Abstract
Objectives
The activator protein-1 (AP-1) transcription factor component c-Fos regulates chondrocyte proliferation and differentiation, but its involvement in osteoarthritis (OA) has not been functionally assessed.
Methods
c-Fos expression was evaluated by immunohistochemistry on articular cartilage sections from patients with OA and mice subjected to the destabilisation of the medial meniscus (DMM) model of OA. Cartilage-specific c-Fos knockout (c-FosΔCh) mice were generated by crossing c-fosfl/fl to Col2a1-CreERT mice. Articular cartilage was evaluated by histology, immunohistochemistry, RNA sequencing (RNA-seq), quantitative reverse transcription PCR (qRT-PCR) and in situ metabolic enzyme assays. The effect of dichloroacetic acid (DCA), an inhibitor of pyruvate dehydrogenase kinase (Pdk), was assessed in c-FosΔCh mice subjected to DMM.
Results
FOS-positive chondrocytes were increased in human and murine OA cartilage during disease progression. Compared with c-FosWT mice, c-FosΔCh mice exhibited exacerbated DMM-induced cartilage destruction. Chondrocytes lacking c-Fos proliferate less, have shorter collagen fibres and reduced cartilage matrix. Comparative RNA-seq revealed a prominent anaerobic glycolysis gene expression signature. Consistently decreased pyruvate dehydrogenase (Pdh) and elevated lactate dehydrogenase (Ldh) enzymatic activities were measured in situ, which are likely due to higher expression of hypoxia-inducible factor-1α, Ldha, and Pdk1 in chondrocytes. In vivo treatment of c-FosΔCh mice with DCA restored Pdh/Ldh activity, chondrocyte proliferation, collagen biosynthesis and decreased cartilage damage after DMM, thereby reverting the deleterious effects of c-Fos inactivation.
Conclusions
c-Fos modulates cellular bioenergetics in chondrocytes by balancing pyruvate flux between anaerobic glycolysis and the tricarboxylic acid cycle in response to OA signals. We identify a novel metabolic adaptation of chondrocytes controlled by c-Fos-containing AP-1 dimers that could be therapeutically relevant.
Keywords: Osteoarthritis; Chondrocytes; Arthritis, Experimental
WHAT IS ALREADY KNOWN ON THIS TOPIC
Anaerobic glycolysis is the primary energy source in healthy articular cartilage chondrocytes, but the early metabolic response of chondrocytes at the onset of osteoarthritis (OA) and how this could affect disease outcome is not known.
WHAT THIS STUDY ADDS
First time in vivo investigation of the role of c-Fos/AP-1 (activator protein-1) in chondrocyte metabolism using genetically modified mouse models, in situ metabolic enzyme assays and comparative OMIC analyses, providing insights into the early events of OA pathogenesis.
Demonstration that c-Fos, highly expressed in human and mouse OA samples, is essential for the cartilage-protective response of chondrocytes to stress.
Discovering that c-Fos regulates cellular bioenergetics and cartilage integrity in OA chondrocytes by modulating the activity of enzyme complexes that control pyruvate usage between anaerobic glycolysis and the tricarboxylic acid (TCA)-cycle/oxidative phosphorylation (OXPHOS).
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study provides new insights into how articular chondrocytes adapt to OA-associated signals and demonstrates a crucial role of cellular bioenergetics in cartilage integrity. Testing whether therapeutic intervention aimed at boosting the early metabolic stress response of chondrocytes to OA signals in larger and appropriately designed studies is crucial to improve disease outcomes, prevent disability and reduce healthcare and societal costs.
Introduction
Osteoarthritis (OA) is the most common joint disease and its prevalence is growing in developed countries due to population ageing, more frequent biomechanical trauma and obesity.1 2 OA has long been considered the consequence of a ‘wear and tear’ process leading to loss of articular cartilage. However, accumulating clinical and experimental data have changed this perception. Synovial inflammation is frequently present in clinical OA and in animal models3 4, and metabolic mediators have been implicated in disease onset and/or progression.5–8 These modulate metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) families that breakdown articular cartilage during OA progression.9 10 Obesity and metabolic syndrome are recognized as strong risk factors for hand OA, but also knee OA, where low-grade chronic inflammation and systemic metabolic alterations disrupt joint tissue homoeostasis.2 5 11 Several lines of evidence suggest that cells in the joint are subjected to metabolic alterations and shift from a resting state to a metabolically active state in order to meet the energy requirements of extracellular matrix (ECM) biosynthesis, cell proliferation and survival.7 12 How articular chondrocytes, that primarily rely on glycolysis in low oxygen homoeostatic conditions, respond to joint damage and adapt to microenvironmental changes in articular cartilage is an important yet poorly understood question.
A large fraction (40%) of knee OA is heritable and genome-wide association studies indicated that most OA risk variants are located in non-coding sequences, and enriched close to genes involved in bone and cartilage development.13 14 This suggests an important role for chondrocyte regulatory elements together with non-genetic factors in OA onset and/or development. Importantly, mutations in cartilage matrix genes such as COL2A1, COL9A3, COL11A2 and cartilage oligomeric matrix protein (COMP), which are produced by articular chondrocytes, cause chondrodysplasia with early-onset OA.15 Recent advances in imaging technologies revealed that morphological changes in the collagen network and biomechanical changes in the articular cartilage are already present in early-stage OA.16 This implies that chondrocytes maintain the structural and functional integrity of cartilage and sense microenvironmental changes during OA onset, although the underlying cartilage protective mechanisms remain elusive.
The Jun (c-Jun, JunB, JunD) and Fos (c-Fos, FosB, Fra1, Fra2) proteins are components of the dimeric activator protein-1 (AP-1) transcription factor complex.17 While Jun proteins can form homodimers or heterodimers, Fos proteins can only form heterodimers. AP-1 is activated by various signals, such as growth factors, inflammatory cytokines, mechanical and oxidative stress,18–20 and has an essential role in cartilage and bone physiology.17 21 22 For example, c-Jun is required for joint cell specification and intervertebral disc formation, while Fra2 regulates cartilage development and chondrocyte differentiation.23 24 Importantly, ectopic expression of c-Fos in H2-c-fosLTR transgenic mice drives osteoblast and chondrocyte proliferation resulting in osteosarcoma and chondrogenic sarcoma.25 26 In humans two single nucleotide polymorphisms in the FOS promoter were found to be associated with knee-OA susceptibility.27 While the functional role of AP-1 in OA has not yet been thoroughly assessed in vivo, these data suggest that AP-1 dimers are likely important players in joint and cartilage biology, which led us to hypothesize that c-Fos expression in chondrocytes is functionally relevant in OA.
Here we show that FOS protein expression is increased in OA cartilage. To investigate the role of c-Fos containing AP-1 dimers (c-Fos/AP-1) in cartilage integrity, c-Fos was genetically inactivated in chondrocytes in the context of a well-established experimental OA model. We demonstrate that c-Fos controls chondrocyte response to microenvironmental stress caused by biomechanical and inflammatory cues. Furthermore, we identify a switch in energy metabolism from aerobic glycolysis to pyruvate oxidation and tricarboxylic acid (TCA) cycle in chondrocytes as a central mechanism in the early stages of experimental OA, with pyruvate and lactate dehydrogenases as critical metabolic enzymes downstream of c-Fos.
Results
Damaged human and murine knee cartilage expresses high c-Fos/AP-1
First, FOS protein expression was evaluated in articular cartilage from 20 patients with knee OA. Cartilage destruction was graded using the Mankin score (MS)28 and for each patient, two regions with high (MS≥8) or low (MS≤5) scores were selected and subjected to FOS immunohistochemistry (IHC). Regardless of the overall MS of the section, chondrocytes in the damaged regions close to the cartilage surface displayed more intense FOS staining, compared with cells in the middle and/or deeper areas (figure 1A). Quantitative analysis further revealed that FOS-positive articular chondrocytes were more abundant in severely damaged cartilage areas (MS 8–14) compared with non-damaged and/or mildly-damaged (MS 0–5) regions (figure 1B). Thus FOS expression is most elevated in OA-affected cartilage, compared with less damaged regions.
Figure 1.
c-Fos is activated in articular chondrocytes in patients with OA and a mouse OA. (A and B) Femoral condyles of 20 patients undergoing knee arthroplasty were collected and histopathologically graded using the Mankin score (MS; 0: most intact; 14: most degenerated). From each patient, tissue regions with MS 0–5 and MS 8–14 were selected and histological sections were stained immunohistochemically (IHC) with antibodies against c-Fos. (A) IHC images of c-Fos in representative cartilage regions with MS 4 (left) and MS 9 (right). (B) Quantification of c-Fos-positive in MS 0–5 and MS 8–14 regions. The dotted lines connect the sample pairs from each patient. Statistical differences between groups were analysed by Mann-Whitney test. (C and D) 10 weeks-old wild-type mice were subjected to DMM (n=7)/sham (n=4) and cartilage damage was evaluated by Osteoarthritis Research Society International (OARSI) system 2 and 8 weeks post surgery. Red and black indicate c-Fos positive and negative cells, respectively. (C) Representative images of safranin O/fast green staining of the joint. (D) Quantification of cartilage damage. Black arrows indicate the damaged area. Statistical differences between groups were analysed by Mann-Whitney test. (E) Representative IHC images of c-Fos at 2 and 8 weeks post surgery. (F) Quantification of c-Fos positive cells. Red arrows indicate positive cells. Bar graphs and plots represent or include mean±SD, respectively. *p<0.05, **p<0.01, ***p<0.001, ***p<0.0001. Statistical differences between groups were analysed by non-parametric Mann-Whitney test in B and by two-way ANOVA with Bonferroni post hoc analysis in D and F. ANOVA, analysis of variance; DMM, destabilisation of the medial meniscus; OA, osteoarthritis; OARSI, Osteoarthritis Research Society International.
Next, adult wild-type mice were subjected to a well-established, surgery-induced OA model: destabilisation of the medial meniscus (DMM).29 Articular cartilage damage was observed 8 weeks post DMM (figure 1C and D). IHC revealed increased expression of c-Fos and phosphorylated (activated) c-Fos in articular chondrocytes at 2 and 8 weeks post DMM (figure 1E and F and online supplemental figure 1A,B). c-Jun expression was also significantly elevated, although phosphorylated c-Jun was unchanged (online supplemental figure 1C,D). Sox9, a master regulator of chondrogenesis often cooperating with AP-1 was also increased (online supplemental figure 1E,F).22 Importantly, the number of c-Fos-positive chondrocytes was already increased 2 weeks post DMM (figure 1E and F), before any damage was observed (figure 1D) suggesting that c-Fos/AP-1 could be implicated in the response of chondrocytes to OA-inducing signals.
ard-2023-224002supp001.pdf (1.9MB, pdf)
Articular cartilage is protected by c-Fos in the DMM mouse model
Cartilage-specific tamoxifen (TAM)-inducible c-Fos loss-of-function (LOF) mice were generated combining c-fosflox/flox and Col2a1-CreERT by genetic crosses (c-FosΔCh mice).30 31 In c-FosΔCh mice, a nuclear localisation signal-enhanced green fluorescent protein (nls-EGFP) sequence is inserted in the c-fos locus to place nuclear EGFP expression under the control of the c-fos promoter and regulatory elements on CRE/LoxP deletion (figure 2A). Immunofluorescence (IF) of EGFP in intact knee joints (10 weeks of age) revealed that approximately 70% (62–90%) of articular chondrocytes expressed EGFP 1 week after TAM injection (figure 2B) and a number of c-Fos-deficient (EGFP positive) chondrocytes were still detected in the articular cartilage 1 year later (online supplemental figure 2A,B). While no obvious growth abnormalities were observed in c-FosΔCh mice, safranin O/fast green staining of knee joint sections revealed lower glycosaminoglycans (GAGs) content, but comparable cartilage area in 1-year-old c-FosΔCh compared with c-FosWT mice (online supplemental figure 2B–D).
Figure 2.
c-Fos protects knee cartilage in experimental OA. (A) Targeting strategy and structure of the floxed/deleted c-Fos allele. Cre expression results in deletion of exons 2–4 and expression of nuclear EGFP under the control of the c-fos promoter. Coding areas (exon 1–4) are depicted in grey boxes. Experimental procedure and timeline to delete c-fos in chondrocytes and experimentally induced cartilage damage (DMM). Tamoxifen was injected intraperitoneally at two time points (2.5 and 9 weeks of age, 2 mg/mouse/day, 5 consecutive days) and mice were subjected to DMM/sham at 10 weeks of age and knee joints analysed 8 weeks post surgery. (B) Analysis of c-Fos deletion by anti-GFP immunofluorescence (red) in c-FosWT and c-FosΔCh mice articular cartilage at 10 weeks of age. The left panels are representative images of GFP-positive articular chondrocytes before surgery. (nuclei counterstained with DAPI) while % GFP-positive articular chondrocytes are plotted on the right. Articular cartilage is depicted with white dashed lines. (C) Representative images of safranin O/fast green staining of knee joints from c-FosWT mice and c-FosΔCh mice 8 weeks post surgery. (D) Quantification of cartilage damage on the medial side. (E) Relative cartilage area quantified by ImageJ analysis. Bar graphs and plots represent or include mean±SD, respectively. *p<0.05, **p<0.01, and ***p<0.001. Statistical differences between groups were analysed by non-parametric Mann-Whitney test in B and by two-way ANOVA with Bonferroni post hoc analysis in D and E. ANOVA, analysis of variance; DAPI, 4′,6-diamidino-2-phenylindole; DMM, destabilisation of the medial meniscus; EGFP, enhanced green fluorescent protein; OA, osteoarthritis; OARSI, Osteoarthritis Research Society International; TAM, Tamoxifen.
Next, 10-week-old c-FosWT and c-FosΔCh mice were subjected to DMM. While osteophyte (OP)-like bone/cartilage formations were observed in the medial tibial plateau and OP maturity increased over time, no significant differences in OP size and maturity were noted between operated groups (online supplemental figure 2F–G) and synovial thickening was comparable 2 weeks post DMM (online supplemental figure 2H,I). However, OARSI scoring revealed more severe DMM-induced cartilage damage in c-FosΔCh mice 8 weeks post surgery than those from c-FosWT mice (figure 2C and D). Consistently, the cartilage area after DMM was significantly smaller in c-FosΔCh mice than in c-FosWT mice (figure 2E). These data suggest that c-Fos expression in articular chondrocytes has a protective role during DMM-induced cartilage damage.
Chondrocyte proliferation and collagen production during cartilage degeneration rely on c-Fos expression
DMM-induced articular chondrocyte proliferation was lower in c-FosΔCh compared with c-FosWT mice as shown by Ki67 (figure 3A and B) and PCNA IHC (online supplemental figure 3A,B) and this was consistent with decreased chondrocyte density in Fos mutants (figure 3C). Collagen was next assessed by picrosirius red staining and polarised light visualisation as well as by IF for the Col2a1 subunit of collagen type II, the major cartilage extracellular matrix (ECM) component. Eight weeks post surgery, articular cartilage in DMM/sham-treated c-FosWT mice and sham-treated c-FosΔCh mice was composed of collagen bundles (yellow/green), while collagen fibres in DMM-treated c-FosΔCh appeared less stained with picrosirius red (figure 3D). Digital image analyses (online supplemental figure 3C) further documented decreased collagen area (figure 3E) and reduced fibre length in c-FosΔCh mice after DMM (figure 3F). Consistently, while Col2a1-immunopositive area was increased by DMM in c-FosWT mice, particularly in the calcified cartilage zone, it was lower in DMM-treated c-FosΔCh mice (figure 3G and H). MMP-13 and Adamts-5 are key degrading enzymes for collagen and GAGs, respectively, and their gene inactivation in mice leads to resistance to cartilage damage.32 33 While c-Fos deletion had no notable effect on MMP-13 expression, Adamts-5 was found decreased in knee sections from sham-treated and DMM-treated c-FosΔCh mice (online supplemental figure 3D–G, online supplemental figure 9A and C). Taken together, increased cartilage damage in c-FosΔCh mice is likely due to reduced chondrocyte proliferation and decreased collagen/ECM anabolic reactions rather than increased collagen catabolic processes.
Figure 3.
c-Fos affects chondrocyte proliferation and collagen organisation during cartilage damage progression. (A) Representative images of Ki67 and (B) quantification of Ki67-positive cells. (C) Chondrocyte density in articular cartilage from c-FosWT mice and c-FosΔCh mice at 8 weeks post surgery. (D) Representative images of picrosirius red staining of knee joints from c-FosWT mice and c-FosΔCh mice 8 weeks post surgery. Pictures are taken under the polarised light. (E) Quantification of collagen area in articular cartilage based on picrosirius red staining. (F) Quantification of collagen fibre length. Picrosirius red staining images were analysed using CurveAlign. (G) IF of collagen type 2 (green) in articular cartilage from c-FosWT mice and c-FosΔCh mice 8 weeks post surgery. Col2 positive areas are indicated by white arrows. (H) Quantification of Col2-positive area. Bar graphs and plots represent or include mean±SD, respectively. *p<0.05, **p<0.01, and ***p<0.001. In all panels, statistical differences between groups were analysed by two-way ANOVA with Bonferroni post hoc analysis. ANOVA, analysis of variance; DMM, destabilisation of the medial meniscus; IF, immunofluorescence
Energy metabolism during OA is dependent on c-Fos
To unravel the early molecular events contributing to the c-Fos protective function, articular cartilage was isolated from c-FosWT and c-FosΔCh mice 4 weeks after surgery, when cartilage damage was minimal and similar between the two genotypes (online supplemental figure 4A), and subjected to RNA sequencing (RNA-seq). Two data sets were generated: data set 1 consists of differentially expressed genes (DEGs) between DMM-treated and the non-operated contralateral knee in c-FosWT mice, while data set 2 contains DEGs between knee cartilage samples isolated from DMM-treated c-FosWT and DMM-treated c-FosΔCh mice (online supplemental figure 4B). A total of 801 DEGs were detected in data set 1 and 5167 in data set 2, with 725 DEGs shared between two data sets (online supplemental figure 4B, online supplemental table 3). Heat map representation of the shared upregulated and downregulated genes along the three analysis groups revealed a striking inverse regulation between DMM-treated c-FosWT and c-FosΔCh samples with the expression profile of DMM-treated c-FosΔCh largely comparable to the profile of c-FosWT contralateral knee (online supplemental figure 4C).
ard-2023-224002supp002.xlsx (1.8MB, xlsx)
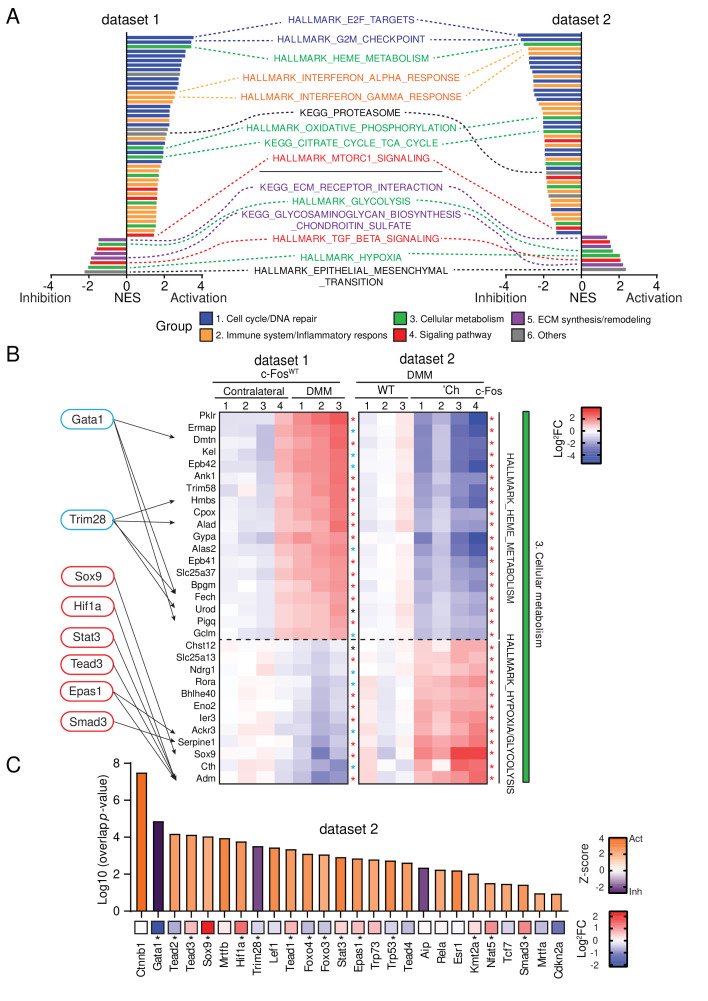
Pathway analysis was next conducted using gene set enrichment analysis.34 Overall, 52 pathways classified in six biological process groups emerged in the two data sets (figure 4A, online supplemental table 4). Cell cycle progression/DNA repair (group 1) and inflammatory response/immune system (group 2) were activated by DMM in c-FosWT (data set 1), while pathways belonging to ECM synthesis/remodelling (group 5) were mostly suppressed (figure 4A, online supplemental figure 4D). In cellular metabolism (group 3), tricarboxylic acid (TCA) cycle, oxidative phosphorylation (OXPHOS) and heme metabolism, three interconnected pathways often increased in cells on high energy demand, were upregulated in data set 1, while glycolysis and hypoxia appeared downregulated (figure 4A and B). Activation of mammalian target of rapamycin (mTOR) and suppression of transforming growth factor-β (TGF-β) signalling pathways (group 4), was also apparent in data set 1 (figure 4A), consistent with their role in cartilage homoeostasis and OA.35 36 Importantly, c-Fos deletion (data set 2) completely inverted the picture of DMM-induced genes and pathways (figure 4A), with a marked suppression of OXPHOS, heme metabolism and TCA cycle, while glycolysis and hypoxia were restored to wild-type contralateral levels (figure 4A and B, online supplemental figure 4D). Finally, when correlating Ingenuity Pathway Analysis (IPA) Z-scores with Log2Fc mRNA expression, several transcription factors and secreted proteins relevant to Fos-dependent chondrocyte response to DMM, were found connected to biological processes in group 3, that is, cellular metabolism pathways. For example, Sox9, Smad3 and Hif-1α and their upstream regulator Tgfβ1/2/3 and Bmp2/4 were also activated/increased in data set 2 (figure 4B and C and (online supplemental figure 4E), while these were decreased in data set 1 (figure 4B, online supplemental figure 4F), consistent with the opposite glycolysis/hypoxia signatures between the two data sets (figure 4B). These data reveal that metabolic responses of chondrocytes during cartilage damage are characterised by a shift from aerobic glycolysis to TCA cycle and these responses are strikingly affected by c-Fos expression.
Figure 4.
c-Fos transcriptionally controls cellular metabolic pathways. Bulk RNA-sequencing of articular cartilage from DMM-treated mice. (A) GSEA analysis indicating common core biological pathways either enriched or downregulated between data set 1 (c-FosWT mice: DMM-treated vs contralateral articular cartilage) and data set 2 (DMM-treated articular cartilage: c-FosWT vs c-FosΔCh mice). NES, normalised enrichment score. Contralateral c-FosWT, n=4, DMM c-FosWT, n=3. DMM c-FosΔCh, n=4. (B) Log2FC-based relative mRNA expression heat map of top-ranked factors enriched in the cellular metabolism (group 3) in figure 4C and online supplemental figure 4A (green) and IPA-predicted transcription factors (TFs) downstream of c-Fos in the DMM-treated side. Target genes of TFs detected by IPA are indicated with arrows. Asterisk indicates p<0.05 (black), p<0.01 (blue) and p<0.001 (red). (C) IPA-predicted upstream TFs from DMM-treated c-FosΔCh vs c-FosWT mice, showing activation Z-score (bars) and Log2FC (asterisk indicates p value<0.05). Statistical evaluation of RNA-seq data was performed as indicated in the methods. DMM, destabilisation of the medial meniscus; GSEA, gene set enrichment analysis; IPA, Ingenuity Pathway Analysis; RNA-seq, RNA sequencing; WT, wild-type.
ard-2023-224002supp003.xlsx (13.1KB, xlsx)
Pyruvate dehydrogenase and lactate dehydrogenase activities are modulated by c-Fos in chondrocytes
We next examined the molecular events mediating the effect of c-Fos on the metabolic pathways affected by DMM. Pyruvate, a cellular metabolite located at the intersection of multiple metabolic pathways, can be either converted to lactate by lactate dehydrogenase (Ldh) to supply glycolysis with NAD+, or to acetyl-coenzyme A by the pyruvate dehydrogenase (Pdh) complex and subsequently oxidized by the TCA cycle. Suppression of Pdh activity by pyruvate dehydrogenase kinases (Pdks) balances carbon flux between the two metabolic circuits. In sharp contrast to the situation observed in the Fos-proficient data set 1 (online supplemental figure 5A), expression of glycolytic enzymes increased in Fos-deficient cartilage after DMM (data set 2), while the majority of TCA cycle enzymes decreased (figure 5A). Increased expression of ldha, encoding for a major subunit of Ldh, in c-FosΔCh samples was confirmed by quantitative PCR (qPCR) (online supplemental figure 5B). RNA-seq and qPCR analyses revealed that pdk1, but not other pdk isoforms, was upregulated in c-FosΔCh mice, while Pdh complex subunits, such as pdha, pdhx and dld were largely unaffected (figure 5A and online supplemental figure 5C). Increased Pdk1 in chondrocytes of DMM-treated c-FosΔCh mice was also apparent by IHC (figure 5B and C). Hif-1α is an important upstream transcriptional regulator of Ldh37–39 and Pdk1,37 40 and a suppressor of chondrocyte proliferation in the hypoxic growth plate.39 41 Consistent with increased Ldh and Pdk1 protein expression but also the IPA prediction, increased Hif-1α protein was apparent in the cartilage of DMM-treated c-FosΔCh mice compared with c-FosWT mice (figure 4C, figure 5D and E and online supplemental table 3). Although following the same direction as the DMM-treated pairs, most of the observed differences between c-FosWT and c-FosΔCh samples were not statistically significant between sham-treated (figure 5C and E) or contralateral groups (online supplemental figure 5B,C). mRNA expression of ldha, pdk1 and hif1a were not affected by in vitro AdenoCre-mediated c-fos deletion in primary articular chondrocytes (online supplemental figure 5D), in line with the rather healthy cartilage displayed by un/sham-operated c-FosΔCh mice. These data collectively indicate that the molecular and cellular events downstream of c-Fos are likely part of the response of chondrocytes to OA-related signals such as biomechanical stress or cartilage damage caused by DMM. Interestingly, while transcripts of Hif-1α modulating factors such as egln1 and vhl, encoding the HIF-prolyl hydroxylase Phd2 and the E3-ubiquitin ligase pVHL respectively, were either increased or unaffected in Fos-deficient cartilage after DMM, proteasome genes and their master transcriptional regulator nrf1 were decreased (online supplemental figure 5E, data set 2) and the reverse expression signature were observed in the Fos-proficient data set 1 (figure 4A, online supplemental figure 5E). These data suggest that the cellular and metabolic changes observed in DMM-treated Fos-deficient mice could be caused, at least in part, by Hif-1α activation, potentially due to decreased proteasome activity.
Figure 5.
c-Fos maintains pyruvate dehydrogenase activity in experimental OA. (A) Relative mRNA expression heat map of factors in glycolysis, PDH-PDK pathway and TCA cycle based on Log2FC in the DMM-treated side compared with those from the contralateral side in c-FosWT mice. Asterisk indicates p<0.05 (black), p<0.01 (blue) and p<0.001 (red). (B) Representative images of Pdk1 and (C) quantification of positive cells. (D) Representative images of HIF-1α and (E) quantification of positive cells. Red arrows indicate positive cells. (F and G) In situ enzyme activity assay by formazan formation for Ldh (F) and Pdh (G). Bar graphs and plots represent or include mean±SD. *p<0.05, **p<0.01 and ***p<0.001. In all panels, statistical differences between groups were analysed by two-way ANOVA with Bonferroni post hoc analysis. ANOVA, analysis of variance; DMM, destabilisation of the medial meniscus; Ldh, lactate dehydrogenase; OA, osteoarthritis; PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase; TCA, tricarboxylic acid; WT, wild-type.
Ldh and Pdh activities were next assessed in articular cartilage tissue sections by in situ enzyme histochemistry.42–45 A strong lactate-dependent activity was detected in articular chondrocytes and quantitative analysis revealed higher amounts of active Ldh in sections from DMM-treated c-FosΔCh mice compared with c-FosWT (figure 5F and online supplemental figure 6A). Conversely, pyruvate-dependent Pdh activity was lower in articular chondrocytes from DMM-treated c-FosΔCh mice (figure 5G and online supplemental figure 6B). Finally, while Pdh activity in murine primary articular chondrocytes was not affected by in vitro c-fos deletion, treatment with dichloroacetic acid (DCA), a PDK inhibitor, led to a more robust increase of Pdh activity in Fos-deficient chondrocytes (online supplemental figure 6C). These results suggest that the Hif-1α/Ldh/Pdk/Pdh complex axis downstream of c-Fos could be a functional determinant for the switch in energy metabolism occurring in chondrocytes in response to OA signals.
DCA treatment reverts the deleterious effects of c-Fos inactivation in experimental OA
Hif-1α and Pdk1 protein expression and glycolytic flux are increased in chondrocytes in mice lacking prolyl hydroxylase 2 (Phd2), the main negative regulator of Hif-1α, leading to various cartilage dysplasias.46 Importantly, in vivo DCA treatment increased collagen synthesis in cartilage by restoring glucose oxidation, oxygen consumption and cell proliferation in Phd2 mutants, while sparing the wild-type littermates.46 Cohorts of c-FosWT and c-FosΔCh mice were therefore subjected to DMM and treated with DCA in a therapeutic setting (figure 6A).
Figure 6.
DCA treatment reduces DMM-induced cartilage damage in c-Fos deficient mice. (A) Experimental procedure. Tamoxifen was injected into mice at two time points (2.5 and 9 weeks of age, 2 mg/mouse/day, 5 consecutive days). Mice were subjected to DMM/sham at 10 weeks of age and treated with DCA from 11 weeks of age for 7 weeks. Knee joints were analysed 8 weeks post surgery. (B) Representative images of safranin O/fast green staining of knee joints from c-FosWT mice and c-FosΔCh mice treated with or without DCA 8 weeks post DMM. (C) Quantification of cartilage damage. (D) Chondrocyte density in articular cartilage from c-FosWT mice and c-FosΔCh mice 8 weeks post surgery. (E) Quantification of Ki67 positive cells. (F) Quantification of collagen area based on picrosirius red staining in the articular cartilage. (G) Quantification of collagen fibre length. The images of collagen fibre from picrosirius red staining were analysed by CurveAlign. (H) Quantification of IF images of collagen type 2 positive area. Bar graphs and plots represent or include mean±SD. *p<0.05, **p<0.01 and ***p<0.001. In all panels, statistical differences between groups were analysed by two-way ANOVA with Bonferroni post hoc analysis. ANOVA, analysis of variance; DCA, dichloroacetic acid; DMM, destabilisation of the medial meniscus; IF, immunofluorescence; TAM, Tamoxifen; WT, wild-type.
After 3 weeks of DCA treatment, in situ Pdh activity was still lower in most c-FosΔCh mutants (online supplemental figure 7A,B), while Ldh activity was restored to the levels measured in c-FosWT samples (online supplemental figure 7C,D). End-point histological analyses 1 month later, revealed that while DCA had no effect on c-FosWT mice, cartilage damage was reduced in DCA-treated c-FosΔCh mice to levels similar to c-FosWT (figure 6B,C). Consistent with a possible implication of Hif-1α downstream of c-Fos, chondrocyte density and Ki67 positivity were increased in DCA-treated c-FosΔCh mice (figure 6D and E, online supplemental figure 8A). Finally, DCA treatment restored collagen area (figure 6F, online supplemental figure 8B), collagen fibre length (figure 6G) and Col2-positive articular chondrocytes with less cartilage damage (figure 6H and online supplemental figure 8C) in c-FosΔCh mice, again reaching values comparable to c-FosWT. Taken together, pyruvate metabolism by Ldh and Pdh is a critical node downstream of c-Fos involved in the response of chondrocytes during OA.
Discussion
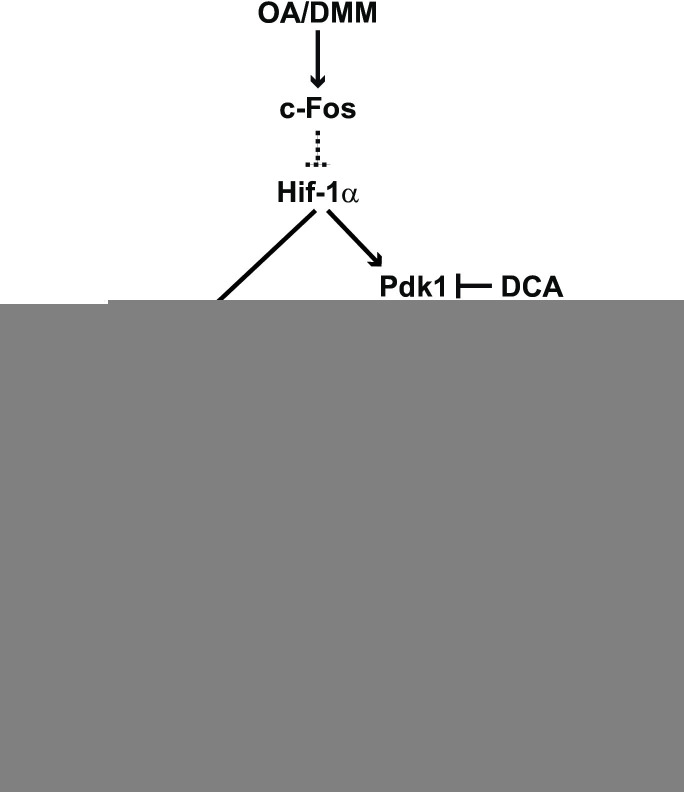
Despite a large number of studies, there is no disease-modifying drug or preventive strategy for OA due to limited mechanistic knowledge of how this heterogeneous disease develops.7 Combining mouse models and patient samples, the present study provides new insights into how articular chondrocytes adapt to OA-associated signals and demonstrates a crucial role of the AP-1-forming protein c-Fos in modulating cellular pyruvate usage and cartilage integrity (figure 7).
Figure 7.
Scheme depicting chondrocyte pyruvate usage pathways modulated by c-Fos/AP-1 in experimental OA. In early OA, the Hif-1α/Pdk1/Pdh and/or Hif-1α/Ldh pathways are suppressed by c-Fos, and pyruvate—acetyl-CoA conversion is predominant, leading to increased TCA cycle/OXPHOS and decreased lactate production. In chondrocytes subjected to DMM, c-Fos/AP-1 modulates pyruvate metabolism through Hif-1α/Pdk1/Pdh and/or Hif-1α/Ldh, thereby controlling cell proliferation and collagen biosynthesis, improving cartilage integrity and counteracting OA progression. The dotted lines between c-Fos and Hif-1α, two nuclear proteins forming heterodimeric transcription factors, indicate yet-to-be-defined pathways, such as the Tgfβ/Smad/Bmp and mTORC1, which are more likely than a direct transcriptional regulation. In Fos-deficient cells, chondrocytes execute these events in the opposite manner, whereby c-Fos-induced protection is lost and the energy deficit leads to decreased proliferation, collagen synthesis and increased OA. Elevated Pdk and Ldh activity can be therapeutically targeted in Fos-deficient cells using DCA to promote TCA cycle/OXPHOS and suppress glycolysis, rescuing the above-mentioned defects in proliferation and cartilage integrity. DCA, dichloroacetic acid; DMM, destabilisation of the medial meniscus; Ldh, lactate dehydrogenase; OA, osteoarthritis; OXPHOS, oxidative phosphorylation; Pdh, pyruvate dehydrogenase; Pdk, pyruvate dehydrogenase kinase; TCA, tricarboxylic acid.
Glycolysis rather than the TCA cycle and subsequent oxidative phosphorylation is the primary energy source for chondrocytes in vivo, likely owing to their hypoxic environment.6 7 47 Our findings provide evidence that the fine-tuning between glycolysis and TCA cycle—OXPHOS that occurs in articular chondrocytes in response to excessive biological/mechanical stress is an important determinant of OA pathogenesis. In the early stages of experimental OA, the shift in cellular energy metabolism in chondrocytes towards increased utilisation of pyruvate in the TCA cycle—OXPHOS is likely necessary to satisfy the high energy demand of early response processes, such as proliferation and collagen/matrix synthesis. Genetic inactivation c-Fos in chondrocytes leads to major impairment of their metabolic response to OA signals, through changes in expression and/or activity of two pyruvate-metabolising enzymes: pyruvate and lactate dehydrogenase. This balance in the activity of these two enzymes determines the usage of rapid and low-energy producing lactic fermentation (glycolysis) or slower but high-energy producing TCA cycle—OXPHOS (figure 7). Decreased Pdh and increased Ldh activities, measured in situ in DMM-treated Fos-deficient articular chondrocytes, are in line with a decreased influx of acetyl-CoA to the TCA cycle and a consequent energy deficit in these mutant cells. Fos-deficient articular chondrocytes subjected to experimental OA proliferate less frequently, and produce less and shorter collagen fibres, resulting in increased overall cartilage damage. Chondrocyte-specific gene inactivation of Ldha is beneficial in experimental OA48 and we demonstrate that restoring Ldh/Pdh activities to levels similar to wild-type by treatment with DCA is beneficial in Fos-deficient mutants, rescuing all above-mentioned phenotypes. While DCA had no therapeutic benefit in wild-type mice, strategies mimicking the effect of DCA might still be of interest to potentiate other therapies. Nevertheless, this striking result indicates that in OA chondrocytes, c-Fos is more essential to modulate pyruvate usage than to control the expression of its classical target genes, such as matrix-degrading enzymes and cell cycle/proliferation proteins. Whether c-Fos, a bona fide oncogene, might also modulate pyruvate usage in highly glycolytic solid tumours is worth exploring, for example, using Fos-dependent experimental models of chondrosarcoma and/or osteosarcoma.
Two studies have documented that T-5224, a broad-spectrum AP-1 dimer inhibitor, ameliorates DMM-induced experimental OA.49 50 While these results might seem in disagreement with our genetic experiment selectively deleting c-Fos in chondrocytes, rather we infer that the role of AP-1 in OA pathogenesis is not limited to the function of Fos-containing AP-1 heterodimers, nor limited to a single cell type in the joint. Broadly inhibiting the binding of AP-1 dimers to DNA can limit the transcription of AP-1 target genes essential to OA pathogenesis in chondrocytes, but also in joint immune and/or synovial cells. Consistently, mice broadly deficient for Batf, a bZIP-containing protein that forms AP-1 dimers only with Jun proteins are resistant to experimental OA.50 We envisage that the disease resistance reflects changes in AP-1 dimer composition and the relevance of AP-1 activity in other cell types beyond chondrocytes during OA. In this regard, it is striking that Fos inactivation in chondrocytes had little effect on DMM-induced osteophyte formation or synovial thickening, two OA-related pathological events that involve other mesenchymal cells. Examining the effects of c-Fos inactivation in other cell types of the joint and identifying among the Jun, ATF and MAF proteins the essential c-Fos dimerising partner(s) in chondrocytes might provide further mechanistic clues to OA pathogenesis. As c-Jun and JunB are both increased during human50 and mouse OA, these are the most likely Fos partners to genetically assess in future in vivo experiments.
Decreased Pdh and increased Ldh activities in DMM-treated, Fos-deficient chondrocytes are the net outcome of increased Hif-1α and Pdk1 mRNA and protein expression. The essential contribution of HIF-1α to the metabolic changes in the absence of c-Fos is not formally demonstrated by in vivo loss and gain of function experiments, but is very likely given the reported role of HIF in chondrocyte metabolism. Beyond its direct control of both Ldh37–39 and Pdk137–40 expression, Hif-1α and HIF signalling, is a crucial determinant of important biological processes in chondrocytes, such as survival and proliferation,39 41 collagen synthesis and matrix quality.46 It is therefore not surprising that genetic or pharmacological manipulation of Hif-1α or its upstream regulator Phd2, lead to pleiotropic effects in mouse cartilage. For example, inappropriate HIF-1α signalling in mice lacking Phd2 in chondrocytes resulted in skeletal dysplasia with increased bone mass46 and reduced articular cartilage thickness.51 HIF-1α stabilisation in Phd2-deficient chondrocytes also resulted in metabolic reprogramming with enhanced glutamine flux and decreased glucose oxidation leading to collagen over-modifications and the formation of a cartilaginous matrix more resistant to protease-mediated degradation, despite decreased collagen synthesis.46 As cartilage stiffness, matrix composition and matrix catabolism are also important determinants of OA, the deleterious effects of Hif-1α gene inactivation in experimental OA52 53 are likely due to the additive effects of imbalanced energy and cartilage matrix metabolism, dysregulated gene expression of matrix-degrading enzymes and acute cell death/autophagy responses.
Several Hif-1α target genes that were reported to increase in Phd-2 deficient chondrocytes are increased by DMM in c-FosΔCh mice: pdk1, ldha, the glucose transporter Glut1 encoded by slc2a1, the glutamine sensor glutaminase 1 (gls1) and the collagen-modifying enzymes p4ha, p4hb, lox and plod2, consistent with increased Hif-1α expression and with the cartilage and collagen defects observed in c-FosΔCh mutants. RNA-seq experiments also revealed increased Phd2 and Vhl mRNA expression in c-FosΔCh DMM samples (data set 2), consistent with Phd2 being a transcriptional Hif-1α target. Increased Hif-1α in c-Fos-deficient chondrocytes is likely not due to decreased prolyl and asparaginyl hydroxylase activity, or direct binding of c-Fos/AP-1 to the hif1a promoter, but could be a result of reduced proteasome activity. Altered signalling activity from the Tgfβ/Smad/Bmp and/or mTORC1 pathway, which have been documented to interact with and modulate both AP-1 and HIF signalling are also attractive candidates to explore functionally, with the prospect to substantiate the envisaged link between c-Fos/AP-1 and Hif-1α.
Overall, this work highlights the function of Fos/AP-1 in OA pathogenesis and provides convincing evidence that early modulation of the balance in pyruvate usage between lactate production and TCA-cycle-OXPHOS in chondrocytes determines OA outcome. Whether boosting this early metabolic stress response of chondrocytes can facilitate the cartilage regenerating or repair capacity at early stages of the disease or whether therapeutic intervention can trigger this response even at later stages of the disease certainly warrants further experimentation.
Acknowledgments
We thank Sarah Sinclair and Christina Bauer for assisting with tissue sections staining and mouse experiments and Dragana Kubatovic for helping with mouse colony management. We are very grateful to Drs Doug Hanahan, Georg Schett, Nabil Djouder and Rudolf Zechner and to the members of the Wagner Laboratory for helpful comments and discussions. Elisabeth Ponweiser at KILM/MUW for help with in situ Pdh/Ldh activity assays; Alexander Stögner and Ruth Grübl-Barabas from the Department of Orthopedics and Trauma Surgery/MUW for help with preparation and IHC of human OA cartilage, Christian Beyer from the University Erlangen for help in establishing the DMM procedure. We thank S Derdak for the help with bulk RNA-seq analysis. RNA-seq was performed at the Core Facilities of the MUW, a member of the Vienna Life-Science Instruments initiative.
Footnotes
Handling editor: Thomas Pap
Contributors: KM designed and performed experiments and wrote the manuscript. LB contributed to mouse colony management, experimental design and manuscript writing. AH contributed to in situ activity assays and MB contributed to bulk RNA-seq analyses of mouse cartilage and primary osteoarthritis (OA) chondrocytes. MK and HY contributed to data mining of single-cell RNA-seq data sets from human OA chondrocytes. ST and RW provided human articular cartilage from patients with OA and analyses of c-Fos and Pdk1 expression and the associated clinical data. EFW directed the study, approved the data and edited the manuscript with input from all the authors. Guarantor: EFW.
Funding: ST gratefully acknowledges support from the AFOR Foundation and from a fellowship by Johnson & Johnson Medical Products GmbH. HY is an H2020 – MSCA fellow (ITN 2019-859860-CANCERPREV). The Wagner Laboratory is supported by the ERC (AdG 2016-741888-CSI-Fun), an H2020 – MSCA grant (ITN 2019-859860-CANCERPREV) and the MUW.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Human articular cartilage was obtained from patients with osteoarthritis during total knee arthroplasty with written informed consent and following the terms of the ethics committee of the Medical University of Vienna (EK-Nr.: 1822/2017, 2166/2020). Participants gave informed consent to participate in the study before taking part.
References
- 1. Charlier E, Deroyer C, Ciregia F, et al. Chondrocyte Dedifferentiation and osteoarthritis (OA). Biochemical Pharmacology 2019;165:49–65. 10.1016/j.bcp.2019.02.036 [DOI] [PubMed] [Google Scholar]
- 2. Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers 2016;2:16072. 10.1038/nrdp.2016.72 [DOI] [PubMed] [Google Scholar]
- 3. Ayral X, Pickering EH, Woodworth TG, et al. Synovitis: A potential predictive factor of structural progression of medial Tibiofemoral knee osteoarthritis - results of a 1 year longitudinal Arthroscopic study in 422 patients. Osteoarthritis and Cartilage 2005;13:361–7. 10.1016/j.joca.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 4. Sellam J, Berenbaum F. The role of Synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 2010;6:625–35. 10.1038/nrrheum.2010.159 [DOI] [PubMed] [Google Scholar]
- 5. Berenbaum F, Griffin TM, Liu-Bryan R. Review: metabolic regulation of inflammation in osteoarthritis. Arthritis Rheumatol 2017;69:9–21. 10.1002/art.39842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mobasheri A, Rayman MP, Gualillo O, et al. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2017;13:302–11. 10.1038/nrrheum.2017.50 [DOI] [PubMed] [Google Scholar]
- 7. Zheng L, Zhang Z, Sheng P, et al. The role of metabolism in Chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res Rev 2021;66:101249. 10.1016/j.arr.2020.101249 [DOI] [PubMed] [Google Scholar]
- 8. Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol 2015;11:35–44. 10.1038/nrrheum.2014.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not Osteoarthrosis. Osteoarthritis and Cartilage 2013;21:16–21. 10.1016/j.joca.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 10. Yang CY, Chanalaris A, Troeberg L. ADAMTS and ADAM Metalloproteinases in osteoarthritis – looking beyond the usual suspects. Osteoarthritis Cartilage 2017;25:1000–9. 10.1016/j.joca.2017.02.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yusuf E, Nelissen RG, Ioan-Facsinay A, et al. Association between weight or body mass index and hand osteoarthritis: A systematic review. Ann Rheum Dis 2010;69:761–5. 10.1136/ard.2008.106930 [DOI] [PubMed] [Google Scholar]
- 12. Loeser RF. The role of aging in the development of osteoarthritis. Trans Am Clin Climatol Assoc 2017;128:44–54. [PMC free article] [PubMed] [Google Scholar]
- 13. Loughlin J. Genetic contribution to osteoarthritis development: Current state of evidence. Curr Opin Rheumatol 2015;27:284–8. 10.1097/BOR.0000000000000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tachmazidou I, Hatzikotoulas K, Southam L, et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet 2019;51:230–6. 10.1038/s41588-018-0327-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aury-Landas J, Marcelli C, Leclercq S, et al. Genetic determinism of primary early-onset osteoarthritis. Trends Mol Med 2016;22:38–52. 10.1016/j.molmed.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 16. Stolz M, Gottardi R, Raiteri R, et al. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy (nature nanotechnology. Nature Nanotech 2009;4:186–92. 10.1038/nnano.2008.410 [DOI] [PubMed] [Google Scholar]
- 17. Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev 2005;208:126–40. 10.1111/j.0105-2896.2005.00332.x [DOI] [PubMed] [Google Scholar]
- 18. Zhou LZH, Johnson AP, Rando TA. NFκB and AP-1 mediate transcriptional responses to oxidative stress in Skeletal muscle cells. Free Radical Biology and Medicine 2001;31:1405–16. 10.1016/S0891-5849(01)00719-5 [DOI] [PubMed] [Google Scholar]
- 19. Kyriakis JM. Activation of the AP-1 transcription factor by inflammatory Cytokines of the TNF family. Gene Expr 1999;7:217–31. [PMC free article] [PubMed] [Google Scholar]
- 20. Ogasawara A, Arakawa T, Kaneda T, et al. Fluid shear stress-induced Cyclooxygenase-2 expression is mediated by C/EBP beta, cAMP-response element-binding protein, and AP-1 in Osteoblastic Mc3T3-E1 cells. J Biol Chem 2001;276:7048–54. 10.1074/jbc.M008070200 [DOI] [PubMed] [Google Scholar]
- 21. Bozec A, Bakiri L, Jimenez M, et al. Fra-2/AP-1 controls bone formation by regulating Osteoblast differentiation and collagen production. J Cell Biol 2010;190:1093–106. 10.1083/jcb.201002111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He X, Ohba S, Hojo H, et al. AP-1 family members act with Sox9 to promote Chondrocyte hypertrophy. Development 2016;143:3012–23. 10.1242/dev.134502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Behrens A, Haigh J, Mechta-Grigoriou F, et al. Impaired Intervertebral disc formation in the absence of Jun. Development 2003;130:103–9. 10.1242/dev.00186 [DOI] [PubMed] [Google Scholar]
- 24. Karreth F, Hoebertz A, Scheuch H, et al. The Ap1 transcription factor Fra2 is required for efficient cartilage development. Development 2004;131:5717–25. 10.1242/dev.01414 [DOI] [PubMed] [Google Scholar]
- 25. Grigoriadis AE, Schellander K, Wang ZQ, et al. Osteoblasts are target cells for transformation in C-Fos transgenic mice. J Cell Biol 1993;122:685–701. 10.1083/jcb.122.3.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang ZQ, Grigoriadis AE, Möhle-Steinlein U, et al. A novel target cell for C-Fos-induced Oncogenesis: development of Chondrogenic tumours in embryonic stem cell Chimeras. EMBO J 1991;10:2437–50. 10.1002/j.1460-2075.1991.tb07783.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huber R, Kirsten H, Näkki A, et al. Association of human FOS promoter variants with the occurrence of knee-osteoarthritis in a case control Association study. IJMS 2019;20:1382. 10.3390/ijms20061382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weinmann D, Kenn M, Schmidt S, et al. Galectin-8 induces functional disease markers in human osteoarthritis and cooperates with Galectins-1 and -3. Cell Mol Life Sci 2018;75:4187–205. 10.1007/s00018-018-2856-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glasson SS, Blanchet TJ, Morris EA. The surgical Destabilization of the medial Meniscus (DMM) model of osteoarthritis in the 129/Svev mouse. Osteoarthritis and Cartilage 2007;15:1061–9. 10.1016/j.joca.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 30. Fleischmann A, Hvalby O, Jensen V, et al. Impaired long-term memory and Nr2A-type NMDA receptor-dependent synaptic plasticity in mice lacking C-Fos in the CNS. J Neurosci 2003;23:9116–22. 10.1523/JNEUROSCI.23-27-09116.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu M, Chen M, Lichtler AC, et al. Tamoxifen-inducible CRE-Recombination in Articular Chondrocytes of adult Col2A1-Creert2 transgenic mice. Osteoarthritis and Cartilage 2008;16:129–30. 10.1016/j.joca.2007.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang M, Sampson ER, Jin H, et al. Mmp13 is a critical target Gene during the progression of osteoarthritis. Arthritis Res Ther 2013;15:R5. 10.1186/ar4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glasson SS, Askew R, Sheppard B, et al. Deletion of active Adamts5 Preventscartilage degradation in a Murinemodel of osteoarthritis. Nature 2005;434:644–8. 10.1038/nature03369 [DOI] [PubMed] [Google Scholar]
- 34. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005;102:15545–50. 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhai G, Doré J, Rahman P. TGF-Β signal Transduction pathways and osteoarthritis. Rheumatol Int 2015;35:1283–92. 10.1007/s00296-015-3251-z [DOI] [PubMed] [Google Scholar]
- 36. Sun K, Luo J, Guo J, et al. The Pi3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthritis and Cartilage 2020;28:400–9. 10.1016/j.joca.2020.02.027 [DOI] [PubMed] [Google Scholar]
- 37. Taylor CT, Scholz CC. The effect of HIF on metabolism and immunity. Nat Rev Nephrol 2022;18:573–87. 10.1038/s41581-022-00587-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Semenza GL, Roth PH, Fang HM, et al. Transcriptional regulation of genes Encoding Glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 1994;269:23757–63. [PubMed] [Google Scholar]
- 39. Yao Q, Khan MP, Merceron C, et al. Suppressing mitochondrial respiration is critical for hypoxia tolerance in the fetal growth plate. Dev Cell 2019;49:748–63. 10.1016/j.devcel.2019.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bentovim L, Amarilio R, Zelzer E. Hif1Α is a central regulator of collagen hydroxylation and secretion under hypoxia during bone development. Development 2012;139:4473–83. 10.1242/dev.083881 [DOI] [PubMed] [Google Scholar]
- 41. Schipani E, Ryan HE, Didrickson S, et al. Hypoxia in cartilage: HIF-1Α is essential for Chondrocyte growth arrest and survival. Genes Dev 2001;15:2865–76. 10.1101/gad.934301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller A, Nagy C, Knapp B, et al. Exploring metabolic configurations of single cells within complex tissue Microenvironments. Cell Metab 2017;26:788–800. 10.1016/j.cmet.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 43. Golias T, Papandreou I, Sun R, et al. Hypoxic repression of pyruvate dehydrogenase activity is necessary for metabolic Reprogramming and growth of model tumours. Sci Rep 2016;6:31146. 10.1038/srep31146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Molenaar RJ, Khurshed M, Hira VVV, et al. Metabolic mapping: quantitative enzyme Cytochemistry and histochemistry to determine the activity of Dehydrogenases in cells and tissues. JoVE 2018. 10.3791/56843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun RC, Koong A, Giaccia A, et al. Measuring the impact of Microenvironmental conditions on mitochondrial dehydrogenase activity in cultured cells. Adv Exp Med Biol 2016;899:113–20. 10.1007/978-3-319-26666-4_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stegen S, Laperre K, Eelen G, et al. HIF-1Α Metabolically controls collagen synthesis and modification in Chondrocytes. Nature 2019;565:511–5. 10.1038/s41586-019-0874-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou S, Cui Z, Urban JPG. Factors influencing the oxygen concentration gradient from the Synovial surface of Articular cartilage to the cartilage-bone interface: A modeling study. Arthritis Rheum 2004;50:3915–24. 10.1002/art.20675 [DOI] [PubMed] [Google Scholar]
- 48. Arra M, Swarnkar G, Ke K, et al. LDHA-mediated ROS generation in Chondrocytes is a potential therapeutic target for osteoarthritis. Nat Commun 2020;11:3427. 10.1038/s41467-020-17242-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Motomura H, Seki S, Shiozawa S, et al. A selective C-Fos/AP-1 inhibitor prevents cartilage destruction and subsequent Osteophyte formation. Biochem Biophys Res Commun 2018;497:756–61. 10.1016/j.bbrc.2018.02.147 [DOI] [PubMed] [Google Scholar]
- 50. Rhee J, Park S-H, Kim S-K, et al. Inhibition of BATF/JUN transcriptional activity protects against Osteoarthritic cartilage destruction. Ann Rheum Dis 2017;76:427–34. 10.1136/annrheumdis-2015-208953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheng S, Pourteymoor S, Alarcon C, et al. Conditional deletion of the Phd2 Gene in Articular Chondrocytes accelerates differentiation and reduces Articular cartilage thickness. Sci Rep 2017;7:45408. 10.1038/srep45408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bouaziz W, Sigaux J, Modrowski D, et al. Interaction of Hif1Α and Β-Catenin inhibits matrix metalloproteinase 13 expression and prevents cartilage damage in mice. Proc Natl Acad Sci U S A 2016;113:5453–8. 10.1073/pnas.1514854113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Okada K, Mori D, Makii Y, et al. Hypoxia-inducible Factor-1 alpha maintains mouse Articular cartilage through suppression of NF-ΚB signaling. Sci Rep 2020;10:5425. 10.1038/s41598-020-62463-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2023-224002supp001.pdf (1.9MB, pdf)
ard-2023-224002supp002.xlsx (1.8MB, xlsx)
ard-2023-224002supp003.xlsx (13.1KB, xlsx)
Data Availability Statement
Data are available upon reasonable request.