Abstract
BACKGROUND:
The current methodology used to detect, diagnose, and monitor many types of cancers requires invasive tissue biopsy testing. Recently, liquid biopsy using blood, plasma, urine, saliva, and various other bodily fluids has shown utility to solve many issues associated with tissue biopsy. Blood/plasma has received most of the attention within the liquid biopsy field, however, obtaining blood samples from patients is still somewhat invasive and requires trained professionals. Using urine to detect cell-free DNA cancer biomarkers offers a truly non-invasive sampling method that can be easily and reproducibly conducted by patients.
CONTENT:
Novel technologies and approaches have made the detection of small quantities of cell-free tumor DNA of varying lengths possible. Recent studies using urine circulating tumor DNA to detect cancer mutations and other biomarkers have shown sensitivity comparable to blood/plasma cell-free DNA liquid biopsy for many cancer types. Thus, urine cell-free DNA liquid biopsy may replace or provide supplementary information to tissue/blood biopsies. Further investigation with larger patient cohorts and standardization of pre-analytical factors is necessary to determine the utility of urine cell-free DNA liquid biopsy for cancer detection, diagnosis, and monitoring in a clinical setting.
SUMMARY:
In this mini-review we discuss the biological aspects of cell-free DNA in urine, numerous studies using urine cell-free DNA to detect urological cancers, and recent studies using urine cell-free DNA to detect and monitor non-urological cancers including lung, breast, colorectal, and other cancers.
Introduction
Following various imaging tests, tissue biopsy is the standard approach for cancer diagnosis and aids in treatment planning. However, tissue biopsy requires invasive procedures; it only provides a snapshot of the molecular aberration in the tumor, which does not reflect tumor heterogeneity. Multiple tissue biopsies are impractical for disease monitoring (1). Liquid biopsy, an emerging field in the past decade, has the potential to solve many of the problems associated with tissue biopsy and could be a better method for cancer screening. Many promising cancer biomarkers are being investigated that may be applicable for liquid biopsy testing. Cell-free DNA (cfDNA) and circulating tumor cells (CTCs) have been the primary biomarker targets that are present in different bodily fluids such as blood/plasma, urine, saliva, cerebral spinal fluid (CSF), and more (2). While CTCs can provide DNA, RNA, and protein for analysis, they are very rare, lack cancer heterogeneity, and there is not a standardized isolation method. Although circulating tumor DNA (ctDNA) can be contaminated with DNA from normal cells including circulating blood cells, it is easier to isolate, represents tumor heterogeneity, and is more sensitive for treatment monitoring and determining tumor burden (1). Due to these factors, ctDNA seems to have greater clinical utility and ease of access than cellular tumor DNA from CTCs. So far, blood has been studied most, but acquiring this source of liquid biopsy material is invasive and requires a trained professional to obtain samples from patients (3). Urine samples are easily accessible, have high patient compliance, and represent the body’s health due to the constant filtration of blood via the kidneys (4). Thus, liquid biopsy using urine cell-free DNA (ucfDNA) is less invasive, and patients can frequently collect large volumes of urine at home (3). Since 2005, the total number of Food and Drug Administration (FDA) and European Medicines Agency (EMA) clinical trials involving ucfDNA analysis is only 20 compared to 404 for serum/plasma cfDNA (5). Overall, cfDNA analysis in urine has received far less attention than in blood, yet some recent studies show similar cancer mutation detection rates between blood and urine cfDNA (6, 7). With continued ucfDNA research and the standardization of pre-analytical and analytical factors for urine processing, this form of liquid biopsy could increase early cancer detection, improve disease monitoring, and provide real-time treatment efficacy to physicians. In this review, we highlight the current understanding of the biology of ucfDNA and recent studies using ucfDNA as a biomarker for urological and non-urological cancers.
THE CHARACTERISTICS AND BIOLOGY OF URINE CFDNA
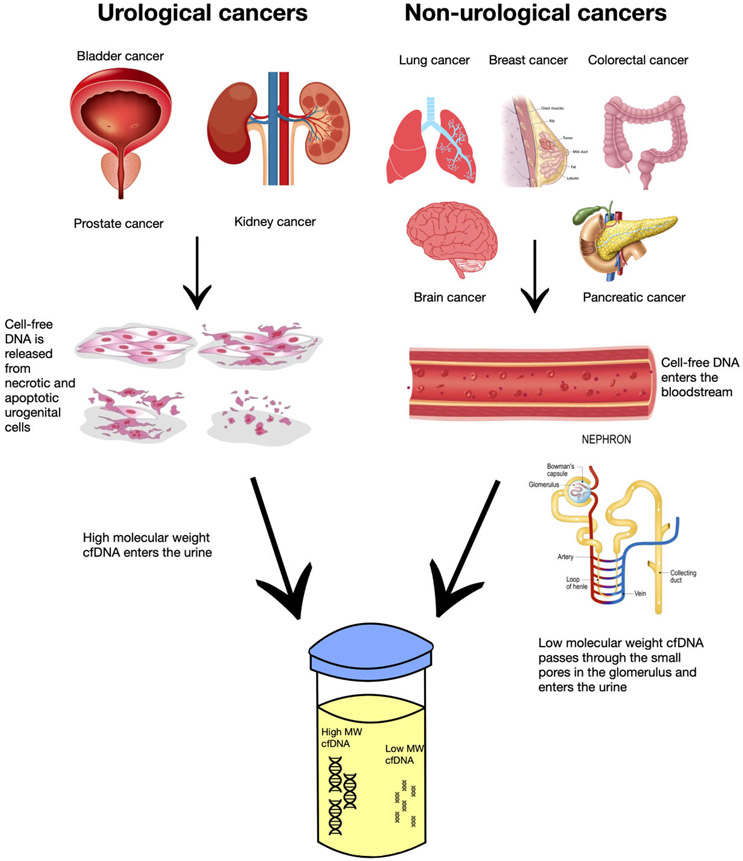
Urine contains many compounds such as cells, salts, and cell-free nucleic acids like DNA, mRNA, micro-RNAs (miRNAs), and long non-coding RNAs (lncRNAs) (8). These circulating cell-free nucleic acids are found at various concentrations in the urine. For example, the concentration of RNA is reported to be 20–140 ng/mL and miRNAs may be more resistant to nucleases because of their short size, but exact concentrations are unknown (9). Thus, additional studies are needed to evaluate the concentrations of various RNAs in urine. Cell-free DNA concentrations range from 1 to 200 ng/mL (10) and they can accumulate in the urine via multiple processes (Fig. 1). The cfDNA can be directly released into the urine by necrotic and apoptotic cells of the urogenital system. Additionally, it can be transported through the blood and filtered into the urine by the kidneys, and cells can secrete them in exosomes. However, the exact biological mechanisms are still unclear and may differ between cancer types, prompting further investigation (9). In healthy individuals, circulating cfDNA concentration is relatively low, but this concentration can increase when the body undergoes various forms of stress such as cancer, resulting in up to a 10 times increase of cfDNA compared to healthy control levels (11). Urine cfDNA has 2 distinct size groups: high molecular weight, greater than 1000 bp, and low molecular weight, typically from 40 to 250 bp (12). Su and coworkers isolated both sizes of urine cfDNA while studying samples from colorectal carcinoma patients and concluded that the 150 to 250 bp DNA originated, at least in part, from circulation, while the large fragments originated from urinary tract cells (13). Because urine can contain both local ctDNA from urological cancer cells and ctDNA in circulation from non-urological cancers, it may be a better alternative for disease monitoring than blood/tissue testing (11). For many non-urological cancers, most urine ctDNA (uctDNA) is below 100 bp in size, likely because glomerular filtration within the kidneys restricts ctDNA fragments greater than 70 kDa (double stranded of 107 bp in size) from passing through the nephron (12). Markus et al. confirmed this by performing whole-genome sequencing and found the modal size of urine cfDNA to be 80 to 81 bp and successive peaks at 10 bp intervals, indicating some form of cfDNA protection via protein/histone association in urine (14). Therefore, because only these limited-sized compounds can pass the barrier, urine is considered “cleaner” than plasma/serum due to lower concentrations of proteins and cells, making ucfDNA isolation less complex (13). However, in urine, nucleic acids are subject to higher levels of DNase activity, which breaks down the cfDNA fragments (8). However, the amount of nucleases present in the urine may provide us with another helpful biomarker. Zhou and coworkers found a greater concentration of ucfDNA with jagged ends (double-stranded DNA with single-stranded overhangs) than plasma cfDNA, likely due to different DNase activity levels. In urine samples from bladder cancer patients, they found lower levels of ucfDNA with jagged ends than healthy volunteers, potentially due to cancer-induced decreased nuclease activity (15). Also, Yao and colleagues studied the half-life of cfDNA in urine, serum, and saliva and found the half-life to be extremely short and immeasurable for urine cfDNA (16). Thus, pre-analytical factors such as sample collection, sample volume, processing time, preservatives, and first void urine may have a large impact on the quantity of cfDNA recovered and thus affect sensitivity and specificity of analytical assays. In a pilot study, Augustus and coworkers have studied many of these pre-analytical variables and offer recommendations to maximize ucfDNA quantity. They found that the first void urine is not the only portion with a high cfDNA concentration, that fresh urine should be processed right away or Streck preservative should be added, and urine samples should be taken more than 1.5 h apart, among other findings (17). Another important pre-analytical factor for ucfDNA liquid biopsy is the method of DNA isolation. Oreskovic and colleagues have studied different cfDNA extraction methods and found the Qiagen QIAamp Circulating Nucleic Acid Kit to poorly recover ucfDNA fragments below 150 bp. They also concluded that the Q Sepharose extraction method can recover ucfDNA over 40 bp and is the best method for next-generation sequencing (NGS) and single-stranded library preparation (10). For ucfDNA isolation in bladder cancer, the QIAamp Circulating Nucleic Acid Kit is the most used, although no consensus has been made on the optimal kit yet (18). This is concerning because the Qiagen kit may not detect ultra-short ucfDNA in non-urological cancer studies. New analytical technologies such as quantitative PCR (qPCR), droplet digital PCR (ddPCR), and NGS have allowed for more accurate detection of smaller cfDNA quantities and fragments, leading to advancements in this field (19). Further standardization of pre-analytical and analytical techniques will allow for the continuation of advances within the ucfDNA analysis field for both urological and non-urological cancers.
Fig. 1.
Urinary cell-free DNA enters the urine through different processes and in various sizes. Cell-free DNA from non-urological cancers enters the bloodstream and passes through the kidneys. Only low molecular weight (typically 40 to 250 bp) cfDNA can pass through the small pores during glomerular filtration into the urine. High molecular weight (typically greater than 1000 bp) cfDNA from urological cancers originates from necrotic and apoptotic cells in the urogenital system and is released directly into the urine.
UROLOGICAL CANCERS
Bladder cancer.
Most ucfDNA liquid biopsy studies have focused on urological cancers because the majority of ucfDNA comes from dying cells of the urogenital system (20). These cancers include bladder and kidney, male reproductive organs, and prostate cancers (Table 1). Bladder cancer cells are in direct contact with urine; therefore, cellular necrosis introduces longer cfDNA fragments that current analytical techniques can more easily detect (31). Casadio and coworkers assessed the integrity of >250 bp ucfDNA in early-stage bladder cancer patients by measuring 3 common bladder cancer gene biomarkers from the urine supernatant: MYC, ERBB2, and BCAS1. DNA integrity is a marker for necrotic cancer cells because DNA from normal apoptotic cells is highly fragmented (typically shorter that 250 bp), while necrotic cancer cells release long, unfragmented DNA. Integrity is a calculated ratio comparing the concentrations of long ucfDNA to short ucfDNA, and in this study, the DNA integrity ratio increased 40-fold in cancer patients. Analysis was performed using qPCR and resulted in a sensitivity of 73% with a specificity of 84% for symptomatic bladder cancer patients, showing promising results for early bladder cancer diagnosis using ucfDNA integrity analysis (21).
Table 1.
Urine cell-free DNA liquid biopsy studies for urological cancers.a
| Cancer type |
Cancer patients enrolled |
Assessed biomarkers | ucfDNA analytical technique | Study purpose | Tumor mutation profiling comparison | Year | Reference |
|---|---|---|---|---|---|---|---|
| Bladder | 51 | ucfDNA integrity | qPCR | Cancer diagnosis | 73% sensitivity and 84% specificity with healthy patients | 2013 | Casadio et al. (21) |
| Bladder | 104 | TERT 228 G > A/T mutation | ddPCR | Cancer detection | 92% concordance with tissue, 96% specificity | 2018 | Russo et al.(22) |
| Bladder | 92 | 48 bladder-cancer specific genes | NGS | Cancer detection | N/A | 2020 | Ou et al.(23) |
| Bladder | 46 | ucfDNA methylation and copy number alterations | Shallow-depth genome-wide bisulfite sequencing | Cancer detection, diagnosis, monitoring | 93.5% sensitivity and 95.8% specificity | 2019 | Cheng et al. (24) |
| Bladder | 54 | utDNA mutations | Novel high-throughput sequencing method (uCAPP-Seq) | Cancer detection & monitoring | 93% sensitivity and 100% specificity for tumor mutation-informed approach, 84% sensitivity and 96% specificity when blinded | 2019 | Dudley et al. (25) |
| Bladder | 47 | 22 bladder cancer-related genes, 740 mutation hotspots | Cell-free single-molecule unique primer extension resequencing (cf-SUPER) | Cancer detection | 82.7% sensitivity and 89.6% specificity | 2020 | Zhao et al.(26) |
| Bladder | 56 | TERT promoter and FGFR3 mutations | ddPCR | Cancer diagnosis | 55.4% sensitivity and 100% specificity | 2019 | Hayashi et al. (27) |
| Renal | 91 | Common renal cancer mutations | sWGS | Characterization of urine cell-free tumor DNA | Roughly 50% for a targeted approach using plasma and urine | 2020 | Smith et al.(28) |
| Renal and Bladder | 120 | cfDNA differentially methylated regions | Cell-free methylated DNA immunoprecipitation and high-throughput sequencing (cfMeDIP-seq) | Cancer detection | 0.858 AUROC | 2020 | Nuzzo et al. (29) |
| Prostate | 67 | ucfDNA integrity | qPCR | Cancer detection | 58% sensitivity and 44% specificity | 2015 | Salvi et al. (30) |
Abbreviations: cfDNA, cell-free DNA; ddPCR, droplet digital PCR; NGS, next-generation sequencing; qPCR, quantitative PCR; sWGS, shallow whole genome sequencing; ucfDNA, urinary cell-free DNA; utDNA, urine tumor DNA; AUROC, area under the receiver operating characteristic; N/A, not performed.
Other studies have analyzed common genomic alterations in bladder cancer and shown that ucfDNA mutation analysis may have clinical relevance. Russo and colleagues attempted to detect TERT promoter mutations in ucfDNA using ddPCR and targeted NGS on matched tumor specimens. This mutation is found in over 75% of bladder tumors, which likely contributed to the observed 92% concordance between tissue samples and ucfDNA having the TERT 228 G > A/T mutation (22). In another study, Ou and coworkers collected 10 to 50 mL of urine from patients and developed a 5 gene panel for detecting bladder cancer from ucfDNA present in 2 mL of urine supernatant using a NGS assay. This pilot study showed a better concordance between this 5 gene ucfDNA panel (TERT, FGFR3, TP53, PIK3CA, and KRAS) and cancer tissue than plasma’s concordance with cancer tissue. This is an important step in the development in a non-invasive diagnostic clinical test because other urine liquid biopsy tests such as bladder tumor antigen, cytology, and fluorescence in situ hybridization show limited sensitivity/specificity and are not accepted for clinical diagnosis (23). Additionally, Hayashi and colleagues had analyzed TERT promoter and FGFR3 mutations in ucfDNA using ddPCR and found a sensitivity of 78% in combination with cytology results for diagnosing patients with upper tract urothelial carcinoma (UTUC). Urine cytology is the only non-invasive diagnostic method that is currently recommended for UTUC detection, yet it has only a 40% sensitivity (27). These findings show promising potential for using non-invasive ucfDNA genomic alteration analysis to detect bladder cancers.
Also, some researchers have developed novel approaches to detect bladder cancers in ucfDNA. For example, Cheng and colleagues used a novel technology called shallow-depth genome-wide bisulfite sequencing to detect copy number alterations and ucfDNA methylation at a 93.5% sensitivity from 20 mL of morning voided urine from bladder cancer patients. These results show an increased sensitivity compared to conventional urine cytology, especially for tumors of low grade (24). Another novel technology designed by Dudley and coworkers for early-stage non-muscle-invasive bladder cancer diagnosis is a high-throughput sequencing method to detect urine tumor DNA, referred to as utDNA cancer personalized profiling by deep sequencing (uCAPP-Seq). This targeted-sequencing, mutation-informed approach utilizes prior sequencing knowledge from patients’ tumors and germline tissue and tests urine samples for these mutations. Conversely, the tumor-naïve approach detects driver mutations without knowing the patient’s tumor genotype. The targeted-sequencing method detected 93% of cancer cases before treatment and the tumor-naïve approach detected 84%. They also found PLEKHS1 gene promoter mutations in 46% of the patients with bladder cancer, indicating the potential clinical utility of these mutations as bladder cancer biomarkers (25). Another method, cell-free single-molecule unique primer extension resequencing (cf-SUPER), was developed by Zhao and coworkers to look for mutation-harboring ucfDNA fragments, using as little as 1 ng of DNA. They used 22 bladder cancer-related genes and analyzed 740 mutation hotspots to detect mutations in ucfDNA and tissue samples with over 82% sensitivity (26). These approaches have attempted to solve problems with the more conventional methods described previously. While they show promise, further validation with independent, larger patient cohorts needs to be conducted to confirm the performance of these advanced approaches.
Renal cell cancer.
A few studies have investigated kidney cancers in the ucfDNA liquid biopsy field. Smith and coworkers used both targeted and untargeted sequencing methods to detect renal cell carcinoma (RCC) from plasma and urine cfDNA. The targeted approach sequenced a 10-gene panel consisting of the most common mutated genes in RCC patients while the untargeted approach employed genome-wide sequencing. Overall, they found that there are low levels of ctDNA for RCC, yet there is some evidence that uctDNA may be better than tissue biopsy at representing tumor heterogeneity (28). Another assay designed by Nuzzo and colleagues called cell-free methylated DNA immunoprecipitation and high-throughput sequencing (cfMeDIP-seq) can detect early-stage RCC from plasma and urine samples using a small amount of DNA (≤10 ng). The ucfDNA from samples was used to correctly classify RCC patients and controls with area under the receiver operating characteristic (AUROC) of 0.858, which is comparable to the 0.99 AUROC shown for plasma cfDNA (29). These preliminary studies show some promise for ucfDNA in RCC detection, however, there was a small number of participants in these studies, and the evidence for clinical usage is not overwhelming.
Prostate cancer.
In prostate cancer, Salvi and coworkers evaluated cfDNA integrity like Casadio and colleagues did in their study on bladder cancer patients and found that ucfDNA integrity only had a 58% sensitivity compared to 95% sensitivity for prostate-specific antigen levels (30). Their results indicate that ucfDNA integrity is not a reliable biomarker for prostate cancer diagnosis compared to conventional methods. Very few studies have tried using ucfDNA as a biomarker for prostate cancer, so further investigation is required to determine if there is a clinical utility for a ucfDNA liquid biopsy detection approach for prostate cancers.
NON-UROLOGICAL CANCERS
Lung cancer.
As the field of urine liquid biopsy expands, researchers have begun exploring the presence of ctDNA in urine for non-urological cancers (Table 2). Interest in detecting uctDNA mutations in lung cancer patients has grown due to the prevalence of lung cancer. For example, Reckamp and coworkers detected EGFR L858R and T790M mutations from ctDNA in the urine of patients with metastatic non-small cell lung cancer (NSCLC) at 75% sensitivity and 72% sensitivity, respectively, compared to tissue samples. This study marks the first use of uctDNA to detect EGFR mutations in NSCLC patients. Additionally, they found increased sensitivity when urine volumes of 90 to 100 mL were collected, opposed to less than 90 mL, showing the need for pre-analytical standardization of urine collection (32). Husain and colleagues also detected T790M mutant DNA fragments and monitored uctDNA levels after the administration of osimertinib, an anti-epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor. Their findings showed that ucfDNA could make the assessment of patient drug response more accessible and could assist in future drug development studies (33). In another lung cancer study, Xie and group detected KRAS mutations in advanced-stage NSCLC patients using ucfDNA at a 93% concordance with tumor tissue and found significantly higher overall cfDNA levels in the KRAS-mutated patients vs the wild-type KRAS patients (34). This study reports one of the highest concordances with tumor tissue for urine cfDNA. Another more recent pilot study conducted by Satapathy and coworkers found a reduced sensitivity of 60% when performing ddPCR analysis of urine samples from adenocarcinoma patients compared to tissue analysis. There is a chance this lower sensitivity could be due to pre-analytical variation because small urine volumes were used (30 mL), and the samples were without preservatives, such as EDTA, yet, testing multiple liquid biopsy samples increased overall sensitivity (35). These preliminary studies show the possible utility of using ucfDNA for lung cancer detection and monitoring in future patients, but further clinical studies are needed to solidify the efficacy of ucfDNA liquid biopsy.
Table 2.
Non-urological cancer urine cell-free DNA liquid biopsy studies.a
| Cancer type |
Cancer patients enrolled |
Assessed biomarkers | ucfDNA analytical technique |
Study purpose | Tumor mutation profiling comparison |
Year | Reference |
|---|---|---|---|---|---|---|---|
| NSCLC | 63 | EGFR (L858R, T790M) mutations | ddPCR, NGS | Rociletinib (EGFR TKI) response | L858R: 75% sensitivity and 100% specificity T790M: 72% sensitivity and 96% specificity |
2016 | Reckamp et al. (32) |
| NSCLC | 9 | EGFR mutations | ddPCR, NGS | Osimertinib (EGFR TKI) response | N/A | 2017 | Husain et al. (33) |
| NSCLC | 150 | KRAS mutation | ddPCR | Predicting mutations and outcomes | 93% concordance with tissue | 2018 | Xie et al. (34) |
| NSCLC | 81 | EGFR mutations | ddPCR | Diagnosis and management | 60% sensitivity and 100% specificity | 2021 | Satapathy et al. (35) |
| Breast | 250 | PIK3CA mutations | ddPCR | Detection, monitoring, disease relapse | 91% sensitivity and 100% specificity | 2020 | Zuo et al. (7) |
| Breast | 200 | PIK3CA mutations | ddPCR | Treatment and disease relapse | 77% sensitivity | 2020 | Zhang et al. (36) |
| Breast | 300 | PIK3CA and TP53 mutations | N/A | Cancer monitoring | 97% concordance with tissue biopsy | 2020 | Guan et al.(37) |
| Colorectal | 20 | KRAS mutations | qPCR, RE-PCR | Cancer detection | 95% sensitivity (200 μL fluid used) | 2008 | Su et al. (6) |
| Colorectal | 92 | cfDNA (SEPTIN9 & SDC2) methylation levels | qMSP | Cancer detection | 70% sensitivity and 86% specificity | 2021 | Bach et al.(38) |
| Glioma | 35 | 52 commonly mutated glioma genes, fragmentation patterns | WES, sWGS | Cancer detection | N/A | 2021 | Mouliere et al. (39) |
| Pancreatic | 56 | KRAS mutations | ddPCR | Cancer detection | 42% sensitivity | 2019 | Terasawa et al. (40) |
Abbreviations: NSCLC, non-small cell lung cancer; ddPCR, droplet digital PCR; NGS, next-generation sequencing; EGFR, epidermal growth factor receptor; cfDNA, cell-free DNA; qPCR, quantitative PCR; RE-PCR, restriction enriched polymerase chain reaction; qMSP, quantitative methylation specific PCR; WES, whole-exome sequencing; sWGS, shallow whole-genome sequencing; N/A, not performed.
Breast cancer.
Urinary cfDNA in breast cancer patients has received increasing attention, with a few recent studies testing ucfDNA liquid biopsy in early breast cancer patients. Zuo and colleagues used ddPCR to detect PIK3CA mutations in ucfDNA with a sensitivity of 91% compared to tissue biopsy analysis, while plasma showed a 93% sensitivity. Thus, urine and plasma samples showed similar rates of ctDNA mutation detection, and 240 of the 250 early-stage breast patients showed identical results between urine and plasma (7). Zhang and coworkers also tested urine and plasma ctDNA PIK3CA mutations with ddPCR and concluded over 77% sensitivity compared to tissue biopsy. Additionally, they showed a significant drop in uctDNA after receiving treatment (36). Guan et al. showed a concordance of over 97% with tissue biopsy samples and early breast cancer patients have higher ucfDNA levels than the healthy controls (37). These studies indicate that ucfDNA quantity and mutations could be useful biomarkers in the early detection of breast cancer and monitoring of disease progression.
Colorectal cancer.
Also, urine cfDNA studies have identified a few biomarkers for colorectal cancer (CRC) detection and have shown promising results. Su and group compared detection rates of KRAS-mutated cfDNA in urine, plasma, and serum of colorectal carcinoma patients and found significantly higher levels of low molecular weight cfDNA in urine and serum compared to plasma. When using 200 μL of bodily fluid, urine had a 95% sensitivity which was significantly higher than serum (35%) and plasma (40%), but when using only 10 μL of bodily fluid, all fluids showed comparable sensitivity. Long circulating DNA and proteins in the blood may account for the lack of sensitivity increase when using greater bodily fluid concentrations, possibly because of PCR amplification inhibition. Another interesting finding was that most of the detected mutated ucfDNA molecules were smaller than 700 bp in length (6). In a more recent study, Bach and coworkers measured the cfDNA methylation levels of 6 CRC-associated markers in 40 mL of urine from colorectal cancer patients and healthy volunteers. Using the SEPTIN9 and SDC2 methylation markers, detection of 70% of CRC cases was possible with 86% specificity from the urine supernatant. These levels of detection came close to the levels seen for SEPTIN9 methylation CRC detection in plasma (75% to 81%), for which there is an FDA-approved test available (38). With further research, a similar, less invasive, urine-based test may soon be available as well.
Other cancers.
Other cancer types, such as brain cancer, have received attention in the urine liquid biopsy field recently. Mouliere and colleagues investigated 35 glioma patients’ ctDNA in CSF, plasma, and urine using shallow whole-genome sequencing and found over a 2-fold increase in ucfDNA concentration for glioma patients vs controls and concluded that ucfDNA is significantly shorter (101 bp) and more fragmented than in healthy (137 bp) controls. While specific mutation detection was not spectacular, the fragmentation pattern seen in uctDNA may be an important diagnostic biomarker for potential glioma patients (39). In pancreatic cancer, Terasawa and group used ddPCR to detect cfDNA KRAS mutations from urine and plasma samples of 56 patients with pancreatic ductal adenocarcinoma. KRAS mutations were found in 42% of cases for both urine and plasma and urine proved to have higher sensitivity in patients suffering from renal function degeneracy (40). This is one of the only studies using ucfDNA to detect pancreatic cancers, yet the evidence shows substituting urine for blood as a liquid biopsy sample may be as sensitive, if not more sensitive. Further investigation is required to determine if there is clinical utility for a urine cfDNA liquid biopsy detection approach of pancreatic cancers.
Conclusion
For both urological and non-urological cancers, urine liquid biopsy using cfDNA has shown its value and promise for cancer detection, monitoring cancer progression, and development of metastases. Compared to blood/plasma cfDNA liquid biopsy, much research still needs to be conducted to fully understand the utility and intricacies of this relatively new liquid biopsy field. For example, the mechanism by which non-urological circulating cfDNA passes through the pores of the glomerulus is still unclear and requires further exploration. Urine liquid biopsy concordance with tissue has shown similar results to blood liquid biopsy, thus warranting the continuation of research because of the non-invasive nature and ease of urine collection as opposed to blood. However, while urine sample collection is simple compared to other bodily fluids, this may result in more pre-analytical variability. Pre-analytical variability coupled with rapid degradation of ucfDNA from high nuclease activity may pose challenges in reproducibility between urine liquid biopsy studies. Exploration and standardization of the best collection devices, preservatives, and extraction methods are still needed to limit this variability. Further, increasing the size of patient cohorts and using new, sensitive genetic analysis technologies may help urine cfDNA liquid biopsy become a regular clinical testing procedure. For example, single-strand library preparation could help us further investigate the newfound jagged ends present in ucfDNA. With supplemental research, new biomarkers and technologies may be discovered, such as cf-SUPER, which has allowed for high-sensitivity detection of bladder uctDNA at low DNA input concentrations. Novel methods designed to extract and evaluate the low molecular weight, ultra-short cfDNA may advance the ucfDNA liquid biopsy field for non-urological cancers as well. In summary, we believe urinary cfDNA is a promising biomarker for liquid biopsy in cancer management.
Research Funding:
D. Wong, NIH U01CA233370, NIH UG3TR002978, NIH R21CA239052, NIH UH3CA206126; F. Li, NIH R21CA239052, R21CA239052.
Nonstandard Abbreviations:
- cfDNA
cell-free DNA
- CTCs
circulating tumor cells
- ctDNA
circulating tumor DNA
- ucfDNA
urine cell-free DNA
- uctDNA
urine circulating tumor DNA
- NGS
next-generation sequencing
- ddPCR
droplet digital PCR
- RCC
renal cell carcinoma
- CRC
colorectal cancer
Human Genes
- MYC
myc proto-oncogene bHLH transcription factor
- ERBB2
erb-b2 receptor tyrosine kinase 2
- BCAS1
brain enriched myelin associated protein 1
- TERT
telomerase reverse transcriptase
- FGFR3
fibroblast growth factor receptor 3
- TP53
tumor protein p53
- PIK3CA
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha
- KRAS
KRAS proto-oncogene, GTPase
- PLEKHS1
pleckstrin homology domain containing S1
- EGFR
epidermal growth factor receptor
- SEPTIN9
septin 9
- SDC2
syndecan 2
Footnotes
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form.
Disclosures and/or potential conflicts of interest:
Employment or Leadership: David Wong has equity in Liquid Diagnostics LLC and RNAmeTRIX. He is consultant to Colgate-Palmolive and GSKDr. Liying Zhang reports that family members hold leadership positions and ownership interests of Decipher Medicine. All other other authors do not have any conflict of interest.
Consultant or Advisory Role: None declared.
Stock Ownership: L. Zhang reports that family members hold ownership interests in Decipher Medicine.
Honoraria: None declared.
Expert Testimony: None declared.
Patents: None declared.
References
- 1.Lim M, Kim CJ, Sunkara V, Kim MH, Cho YK. Liquid biopsy in lung cancer: clinical applications of circulating biomarkers (CTCs and ctDNA). Micromachines (Basel) 2018;9:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardelli A, Pantel K. Liquid biopsies, what we do not know (yet). Cancer Cell 2017;31:172–9. [DOI] [PubMed] [Google Scholar]
- 3.Jain S, Lin SY, Song W, Su YH. Urine-based liquid biopsy for nonurological cancers. Genet Test Mol Biomarkers 2019;23:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara F, Zoupanou S, Primiceri E, Ali Z, Chiriaco MS. Beyond liquid biopsy: toward non-invasive assays for distanced cancer diagnostics in pandemics. Biosens Bioelectron 2022;196:113698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cisneros-Villanueva M, Hidalgo-Pérez L, Rios-Romero M, Cedro-Tanda A, Ruiz-Villavicencio CA, Page K, et al. Cell-free DNA analysis in current cancer clinical trials: a review. Br J Cancer 2022;126:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su YH, Wang M, Brenner DE, Norton PA, Block TM. Detection of mutated K-ras DNA in urine, plasma, and serum of patients with colorectal carcinoma or adenomatous polyps. Ann N Y Acad Sci 2008;1137:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuo Z, Tang J, Cai X, Ke F, Shi Z. Probing of breast cancer using a combination of plasma and urinary circulating cell-free DNA. Biosci Rep 2020;40:BSR20194306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oshi M, Murthy V, Takahashi H, Huyser M, Okano M, Tokumaru Y, et al. Urine as a source of liquid biopsy for cancer. Cancers (Basel) 2021;13:2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryzgunova OE, Laktionov PP. Extracellular nucleic acids in urine: sources, structure, diagnostic potential. Acta Naturae 2015;7:48–54. [PMC free article] [PubMed] [Google Scholar]
- 10.Oreskovic A, Brault ND, Panpradist N, Lai JJ, Lutz BR. Analytical comparison of methods for extraction of short cell-free DNA from urine. J Mol Diagn 2019;21:1067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner B, Warton K, Ford CE. Transcending blood–opportunities for alternate liquid biopsies in oncology. Cancers (Basel) 2022;14:1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Udomruk S, Orrapin S, Pruksakorn D, Chaiyawat P. Size distribution of cell-free DNA in oncology. Crit Rev Oncol Hematol 2021;166:103455. [DOI] [PubMed] [Google Scholar]
- 13.Su YH, Wang M, Brenner DE, Ng A, Melkonyan H, Umansky S, et al. Human urine contains small, 150 to 250 nucleotide-sized, soluble DNA derived from the circulation and may be useful in the detection of colorectal cancer. J Mol Diagn 2004;6:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markus H, Zhao J, Contente-Cuomo T, Stephens MD, Raupach E, Odenheimer-Bergman A, et al. Analysis of recurrently protected genomic regions in cell-free DNA found in urine. Sci Transl Med 2021;13:eaaz3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Z, Cheng SH, Ding SC, Heung MMS, Xie T, Cheng THT, et al. Jagged ends of urinary cell-free DNA: characterization and feasibility assessment in bladder cancer detection. Clin Chem 2021;67:621–30. [DOI] [PubMed] [Google Scholar]
- 16.Yao W, Mei C, Nan X, Hui L. Evaluation and comparison of in vitro degradation kinetics of DNA in serum, urine and saliva: a qualitative study. Gene 2016; 590:142–8. [DOI] [PubMed] [Google Scholar]
- 17.Augustus E, Van Casteren K, Sorber L, van Dam P, Roeyen G, Peeters M, et al. The art of obtaining a high yield of cell-free DNA from urine. PLoS One 2020;15:e0231058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herranz R, Oto J, Plana E, Fernández-Pardo Á, Cana F, Martínez-Sarmiento M, et al. Circulating cell-free DNA in liquid biopsies as potential biomarker for bladder cancer: a systematic review. Cancers (Basel) 2021;13:1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha S, Araf Y, Promon SK. Circulating tumor DNA in cancer diagnosis, monitoring, and prognosis. J Egypt Natl Canc Inst 2022;34:8. [DOI] [PubMed] [Google Scholar]
- 20.Lu T, Li J. Clinical applications of urinary cell-free DNA in cancer: current insights and promising future. Am J Cancer Res 2017;7:2318–32. [PMC free article] [PubMed] [Google Scholar]
- 21.Casadio V, Calistri D, Tebaldi M, Bravaccini S, Gunelli R, Martorana G, et al. Urine cell-free DNA integrity as a marker for early bladder cancer diagnosis: preliminary data. Urol Oncol 2013;31:1744–50. [DOI] [PubMed] [Google Scholar]
- 22.Russo IJ, Ju Y, Gordon NS, Zeegers MP, Cheng KK, James ND, et al. Toward personalised liquid biopsies for urothelial carcinoma: characterisation of ddPCR and urinary cfDNA for the detection of the TERT 228 G > A/T mutation. Bladder Cancer 2018;4:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ou Z, Li K, Yang T, Dai Y, Chandra M, Ning J, et al. Detection of bladder cancer using urinary cell-free DNA and cellular DNA. Clin Transl Med 2020;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng THT, Jiang P, Teoh JYC, Heung MMS, Tam JCW, Sun X, et al. Noninvasive detection of bladder cancer by shallow-depth genome-wide bisulfite sequencing of urinary cell-free DNA for methylation and copy number profiling. Clin Chem 2019;65:927–36. [DOI] [PubMed] [Google Scholar]
- 25.Dudley JC, Schroers-Martin J, Lazzareschi DV, Shi WY, Chen SB, Esfahani MS, et al. Detection and surveillance of bladder cancer using urine tumor DNA. Cancer Discov 2019;9:500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao C, Pan Y, Wang Y, Li Y, Han W, Lu L, et al. A novel cell-free single-molecule unique primer extension resequencing (cf-SUPER) technology for bladder cancer non-invasive detection in urine. Transl Androl Urol 2020;9:1222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi Y, Fujita K, Matsuzaki K, Matsushita M, Kawamura N, Koh Y, et al. Diagnostic potential of TERT promoter and FGFR3 mutations in urinary cell-free DNA in upper tract urothelial carcinoma. Cancer Sci 2019;110:1771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith CG, Moser T, Mouliere F, Field-Rayner J, Eldridge M, Riediger AL, et al. Comprehensive characterization of cell-free tumor DNA in plasma and urine of patients with renal tumors. Genome Med 2020;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuzzo PV, Berchuck JE, Korthauer K, Spisak S, Nassar AH, Abou Alaiwi S, et al. Detection of renal cell carcinoma using plasma and urine cell-free DNA methylomes. Nat Med 2020;26:1041–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvi S, Gurioli G, Martignano F, Foca F, Gunelli R, Cicchetti G, et al. Urine cell-free DNA integrity analysis for early detection of prostate cancer patients. Dis Markers 2015;2015:574120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvi S, Casadio V. Urinary cell-free DNA: potential and applications. Methods Mol Biol 2019;1909:201–9. [DOI] [PubMed] [Google Scholar]
- 32.Reckamp KL, Melnikova VO, Karlovich C, Sequist LV, Camidge DR, Wakelee H, et al. A highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma. J Thorac Oncol 2016;11:1690–700. [DOI] [PubMed] [Google Scholar]
- 33.Husain H, Melnikova VO, Kosco K, Woodward B, More S, Pingle SC, et al. Monitoring daily dynamics of early tumor response to targeted therapy by detecting circulating tumor DNA in urine. Clin Cancer Res 2017;23:4716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie F, Li P, Gong J, Tan H, Ma J. Urinary cell-free DNA as a prognostic marker for KRAS-positive advanced-stage NSCLC. Clin Transl Oncol 2018;20:591–8. [DOI] [PubMed] [Google Scholar]
- 35.Satapathy S, Singh V, Nambirajan A, Malik PS, Tanwar P, Mehta A, et al. EGFR Mutation testing on plasma and urine samples: a pilot study evaluating the value of liquid biopsy in lung cancer diagnosis and management. Curr Probl Cancer 2021;45:100722. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Zhang X, Shen S. Treatment and relapse in breast cancer show significant correlations to noninvasive testing using urinary and plasma DNA. Future Oncol 2020;16:849–58. [DOI] [PubMed] [Google Scholar]
- 37.Guan G, Wang Y, Sun Q, Wang L, Xie F, Yan J, et al. Utility of urinary ctDNA to monitoring minimal residual disease in early breast cancer patients. Cancer Biomark 2020;28:111–9. [DOI] [PubMed] [Google Scholar]
- 38.Bach S, Paulis I, Sluiter NR, Tibbesma M, Martin I, van de Wiel MA, et al. Detection of colorectal cancer in urine using DNA methylation analysis. Sci Rep 2021;11:2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mouliere F, Smith CG, Heider K, Su J, van der Pol Y, Thompson M, et al. Fragmentation patterns and personalized sequencing of cell-free DNA in urine and plasma of glioma patients. EMBO Mol Med 2021;13:e12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terasawa H, Kinugasa H, Ako S, Hirai M, Matsushita H, Uchida D, et al. Utility of liquid biopsy using urine in patients with pancreatic ductal adenocarcinoma. Cancer Biol Ther 2019;20:1348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]