mykytczuk NCS, Yergeau Éet al. Defining the functional potential and active community members of a sediment microbial community in a high-Arctic hypersaline subzero spring. Appl Environ Microbiol. 2013;79:3637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Francis CA. Spatiotemporal characterization of San Francisco Bay denitrifying communities: a comparison of nirK and nirS diversity and abundance. Microb Ecol. 2017;73:271–84. [DOI] [PubMed] [Google Scholar]
- Li M, Hong Y, Cao Het al. Diversity, abundance, and distribution of NO-forming nitrite reductase-encoding genes in deep-sea subsurface sediments of the South China Sea. Geobiology. 2013;11. 10.1111/gbi.12020. [DOI] [PubMed] [Google Scholar]
- Li R, Wu S, Chai Met al. Denitrifier communities differ in mangrove wetlands across China. Mar Pollut Bull. 2020;155. 10.1016/j.marpolbul.2020.111160. [DOI] [PubMed] [Google Scholar]
- Lledó B, Martínez-Espinosa RM, Marhuenda-Egea FCet al. Respiratory nitrate reductase from haloarchaeon Haloferax mediterranei: biochemical and genetic analysis. Biochim Biophys Acta. 2004;1674:50–9. [DOI] [PubMed] [Google Scholar]
- Luo Z, Zhong Q, Han Xet al. Depth-dependent variability of biological nitrogen fixation and diazotrophic communities in mangrove sediments. Microbiome. 2021;9. 10.1186/s40168-021-01164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant HK, Ahmerkamp S, Lavik Get al. Denitrifying community in coastal sediments performs aerobic and anaerobic respiration simultaneously. ISME J. 2017;11. 10.1038/ismej.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Espinosa RM, Dridge EJ, Bonete MJet al. Look on the positive side! the orientation, identification and bioenergetics of “Archaeal” membrane-bound nitrate reductases. FEMS Microbiol Lett. 2007;276:129–39. [DOI] [PubMed] [Google Scholar]
- Martínez-Espinosa RM, Zafrilla B, Camacho Met al. Nitrate and nitrite removal from salted water by Haloferax mediterranei. Biocatal Biotransform. 2007;25:295–300. [Google Scholar]
- Marton JM, Herbert ER, Craft CB. Effects of salinity on denitrification and greenhouse gas production from laboratory-incubated tidal forest soils. Wetlands. 2012;32. 10.1007/s13157-012-0270-3. [DOI] [Google Scholar]
- Miralles-Robledillo JM, Bernabeu E, Giani Met al. Distribution of denitrification among haloarchaea: a comprehensive study. Microorganisms. 2021;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles-Robledillo JM, Martínez-Espinosa RM, Pire C. Analysis of the external signals driving the transcriptional regulation of the main genes involved in denitrification in Haloferax mediterranei. Front Microbiol. 2023;14:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier AC, Francis CA. Denitrier abundance and activity across the San Francisco Bay estuary. Environ Microbiol Rep. 2010a;2. 10.1111/j.1758-2229.2010.00156.x. [DOI] [PubMed] [Google Scholar]
- Nogales B, Timmis KN, Nedwell DBet al. Detection and diversity of expressed denitrification genes in estuarine sediments after reverse transcription-PCR amplification from mRNA. Appl Environ Microbiol. 2002;68. 10.1128/AEM.68.10.5017-5025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Hueso R, Arróniz-Crespo M, Bowker MAet al. Biogeochemical indicators of elevated nitrogen deposition in semiarid mediterranean ecosystems. Environ Monit Assess. 2014;186. 10.1007/s10661-014-3822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A. Diversity of halophilic microorganisms: environments, phylogeny, physiology, and applications. J Ind Microbiol Biotechnol. 2002;28. 10.1038/sj/jim/7000176. [DOI] [PubMed] [Google Scholar]
- Pavloudi C, Zafeiropoulos H. Deciphering the community structure and the functional potential of a hypersaline marsh microbial mat community. FEMS Microbiol Ecol. 2022;98:1–18. [DOI] [PubMed] [Google Scholar]
- Pennings SC. Ecology: the big picture of marsh loss. Nature. 2012;490. 10.1038/490352a. [DOI] [PubMed] [Google Scholar]
- Perry E, Velazquez-Oliman G, Marin L. The hydrogeochemistry of the karst aquifer system of the Northern Yucatan Peninsula, Mexico. Int Geol Rev. 2010;44:191–221. http://dx.doi.org/102747/0020-6814443191. [Google Scholar]
- Philippot L, Hallin S, Schloter M. Ecology of denitrifying prokaryotes in agricultural soil. Adv Agron. 2007;96. 10.1016/S0065-2113(07)96003-4. [DOI] [Google Scholar]
- Pruitt KD, Tatusova T, Maglott DR. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007;35. 10.1093/nar/gkl842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raboni M, Viotti P, Rada ECet al. The sensitivity of a specific denitrification rate under the dissolved oxygen pressure. Int J Environ Res Public Health. 2020;17. 10.3390/ijerph17249366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DJ. Bacterial respiration: a flexible process for a changing environment. Microbiology. 2000;146. 10.1099/00221287-146-3-551. [DOI] [PubMed] [Google Scholar]
- Seitzinger S. Nitrogen cycle: out of reach. Nature. 2008;452. 10.1038/452162a. [DOI] [PubMed] [Google Scholar]
- Seitzinger S, Harrison JA, Böhlke JKet al. Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl. 2006;16. 10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sheng Q, Wang L, Wu J. Vegetation alters the effects of salinity on greenhouse gas emissions and carbon sequestration in a newly created wetland. Ecol Eng. 2015;84. 10.1016/j.ecoleng.2015.09.047. [DOI] [Google Scholar]
- Smith CJ, Nedwell DB, Dong LFet al. Diversity and abundance of nitrate reductase genes (narG and napA), nitrite reductase genes (nirS and nrfA), and their transcripts in estuarine sediments. Appl Environ Microbiol. 2007;73. 10.1128/AEM.02894-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM, Mosier AC, Francis CA. Spatiotemporal relationships between the abundance, distribution, and potential activities of ammonia-oxidizing and denitrifying microorganisms in intertidal sediments. Microb Ecol. 2014;69. 10.1007/s00248-014-0450-1. [DOI] [PubMed] [Google Scholar]
- Stocker TF, Qin D, Plattner G-Ket al. IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. AR5. Cambridge: Cambridge University Press, 2013. [Google Scholar]
- Torregrosa-Crespo J, Bergaust L, Pire Cet al. Denitrifying haloarchaea: sources and sinks of nitrogenous gases. FEMS Microbiol Lett. 2018;365. 10.1093/femsle/fnx270. [DOI] [PubMed] [Google Scholar]
- Torregrosa-Crespo J, Pire C, Bergaust L. et al. Haloferax mediterranei, an archaeal model for denitrification in saline systems, characterized through integrated physiological and transcriptional analyses. Front Microbiol. 2020a;11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrosa-Crespo J, Pire C, Richardson DJet al. Exploring the molecular machinery of denitrification in Haloferax mediterranei through proteomics. Front Microbiol. 2020b;11:605859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes DS, Real E. Nitrogen and phosphorus in water and sediments at Ria Lagartos coastal lagoon, Yucatan, Gulf of Mexico. Ind J Mar Sci. 2004;33:338–45. [Google Scholar]
- Wang H, Gilbert JA, Zhu Yet al. Salinity is a key factor driving the nitrogen cycling in the mangrove sediment. Sci Total Environ. 2018;631-632. 10.1016/j.scitotenv.2018.03.102. [DOI] [PubMed] [Google Scholar]
- Wang J, Kan J, Qian Get al. Denitrification and anammox: understanding nitrogen loss from Yangtze Estuary to the east China sea (ECS). Environ Pollut. 2019a;252. 10.1016/j.envpol.2019.06.025. [DOI] [PubMed] [Google Scholar]
- Wang J, Yang D, Zhang Yet al. Do patterns of bacterial diversity along salinity gradients differ from those observed for macroorganisms?. PLoS ONE. 2011;6. 10.1371/journal.pone.0027597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Tang T, Burek Pet al. Increasing nitrogen export to sea: a scenario analysis for the Indus River. Sci Total Environ. 2019b;694:133629. [DOI] [PubMed] [Google Scholar]
- Wei W, Isobe K, Nishizawa Tet al. Higher diversity and abundance of denitrifying microorganisms in environments than considered previously. ISME J. 2015;9:1954–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VNL, Dalal RC, Greene RSB. Salinity and sodicity effects on respiration and microbial biomass of soil. Biol Fertil Soils. 2008;44. 10.1007/s00374-008-0279-1. [DOI] [Google Scholar]
- Xiao K, Wu J, Li Het al. Nitrogen fate in a subtropical mangrove swamp: potential association with seawater-groundwater exchange. Sci Total Environ. 2018;635. 10.1016/j.scitotenv.2018.04.143. [DOI] [PubMed] [Google Scholar]
- Xie H, Hong Y, Liu Het al. Spatio-temporal shifts in community structure and activity of nirS-type denitrifiers in the sediment cores of Pearl River Estuary. PLoS ONE. 2020;15. 10.1371/journal.pone.0231271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Zhang C, Zhou Xet al. Salinity-dominated change in community structure and ecological function of Archaea from the lower Pearl River to coastal South China Sea. Appl Microbiol Biotechnol. 2014;98. 10.1007/s00253-014-5838-9. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Ishii S, Otsuka Set al. Temporal shifts in diversity and quantity of nirS and nirK in a rice paddy field soil. Soil Biol Biochem. 2009;41. 10.1016/j.soilbio.2009.07.012. [DOI] [Google Scholar]
- Zheng Y, Hou L, Liu Met al. Diversity, abundance, and distribution of nirS-harboring denitrifiers in intertidal sediments of the Yangtze Estuary. Microb Ecol. 2015;70. 10.1007/s00248-015-0567-x. [DOI] [PubMed] [Google Scholar]
- Zhou M, Butterbach-Bahl K, Vereecken Het al. A meta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems. Glob Chang Biol. 2017;23. 10.1111/gcb.13430. [DOI] [PubMed] [Google Scholar]
- Zhou S, Huang T, Zhang Cet al. Illumina MiSeq sequencing reveals the community composition of NirS-Type and NirK-Type denitrifiers in Zhoucun reservoir-a large shallow eutrophic reservoir in northern China. RSC Adv. 2016;6. 10.1039/c6ra18017e. [DOI] [Google Scholar]
- Zumft WG. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev. 1997;61. 10.1128/.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]