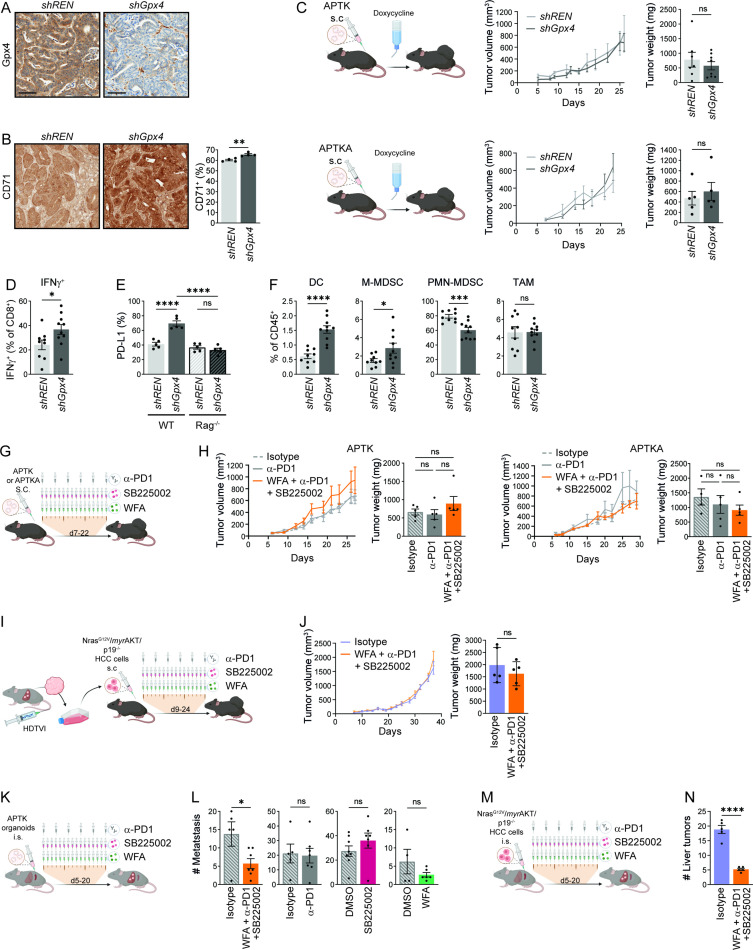
Figure 4.
Ferroptosis effect on colorectal cancer. (A) Representative immunohistochemical Gpx4 staining of shREN or shGPX4 APTKA s.c tumours. (B) Immunohistochemical quantification of CD71+ cells in shREN and shGpx4 APTKA s.c. tumours (n=4). (C) Tumour volumes and weights at end point of shREN and shGpx4 APTK and ATPKA subcutaneous tumours (n≥5). (D) Flow cytometry analysis of IFNγ expression in CD8+ T cells from shREN and shGpx4 s.c. APTKA tumours, after ex vivo PMA/Ionomycin stimulation (n≥9). (E) Immunofluorescence quantification of PD-L1 expression in s.c shREN and shGpx4 APTKA tumours from WT or Rag-/- mice (n=5). (F) Percentages of DC, M-MDSC, PMN-MDSC in immune infiltrates of shREN and shGpx4 s.c. APTKA tumours analysed by flow cytometry (n≥9). (G) Treatment scheme after s.c. transplantation of APTK or APTKA organoids. (H) Tumour volumes and end point weights of s.c APTK and APTKA treated with the indicated compounds (n≥5). (I) Scheme for the isolation of NrasG12V /myrAKT/p19 -/- HCC cells followed by subcutaneous transplantation and treatments. (J) Tumour volumes and weights at end-point of subcutaneously transplanted NrasG12V /myrAKT/p19 -/- HCC cells with the indicated treatment. (K) Treatment scheme after intrasplenic (i.s.) injection of APTK organoids. (L) Numbers of liver metastasis after intrasplenic injection of APTK organoids and treatment as indicated (n≥4). (M) Treatment scheme after intrasplenic (i.s.) injection of NrasG12V /myrAKT/p19 -/- HCC cells. (N) Number of liver tumours after intrasplenic transplantation of NrasG12V /myrAKT/p19 -/- HCC cells and the indicated treatment (n≥5). (G, I, K, M) Scheme for i.p. injections, each represented by a syringe, with 250 µg of α-PD-1 or isotype (Rat IgG2a,κ), 2.5 mg/kg of Withaferin A (WFA) or 1 mg/kg of SB225002. (B–M) Data are mean±SEM, n.s not significant, *p≤0.05, ***p≤0.001, ****p≤0.0001 by t-test (B–F, J, L, N) one-way ANOVA with Šídák’s multiple comparisons test (H) of the indicated pairs. ANOVA, analysis of variance; DC, dendritic cell; HCC, hepatocellular carcinoma; MDSC, myeloid derived suppressor cell; PMA, phorbol myristate acetate; s.c., subcutaneous.