Abstract
Tumor mutational burden (TMB) is an emerging biomarker for the prediction of immunotherapy success in solid tumors. Gliomas, however, do not demonstrate a correlation between TMB and immunotherapy efficacy. Here, we discuss the potential factors influencing this discordance, focusing on the impact of neoantigen immunogenicity, clonality, expression, and presentation.
Background on immunotherapy and TMB
Immunotherapy (see Glossary) has emerged as an effective therapeutic option for several cancers. While immunotherapy is effective in certain subsets of patients, it has not improved clinical outcomes for others, thus underscoring the importance of identifying predictive biomarkers of immunotherapy response [1].
TMB, as a biomarker of response, has consequently become a focus of interest. Somatic TMB is usually calculated using DNA-based sequencing strategies with both tumor and blood samples. However, variance in testing platforms, bioinformatics pipelines, tumor cell content, DNA quality, and TMB cutoff definitions according to tumor type have prevented the standardization and clinical utility of TMB measurements [2]. Nevertheless, TMB is proportional to the predicted neoantigen burden and tumors with a high TMB are more likely to have a high neoantigen burden that may induce an antitumor immune response [2]. Recently, the FDA approved the use of pembrolizumab, an anti-PD-1 inhibitor, for the treatment of solid tumors with a high TMB (ten or more mutations per megabase) based on the KEYNOTE-158 study, highlighting the importance of TMB as a biomarker in predicting response to immunotherapy.
By contrast, a high TMB in gliomas has not been reported to correlate with better survival outcomes in response to immunotherapy, as summarized in Table 1. One exception to this is in the reported cases of germline biallelic mismatch repair (MMR) deficiency (bMMRD) in children, which results in glioblastoma (GBM) with an extremely high TMB [3]. bMMRD GBMs, which have the highest known TMB among all human cancers and are unique for their ultrahypermutation, respond favorably to nivolumab treatment [3]. Otherwise, a high TMB in gliomas is not associated with improved survival following immunotherapy [1].
Table 1.
Summary of studies that disqualify TMB as a biomarker for immunotherapy efficacy in gliomas
| Study | Relevant study goal | Method | Relevant result | Conclusion |
|---|---|---|---|---|
| Select studies relating TMB and neoantigen immunogenicity | ||||
| Zhang et al. [5] | To identify a subgroup of IDH wild-type GBM patients that will show longest survival and benefit most from immunotherapy | Application of a neoantigen fitness model to 238 IDH wild-type GBM patients | High-quality neoantigen model and quantification of CD8+ T cell infiltration were used to identify a subset of patients that display a higher likelihood of response to immunotherapy, while TMB independently could not | TMB alone is inadequate in determining immunotherapy efficacy in GBMs |
| Rech et al. [6] | To characterize a new subset of neoantigens that display higher immunogenicity than classically defined neoantigens (CDNs) | Analysis of predicted neoantigens and immune activity in 6324 patients across 27 tumor types from The Cancer Genome Atlas (TCGA) | Characterized alternatively defined neoantigens (ADNs), which display higher affinity to MHC-I and MHC-II molecules than CDNs and can predict immune phenotype and patient survival LGGs and GBMs displayed comparatively lower numbers of mean ADNs (28 and 38, respectively) |
LGGs and GBMs tend to harbor lower numbers of immunogenic neoantigens, likely because they produce fewer ADNs per mutation |
| Select studies relating TMB and neoantigen clonality | ||||
| Touat et al. [10] | To understand mutational landscape differences between gliomas and other cancers and their impact on immunotherapy response | Analysis of mutational burden and signatures in 10294 gliomas | MMR-deficient gliomas lacked T cell infiltration despite TMB being similar to other hypermutated cancers Hypermutated gliomas demonstrated greater subclonal neoantigens than other hypermutated cancers | GBMs, especially post-TMZ treatment, with high TMB harbor large subclonal neoantigen populations |
| Kim et al. [9] | To understand the intratumoral clonal composition of GBMs and the impact of therapeutic intervention | Analysis of 252 GBM samples from TCGA and 60 biopsies from 23 pairs of pre- and post-treatment GBMs | Two independently sequenced biopsies from a single post-TMZ hypermutated recurrent glioma revealed 2429 and 5980 mutations, respectively, but only 163 shared mutations Presence of TP53 mutations in both primary and recurrent tumor samples was associated with increased frequency of subclonal neoantigens in samples | Post-TMZ GBMs with high TMB carry large subclonal neoantigen populations TP53-mutated GBMs may show favorable outcomes with combination therapies targeting subclonal neoantigens |
| Select studies relating TMB and neoantigen expression and presentation | ||||
| Nejo et al. [11] | To investigate changes in the neoantigen landscape during glioma progression | Exome and RNA-seq analysis of 25 pairs of primary and recurrent grade II–IV gliomas | No difference in the total number of neoantigens between primary and recurrent samples Neoantigen expression ratio decreased significantly in the recurrent tumor samples for neoantigens predicted to be highly immunogenic and clonal | A decrease in neoantigen expression ratio occurs due to immune selective pressure against highly immunogenic neoantigens, resulting in neoantigen ‘invisibility’ Treatments that overcome this immune evasion mechanism are required in addition to immunotherapy |
| Facoetti et al. [12] | To determine frequency of HLA and APM component abnormalities in malignant brain tumors | Analysis of 88 surgically removed malignant astrocytic tumors | ~50% of GBM lesions and ~20% of grade II astrocytoma lesions had HLA-I loss ~70% oftumors showed selective HLA-A downregulation; this is higher than in other malignancies ~20% of GBMs showed downregulation of tapasin, an APM component |
Presence of HLA-I and APM defects in malignant brain tumors may explain lack of immunotherapy efficacy in gliomas with high TMB |
| Yeung et al. [13] | To determine prevalence of LOH in HLA-I and B2M and its impact on survival in GBM patients | Cross-sectional analysis of 60 adult GBM patients | 41.4% of cases displayed LOH in HLA-I, which was associated with poorer survival 18.2% ofcases displayed LOH in B2M HLA-I downregulation seen in 22–43% of cases |
LOH in HLA-I and B2M is common in GBM patients and may reflect another mechanism of immune escape employed by tumors |
| Mehling et al. [14] | To investigate impact of APM component defects in astrocytomas with intact HLA-I expression | Analysis of 16 WHO grade I–IV astrocytomas | Downregulation of APM component (LMP2, TAP1, B2M) expression in astrocytomas, in contrast to normal expression of components in non-pathological astrocytes | Peptide-free expression of HLA-I molecules (due to APM impairment) in astrocytomas prevents activation of CD8+ T cells and inhibits NK cells |
The disconnect observed between TMB and immunotherapy response in gliomas can potentially be attributed to the lack of efficacy of immunotherapy in general. This lack of efficacy can be explained by several factors including, but not limited to, an immunosuppressive tumor microenvironment (TME), the presence of the blood–brain barrier, T cell exhaustion, and epigenetic silencing events that modulate immune responses, especially in isocitrate dehydrogenase (IDH)-mutant gliomas [4]. Importantly, the high discordance observed between TMB and immunotherapy response in gliomas may also be a consequence of neoantigen-specific factors, such as low neoantigen immunogenicity, high neoantigen heterogeneity, and the lack of neoantigen expression and presentation.
TMB and neoantigen immunogenicity
One would expect that the tumors with a high TMB bear a higher quantity of neoantigens, but these neoantigens are not always equal in their ability to generate an effective antitumor response (Figure 1A). Studies have suggested that a large pool of neoantigens may generate a significantly weaker immune response than a much smaller pool of highly immunogenic neoantigens [5,6]. This highlights the importance of the correlation between the quality, rather than the quantity, of a neoantigen and its generated immune response, and demonstrates a critical disconnect between TMB and the immune response since TMB measurements reflect the quantity but not quality of neoantigens.
Figure 1. Neoantigen-related reasons for discordance between tumor mutational burden and immunotherapy efficacy in gliomas.
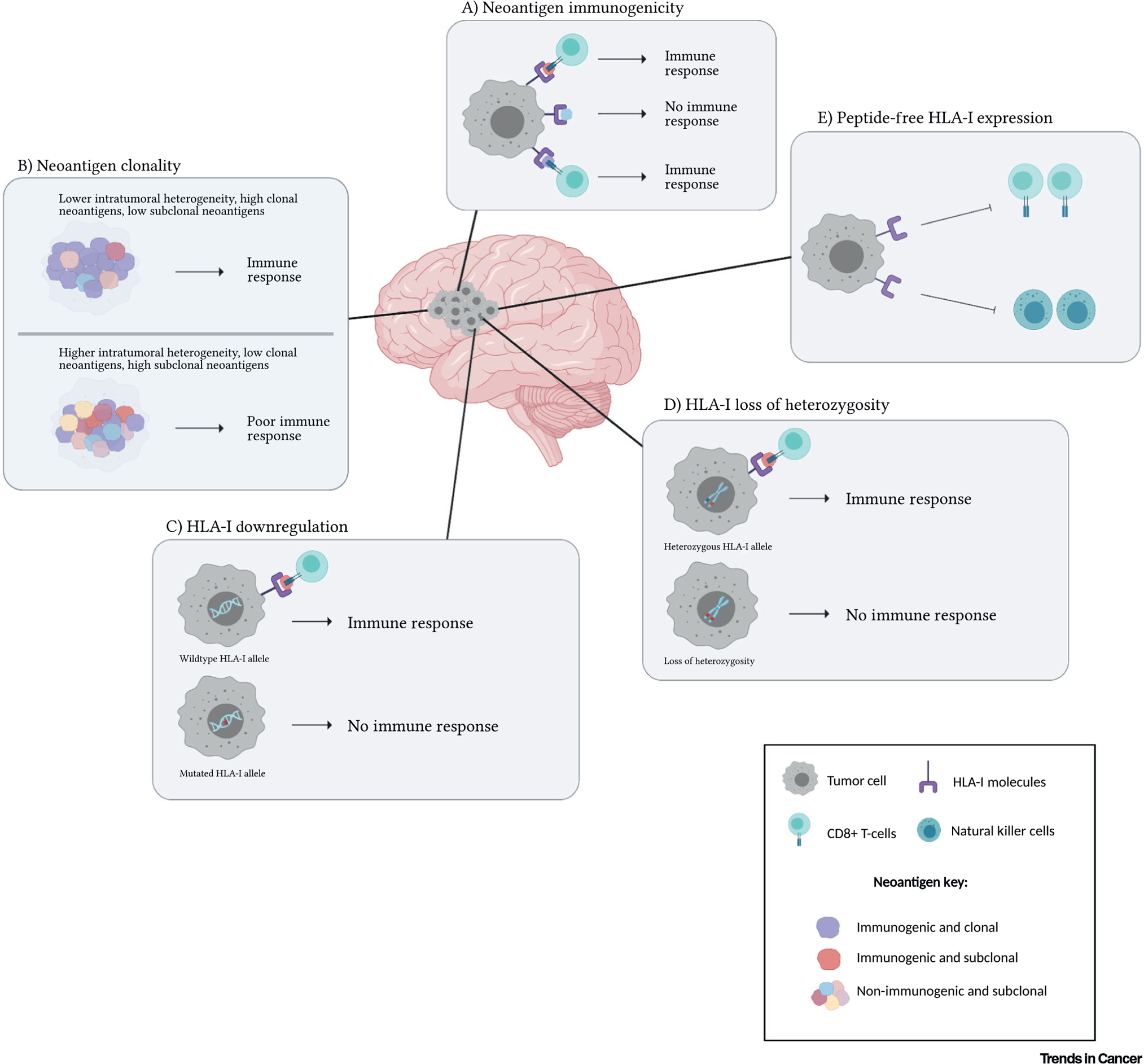
(A) Neoantigen immunogenicity: Immunogenic neoantigens (red and purple) show high-affinity binding to human leukocyte antigen class I (HLA-I) molecules and allow T cell recognition. Non-immunogenic neoantigens (blue) do not elicit an immune response, resulting in immune evasion. (B) Neoantigen clonality: High neoantigen heterogeneity in gliomas (below) gives rise to several subclonal neoantigens populations (pink, blue, yellow, red) and very few clonal neoantigens (purple) compared to tumors with lower neoantigen heterogeneity (above). (C) HLA-I downregulation: Wild-type HLA-I results in the expression of HLA-I molecules and the presentation of neoantigens to cytotoxic T cells (above). Mutations in the HLA-I genes may cause downregulation of HLA-I molecule expression, in turn hindering the presentation of neoantigens (below). (D) HLA-I loss of heterozygosity (LOH): Heterozygous HLA-I alleles allow normal expression of HLA-I molecules and presentation of neoantigens (above). However, LOH in HLA-I alleles can prevent the expression of HLA-I molecules and affect neoantigen presentation (below). (E) Peptide-free HLA-I expression: Peptide-free intact HLA-I expression inhibits CD8+ T cell and natural killer (NK) cell activation.
Generally, neoantigens are computationally identified by predicting their ability to bind with high affinity to MHC molecules. However, these predicted neoantigens may not be immunogenic in vivo due to additional characteristics involved in determining their true immunogenicity [7]. A neoantigen fitness model has thus been developed to incorporate these characteristics and better predict the immunogenicity of neoantigens. This model is based on the product of two factors, A*R. The amplitude (A), also commonly referred to as the differential agretopicity index (DAI), measures the ratio of the MHC molecule-binding affinity of the mutant peptide to the binding affinity of the non-mutant counterpart [7]. The intrinsic T cell receptor (TCR) recognition probability (R) is determined by aligning the neoantigen to other positively recognized MHC-I-restricted T cell antigens from the Immune Epitope Database (IEDB) [7]. This model has been shown to better predict survival after immunotherapy in gliomas and other cancer types (Table 1, Studies by Zhang et al. and Rech et al.) [5–7].
Overall, TMB and affinity for MHC-I molecules do not fully reflect the functional immunogenicity of neoantigens. Information about the quality or ability of a neoantigen to generate an immune response is crucial in predicting immunotherapy efficacy.
Subclonal neoantigens and immunotherapy resistance
Another important factor influencing the discordance between TMB and immunotherapy efficacy in gliomas is the high degree of subclonal neoantigens, which arises due to extensive neoantigen heterogeneity. In a study conducted to understand the impact of neoantigen heterogeneity on antitumor immunity, a significant correlation was found between high clonal neoantigen burden and longer overall survival in patients with lung adenocarcinoma [8]. Furthermore, 12 of 13 patients with a high TMB and high clonal neoantigen population exhibited durable clinical benefit (DCB) with anti-PD-1 therapy, while only 2 of 18 patients with a high TMB and high subclonal population showed DCB. This suggests that tumors with more clonal neoantigens respond favorably to immunotherapy [8]. Gliomas harbor large subclonal neoantigen populations, which may contribute to a high TMB but are likely to not translate into effective immunotherapy responses (Figure 1B) [8–10].
Hypermutation can be acquired both de novo and post-treatment. While hypermutation is rare in primary gliomas, it is more common in recurrent gliomas that have been treated with temozolomide (TMZ) and thus its impact on neoantigen populations in GBMs has been studied extensively [9,10]. Interestingly, treatment with TMZ promotes the expansion of MMR-deficient cells and causes the late accumulation of random TMZ-induced mutations. Therefore, TMZ-induced hypermutated GBMs may also harbor several subclonal neoantigens, which are unable to generate a sufficient antitumor immune response, leading to a lack of immunotherapy efficacy (Table 1, Studies by Touat et al. and Kim et al.) [10].
In summary, the high degree of neoantigen heterogeneity in gliomas may lead to a high TMB and promote the emergence of several subclonal neoantigen populations, thus contributing to immunotherapy resistance and the discordance between TMB and immunotherapy efficacy in gliomas.
Effect of neoantigen expression and presentation on immune recognition
While neoantigen characteristics such as immunogenicity and clonality play vital roles in the ability to generate an immune response, the expression and presentation of these neoantigens are equally important. Antigen processing and presentation is a complex, multistep process that can be dysregulated at various levels, potentially affecting response to immunotherapy. Since TMB calculations do not include a measurement of these pathways, this could further contribute to the discordance between TMB and immunotherapy efficacy.
First, selective immune pressure may decrease the expression of highly immunogenic and clonal neoantigen populations, thereby causing neoantigen ‘invisibility’ and tumor evasion (Table 1, Study by Nejo et al.) [11]. Second, human leukocyte antigen class I (HLA-I) molecules may be reversibly downregulated secondary to impaired antigen presentation machinery (APM) or epigenetic silencing, resulting in reduced presentation of neoantigens (Table 1, Study by Facoetti et al.) (Figure 1C) [12]. Furthermore, selective immune pressure may also result in loss of heterozygosity (LOH) of HLA-I and β-2-microglobulin genes, which can lead to their irreversible downregulation, thus preventing immunogenic neoantigens from being presented to the immune system (Table 1, Study by Yeung et al.) (Figure 1D) [13]. This may also be a mechanism of treatment resistance due to tumor evolution and selective immune pressure. Finally, although only preliminary findings have been published, impaired APM may also result in peptide-free HLA-I expression, thus preventing CD8+ T cell activation and inhibiting natural killer (NK) cells (Table 1, Study by Mehling et al.) (Figure 1E) [14].
Together, the effect of selective immune pressure and HLA-I or APM dysregulation can prevent adequate presentation of neoantigens to the immune system, thus affecting their recognition by cytotoxic T cells and contributing to the lack of immunotherapy efficacy despite a potentially high TMB.
Concluding remarks and future directions
Overall, a high TMB is not indicative of response to immunotherapy for gliomas due to the complexities of neoantigen immunogenicity, clonality, presentation, and expression. Furthermore, TMB calculations do not capture the impact of other factors, such as the immunosuppressive TME and T cell exhaustion, on the response to immunotherapy and thus do not provide an accurate measurement of immunotherapy efficacy in gliomas.
It remains to be further explored whether alternative biomarkers can be used to better predict the response to immunotherapy. Cancer germline antigens (CGAs), for example, have similar expression levels across tumor types with different genomic features and TMBs. They may be of value as biomarkers once the quality of immune responses against neoantigens and CGAs are well studied [15].
Since studies that have examined the correlation between TMB and immunotherapy response in gliomas have largely been retrospective analyses, prospective clinical trials that standardize TMB calculation and use carefully designed correlative studies in glioma patients are needed to provide further insight into the use of TMB as a predictive biomarker of immunotherapy efficacy. Furthermore, while TMB may become of value as a biomarker in some capacity on further investigation, it is unlikely that a single biomarker will be able to predict immunotherapy efficacy in gliomas. Instead, it is more probable that composite biomarkers, including those describing immune infiltrates and TME composition, will be required to successfully predict immunotherapy response in glioma patients.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health (NIH) and the NIH Lasker Clinical Research Scholars Program. The authors also thank all of the patients and families for their participation in brain tumor clinical trials and for their support of our clinical research.
Glossary
- ß-2-Microglobulin
an important component of the HLA-I complex that is involved in presentation of neoantigens
- Alternatively defined neoantigens (ADNs)
the neoantigens with a greater than ten times improvement in MHC-I binding affinity and a greater than four times improvement in MHC-II binding affinity compared with their non-mutant counterpart
- Antigen presentation machinery (APM)
the components involved in the processing and presentation of antigens
- Cancer germline antigens (CGAs)
a class of immunogenic antigens that are not expressed in normal human tissue (other than testes) but are often expressed on cancer cells
- Classically defined neoantigens (CDNs)
the neoantigens computationally identified by predicting their ability to bind with high affinity to MHC molecules
- Clonal neoantigens
the neoantigens that are derived from the mutations that occur early in tumorigenesis and are found in most tumor cells
- Human leukocyte antigen class I (HLA-I)
the MHC-I molecules in humans that are involved in antigen processing and presentation in humans. HLA gene products are divided into classes I, II, and III based on their structure and function, and HLA-I molecules are responsible for presenting endogenous peptides to CD8+ T-cells. Furthermore, the diversity of HLA-I molecules (HLA-A, HLA-B, HLA-C) influences the number of unique neoantigens that are presented, as they are all responsible for binding specific peptides
- Hypermutation
the process of accumulating an unusually high number of somatic mutations
- Immunogenic neoantigen
a neoantigen that is capable of being recognized by the host immune system and eliciting an immune response
- Immunotherapy
a form of treatment that activates the immune system to attack the disease
- Loss of heterozygosity (LOH)
the loss of an allele at a heterozygous locus either via simple deletion of an allele or by deletion of an allele followed by the duplication of the remaining allele
- Neoantigen expression ratio
the ratio of expressed neoantigens to predicted neoantigens
- Neoantigens
the tumor-specific peptides derived from somatic mutations that are absent in normal human cells
- Selective immune pressure
the process of elimination of certain immunogenic neoantigens, which leads to the accumulation of neoantigens that are advantageous to the tumor cells and can promote immune evasion
- Subclonal neoantigens
the neoantigens that are derived from the mutations that occur later in malignant transformation and are found in only a fraction of tumor cells
- tumor mutational burden (TMB)
the total number of mutations found in the DNA of cancer cells
Footnotes
Declaration of interests
No interests are declared.
References
- 1.Samstein RM et al. (2019) Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 51, 202–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melendez B et al. (2018) Methods of measurement for tumor mutational burden in tumor tissue. Transl. Lung Cancer Res. 7, 661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouffet E et al. (2016) Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J. Clin. Oncol. 34, 2206–2211 [DOI] [PubMed] [Google Scholar]
- 4.Sampson JH et al. (2020) Brain immunology and immunotherapy in brain tumours. Nat. Rev. Cancer 20, 12–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J et al. (2019) The combination of neoantigen quality and T lymphocyte infiltrates identifies glioblastomas with the longest survival. Commun. Biol. 2, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rech AJ et al. (2018) Tumor immunity and survival as a function of alternative neopeptides in human cancer. Cancer Immunol. Res. 6, 276–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luksza M et al. (2017) A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 551, 517–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGranahan N et al. (2016) Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H et al. (2015) Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res. 25, 316–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Touat M et al. (2020) Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 580, 517–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nejo T et al. (2019) Reduced neoantigen expression revealed by longitudinal multiomics as a possible immune evasion mechanism in glioma. Cancer Immunol. Res. 7, 1148–1161 [DOI] [PubMed] [Google Scholar]
- 12.Facoetti A et al. (2005) Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin. Cancer Res. 11, 8304–8311 [DOI] [PubMed] [Google Scholar]
- 13.Yeung JT et al. (2013) LOH in the HLA class I region at 6p21 is associated with shorter survival in newly diagnosed adult glioblastoma. Clin. Cancer Res. 19, 1816–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehling M et al. (2007) WHO grade associated downregulation of MHC class I antigen-processing machinery components in human astrocytomas: does it reflect a potential immune escape mechanism? Acta Neuropathol. 114, 111–119 [DOI] [PubMed] [Google Scholar]
- 15.Angelova M et al. (2015) Characterization of the immunophenotypes and antigenomes of colorectal cancers reveals distinct tumor escape mechanisms and novel targets for immunotherapy. Genome Biol. 16, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]


