Abstract
Purpose
ASTRIS study aimed the largest to evaluate the effectiveness and safety of second- or higher-line osimertinib in patients with advanced/metastatic epidermal growth factor receptor (EGFR) T790M mutation-positive non–small cell lung cancer (NSCLC) in the real-world setting. Here we report the results of Chinese patients in ASTRIS study.
Methods
Adults with EGFR T790M-positive advanced NSCLC pretreated with EGFR-tyrosine kinase inhibitor (EGFR-TKI), having a WHO performance status score of 0–2 and asymptomatic, stable central nervous system (CNS) metastases were included. All patients received once-daily osimertinib 80 mg orally. The outcomes included investigator-assessed clinical response, progression-free survival (PFS), time-to-treatment discontinuation (TTD), and safety.
Results
A total of 1350 patients were included. Response rate was 55.7% (95% confidence interval [CI] 0.53–0.58). The median PFS and the median TTD were 11.7 months (95% CI 11.1–12.5) and 13.9 months (95% CI 13.1–15.2), respectively. Overall, 389 patients (28.8%) had at least one protocol-specified adverse event (AE); AEs of interstitial lung diseases/pneumonitis-like events and QT prolongation were reported in 3 (0.2%) and 59 (4.4%) patients, respectively.
Conclusion
Osimertinib was effective in Chinese patients with T790M-positive NSCLC who had progressed after first- or second-generation EGFR-TKI in real-word setting and the results were consistent with ASTRIS study overall population and AURA studies. No new safety signals or events were identified.
Clinical trial number
Keywords: Epidermal growth factor receptor, T790M, Non-small cell lung cancer, Osimertinib, Real-world
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, with a mortality rate of 18% (Sung et al. 2021). In China, lung cancer is the most prevalent cancer, with an estimated 0.82 million new cases in 2020 and 40% of global deaths (Cao et al. 2021). China is expected to witness an increase in magnitude in the lung cancer mortality with 5.07 million deaths in 2040 (Cao et al. 2021). Non-small cell lung cancer (NSCLC) is the most common histologic subtype and accounts for 85% of lung cancer cases in China with one-third of patients having locally or regionally advanced disease at the time of diagnosis (Gan et al. 2021; Govindan et al. 2008). Despite rapid advancements in the diagnosis and treatment of lung cancer, the prognosis for patients with advanced NSCLC remains poor globally, and the 5-year survival rate is less than 10% (Ricciuti et al. 2018).
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) are the recommended first-line treatment strategy for patients with advanced NSCLC harboring the EGFR sensitizing mutations. However, the majority of the patients develop resistance following treatment with first- or second-generation EGFR-TKIs after a median progression-free survival (PFS) of 9–14 months (Morgillo et al. 2016). The most common mechanism for resistance is the development of “gatekeeper” T790M mutation in exon 20 (converting threonine 790 of the EGFR kinase domain to methionine), which is identified in around 50–70% of resistant cases (Kohsaka et al. 2019; Peng et al. 2021).
Osimertinib is an oral, irreversible EGFR-TKI that selectively inhibits both EGFR sensitizing and T790M resistance mutations and has proven to be efficacious in patients with central nervous system (CNS) metastases (Marinis et al. 2019). In a randomized, Phase III AURA3 clinical trial comparing osimertinib with platinum-based doublet chemotherapy in T790M-positive advanced NSCLC patients pre-treated with EGFR-TKI therapy, osimertinib significantly prolonged the median PFS (10.1 vs 4.4 months, 95% confidence interval [CI] 0.23–0.41; P < 0.001) in overall patients and also in patients with CNS metastases (8.5 vs 4.2 months; 95% CI 0.21–0.49) (Mok et al. 2017). The objective response rate (ORR) was also significantly better with osimertinib compared with the chemotherapy (71% vs 31%; P < 0.001). Hence, the AURA3 study established the superiority of osimertinib as the standard of care for patients with confirmed T790M-positive advanced NSCLC after the first-line EGFR-TKI (Mok et al. 2017). Other clinical trials of osimertinib as a second line of treatment for T790M-positive advanced NSCLC, such as AURA2 (Goss et al. 2016), AURA extension (Yang et al. 2017) and a pooled analysis of AURA2/AURA extension trials (Ahn et al. 2019) have also reported a prolonged median PFS of 9.9–12.3 months in overall population. Although the above trials have shown promising efficacy of osimertinib in patients with EGFR T790M-positive advanced NSCLC, further evaluation is warranted through a real-world study that will help clinicians gain more effectiveness and safety profile in a large, varied patient population. ASTRIS was aimed at supplementing the existing clinical evidence for the use of osimertinib monotherapy in the EGFR T790M-positive advanced NSCLC patients in the real-world setting. Here, we report the results of osimertinib monotherapy in Chinese adults with EGFR T790M-positive advanced or metastatic NSCLC from ASTRIS study.
Materials and methods
Study design
ASTRIS is an open-label, single-arm, multinational, real-world study. The study was approved by the Institutional Review Board/Ethics Committee and conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the Declaration of Helsinki, local regulatory requirements, and the policy on bioethics and human biologic samples of AstraZeneca. The study protocol was designed by the sponsor (AstraZeneca, Södertälje, Sweden) in collaboration with investigators and is registered at clinicaltrials.gov (NCT02474355). All patients provided their written informed consent.
Participants
Chinese adults (≥ 18 years) with locally advanced (stage IIIB) or metastatic (stage IV) NSCLC (based on TNM staging, AJCC 7th edition), WHO performance status (PS) score of 0–2, confirmed T790M mutation and who had received prior EGFR-TKI therapy in any treatment line were included in the study. Patients with asymptomatic or stable CNS metastases were allowed.
Patients with a history of interstitial lung disease (ILD) or symptoms of uncontrolled/severe systemic disease were excluded. The cardiac exclusion criteria were either a mean resting corrected QT interval > 470 ms as per Fredericia formula (Cadogan 2020), factors increasing the risk of arrhythmic events/QTc prolongation, or clinically significant resting electrocardiogram morphological abnormalities.
Interventions
Patients were given 80 mg osimertinib orally once daily and were continued on treatment if they still receiving clinical benefit, as judged by the investigator.
Study outcomes and endpoints
The effectiveness and safety data were collected at the treatment visit (every 6 weeks) and termination visit (30 days post last dose). The outcomes included investigator-assessed response rate, PFS, time-to-treatment discontinuation (TTD) and safety profile. This study only collected the incidence of protocol-defined adverse events (AEs), including serious adverse events (SAEs), AEs of special interest (ILD or pneumonitis-like events and QTs prolongation events), and AEs leading to dose modification or discontinuation or death.
Statistical analysis
All time-to-event outcomes and safety analyses were performed on the full analysis set (FAS) that included all patients receiving at least one dose of osimertinib. The response rate analyses were assessed on the response evaluable set, defined as those patients in the FAS who had at least one documented response assessment by the investigator. Patients who transitioned to a commercial product following national reimbursement were censored at the time of this transition. Time-to-event outcomes (PFS and TTD), including their 95% CIs were estimated using the Kaplan–Meier plot. All AEs and SAEs leading to study drug discontinuation or dose modification were summarized by Medical Dictionary for Regulatory Activities (MedDRA) preferred term (Version 23.1) and Common Terminology Criteria for Adverse Events (CTCAE) class. All statistical analyses were performed using SAS® (SAS Institute, North Carolina) version 9.2 (or later).
Results
Patient characteristics
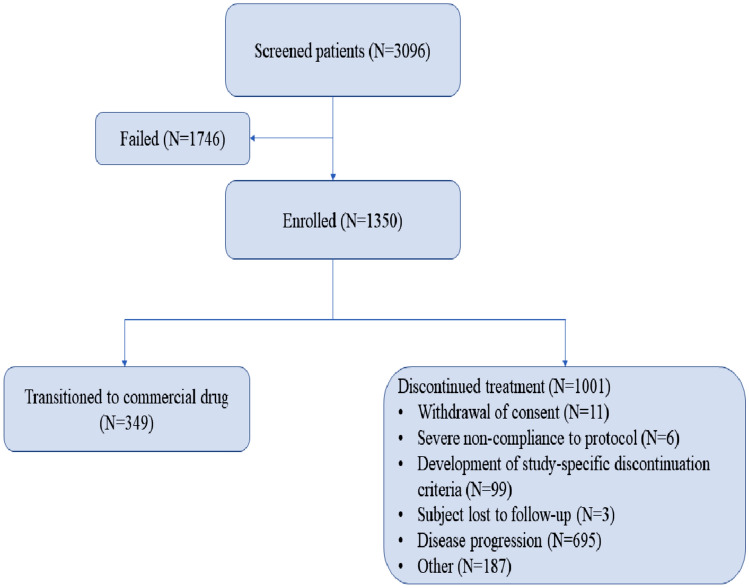
A total of 1350 Chinese patients were included from September 27, 2016 to September 22, 2017, and received at least one dose of osimertinib. Patient disposition is given in Fig. 1, and the patient demographics and baseline characteristics are summarized in Table 1. Of 1350, 806 (59.7%) patients were female. The median age was 60 years (range 29–87), with 404 (29.9%) patients aged ≥ 65 years. The majority of the patients had stage IV lung cancer (1113; 82.8%) at diagnosis and WHO PS of 1 (973; 72.1%). 774 of 1350 (57.3%) patients had a brain scan available at baseline; 341 of 1350 (25.3%) patients with CNS lesions. All the patients had previously received prior EGFR-TKI treatment; the most commonly used EGFR-TKI was gefitinib in 721 patients (53.4%) followed by icotinib in 371 patients (27.5%) and erlotinib in 286 patients (21.2%). A considerable number of patients had also received prior chemotherapy (651; 48.2%) and radiotherapy (352; 26.1%) (Table 1).
Fig. 1.
Patient disposition
Table 1.
Baseline demographics and disease characteristics of full analysis set
| Characteristics | Osimertinib (N = 1350) |
|---|---|
| Median age, years (range) | 60.0 (29–87) |
| Age < 65 years, n (%) | 946 (70.1) |
| Age ≥ 65 years, n (%) | 404 (29.9) |
| Gender, n (%) | |
| Female | 806 (59.7) |
| Male | 544 (40.3) |
| Ethnicity, n (%) | |
| Asian | 1349 (99.9) |
| Non-Asian | 1 (0.1) |
| WHO performance status, n (%) | |
| 0 | 268 (19.9) |
| 1 | 973 (72.1) |
| 2 | 109 (8.1) |
| Disease stage at diagnosis, n (%) | 1345 |
| Stage 0 | 3 (0.2%) |
| Stage IA–IB | 61 (4.6%) |
| Stage IIA–IIB | 34 (2.5%) |
| Stage IIIA | 68 (5.1%) |
| Stage IIIB–IV | 1179 (87.7%) |
| Median duration between diagnosis and enrolment, months, median (range) | 23 (1–225) |
| BM/LM metastases at baseline, n (%) | |
| Yes | 341 (25.3) |
| BM only | 317 (23.5) |
| LM only | 8 (0.6) |
| Both BM and LM | 16 (1.2) |
| No | 433 (32.0) |
| Not assessed | 576 (42.7) |
| Previous EGFR-TKI#, n (%) | |
| Gefitinib | 721 (53.4) |
| Icotinib | 371 (27.5) |
| Erlotinib | 286 (21.2) |
| Others | 35 (2.6) |
| Prior anticancer therapy*, n (%) | |
| Chemotherapy | 651 (48.2) |
| First line | 474 (72.8%) |
| Second line | 266 (40.9%) |
| ≥ Third line | 169 (25.9%) |
| Adjuvant | 54 (8.3%) |
| Neo adjuvant | 9 (1.4%) |
| Palliative | 5 (0.8%) |
| Maintenance | 16 (2.5%) |
| Radiotherapy | 352 (26.1) |
| Other | 128 (9.5) |
| Median duration between last anticancer therapy and enrollment, months, median (range) | 0.4 (0–39) |
Data are presented as n (%), unless otherwise stated;
BM brain metastases, LM leptomeningeal metastases, EGFR-TKI epidermal growth factor receptor-tyrosine kinase inhibitor, SD standard deviation
#Patients may have received more than 1 prior EGFR-TKI
*Patients may have no, one, or more than one previous anti-cancer treatment/radiotherapy/surgery
EGFR T790M testing
EGFR T790M molecular testing was performed either on tissue (783; 58%) or blood (567; 42%) samples using different platforms, of which Roche Cobas EGFR assay (Roche Molecular Diagnostics, Inc., CA, USA) was the most commonly used one (1269; 94%). Most of the patients had common mutations (1244, 92.1%), which included T790M either with exon 19 deletion (793, 63.4%) or L858R (451, 36.1%) (Table 2).
Table 2.
Specimens and platforms used for baseline EGFR T790M molecular testing (full analysis set)
| Specimen source/testing platform | Full analysis set (N = 1350) |
|---|---|
| Specimen source and testing platform, n (%) | |
| Tissue | 783 (58.0) |
| Roche Cobas EGFR assay | 783 (100.0) |
| Blood | 567 (42.0) |
| Roche Cobas EGFR assay | 486 (85.7) |
| ddPCR | 56 (9.9) |
| AMOY | 16 (2.8) |
| EGFR mutation positive status, n (%) | |
| T790M | 1350 (100) |
| T790M + Exon 19 deletion | 793 (63.4) |
| T790M + L858R | 451 (36.1) |
| T790M + G719X | 6 (0.5) |
| Mutation combinations, n (%) | |
| T790M only | 89 (6.6) |
| T790M + common mutations | 1244 (92.1) |
| T790M + uncommon/compound mutations | 17 (1.3) |
| T790M + uncommon mutations | 6 (0.4) |
| T790M + common compound mutations | 5 (0.4) |
| T790M + uncommon compound mutations | 6 (0.4) |
Common mutations are T790M + Exon 19 Deletion only; T790M + L858R only. Uncommon mutations are T790M + G719X only; T790M + S768I only; T790M + Exon 20 Insertion only. Common compound mutations are T790M + 2 common mutations. Uncommon compound mutations are T790M + 2 or more mutations including at least 1 uncommon mutation
ddPCR droplet digital polymerase chain reaction, EGFR epidermal growth factor receptor
Effectiveness
Progression-Free survival
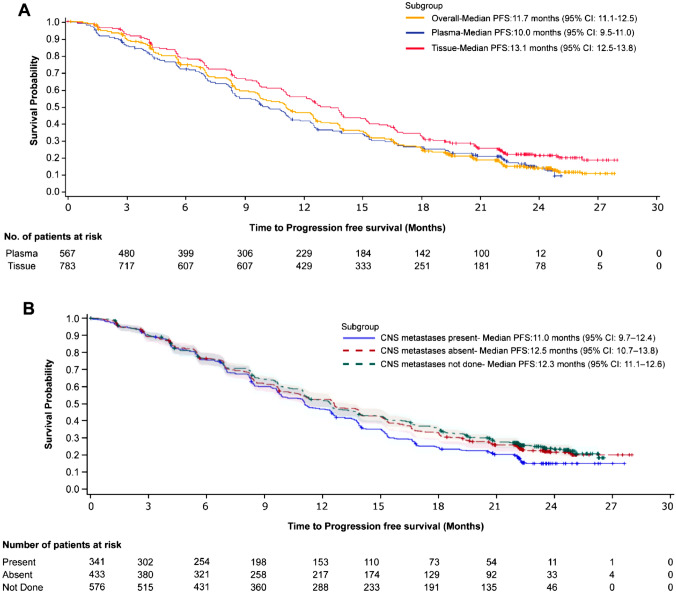
Disease progression or death was reported in 1059 patients (78.4%). The median PFS was 11.7 months (95% CI 11.1–12.5) for the overall population. The median PFS by tissue testing and plasma testing was 13.1 months (95% CI 12.5–13.8) and 10.0 months (95% CI 9.5–11.0), respectively (Fig. 2a). In patients with and without CNS metastases at baseline, the median PFS was 11.0 months (95% CI 9.7–12.4) and 12.5 months (95% CI 10.7–13.8), respectively (Fig. 2b).
Fig. 2.
Kaplan-Meir plot for PFS in a full analysis set, plasma (Roche Cobas and ddPCR) and tissue (Roche Cobas) and b CNS metastases stratified patients. CNS, central nervous system; ddPCR, droplet digital polymerase chain reaction; PFS, progression-free survival
Clinical response rate
Response evaluation was available from 1322 patients; of them, 736 patients (55.7%) had an investigator-assessed best overall response as “responding” (95% CI 52.9–58.4). The response rate in patients with T790M-positive status by tissue testing and plasma testing was 60.4% (95% CI 56.9–63.9) and 48.9% (95% CI 44.6–53.2), respectively. The response rate in patients with and without CNS metastases was 58.2% (95% CI 52.7–63.6) and 57.9% (95% CI 53.0–62.6).
Time to treatment discontinuation
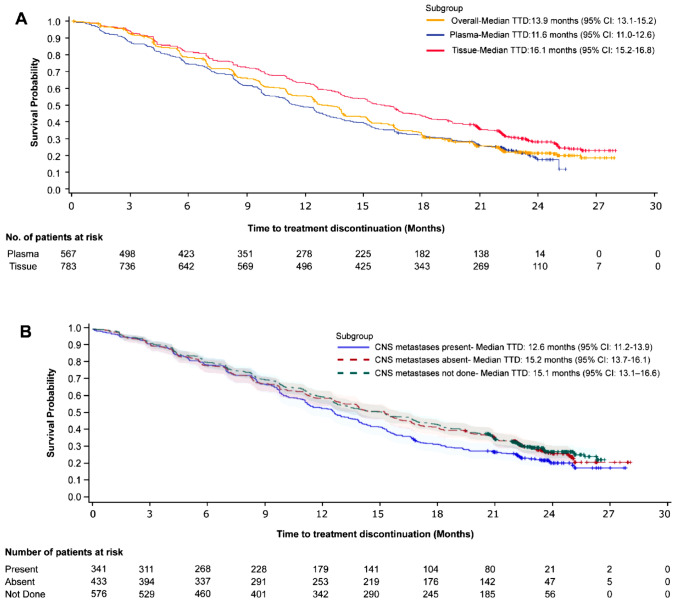
Of 1350 enrolled patients, 349 patients (25.9%) transitioned to the commercial drug at the time of national reimbursement; the remaining 1001 patients (74.1%) discontinued the treatment, with a median TTD of 13.9 months (95% CI 13.1–15.2). The median TTD in patients with T790M-positive status by tissue testing and plasma testing was 16.1 months (95% CI 15.2–16.8) and 11.6 months (95% CI 11.0–12.6), respectively (Fig. 3a). The prime reason for discontinuation were disease progression (695/1001; 69.4%). The median TTD in patients stratified by the presence or absence of CNS metastases was 12.6 months (95% CI 11.2–13.9) and 15.2 months (95% CI 13.7–16.1), respectively (Fig. 3b).
Fig. 3.
Kaplan-Meir plot for TTD in a full analysis set, plasma (Roche Cobas and ddPCR) and tissue (Roche Cobas) and b CNS metastases stratified patients. CNS, central nervous system; ddPCR, droplet digital polymerase chain reaction; TTD, time to treatment discontinuation
Safety
A total of 389 of 1350 (28.8%) patients had at least one protocol-defined AE during the study (Table 3), and 308 of 1350 (22.8%) patients reported an AE with CTCAE grade ≥ 3. Dose modification and discontinuation due to AEs were observed in 124 (9.2%) and 95 (7.0%) patients, respectively. A total of 297 (22.0%) patients had SAEs and were possibly related to osimertinib in 52 (3.9%) patients. Deaths were reported in 92 (6.8%) patients and were possibly related to osimertinib in 14 (1.0%) patients. AEs of special interest were reported in a total of 62 of 1350 (4.6%) patients. Three (0.2%) patients had lung disease/pneumonitis-like events and 59 (4.4%) patients had QTc prolongation events.
Table 3.
Overall summary of AEs
| Adverse events, n (%) | Osimertinib (N = 1350) |
|---|---|
| Any protocol-specified AE# | 389 (28.8) |
| AE leading to dose modification | 124 (9.2) |
| AE leading to discontinuation | 95 (7.0) |
| AE of special interest* | 62 (4.6) |
| Lung disease/pneumonitis like event | 3 (0.2) |
| QTc prolongation event | 59 (4.4) |
| Serious AE | 297 (22.0) |
| Treatment related serious AE | 52 (3.9) |
| AE leading to death | 92 (6.8) |
| Treatment related AE leading to death | 14 (1.0) |
| Protocol-specified AEs by preferred term, reported in ≥ 1% of patients | |
| Electrocardiogram QT prolonged | 65 (4.8) |
| Pneumonia | 39 (2.9) |
| Death | 27 (2.0) |
| Cerebral infarction | 18 (1.3) |
| Pulmonary embolism | 16 (1.2) |
Data are presented as n (%)
AE adverse event
#Safety assessment included serious AEs, AEs leading to dose modification, AEs leading to treatment discontinuation, and AEs of special interest (interstitial lung disease/pneumonitis-like events and QTc prolongation events)
*Interstitial lung disease/pneumonitis-like events and QTc prolongation events
Discussion
This study aimed to assess the effectiveness and safety of second-line or higher osimertinib in a real-world setting in patients with EGFR T790M-positive advanced NSCLC who had received prior EGFR-TKI therapy. Our analysis of Chinese patients is consistent with the ASTRIS global population. OS data in Chinese patients were of low maturity and not reported here. In China, until January 5, 2022, 80/1350 (6%) patients still benefit from the commercial drug and have a duration of osimertinib treatment of more than 4 years from the last patient enrolled.
The median PFS observed in our study was 11.7 months (95% CI 11.1–12.5), which was comparable with that reported in ASTRIS global population (Marinis et al. 2019) (11.1 months; 95% CI 11.0–12.0) and in the previous osimertinib studies, notably AURA3 (Mok et al. 2017) (10.1 months; 95% CI 8.3–12.3), AURA extension (Yang et al. 2017) (12.3 months; 95% CI 9.5–13.8), AURA2(Goss et al. 2016) (9.9 months; 95% CI 8.5–12.3), retrospective study by Peng et al. (Peng et al. 2021) (12.0 months; 95% CI 10.5–13.5), ASTRIS Korean subgroup (Cho et al. 2020) (12.4 months; 95% CI 11.1–13.6), AURA3 Japanese subgroup (Akamatsu et al. 2018) (12.5 months; 95% CI 6.9 to not calculated), and in a Phase III open-label study (Nie et al. 2018) (10.2 months; 95% CI 8.27–9.75) but a recently published Korean real-world study reported a longer PFS (Lee et al. 2023) (14.2 months; 95% CI 13.0–16.4) Several methods are available to detect the presence of EGFR T790M from tumor tissue or circulating tumor DNA from plasma (Spence et al. 2021). Tumor tissue is generally preferred and is the current gold standard for detecting resistance mutations (John et al. 2017). For initial EGFR T790M testing, however, liquid biopsy is recommended by the National Comprehensive Cancer Network guidelines as an alternative to tissue biopsy (John et al. 2017). The median PFS observed in patients with T790M-positive status by plasma testing in the ASTRIS global study (9.7 months; 95% CI 8.6–10.3), AURA3 study (8.2 months; 95% CI: 6.8–9.7), Korean real-world study (11.0 months; 95% CI 9.0–12.6) was also comparable to our study (10.0 months; 95% CI 9.5–11.0) (Marinis et al. 2019; Mok et al. 2017; Lee et al. 2023). A shorter PFS was observed in plasma EGFR T790M-positive patients in the ASTRIS Korean subgroup study (Cho et al. 2020) (6.9 months; 95% CI 2.5–10.9). However, in terms of testing of T790M on tissue, the study reported a median PFS of 13.1 months (95% CI 12.5–13.8) which was in good agreement with the ASTRIS global study (median PFS: 12.7 months; 95% CI 12.4–13.8) (Marinis et al. 2019) and ASTRIS Korean subgroup study (median PFS: 13.6 months; 95% CI 12.0–14.5) (Cho et al. 2020).
The effectiveness was also analyzed by the presence of CNS metastases. The benefit of osimertinib treatment was also found to be extended to the subgroup of Chinese EGFR T790M mutation positive NSCLC patients with brain/leptomeningeal metastases reporting a median PFS of 11.0 months (95% CI 9.7–12.4). The findings were consistent with previous reports – AURA extension (Yang et al. 2017) (7.1 months, 95% CI 4.2–12.3), pooled analysis of AURA2/AURA extension (Ahn et al. 2019) (8.2 months, 95% CI 6.9–9.7 months) and AURA3 study (Mok et al. 2017) (8.5 months, 95% CI 6.8–12.3), ASTRIS Korean subgroup study (Cho et al. 2020) (median PFS: 10.8 months; 95% CI 9.5–11.5) and Korean real-world study (Lee et al. 2023) (12.1 months, 95% CI 10.3–14.7). On similar lines, a good response rate of 58.2% (95% CI 52.7–63.6) was achieved in the CNS metastases subgroup in our study which was in line with the AURA extension (Yang et al. 2017) (64%, 95% CI 43–82), pooled analysis of AURA2/AURA extension (Ahn et al. 2019) (59%, 95% CI 51–67) and ASTRIS Korean subgroup study (Cho et al. 2020) (68%, 95% CI 61–74.5). The median TTD in patients with and without CNS metastases was found to be 12.6 months (95% CI: 11.2–13.9) and 15.2 months (95% CI 13.7–16.1), respectively in the present study which was consistent with ASTRIS Korean subgroup study (Cho et al. 2020) (with CNS metastases: 11.2 months, 95% CI 9.4–14.8; without CNS metastases: 14.7 months, 95% CI 12.2–not reached) and Korean real-world study (Lee et al. 2023) (with CNS metastases: 12.5 months, 95% CI 11.0–14.0; without CNS metastases: 15.9 months, 95% CI 14.8–17.0).
Osimertinib was well-tolerated and no new safety signals were observed in our study. The safety profile was similar to that observed in the ASTIRS global population (Marinis et al. 2019), which is an interim analysis of the global study, and AURA clinical studies. The overall incidence rate of adverse events observed in Chinese patients was 28.8% which was comparable to ASTRIS global (30%), and ASTRIS Korean subgroup study (Cho et al. 2020) (31.1%). In these protocol-defined AEs, the most frequent events (> 1% patients) reported in the Chinese population were prolonged electrocardiogram QT (4.8%), pneumonia (2.9%) and death (2.0%). Besides, the incidence of SAEs was also comparable between our study (22%) and ASTRIS global (21%) (Marinis et al. 2019), AURA2 (25%) (Goss et al. 2016) and AURA extension (27%) (Yang et al. 2017) and ASTRIS Korean subgroup study (24.9%) (Cho et al. 2020). Lung disease/pneumonitis-like event and QTc prolongation were the AEs of special interest observed at a rate of 0.2% and 4.4%, respectively in our study compared to 1% and 3%, respectively seen in ASTIRS global (Marinis et al. 2019), 2% and 6% respectively in AURA2 (Goss et al. 2016), 4% each in AURA3 (Mok et al. 2017) study and 1.7% and 1.5%, respectively in ASTRIS Korean subgroup study (Cho et al. 2020).
Although our study gives a better understanding of the effectiveness and safety aspects of osimertinib in a real-world scenario in a Chinese setting complementing the results of a global setting, certain limitations must be taken care of before drawing any conclusion. Considering the fact that due to many patients transitioning to commercial supply following national reimbursement as per ASTRIS protocol, the follow-up was incomplete and the exposure may have been underestimated. Also, the response rates were investigator-assessed as per institutional standards. Not all patients were assessed at baseline for the CNS metastases and the types of samples used and the availability of the type of testing methods for T790M at different study sites could also have introduced bias in the study results.
Conclusion
The real-world effectiveness and safety results in Chinese patients supporting osimertinib as a standard second-line treatment in patients with T790M-positive advanced NSCLC.
Acknowledgements
The authors thank all the patients and their families, investigators, trial staff, and participants of the study. The authors also thank Dr. Tanushree Goswami and Dr. Ramandeep Singh from Indegene Pvt. Ltd. for providing medical writing and editorial services in accordance with Good Publication Practice (GPP3) guidelines.
Author contributions
QZ conceptualization, methodology, and writing-review and editing. H-LZ writing-review and editing. L-YJ writing-review and editing. Y-KS writing-review and editing. YC Writing-review and editing. J-MY writing-review and editing. C-CZ writing-review and editing. YH writing-review and editing. Y-PH writing-review and editing. Z-AL writing-review and editing. Y-YP writing-review and editing. W-LZ writing-review and editing. Yong Song: writing-review and editing. GW writing-review and editing. G-YC writing-review and editing. YL writing-review and editing. C-YZ writing-review and editing. Y-PZ writing-review and editing. YC writing-review and editing. SL writing-review and editing. C-LW writing-review and editing. J-YZ writing-review and editing. Y-PL writing-review and editing. J-XH writing-review and editing. JW writing-review and editing. Y-LW writing-review and editing.
Funding
This study was supported by AstraZeneca (Södertälje, Sweden).
Data availability
The datasets that were used and analysed during the current study are available from the corresponding authors on reasonable request.
Declarations
Conflict of interest
QZ reports honoraria from AstraZeneca, Boehringer Ingelheim, BMS, Eli Lilly, MSD, Pfizer, Roche, and Sanofi. C–C Z reports personal fees from Lily China, Sanofi, BI, Roche China, MSD, Qilu, Hengrui, Innovent Biologics, C-stone, LUYE Pharma, TopAlliance Biosciences Inc, Amoy Diagnostics. SL received research support and speaker fees from AstraZeneca, Hutchison, BMS, Heng Rui, Beigene, Roche, and Hansoh and acted as an advisor and consultant with AstraZeneca, Pfizer, Boehringer Ingelheim, Hutchison MediPharma, Simcere, ZaiLab, GenomiCare, Yuhan Corporation, PrIME Oncology, Menarini, and Roche. Y-LW reports advisory services for AstraZeneca, Boehringer Ingelheim, Novartis, Takeda; personal fees from AstraZeneca, Beigene, Boehringer Ingelheim, BMS, Eli Lilly, MSD, Pfizer, Roche, Sanofi; and grants from AstraZeneca, Boehringer Ingelheim, BMS, Hengrui, and Roche. The remaining authors have no conflicts of interest to declare.
Ethical approval
The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the Declaration of Helsinki, local regulatory requirements, and the policy on bioethics and human biologic samples of AstraZeneca.
Consent to participate
Informed consent was obtained from all the participants included in the study.
Consent to publish
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahn M-J, Tsai C-M, Shepherd FA, et al. Osimertinib in patients with T790M mutation-positive, advanced non-small cell lung cancer: long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer. 2019;125:892–901. doi: 10.1002/cncr.31891. [DOI] [PubMed] [Google Scholar]
- Akamatsu H, Katakami N, Okamoto I, et al. Osimertinib in Japanese patients with EGFR T790M mutation-positive advanced non-small-cell lung cancer: AURA3 trial. Cancer Sci. 2018;109:1930–1938. doi: 10.1111/cas.13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadogan M (2020) Fridericia formula. In: Life in the Fast Lane • LITFL. https://litfl.com/fridericia-formula/. Accessed 20 Apr 2022
- Cao W, Chen H-D, Yu Y-W, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134:783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho BC, Kim D-W, Park K, et al. Real-world use of osimertinib in non–small cell lung cancer: ASTRIS study Korean subgroup analysis. Curr Med Res Opin. 2020;36:477–482. doi: 10.1080/03007995.2019.1676708. [DOI] [PubMed] [Google Scholar]
- de Marinis F, Wu Y-L, de Castro G, et al. ASTRIS: a global real-world study of osimertinib in >3000 patients with EGFR T790M positive non-small-cell lung cancer. Future Oncol. 2019;15:3003–3014. doi: 10.2217/fon-2019-0324. [DOI] [PubMed] [Google Scholar]
- Gan J, Fang W, Zhang L. Therapy of lung cancer in China: introducing the special collection. Ther Adv Med Oncol. 2021;13:17588359211038200. doi: 10.1177/17588359211038199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss G, Tsai C-M, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17:1643–1652. doi: 10.1016/S1470-2045(16)30508-3. [DOI] [PubMed] [Google Scholar]
- Govindan R, Bogart J, Vokes EE. Locally advanced non-small cell lung cancer: the past, present, and future. J Thorac Oncol. 2008;3:917–928. doi: 10.1097/JTO.0b013e318180270b. [DOI] [PubMed] [Google Scholar]
- John T, Bowden JJ, Clarke S, et al. Australian recommendations for EGFR T790M testing in advanced non-small cell lung cancer. Asia Pac J Clin Oncol. 2017;13:296–303. doi: 10.1111/ajco.12699. [DOI] [PubMed] [Google Scholar]
- Kohsaka S, Petronczki M, Solca F, Maemondo M. Tumor clonality and resistance mechanisms in EGFR mutation-positive non-small-cell lung cancer: implications for therapeutic sequencing. Future Oncol. 2019;15:637–652. doi: 10.2217/fon-2018-0736. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim EY, Park C-K, et al. Real-world study of osimertinib in korean patients with epidermal growth factor receptor T790M mutation-positive non-small cell lung cancer. Cancer Res Treat. 2023;55:112–122. doi: 10.4143/crt.2022.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgillo F, Della Corte CM, Fasano M, Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open. 2016;1:e000060. doi: 10.1136/esmoopen-2016-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie K, Zhang Z, Zhang C, et al. Osimertinib compared docetaxel-bevacizumab as third-line treatment in EGFR T790M mutated non-small-cell lung cancer. Lung Cancer. 2018;121:5–11. doi: 10.1016/j.lungcan.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Peng D, Shan D, Dai C, et al. Real-world data on osimertinib in Chinese patients with pretreated, EGFR T790M mutation positive, advanced non-small cell lung cancer: a retrospective study. Cancer Manag Res. 2021;13:2033–2039. doi: 10.2147/CMAR.S287466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciuti B, Baglivo S, De Giglio A, Chiari R. Afatinib in the first-line treatment of patients with non-small cell lung cancer: clinical evidence and experience. Ther Adv Respir Dis. 2018;12:1753466618808659. doi: 10.1177/1753466618808659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence T, Perera S, Weiss J, et al. Clinical implementation of circulating tumour DNA testing for EGFR T790M for detection of treatment resistance in non-small cell lung cancer. J Clin Pathol. 2021;74:91–97. doi: 10.1136/jclinpath-2020-206668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Yang JC-H, Ahn M-J, Kim D-W, et al. Osimertinib in pretreated T790M-positive advanced non–small-cell lung cancer: AURA study phase II extension component. JCO. 2017;35:1288–1296. doi: 10.1200/JCO.2016.70.3223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets that were used and analysed during the current study are available from the corresponding authors on reasonable request.