Abstract
A new study provides a comprehensive molecular mechanism that controls interspecific incompatibility of self-incompatible (SI) plants in the Brassicaceae. This finding points to a potentially promising path to break interspecific barriers and achieve introgression of desirable traits into crops from distant species among SI crops in the Brassicaceae.
Keywords: Pollen-stigma recognition, Self-incompatibility, Interspecific barriers, ROS
One of the biggest challenges in plant breeding is the difficulty to develop new crop cultivars, through hybridization between two different species, due to the presence of reproductive barriers. Therefore, understanding the molecular mechanisms controlling these reproductive barriers is of profound importance. In recent years, many studies have focused on the prezygotic barriers, which are mediated by pollen-pistil interactions, to identify targets and methods for overcoming these barriers.
Pollen grains encounter three reproductive checkpoints as they interact with the pistil: the stigma, the transmitting tract, and the ovule. The recognition of pollen by the stigma constitutes the first crucial recognition step in this process. The molecular mechanism underlying the self-incompatibility (SI) response, at the stigma, has been well elucidated in various plant species. Within the Brassicaceae, the hydration and germination of self-pollen are prevented by a ligand–receptor interaction between the pollen-expressed S-locus cysteine-rich protein/S-locus protein 11 (SCR/SP11) and its receptor, the stigma-expressed S-locus receptor kinase (SRK) (Kachroo et al. 2001; Nasrallah 2019; Shimosato et al. 2007; Takayama et al. 2001). Several components, including the M-locus protein kinase (MLPK) and ARM-repeat containing 1 (ARC1) E3 ubiquitin ligase, have been identified as direct downstream effectors of SRK (Gu et al. 1998; Murase et al. 2004). Although the MLPK has been identified as a positive regulator of the SI response, in Brassica rapa (B. rapa), yet its specific functions and requirement throughout the Brassicaceae remain unclear. In contrast, ARC1 is phosphorylated by SRK and targets proteins required for compatible (CP) responses for degradation, thereby blocking the hydration and metabolic activation of self-pollen.
Reactive oxygen species (ROS) levels in stigmas also experience alterations after pollination in SI species (Zhang et al. 2021). Basal ROS levels in the stigma decrease following cross-pollination in SI species, but increase significantly after self-pollination, playing a critical role in mediating the inhibition and rejection of incompatible pollen. It has been reported that the alteration of ROS levels, during the SI response, is regulated by the stigma receptors SRK and a Catharanthus roseus receptor-like kinase-like 1 (CrRLKL1) family receptor, FERONIA (FER). In fact, suppressing the expression of SRK or the FER homolog in B. rapa effectively reduces stigmatic ROS and interferes with SI. In contrast, little is known about pollen recognition at the stigmas of SC plants. Recently, the ANJEA-FERONIA receptor kinase complex, together with the RAPID ALKALINIZATION FACTOR (RALF) peptides, were reported to act as an important stigmatic gatekeeper. The pollen coat protein B-class peptides (PCP-Bs) from the pollen competitively bind the ANJEA (ANJ)-FER receptor kinase complex and promote pollen hydration and germination, during which process ROS content also plays important roles. Pollination, or PCP-Bs treatment, reduces the content of stigmatic ROS and enables pollen hydration (Liu et al. 2021). Nevertheless, it remains unclear whether this molecular mechanism plays a role in the establishment of interspecific barriers.
It has long been an interesting question to study the role of SI systems in interspecific reproductive barriers. The SI × SC rule, documented by Lewis and Crowe, suggests that the self-incompatibility machinery can be utilized in interspecific recognition (Lewis and Crowe 1958). However, there are exceptions to this rule, particularly in some plants from the Brassicaceae and Poaceae families (Li et al. 2018; Sampson 1962). Thus, whether and to which extent SI and hybridization barriers overlap remains a long unanswered question. The evidence to support a mechanistic overlap between SI and hybridization barriers remains indirect. On one hand, it was shown that a self-incompatible population of A. lyrata not only rejected self-pollen but also rejected interspecific pollen from three self-compatible species, all of which were accepted by self-compatible A. lyrata (Li et al. 2018). On the other hand, however, it was reported in other studies that SI factors are not involved in the barriers to hybridization on the stigma. Transformation of self-compatible A. thaliana with the male (SCR) and female (SRK) S-determinants of self-incompatible A. lyrata produces a full allele-specific SI response, but does not lead to pollen rejection of A. thaliana (Nasrallah 2002), suggesting that additional mechanisms are required for the observed hybridization barrier between these two species. Moreover, in A. thaliana, a stigmatic plasma membrane-localized protein, STIGMATIC PRIVACY 1 (SPRI1), was identified as an interspecies incompatibility factor that functions in an SI-independent manner (Fujii et al. 2019). Therefore, the current understanding of self-incompatibility suggests that a SI mechanism possibly plays a role in interspecific reproductive barriers, and that unknown downstream signaling or regulatory components are shared between the self-incompatibility and interspecies pollen rejection pathways. It is also clear that other SI-independent mechanisms are also essential for the establishment of the stigmatic reproductive barrier.
The recent paper “Stigma Receptors Control Intraspecies and Interspecies Barriers in Brassicaceae”, published in Nature (Huang et al. 2023), sheds light on the molecular mechanisms behind interspecific incompatibility in the Brassicaceae. The study established that the regulators involved in an SI response also participate in the recognition of interspecies pollen in the Brassicaceae. The authors provided evidence that, in SI species (e.g., B. rapa), the pollen determinant SCR/SP11, or signals from interspecies unilaterally incompatible (UI) pollen, bind to the stigmatic SRK receptor, and then recruits the receptor FER, which activates ROS production in the stigma, thereby aiding in the rejection of self-pollen or interspecies pollen (Fig. 1).
Fig. 1.
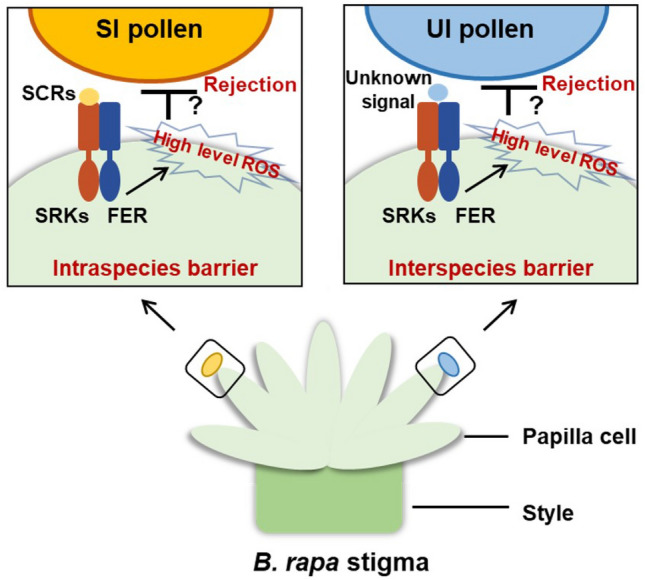
Schematic diagram of intraspecific and interspecific barrier establishment in SI species Brassica rapa. In stigmas from SI species, the intraspecies self-incompatible pollen (SI pollen) is rejected by SCRs-SRKs/FER activated ROS, and the rejected interspecies unilaterally incompatible pollen (UI pollen) also activates ROS via the interaction between unknown signals from UI pollen and SRKs-FER
The results of this study provide important insights into the molecular mechanisms underlying the regulation of intraspecific and interspecific barriers of SI plants in the Brassicaceae. Specifically, the authors have shown that the stigmatic receptors, SRK and FER, are integral to both types of barriers in the self-incompatible species B. rapa. The role of redox conditions, in the stigma, in determining the fate of pollen is also highlighted in this study. Here, they established that ROS levels increase significantly in response to both self-pollen and interspecific pollen, but decrease in response to compatible pollen in both self-incompatible and self-compatible species. In addition, regulation of ROS levels is dependent on the function of the receptor FER. These findings point to new opportunities for cross breeding in Brassicaceae crops by manipulating the components involved in pollen-stigma recognition, such as reducing ROS levels, increasing NO levels, or disrupting the BrSRK–BrFER interaction and BrFER-to-BrRBOH signaling. Breaking the stigmatic barrier to achieve interspecific and intergeneric fertilization, in the SI plant B. rapa, represents a major breakthrough in crop improvement.
However, there remain several key unresolved issues that require further clarification. This study emphasizes, once again, the crucial role played by ROS in the recognition of pollen by the pistil. The study reveals that in SI plant species from the Brassicaceae, interspecies pollen is rejected through the activation of the SI-dependent increase of ROS production. Here, it should be noted that distinct SI mechanisms are employed in different plant species, such as those observed in the Papaveraceae and Solanaceae families (Takayama and Isogai 2005; Wilkins et al. 2014), and it remains to be determined whether ROS levels play a critical role in establishing the interspecific barriers in all these different plant families. In addition, ROS has been reported to play an important role in the SI response (Zhang et al. 2021) in SI plants as well as in pollen hydration in SC plants (Liu et al. 2021).
Based on the conclusions from these studies, two important questions that need to be answered. The first is what is the exact role of ROS in inhibiting incompatible pollen? The second is what cellular events are affected by ROS that are necessary for pollen hydration and germination? We assume that another potential downstream component in pollen-stigma recognition is Ca2+. Notably, the cytoplasmic Ca2+ levels in papilla cells undergo a remarkable increase after self-pollination, in SI responses, whereas only occasional and minimal increases in cytoplasmic Ca2+ levels were observed after cross-pollination (Iwano et al. 2015). Stigmatic Ca2+ is also implicated downstream of the SP11/SCR-SRK interaction, as the interaction between SP11/SCR and SRK triggers an increase in cytoplasmic Ca2+ in papilla cell protoplasts (Iwano et al. 2015). Whether Ca2+ is part of the interspecific barriers, and whether the role of Ca2+ is like that for ROS, remains an open question.
Another key challenge is the identification of the new signals involved in the establishment of a stigmatic reproductive barrier. Although it was shown that proteins, extracted from UI pollen, enhance the BrSRK46 interaction with BrFER1, and increase the production of ROS, it is still unclear whether the signal is an entirely different signal or a signal similar to SCR/SP11. The authors determined that, in the SC species A. thaliana, the higher binding affinity of stigmatic receptor AtFER to the intraspecific AtPCP-Bγ, rather than the interspecific BrPCP-Bγ, led to a more rapid hydration of pollen and longer pollen tubes, upon intraspecific pollination, revealing the vital role of FER in the establishment of the interspecific barrier.
Given that the events occurring at the pollen–stigma interface include pollen adhesion, hydration, pollen tube germination, penetration of the stigmatic cuticle, and growth into the transmitting tissue, and that PCP-Bs are only the signals involved in pollen hydration (Liu et al. 2021; Wang et al. 2017), it is therefore reasonable to predict the existence of additional signals involved in the other events that are potentially important to serve as interspecific barriers (Zhong et al. 2019). The discovery of novel signals would facilitate a better understanding of the pollen–stigma recognition, which provides new reproductive barriers for us to break for agriculture utility. While the reproductive barrier was partially alleviated in SI plants, via the reduction in ROS levels, or disruption of the BrSRK-BrFER interaction, the mutation of either SRK or FER receptors could not fully break the interspecific barrier. Therefore, there must be other mechanisms involved in interspecific pollen recognition that are yet to be discovered, particularly those SI-independent mechanisms in the SC plants.
It is important to note that, prezygotic barriers include not only pollen recognition, by the pistil, but also pollen tube guidance, as well as reception, by the synergid cells (Hater et al. 2020). Breaking each of these barriers is essential for achieving interspecific hybridization. Key factors that play a role in pollen tube guidance and reception have been identified (Escobar-Restrepo et al. 2007; Liu et al. 2013; Okuda et al. 2009; Takeuchi and Higashiyama 2016; Zhong et al. 2019; Zhong et al. 2022; Zhong and Qu 2019), and manipulation of these factors will significantly improve the efficiency of breaking corresponding reproductive barriers. In addition, future studies should focus on the signals involved in the male–female interaction, i.e., to identify more recognition-involved signals. Despite these unresolved questions, the findings reported by Huang et al. (2023) have significant implications for the field and will help deepen our understanding of pollen–stigma recognition and offer new targets for the dismantling of reproductive barriers.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no conflicts of interest. Author Li-Jia Qu Xie was not involved in the journal’s review of the manuscript.
Contributor Information
Sheng Zhong, Email: shengshengzz@pku.edu.cn.
Li-Jia Qu, Email: qulj@pku.edu.cn.
References
- Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- Fujii S, et al. A stigmatic gene confers interspecies incompatibility in the Brassicaceae. Nat Plants. 2019;5:731–741. doi: 10.1038/s41477-019-0444-6. [DOI] [PubMed] [Google Scholar]
- Gu TS, Mazzurco M, Sulaman W, Matias DD, Goring DR. Binding of an arm repeat protein to the kinase domain of the S-locus receptor kinase. Proc Natl Acad Sci USA. 1998;95:382–387. doi: 10.1073/pnas.95.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hater F, Nakel T, Groß-Hardt R. Reproductive multitasking: the female gametophyte. Annu Rev Plant Biol. 2020;71:517–546. doi: 10.1146/annurev-arplant-081519-035943. [DOI] [PubMed] [Google Scholar]
- Huang J, et al. Stigma receptors control intraspecies and interspecies barriers in Brassicaceae. Nature. 2023;614:303–308. doi: 10.1038/s41586-022-05640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M, et al. Calcium signalling mediates self-incompatibility response in the Brassicaceae. Nat Plants. 2015;1:15128. doi: 10.1038/nplants.2015.128. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Schopfer CR, Nasrallah ME, Nasrallah JB. Allele-specific receptor-ligand interactions in Brassica self-incompatibility. Science. 2001;293:1824–1826. doi: 10.1126/science.1062509. [DOI] [PubMed] [Google Scholar]
- Lewis D, Crowe LK. Unilateral interspecific incompatibility in flowering plants. Heredity. 1958;12:233–256. doi: 10.1038/hdy.1958.26. [DOI] [Google Scholar]
- Li L, et al. Evolution of interspecies unilateral incompatibility in the relatives of Arabidopsis thaliana. Mol Ecol. 2018;27:2742–2753. doi: 10.1111/mec.14707. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. Membrane-bound RLCKs LIP1 and LIP2 are essential male factors controlling male-female attraction in Arabidopsis. Curr Biol. 2013;23:993–998. doi: 10.1016/j.cub.2013.04.043. [DOI] [PubMed] [Google Scholar]
- Liu C, et al. Pollen PCP-B peptides unlock a stigma peptide-receptor kinase gating mechanism for pollination. Science. 2021;372:171–175. doi: 10.1126/science.abc6107. [DOI] [PubMed] [Google Scholar]
- Murase K, Shiba H, Iwano M, Che FS, Watanabe M, Isogai A, Takayama S. A membrane-anchored protein kinase involved in Brassica self-incompatibility signaling. Science. 2004;303:1516–1519. doi: 10.1126/science.1093586. [DOI] [PubMed] [Google Scholar]
- Nasrallah JB. Recognition and rejection of self in plant reproduction. Science. 2002;296:305–308. doi: 10.1126/science.296.5566.305. [DOI] [PubMed] [Google Scholar]
- Nasrallah JB. Self-incompatibility in the Brassicaceae: regulation and mechanism of self-recognition. Plant Dev Evo. 2019;131:435–452. doi: 10.1016/bs.ctdb.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Okuda S, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–361. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- Sampson DR. Intergeneric pollen-stigma incompatibility in the Cruciferae. Can J Genet Cytol. 1962;4:38–49. doi: 10.1139/g62-006. [DOI] [Google Scholar]
- Shimosato H, et al. Characterization of the SP11/SCR high-affinity binding site involved in self/nonself recognition in Brassica self-incompatibility. Plant Cell. 2007;19:107–117. doi: 10.1105/tpc.105.038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, et al. Direct ligand-receptor complex interaction controls Brassica self-incompatibility. Nature. 2001;413:534–538. doi: 10.1038/35097104. [DOI] [PubMed] [Google Scholar]
- Takayama S, Isogai A. Self-incompatibility in plants. Annu Rev Plant Biol. 2005;56:467–489. doi: 10.1146/annurev.arplant.56.032604.144249. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Higashiyama T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature. 2016;531:245–248. doi: 10.1038/nature17413. [DOI] [PubMed] [Google Scholar]
- Wang L, Clarke LA, Eason RJ, Parker CC, Qi B, Scott RJ, Doughty J. PCP-B class pollen coat proteins are key regulators of the hydration checkpoint in Arabidopsis thaliana pollen-stigma interactions. New Phytol. 2017;213:764–777. doi: 10.1111/nph.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins KA, Poulter NS, Franklin-Tong VE. Taking one for the team: self-recognition and cell suicide in pollen. J Exp Bot. 2014;65:1331–1342. doi: 10.1093/jxb/ert468. [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. FERONIA receptor kinase-regulated reactive oxygen species mediate self-incompatibility in Brassica rapa. Curr Biol. 2021;31:3004–3072. doi: 10.1016/j.cub.2021.04.060. [DOI] [PubMed] [Google Scholar]
- Zhong S, et al. Cysteine-rich peptides promote interspecific genetic isolation in Arabidopsis. Science. 2019;364:eaau9564. doi: 10.1126/science.aau9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, et al. RALF peptide signaling controls the polytubey block in Arabidopsis. Science. 2022;375:290–296. doi: 10.1126/science.abl4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Qu L-J. Cysteine-rich peptides: signals for pollen tube guidance, species isolation and beyond. Sci China Life Sci. 2019;62:1243–1245. doi: 10.1007/s11427-019-9819-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


