Abstract
Genetically engineered live Plasmodium sporozoites constitute a platform for creating consistently attenuated, genetically-defined, whole parasite vaccines through targeted gene deletions. Such genetically attenuated parasites (GAP) do not require attenuation by irradiation or concomitant drug treatment. We previously developed a P. falciparum (Pf) GAP with deletions in three pre-erythrocytic stage-expressed genes P52, P36 and SAP1 (PfGAP3KO) and demonstrated its safety and immunogenicity in humans. Here, we further assessed safety, tolerability and immunogenicity of the PfGAP3KO vaccine and tested efficacy against controlled human malaria infection (CHMI) in malaria-naïve subjects. The vaccine was delivered by three (n=6) or five (n=8) immunizations with ~200 PfGAP3KO-infected mosquito bites per immunization. PfGAP3KO was safe and well-tolerated with no breakthrough blood stage infections as determined by Plasmodium 18S rRNA biomarker testing. Vaccine-related adverse events were predominately localized urticaria related to the many mosquito bites administered per vaccination. Upon mosquito bite CHMI with fully infectious Pf NF54 parasites one month after the last immunization, half of the study participants who received either three or five PfGAP3KO immunizations remained blood stage negative, as shown by a lack of Plasmodium 18S rRNA in the blood for 28 days post-CHMI. Six protected study participants received a second CHMI six months later and one remained completely protected. Thus, the PfGAP3KO vaccine was safe, immunogenic, and capable of inducing protection against sporozoite infection. These results warrant further evaluation of PfGAP3KO vaccine efficacy in dose range finding trials with an injectable formulation.
Keywords: controlled human malaria infection, Plasmodium falciparum, malaria, vaccine, sporozoite, liver stage, genetic attenuations
One Sentence Summary:
The genetically attenuated Plasmodium falciparum strain, PfGAP3KO (Pf P52−/P36−/SAP1−) was safe and immunogenic and achieved protection against controlled human malaria infection in half of tested malaria-naïve study participants.
Introduction
Malaria is a major cause of morbidity and mortality globally. Despite extensive malaria control efforts and major reductions in malaria prevalence in many parts of the world, the WHO reported 241 million cases and 627,000 malaria deaths in 2020 (1), significant increases over the preceding years. Current public health interventions are under constant threat from the specter of resistance to both insecticides that target the mosquito vector and anti-malarial drugs that target the blood stage parasite and COVID-associated disruptions have become an additional threat to malaria control. A safe and highly effective malaria vaccine is thus urgently needed to combat Plasmodium falciparum (Pf), which is the major cause of morbidity and mortality among children and pregnant women in sub-Saharan Africa.
Human malaria infection begins when Plasmodium sporozoite stage parasites are transmitted to humans via the bites of infected Anopheles mosquitoes. Motile sporozoites leave the bite site, enter the bloodstream and travel to the liver. Sporozoites then traverse the liver sinusoidal endothelium and infect hepatocytes, initiating the asymptomatic liver stages of infection. Liver stage parasites undergo asexual replication within the infected hepatocyte and each liver stage parasite can ultimately generate and release tens of thousands of exo-erythrocytic merozoites into the bloodstream that then invade red blood cells. This invasion initiates the blood stages of the life cycle, leading to malaria disease and its associated morbidity and mortality as well as the transmission of sexual gametocyte stages back to the mosquito. The pre-erythrocytic stages (sporozoite and liver stages) of the life cycle are an extreme bottleneck, since infectious bites likely only deposit tens to hundreds of sporozoites and only a fraction of these sporozoites reach the liver (2). Vaccines that target the pre-erythrocytic stages can prevent sporozoite entry into hepatocytes and/or kill liver stage parasites. This in turn would prevent the liver stage-to-blood stage transition of the parasite and thus, avert all disease, and importantly, onward transmission to the mosquito vector (3). Vaccine efforts to target pre-erythrocytic stages have concentrated on subunit vaccines and attenuated whole parasite vaccines. The most clinically advanced subunit vaccines target the major surface protein of the sporozoite, the circumsporozoite protein (CSP), and include RTS,S and R21. The recently WHO-approved vaccine RTS,S confers significant but limited efficacy over time against naturally-transmitted malaria (4, 5). Initial results from studies using R21, a modified CSP-based vaccine, showed modestly higher efficacy (6) in a clinical trial carried out in young children in Burkina Faso.
As an alternative to subunit vaccines, attenuated pre-erythrocytic parasites are promising vaccine candidates, and numerous animal studies and human clinical studies have demonstrated that complete sterilizing immunity can be achieved with these vaccines (7–16). Whole parasite vaccines are administered in the form of sporozoites stages that infect the liver but their attenuation halts liver stage development and prevents progression of the parasite to blood stage infection (17). However, if attenuation is incomplete, breakthrough blood stage infections will occur, which is a formidable safety challenge to overcome for live sporozoite vaccines.
Attenuation of whole parasites has successfully been achieved in three distinct ways – irradiation, antimalarial drugs and genetic engineering. Irradiated sporozoites were originally tested in trials using administration by mosquito bite and have over the past decade been used in dose ranging clinical trials with direct venous injection (DVI) of purified and cryopreserved radiation-attenuated sporozoites (RAS) called the PfSPZ vaccine (7). Trials administering RAS by mosquito bite showed that >1,000 immunizing mosquito bites were required to achieve 50–100% protection against controlled human malaria infection (CHMI) with fully infectious Pf sporozoites (8, 9, 18–22). To achieve complete protection against CHMI, at least three DVI immunizations with 900,000 PfSPZ for each dose are required (20). Immunization with non-attenuated sporozoites with concurrent administration of antimalarial drugs, referred to as chemoprophylaxis vaccination (CVac), has also been explored. The antimalarial chemoprophylaxis is selected to either kill liver stage parasites by administering the drug pyrimethamine or the first wave of asexual blood stage parasites after exo-erythrocytic merozoite release from the liver by administration of chloroquine. CVac recently demonstrated sterile protection from CHMI with both homologous NF54 strain and heterologous 7G8 strain Pf parasites (23) and requires dosing with far fewer sporozoites than RAS to induce complete protection (24). However, since CVac requires antimalarial drug treatment during immunization, its use for vaccination is somewhat more complex than when using an inherently attenuated parasite that would not require such drug coverage.
Continued advances in parasite genetic engineering have enabled the creation of genetically attenuated parasites (GAP) by deletion of genes essential for Plasmodium liver or blood stage development. GAPs have been extensively evaluated in rodent malaria parasite models, and numerous gene knockout parasites have been created that arrest the during liver stage infection [reviewed in (25)]. Like RAS and CVac, immunizations with GAPs confer durable, completely sterilizing pre-erythrocytic immunity in mouse models (26). As a human vaccine, the significant advantages of GAP over RAS are that the former are comprised of a homogeneous population of parasites with consistent and defined genetic composition and attenuation phenotype, and their considerable advantage over CVac is that they do not require administration of an antimalarial drug during or after immunization. Furthermore, with the advent of CRISPR/Cas9 technology, GAP can be further genetically engineered and optimized to iteratively improve vaccine safety and efficacy. In addition, efforts are also underway to create and evaluate blood stage GAP vaccines (27).
We previously created a Pf GAP called PfGAP3KO with deletions in three genes (P52, P36 and SAP1), which are critical for early liver stage parasite development within hepatocytes (28). In a first-in-human safety trial where ten study participants were each inoculated with PfGAP3KO via ~200 infectious mosquito bites, PfGAP3KO was safe with no evidence of breakthrough blood stage infection (29). Because of the cost and large effort associated with the development of an injectable formulation of whole sporozoite vaccines, we here intended to establish as proof-of-concept that the PfGAP3KO vaccine administered by mosquito bites could protect human subjects from subsequent CHMI.
Results
The PfGAP3KO genome has deletions in three genes that are critical for early pre-erythrocytic infection: P52, P36 and SAP1. These deletions were sufficient to completely attenuate the parasite and resulted in a safe and immunogenic candidate vaccine when administered by mosquito bite in a first-in-human clinical safety study (29). Here, we conducted a CHMI trial of 16 study participants in Study Arms 1 (n=10) and 2 (n=6) who were vaccinated five times or three times, respectively, with the PfGAP3KO vaccine administered by the bite of ~200 infected A. stephensi mosquitoes for each vaccination (Figures 1–2). The mean age for the subjects was 31.1 years (median 29 years; range 22–48 years); age was comparable across treatment groups. Additional demographic parameters are in Table S1. One month after the last vaccination, all study arm participants, and additional malaria-naïve infectivity control study participants, were exposed to CHMI by the bite of five Pf NF54-infected mosquitoes and vaccine efficacy was determined over a 28-day period. All protected study participants were then exposed to a second CHMI six months later, alongside additional malaria-naïve infectivity control study participants.
Figure 1. Study design for vaccination and both CHMI phases.
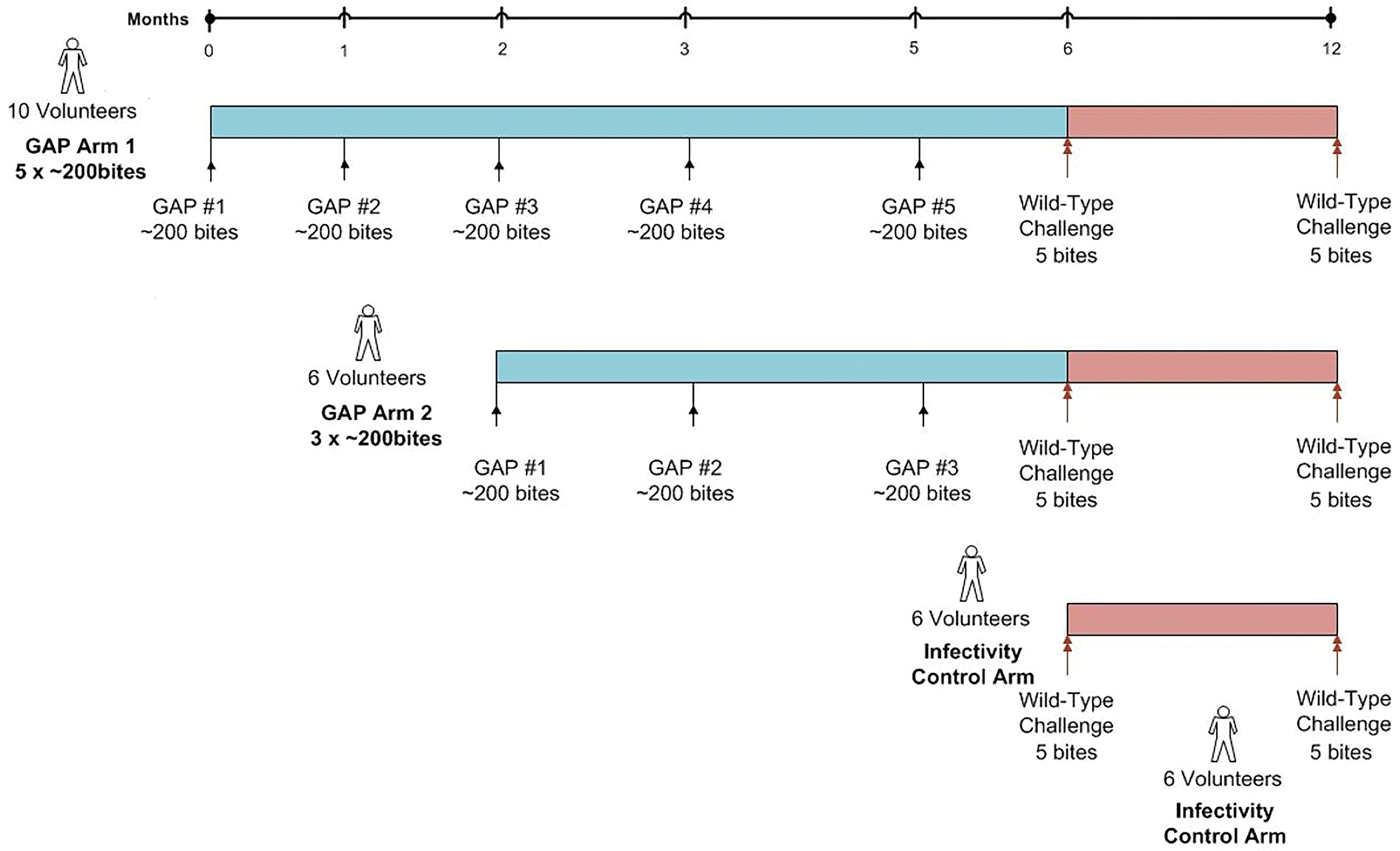
PfGAP3KO sporozoites were delivered to eligible study participants five (Aim 1) or three times (Arm 2) via ~200 PfGAP3KO infected mosquito bites per administration. The interval between doses was one month, except for the interval between the last two doses, which was two months. One month after the last immunization, vaccinated study participants and a group of malaria-naïve study participants as infectivity controls underwent CHMI induced by five wild-type Pf NF54-infected mosquito bites. Vaccinated study participants who were protected against the first CHMI were eligible for a second CHMI six months later, and these study participants were accompanied at CHMI by another group of malaria-naïve study participants as infectivity controls.
Figure 2. Consort diagram for enrollment through study completion.
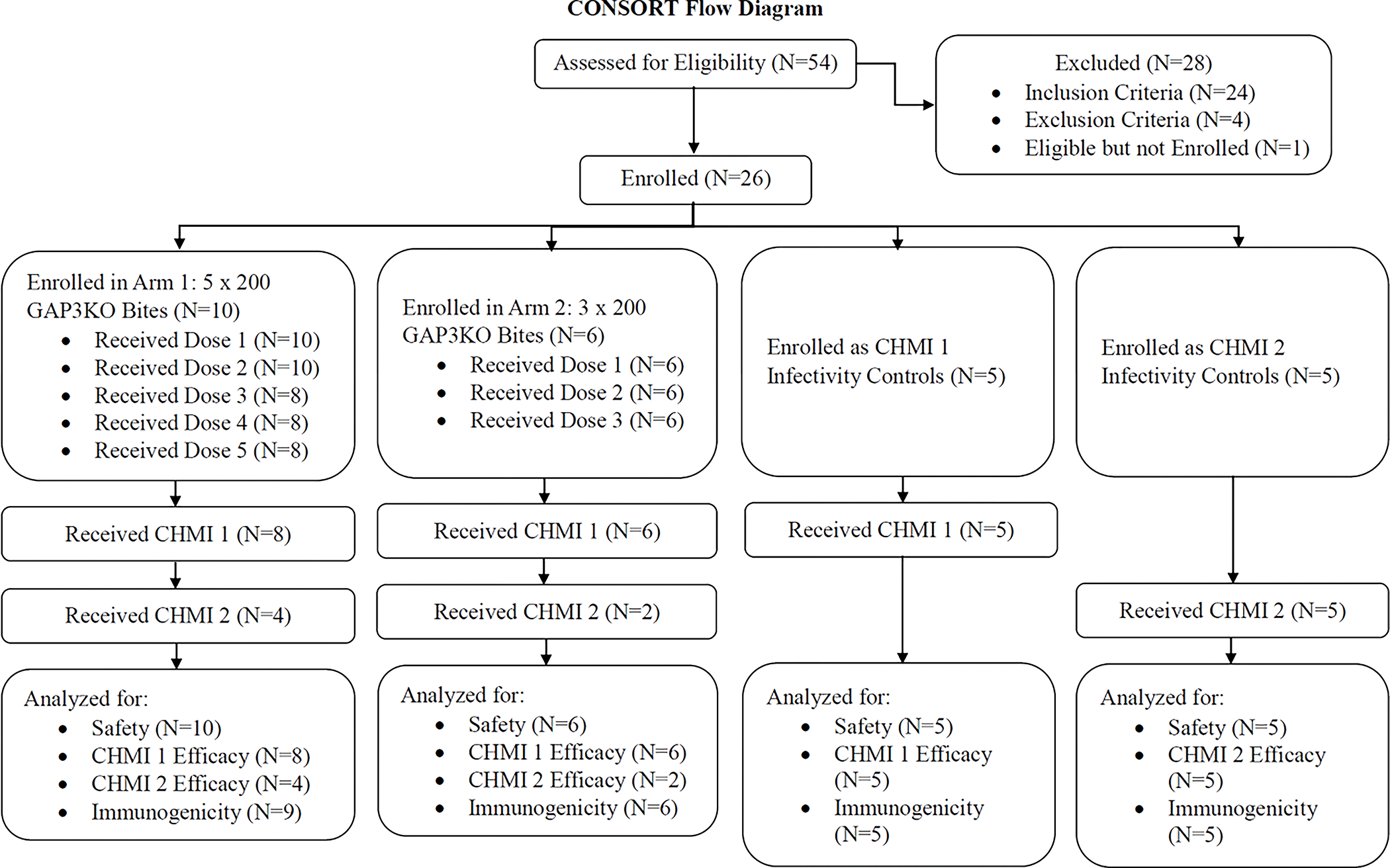
The number of study participants who were eligible, enrolled, and completed aspects of the trial are listed by study arm.
Production of PfGAP3KO and experimental vaccination of study participants
Table 1 shows the intended and estimated number of infectious bites that each study participant received during the course of vaccination. Of note, the estimated number for each study participant was between 88% and 107% of the planed dose with an overall average of 95%.
Table 1.
Cumulative mosquito bites during vaccination and lack of post-vaccination qRT-PCR positivity.
| Group | n | Intended number of mosquito bites per study participant | Range of estimated number of mosquito bites per study participant | # of persons with qRT-PCR positivity |
|---|---|---|---|---|
| Arm 1 | 8* | 1,000 (5 × 200 bites) | 882 – 1,069 | 0 |
| Arm 2 | 6 | 600 (3 × 200 bites) | 468 – 645 | 0 |
Range estimates do not include two subjects in Arm 1 who were discontinued early and therefore did not undergo all mosquito bite administration events.
Safety and tolerability of repeated PfGAP3KO dosing
The study was conducted in accordance with the clinical trial protocol. There were 48 subject-specific protocol deviations for the 20 subjects; no protocol deviations resulted in subject termination and no protocol deviations were believed to have resulted in an adverse event (AE) or to have compromised subject safety or study outcome.
As expected with a study where participants were receiving large numbers of mosquito bites, all study participants reported at least one systemic solicited AE and one local solicited AE (Figures 3 and S1 and Table S2). The most common AEs (occurring in the majority of subjects in Arm 1 and 2) were local bite-site erythema, pruritis, edema, induration, discoloration, tenderness, and pain, as well as frequent fatigue, headache, and malaise. These AEs were likely related to the very large number of mosquito bites required for vaccine administration, which has also been seen in other mosquito bite-delivered sporozoite vaccine studies (19). One Arm 1 participant experienced more frequent Grade 2 AEs than any other participant and nearly all of the Grade 3 AEs recorded in the study. One participant in Arm 1 was discontinued for bite site edema and a further participant in Arm 1 was discontinued for the allergic reaction AE described below. Safety visits and qRT-PCR monitoring for discontinued participants were maintained per protocol. No deaths or other serious AEs (SAEs) were reported during the study.
Figure 3. Frequency and maximum severity of local and systemic AEs during vaccination phase.
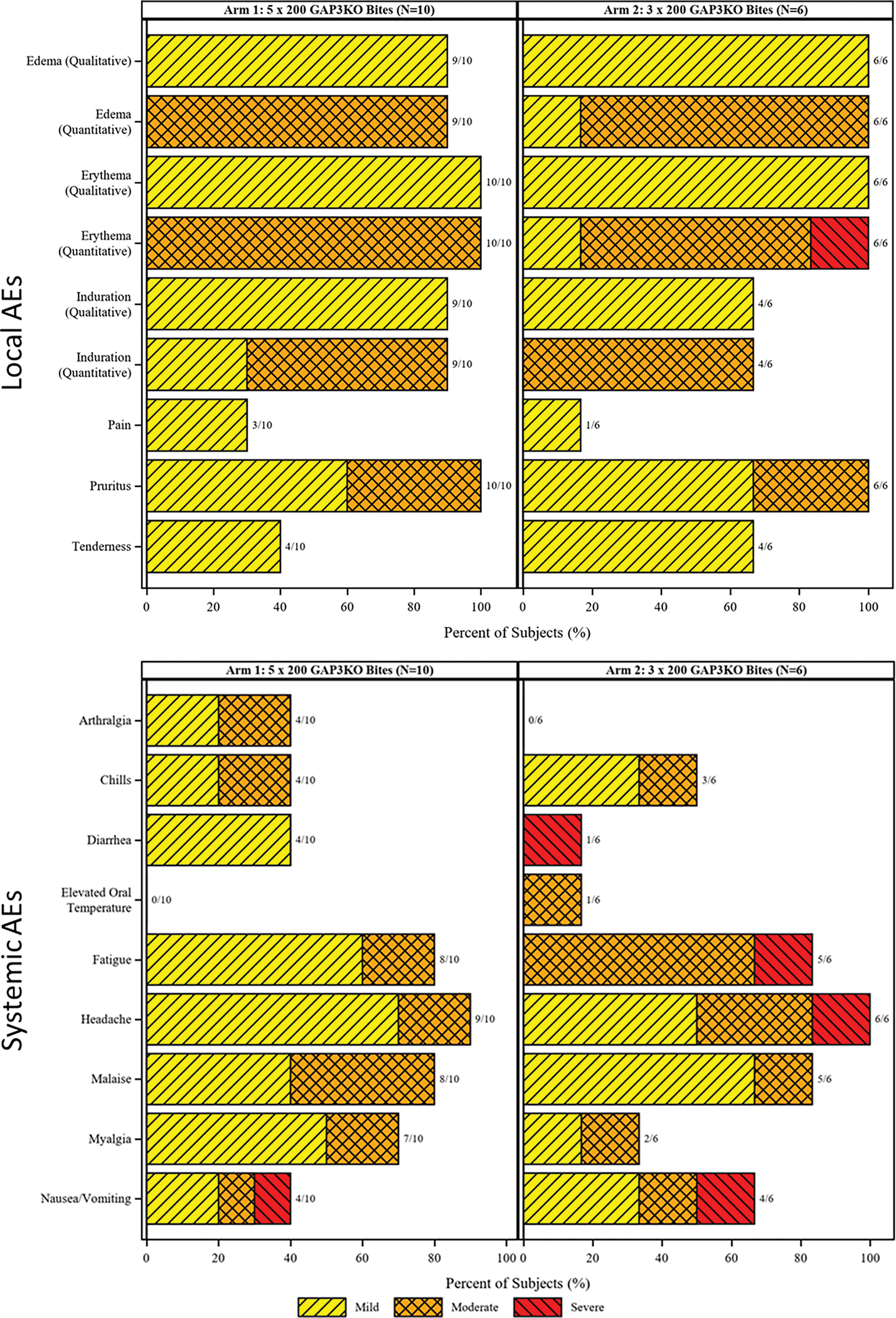
Local (top) and systemic (bottom) AEs are shown as a histogram for study participants in Arm 1 (left) and Arm 2 (right). Grading is shown as in the key and the number of study participants with each listed AE are listed in the bar labels.
One participant was discontinued due to a severe allergic reaction after the second round of mosquito bite administration. The study participant developed bite site erythema, edema, and pruritus within minutes without immediate systemic symptoms. Twenty minutes later, the study participant developed generalized hives and reported a tickling sensation in her throat that caused a slight cough. The study participant consequently received oral diphenhydramine (50 mg) and later one dose of injectable epinephrine with improvement in symptoms and was transported to a medical facility for observation, was diagnosed with an allergic reaction, and was prescribed prednisone (40 mg) for 12 days. Hives were completely resolved three hours after transfer. This AE was labeled as a severe allergic reaction related to the study product but not an SAE as defined in the protocol.
With respect to laboratory abnormalities, all chemistry abnormalities observed post-vaccination were graded as mild and determined to be unrelated to PfGAP3KO (Figure S2). Six hematologic abnormalities (five decreased leukocyte counts and two decreased hemoglobin levels) observed post-vaccination were mild (Figure S3) and all were considered related to the study product except one low hemoglobin level attributed to menstrual blood loss. No vaccinations were discontinued due to hematology laboratory results.
Full attenuation of PfGAP3KO confirmed by qRT-PCR
All study participants that received PfGAP3KO completed the vaccinations without exhibiting any malaria symptoms and remained negative for blood stage parasitemia as demonstrated by qRT-PCR that detected parasite nucleic acids from blood sampling (Table 1 and Figure S1). At a limit of detection of 20 parasites per mL of whole blood, Pf 18S rRNA was not detected in any of the post-vaccination blood samples tested, including for the two participants who discontinued prior to completing all vaccinations. These findings demonstrated complete attenuation of PfGAP3KO during pre-erythrocytic infection in accordance with our previous findings (29).
Efficacy against NF54 challenge by mosquito-bite delivered CHMI
One month after the final scheduled vaccination, all study participants who had completed the vaccination series underwent CHMI 1 by the bite of five infected mosquitoes harboring infectious Pf NF54 wildtype sporozoites. In addition, a group of five unvaccinated infectivity control study participants were subjected to the same CHMI. Four of the five infectivity controls became blood stage patent, based on qRT-PCR, on Days 7 and 8 after CHMI but surprisingly, the fifth control study participant did not become blood stage patent over 14 days (Figure 4A and Table 2). In Study Arm 1, where study participants received five vaccinations, four of eight remained blood stage negative post CHMI. The remaining four study participants became blood stage positive between days seven and nine after CHMI (Figure 4A and Table 2). Three of six study participants in Study Arm 2 (three vaccinations) also remained blood stage negative post CHMI and the remaining three study participants became blood stage positive between days seven and eight after CHMI (Figure 4A and Table 2). Thus overall, half of vaccinated study participants appeared to be protected at the pre-erythrocytic phase of infection post CHMI. Six months after CHMI 1, four protected study participants from Study Arm 1 and two from Study Arm 2, as well as another set of five infectivity controls were subjected to CHMI 2, again with the bite of five Pf NF54-infected mosquitoes (Figure 4B). One of the six protected study participants (the study participant was in Study Arm 1) from CHMI 1 again remained blood stage negative post CHMI 2 and the remaining five became blood stage positive between days seven and nine after the CHMI. All five infectivity controls became blood stage positive between seven and nine days after CHMI 2 (Figure 4B).
Figure 4. Infection rate following the first and second CHMI.
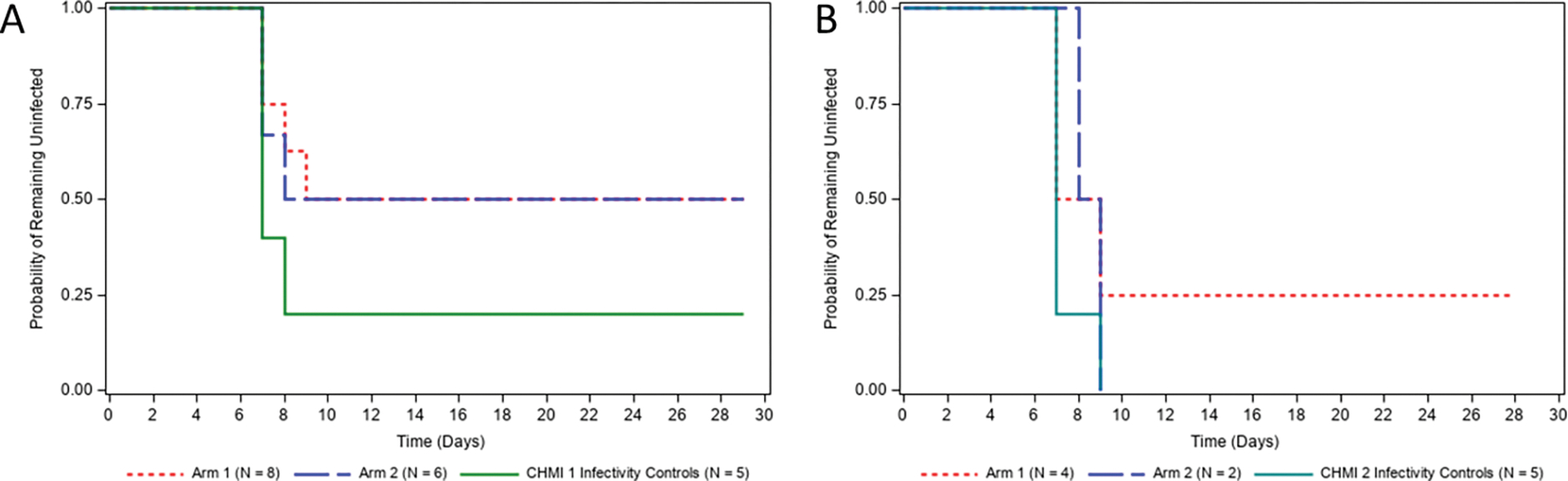
Time-to-event analysis for the protocol-defined primary efficacy threshold of documented Plasmodium infection after the first (A) and second CHMI (B). The y-axis represents the percentage of study participants that had not reached the definition of infection post-CHMI (one positive qRT-PCR result ≥20 estimated parasites/mL). Days are numbered post-CHMI.
Table 2.
Detection of patency after CHMI.
| CHMI | Statistic | Arm 1: 5×200 GAP3KO bites | Arm 2: 3×200 GAP3KO bites | CHMI infectivity controls |
|---|---|---|---|---|
|
| ||||
| 1 | n/Na | 4/8 | 3/6 | 4/5 |
|
| ||||
| Infection rate | 0.50 | 0.50 | 0.80 | |
| (95% CI)b | (0.22, 0.78) | (0.19, 0.81) | (0.38, 0.96) | |
|
| ||||
| Vaccine efficacyc | 0.38 | 0.38 | N/A | |
| (95% CI)d | (−0.75, 0.79) | (−1.13, 0.83) | ||
|
| ||||
| 2 | n/Na | ¾ | 2/2 | 5/5 |
|
| ||||
| Infection rate | 0.75 | 1.00 | 1.00 | |
| (95% CI)b | (0.30, 0.95) | (0.34, 1.00) | (0.57, 1.00) | |
|
| ||||
| Vaccine efficacyc | 0.25 | 0 | N/A | |
| (95% CI)d | (−0.57, 0.81) | |||
n=number of subjects with malaria infection defined as a positive qRT-PCR assay with parasite density of ≥20 parasites/mL.
N=total number of subjects in the specified group who received the specified challenge.
Wilson score interval used for the confidence interval of the proportion.
Vaccine efficacy is defined as 1-pv/pc, where pv is the infection rate in the vaccinated group, and pc is the infection rate in the control group.
Confidence intervals for vaccine efficacy are calculated from exact unconditional confidence limits for relative risk based on the Farrington-Manning score statistic.
N/A=Not applicable.
Immunogenicity of three or five doses of PfGAP3KO induces IgG antibodies to CSP, TRAP and MSP3
Antibodies that bind to the sporozoite can prevent infection of the liver and thus prevent life cycle progression. Specifically, clinical studies have shown that antibodies to CSP are associated with the level of protection after vaccination (30). Thus, IgG antibody levels to CSP as well as additional antigens (TRAP, MSP1, MSP3, GLURP, AMA1) were measured by ELISA after vaccination in both Study Arms (Figure 5). Antibody titers to CSP were evident after only one vaccination in both Study Arms and further increased but to a lesser degree after the second and third vaccination to reach high levels (Figure 5A). No significant increase in CSP titers was seen in Study Arm 1 after the fourth and fifth vaccination as compared to vaccination three and the titers were not significantly different after three vaccinations when the two Study Arms were compared. Antibody titers to CSP at the pre-CHMI 1 time point ranged widely in all Study Arm study participants, and there was no apparent correlation between antibody titer and protection (Figure 5B); infectivity controls had negligible titers.
Figure 5. Antibody immunogenicity to pre-erythrocytic antigens.
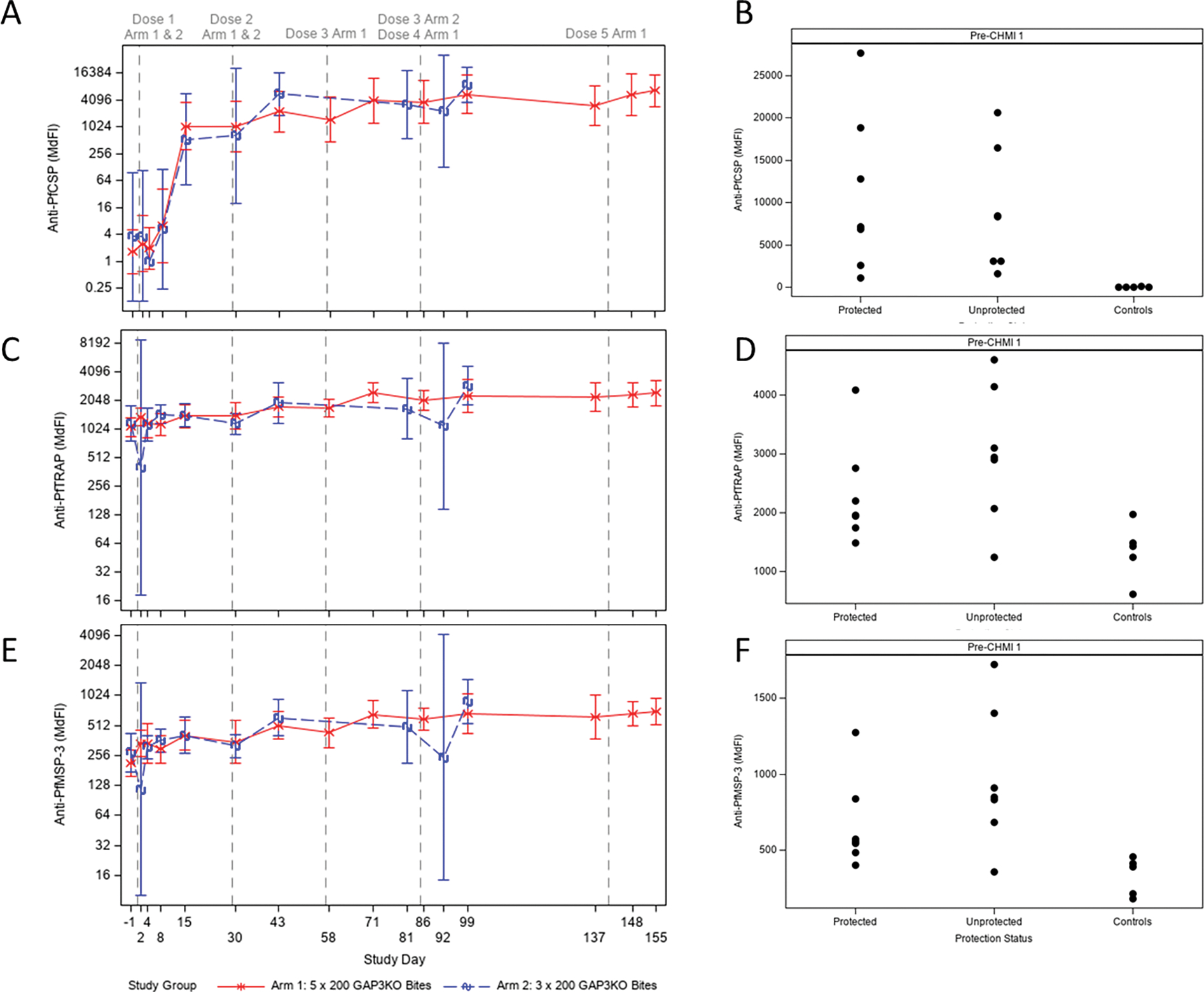
Vaccine-induced antibodies recognizing full-length Pf CSP (A), Pf TRAP (C), and Pf MSP3 (E) were quantified by ELISA at each time point as shown. Arm 1 is shown in the solid red line, and Arm 2 is shown in the dashed blue line. Antibody levels are expressed in median fluorescence intensity (MdFI). Vertical dashed lines correspond to immunization procedures as noted at the top. Each data point represents the geometric mean for each Study Arm at each time point; error bars represent 95% confidence intervals. When pre-CHMI antibody levels were compared for protected vs. unprotected vs. naïve controls for Pf CSP (B), Pf TRAP (D), and Pf MSP3 (F), there was no significant difference between protected and unprotected groups for responses to any antigen.
The single infectivity control subject who failed to become infected following CHMI 1 (VMP.01366) had a detectable but extremely low anti-PfCSP titer (MdFI at screening = 128), which was higher than the other four infectivity control subjects in CHMI 1 (one had MdFI = 31, three had undetectable CSP titers at screening). It is unclear whether this higher baseline level may explain the subject’s protection from CHMI, as vaccinated subjects who were not protected following CHMI 1 all had anti-PfCSP antibody levels substantially higher than an MdFI of 128 at the pre-CHMI timepoint.
Vaccination also increased antibodies titers to the pre-erythrocytic antigen TRAP and the sporozoite antigen MSP3 -- titers ranged widely between individual study participants (Figures 5C and E) but did not correlate with protection status and were not increased significantly as compared to the infectivity controls (Figure 5D and F). Responses to predominately Pf blood stage antigens (MSP1, GLURP, AMA1) were not statistically different when compared to controls (Figure S4).
PfGAP3KO-elicited antibodies have functional activity against Pf sporozoites
Since the serum of vaccinated study participants in both Study Arms showed considerable IgG antibody titers to CSP, we determined whether serum antibodies were functional in blocking sporozoite infection using an in vitro inhibition of sporozoite cell traversal and host cell invasion (ISTI) assay. In this assay, serum is incubated with Pf NF54 sporozoites and the sporozoites are then allowed to invade a hepatocyte cell line, HC04 over a period of 90 minutes. Invasion rates are measured based on the number of infected HC04 cells using an antibody to CSP to detect the parasite within the HC04 cell and flow cytometry. Sera from the unvaccinated infectivity control study participants were used as a negative control in the assessment of post- CHMI responses.
Before vaccination, serum inhibited invasion by approximately 30% (Figure 6A). 15 days after the first vaccination, invasion was inhibited between 65–75% for both Study Arms (Figure 6A), strongly suggesting that antibody responses elicited by vaccination are inhibiting sporozoite invasion of HC04 cells and by association, would prevent their entry into the liver. Inhibition of invasion did not significantly increase with further vaccinations. In addition, inhibition of invasion was measured using serum taken from study participants one day before and eight days after both CHMI 1 and CHMI 2 (Figure 6B). Serum from vaccinated study participants in both Study Arms significantly inhibited invasion as compared to the infectivity controls and inhibition did not significantly increase from day −1 to day 8 of CHMI 1 and CHMI 2.
Figure 6. Inhibition of sporozoite invasion.
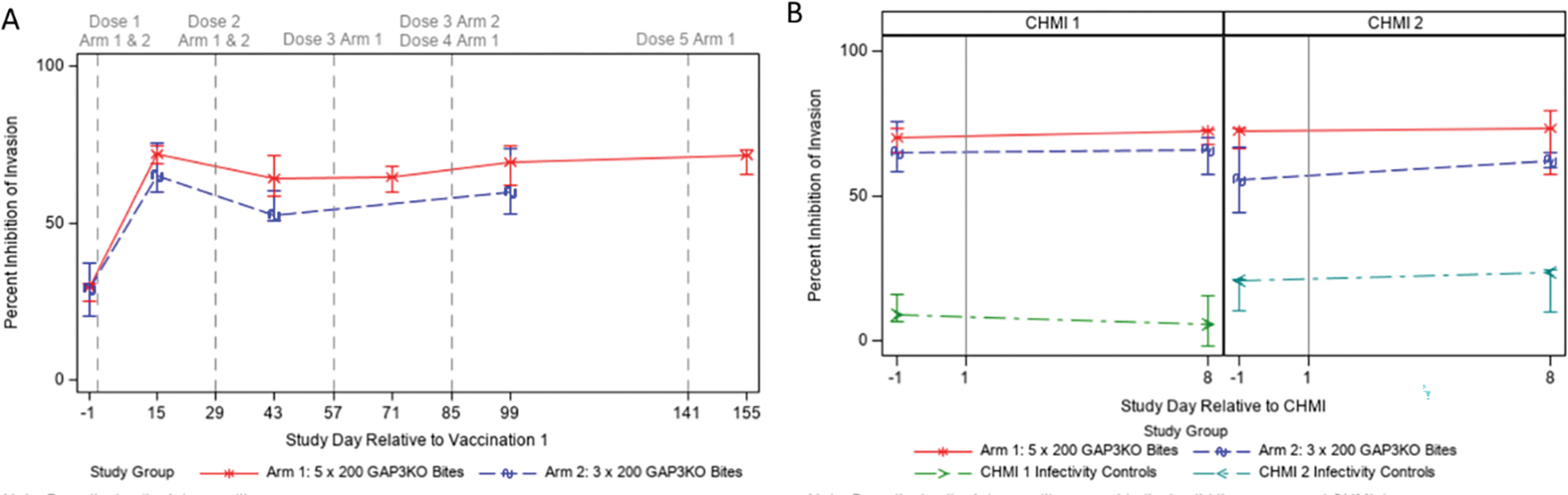
Sera from immunized study participants were also evaluated for functional inhibition of sporozoite invasion and are displayed relative to vaccination (A) or CHMI (B) using an in vitro ISTI assay. Each data point represents the median inhibition for each Study Arm at each time point; error bars represent the interquartile range. Vertical dashed lines in A correspond to immunization procedures as noted at the top; vertical solid lines in B represent CHMI days.
Discussion
In the present study, we demonstrate that vaccination with mosquito bite-delivered PfGAP3KO was safe and conferred sterilizing protection against CHMI with wild-type Pf NF54 sporozoites during the pre-erythrocytic phase of infection in a subset of participants. One month after the last of either five or three vaccinations, half of study participants remained blood stage negative post CHMI. Sterile protection was not consistently durable, as evidenced by the findings from the second CHMI, given six months after the first CHMI, which found that only one of the six study participants protected against the first CHMI was completely protected against a second CHMI. Since half of study participants were protected from the first CHMI in both Study Arms 1 (five vaccinations) and 2 (three vaccinations), the fourth and fifth vaccination appeared to not increase vaccine efficacy. Notably, there was no increase in protective antibody responses targeting the sporozoite after the third vaccination as indicated by no further increases in anti-CSP antibody titers or inhibition of sporozoite invasion measurements.
Studies of attenuated sporozoite vaccination in rodent models have shown that vaccination by mosquito bites induces antibody responses that can target the sporozoite at the site of inoculation and thus after each successive vaccination, sporozoites are inhibited in their ability to egress from the bite site (31–34). When vaccine-stage sporozoites are prevented from exiting the bite site, entering the vasculature, and infecting hepatocytes, resulting cellular immune responses are reduced, resulting in fewer critically protective CD8+ T cells. Studies in rodent models have shown that protective liver resident memory CD8+ T (CD8+ TRM) cells are essential for the killing of liver stage parasite-infected hepatocytes after sporozoite challenge (35–37), but sporozoites must reach the liver and infect hepatocytes to induce protective CD8+ TRM responses. Furthermore, vaccination of rodents with attenuated sporozoites has demonstrated that, after only one vaccination, there is a significant decrease in the liver stage burden achieved upon subsequent vaccinations (38). Thus, in the current study, it is likely that with each successive vaccination, the number of PfGAP3KO sporozoites reaching the liver rapidly declined and thus limited the generation of protective liver resident memory CD8+ TRM cells essential for complete pre-erythrocytic protection and effectiveness against CHMI.
PfGAP3KO suffer a very early arrest in liver stage development and thus this clinical trial allows the results to be compared against other clinical trials that utilized attenuated parasites that arrest similarly early after hepatocyte infection. These include RAS and GA1, the latter of which is a genetically attenuated parasite with deletions in both SAP1 and B9 (30). RAS-based vaccines have often been delivered by the bites of irradiated mosquitoes (9, 18, 20). More recently, numerous studies have begun to use purified cryopreserved RAS, referred to as PfSPZ Vaccine, which is inoculated by DVI and thereby bypasses the skin, and this formulation is intended to be licensed as a malaria vaccine. Although RAS are now routinely delivered by DVI, two previous trials that delivered RAS via 800–1200 total infected mosquito bites over five immunizations, achieved ~50% protection against CHMI (19, 20). Our trial described herein was also based on administering ~600 or ~1000 total infectious mosquito bites over three or five immunizations, respectively, and we also observed that half of PfGAP3KO-immunized study participants remained free of blood stage parasitemia. This suggests that PfGAP3KO can be as efficacious as RAS. A recent trial with mosquito bite-delivered RAS intentionally aimed for ~50% efficacy upon CHMI challenge in an effort to discover immune correlates of protection, which could then be used as comparators in further trials (18). In this trial known as the IMRAS trial (immunization with live, radiation-attenuated Pf sporozoites), study participants were divided into two cohorts (n=10–11 vaccinated study participants per cohort) and immunized separately on five occasions with the aim of delivering ~200 infectious bites per immunization (the same number of bites we also aimed for in our trial). In one cohort, the protective efficacy was 55% as expected, but surprisingly, in the second cohort, the efficacy was 90%. The authors speculated that an inadvertently higher first dose of sporozoites in the second cohort and/or higher per-mosquito dosages of sporozoites may have been responsible for the increased protection. Specifically, in IMRAS cohort 1, 11 subjects received 810–1235 infected bites with 6/11 (55%) protected against CHMI. In IMRAS cohort 2, 10 subjects received 839–1131 infected bites (similar numbers to cohort 1) but the first dose was significantly higher than in cohort 1 and there was also a significantly reduced fifth dose compared to cohort 1. In addition, the median salivary gland sporozoite loads of all immunizations in IMRAS cohort 2 were significantly higher than cohort 1, suggesting that the second cohort received more sporozoites even though the number of mosquito bites were comparable. Based on these findings, we speculate that the doses of PfGAP3KO administered in our trial did not reach the levels seen in IMRAS cohort 2. It remains unclear whether we could have improved protection by either increasing the first dose and/or decreasing the last dose, as suggested by the IMRAS trial. It is clear however from both our trial presented herein and the previously reported trials using mosquito bite delivery of RAS, that sporozoite dosage by mosquito bite cannot be well controlled and thus, dose relationships to protective efficacy against CHMI are difficult to determine. Therefore, while constituting a proof-of-concept first step in clinical evaluation, mosquito bite-based immunization with sporozoites is variable and cannot be used as a definitive method to establish vaccine efficacy.
Results from trials using the purified and cryopreserved PfSPZ RAS Vaccine administered by DVI have yielded more consistent results with higher rates of complete protection from CHMI and natural infection. The initial study using the PfSPZ Vaccine relied on subcutaneous or intradermal injection in an effort to mimic the natural route of infection, but this led to only low levels of protection (39). When DVI was compared to subcutaneous immunization of PfSPZ in non-human primates (NHPs), increased immune responses to the DVI-administered PfSPZ vaccination were observed (40), particularly better CD8+ cell responses. In the follow-up trial in humans, DVI of PfSPZ achieved complete protection from CHMI (40). Thus, DVI of purified, cryopreserved sporozoites provides the opportunity to consistently administer exact dosages and to avoid inoculation into the skin, thereby increasing the likelihood of the sporozoite vaccine reaching the liver and eliciting protective CD8+ TRM responses. As such, future trials using PfGAP3KO will ideally use DVI of a vialed, cryopreserved formulation and this will certainly be essential for its development as a licensed vaccine as well.
Recently, PfSPZ-GA1, a GAP with deletions in SAP1 and B9, was directly compared with PfSPZ Vaccine in a trial where study participants were immunized thrice with either 0.45 × 106 (n=13) or 0.9 × 106 PfSPZ-GA1 (n=13) or with 0.45 × 106 PfSPZ Vaccine (n=13) as a control and challenged by mosquito bite CHMI (30). Protective efficacy of GA1 vaccinations was surprisingly low with 3/25 study participants protected across the two different dosage groups combined. However, this trial faced some unexpected issues with the finding that PfSPZ Vaccine in the control arm did not protect any of the 13 vaccinated participants. This was very different from an earlier study where three doses of PfSPZ Vaccine protected 13/15 study participants (41). Thus, although the GA1 trial is inherently difficult to interpret, the observation that PfSPZ-GA1 provided some protection with three doses is not inconsistent with our finding here that PfGAP3KO protected with three doses of immunizing mosquito bites, and further suggests that GAPs might be as immunogenic and protective as the irradiated PfSPZ vaccine.
Our study had several limitations. During the first CHMI, one of five infectivity control group participants did not develop a blood stage infection, even though mosquito bites used for that particular CHMI were shown to be infectious. It is not possible to determine why this study participant failed to develop a blood stage infection. However, <100% infection in the infectivity control group has been reported in other mosquito bite challenge studies (40, 42, 43). The lack of parasitemia post-CHMI in an infectivity control does, however, limit interpretation of the role of vaccination in protection of vaccinated study participants. A second limitation as discussed is that the exact dose PfGAP3KO is unknowable due to the mosquito bite delivery method. A third limitation is that it remains unknown whether PfGAP3KO immunization elicited protective T cell responses in subjects mainly because we do not yet have a direct peripheral blood measure to track liver CD8+ TRM cells. As such, it is difficult to know how T cell immunogenic PfGAP3KO is and whether there are any important differences between the irradiated PfSPZ vaccine and PfGAP3KO vaccine in this regard. Future comparative DVI immunization studies may help to address these outstanding questions.
In conclusion, our study establishes proof-of-concept for the safety, immunogenicity, and efficacy of PfGAP3KO, an early liver stage-arresting GAP, in humans. Based on numerous trials carried out with mosquito bite-administered RAS, PfSPZ RAS vaccine and more recently with the GAP PfSPZ-GA1, PfGAP3KO is likely as potent an immunogen as the PfSPZ Vaccine and thus warrants further clinical trial investigation. Furthermore, the predominant AEs observed in our study were related to the mosquito bite administration, and transition to DVI will reduce such AEs. Thus, an aseptic, purified, cryopreserved, vialed, and DVI-administered formulation PfGAP3KO will allow for safe and precise dose finding trials to achieve complete sterilizing protection with this candidate vaccine, as it has been done with PfSPZ RAS vaccine (44–46). At the same time, ongoing research utilizing the GAP platform might lead to creation of genetically attenuated Pf vaccine strains with optimized immunogenicity and potentially better efficacy such as later liver stage-arresting GAPs (47) and blood stage GAPs (27). These efforts will further improve the prospects of a highly efficacious, live-attenuated malaria vaccine.
Materials and Methods
Study design and ethical approval
This clinical trial (VTEU 14-0088) was a single center, open-label, phase 2 experimental medicine study designed to evaluate the safety, tolerability, immunogenicity, and efficacy of the genetically attenuated Pf P52−/P36−/SAP1−, early liver stage-arresting triple knockout vaccine, known as PfGAP3KO. The overall study design is depicted in Figure 1. Prior to initiation, the study was Investigational Review Board approved by Quorum Review (File # 32189/1). All study participants were screened for eligibility up to 90 days prior to enrollment (see Table S3 and Supplemental Methods for eligibility criteria). Screening included informed consent, assessment of understanding, and laboratory and clinical assessments.
The trial was to include 16 study participants in Study Arms 1 and 2 who received the PfGAP3KO vaccine administered by the bite of ~200 infected Anopheles stephensi mosquitoes in a controlled clinical environment, and 12 CHMI infectivity controls (six for each of the two CHMIs). The 10 study participants in Study Arm 1 were to receive five vaccinations, with the first four vaccinations given four weeks apart and the fifth vaccination given eight weeks after the fourth vaccination, for a maximum cumulative dose of ~1,000 PfGAP3KO infected bites per study participant. The six study participants in Arm 2 were to receive three vaccinations, with the second vaccination given four weeks after the first vaccination and the third vaccination given eight weeks after the second vaccination, for a maximum cumulative dose of ~600 PfGAP3KO bites per study participant. The scheduled vaccinations were to be given to all Study Arm study participants on the same day. To allow the first and second CHMI administrations to be given concurrently to study participants in both Study Arms, study participants in Study Arm 2 initiated the vaccination series on the same day that Study Arm 1 study participants received their third vaccination.
The first CHMI was given four weeks after the last vaccination for study participants in both Study Arms and included five infectivity controls. The second CHMI was given ~26 weeks (six months) after the first CHMI and included study participants in Study Arms 1 and 2 who did not have any detectable Pf infection following the first CHMI plus six new infectivity controls.
Mosquito bite procedures
All vaccinations and challenges were administered via the bites of Anopheles stephensi mosquitoes under controlled conditions. PfGAP3KO was produced as below and as previously described (29); further details are in the Supplemental Methods. Mosquitoes were raised under phase-appropriate cGMP conditions at the Center for Mosquito Production and Malaria Infection Research (CeMPMIR) facility at the Seattle Children’s Research Institute.
For vaccination by mosquito bites, PfGAP3KO asexual blood stage parasites were thawed from a Working Cell Bank and expanded in normal human erythrocytes using standard culture conditions. Laboratory-reared A. stephensi mosquitoes were infected by membrane feeds on mature gametocyte cultures. The evaluation of mosquito PfGAP3KO infection before the experimental bite exposures assessed midgut oocyst infection by midgut removal and oocyst enumeration and on salivary gland infection, based on smash tests one day before vaccination, in each infectious cage. Cages were scored for infectivity and the most robustly infected cages were used to create pools of mosquitoes in cups for vaccination of study participants with the aim of achieving 200 infected bites per immunization. After vaccination, 20 fed mosquitoes were analyzed for infection and the total number of infectious bites was estimated based on the total number of mosquitoes that fed. For wild-type challenge by mosquito bites, five infectious bites were administered and verified as described (48).
For administration of the vaccine, PfGAP3KO-infected mosquitoes were allowed to feed on the study participant’s arm for 10 minutes. The total number of mosquitoes placed in the carton was adjusted to achieve the target number of 200 infectious bites and allows for adjustment based on lot-specific infection rates. An estimate of the lot-specific infection rate was made one day prior to administration by dissecting the salivary glands from a sample of mosquitoes taken from the source cages. Infection rates were scored (Table S4) with gland grades of 2+ or higher considered infected. Following the 10-minute feed of mosquitos, 20 blood-engorged mosquitoes were evaluated to determine study participant-specific infection rates. Infection rates were estimated based on the percentage of blood-fed mosquitoes demonstrating ≥11 sporozoites upon dissection under a stereoscopic microscope. The remaining mosquitoes in the feeding carton were killed and the total number of mosquitoes with blood-engorged abdomens counted. This provided an estimate of the study participant-specific total number of infectious bites. The number of infectious bites for each study participant was then determined by multiplying the total number of bites by the estimated proportion of fed mosquitoes infected.
Safety assessments
After administration, study participants were closely monitored for acute reactogenicity at 30 and 60 minutes post-administration. Clinical visits continued thereafter to assess safety, including solicited local and systemic reactogenicity as detailed in the Supplemental Methods. Any study participant that demonstrated patent parasitemia after vaccination was to be treated with a standard oral regimen of atovaquone/proguanil (Malarone®).
Sample processing
Blood samples were collected from study participants at predefined time points in accordance with the study design depicted in Figure 1 and Supplemental Methods.
Diagnostic quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Fifty μL aliquots of EDTA-anticoagulated whole blood were preserved in 2 mL of NucliSens lysis buffer (bioMérieux) for same day extraction and qRT-PCR testing. Additional aliquots in lysis buffer were also stored at −80°C as backup samples. Nucleic acid extraction and amplification were performed on the Abbott m2000sp/rt system as previously described (49). This assay sensitively detects and quantifies blood-stage Pf parasites based on detection of the Pf 18S rRNA/rDNA with a limit of detection of 20 estimated parasites/mL.
ELISAs
Sera obtained pre- and post- vaccination and pre- and post-CHMI were assessed by ELISA for antibodies to Pf CSP (amino acids 21–289), glutamate-rich protein (GLURP), merozoite surface protein-1 (MSP1), merozoite surface protein-3 (MSP3), thrombospondin-related anonymous protein (TRAP), and apical membrane antigen-1 (AMA1). ELISAs were conducted by coating ELISA plates (Corning) with each protein in sodium-carbonate/calcium-bicarbonate coating buffer overnight at 4°C. Plates were washed three times in PBS with 0.05% Tween-20 prior to blocking with dilution/blocking buffer (PBS containing 0.05% Tween-20 and 6% BSA) for 1 hr at room temperature. After washing, sera from study participants were diluted 1:200 in dilution/blocking buffer and tested in triplicate. A standard positive sample was used to create a 10-point two-fold dilution series and additional positive and negative control sera were also included sample at 1:200 dilutions. Samples were incubated on the plate at room temperature (RT) for 2 hr prior to washing and application of an HRP-conjugated anti-human IgG secondary antibody (Life Technologies) at a 1:200 dilution in dilution/blocking buffer. The secondary antibody was incubated at RT 2 hr. Plates were washed three times, developed in SigmaFast OPD (Sigma) for 4 minutes and read for absorbance at 450 nm. Arbitrary units (AU) were determined by interpolating values based on the standard curve using nonlinear regression with Prism 6 software and multiplying by the dilution factor. An AU value of 2000 was used as the cutoff for positivity and sample data was not accepted if it was outside the standard curve range, if the OD value was ≥3.5 or if the %CV between triplicate values was >30%. All ELISA values were repeated and are reported as the mean of two independent runs.
Inhibition of sporozoite traversal and invasion (ISTI) assay
An assessment of the ability of serum from immunized study participants to inhibit sporozoite traversal and invasion was performed as described previously (50, 51). Briefly, Pf salivary gland sporozoites were incubated with sera from study participants at a 1:20 dilution in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% FBS, 2.5mM glutamine, penicillin/streptomycin, amphotericin B (Fungizone) and 10 mg/mL FITC-dextran for 15 minutes at 37°C. Sporozoites were then applied to HC04 cells in triplicate in 96 well plates in a total volume of 100 μL/well at a MOI of 0.3. The plate was then centrifuged at 400 × g for 3 minutes and then incubated at 37°C for 90 minutes to facilitate sporozoite/hepatoma interaction. Cells were then fixed, permeabilized and stained with an Alexa fluor-647-labeled anti-CSP monoclonal antibody (clone 2A10). Cells were analyzed for invasion (CSP+) and traversal (dextran+) by flow cytometry. Percent inhibition of invasion and traversal for each study participant was determined by normalizing to the CSP/dextran-positive cells in triplicate wells containing study participant-matched pre-immune serum. Sample data was rejected if the %CV for triplicates was >30% or if invasion of cells in untreated wells was <0.5%. Data are reported as the average of three independent experiments.
Statistics
Since the study was designed to characterize safety, attenuation, and immunogenicity of PfGAP3KO, most data were descriptive. Where performed, statistical analyses included all evaluable study participants at each time point. For immunology assays, qualitative assay data analysis was performed by tabulating the frequency of positive responses for each assay at each time-point that an assessment was performed with crude response rates presented with corresponding exact 95% confidence interval estimates. No formal sample size calculation was conducted as the number of subjects that could be evaluated was limited by logistical considerations related to the large number of mosquitoes needed for vaccine administration. The ability of the study to detect parasitemia was expressed by the true event rate above which at least one study participant would likely be confirmed, via biomarker qRT-PCR, to have experienced parasitemia and the true event rate below which no such biomarker-based parasitemic events would likely be observed.
Supplementary Material
Acknowledgments:
We thank the study participants in the clinical trial as well as the staff of the Kaiser Permanente Washington Health Research Institute and the Seattle Children’s Research Institute Center for Mosquito Production and Malaria Infection Research for their critical contributions to the study.
Funding:
This work was supported by contract HHSN272201300019I from the National Institutes of Health.
Footnotes
Competing interests: AMV and SHIK hold a patent related to GAP creation, PCT Application No. PCT/US2018/015096; remaining authors have no stated conflicts.
Clinical Trials Registration: VTEU 14-0088 (Clinicaltrials.gov NCT03168854)
Data and materials availability:
All relevant data is described in the manuscript.
References:
- 1.World Malaria Report 2021 (2021) https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021). [Google Scholar]
- 2.Kappe SH, Vaughan AM, Boddey JA, Cowman AF, That was then but this is now: Malaria research in the time of an eradication agenda. Science 328, 862–866 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Draper SJ, Sack BK, King CR, Nielsen CM, Rayner JC, Higgins MK, Long CA, Seder RA, Malaria vaccines: Recent advances and new horizons. Cell Host Microbe 24, 43–56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RTS,S Clinical Trials Partnership [Agnandji, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Machevo S, Acacio S, Bulo H, Sigauque B, Macete E, Alonso P, Abdulla S, Salim N, Minja R, Mpina M, Ahmed S, Ali AM, Mtoro AT, Hamad AS, Mutani P, Tanner M, Tinto H, D’Alessandro U, Sorgho H, Valea I, Bihoun B, Guiraud I, Kabore B, Sombie O, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Oneko M, Odero C, Otieno K, Awino N, McMorrow M, Muturi-Kioi V, Laserson KF, Slutsker L, Otieno W, Otieno L, Otsyula N, Gondi S, Otieno A, Owira V, Oguk E, Odongo G, Woods JB, Ogutu B, Njuguna P, Chilengi R, Akoo P, Kerubo C, Maingi C, Lang T, Olotu A, Bejon P, Marsh K, Mwambingu G, Owusu-Agyei S, Asante KP, Osei-Kwakye K, Boahen O, Dosoo D, Asante I, Adjei G, Kwara E, Chandramohan D, Greenwood B, Lusingu J, Gesase S, Malabeja A, Abdul O, Mahende C, Liheluka E, Malle L, Lemnge M, Theander TG, Drakeley C, Ansong D, Agbenyega T, Adjei S, Boateng HO, Rettig T, Bawa J, Sylverken J, Sambian D, Sarfo A, Agyekum A, Martinson F, Hoffman I, Mvalo T, Kamthunzi P, Nkomo R, Tembo T, Tegha G, Tsidya M, Kilembe J, Chawinga C, Ballou WR, Cohen J, Guerra Y, Jongert E, Lapierre D, Leach A, Lievens M, Ofori-Anyinam O, Olivier A, Vekemans J, Carter T, Kaslow D, Leboulleux D, Loucq C, Radford A, Savarese B, Schellenberg D, Sillman M, Vansadia P, A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 367, 2284–2295 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olotu A, Fegan G, Wambua J, Nyangweso G, Awuondo KO, Leach A, Lievens M, Leboulleux D, Njuguna P, Peshu N, Marsh K, Bejon P, Four-year efficacy of RTS,S/AE01e and its interaction with malaria exposure. N Engl J Med 368, 1111–1120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datoo MS, Natama MH, Some A, Traore O, Rouamba T, Bellamy D, Yameogo P, Valia D, Tegneri M, Ouedraogo F, Soma R, Sawadogo S, Sorgho F, Derra K, Rouamba E, Orindi B, Ramos Lopez F, Flaxman A, Cappuccini F, Kailath R, Elias S, Mukhopadhyay E, Noe A, Cairns M, Lawrie A, Roberts R, Valea I, Sorgho H, Williams N, Glenn G, Fries L, Reimer J, Ewer KJ, Shaligram U, Hill AVS, Tinto H, Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: A randomised controlled trial. Lancet 397, 1809–1818 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nussenzweig RS, Vanderberg J, Most H, Orton C, Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature 216, 160–162 (1967). [DOI] [PubMed] [Google Scholar]
- 8.Clyde DF, Most H, McCarthy VC, Vanderberg JP, Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci 266, 169–177 (1973). [DOI] [PubMed] [Google Scholar]
- 9.Clyde DF, Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg 24, 397–401 (1975). [DOI] [PubMed] [Google Scholar]
- 10.Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, Matuschewski K, Kappe SH, Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci U S A 102, 3022–3027 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller AK, Labaied M, Kappe SH, Matuschewski K, Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433, 164–167 (2005). [DOI] [PubMed] [Google Scholar]
- 12.van Dijk MR, Douradinha B, Franke-Fayard B, Heussler V, van Dooren MW, van Schaijk B, van Gemert GJ, Sauerwein RW, Mota MM, Waters AP, Janse CJ, Genetically attenuated, p36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc Natl Acad Sci U S A 102, 12194–12199 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douradinha B, van Dijk MR, Ataide R, van Gemert GJ, Thompson J, Franetich JF, Mazier D, Luty AJ, Sauerwein R, Janse CJ, Waters AP, Mota MM, Genetically attenuated p36p-deficient Plasmodium berghei sporozoites confer long-lasting and partial cross-species protection. Int J Parasitol 37, 1511–1519 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Vaughan AM, O’Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, Cowman AF, Kappe SH, Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol 11, 506–520 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishizuka AS, Lyke KE, DeZure A, Berry AA, Richie TL, Mendoza FH, Enama ME, Gordon IJ, Chang LJ, Sarwar UN, Zephir KL, Holman LA, James ER, Billingsley PF, Gunasekera A, Chakravarty S, Manoj A, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, K CN, Murshedkar T, DeCederfelt H, Plummer SH, Hendel CS, Novik L, Costner PJ, Saunders JG, Laurens MB, Plowe CV, Flynn B, Whalen WR, Todd JP, Noor J, Rao S, Sierra-Davidson K, Lynn GM, Epstein JE, Kemp MA, Fahle GA, Mikolajczak SA, Fishbaugher M, Sack BK, Kappe SH, Davidson SA, Garver LS, Bjorkstrom NK, Nason MC, Graham BS, Roederer M, Sim BK, Hoffman SL, Ledgerwood JE, Seder RA, Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 22, 614–623 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sissoko MS, Healy SA, Katile A, Omaswa F, Zaidi I, Gabriel EE, Kamate B, Samake Y, Guindo MA, Dolo A, Niangaly A, Niare K, Zeguime A, Sissoko K, Diallo H, Thera I, Ding K, Fay MP, O’Connell EM, Nutman TB, Wong-Madden S, Murshedkar T, Ruben AJ, Li M, Abebe Y, Manoj A, Gunasekera A, Chakravarty S, Sim BKL, Billingsley PF, James ER, Walther M, Richie TL, Hoffman SL, Doumbo O, Duffy PE, Safety and efficacy of pfspz vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: A randomised, double-blind phase 1 trial. Lancet Infect Dis 17, 498–509 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler NS, Vaughan AM, Harty JT, Kappe SH, Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol 33, 247–254 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Hickey B, Teneza-Mora N, Lumsden J, Reyes S, Sedegah M, Garver L, Hollingdale MR, Banania JG, Ganeshan H, Dowler M, Reyes A, Tamminga C, Singer A, Simmons A, Belmonte M, Belmonte A, Huang J, Inoue S, Velasco R, Abot S, Vasquez CS, Guzman I, Wong M, Twomey P, Wojnarski M, Moon J, Alcorta Y, Maiolatesi S, Spring M, Davidson S, Chaudhury S, Villasante E, Richie TL, Epstein JE, Imras-a clinical trial of mosquito-bite immunization with live, radiation-attenuated P. falciparum sporozoites: Impact of immunization parameters on protective efficacy and generation of a repository of immunologic reagents. PLoS One 15, e0233840 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hickey BW, Lumsden JM, Reyes S, Sedegah M, Hollingdale MR, Freilich DA, Luke TC, Charoenvit Y, Goh LM, Berzins MP, Bebris L, Sacci JB Jr., De La Vega P, Wang R, Ganeshan H, Abot EN, Carucci DJ, Doolan DL, Brice GT, Kumar A, Aguiar J, Nutman TB, Leitman SF, Hoffman SL, Epstein JE, Richie TL, Mosquito bite immunization with radiation-attenuated Plasmodium falciparum sporozoites: Safety, tolerability, protective efficacy and humoral immunogenicity. Malar J 15, 377 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, Gordon DM, Stoute JA, Church LW, Sedegah M, Heppner DG, Ballou WR, Richie TL, Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis 185, 1155–1164 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Rieckmann KH, Human immunization with attenuated sporozoites. Bull World Health Organ 68 Suppl, 13–16 (1990). [PMC free article] [PubMed] [Google Scholar]
- 22.Rieckmann KH, Beaudoin RL, Cassells JS, Sell KW, Use of attenuated sporozoites in the immunization of human volunteers against falciparum malaria. Bull World Health Organ 57 Suppl 1, 261–265 (1979). [PMC free article] [PubMed] [Google Scholar]
- 23.Mwakingwe-Omari A, Healy SA, Lane J, Cook DM, Kalhori S, Wyatt C, Kolluri A, Marte-Salcedo O, Imeru A, Nason M, Ding LK, Decederfelt H, Duan J, Neal J, Raiten J, Lee G, Hume JCC, Jeon JE, Ikpeama I, Kc N, Chakravarty S, Murshedkar T, Church LWP, Manoj A, Gunasekera A, Anderson C, Murphy SC, March S, Bhatia SN, James ER, Billingsley PF, Sim BKL, Richie TL, Zaidi I, Hoffman SL, Duffy PE, Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity. Nature 595, 289–294 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, Spaarman L, de Mast Q, Roeffen W, Snounou G, Renia L, van der Ven A, Hermsen CC, Sauerwein R, Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 361, 468–477 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Khan SM, Janse CJ, Kappe SH, Mikolajczak SA, Genetic engineering of attenuated malaria parasites for vaccination. Curr Opin Biotechnol 23, 908–916 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Bijker EM, Borrmann S, Kappe SH, Mordmuller B, Sack BK, Khan SM, Novel approaches to whole sporozoite vaccination against malaria. Vaccine 33, 7462–7468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webster R, Sekuloski S, Odedra A, Woolley S, Jennings H, Amante F, Trenholme KR, Healer J, Cowman AF, Eriksson EM, Sathe P, Penington J, Blanch AJ, Dixon MWA, Tilley L, Duffy MF, Craig A, Storm J, Chan JA, Evans K, Papenfuss AT, Schofield L, Griffin P, Barber BE, Andrew D, Boyle MJ, de Labastida Rivera F, Engwerda C, McCarthy JS, Safety, infectivity and immunogenicity of a genetically attenuated blood-stage malaria vaccine. BMC Med 19, 293 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikolajczak SA, Lakshmanan V, Fishbaugher M, Camargo N, Harupa A, Kaushansky A, Douglass AN, Baldwin M, Healer J, O’Neill M, Phuong T, Cowman A, Kappe SH, A next-generation genetically attenuated Plasmodium falciparum parasite created by triple gene deletion. Mol Ther 22, 1707–1715 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kublin JG, Mikolajczak SA, Sack BK, Fishbaugher ME, Seilie A, Shelton L, VonGoedert T, Firat M, Magee S, Fritzen E, Betz W, Kain HS, Dankwa DA, Steel RW, Vaughan AM, Noah Sather D, Murphy SC, Kappe SH, Complete attenuation of genetically engineered Plasmodium falciparum sporozoites in human subjects. Sci Transl Med 9, eaad9099 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Roestenberg M, Walk J, van der Boor SC, Langenberg MCC, Hoogerwerf MA, Janse JJ, Manurung M, Yap XZ, Garcia AF, Koopman JPR, Meij P, Wessels E, Teelen K, van Waardenburg YM, van de Vegte-Bolmer M, van Gemert GJ, Visser LG, van der Ven A, de Mast Q, Natasha KC, Abebe Y, Murshedkar T, Billingsley PF, Richie TL, Sim BKL, Janse CJ, Hoffman SL, Khan SM, Sauerwein RW, A double-blind, placebo-controlled phase 1/2a trial of the genetically attenuated malaria vaccine PfSPZ-GA1. Sci Transl Med 12, eaaz5629 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Kebaier C, Voza T, Vanderberg J, Kinetics of mosquito-injected Plasmodium sporozoites in mice: Fewer sporozoites are injected into sporozoite-immunized mice. PLoS Pathog 5, e1000399 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanderberg JP, Frevert U, Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol 34, 991–996 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Flores-Garcia Y, Nasir G, Hopp CS, Munoz C, Balaban AE, Zavala F, Sinnis P, Antibody-mediated protection against Plasmodium sporozoites begins at the dermal inoculation site. mBio 9, e02194–02118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart MJ, Nawrot RJ, Schulman S, Vanderberg JP, Plasmodium berghei sporozoite invasion is blocked in vitro by sporozoite-immobilizing antibodies. Infect Immun 51, 859–864 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsen TM, Stone BC, Chuenchob V, Murphy SC, Prime-and-trap malaria vaccination to generate protective CD8(+) liver-resident memory T cells. J Immunol 201, 1984–1993 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Fernandez-Ruiz D, Ng WY, Holz LE, Ma JZ, Zaid A, Wong YC, Lau LS, Mollard V, Cozijnsen A, Collins N, Li J, Davey GM, Kato Y, Devi S, Skandari R, Pauley M, Manton JH, Godfrey DI, Braun A, Tay SS, Tan PS, Bowen DG, Koch-Nolte F, Rissiek B, Carbone FR, Crabb BS, Lahoud M, Cockburn IA, Mueller SN, Bertolino P, McFadden GI, Caminschi I, Heath WR, Liver-resident memory CD8+ T cells form a front-line defense against malaria liver-stage infection. Immunity 45, 889–902 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Gola A, Silman D, Walters AA, Sridhar S, Uderhardt S, Salman AM, Halbroth BR, Bellamy D, Bowyer G, Powlson J, Baker M, Venkatraman N, Poulton I, Berrie E, Roberts R, Lawrie AM, Angus B, Khan SM, Janse CJ, Ewer KJ, Germain RN, Spencer AJ, Hill AVS, Prime and target immunization protects against liver-stage malaria in mice. Sci Transl Med 10, eaap9128 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Murphy SC, Kas A, Stone BC, Bevan MJ, A T-cell response to a liver-stage plasmodium antigen is not boosted by repeated sporozoite immunizations. Proc Natl Acad Sci U S A 110, 6055–6060 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, Richman A, Velmurugan S, Reyes S, Li M, Tucker K, Ahumada A, Ruben AJ, Li T, Stafford R, Eappen AG, Tamminga C, Bennett JW, Ockenhouse CF, Murphy JR, Komisar J, Thomas N, Loyevsky M, Birkett A, Plowe CV, Loucq C, Edelman R, Richie TL, Seder RA, Hoffman SL, Live attenuated malaria vaccine designed to protect through hepatic CD8 T cell immunity. Science 334, 475–480 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJ, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BK, Ledgerwood JE, Graham BS, Hoffman SL, Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341, 1359–1365 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Epstein JE, Paolino KM, Richie TL, Sedegah M, Singer A, Ruben AJ, Chakravarty S, Stafford A, Ruck RC, Eappen AG, Li T, Billingsley PF, Manoj A, Silva JC, Moser K, Nielsen R, Tosh D, Cicatelli S, Ganeshan H, Case J, Padilla D, Davidson S, Garver L, Saverino E, Murshedkar T, Gunasekera A, Twomey PS, Reyes S, Moon JE, James ER, Kc N, Li M, Abot E, Belmonte A, Hauns K, Belmonte M, Huang J, Vasquez C, Remich S, Carrington M, Abebe Y, Tillman A, Hickey B, Regules J, Villasante E, Sim BK, Hoffman SL, Protection against plasmodium falciparum malaria by PfSPZ vaccine. JCI Insight 2, e89154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roestenberg M, Teirlinck AC, McCall MB, Teelen K, Makamdop KN, Wiersma J, Arens T, Beckers P, van Gemert G, van de Vegte-Bolmer M, van der Ven AJ, Luty AJ, Hermsen CC, Sauerwein RW, Long-term protection against malaria after experimental sporozoite inoculation: An open-label follow-up study. Lancet 377, 1770–1776 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, de Barra E, Havelock T, Bowyer G, Poulton ID, de Cassan S, Longley R, Illingworth JJ, Douglas AD, Mange PB, Collins KA, Roberts R, Gerry S, Berrie E, Moyle S, Colloca S, Cortese R, Sinden RE, Gilbert SC, Bejon P, Lawrie AM, Nicosia A, Faust SN, Hill AV, Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and me-trap against controlled human malaria infection in malaria-naive individuals. J Infect Dis 211, 1076–1086 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinhardt LC, Richie TL, Yego R, Akach D, Hamel MJ, Gutman JR, Wiegand RE, Nzuu EL, Dungani A, Kc N, Murshedkar T, Church LWP, Sim BKL, Billingsley PF, James ER, Abebe Y, Kariuki S, Samuels AM, Otieno K, Sang T, Kachur SP, Styers D, Schlessman K, Abarbanell G, Hoffman SL, Seder RA, Oneko M, Safety, tolerability, and immunogenicity of Plasmodium falciparum sporozoite vaccine administered by direct venous inoculation to infants and young children: Findings from an age de-escalation, dose-escalation, double-blind, randomized controlled study in western Kenya. Clin Infect Dis 71, 1063–1071 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sissoko MS, Healy SA, Katile A, Zaidi I, Hu Z, Kamate B, Samake Y, Sissoko K, Mwakingwe-Omari A, Lane J, Imeru A, Mohan R, Thera I, Guindo CO, Dolo A, Niare K, Koita F, Niangaly A, Rausch KM, Zeguime A, Guindo MA, Bah A, Abebe Y, James ER, Manoj A, Murshedkar T, Kc N, Sim BKL, Billingsley PF, Richie TL, Hoffman SL, Doumbo O, Duffy PE, Safety and efficacy of a three-dose regimen of Plasmodium falciparum sporozoite vaccine in adults during an intense malaria transmission season in Mali: A randomised, controlled phase 1 trial. Lancet Infect Dis 22, 377–389 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyke KE, Singer A, Berry AA, Reyes S, Chakravarty S, James ER, Billingsley PF, Gunasekera A, Manoj A, Murshedkar T, Laurens MB, Church WP, Garver Baldwin LS, Sedegah M, Banania G, Ganeshan H, Guzman I, Reyes A, Wong M, Belmonte A, Ozemoya A, Belmonte M, Huang J, Villasante E, Sim BKL, Hoffman SL, Richie TL, Epstein JE, Warfighter IIST, Multidose priming and delayed boosting improve Plasmodium falciparum sporozoite vaccine efficacy against heterologous P. falciparum controlled human malaria infection. Clin Infect Dis 73, e2424–e2435 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Goswami D, Betz W, Locham NK, Parthiban C, Brager C, Schafer C, Camargo N, Nguyen T, Kennedy SY, Murphy SC, Vaughan AM, Kappe SH, A replication-competent late liver stage-attenuated human malaria parasite. JCI Insight 5, e135589 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talley AK, Healy SA, Finney OC, Murphy SC, Kublin J, Salas CJ, Lundebjerg S, Gilbert P, Van Voorhis WC, Whisler J, Wang R, Ockenhouse CF, Heppner DG, Kappe SH, Duffy PE, Safety and comparability of controlled human Plasmodium falciparum infection by mosquito bite in malaria-naïve subjects at a new facility for sporozoite challenge. PLoS One 9, e109654 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy SC, Daza G, Chang M, Coombs R, Laser cutting eliminates nucleic acid cross-contamination in dried-blood-spot processing. J Clin Microbiol 50, 4128–4130 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaushansky A, Rezakhani N, Mann H, Kappe SH, Development of a quantitative flow cytometry-based assay to assess infection by Plasmodium falciparum sporozoites. Mol Biochem Parasitol 183, 100–103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finney OC, Keitany GJ, Smithers H, Kaushansky A, Kappe S, Wang R, Immunization with genetically attenuated P. falciparum parasites induces long-lived antibodies that efficiently block hepatocyte invasion by sporozoites. Vaccine 32, 2135–2138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chulay JD, Schneider I, Cosgriff TM, Hoffman SL, Ballou WR, Quakyi IA, Carter R, Trosper JH, Hockmeyer WT, Malaria transmitted to humans by mosquitoes infected from cultured Plasmodium falciparum. Am J Trop Med Hyg 35, 66–68 (1986). [DOI] [PubMed] [Google Scholar]
- 53.O’Neill MT, Phuong T, Healer J, Richard D, Cowman AF, Gene deletion from Plasmodium falciparum using Flp and Cre recombinases: Implications for applied site-specific recombination. Int J Parasitol 41, 117–123 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Maier AG, Braks JA, Waters AP, Cowman AF, Negative selection using yeast cytosine deaminase/uracil phosphoribosyl transferase in Plasmodium falciparum for targeted gene deletion by double crossover recombination. Mol Biochem Parasitol 150, 118–121 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data is described in the manuscript.


