Abstract
Dual-tropic human immunodeficiency virus type 1 (HIV-1) strains infect both primary macrophages and transformed T-cell lines. Prototype T-cell line-tropic (T-tropic) strains use CXCR4 as their principal entry coreceptor (X4 strains), while macrophagetropic (M-tropic) strains use CCR5 (R5 strains). Prototype dual tropic strains use both coreceptors (R5X4 strains). Recently, CXCR4 expressed on macrophages was found to support infection by certain HIV-1 isolates, including the dual-tropic R5X4 strain 89.6, but not by T-tropic X4 prototypes like 3B. To better understand the cellular basis for dual tropism, we analyzed the macrophage coreceptors used for Env-mediated cell-cell fusion as well as infection by several dual-tropic HIV-1 isolates. Like 89.6, the R5X4 strain DH12 fused with and infected both wild-type and CCR5-negative macrophages. The CXCR4-specific inhibitor AMD3100 blocked DH12 fusion and infection in macrophages that lacked CCR5 but not in wild-type macrophages. This finding indicates two independent entry pathways in macrophages for DH12, CCR5 and CXCR4. Three primary isolates that use CXCR4 but not CCR5 (tybe, UG021, and UG024) replicated efficiently in macrophages regardless of whether CCR5 was present, and AMD3100 blocking of CXCR4 prevented infection in both CCR5 negative and wild-type macrophages. Fusion mediated by UG021 and UG024 Envs in both wild-type and CCR5-deficient macrophages was also blocked by AMD3100. Therefore, these isolates use CXCR4 exclusively for entry into macrophages. These results confirm that macrophage CXCR4 can be used for fusion and infection by primary HIV-1 isolates and indicate that CXCR4 may be the sole macrophage coreceptor for some strains. Thus, dual tropism can result from two distinct mechanisms: utilization of both CCR5 and CXCR4 on macrophages and T-cell lines, respectively (dual-tropic R5X4), or the ability to efficiently utilize CXCR4 on both macrophages and T-cell lines (dual-tropic X4).
Prototype macrophagetropic (M-tropic) non-syncytium-inducing (NSI) variants of human immunodeficiency virus type 1 (HIV-1) replicate in primary lymphocytes and macrophages but not in transformed cell lines, are poorly cytopathic in vitro, and use CCR5 as their principal coreceptor for entry (R5 strains) (reviewed in references 4, 11, 23, and 31). In contrast, prototype T-cell line-tropic (T-tropic) syncytium-inducing (SI) variants replicate in lymphocytes and CD4+ transformed cell lines but not macrophages, are highly cytopathic in vitro, and use CXCR4 as their principal coreceptor (X4 strains). M-tropic NSI strains which use CCR5 are responsible for person-to-person viral transmission and are the predominant species during the asymptomatic phase of infection (48, 52). SI strains that replicate in transformed cell lines and use CXCR4 for entry frequently emerge later in infection and are linked with disease progression (15, 39). Other chemokine or orphan receptors in addition to the major coreceptors CCR5 and CXCR4 can be used by certain strains in transfected cells, but whether they are used in vivo or for infection of primary cells is uncertain (4, 11, 23, 31).
Dual-tropic HIV-1 isolates can infect both macrophages and CD4+ T-cell lines, in addition to primary lymphocytes. Dual-tropic isolates may be intermediates in the evolution from M-tropic/NSI to T-tropic/SI that occurs in vivo (39). Alternatively, some studies have reported that late-stage SI strains retain the capacity to infect macrophages, which suggests that late-stage variants are more similar to dual-tropic than to T-tropic prototypes (15, 45, 47). Prototype dual-tropic HIV-1 isolates such as strains 89.6 and DH12 can use both CCR5 and CXCR4 as fusion coreceptors (R5X4 strains) (10, 18). The use of both coreceptors by these strains provides an explanation for dual tropism, whereby CCR5 would mediate infection of macrophages and lymphocytes, while CXCR4 would enable infection of cell lines and lymphocytes and result in the SI phenotype.
The relationship between M versus T tropism and CCR5 versus CXCR4 utilization initially suggested that the cellular determinants of tropism would be linked in a straightforward manner to selective expression of CCR5 on macrophages, CXCR4 on cell lines, and both on lymphocytes. However, we and several other groups have found that macrophages express CXCR4 and that it can be used for entry by some HIV-1 isolates, even though macrophages are not permissive for prototype T-tropic X4 strains (44, 49, 51). Importantly, X4 prototypes are generally T-cell line-adapted (TCLA) strains and may differ from X4 primary isolates that have not been subjected to extensive passage in vitro. Thus, the relationship between coreceptor expression, HIV-1 permissiveness, and the cellular determinants of tropism are complex and may differ between prototype and primary isolates.
This study was designed to address the cellular basis for dual tropism among primary isolates of HIV-1. In addition, it was aimed at better understanding the role of macrophage CXCR4 in HIV-1 entry. Because there may be multiple levels in the viral life cycle at which permissive versus restricted infection may be determined, we studied cell-cell fusion mediated by the Env glycoprotein and specific coreceptors on macrophages, as well as coreceptor-mediated macrophage infection. To this end, we examined fusion and infection in primary macrophages from normal donors in parallel with macrophages lacking CCR5 derived from donors homozygous for the CCR5 Δ32 deletion allele (35). In conjunction, we used a highly specific CXCR4 inhibitor, AMD3100 (38). The results indicate that both CCR5 and CXCR4 on macrophages can support fusion and infection by some primary HIV-1 strains and that there are strain-specific differences in the ability to use macrophage CXCR4 for fusion. They also show that some dual-tropic isolates use both coreceptors on macrophages, but that other dual-tropic primary isolates use CXCR4 only and are dual tropic because they are able to use CXCR4 on macrophages as well as on cell lines.
MATERIALS AND METHODS
Viruses and env expression vectors.
Prototype viruses used were the M-tropic strain JRFL, T-tropic strain 3B, and dual-tropic strain 89.6 (12, 25, 40). The dual-tropic strain DH12 was kindly provided by M. Cho, National Institutes of Health (NIH) (41). Clade D strains UG021 and UG024 were obtained from the NIH AIDS Reagent Repository (21). The primary isolate tybe was isolated from the cerebrospinal fluid cell pellet of a subject with AIDS by coculture with seronegative donor peripheral blood mononuclear cells that had been stimulated with phytohemagglutinin and interleukin-2. The plasmid-encoded JRFL, 89.6, and 3B (clone BH8) env clones have been described previously (18). The UG021.16 and UG024.2 env clones were obtained from the NIH AIDS Reagent Repository (6, 21). In some experiments, recombinant vaccinia viruses were used to express Env proteins from 3B (BH8), 89.6, JRFL, and DH12 (10, 18).
Isolation of MDM and macrophage infections.
Healthy volunteers were screened by PCR for the presence or absence of the CCR5 Δ32 deletion allele as previously described (35). Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque separation from heparinized blood, and monocytes were purified by a stringent two-step selective adherence procedure as previously described (13). Cells were plated at 2 × 105 cells per well in 48-well tissue culture plates and were cultured for 1 week before use to allow differentiation into monocyte-derived macrophages (MDM). Cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, 10% horse serum, glutamine (1 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), and macrophage colony-stimulating factor (100 U/ml; Genetics Institute), and 50 to 100% of medium was replaced with fresh medium twice weekly.
One-week-old cultures of MDM were infected overnight with 20 ng of p24 antigen of each virus and then washed extensively. Supernatant was sampled periodically for p24 antigen production by enzyme-linked immunosorbent assay (Dupont). To block CXCR4, MDM were incubated with AMD3100 (1 μg/ml) for 1 h before and throughout the infection and were replaced each time medium was replaced (38).
Cell-cell fusion with primary macrophages mediated by Env.
Two assays were used to analyze cell-cell fusion with primary macrophages mediated by Env. For plasmid-encoded T7-driven env genes, we used a fusion assay that employs two different recombinant vaccinia viruses expressing distinct RNA polymerases (24). MDM were infected for 1 h at a multiplicity of infection (MOI) of 5 with recombinant vaccinia virus vSIMBE/L (46), which expresses the SP6 RNA polymerase. They were then incubated overnight at 32°C in medium supplemented with rifampin (100 μg/ml; Sigma). Effector 293T cells were infected for 1 h at an MOI of 10 with recombinant vaccinia virus vP11T7gene1 (1), which expresses the T7 polymerase, and then cotransfected by the calcium phosphate method with a plasmid bearing env genes under control of the T7 promoter and a reporter plasmid bearing the luciferase reporter gene under control of SP6 promoter (Promega). Following transfection, the effector cells were incubated overnight at 32°C in medium containing rifampin. Env-expressing effector cells were then mixed with target macrophages in the presence of rifampin and Ara-C (cytosine β-d-arabinofuranoside; 0.1 μM; Sigma), and cell-cell fusion was quantified by measuring luciferase activity (expressed as relative light units [RLU]) in cell lysates 6 h later as previously described (18).
For vaccinia virus-encoded env genes, we used a macrophage cell-cell fusion assay modified after one described by Broder et al. (7). Macrophages were infected with the T7 polymerase-expressing recombinant vaccinia virus vTF7.3 (20) for 1 h at an MOI of 5. They were then incubated at 32°C overnight in macrophage medium supplemented with rifampin. Effector 293T cells were infected with Env-expressing recombinant vaccinia viruses for 1 h at an MOI of 10. They were then transfected with a plasmid encoding the luciferase reporter gene under control of the T7 promoter (Promega) and incubated in rifampin-containing medium at 32°C overnight. Env-expressing effector cells and macrophages were then mixed in the presence of both rifampin and Ara-C. Six hours later, the cultures were lysed for measurement of luciferase activity.
In the macrophage fusion assays, we used AMD3100 (10 μg/ml) to test CXCR4-mediated fusion. AMD3100 was added to MDM 1 h before the addition of effector cells and maintained throughout the period of cell mixing. To test for CCR5-mediated fusion, MDM were similarly incubated with a mixture of three anti-CCR5 monoclonal antibodies (MAbs). MAb 2D7 (50) (Pharmingen) was used at 20 μg/ml, and MAbs CTC5 and 45529 (28) (kindly provided by B. Lee and R. Doms, University of Pennsylvania) were used at 10 μg/ml.
Coreceptor use in heterologous systems.
To determine coreceptor usage of viruses, both CD4-coreceptor-transfected quail QT6 cells and HOS-CD4-coreceptor cells were used. QT6 cells were transfected with CD4 and coreceptor, infected with DNase-treated viruses, and subjected to PCR 2 days later to detect viral reverse transcription products as previously described (18). HOS-CD4 cells expressing various coreceptors (16), obtained from the NIH AIDS Reagent Repository, were infected with equal amounts of virus stocks based on p24 antigen content, and replication was monitored by p24 levels in the supernatant. Coreceptor utilization by env clones contained in plasmid or recombinant vaccinia virus vectors was determined by measuring cell-cell fusion between Env-expressing 293T cells and QT6 cells transfected with CD4 and coreceptor as previously described (18).
RESULTS
CXCR4- and CCR5-mediated infection of macrophages by prototype strains.
To define the roles played by CXCR4 and CCR5 in HIV-1 entry into macrophages, we first tested several prototype HIV-1 strains that have been well characterized for cofactor usage, syncytium-inducing characteristics, and tropism (Table 1). To test the contribution of CCR5, we used MDM lacking functional CCR5 obtained from individuals homozygous for the Δ32 deletion allele (35). To examine CXCR4, we used the low-molecular-weight inhibitor AMD3100, which specifically blocks CXCR4 (38).
TABLE 1.
Prototype and primary isolate strains used in this study
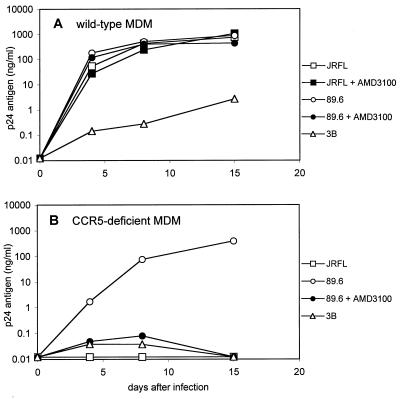
As shown in Fig. 1, the M-tropic prototype JRFL infected wild-type but not CCR5-deficient macrophages. JRFL was not affected by AMD3100, which is expected for a CCR5-dependent strain and also indicates that AMD3100 does not have nonspecific inhibitory or toxic effects in macrophages. In contrast, the dual-tropic strain 89.6 infected both wild-type and CCR5-deficient macrophages (Fig. 1). We previously showed that 89.6 infection of CCR5-deficient macrophages was inhibited by the CXCR4 ligand SDF-1α and CXCR4 MAb 12G5 (51). Here we found that AMD3100 completely blocked infection of macrophages that lack CCR5 but not wild-type macrophages. These results confirm that strain 89.6 can use either CXCR4 or CCR5 to infect macrophages. We consistently observed approximately fourfold-lower peak antigen levels or delayed replication kinetics for 89.6 in CCR5-deficient compared with wild-type macrophages (Fig. 1 and data not shown). In contrast, blocking CXCR4 in wild-type macrophages had no detectable effect on 89.6 replication patterns. This result suggests that CCR5 is probably quantitatively more important than CXCR4 for 89.6 entry into macrophages, although donor-to-donor variability limits the ability to make quantitative comparisons between different macrophage cultures.
FIG. 1.
Blocking CXCR4-mediated infection of wild-type and CCR5-deficient primary macrophages. Monocytes from blood donors homozygous for the CCR5 wild-type (A) or Δ32 deletion (B) alleles were cultured for 1 week to allow differentiation into MDM. Cultures were infected overnight with equal amounts of M-tropic strain JRFL, T-tropic strain 3B, or dual-tropic isolate 89.6 (20 ng of p24 antigen) in the presence or absence of the CXCR4 inhibitor AMD3100 (1 μg/ml). Cultures were sampled periodically for p24 antigen in the supernatant. Data shown are means of duplicate wells and are representative of multiple independent experiments with different blood donors, except that this experiment shows the highest levels of 3B replication observed.
Unlike 89.6, the CXCR4-dependent prototype 3B was restricted in both macrophage types. As shown previously (13, 32), there is considerable variability in the absolute levels to which T-tropic prototypes replicate in macrophages. Peak 3B p24 antigen levels were generally ≤0.1 ng/ml and often below the threshold of detection. However, modest levels were occasionally produced, and Fig. 1A shows the highest 3B p24 antigen levels (∼1 ng/ml) that we found in macrophages. Nevertheless, these levels were several orders of magnitude lower than in parallel wells infected with M-tropic prototypes. Thus, while different macrophage cultures were more or less permissive, within each experiment there was a consistent gradation of restricted to productive infection by T- and M-tropic strains, respectively. These findings emphasize that macrophage tropism is relative rather than absolute. Despite differences in replication among specific experiments and donors, there was no pattern suggesting more efficient 3B replication in macrophages of either CCR5 genotype.
Cell-cell fusion with primary macrophages mediated by Env and CXCR4 or CCR5.
To specifically address coreceptor utilization in Env-mediated fusion with macrophages, we employed a macrophage cell fusion assay modified after several widely used cell line-based assays (18, 19, 33) and a macrophage fusion assay previously described (2, 7). Since macrophage resistance to transfection precludes the direct introduction of a reporter plasmid, and because several primary env genes that we eventually wished to test are cloned into T7 promoter-driven plasmids, we developed an approach that utilizes dual recombinant vaccinia viruses expressing distinct RNA polymerases (24). Effector 293T cells were infected with a recombinant vaccinia virus that expresses T7 RNA polymerase and then cotransfected with T7 promoter-driven env gene clones and an SP6 promoter-driven luciferase reporter plasmid. These cells were then mixed with primary macrophages that had been infected with a recombinant vaccinia virus that expresses the SP6 RNA polymerase. Fusion mediated by effector cell Env and endogenous macrophage chemokine receptors and CD4 results in cytoplasmic mixing and SP6-driven reporter gene expression.
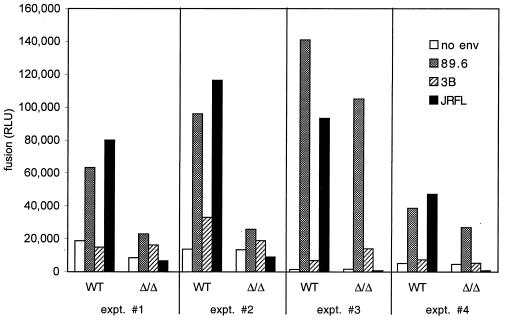
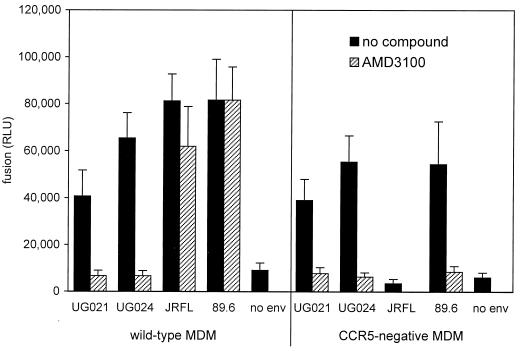
Figure 2 shows the results of four representative fusion experiments in which macrophages from CCR5 wild-type and Δ32 homozygous individuals were studied in parallel. Both JRFL and 89.6 Envs fused with wild-type macrophages, while 3B gave luciferase levels that were marginally if at all higher than control cells lacking Env. In CCR5-deficient macrophages, no fusion was seen with JRFL, while fusion with 89.6 varied. 89.6 produced high levels of luciferase relative to background in some CCR5-negative macrophages and intermediate levels in some cultures, while levels in other cultures were only twice background and only slightly greater than that seen with 3B. 89.6 luciferase levels were always lower in CCR5-deficient macrophages than in wild-type cells examined in parallel, although the difference was not statistically significant owing to the large experiment-to-experiment variability (Table 2). Together, these data indicate exclusive CCR5 dependence for JRFL fusion and a CCR5-independent fusion pathway for 89.6 that is variable but very likely less efficient than that mediated by CCR5. In additional experiments, we found that CCR5-independent 89.6 fusion was reduced to background levels by AMD3100 at 10 μg/ml (data not shown), confirming that it was mediated by CXCR4. Luciferase levels produced by 3B were low and inconsistent (Fig. 2 and Table 2), and as a result we were unable to confirm by blocking whether it reflected real albeit inefficient fusion mediated by CXCR4. Of note, the dual recombinant vaccinia virus system used here results in somewhat higher levels of background (no env) luciferase expression compared with single vaccinia virus systems used in transformed cell line-based assays and so may be less sensitive in detecting low levels of luciferase expression that results from very inefficient fusion. Whether levels of 89.6-mediated fusion in CCR5-negative macrophages correlate with permissiveness to infection, and whether they are related to levels of CXCR4 expression, is under investigation.
FIG. 2.
Cell-cell fusion with primary macrophages mediated by HIV-1 Env. Effector 293T cells were infected with recombinant vaccinia virus vP11T7gene1, which expresses T7 polymerase, and then cotransfected with a plasmid encoding env and a reporter plasmid containing the luciferase gene under control of the SP6 promoter. These cells were then mixed with week-old MDM from CCR5 wild-type donors (WT) or Δ32 homozygous donors (Δ/Δ) that had been infected with recombinant vaccinia virus vSIMBE/L, which expresses the SP6 polymerase. Luciferase levels (RLU) in cell lysates were measured 6 h later as an indication of cell-cell fusion, which results in SP6-driven luciferase expression. Data represent means of duplicate wells, and results of four independent experiments are shown.
TABLE 2.
Fusion mediated by HIV-1 Envs with macrophages from wild-type and CCR5 Δ32 homozygous donors
| Env | Mean RLU ± SEM (median)a
|
|
|---|---|---|
| Wild-type MDM | CCR5-negative MDM | |
| JRFL | 81,245 ± 11,557* (80,021) | 3,564 ± 1,740† (1,444) |
| 3B | 17,827 ± 5,340 (14,703) | 16,447 ± 3,739 (16,018) |
| 89.6 | 81,637 ± 17,410* (69,466) | 54,418 ± 18,100* (26,803) |
| No env | 9,128 ± 3,089 (7,167) | 6,005 ± 2,162 (4,517) |
*, significant differences (P < 0.05) compared with “No env” in the same MDM type; †, significant difference (P < 0.05) compared with fusion for that Env in wild-type MDM. Data represent results of five to eight experiments in each category.
Use of macrophage CCR5 and CXCR4 for fusion and infection by dual-tropic strain DH12.
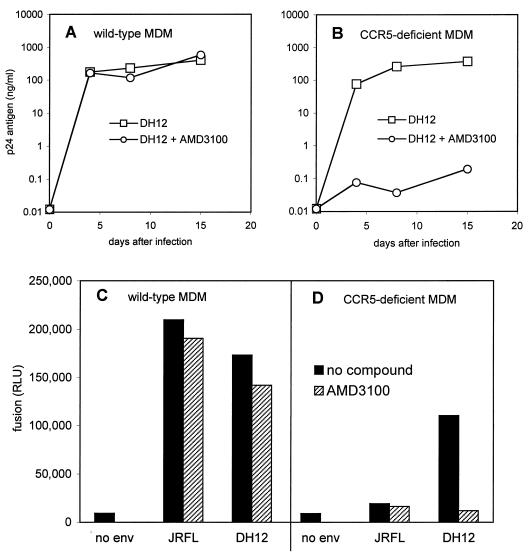
We next tested another dual-tropic R5X4 HIV-1 isolate, DH12, that is widely used in in vitro and in vivo studies (10, 41, 42). Like 89.6, DH12 also replicated in macrophages whether or not CCR5 was present (Fig. 3). Blocking CXCR4 with AMD3100 also had no effect on DH12 infection of wild-type macrophages but inhibited replication in macrophages lacking CCR5 (Fig. 3A and B). The DH12 Env glycoprotein also mediated fusion with both wild-type and CCR5-deficient macrophages. Fusion was completely blocked by inhibiting CXCR4 with AMD3100 in macrophages that lacked CCR5, but there was no consistent effect on fusion in wild-type cells (Fig. 3C and D). Thus, DH12 can enter and replicate in macrophages if either CCR5 or CXCR4 is available. These results also show that DH12 does not utilize a distinct coreceptor other than CCR5 or CXCR4 for entry into macrophages, since neither fusion nor productive infection was seen if both CCR5 and CXCR4 were unavailable.
FIG. 3.
Macrophage infection and cell-cell fusion with the dual-tropic isolate DH12. Wild-type (A) or CCR5-deficient MDM (B) were infected with the clade B isolate DH12, which uses both CCR5 and CXCR4 in heterologous systems. Infections were done in the presence or absence of the CXCR4 inhibitor AMD3100 (1 μg/ml) as described in the legend to Fig. 1, and supernatant was sampled periodically for p24 antigen. Data are means of duplicate wells and are representative of three independent experiments. To address cell-cell fusion, 293T effector cells were infected with recombinant vaccinia viruses expressing the Env glycoprotein of DH12 or the M-tropic prototype JRFL or with control vaccinia virus and then cotransfected with a T7-driven luciferase reporter gene. These cells were mixed with CCR5 wild-type (C) or Δ32 homozygous (D) MDM that had been infected with recombinant vaccinia virus vTF7.3, which expresses the T7 polymerase. Luciferase expression (RLU) was measured in cell lysates 6 h later as an indication of cell-cell fusion and T7-driven reporter gene expression. Data are means of duplicate wells and are representative of three independent experiments.
Exclusively CXCR4-mediated macrophage infection and fusion by X4 primary isolates.
To determine whether the use of macrophage CXCR4 was restricted to the subset of isolates like 89.6 and DH12 that had the ability to use both major coreceptors or whether it might be a feature of CXCR4-restricted isolates as well, we studied several X4 primary isolates that use CXCR4 but not CCR5 for fusion in heterologous systems (Table 1). Strain tybe is a primary isolate obtained in our laboratory from cerebrospinal fluid of an individual with AIDS. We selected this isolate for study because it had a phenotype that was unusual for HIV-1 strains derived from the central nervous system (CNS), since CNS isolates are typically NSI do not replicate in cell lines (9) but strain tybe replicated and produced syncytia in MT-2 cells. Strain tybe used CXCR4 but not CCR5 for entry and infection both in HOS-CD4-coreceptor cells and in CD4-coreceptor-transfected QT6 cells (Table 1). We also studied two international isolates with clade D envelopes (UG021 and UG024) because in preliminary experiments we found that they replicated in both macrophages and MT-2 cells (data not shown) yet had been reported to be X4 isolates (6). Using CD4-coreceptor-transfected QT6 cells, we confirmed that these two strains also used CXCR4 but not CCR5 for entry (Table 1).
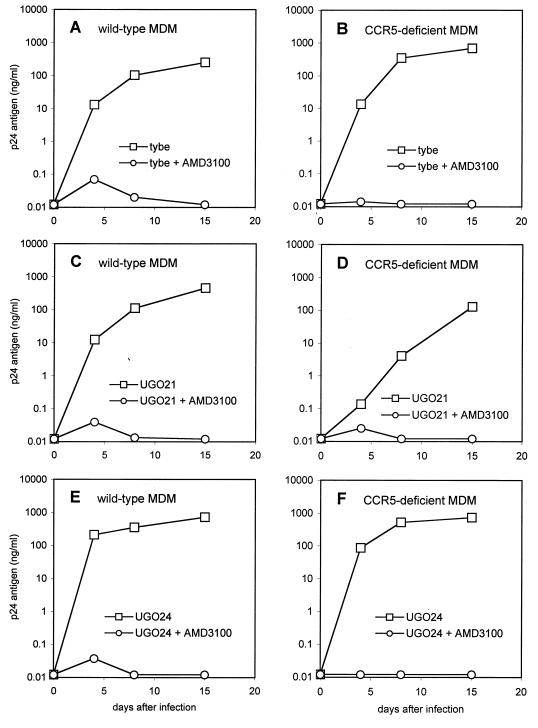
As shown in Fig. 4, all three isolates productively infected both CCR5 wild-type and Δ32 homozygous macrophages. Like 89.6 and DH12, infection of CCR5-deficient macrophages was blocked by AMD3100. In contrast to those R5X4 strains, however, infection of wild-type cells by UG021, UG024, and tybe was also blocked by AMD3100. This indicated that CXCR4 was essential for infection of macrophages regardless of whether CCR5 was present.
FIG. 4.
Infection of macrophages by SI primary isolates that use CXCR4 but not CCR5 in heterologous systems. Macrophages from CCR5 wild-type donors (A, C, and E) or Δ32 homozygous donors (B, D, and E) were infected with the primary isolate tybe (A and B) and two clade D primary isolates, UG021 (C and D) and UG024 (E and F). Infections were done in the presence or absence of the CXCR4 inhibitor AMD3100 (1 μg/ml) as described in the legend to Fig. 1, and supernatant was sampled periodically for p24 antigen. Data are means of duplicate wells and are representative of three independent experiments.
We then used the cell-cell fusion assay to examine coreceptor-specific macrophage fusion mediated by the UG021 and UG024 Env glycoproteins (Fig. 5). Both strains mediated fusion with wild-type and CCR5-deficient macrophages, and AMD3100 completely blocked UG021 and UG024 fusion whether or not CCR5 was present. UG021 and UG024 also mediated similar levels of luciferase expression when macrophages from CCR5 wild-type and Δ32 homozygous donors were examined in parallel (mean ± standard error of the mean [SEM] in wild-type MDM versus CCR5-deficient MDM of 40,621 ± 11,074 versus 39,038 ± 8,861 for UG021, and 65,357 ± 10,752 versus 55,329 ± 11,090 for UG024; P = not significant). These results confirm that CXCR4 is the only entry pathway for UG021 and UG024 in macrophages.
FIG. 5.
Cell-cell fusion with primary macrophages mediated by Env glycoproteins of X4 primary isolates. Effector 293T cells were infected with T7 polymerase-expressing vaccinia virus vP11T7gene1 and then cotransfected with T7-driven env genes and an SP6-driven luciferase reporter gene. Wild-type or CCR5-deficient macrophages were infected with the SP6 polymerase-expressing recombinant vaccinia virus vSIMBE/L. Cells were mixed in the presence or absence of the CXCR4 inhibitor AMD3100 (10 μg/ml), and luciferase activity (RLU) was measured in cell lysates 6 h later as described for Fig. 2. Data represent means ± SEM of five experiments.
CXCR4-mediated fusion in wild-type MDM.
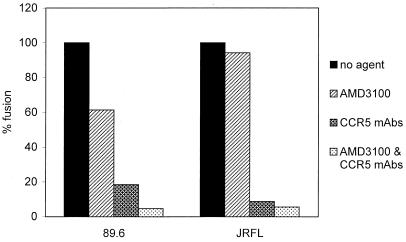
The ability of UG021, UG024, and tybe to use CXCR4 in both wild-type and CCR5 Δ32 homozygous MDM indicates that CXCR4 can function as a coreceptor on macrophages whether or not CCR5 is present. However, to determine whether a strain capable of using both CXCR4 and CCR5 in macrophages actually uses both coreceptors if both are present, we examined wild-type MDM in fusion experiments using agents that block CCR5 and CXCR4 (Fig. 6). To block CXCR4 we used AMD3100, and for CCR5 we used a combination of MAbs previously shown to be effective at inhibiting HIV-1 env fusion mediated by CCR5 (28). In wild-type MDM, AMD3100 had no effect on JRFL-mediated fusion, while 89.6 fusion was reduced to a variable and modest degree (0 to 40% inhibition). The anti-CCR5 MAbs reduced JRFL fusion to near-background levels, and no further reduction was seen when AMD3100 was used in combination. In contrast, blocking CCR5 reduced 89.6 fusion by about 80%, and the addition of AMD3100 reduced the levels of fusion further (Fig. 6). This finding suggests that in macrophages expressing both CCR5 and CXCR4, 89.6 uses both pathways for fusion.
FIG. 6.
Blocking CCR5 and CXCR4-mediated fusion in wild-type macrophages. MDM from donors with the CCR5 wild-type genotype were used as targets for fusion with effector cells expressing the 89.6 or JRFL envs, as described in the legends to Fig. 1 and 5 and in Materials and Methods. One hour before the addition of effector cells and during the period of cell mixing, target MDM were supplemented with AMD3100 (10 μg/ml) and/or with a mixture of three anti-CCR5 MAbs, 2D7 (20 μg/ml), CTC5 (10 μg/ml), and 45529 (10 μg/ml). Data are means of duplicate wells and are representative of two experiments with different donors that gave similar results.
DISCUSSION
In this study, we showed that several primary HIV-1 isolates are able to efficiently utilize CXCR4 on macrophages for fusion as well as productive infection. This included isolates that use CXCR4 but not CCR5 and rely on CXCR4 exclusively for macrophage entry, as well as strains that can use either one for entry into macrophages. All of these strains have a dual-tropic phenotype since they replicate in macrophages and in cell lines and induce syncytia in infected lymphoid cells. This indicates that there are two distinct mechanisms of dual tropism: utilization of CCR5 and CXCR4 to enter macrophages and T-cell lines, respectively (dual-tropic R5X4), and the ability to efficiently utilize CXCR4 on both macrophages and T-cell lines (dual-tropic X4). These results extend previous observations on macrophage CXCR4 by our group and others (44, 49, 51) by demonstrating dual entry pathways into macrophages for primary isolates and strain-specific differences in macrophage CXCR4 utilization at the level cell-cell fusion as well as infection.
HIV-1 isolates have long been classified by tropism based on replication patterns in vitro. The identification of CCR5 and CXCR4 as distinct coreceptors for M- and T-tropic prototypes has led to the classification of strains based on major coreceptor selectivity (5). However, these and other recent results (8, 44, 49) highlight the fact that coreceptor use does not always predict tropism and emphasize that the designation of a strain as R5, X4, or R5X4 based on coreceptor utilization provides valuable information which is complementary to, but does not substitute for, biological characterization based on target cell tropism. Thus, it may be useful to characterize X4 isolates by tropism as well as coreceptor usage, as T-tropic X4 (T-X4) or dual tropic X4 (D-X4). Similarly, some NSI strains do not replicate in primary macrophages, and so not all R5 isolates are necessarily M-tropic, although whether their block is at the level of CCR5 utilization is not known.
Either CCR5 or CXCR4 can mediate entry into macrophages, and some strains use both pathways. For the dual coreceptor strains DH12 and 89.6, we found that blocking CXCR4 in wild-type macrophages that expressed both coreceptors did not have a consistent effect on either fusion or replication. In contrast, when CCR5-negative macrophages were infected in parallel with wild-type cells, we did see a trend toward lower peak p24 antigen levels or slower replication kinetics in cells lacking CCR5. While it is difficult to directly compare HIV-1 replication in cells from different donors, this implies that for strains that use both pathways, CCR5 probably makes a greater contribution than CXCR4, although more quantitative studies will be needed to confirm this. On the other hand, strains UG021, UG024, and tybe used CXCR4 exclusively and resulted in levels of fusion and/or infection that were comparable to those mediated by CCR5 for M-tropic prototypes, which indicates that CXCR4 can be a highly efficient entry pathway in macrophages. We also found considerable donor to donor differences in the absolute levels of fusion mediated by CXCR4, but the pattern of permissive versus nonpermissive CXCR4 utilization was consistent. Studies are under way to determine whether these donor differences are linked to differences in coreceptor or CD4 expression levels.
Some investigators have long held that virtually all primary isolates are M-tropic whether or not they replicate in cell lines and induce syncytia (47). One suggested explanation is that SI primary isolates generally use CXCR4 in addition to, rather than instead of, CCR5 (15, 45). Another explanation, suggested by our data, is that even CXCR4-restricted primary strains may fuse with and infect macrophages through CXCR4. Together with other recent reports (44, 49), these findings suggest that the ability to utilize CXCR4 on macrophages may be a relatively common feature of X4 primary isolates and that the failure of prototype TCLA T-tropic X4 strains to use macrophage CXCR4 may be related, in part, to changes incurred during T-cell line passage of those strains. On the other hand, the ability to utilize macrophage CXCR4 is not a universal feature of X4 primary isolates, as we and others have evaluated several other X4 isolates that do not replicate in macrophages ((14, 35); data not shown). Further studies are required to determine what proportion of primary strains efficiently use macrophage CXCR4 and whether the presence of dual-tropic X4 variants correlates with particular aspects of pathogenesis, such as CNS infection by SI X4 variants as was the case for isolate tybe. Person-to-person transmission of SI X4 variants, an uncommon event, is another area in which this phenotype might potentially play a role, although one X4 SI strain isolated from an infected CCR5 Δ32 homozygous individual did not replicate in macrophages (30). If the ability to infect macrophages via CXCR4 is a common property of primary isolates, then possibly factors other than macrophage infection per se are responsible for the central role of CCR5-dependent M-tropic variants in HIV-1 transmission (29, 36, 48, 52).
In contrast to the three X4 primary isolates evaluated here, the T-tropic X4 prototype fused poorly with primary macrophages, which indicates that inefficient entry is a major element in its restriction in macrophages, consistent with many similar reports (2, 7, 27, 34, 51). However, while macrophage tropism is often categorized as all or none, T-tropic prototype strains may infect macrophages inefficiently (13, 32), and tropism is most accurately considered relative rather than absolute. In fact, overexpressing CD4 on macrophages can enable higher levels of T-tropic prototype fusion, although it also boosts fusion of M-tropic strains and thus does not abrogate the relative differences in fusion linked to tropism (3, 7). In contrast, postentry restrictions have also been described for these strains in macrophages (22, 37, 43). Thus, it is likely that inefficient fusion is one important level at which these strains are restricted but that low-level entry can occur and blocks exist at other levels which may be more apparent depending on the inocula used, culture conditions, and other experimental differences.
The reason that some X4 strains efficiently use CXCR4 on macrophages but prototype TCLA X4 strains do not is unclear. Macrophage CXCR4 differs from that on other cell types in a number of ways. The levels of both CXCR4 and CD4 are lower in macrophages than in lymphocytes and transformed cell lines, and strains may differ in the ability to utilize these molecules expressed at limiting concentrations (27, 51). However, this explanation is not entirely consistent with the observations that TCLA X4 strains can use lower levels of CD4 than X4 primary isolates (26) and that macrophage CD4 overexpression boosts both T-tropic and M-tropic fusion but does not alter relative fusion selectivity (7). Recently, Lapham et al. identified biochemical differences between CXCR4 in macrophages and other cell types, including a higher apparent molecular weight, expression in multimeric form, and differential recognition by antibodies (27). They also found that CD4 coprecipitated with CCR5 in macrophages but not with the predominant CXCR4 multimer, suggesting that the coreceptors in macrophages differ in the ability to associate with CD4. Furthermore, Dimitrov et al. have found that gp120 from TCLA HIV-1 enables CD4-CXCR4 coimmunoprecipitation in lymphocytes but not in macrophages (17). It remains to be determined whether differences between X4 isolates in the ability to utilize macrophage CXCR4 is linked to the ability to use posttranslationally modified or multimeric CXCR4 or to induce CD4-CXCR4 associations needed to generate the trimeric Env-CD4-coreceptor fusion complex.
The role of dual-tropic strains in HIV-1 pathogenesis is yet to be clarified. Initial reports suggested that early strains were uniformly M-tropic/NSI and that late-stage isolates were frequently comprised of a mixture of M-tropic/NSI and T-tropic/SI variants (39). Dual-tropic variants, with features of both categories, might thus represent transitional isolates in the in vivo evolution of viral phenotypes (12). On the other hand, some studies have suggested that late-stage SI variants are more often dual tropic, replicating in both macrophages and cell lines as well as lymphocytes (15, 45). In either case, isolates with the dual-tropic phenotype may be important in pathogenesis, and it will be important to develop a better understanding of the cellular basis for dual tropism.
ACKNOWLEDGMENTS
We thank A. Abdool and L. Wojcik for expert technical assistance, R. Doms for valuable advice, J. Joseph for critical reading of the manuscript, and the blood donors who generously provided cells. We also thank M. Cho for strain DH12, R. Doms and B. Lee for env expression vectors and MAbs, B. Hahn for clade D env isolates, and N. Landau for HOS cells obtained through the NIH AIDS Reagent Program, and C. Wood for M-CSF.
This work was supported by NIH grants AI 35502 and HL 58004 to R.G.C., AI 40957 to S.N.I., and NS 37651 to D.L.K.
REFERENCES
- 1.Alexander W A, Moss B, Fuerst T R. Regulated expression of foreign genes in vaccinia virus under the control of bacteriophage T7 RNA polymerase and the Escherichia coli lac repressor. J Virol. 1992;66:2934–2942. doi: 10.1128/jvi.66.5.2934-2942.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Broder C C, Berger E A. Cell type-specific fusion cofactors determine human immunodeficiency virus type 1 tropism for T-cell lines versus primary macrophages. J Virol. 1996;70:5487–5494. doi: 10.1128/jvi.70.8.5487-5494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazan H A, Alkhatib G, Broder C C, Berger E A. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates. J Virol. 1998;72:4485–4491. doi: 10.1128/jvi.72.5.4485-4491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger E A. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11(Suppl. A):S3–S16. [PubMed] [Google Scholar]
- 5.Berger E A, Doms R W, Fenyö E M, Korber B T M, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 6.Björndal Å, Deng H K, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyö E M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broder C C, Berger E A. Fusogenic selectivity of the envelope glycoprotein is a major determinant of human immunodeficiency virus type 1 tropism for CD4+ T-cell lines vs primary macrophages. Proc Natl Acad Sci USA. 1995;92:9004–9008. doi: 10.1073/pnas.92.19.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng-Mayer C, Liu R, Landau N R, Stamatatos L. Macrophage tropism of human immunodeficiency virus type 1 and utilization of the CC-CKR5 coreceptor. J Virol. 1997;71:1657–1661. doi: 10.1128/jvi.71.2.1657-1661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng-Mayer C, Weiss C, Seto D, Levy J A. Isolates of human immunodeficiency virus type 1 from the brain may constitute a special group of the AIDS virus. Proc Natl Acad Sci USA. 1989;86:8575–8579. doi: 10.1073/pnas.86.21.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho M W, Lee M K, Carney M C, Berson J F, Doms R W, Martin M A. Identification of determinants on a dualtropic human immunodeficiency virus type 1 envelope glycoprotein that confer usage of CXCR4. J Virol. 1998;72:2509–2515. doi: 10.1128/jvi.72.3.2509-2515.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clapham P R. HIV and chemokines: ligands sharing cell-surface receptors. Trends Cell Biol. 1997;7:264–268. doi: 10.1016/S0962-8924(97)01075-1. [DOI] [PubMed] [Google Scholar]
- 12.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collman R, Hassan N F, Walker R, Godfrey B, Cutilli J, Hastings J C, Friedman H, Douglas S D, Nathanson N. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989;170:1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor R I, Paxton W A, Sheridan K E, Koup R A. Macrophages and CD4+ T lymphocytes from two multiply exposed, uninfected individuals resist infection with primary non-syncytium-inducing isolates of human immunodeficiency virus type 1. J Virol. 1996;70:8758–8764. doi: 10.1128/jvi.70.12.8758-8764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng H, Liu R, Ellmeir W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 17.Dimitrov D S, Norwood D, Stantchev T S, Feng Y, Xiao X, Broder C C. A mechanism of resistance to HIV-1 entry: inefficient interactions of CXCR4 with CD4 and gp120 in macrophages. Virology. 1999;259:1–6. doi: 10.1006/viro.1999.9747. [DOI] [PubMed] [Google Scholar]
- 18.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3 and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 19.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–876. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 20.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao F, Morrison S G, Robertson D L, Thornton C L, Craig S, Karlsson G, Morgado M, Galvao-Castro B, von Briesen H, et al. Molecular cloning and analysis of functional envelope genes from human immunodeficiency virus type 1 sequence subtypes A through G. The WHO and NIAID Networks for HIV Isolation and Characterization. J Virol. 1996;70:1651–1667. doi: 10.1128/jvi.70.3.1651-1667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinzinger N, Baca-Regen L, Stevenson M, Gendelman H E. Efficient synthesis of viral nucleic acids following monocyte infection by HIV-1. Virology. 1995;206:731–735. doi: 10.1016/s0042-6822(95)80097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman T L, Doms R W. Chemokines and coreceptors in HIV/SIV-host interactions. AIDS. 1998;12(Suppl. A):S17–S26. [PubMed] [Google Scholar]
- 24.Isaacs, S. N., Y. Yi, A. Singh, and R. G. Collman. A macrophage fusion assay for rapid screening of cloned HIV-1 Env using dual recombinant vaccinia viruses expressing distinct RNA polymerases. J. Virol. Methods, in press. [DOI] [PubMed]
- 25.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S Y. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 26.Kozak S L, Platt E J, Madani N, Ferro F E, Jr, Peden K, Kabat D. CD4, CXCR-4, and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J Virol. 1997;71:873–882. doi: 10.1128/jvi.71.2.873-882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lapham C K, Zaitseva M B, Lee S, Romanstseva T, Golding H. Fusion of monocytes and macrophages with HIV-1 correlates with biochemical properties of CXCR4 and CCR5. Nat Med. 1999;5:303–308. doi: 10.1038/6523. [DOI] [PubMed] [Google Scholar]
- 28.Lee B, Sharron M, Blanpain C, Doranz B J, Vakili J, Setoh P, Berg E, Liu G, Guy H R, Durell S R, Parmentier M, Chang C N, Price K, Tsang M, Doms R W. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274:9617–9626. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- 29.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 30.Michael N L, Nelson J A E, KewalRamani V N, Chang G, O’Brien S J, Mascola J R, Volsky B, Louder M, White II G C, Litman D R, Swanstrom R, O’Brien T R. Exclusive and persistent use of the entry coreceptor CXCR4 by human immunodeficiency virus type 1 from a subject homozygous for CCR5 Δ32. J Virol. 1998;72:6040–6047. doi: 10.1128/jvi.72.7.6040-6047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson J K A, Cross G D, Callaway C S, McDougal J S. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) J Immunol. 1986;137:323–329. [PubMed] [Google Scholar]
- 33.Nussbaum O, Broder C C, Berger E A. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J Virol. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Brien W A, Koyanagi Y, Namazie A, Zhao J-Q, Diagne A, Idler K, Zack J A, Chen I S Y. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 35.Rana S, Besson G, Cook D G, Rucker J, Smyth R J, Yi Y, Turner J D, Guo H-H, Du J-G, Peiper S C, Lavi E, Samson M, Libert F, Liesnard C, Vassart G, Doms R W, Parmentier M, Collman R G. Role of CCR5 in infection of primary macrophages and lymphocytes by macrophage-tropic strains of human immunodeficiency virus: resistance to patient-derived and prototype isolates resulting from the Δccr5 mutation. J Virol. 1997;71:3219–3227. doi: 10.1128/jvi.71.4.3219-3227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C M, Saragosti S, Lapoumeroulie C, Cogniaux J, Forceille C, Muyldermans G, Verhofstede C, Bortonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection of Caucasian individuals bearing mutant alleles of the CCR5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 37.Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol. 1998;72:4633–4642. doi: 10.1128/jvi.72.6.4633-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schols D, Struyf S, Van Damme J, Esté J A, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E Y, van Steenwijk R P, Lange J M A, Eeftink Schattenkerk J K M, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus populations. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw G M, Hahn B H, Arya S K, Groopman J E, Gallo R C, Wong-Staal F. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science. 1984;226:1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- 41.Shibata R, Hoggan M D, Broscius C, Englund G, Theodore T S, Buckler-White A, Arthur L O, Israel Z, Schultz A, Lane H C, Martin M A. Isolation and characterization of a syncytium-inducing, macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J Virol. 1995;69:4453–4462. doi: 10.1128/jvi.69.7.4453-4462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibata R, Maldarelli F, Siemon C, Matano T, Parta M, Miller G, Fredrickson T, Martin M A. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J Infect Dis. 1997;176:362–373. doi: 10.1086/514053. [DOI] [PubMed] [Google Scholar]
- 43.Simmons G, McKnight A, Takeuchi Y, Hoshino H, Clapham P R. Cell-to-cell fusion, but not virus entry in macrophages by T-cell line tropic HIV-1 strains: a V3 loop-determined restriction. Virology. 1995;209:696–700. doi: 10.1006/viro.1995.1307. [DOI] [PubMed] [Google Scholar]
- 44.Simmons G, Reeves J D, McKnight A, Dejucq N, Hibbitts S, Power C A, Aarons E, Schols D, De Clercq E, Proudfoot A E, Clapham P R. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J Virol. 1998;72:8453–8457. doi: 10.1128/jvi.72.10.8453-8457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Usdin T B, Brownstein M J, Moss B, Isaacs S N. SP6 RNA polymerase containing vaccinia virus for rapid expression of cloned genes in tissue culture. BioTechniques. 1993;14:222–224. [PubMed] [Google Scholar]
- 47.Valentin A, Albert J, Fenyo E M, Asjo B. Dual tropism for macrophages and lymphocytes is a common feature of primary human immunodeficiency virus type 1 and 2 isolates. J Virol. 1994;68:6684–6689. doi: 10.1128/jvi.68.10.6684-6689.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van’t Wout A B, Kootstra N A, Mulder-Kampinga G A, Albrecht-van Lent N, Scherpbier H J, Veenstra J, Boer K, Coutinho R A, Miedema F, Schuitemaker H. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J Clin Investig. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verani A, Pesenti E, Polo S, Tresoldi E, Scarlatti G, Lusso P, Siccardi A G, Vercelli D. CXCR4 is a functional coreceptor for infection of human macrophages by CXCR4-dependent primary HIV-1 isolates. J Immunol. 1998;161:2084–2088. [PubMed] [Google Scholar]
- 50.Wu L J, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yi Y, Rana S, Turner J D, Gaddis N, Collman R G. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:772–779. doi: 10.1128/jvi.72.1.772-777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]