Abstract
Heart failure is a significant public health concern characterized by notable rates of morbidity and mortality. Despite the presence of guideline-directed medical therapy (GDMT), its utilization remains inadequate. This practical recommendation paper focuses on the utilization of angiotensin receptor–neprilysin inhibitor (ARNI) as a pivotal treatment for heart failure with reduced ejection fraction (HFrEF), heart failure with preserved ejection fraction (HFpEF), and heart failure with improved ejection fraction (HFimpEF). The recommendations presented in this paper have been developed by a group of cardiologists in India who convened six advisory board meetings to discuss the utilization of ARNI in the management of heart failure. The paper emphasizes the importance of accurate biomarkers for diagnosing heart failure, particularly N-terminal pro-B-type natriuretic peptide (NT-proBNP) and B-type natriuretic peptide (BNP), which are commonly used. Additionally, the paper advocates the use of imaging, specifically echocardiography, in diagnosing and monitoring heart failure patients. Moreover, the paper highlights the role of ARNI in heart failure management, with numerous clinical trials that have demonstrated its effectiveness in reducing cardiovascular death or heart failure hospitalization, enhancing quality of life, and diminishing the risk of ventricular arrhythmias. This practical recommendation paper offers valuable insights into the utilization of ARNI in the management of heart failure, aiming to enhance the implementation of GDMT and ultimately alleviate the burden of heart failure on society.
Keywords: Heart failure, Angiotensin receptor-neprilysin inhibitor, Guideline-directed medical therapy, Heart failure with preserved ejection fraction, Heart failure with reduced ejection fraction
Key Summary Points
| The document is a practical recommendation paper based on a series of advisory board meetings held by a group of cardiologists in India to discuss the use of ARNI in the management of heart failure. |
| The document emphasizes the importance of implementing guideline-directed medical therapy (GDMT) for heart failure, as there is insufficient implementation of GDMT according to the National Heart Failure Register. |
| The recommendations cover several aspects of heart failure management, including biomarkers, imaging, initiation and titration of guideline-directed therapy, the role of ARNI in HFrEF, HFpEF, and HFmrEF, dose titration, and in-hospital initiation of ARNI. |
| The use of ARNI in heart failure is supported by clinical trials and guidelines, with ARNI being one of the four medications recommended for treating heart failure with reduced ejection fraction (HFrEF). |
| ARNIs can improve diastolic and left ventricular function, quality of life, and reduce the risk of ventricular arrhythmias. |
Practical recommendations: summary
| 1 | Recommendations on the use of biomarkers for the diagnosis of HF |
| BNP and NT-proBNP are used for heart failure diagnosis and prognosis, but renal dysfunction may affect results and there is no evidence to target treatment to specific levels. NTproBNP is the more accurate biomarker in patients on sacubitril/valsartan. | |
| 2 | Recommendation on imaging for diagnosis and follow-up of HF patients |
| Patients with new HF should have an echocardiogram (with strain imaging if available) to aid in the correct diagnosis and after 3–6 months of GDMT, repeat imaging can help in treatment decisions, with alternative modalities like cardiac MRI considered if echocardiography fails to assess LVEF. | |
| 3 | Recommendation on initiation, addition, or switching to new evidence-based guideline-directed therapy for HFrEF and its titration |
| Established treatments for HFrEF include ARNIs, SGLT2i, ACEIs, ARBs, beta-blockers, aldosterone antagonists, loop diuretics, hydralazine/isosorbide dinitrate, and ivabradine, except for loop diuretics, which have been proven to improve symptoms, reduce hospital stays, and increase survival rates in clinical trials. The choice of initial treatment is based on various factors, with all treatments eventually increased to the maximum tolerated or targeted dose. | |
| 4 | Recommendation on the role of ARNI in HFrEF |
| The guidelines recommend a combination of four medications for treating heart failure with reduced ejection fraction (HFrEF): ARNIs, mineralocorticoid receptor antagonists, beta-blockers, and sodium glucose cotransporter-2 inhibitors. The ACCF/AHA/HFSA guidelines give ARNI a Class 1A recommendation and the ESC guidelines give it a 1B recommendation. The PARADIGM HF trial demonstrated that sacubitril/valsartan had a lower rate of cardiovascular death hospitalization compared to enalapril, with better improvement in quality of life. | |
| 5 | Recommendation on the role of ARNI in HFpEF |
| The ACC guidelines have given a Class IIb recommendation for using ARNI in HFpEF. In the PARAGON-HF trial done in patients with EF of 45% or higher, the main outcome showed a non-significant reduction in cardiovascular deaths or heart failure hospitalizations in the sacubitril-valsartan group. Secondary outcomes confirmed a significant improvement in NYHA class and renal function, with more benefits seen in female patients and patients with EF between 45 to 57%, highlighting need for personalized therapy with ARNI in HFpEF. | |
| 6 | Recommendation on continuing GDMT in HFimpEF |
| Continue current GDMT in HFrEF patients with recovered LVEF unless there is a clear reversible cause, as discontinuing it leads to high rates of HF events (per TRED-HF study). | |
| 7 | Recommendations on the role of ARNI in cardiac reverse remodeling |
| Studies have shown that ARNIs can improve LV and diastolic function, quality of life, and reduce risk of ventricular arrhythmias, with positive results seen in trials such as PROVE-HF and EVALUATE-HF. | |
| 8 | Recommendations on dose of ARNI |
| The suggested initial dose of sacubitril/valsartan is 50 mg, to be taken orally twice daily. After a period of 2–4 weeks, the dosage can be increased to 100 mg and further escalated to 200 mg, based on the individual's tolerance. However, certain circumstances may require a reduction in the starting dose. These include patients who are not currently receiving an ACEi or ARB, individuals with severe renal impairment, individuals with blood pressure below 90/60, or patients with moderate hepatic impairment. | |
| 9 | Recommendation on ARNI dose titration |
| The initiation and dose increase of sacubitril/valsartan over 3 (for 100 mg bd starting dose) or 6 weeks (for 50 mg BD starting dose) has a tolerable profile and a more gradual increase maximized the target dose attainment in low-dose ACEI/ARB patients (TITRATION trial). | |
| 10 | Recommendation on in-hospital initiation of ARNI |
| The TRANSITION trial found initiation of sacubitril/valsartan to be feasible in patients with HFrEF who had stabilized after an acute heart failure event. The PIONEER-HF trial showed that sacubitril–valsartan was more effective than enalapril in reducing NT-proBNP levels in patients with HFrEF hospitalized for acute decompensation. | |
| 11 | Recommendation on initiation of an ARNI de novo |
| Starting directly on ARNI is safe and effective with improved cardiac function and tolerability and is recommended with monitoring and assessment considering the risk of angioedema or hypotension. | |
| 12 | Recommendation of using ARNI with SGLT2 inhibitor |
| ARNI may be combined with SGLT2i for the treatment of heart failure. Whenever diuretics are used, the dosage needs to be adjusted. | |
| 13 | Recommendation on ARNI dose modification for hepatic dysfunction patients |
| In HF patients with moderate hepatic impairment (Child–Pugh B), the loading dose of ARNI should be halved and the subsequent doses should be gradually increased to reach the maximum tolerated dose. No dose adjustment is needed in mild hepatic impairment (Child–Pugh A). ARNI should not be prescribed in patients with severe hepatic impairment. | |
| 14 | Recommendation on ARNI in patients with CKD |
| ARNI can be prescribed to non-dialysis patients with CKD and heart failure. Use of ARNI in such patients reduces cardiovascular risk and improves eGFR compared to ACEI/ARBs. | |
| 15 | Recommendation on the dose titration of ARNI in renal impairment |
| ARNI dose adjustment is not required in HF patients with mild to moderate renal impairment. Depending on the patient's blood pressure, a loading dose of 25–50 mg BID is advised in severe renal impairment. The dose of ARNI should be gradually increased every 2–4 weeks to reach the maintenance dose of 200 mg BID (maximum tolerated dose). | |
| 16 | Recommendations on management of hypovolemia and hypotension in patients initiated on ARNI |
| If low blood pressure or symptoms of hypotension are present, adjust diuretic and anti-hypertensive medication, address other causes, and rehydrate. Correct hypovolemia before starting ARNI or use low-dose ARNI to reduce the risk of hypotension. If the patient is still hypotensive, reduce the dose or temporarily discontinue ARNI; permanent discontinuation is usually not required. | |
| 17 | Recommendation on management of hyperkalemia on initiating ARNI |
| ARNI therapy may cause elevated potassium levels. Studies show slightly lower hyperkalemia incidence with ARNI compared to enalapril. If hyperkalemia occurs, address risk factors, discontinue potassium supplements and MRA, and use potassium-lowering drugs. ARNI may require temporary adjustment or discontinuation with close potassium monitoring. ARNI can be gradually resumed when potassium levels normalize. | |
| 18 | Recommendation on management of patients with rapid decline in renal function on starting ARNI |
| Patients with rapid decline in renal function from ARNI therapy have no specific treatment data, but RAAS inhibitor treatment methods can be used. Determine the cause of the decline, and if creatinine increases less than 30% from baseline, ARNI can continue. Adjust or discontinue ARNI and investigate underlying causes if creatinine exceeds baseline by 30%. Discontinue ARNI if creatinine exceeds baseline by 50%. Address other potential causes before considering ARNI adjustment or discontinuation. | |
| 19 | Recommendations of ARNI in patients on maintenance dialysis |
| ARNI is advised for heart failure patients receiving maintenance dialysis in order to enhance myocardial remodeling, safeguard remaining renal function, manage heart failure symptoms, and lower the risk of CV events. | |
| 20 | Recommendations on improving medication adherence |
| Dedicated heart failure clinics with regular follow-up with a heart failure nurse can help improve medication adherence. The use of a pre-discharge medication checklist is another helpful tool for improving GDMT implementation. |
Introduction
Heart failure (HF) is a clinical syndrome that occurs due to structural and functional abnormalities in the myocardium, leading to the impaired filling or ejection of blood from the ventricles. Impaired relaxation or contractility of the heart leads to elevated cardiac filling pressure, elevated jugular venous pressure (JVP), physical symptoms, and lung crackles [1]. Heart failure can be classified into different categories based on the left ventricular ejection fraction (LVEF). HFrEF refers to heart failure with reduced ejection fraction, where the LVEF is equal to or below 40%. HFpEF, on the other hand, represents heart failure with preserved ejection fraction, indicating an LVEF of 50% or higher. There is also a category called HFmrEF, which corresponds to heart failure with mildly reduced ejection fraction, with the LVEF ranging from 41 to 49% [2].
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Burden of Heart Failure: Global and India
Heart failure affects an estimated 26 million people globally, with 1 million hospitalizations annually and a 1-year mortality rate of 23.6% in high-income countries [3]. HF prevalence is increasing due to an aging population despite improved cardiovascular care. HF is estimated to affect 12% of adults, with an incidence of 3/1000 person-years in Europe [4, 5] HF deaths in the US increased from 275,000 in 2009 to 310,000 in 2014 [6].
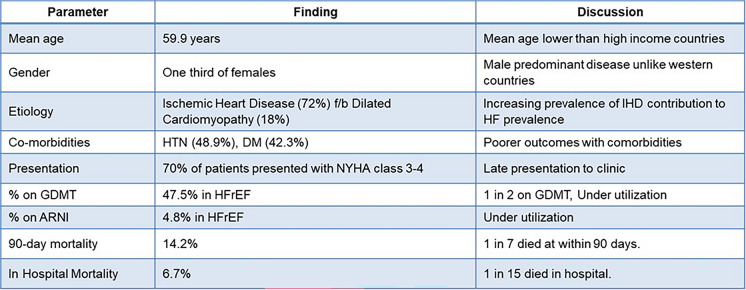
As per the INDUS study, HF is estimated to affect 8–10 million people in India, or 1% of the population, with an estimated mortality rate of 0.1–0.16 million per year [7]. The National Heart Failure Registry (NHFR) published in 2022 followed 10,851 patients across 53 hospitals in 21 states, providing valuable insights into the burden of heart failure in India [8]. The NHFR findings for India are summarized in Fig. 1.
Fig. 1.
Findings from the National Heart Failure Registry of India (2022). ARNI angiotensin receptor–neprilysin inhibitors (ARNI), HTN hypertension, HFrEF heart failure with reduced ejection fraction, DM diabetes mellitus, IHD ischemic heart disease, GDMT guideline-directed medical therapy
Heart failure (HF) leads to 1.8 million hospitalizations annually in India. The Manipal Heart Failure Registry (MHFR) found that the average duration of hospitalization for HF during the first admission is 5.3 ± 2.9 days. On average, the total cost per patient was INR 133,663, with an average out-of-pocket expense of INR 82,766 and an average insurance coverage of INR 50,896 [9].
Neurohormonal Activation in Heart Failure and Its Therapeutic Antagonism
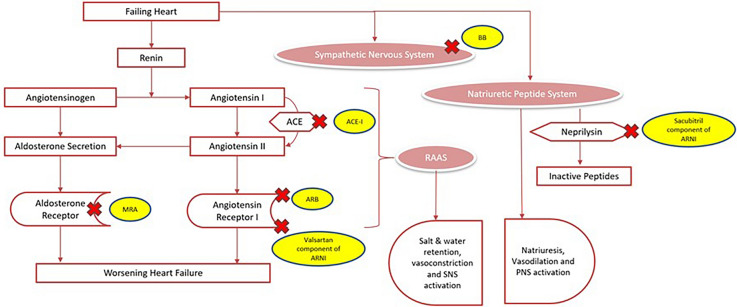
Reduced cardiac output as seen in HFrEF can cause hypotension and reduced organ perfusion, activating compensatory mechanisms such as the sympathetic system, renin–angiotensin–aldosterone system (RAAS), and the natriuretic peptide system. The RAAS plays a key role in the pathophysiology of heart failure, with its main product, angiotensin II (ATII), having initially compensatory effects that later worsen heart failure. In HF, the sympathetic nervous system (SNS) shows an increase in activity, which is indicated by dysfunctional baroreceptor and chemoreceptor reflexes, elevated levels of circulating and neuronal catecholamines, a weakened parasympathetic response, and a heightened sympathetic outflow to the heart, kidneys, and skeletal muscles. Counterregulatory mediators, such as natriuretic peptides (NPs), oppose the SNS and RAAS systems. A-type and B-type NPs promote vasodilation and natriuresis while inhibiting the RAAS and SNS. Neprilysin, an enzyme whose levels increase in heart failure patients, partly breaks down these peptides. In summary, SNS and RAAS adaptations may primarily help cardiac output, which can eventually overpower natriuretic mediators and opposing vasodilator, ultimately worsening HF [10, 11]. This is further represented in Fig. 2.
Fig. 2.
Neurohormonal activation in heart failure and its therapeutic antagonism
In patients experiencing HFpEF, the activation of the RAAS contributes to cardiac fibrosis and rigidity, impaired diastolic function, and corresponding alterations in myocardial systolic function. It also leads to elevated systolic and diastolic blood pressures, unfavorable interaction between arterial and ventricular pulsatile load, and arterial stiffening accompanied by decreased arterial compliance, as evidenced by an increase in pulse wave velocity.
Effective treatment is based on understanding the compensatory mechanisms involved. Medical therapy consists of diuretics, suppression of overactive neurohormonal systems, and increased contractility. The key drugs that cause therapeutic antagonism of neurohormonal activation includes ARNI, beta-blockers, and mineralocorticoid receptor antagonists (MRAs).
Diagnosis of HF
The diagnosis of CHF involves identifying symptoms and signs of HF, as well as evidence of cardiac dysfunction. The diagnostic algorithm is represented in Fig. 3 [1].
Fig. 3.
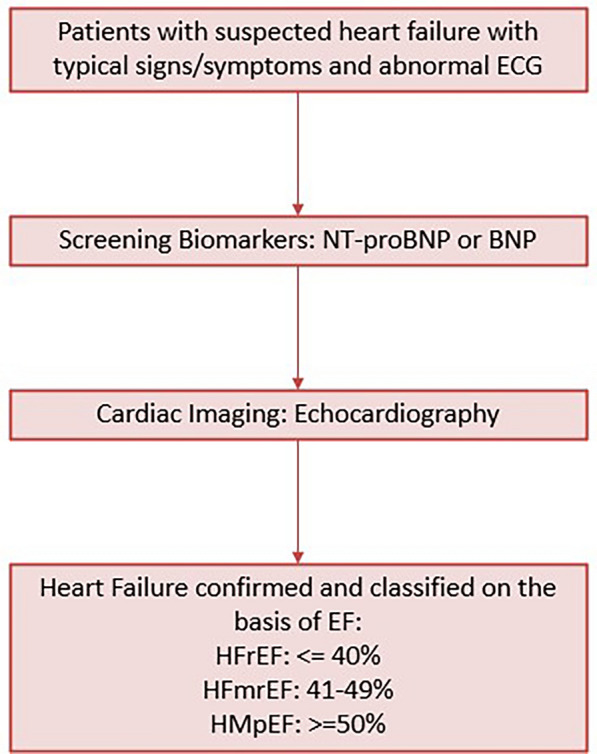
Diagnosis of HF
Role of Biomarkers and Echocardiography
Measuring BNP levels is highly recommended for risk stratification of chronic heart failure patients according to current guidelines (Class 1A recommendation). Furthermore, research has demonstrated that variations in BNP levels over a 6-month period can independently predict unfavorable outcomes, regardless of initial BNP levels. Soluble suppression of tumorigenesis-2 (sST2) is a favorable biomarker of cardiac remodeling [12].
Echocardiography (ECG) is considered the gold standard for diagnosing HF and it requires valuable prognostic data for HFrEF and HFpEF. ECG evaluates various aspects of heart function, including diastolic, systolic, right ventricular, and left atrial functions. Global longitudinal strain (GLS) measurement using 2D speckle-tracking is a useful echocardiographic tool that provides additional knowledge on the risk of HF decompensation in stable patients with left ventricular systolic dysfunction caused by ischemia. Although natriuretic peptides (NPs) may assist in determining a preliminary diagnosis of HF, ECG offers a more comprehensive evaluation. The measurement of LVEF is also important in predicting cardiovascular outcomes [13].
Diagnosis of HFrEF and HFmrEF
The diagnosis of HFrEF and HFmrEF is established through the detection of symptoms and signs of HF and ejection fraction < = 40% and 41–49%, respectively. Increased levels of NPs (BNP ≥ 35 pg/ml or NT-proBNP ≥ 125 pg/ml) and further indications of structural heart disease, such as a larger left atrial size, left ventricular hypertrophy (LVH), or echocardiographic assessments of left ventricular filling, greatly increase the likelihood of a diagnosis of HFmrEF, but are not essential if the EF measurement is certain [1].
Diagnosis of HFpEF
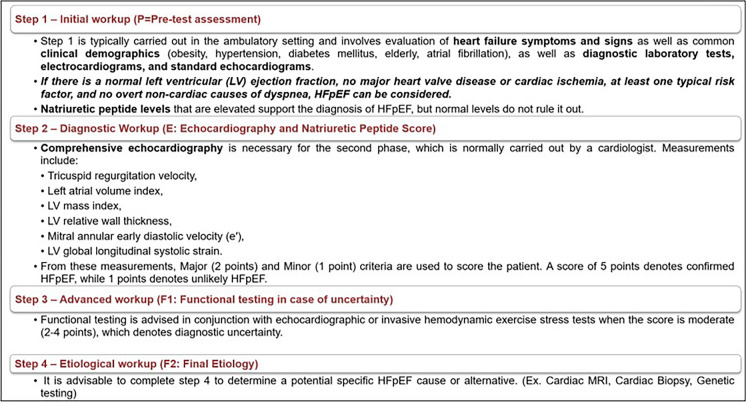
Chronic HFpEF is still difficult to diagnose with certainty. The "HFA-PEFF diagnostic algorithm," a novel stepwise diagnostic procedure is proposed to help improve diagnosis (Fig. 4) [14].
Fig. 4.
HFA-PEFF score for HFpEF diagnosis
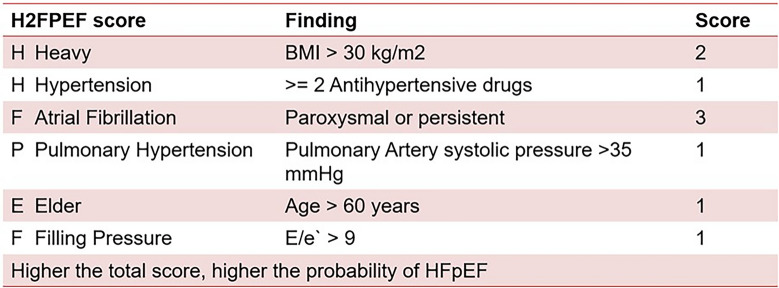
The H2FPEF score is another diagnostic tool for HFpEF (Fig. 5). It determines a composite score based mainly on clinical characteristics and ECG to determine a low, intermediate, and high probability of HFpEF.
Fig. 5.
H2FPEF score
However, its capability to accurately represent HFpEF in well-defined populations is not known, and other studies exploring its association with the functional and clinical markers of HF severity and coronary microvascular dysfunction are limited [1].
Current Guideline Recommendations for the Management of Heart Failure
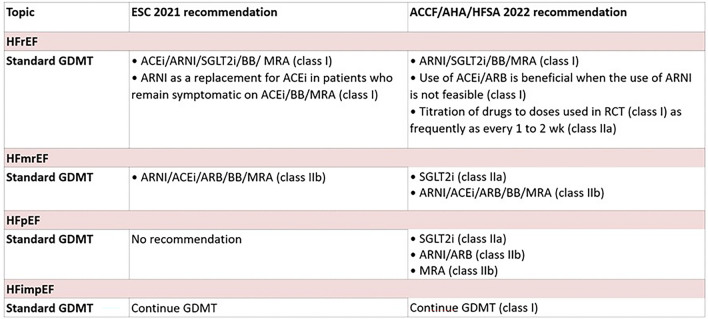
The outline of current recommendations on the medical therapy of heart failure is mentioned in Fig. 6.
Fig. 6.
Current guideline recommendations for the management of heart failure. ARNI angiotensin receptor–neprilysin inhibitors, ACE angiotensin-converting enzyme, ARB angiotensin receptor blocker, MRA mineralocorticoid receptor antagonists, SGLT2i sodium glucose cotransporter-2 inhibitors, GDMT guideline-directed medical therapy, BB beta-blockers, RCT randomized controlled trial, ACCF/AHA/HFSA American Heart Association/American College of Cardiology/Heart Failure Society of America, ESC European Society of Cardiology
Beyond RAAS Inhibition: Sacubitril/Valsartan
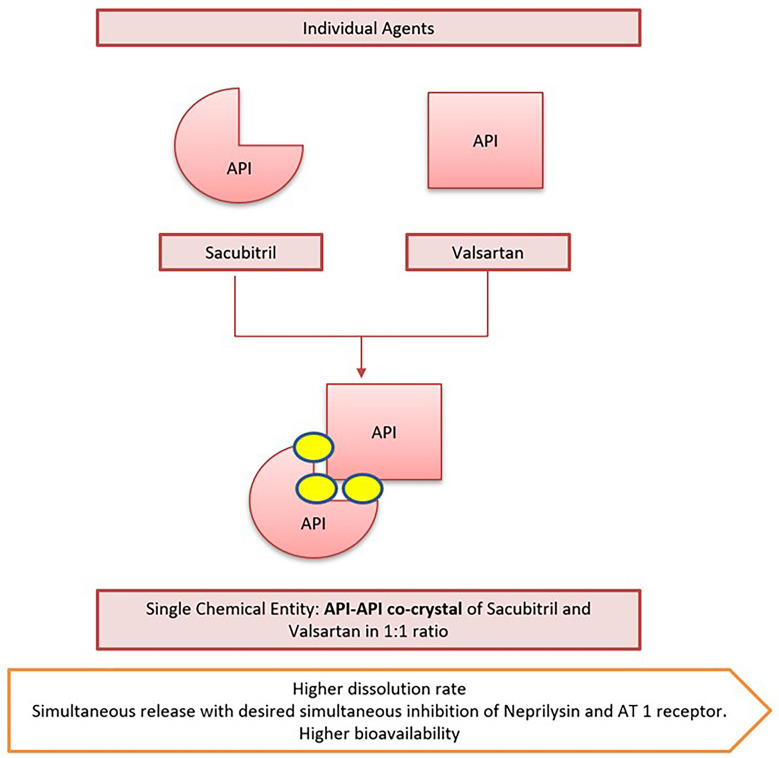
The drug sacubitril/valsartan (Fig. 7) contains two active ingredients, valsartan and sacubitril, in equal amounts by molecule count, and targets two aspects of heart failure treatment: the NPs system through sacubitril, and the RAAS through valsartan [15]. The innovator formulated sacubitril/valsartan as a unique crystalline salt complex having high stability and favourable physicochemical, pharmacokinetic properties. Sacubitril and valsartan are present in their anionic forms with water molecules and sodium cations in the molar ratio of 1:1:3:2.5. This unique crystalline structure provides a higher dissolution rate than a physical mixture of valsartan and sacubitril calcium and desired simultaneous inhibition of neprilysin and angiotensin I receptor. The complex also enables higher bioavailability of valsartan than the other marketed valsartan formulations. Approved 26-, 51-, and 103-mg valsartan doses in co-crystal formulations are equal to 40-, 80-, and 160-mg valsartan doses, respectively, of commercially accessible reference product [16].
Fig. 7.
Co-crystal formulation of sacubitril and valsartan
Need for a Practical Recommendation Paper on ARNI
According to the National Heart Failure Register [17], there is an insufficient application of guideline-directed medical therapy (GDMT), which calls a nationwide quality improvement initiative. This practical recommendation paper for the use of ARNI, one of the crucial treatments for heart failure, is a step in that direction.
Methodology
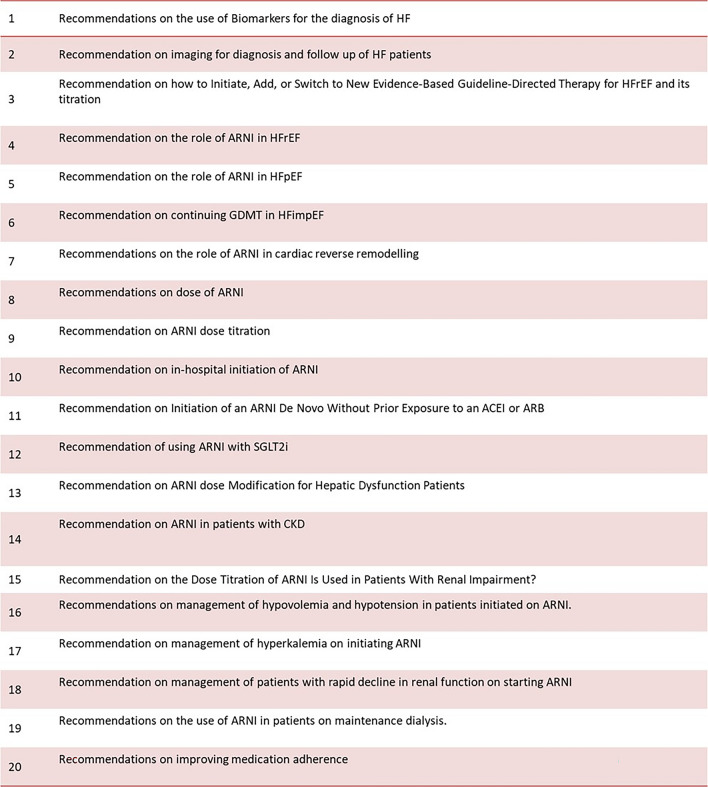
A group of 94 cardiologists and physicians in India convened for seven advisory board meetings to discuss the use of ARNI for the management of HF. The discussions focused on ARNI in the treatment of HFrEF, HFpEF, and HFimpEF. The meetings were moderated by leading cardiologists in the country and included a panel of advisors from across India. The development of this recommendation paper involved identifying evidence gaps and gaining insights on real-world practice patterns. The points discussed by the panel are listed in Fig. 8.
Fig. 8.
Points of discussion at the board meetings
A team conducted a literature review to gather relevant evidence on the above pre-decided topic of contemporary HF care with emphasis on ARNI. This document does not contain any new studies with human participants or animals performed by any of the authors. The findings from this review were presented during an interactive roundtable meeting attended by cardiologists. The meeting involved structured discussions covering various aspects such as new therapies, addressing queries, patient adherence, and strategies for implementation. A distinguished expert served as the moderator for these discussions. Based on the insights gathered from the meeting, a writing committee was established to formulate practical recommendations for the optimal management of heart failure. The resulting document underwent a thorough review process involving the authors and incorporating feedback and comments. After considering all inputs, the document received approval for publication.
Practical Recommendations and Related Evidence
Recommendations on the Use of Biomarkers for the Diagnosis of HF
Recommendation: BNP and NT-proBNP are used for heart failure diagnosis and prognosis, but renal dysfunction may affect results and there is no evidence to target treatment to specific levels. NTproBNP is the more accurate biomarker in patients on sacubitril/valsartan.
Discussion of evidence: BNP and NT-proBNP are the most studied biomarkers in heart failure (HF). They assist in the diagnosis and prediction of the disease. If a patient with HFrEF has high levels of NT-proBNP or BNP, they are at high risk, especially if the levels are increasing. Clinical practice guidelines recommend measuring NT-proBNP or BNP to confirm a diagnosis of HF, determine its severity, or predict its outcome [18].
Recently, biomarkers have been evaluated for their role in determining the response of patients to GDMT. It has been observed that patients do not experience a reduction in their NP levels despite GDMT, known as "nonresponders," tend to have a worse prognosis and more severe left ventricular remodeling. These patients face challenges in achieving favorable outcomes [19]. In an effort to explore the potential benefits of NT-proBNP-guided treatment, the Guiding Evidence-Based Therapy Using Biomarker-Intensified Treatment in Heart Failure (GUIDE-IT) study was conducted between 2013 and 2016. This randomized multicenter clinical trial aimed to determine whether a treatment strategy guided by NT-proBNP levels could improve clinical outcomes in high-risk patients with HFrEF compared to usual care. However, the results of the study indicated that NT-proBNP-guided therapy did not demonstrate superior effectiveness over the usual care strategy in improving outcomes. Overall, while biomarkers such as BNP and NT-proBNP are valuable in assessing patients' response to GDMT, the GUIDE-IT study did not find evidence supporting the use of an NT-proBNP-guided treatment approach as more effective than standard care in enhancing clinical outcomes [20].
Renal dysfunction may affect the analysis of NP levels. Currently, there is no evidence showing that treatment should be targeted at specific levels of BNP or NT-proBNP. The relationship between rising natriuretic peptide levels and adverse outcomes may be influenced by the use of sacubitril/valsartan. BNP levels may increase modestly in patients treated with this drug, while NT-proBNP levels typically decrease more consistently [21].
When assessing NP levels in patients undergoing GDMT for HF, clinicians should consider the treatment context and exercise caution, particularly when interpreting BNP levels in patients using sacubitril/valsartan. In such cases, measuring NT-proBNP may be a preferred option. However, due to potential interference from sacubitril/valsartan, it is important to approach BNP measurements with caution, and NT-proBNP measurement may provide more reliable results in this specific treatment setting.
Congestion in heart failure patients is a significant indicator of poor prognosis. Besides the established marker, BNP emerging markers such as estimated plasma volume status (ePVS), bioimpedance vector analysis (BIVA), and blood urea nitrogen/creatinine ratio (BUN/Cr) are also being recognized for assessing congestion. These markers represent different aspects of congestion in HF, including hemodynamic, intravascular, peripheral hydration, and venous congestion. Each biomarker independently provides prognostic information for all-cause mortality in HF patients, and when combined, they explain 40% of the risk of death. Using a multi-marker score, it is possible to identify HF patients at low and high risk of death. Signs of congestion have an adverse prognosis in HF patients and require prompt recognition to provide targeted therapies. Therefore, they can be considered for rapid stratification of HF patients [22].
Recommendation on Imaging for Diagnosis and Follow-Up of HF Patients
Recommendation: Patients with new HF should have an echocardiogram (with strain imaging if available) to aid in the correct diagnosis and after 3–6 months of GDMT, repeat imaging can help in treatment decisions, with alternative modalities like cardiac MRI considered if echocardiography fails to assess LVEF.
Discussion of evidence: A patient with recently diagnosed HF should undergo ECG, including strain imaging if available, to assess various factors such as LVEF, chamber size, diastolic function, valvular abnormalities, ventricular wall thickness, and hemodynamic parameters. After 3–6 months of receiving optimal GDMT, repeat ECG, which helps in making decisions about advanced therapies or device therapies like transplant or ventricular assist device. However, in a few cases, it is rational to wait longer for such results if there is a chance of progression of LV remodeling. Repeat imaging may also be done if there are significant changes in the patient's clinical status. Routine surveillance echocardiograms are not recommended if there is no change in the patient's clinical status. Echocardiography, however, fails to assess LVEF, radionuclide ventriculography or magnetic resonance imaging may be recommended [23].
Recommendation on Initiation, Addition, or Switching to New Evidence-Based GDMT for HFrEF and Its Titration
Recommendation: Established treatments for HFrEF include ARNIs, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), beta-blockers, aldosterone antagonists, loop diuretics, hydralazine/isosorbide dinitrate, and ivabradine, except for loop diuretics, which is proven to improve symptoms, decrease hospital stays, and greatly increase survival in clinical trials. The selection of initial treatment is based on various factors, with all treatments eventually increased to the maximum tolerated or targeted dose.
Discussion of evidence: The commonly used established treatments for HFrEF are ARNIs, ACEIs, beta-blockers, ARBs, aldosterone antagonists, loop diuretics, hydralazine/isosorbide dinitrate, and ivabradine. Except for loop diuretics, all of these therapies have proven to reduce symptoms, lower hospital stays, or improve survival in clinical trials. The use of digoxin for HFrEF is not supported by recent data and is mainly used for controlling the heart rate in AF in those with low blood pressure. HFrEF often occurs with multiple other conditions and patients are frequently on multiple medications, making it important to have frequent follow-ups to monitor their tolerance and progress [23].
When faced with a new stage C HFrEF diagnosis, there is often a question of whether to begin with a, MRA, beta-blocker, SGLT2i, or ARNI/ACEI/ARB. The decision is based on various factors and all four options can be started simultaneously if eligible. Regardless of the starting sequence, all treatments should be increased to the maximum tolerated or targeted doses as soon as possible. Starting an ARNI/ACEI/ARB is usually easier if the patient is congested, while beta-blockers are better tolerated in less-congested patients with a normal resting heart rate. Beta-blockers should not be started in patients having decompensated symptoms/signs. Beta-blockers is the only drug that has been proven effective in HFrEF patients [23].
After a diagnosis of HF, therapy adjustments should be made every 2 weeks, aiming for a rapid titration of GDMT within 3–6 months. However, it is important to note that achieving rapid titration may not be feasible for all patients. The goal is to up-titrate GDMT to reach targeted or maximum tolerated doses, with frequent reassessments of patient status, kidney function, blood pressure, and electrolytes. Implementing a structured medication plan as part of a disease management program can help in reaching target doses within 6 months of hospital discharge. Reassessment of ventricular function takes place 3–6 months after reaching target doses of GDMT. This evaluation helps determine if device therapies are necessary, particularly for patients at higher risk of sudden death, such as those with ischemic cardiomyopathy and low LVEF [23].
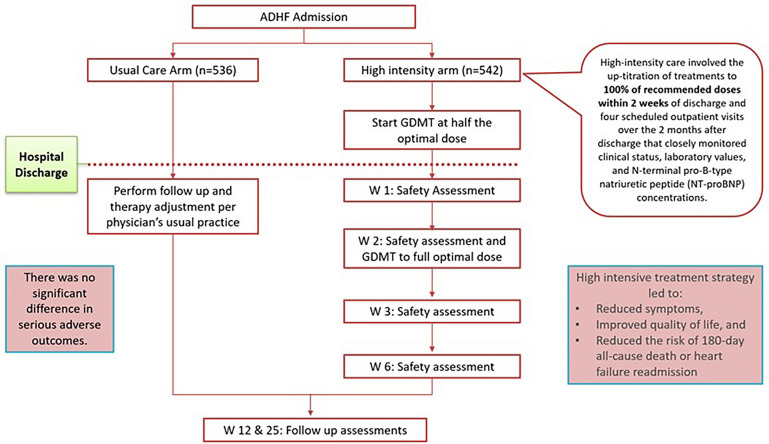
The STRONG-HF trial examined the effectiveness and safety of an intensive treatment strategy for patients admitted to the hospital with acute HF. The study involved 1078 patients from 14 countries and compared usual care with high-intensity care. Usual care followed local practice, while high-intensity care involved up-titrating treatments to 100% of recommended doses within 2 weeks of discharge and conducting four scheduled outpatient visits over 2 months to closely monitor clinical status, laboratory values, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations. The trial was terminated early due to greater-than-expected differences between the two groups. By day 90, a higher proportion of patients in the high-intensity care group had reached full doses of prescribed drugs, experienced symptom reduction, and improved quality of life. The primary endpoint of 180-day all-cause death or heart failure readmission was significantly lower in the high-intensity care group compared to the usual care group. However, it is worth noting that the high-intensity care group also reported more adverse events [24] (Fig. 9).
Fig. 9.
Safety, tolerability, and efficacy of up-titration of guideline-directed medical therapies for acute heart failure—STRONG-HF Trial
HF progression is punctuated by repeated worsening HF events, each resulting in reduced cardiac function. The definition of worsening heart failure (WHF) has evolved from just considering worsening of symptoms requiring hospitalization to escalation of oral diuretics. WHF should be recognized as a clinical condition to firstly optimize existing GDMT (ARNI, beta-blocker, SGLT2 inhibitor and MRA) if possible and secondly add novel drug that improve patient outcomes [25]. Based on the findings of the VICTORIA trial, vericiguat has been specifically recognized as a treatment option for worsening heart failure (WHF) in the recent guidelines for HFrEF. Vericiguat is a novel oral soluble guanylate cyclase stimulator (sGCS) that has shown promise as a disease-modifying therapy for WHF. The VICTORIA trial provided evidence of the efficacy and benefits of vericiguat in improving outcomes for patients with HFrEF and WHF [26].
Recommendation on the Role of ARNI in HFrEF
Recommendation: The guidelines recommend a combination of four medications for treating HFrEF: ARNIs, MRAs, beta-blockers, and SGLT2i. The ACCF/AHA/HFSA guidelines give ARNI a Class 1A recommendation and the ESC guidelines give it a 1B recommendation. The PARADIGM HF trial showed that sacubitril/valsartan had a lower rate of CV death or HF hospitalization compared to enalapril, with improvement in quality of life.
Discussion of evidence: The ACC/AHA/HFSA and ESC guidelines both clearly state that a combination of four medications—ARNI, evidence-based beta-blockers, MRAs, and SGLT2 inhibitors is now the standard for treating HFrEF. The ACCF/AHA/HFSA guidelines give a Class 1A recommendation for using ARNI as the preferred renin–angiotensin modulator, with the use of ACEI or ARB being allowed only if ARNI is not available [18]. The ESC guidelines give ARNI a class 1B recommendation, suggesting it as an alternative for ACEI in appropriate patients experiencing symptoms, but ARNI can also be used as a first-line treatment [1].
The PARADIGM-HF trial enrolled 8442 participants with a mean age of 64, of whom 21% were women. The average BMI was 28 kg/m2, and 60% of participants had an ischemic origin for HF. Around 43% had a prior heart attack, and 35% had diabetes. The average LVEF was 30%. After 8 months of treatment, the group receiving sacubitril/valsartan showed a reduction in SBP by 3.2 mmHg compared to the group taking enalapril. The trial was terminated early based on predefined rules for benefit. After 27 months, the sacubitril/valsartan group demonstrated a lower rate of cardiovascular death or heart failure hospitalization (21.8%) compared to the enalapril group (26.5%, p < 0.001). This benefit was consistent across all subgroups and did not change with time from heart failure hospitalization to screening. The sacubitril/valsartan group also experienced lower rates of CV death, all-cause death, hospitalization for HF, and symptomatic hypotension, along with reduced emergency room visits and heart failure admissions. Among patients with diabetes, those in the sacubitril/valsartan group showed a greater decrease in HbA1c during the first year of follow-up and had less use of insulin. Additionally, sacubitril/valsartan had a positive impact on estimated GFR and urinary albumin/creatinine ratio. The benefits of sacubitril/valsartan varied based on left ventricular ejection fraction, with the greatest benefit observed in women with higher LVEF. During the initial 8–10 weeks of treatment with sacubitril/valsartan, BNP levels increased by 19%, while NT-proBNP levels decreased by 28%. Both biomarkers remained accurate in predicting cardiovascular death or heart failure hospitalization [27].
Patients with HFrEF commonly experience impaired health-related quality of life (HRQL). In the PARADIGM-HF trial, sacubitril/valsartan was found to reduce morbidity and mortality compared to enalapril. An analysis focusing on survivors assessed the impact of therapy on HRQL. Patients completed the Kansas City Cardiomyopathy Questionnaire (KCCQ) at multiple time points. The sacubitril/valsartan group showed improvements in both the KCCQ clinical summary score and KCCQ overall summary score compared to the enalapril group. This indicates that sacubitril/valsartan leads to better HRQL in surviving patients with heart failure [28].
Several gender-related differences in terms of pharmacokinetic and pharmacodynamic characteristics of the drugs have also been observed. These differences may contribute to varying responses and tolerability between men and women, even when following the recommended treatment for HFrEF. Some studies have indicated that lower doses of beta-blockers and renin–angiotensin–aldosterone system inhibitors could be equally effective in women compared to higher doses in men. Additionally, the beneficial effects of sacubitril/valsartan are higher in women with HFpEF. However, current European and American guidelines do not recommend personalized treatment based on gender and body composition in HFrEF therapies [29].
Recommendation on the Role of ARNI in HFpEF
Recommendation: The ACC guidelines have given a Class IIb recommendation for using sacubitril/valsartan in HFpEF. In the PARADIGM HF trial done in patients with EF of 45% or higher, the main outcome showed a non-significant reduction in CV deaths or hospitalizations due to HF in the sacubitril/valsartan group. Secondary outcomes confirmed a significant increase in improvement of NYHA class and renal function, with more benefits seen in female patients and patients with EF between 45 and 57%, highlighting the need for personalized therapy with ARNI in HFpEF [22, 27].
Discussion of evidence: The use of ARNIs in HFpEF has received a Class IIb recommendation in the ACC guidelines. The PARAGON-HF trial involved 4822 patients with New York Heart Association (NYHA) class II to IV heart failure, ejection fraction of 45% or higher, elevated levels of NP, and structural heart disease. These patients received either sacubitril/valsartan (97 mg sacubitril with 103 mg valsartan twice daily) or valsartan (160 mg twice daily). The primary outcome of the trial, the rate of cardiovascular deaths or hospitalizations due to heart failure, did not show a significant difference between the sacubitril/valsartan group (12.8 events per 100 patient-years) and the valsartan group (14.6 events per 100 patient-years) [27].
Regarding secondary outcomes, there was a 15.0% improvement in NYHA class in the sacubitril/valsartan group compared to 12.6% in the valsartan group (p < 0.05). The renal composite outcome was 1.4% in the sacubitril/valsartan group compared to valsartan group (2.7%) (p < 0.05). The benefits of sacubitril/valsartan were found to be different based on sex. Women had a HR of 0.73 for the primary outcome, while men had a HR of 1.03 (p for interaction = 0.017). Renal function and NYHA class improvement was similar in both genders, but symptom improvement was less pronounced in women. The timing of initiating sacubitril/valsartan after a heart failure hospitalization showed varying benefits. Initiating the medication within 30 days after the index hospitalization resulted in an absolute 6.4% reduction in cardiovascular death or hospitalization for heart failure. The reduction was 4.6% when initiated 30–90 days after, 3.4% when initiated 91–180 days after, and no significant benefit was found when initiated more than 180 days after the index hospitalization (p for interaction = 0.05). [27] While the PARADIGM-HF trial showed potential benefits across various subgroups, the PARAGON-HF trial identified only two subgroups that showed a possible treatment advantage. These were patients with an ejection fraction between 45 and 57% and women, who are more likely to have HFpEF than men [30].
A meta-analysis was conducted to assess the effects of ARNI therapy in patients with HFmrEF. ARNI treatment resulted in improved left ventricular function, stroke volume (SV), and fractional shortening (FS), as well as decreased left ventricular end-diastolic diameter (LVEDD), left atrial diameter (LAD), C-reactive protein (CRP), and N-terminal pro-B-type natriuretic peptide (NT-proBNP). Moreover, patients receiving ARNI had higher total effective rates, as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) and 6-Minute Walk Test. Additionally, ARNI treatment was associated with a reduced readmission rate [31]. PARAGLIDE-HF and PARAGON-HF trials revealed that sacubitril/valsartan demonstrated a reduction of 33 cardiovascular and renal events in patients with HFmrEF and HFpEF. These findings offer strong evidence for the utilization of sacubitril/valsartan in patients with HF, particularly those with an EF below the normal range, irrespective of the care setting [32].
Recommendation on Continuing GDMT in HFimpEF
Recommendation: Continue current GDMT in HFrEF patients with recovered LVEF unless there is a clear reversible cause, as discontinuing it leads to high rates of HF events (per TRED-HF study).
Discussion of evidence: The decision of whether to maintain GDMT or decrease/stop it in patients who have fully recovered left ventricular ejection fraction (LVEF) can be challenging for clinicians. The TRED-HF study, an open-label, pilot, randomized study, aimed to investigate the safety of withdrawing heart failure medications in patients with previous dilated cardiomyopathy who had recovered and were asymptomatic. Patients were recruited from a network of hospitals in the UK and were randomly assigned to either phased withdrawal or continuation of treatment. The primary endpoint was the relapse of dilated cardiomyopathy within 6 months. The results showed that 44% of patients in the treatment withdrawal group experienced a relapse of dilated cardiomyopathy, whereas none in the continued treatment group met the primary endpoint. After 6 months, 96% of patients in the continued treatment group attempted the withdrawal of heart failure medications, and nine patients in this group experienced a relapse of dilated cardiomyopathy. These findings suggest that continued treatment with GDMT is correlated with a lower risk of relapse in patients who have recovered LVEF [33].
Recommendations on the Role of ARNI in Cardiac Reverse Remodeling
Recommendation: Studies have shown that ARNIs can improve diastolic function, LV function, reduce risk of ventricular arrhythmias and quality of life, with positive results seen in trials such as PROVE-HF and EVALUATE-HF.
Discussion of evidence: ARNIs have indeed been shown to be benefit on various aspects of HF, including left ventricular function, diastolic function, quality of life, and reduced risk of ventricular arrhythmias. The PROVE-HF study investigated the effects of sacubitril/valsartan therapy on left ventricular function in 794 patients with heart failure. The mean age of study participants were 65.1 years and a mean baseline LVEF of 28.2%. After 1 year of therapy with sacubitril/valsartan, the study found a significant increase in median LVEF from 28.2 to 37.8%. This improvement in LVEF indicates a positive impact on left ventricular function. Furthermore, the study observed a significant decrease in median NT-proBNP concentration from baseline to 12 months. The change in log2-NT-proBNP concentration was found to be significantly correlated with changes in LVEF, left ventricular end-diastolic volume index (LVEDVI), left ventricular end-systolic volume index (LVESVI), left atrial volume index (LAVI), and E/e′ ratio. These correlations suggest that the improvements in LVEF and other echocardiographic parameters are associated with a decrease in NT-proBNP levels, indicating a favorable response to sacubitril/valsartan therapy. The findings of the PROVE-HF study were supported by the EVALUATE-HF trial, which compared sacubitril/valsartan to enalapril. The EVALUATE-HF trial demonstrated early improvement in echocardiographic parameters as early as 12 weeks with sacubitril/valsartan treatment, further affirming the positive effects of this therapy on left ventricular function [34, 35].
Recommendations on Dose of ARNI
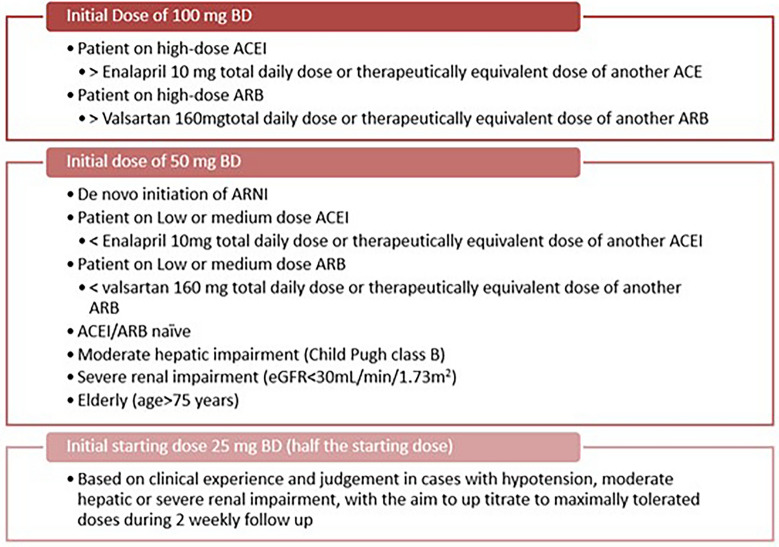
Recommendation: The recommended initial dose of sacubitril/valsartan is 50 mg, taken orally twice a day. After 2–4 weeks, the dose can be doubled to 100 mg and subsequently to 200 mg, as tolerated. In some cases, the starting dose may need to be reduced, such as for patients who are not taking an ACEI or ARB, patients with severe renal dysfunction, blood pressure < 90/60, or patients with moderate hepatic impairment (Fig. 10).
Fig. 10.
Dose adjustments of ARNI for specific patient populations. When making the transition from an ACEI to an ARNI, a 36-h washout period should be strictly observed to avoid angioedema, a delay that is not required when switching from an ARB to an ARNI. Please note that though the recommended starting dose for ARNI as per prescribing information by the innovator is 100 mg BD, Indian doctors recommend using 50 mg BD as the starting dose in Indian patients as per their clinical experience. eGFR estimated glomerular filtration rate, ARNI angiotensin receptor–neprilysin inhibitors, ACE angiotensin-converting enzyme, ARB angiotensin-receptor blocker-mineralocorticoid receptor antagonists, bd twice daily
Discussion of evidence: Caution should be exercised when using ARNI in combination with other medications. It is not recommended to use ARNI together with ACE inhibitors (ACEIs), and a 36-h interval should be observed after discontinuing ACEIs before initiating ARNI. This precaution is necessary due to a high risk of angioedema, as shown in trials involving similar drugs. Patients having history of angioedema are contraindicated from using ARNI, as they may have a higher risk of developing the condition while on ARNI. Additionally, combining ARNI with aliskiren is not recommended. The concurrent use of ARNI with medications such as spironolactone, amiloride, or potassium salts may elevate the risk of hyperkalemia. Pregnant women should avoid using ARNI due to potential teratogenic effects and the inconvenience of subsequent medication adjustments the use of ARNI in combination with other drugs should be approached with caution. Concomitant use of ARNI and ACEI is not recommended, and a 36-h wait is advised after discontinuing ACEI before starting ARNI. Increased risk of angioedema was reported in several clinical studies of ACEI and ARNI. ARNI is contraindicated in patients with a history of angioedema, as they may have an increased risk of developing the condition while using ARNI. The use of ARNI in combination with aliskiren is also not recommended. ARNI combined with spironolactone, amiloride, or potassium salts may increase the risk of hyperkalemia. ARNI should not be used by pregnant women due to the risk of teratogenicity and inconvenience of subsequent drug adjustments [1, 18]. In a particular study, only 32% of patients with HFrEF received high doses of ARNI. While many patients in large pivotal trials were prescribed high doses, adhering to multidrug therapy remains challenging in clinical practice, especially for adults with cardiovascular diseases who have multiple comorbidities and complex medication regimens. However, low-dose ARNI treatment for 1 year proved effective in real-world HFrEF patients, regardless of the specific dosage of sacubitril/valsartan. Enhancements in prognostic biomarkers, health status, and cardiac remodeling were comparable among patients with HFrEF, irrespective of the different dosages of the medication [36].
A study published in Nature examined the feasibility of initiating sacubitril/valsartan at a very low dose (VLD) of 25 mg twice daily in potentially intolerant patients with HFrEF, followed by dose up-titration. In contrast, patients in the standard-dose group started with a dosage of ≥ 50 mg twice daily. The results indicated that initiating treatment with 25 mg twice daily was generally achievable, and patients remained on the treatment with similar reductions in NT-proBNP levels and improvements in left ventricular ejection fraction (LVEF) compared to those on the standard dose of sacubitril/valsartan. Throughout the follow-up period, there were no significant differences between the two groups regarding symptomatic hypotension, worsening renal function, hyperkalemia, cardiovascular mortality, or rehospitalization due to heart failure [37].
Recommendation on ARNI Dose Titration
Recommendation: The initiation and dose increase of sacubitril/valsartan over 3 (for 100 mg bd starting dose) or 6 weeks (for 50 mg BD starting dose) has a tolerable profile and a more gradual increase maximized the target dose attainment in low-dose ACEI/ARB patients (TITRATION trial).
Discussion of evidence: The TITRATION trial analyzed two strategies for starting and increasing the dose of sacubitril/valsartan (LCZ696) in patients with HFrEF and systolic blood pressure of 100 mmHg or more. Patients were allocated into four groups based on their baseline SBP at screening: 100–110, 111–120, 121–139, and 140 mmHg or more. The majority of patients in each SBP group achieved the target dose of LCZ696 without dose interruption or reduction (72.7–82.9% success rate). The success rate was higher with gradual up-titration over 6 weeks compared to rapid up-titration over 3 weeks for patients with SBP 100–110 mmHg. Low SBP did not significantly impact success rate. These findings suggest that low SBP should not prevent the initiation of sacubitril/valsartan [38].
The TITRATION trial also assessed the tolerability of initiating and increasing the dose of sacubitril/valsartan from 50 to 200 mg twice daily in heart failure patients with ejection fraction less than or equal to 35%. The study was conducted over 11 weeks, including a 5-day open-label run-in, and had two regimens for dose increase: a "condensed" regimen (100 mg twice daily for 2 weeks followed by 200 mg twice daily) and a "conservative" regimen (50 mg twice daily for 2 weeks, 100 mg twice daily for 3 weeks, followed by 200 mg twice daily). Of the 540 patients that entered the run-in, 498 (92%) were randomized and 429 completed the study. The rate of adverse events, for example hypotension, renal dysfunction, hyperkalemia, and angioedema, was similar between the two regimens. A higher proportion of low-dose ACEI/ARB patients achieved the target dose with the conservative regimen, while there was no difference in the high-dose ACEI/ARB group. In conclusion, the initiation and dose increase of sacubitril/valsartan over 3 or 6 weeks had a tolerable profile and a more gradual increase maximized the target dose attainment in low-dose ACEI/ARB patients [38].
Recommendation on in-Hospital Initiation of ARNI
Recommendation: The TRANSITION trial found initiation of sacubitril/valsartan to be feasible in patients with HFrEF who had stabilized after an acute heart failure event. The PIONEER-HF trial showed that sacubitril–valsartan was more effective than enalapril in reducing NT-proBNP levels in patients with HFrEF hospitalized for acute decompensation.
Discussion of evidence: The PIONEER HF Trial enrolled patients with HFrEF who were hospitalized for acute decompensated heart failure and randomly assigned them to receive either sacubitril-valsartan or enalapril. The primary outcome was the difference in the N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration, which was found to be significantly greater in the sacubitril-valsartan group than the enalapril group. The study also found that the rates of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema did not significantly differ between both the groups [39].
The TRANSITION trial was a study that aimed to assess the tolerability and optimal time point for initiation of sacubitril/valsartan in patients who had stabilized after an acute heart failure event. Patients were randomly assigned to initiate sacubitril/valsartan either before discharge or between days 1–14 after discharge. Results showed that initiation of sacubitril/valsartan was feasible for patients, with about half of the patients achieving the target dose within 10 weeks, regardless of initiation time. Discontinuation due to adverse events occurred in 7.3 and 4.9% of patients in the pre-discharge and post-discharge groups, respectively [40].
Starting ARNI in a stabilized patient within the last 5 days of hospitalization or in the first days of discharge, does not make any difference, although introducing the therapy during hospitalization, could limit, for various reasons, the therapeutic inertia, which could influence the subsequent implementation in the outpatient phase. All patients hospitalized for acute HF and an EF < 40% should be considered potential candidates for S/V treatment, except for patients with evident contraindications or history of angioedema.
The timing of initiating sacubitril/valsartan therapy in stabilized heart failure patients does not significantly impact outcomes, whether it is within the last 5 days of hospitalization or in the early days of discharge. However, starting the therapy during hospitalization may help overcome therapeutic inertia and facilitate its continuation in the outpatient phase. It is recommended that all patients hospitalized for acute HF with EF below 40% should be considered potential candidates for sacubitril/valsartan treatment, after the patient is hemodynamically stable [41].
Recommendation for Starting ARNI In Patients Without Previous Use of an ACEI or ARB
Recommendation: Starting directly on ARNI is safe and effective with improved cardiac function and tolerability and is recommended with monitoring and assessment considering the risk of angioedema or hypotension.
Discussion of evidence: Initiating ARNI without a pre-treatment period with ACEI or ARB is a safe and effective strategy, based on recent clinical studies and clinical experience. In studies, patients with HFrEF who were not previously taking an ACEI or ARB had no unexpected adverse effects with the direct-to-ARNI approach and showed significant improvement in cardiac function and tolerability. In a sub-analysis of PIONEER-HF, the use of ARNI in hospitalized patients demonstrated a greater reduction in natriuretic peptide levels and better early outcomes compared to those taking enalapril. Direct initiation of ARNI is now recommended, with close monitoring and assessment of blood pressure, electrolytes, and renal function, considering the risk of angioedema or hypotension [39].
Recommendation of Using ARNI with SGLT2 Inhibitor
Recommendation: ARNI may be combined with SGLT2i for the treatment of heart failure. Whenever diuretics are used, the dosage needs to be adjusted.
Discussion of evidence: ARNI and SGLT2 inhibitor have fairly obvious mechanisms of action. The interaction between ARNI, natriuretic peptides, and the RAAS system leads to a reduction in the negative effects of RAAS and an enhancement of the positive effects of the natriuretic peptide system. This interaction aids in the reversal of cardiac remodeling and the improvement of heart failure [42]. SGLT2 inhibitors like dapagliflozin and empagliflozin act on myocardial cells, increase cardiac function, and also stimulate natriuresis, osmotic diuresis, and urine glucose excretion [43]. As ARNI and SGLT2 inhibitor work via separate processes that do not overlap, they can be used in conjunction. A safe and effective combination of ARNI and SGLT2 inhibitor (dapagliflozin), was taken by 11% of patients in the DAPA-HF study, a randomised controlled trial of the drug [44]. Patients with heart failure can therefore combine the two medications. For T2DM patients with HFrEF, combining ARNI to SGLT2 inhibitor provided superior protection against renal function decline in a multicenter observational analysis [45]. Additionally, a study that assessed the ARNI + SGLT2i combination's safety and effectiveness in HFrEF patients with diabetes, showed that the combination significantly improved the clinical and renal prognosis compared to the combination of ACEI/ARB and antidiabetic regimen. The combination was well tolerated, and there were fewer concerns of hyperkalemia and creatinine increase than with ACEI/ARB.
The treating clinician must consider the increased risk of hypovolemia when ARNI and SGLT2 inhibitor are combined, since significant hypovolemia was observed in 10.8% of patients in the DAPA-HF study. Diuretics may work synergistically with ARNI, MRA and SGLT2i to increase the effects on natriuresis and diuresis when used together [46]. As a result, it is important to change the diuretic dosage in due time before adjusting the dosage of ARNI and SGLT2 inhibitor as needed.
Recommendation on ARNI Dose Modification for Hepatic Dysfunction Patients
Recommendation: In HF patients with moderate hepatic impairment (Child–Pugh B), the loading dose of ARNI should be halved and the subsequent doses should be gradually increased to reach the maximum tolerated dose. No dose adjustment is needed in mild hepatic impairment (Child–Pugh A). ARNI should not be prescribed in patients with severe hepatic impairment.
Discussion of evidence: Compared to healthy subjects, patients with mild hepatic impairment have a higher exposure to sacubitril (1.5 times), LBQ657 (1.5 times), and valsartan (1.2 times). Patients with notable liver dysfunction encountered exposure elevations of 3.4, 1.9, and 2.1 times, correspondingly. Another clinical research with 32 patients with mild to moderate hepatic impairment revealed increases in the AUC of sacubitril (53–245%), LBQ657 (48–90%), and valsartan (19–109%). Likewise, there was no obvious difference in the Cmax of valsartan and LBQ657 between those who have mild to moderate liver dysfunction. Hence, the loading dose does not require modification in patients with mild hepatic impairment, but it should be reduced by 50% in individuals with significant hepatic impairment. ARNI is not advised to be prescribed in severe hepatic impairment, biliary cirrhosis, or cholestasis due to lack of pharmacokinetic data [47].
Recommendation on ARNI in Patients with CKD
Recommendation: ARNI can be prescribed to non-dialysis patients with CKD and heart failure. The use of ARNI in such patients reduces cardiovascular risk and improves eGFR compared to ACEI/ARBs.
Discussion of Evidence: Analysis of CKD patients with eGFR of 30–60 ml/min/1.73 m2 in the PARADIGM-HF study demonstrated that the use of ARNI resulted in a decreased risk of cardiovascular events compared to ACEI treatment. This included a 24% reduction in the risk of cardiovascular death, a 21% decrease in the risk of hospitalization due to heart failure, and a 36% decrease in the risk of composite kidney events [48]. It was observed that ARNI treatment led to a slight increase in urine albumin creatinine ratio (UACR), which could be attributed to improved renal perfusion resulting from enhanced cardiac function. Real-world studies conducted in Taiwan and Italy also supported the findings of reduced risk of cardiovascular death and hospitalization, along with improved eGFR, in patients treated with ARNI [49, 50]. In patients with HFpEF, an analysis of the CKD subgroup in the PARAGON-HF study revealed a 50% reduction in the risk of the renal composite endpoint when comparing ARNI to ARB [51]. A recent retrospective cohort study conducted in Taiwan indicated that patients with CKD and HFpEF treated with ARNI had higher eGFR compared to those treated with valsartan. Furthermore, a meta-analysis demonstrated that ARNI improved eGFR and reduced NT-proBNP levels in comparison to ACEI/ARBs [52].
Recommendation for ARNI Dose Titration in Renal Impairment
Recommendation: ARNI dose adjustment is not required in HF patients with mild to moderate renal impairment. Depending on the patient's blood pressure, a loading dose of 25–50 mg BID is advised in severe renal impairment. The dose of ARNI should be gradually increased every 2–4 weeks to reach the maintenance dose of 200 mg BID (maximum tolerated dose).
Summary of evidence: There was no change in the blood levels of sacubitril and valsartan in renally impaired patients. Similarly, no statistically significant difference was seen in LBQ657 exposure among patients with mild renal impairment. The exposure increased in moderate (2.29 times), severe (2.90 times) and non-dialysis ESRD (3.27 times). Furthermore, the half-life of LBQ657 increased from 12 to 21.1, 23.7, and 38.5 h in individuals with mild, moderate, and severe renal impairment, respectively. Consequently, patients with mild renal impairment do not require a dosage adjustment of ARNI. Thus, in patients with moderate to severe renal impairment treatment initiation with 25–50 mg BID is recommended; the dose is increased gradually [53].
Recommendations on Management of Hypovolemia and Hypotension in Patients Initiated on ARNI
Recommendation: If low blood pressure or symptoms of hypotension are present, adjust diuretic and anti-hypertensive medication, address other causes, and rehydrate. Correct hypovolemia before starting ARNI or use low-dose ARNI to reduce the risk of hypotension. If the patient is still hypotensive, reduce the dose or temporarily discontinue ARNI; permanent discontinuation is usually not required.
Summary of evidence: For patients experiencing hypovolemia, it is important to monitor their blood pressure. In case of symptomatic or asymptomatic hypotension (SBP < 100 mmHg) or at risk of hypotension, it is recommended to (a) adjust the dose of anti-hypertensives and diuretics, (b) treat the underlying cause of hypotension, and (c) correct hypovolemia through oral or intravenous rehydration. To reduce the risk of hypotension, it is suggested to correct hypovolemia before starting ARNI or use a low loading dose. Dose reduction or temporary discontinuation of ARNI may be necessary, but permanent discontinuation is usually not required [11, 52].
Recommendation on Management of Hyperkalemia on Initiating ARNI
Recommendation: ARNI therapy may cause elevated potassium levels. Studies show slightly lower hyperkalemia incidence with ARNI compared to enalapril. If hyperkalemia occurs, address risk factors, discontinue potassium supplements and MRA, and use potassium-lowering drugs. ARNI may require temporary adjustment or discontinuation with close potassium monitoring. ARNI can be gradually resumed when potassium levels normalize.
Summary of evidence: The use of ARNI therapy can result in hyperkalemia due to its action on the RAAS system. Hyperkalemia (serum potassium levels above 5 mmol/l) was reported in 12.3% of the PARADIGM-HF patients, while This occurrence was slightly lower than the incidence seen in patients in the enalapril group (13.5%). Similar incidence of hyperkalemia was observed in CKD patients treated with ARNI (32%) or irbesartan (24%, P = 0.10) in the UK HARP-III study. If a patient experiences hyperkalemia, steps should be taken to address any risk factors, discontinue potassium supplementation and MRA use, and utilize potassium-lowering medications as needed. In some cases, it is needed to adjust or stop ARNI therapy until potassium levels return to normal. Close monitoring of serum potassium should be on the clinical checklist. The treating clinician can resume ARNI treatment once potassium levels return to normal (< 5.0 mmol/l) [54, 55].
Recommendation on Management of Patients with Rapid Decline in Renal Function on Starting ARNI
Recommendation: Patients with rapid decline in renal function from ARNI therapy have no specific treatment data, but RAAS inhibitor treatment methods can be used. Determine the cause of the decline, and if creatinine increases less than 30% from baseline, ARNI can continue. Adjust or discontinue ARNI and investigate underlying causes if creatinine exceeds baseline by 30%. Discontinue ARNI if creatinine exceeds baseline by 50%. Address other potential causes before considering ARNI adjustment or discontinuation.
Summary of evidence: Clinical data of ARNI in patients with rapid declining renal function is lacking. Thus, such patients can be treated with ACEIs or ARBs. Step one includes determination of the cause of the declining renal function. If renal artery stenosis is ruled out and the serum creatinine level increases by less than 30% compared to the baseline, ARNI can be continued. However, if serum creatinine increased by 30% from baseline levels, reduce the ARNI dose or discontinue ARNI. In case serum creatinine level increased by 50%, ARNI should be discontinued in such patients. This is based on the results from the PARADIGM-HF study in which renal failure was reported in 5% of ARNI treated patients. When a clinician is treating a patient with rapid declining renal function, other potential causes of renal dysfunction must be treated including hypovolemia, urinary tract infection, before considering a reduction or discontinuation of ARNI therapy [56].
Recommendations on the Use of ARNI in Patients on Maintenance Dialysis
Recommendation: ARNI is advised for heart failure patients receiving maintenance dialysis in order to enhance myocardial remodeling, manage heart failure symptoms, safeguard remaining renal function, and lower the risk of cardiovascular events.
Summary of evidence: There is information on the usage of ARNI in heart failure patients on maintenance dialysis. The maximal blood concentration of LBQ657 was within the safe medication concentration range when ARNI 100 mg BID was used to treat hemodialysis patients with HFrEF or heart failure with midrange ejection fraction, according to clinical research carried out in hemodialysis patients in China (P 0.05) [57]. Another retrospective study with 23 patients further demonstrated that ARNI could increase the left ventricular ejection fraction in dialysis patients with HFrEF [58]. Twenty-one HFpEF patients on peritoneal dialysis patients from a Chinese study with ARNI was found to have a tendency to enhance cardiac function and to considerably improve heart failure symptoms and signs as well as the levels of heart failure markers [59].
Recommendations on Improving Medication Adherence
Recommendations: Dedicated heart failure clinics with regular follow-up with a heart failure nurse can help improve medication adherence. Use of pre-discharge medication checklist is another helpful tool to help improve GDMT implementation.
Summary of evidence: Factors contributing to nonadherence to medication include limited health education, physical limitations, mental health issues, social isolation, specific medical conditions, complex heart failure treatment regimens, polypharmacy, medication side effects, financial burden, lack of social support, and inadequate communication.
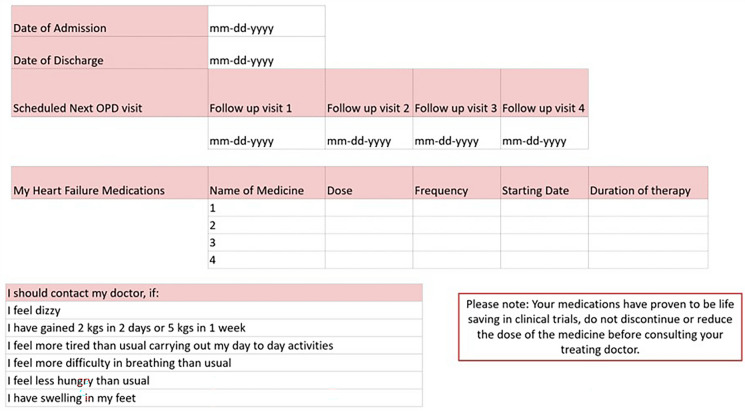
One of the important ways to improve adherence is to take advantage of opportunities to initiate therapy when patients are most likely to adhere to therapy i.e., in-hospital/pre-discharge initiation following decompensation. A pre-discharge medication tracker such as one shown in Fig. 11 will help build accountability [60].
Fig. 11.
Checklist for GDMT for heart failure
Conclusions
The goal of medical management in heart failure is to provide personalized therapy that allows for timely initiation and up-titration of guideline-directed medical treatment to achieve optimally tolerated doses.
Acknowledgements
The authors express their gratitude to Dr. Vidhya Natarajan, Dr. Syed Mujtaba H Naqvi, Dr. Kumar Gaurav, and Dr. Rohit Kumar from the Department of Medical Affairs, Global Generics India (HO), Dr. Reddy's Laboratories, Hyderabad, for organizing and conducting the advisory board meetings, as well as for their editorial and medical writing support. The authors also extend their appreciation to all the doctors who took part in the Advisory Board meetings, as their valuable contributions were instrumental in the development of the recommendations and the final manuscript: Dr A. Purnanand, Dr J Srimannarayana, Dr Anjith. V, Dr P. N. S. Haritha, Dr John Satish, Dr P. Sudarshan, Dr K. Poornachandra Rao, Dr P. Bhaskar Naidu, Dr G. Krishna Mohan, Dr Karthik Naidu, Dr G Somasekhar, Dr S. Madhuri, Dr. GG Shetty, Dr. J Kannan, Dr. Vikram B Kolhari, Dr. Prassanna Katti, Dr. BK Raghunandan, Dr. Shyam Sundar, Dr. Sridhar, Dr. Prabhakar Koregol, Dr. Mahantesh, Dr Kumar Kenchappa, Dr. Niteen Deshpande, Dr. Deepak Bohara, Dr. Rishi Lohiya, Dr. Aziz Khan, Dr. Pankaj Harkut, Dr. Pankaj Manoria, Dr. Ajay Sharma, Dr. Brijesh Shrivastava, Dr. Shriram Agarwal, Dr. Javed Ali Khan, Dr. Smit Shrivastava, Dr. Arvind Pancholia, Dr. Rajeev Kumar Khare, Dr. Siddhant Jain, Dr. Ajit Kumar Sinha, Dr. Satish Kumar, Dr. Priya Ranjan Parida, Dr. Chandrakanta Mishra, Dr. Anup Singh, Dr. KC Mishra, Dr. Giridhari Jena, Dr. Panchanan Sahoo, Dr. Debatra Bose, Dr. Biswakesh Majumder, Dr. Chandan Modak, Dr. Chandra Das, Dr. S Ramamurthy, Dr. Arnab Ganguly, Dr. Bimal Kahar, Dr. D P Raai, Dr. Dipankar Das, Dr. Mrutyunjaya Behera, Dr. Varun Kumar, Dr. Birendra Kumar, Dr. Ashis Ranjan, Dr. Anshu Kumar Agrawal, Dr. Mahesh Agarwal, Dr. Reja MD Wasif Bora, Dr. JK Padhi, Dr. Mayank Agarwal, Dr. Arindam Pande, Dr. Devanu Ghosh Roy, Dr. Dilip Kumar, Dr. Sunil Kumar Lhila, Dr. Kajal Ganguly, Dr. Bijay Prakash Pandey, Dr. Suvro Banerjee, Dr. Dipankar Ghosh Dastidar, Dr. Soumik Chaudhuri, Dr. Bijay Narayan Jha, Dr. Biswajit Majumder, Dr. Aftab Khan, Dr. SK Dutta, Dr. Brajesh Kunwar, Dr Anuj Sathe, Dr Bhaskar Shah, Dr. Anup Taksande, Dr. Sunil Pandrinath Wani, Dr. Ankeet Dedhia, Dr. Zohaib Shaikh, Dr Deepak Shinde, Dr Rakesh Tirmale, Dr. Shakil Shaikh, Dr. Ruchit Shah and Dr. Milan Ghadei
Funding
The advisory board meetings were conducted by Dr. Reddy's Laboratories, Hyderabad, India. The journal’s Rapid Service Fee was supported by Dr. Reddy’s Laboratories.
Medical Writing Assistance
The authors thank IntelliMed Healthcare Solutions, Mumbai, for medical writing support funded by Dr. Reddy’s Laboratories.
Authorship
All authors listed in this article fulfil the criteria set by the International Committee of Medical Journal Editors (ICMJE) for authorship, take responsibility for the integrity of the work, and have provided their consent for the publication of this version.
Author Contributions
Jamshed Dalal, Praveen Chandra and Saumitra Ray substantially contributed to the conception and design of the article. PK Hazra, Vivek Kumar, Jagdish Hiremath, Jabir Abdulla Kutty, Debasis Ghosh, Mahesh K Shah, Karthik Vasudevan and Panchanan Sahoo revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Disclosure
Jamshed Dalal, Praveen Chandra, Saumitra Ray, PK Hazra, Jagdish Hiremath, Vivek Kumar, Mahesh K Shah, Jabir Abdulla Kutty, Debasis Ghosh, Karthik Vasudevan, Panchanan Sahoo have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
- 1.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 3.Harikrishnan S, Bahl A, Roy A, et al. Clinical profile and 90-day outcomes of 10,851 heart failure patients across India: National Heart Failure Registry. ESC Heart Fail. 2022;9:3898–3908. doi: 10.1002/ehf2.14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. [DOI] [PMC free article] [PubMed]
- 6.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304–2322. doi: 10.1016/j.jacc.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi V, Parakh N, Seth S, et al. Heart failure in India: the INDUS (INDia Ukieri Study) study. J Pract Cardiovasc Sci. 2016;2:28. doi: 10.4103/2395-5414.182988. [DOI] [Google Scholar]
- 8.Clinical profile and 90-day outcomes of 10,851 heart failure patients across India: National Heart Failure Registry—Harikrishnan—ESC Heart Failure—Wiley Online Library. 10.1002/ehf2.14096. Accessed 24 Nov 2022. [DOI] [PMC free article] [PubMed]
- 9.Financial burden of heart failure in a developing country: cost analysis from Manipal Heart Failure Registry, India | SpringerLink. 10.1007/s10389-019-01141-w. Accessed 24 Nov 2022.
- 10.Orsborne C, Chaggar PS, Shaw SM, et al. The renin-angiotensin-aldosterone system in heart failure for the non-specialist: the past, the present and the future. Postgrad Med J. 2017;93:29–37. doi: 10.1136/postgradmedj-2016-134045. [DOI] [PubMed] [Google Scholar]
- 11.McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 12.Modin D, Andersen DM, Biering-Sørensen T. Echo and heart failure: when do people need an echo, and when do they need natriuretic peptides? Echo Res Pract. 2018;5:R65–R79. doi: 10.1530/ERP-18-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufmann D, Szwoch M, Kwiatkowska J, et al. Global longitudinal strain can predict heart failure exacerbation in stable outpatients with ischemic left ventricular systolic dysfunction. PLoS ONE. 2019;14:e0225829. doi: 10.1371/journal.pone.0225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur J Heart Fail. 2020;22:391–412. doi: 10.1002/ejhf.1741. [DOI] [PubMed] [Google Scholar]
- 15.Pascual-Figal D, Bayés-Genis A, Beltrán-Troncoso P, et al. Sacubitril-valsartan, clinical benefits and related mechanisms of action in heart failure with reduced ejection fraction. A review. Front Cardiovasc Med. 2021;8:754499. doi: 10.3389/fcvm.2021.754499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.entresto.pdf. https://www.novartis.com/us-en/sites/novartis_us/files/entresto.pdf. Accessed 6 Mar 2023.
- 17.Harikrishnan S, Bahl A, Roy A, et al. National Heart Failure Registry, India: design and methods. Indian Heart J. 2019;71:488–491. doi: 10.1016/j.ihj.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 19.Karlström P, Alehagen U, Boman K, et al. Brain natriuretic peptide-guided treatment does not improve morbidity and mortality in extensively treated patients with chronic heart failure: responders to treatment have a significantly better outcome. Eur J Heart Fail. 2011;13:1096–1103. doi: 10.1093/eurjhf/hfr078. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim NE, Januzzi JL. The future of biomarker-guided therapy for heart failure after the guiding evidence-based therapy using biomarker intensified treatment in heart failure (GUIDE-IT) study. Curr Heart Fail Rep. 2018;15:37–43. doi: 10.1007/s11897-018-0381-0. [DOI] [PubMed] [Google Scholar]
- 21.Langenickel TH, Dole WP. Angiotensin receptor-neprilysin inhibition with LCZ696: a novel approach for the treatment of heart failure. Drug Discov Today Ther Strateg. 2012;9:e131–e139. doi: 10.1016/j.ddstr.2013.11.002. [DOI] [Google Scholar]
- 22.Massari F, Scicchitano P, Iacoviello M, et al. Multiparametric approach to congestion for predicting long-term survival in heart failure. J Cardiol. 2020;75:47–52. doi: 10.1016/j.jjcc.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. https://www.thelancet.com/article/S0140-6736(22)02076-1/fulltext. Accessed 6 Mar 2023. [DOI] [PubMed]
- 25.Greene SJ, Bauersachs J, Brugts JJ, et al. Worsening heart failure: nomenclature, epidemiology, and future directions. J Am Coll Cardiol. 2023;81:413–424. doi: 10.1016/j.jacc.2022.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–1893. doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- 27.Krum H. Prospective comparison of ARNi with ACE-I to determine impact on global mortality and morbidity in heart failure (PARADIGM-HF): paragon of a study or further investigation paramount? Circulation. 2015;131:11–12. doi: 10.1161/CIRCULATIONAHA.114.013887. [DOI] [PubMed] [Google Scholar]
- 28.Lewis EF, Claggett BL, McMurray JJV, et al. Health-related quality of life outcomes in PARADIGM-HF. Circ Heart Fail. 2017;10:e003430. doi: 10.1161/CIRCHEARTFAILURE.116.003430. [DOI] [PubMed] [Google Scholar]
- 29.Iacoviello M, Pugliese R, Correale M, et al. Optimization of drug therapy for heart failure with reduced ejection fraction based on gender. Curr Heart Fail Rep. 2022;19:467–475. doi: 10.1007/s11897-022-00583-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gronda E, Vanoli E, Iacoviello M. The PARAGON-HF trial: the sacubitril/valsartan in heart failure with preserved ejection fraction. Eur Heart J Suppl. 2020;22:L77–L81. doi: 10.1093/eurheartj/suaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin J, Wang W, Wei P, et al. Effects of sacubitril-valsartan on heart failure patients with mid-range ejection fractions: a systematic review and meta-analysis. Front Pharmacol. 2022 doi: 10.3389/fphar.2022.982372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaduganathan M, Mentz RJ, Claggett BL, et al. Sacubitril/valsartan in heart failure with mildly reduced or preserved ejection fraction: a pre-specified participant-level pooled analysis of PARAGLIDE-HF and PARAGON-HF. Eur Heart J. 2023:ehad344. [DOI] [PMC free article] [PubMed]
- 33.Halliday BP, Wassall R, Lota AS, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet. 2019;393:61–73. doi: 10.1016/S0140-6736(18)32484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Januzzi JL, Butler J, Fombu E, et al. Rationale and methods of the prospective study of biomarkers, symptom improvement, and ventricular remodeling during sacubitril/valsartan therapy for heart failure (PROVE-HF) Am Heart J. 2018;199:130–136. doi: 10.1016/j.ahj.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Myhre PL, Claggett BL, Shah AM, et al. Changes in cardiac biomarkers in association with alterations in cardiac structure and function, and health status in heart failure with reduced ejection fraction: the EVALUATE-HF trial. Eur J Heart Fail. 2022;24:1200–1208. doi: 10.1002/ejhf.2541. [DOI] [PubMed] [Google Scholar]
- 36.Mohebi R, Liu Y, Piña IL, et al. Dose-response to sacubitril/valsartan in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2022;80:1529–1541. doi: 10.1016/j.jacc.2022.08.737. [DOI] [PubMed] [Google Scholar]
- 37.Kim H, Oh J, Lee S, et al. Clinical evidence of initiating a very low dose of sacubitril/valsartan: a prospective observational analysis. Sci Rep. 2021;11:16335. doi: 10.1038/s41598-021-95787-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senni M, McMurray JJV, Wachter R, et al. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double-blind, randomized comparison of two uptitration regimens. Eur J Heart Fail. 2016;18:1193–1202. doi: 10.1002/ejhf.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrow DA, Velazquez EJ, DeVore AD, et al. Clinical outcomes in patients with acute decompensated heart failure randomly assigned to sacubitril/valsartan or enalapril in the PIONEER-HF Trial. Circulation. 2019;139:2285–2288. doi: 10.1161/CIRCULATIONAHA.118.039331. [DOI] [PubMed] [Google Scholar]
- 40.Wachter R, Senni M, Belohlavek J, et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019;21:998–1007. doi: 10.1002/ejhf.1498. [DOI] [PubMed] [Google Scholar]
- 41.Di Tano G, Di Lenarda A, Iacoviello M, et al. ANMCO position paper: use of sacubitril/valsartan in hospitalized patients with acute heart failure. Eur Heart J Suppl. 2021;23:C176–C183. doi: 10.1093/eurheartj/suab078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Docherty KF, Vaduganathan M, Solomon SD, et al. Sacubitril/valsartan: neprilysin inhibition 5 years after PARADIGM-HF. JACC Heart Fail. 2020;8:800–810. doi: 10.1016/j.jchf.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pradhan A, Vohra S, Vishwakarma P, et al. Review on sodium-glucose cotransporter 2 inhibitor (SGLT2i) in diabetes mellitus and heart failure. J Family Med Prim Care. 2019;8:1855–1862. doi: 10.4103/jfmpc.jfmpc_232_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomon SD, Jhund PS, Claggett BL, et al. Effect of dapagliflozin in patients with HFrEF treated with sacubitril/valsartan: the DAPA-HF Trial. JACC Heart Fail. 2020;8:811–818. doi: 10.1016/j.jchf.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 45.de la Espriella R, Bayés-Genís A, Morillas H, et al. Renal function dynamics following co-administration of sacubitril/valsartan and empagliflozin in patients with heart failure and type 2 diabetes. ESC Heart Fail. 2020;7:3792–3800. doi: 10.1002/ehf2.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsiao F-C, Lin C-P, Tung Y-C, et al. Combining sodium-glucose cotransporter 2 inhibitors and angiotensin receptor-neprilysin inhibitors in heart failure patients with reduced ejection fraction and diabetes mellitus: a multi-institutional study. Int J Cardiol. 2021;330:91–97. doi: 10.1016/j.ijcard.2021.02.035. [DOI] [PubMed] [Google Scholar]
- 47.Kulmatycki KM, Langenickel T, Ng WH, et al. Pharmacokinetics and safety of sacubitril/valsartan (LCZ696) in patients with mild and moderate hepatic impairment. Int J Clin Pharmacol Ther. 2017;55:728–739. doi: 10.5414/CP202988. [DOI] [PubMed] [Google Scholar]
- 48.Damman K, Gori M, Claggett B, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6:489–498. doi: 10.1016/j.jchf.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Chang H-Y, Feng A-N, Fong M-C, et al. Sacubitril/valsartan in heart failure with reduced ejection fraction patients: real world experience on advanced chronic kidney disease, hypotension, and dose escalation. J Cardiol. 2019;74:372–380. doi: 10.1016/j.jjcc.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Spannella F, Marini M, Giulietti F, et al. Renal effects of sacubitril/valsartan in heart failure with reduced ejection fraction: a real life 1-year follow-up study. Intern Emerg Med. 2019;14:1287–1297. doi: 10.1007/s11739-019-02111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 52.Kang H, Zhang J, Zhang X, et al. Effects of sacubitril/valsartan in patients with heart failure and chronic kidney disease: a meta-analysis. Eur J Pharmacol. 2020;884:173444. doi: 10.1016/j.ejphar.2020.173444. [DOI] [PubMed] [Google Scholar]
- 53.Ayalasomayajula SP, Langenickel TH, Jordaan P, et al. Effect of renal function on the pharmacokinetics of LCZ696 (sacubitril/valsartan), an angiotensin receptor neprilysin inhibitor. Eur J Clin Pharmacol. 2016;72:1065–1073. doi: 10.1007/s00228-016-2072-7. [DOI] [PubMed] [Google Scholar]
- 54.Haynes R, Judge PK, Staplin N, et al. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation. 2018;138:1505–1514. doi: 10.1161/CIRCULATIONAHA.118.034818. [DOI] [PubMed] [Google Scholar]
- 55.Ferreira JP, Mogensen UM, Jhund PS, et al. Serum potassium in the PARADIGM-HF trial. Eur J Heart Fail. 2020;22:2056–2064. doi: 10.1002/ejhf.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fala L. Entresto (sacubitril/valsartan): first-in-class angiotensin receptor neprilysin inhibitor FDA approved for patients with heart failure. Am Health Drug Benefits. 2015;8:330–334. [PMC free article] [PubMed] [Google Scholar]
- 57.Lee S, Oh J, Kim H, et al. Sacubitril/valsartan in patients with heart failure with reduced ejection fraction with end-stage of renal disease. ESC Heart Fail. 2020;7:1125–1129. doi: 10.1002/ehf2.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu S, Xu Z, Lin B, et al. Effects of sacubitril-valsartan in heart failure with preserved ejection fraction in patients undergoing peritoneal dialysis. Front Med (Lausanne) 2021;8:657067. doi: 10.3389/fmed.2021.657067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin J, Ding X, Lin P, et al. A multi-center survey of hypertension and its treatment in patients with maintenance hemodialysis in Shanghai. Zhonghua Nei Ke Za Zhi. 2010;49:563–567. [PubMed] [Google Scholar]
- 60.Schofield T, Bhatia RS, Yin C, et al. Patient experiences using a novel tool to improve care transitions in patients with heart failure: a qualitative analysis. BMJ Open. 2019;9:e026822. doi: 10.1136/bmjopen-2018-026822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.