Abstract
Objective
Increased risk of serious adverse events (AEs) was reported for tofacitinib relative to tumour necrosis factor inhibitor therapy in patients with rheumatoid arthritis (RA) aged ≥50 years enriched for cardiovascular (CV) risk (ORAL Surveillance). We assessed post hoc the potential risk of upadacitinib in a similar RA population.
Methods
Pooled safety data from six phase III trials were evaluated post hoc for AEs in patients receiving upadacitinib 15 mg once a day (with or without conventional synthetic disease-modifying antirheumatic drugs), adalimumab 40 mg every other week with concomitant methotrexate (MTX), or MTX monotherapy in the overall trial population and in a subset of patients with higher CV risk (aged ≥50 years, ≥1 CV risk factor). Higher-risk patients from a head-to-head study of upadacitinib 15 mg versus adalimumab (SELECT-COMPARE) were assessed in parallel. Exposure-adjusted incidence rates for treatment-emergent AEs were summarised based on exposure to upadacitinib or comparators.
Results
A total of 3209 patients received upadacitinib 15 mg, 579 received adalimumab and 314 received MTX monotherapy; ~54% of the patients were included in the overall and SELECT-COMPARE higher-risk populations. Major adverse cardiovascular events (MACE), malignancy (excluding non-melanoma skin cancer (NMSC)) and venous thromboembolism (VTE) were more frequent in the higher-risk cohorts versus the overall population but were generally similar across treatment groups. Rates of serious infections in higher-risk populations and herpes zoster (HZ) and NMSC in all populations were higher with upadacitinib 15 mg than comparators.
Conclusions
An increased risk of MACE, malignancy (excluding NMSC) and VTE was observed in higher-risk populations with RA, yet risk was comparable between upadacitinib-treated and adalimumab-treated patients. Higher rates of NMSC and HZ were observed with upadacitinib versus comparators across all populations, and increased rates of serious infections were detected in upadacitinib-treated patients at higher CV risk.
Trial registration numbers
NCT02706873, NCT02675426, NCT02629159, NCT02706951, NCT02706847 and NCT03086343.
Keywords: arthritis, cardiovascular diseases, tumor necrosis factor inhibitors, antirheumatic agents, methotrexate
WHAT IS ALREADY KNOWN ON THIS TOPIC
Differential safety risks were recently reported in ORAL Surveillance, a postapproval head-to-head and event-driven randomised trial comparing the janus kinase inhibitor (JAKi) tofacitinib to tumour necrosis factor inhibitor therapy in a population with rheumatoid arthritis (RA) enriched for cardiovascular (CV) risk (aged ≥50 years old with ≥1 additional CV risk factor).
WHAT THIS STUDY ADDS
To better understand whether these differential risks are common to all members of the JAKi class, this post hoc analysis evaluated the safety of upadacitinib across the SELECT RA programme, focusing on patients similar to those enrolled in ORAL Surveillance.
The incidence of major adverse cardiovascular events (MACE), malignancy (excluding non-melanoma skin cancer (NMSC)), venous thromboembolism (VTE) and deaths was generally higher in the increased CV risk populations; however, incidence rates were comparable between upadacitinib 15 mg, adalimumab and methotrexate (MTX) monotherapy.
In contrast, rates of herpes zoster (HZ) and NMSC were higher in upadacitinib-treated patients relative to comparators across populations, and rates of serious infections were elevated in patients at higher CV risk. COVID-19-related deaths were only reported in patients receiving upadacitinib; however, the overall mortality rates were comparable between upadacitinib, adalimumab and MTX monotherapy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings in patients with RA at increased risk of CV events help to contextualise the overall risk profile of upadacitinib; while HZ and NMSC were observed at higher rates with upadacitinib versus adalimumab or MTX, rates of MACE, malignancy (excluding NMSC) and VTE were similar across treatments, although numerically higher in patients at increased CV risk.
Introduction
Janus kinase inhibitors (JAKis) are targeted synthetic disease-modifying antirheumatic drugs indicated for the treatment of a broad range of immune-mediated inflammatory diseases, including rheumatoid arthritis (RA). In RA, JAKi provide a treatment option for patients who do not adequately respond to, or are intolerant of, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) or biological disease-modifying antirheumatic drugs (bDMARDs).1
Most published safety data for JAKi have been gleaned from clinical trials, where the clinical characteristics of the patients enrolled are heterogenous and do not fully mirror those in clinical practice. Moreover, there are sparse registry data with JAKi therapy published to date. The JAKi clinical trial programmes have shown the safety of JAKi to be generally similar to tumour necrosis factor inhibitor (TNFi) therapy, except for increased rates of herpes zoster (HZ) and elevations of lipids and creatine phosphokinase.2 More recently, differential safety risks were reported in ORAL Surveillance, a mandated postapproval, head-to-head and event-driven safety study of tofacitinib compared with TNFi therapy in patients with RA aged ≥50 years who had ≥1 additional cardiovascular (CV) risk factor.3 The study demonstrated an increased risk of malignancies (HR 1.48, 95% CI 1.04 to 2.09) and major adverse cardiovascular events (MACE) (HR 1.33, 95% CI 0.91 to 1.94) with tofacitinib compared with TNFi therapy,3 which prompted JAKi label re-evaluations from regulatory authorities across the globe with differing outcomes.4–6
The efficacy and safety of the JAKi upadacitinib have been evaluated in a broad range of patients with RA across the SELECT clinical trial programme,7–13 including long-term head-to-head studies of upadacitinib versus adalimumab (SELECT-COMPARE) and upadacitinib versus methotrexate (MTX, SELECT-EARLY). In this post hoc analysis, we examined whether adverse events (AEs) of special interest occur more frequently among patients with an elevated risk of CV events and assessed the relative risk of these events with upadacitinib versus adalimumab and MTX monotherapy. Importantly, while we attempted to replicate the population criteria of ORAL Surveillance, it should be emphasised that this is a post hoc analysis and not a prospective, controlled safety study; thus, caution should be taken when results between the two studies are compared.
Methods
Studies and patient populations
Data were pooled from six phase III trials in the SELECT RA programme, which evaluated upadacitinib given either as monotherapy or in combination with csDMARDs in both bDMARD-inadequate response (IR) and bDMARD-naïve patients (online supplemental table 1). Trial designs and the eligibility criteria for each study have been described previously.7–12 Patients with previous malignancies (except for successfully treated non-melanoma skin cancer (NMSC) or localised carcinoma in situ of the cervix) and patients with moderate to severe congestive heart failure, uncontrolled hypertension, recent (ie, within the past 6 months) myocardial infarction or stroke, and some other CV conditions were excluded (detailed in online supplemental materials).
ard-2023-223916supp001.pdf (1.3MB, pdf)
Patients received upadacitinib 15 mg once daily (QD) (the approved dose for RA, with or without background csDMARDs) or adalimumab 40 mg every other week plus MTX (SELECT-COMPARE) or MTX monotherapy (SELECT-EARLY). MTX monotherapy was titrated to 20 mg/week (15 mg/week in Japan). Separate safety analyses were also conducted in patients who received upadacitinib 30 mg QD.
Three populations were assessed for AEs of interest: (1) overall population, (2) patients with higher CV risk from the overall population, and (3) patients with higher CV risk specifically from SELECT-COMPARE, permitting a direct evaluation of upadacitinib 15 mg and adalimumab (both receiving concomitant MTX). The higher-risk populations were defined as patients aged ≥50 years with ≥1 CV risk factor. CV risk factors were selected based on the ORAL Surveillance inclusion criteria, within the confines of information collected across the SELECT RA clinical programme, and comprised medical history of (1) prior CV event (defined as any medical history event with a system organ class of ‘cardiac disorders’ per MedDRA V.25.0), (2) hypertension as recorded in the medical history but not based on measured blood pressure values in the trial, (3) diabetes mellitus, (4) current or former tobacco/nicotine use and (5) baseline high-density lipoprotein cholesterol (HDL-C) levels of <40 mg/dL. In contrast to ORAL Surveillance, information regarding family history of premature coronary heart disease and presence of extra-articular RA were not available within the upadacitinib RA clinical trial programme and could not be used to identify patients at increased CV risk.
Patient and public involvement
Patients and the public were not involved in the design or analysis of this study.
Safety assessments
Treatment-emergent adverse events (TEAEs) examined in this analysis included malignancy (excluding NMSC), NMSC, MACE, venous thromboembolism (VTE), serious infectious events (SIE) and HZ. TEAEs were defined as any AE with an onset date on or after the first dose of study drug and, as of the data cut-off for this analysis (15 February 2022), no more than 30 days after the last dose of study drug for upadacitinib or MTX and up to 70 days for adalimumab if patients discontinued prematurely from the study. However, mortality assessment also included deaths that occurred beyond the 30 days (upadacitinib or MTX) or 70 days (adalimumab) after the last dose of study drug.
MACE and VTE were adjudicated by an independent CV adjudication committee in a blinded manner. MACE included CV death, non-fatal myocardial infarction and non-fatal stroke. VTE events included deep vein thrombosis and pulmonary embolism. Malignancy events were reported by investigators and medically reviewed by AbbVie study physicians.
Statistical analysis
Baseline characteristics and safety outcomes were analysed in each of the three population sets. In both the overall population and the overall higher-risk population, upadacitinib 15 mg data included patients with any upadacitinib 15 mg exposure, including those randomised to upadacitinib 15 mg in all trials and those who switched to upadacitinib 15 mg (only data after the switch were included). Adalimumab data reported in the overall and overall higher-risk populations were from patients enrolled in SELECT-COMPARE who were initially randomised to adalimumab or after being rescued to adalimumab from upadacitinib. The MTX treatment group includes data from patients starting MTX monotherapy in SELECT-EARLY, censored at the time of rescue to upadacitinib. In the SELECT-COMPARE higher-risk population set, data are from patients initially randomised to upadacitinib 15 mg and adalimumab, as well as those who switched from placebo to upadacitinib; however, to estimate HRs between upadacitinib 15 mg and adalimumab, patients who switched from their randomised treatment with either upadacitinib or adalimumab were censored at the first dose of their switch therapy. Additional safety analyses were also completed for patients who received upadacitinib 30 mg and included those randomised to upadacitinib 30 mg across four SELECT trials (SELECT-BEYOND, SELECT-EARLY, SELECT-NEXT and SELECT-MONOTHERAPY) and those who switched to upadacitinib 30 mg from placebo or active comparator (only postswitch data were included).
Exposure-adjusted incidence rates (EAIRs) per 100 patient-years (PY) were summarised based on the treatment received at the time of each TEAE, with exposure time calculated as the time to the first event. In patients who did not experience an event, the exposure time was censored on the day of the patient’s last assessment or the cut-off date, whichever occurred first. Additionally, 95% CIs were calculated based on the exact method for the Poisson mean. EAIRs for AEs of interest were descriptively analysed in the higher-risk populations by additional baseline factors, including age (50–<65 years versus ≥65 years) and age plus smoking status (50–<65 years old and never smoked versus ≥65 years or current/former smoker). EAIRs of MACE and VTE were further stratified by medical history of CV or VTE event, respectively. The incidence of AEs leading to death was also determined. The standardised mortality ratio (SMR) was computed using country-specific, age-specific and sex-specific mortality estimates from the WHO. This ‘general population’ estimate was not enriched for additional risk factors; 95% CIs were calculated using Byar’s approximation. The association between disease activity and AE occurrence was analysed using time-weighted area under the curve from baseline for MACE, VTE and malignancy (excluding NMSC); p values were generated using analysis of covariance with the event status as a factor and the baseline disease activity value as a covariate. In the SELECT-COMPARE higher-risk population, HRs for upadacitinib 15 mg versus adalimumab were generated using univariable Cox proportional hazard models for the time from the first dose of study drug to the first AE.
Results
Patients
The overall population included 4102 patients (upadacitinib 15 mg, n=3209; adalimumab, n=579; and MTX monotherapy, n=314), with a cumulative exposure of 10135 PY for upadacitinib 15 mg, 1459 PY for adalimumab and 835 PY for MTX (online supplemental figure 1). The median durations of exposure were 3.7 (maximum (max) 6.1) years on upadacitinib 15 mg, 2.2 (max 6.1) years on adalimumab and 2.6 (max 5.2) years on MTX. More than one-half of patients from the overall population were identified as being at higher CV risk (aged ≥50 years with ≥1 CV risk factor) in each treatment group (upadacitinib 15 mg, n=1717 (54%); adalimumab, n=320 (55%); and MTX monotherapy, n=162 (52%)). The higher CV risk population from SELECT-COMPARE included 649 patients (1852 PY) on upadacitinib 15 mg and 177 patients (339 PY) on adalimumab. The most common comorbidity that qualified patients for inclusion in the higher-risk population was hypertension (~40%), followed by smoking (~37%) and low HDL-C (~11%).
Patient demographics and disease characteristics were generally comparable between treatment groups (table 1), with exceptions for patients who received MTX monotherapy, including shorter time since RA diagnosis and most being csDMARD-naïve. Across populations and treatment groups, most patients were female with high disease activity (mean Clinical Disease Activity Index (CDAI) at baseline: 39.7–41.6). The baseline characteristics of patients in the higher CV risk populations were generally similar to those in ORAL Surveillance,3 although the proportion of bDMARD-IR patients was higher in our datasets. The proportions of patients from each clinical trial who contributed to the overall and higher-risk populations are shown in online supplemental table 1.
Table 1.
Baseline demographics and disease characteristics
| n (%), unless specified | Overall population | Higher CV risk population | SELECT-COMPARE higher CV risk population | |||||
| upadacitinib 15 mg QD±csDMARDs (n=3209) |
adalimumab 40 mg EOW+MTX (n=579) |
MTX monotherapy (n=314) |
upadacitinib 15 mg QD±csDMARD(s) (n=1717) |
adalimumab 40 mg EOW+MTX (n=320) |
MTX monotherapy (n=162) |
upadacitinib 15 mg QD+MTX (n=649) |
adalimumab 40 mg EOW+MTX (n=177) |
|
| Female | 2581 (80.4) | 470 (81.2) | 240 (76.4) | 1331 (77.5) | 254 (79.4) | 114 (70.4) | 501 (77.2) | 133 (75.1) |
| Mean (SD) age (years) | 54.3 (12.0) | 54.2 (11.7) | 53.3 (12.9) | 61.4 (7.3) | 60.6 (7.2) | 61.6 (7.7) | 61.1 (7.2) | 60.9 (7.6) |
| Age ≥65 years | 643 (20.0) | 106 (18.3) | 58 (18.5) | 543 (31.6) | 89 (27.8) | 54 (33.3) | 202 (31.1) | 52 (29.4) |
| Mean (SD) BMI (kg/m2) | 29.1 (6.7)* | 29.4 (7.1) | 28.0 (6.3) | 29.8 (6.5)* | 30.4 (6.9) | 28.7 (6.6) | 29.7 (6.4) | 29.4 (6.3) |
| BMI ≥30 kg/m2 | 1200 (37.4)* | 227 (39.2) | 97 (30.9) | 722 (42.1)* | 144 (45.0) | 56 (34.6) | 267 (41.1) | 67 (37.9) |
| Race | ||||||||
| White | 2784 (86.8) | 504 (87.0) | 256 (81.5) | 1480 (86.2) | 284 (88.8) | 129 (79.6) | 570 (87.8) | 162 (91.5) |
| Black or African–American | 170 (5.3) | 39 (6.7) | 12 (3.8) | 118 (6.9) | 27 (8.4) | 9 (5.6) | 41 (6.3) | 11 (6.2) |
| Asian | 191 (6.0) | 30 (5.2) | 37 (11.8) | 86 (5.0) | 7 (2.2) | 21 (13.0) | 24 (3.7) | 3 (1.7) |
| Other | 64 (2.0) | 6 (1.0) | 9 (2.9) | 33 (1.9) | 2 (0.6) | 3 (1.9) | 14 (2.2) | 1 (0.6) |
| Geographical region | ||||||||
| North America | 815 (25.4) | 122 (21.1) | 46 (14.6) | 537 (31.3) | 79 (24.7) | 29 (17.9) | 148 (22.8) | 39 (22.0) |
| Rest of the world | 2394 (74.6) | 457 (78.9) | 268 (85.4) | 1180 (68.7) | 241 (75.3) | 133 (82.1) | 501 (77.2) | 138 (78.0) |
| Mean (SD) time since diagnosis (years) | 8.5 (8.4)† | 8.2 (8.0) | 2.6 (5.1) | 9.5 (9.0) | 8.9 (8.8) | 2.9 (6.1) | 8.9 (8.4) | 8.8 (9.4) |
| Mean (SD) CDAI‡ | 39.7 (12.7) | 41.1 (13.3) | 40.5 (13.3) | 39.8 (12.6) | 41.6 (13.4) | 39.8 (13.8) | 40.4 (12.7) | 40.2 (13.9) |
| Mean (SD) DAS28(CRP)§ | 5.8 (1.0) | 5.9 (1.0) | 5.9 (1.0) | 5.8 (0.9) | 5.9 (1.0) | 5.8 (1.0) | 5.8 (0.9) | 5.9 (1.0) |
| RF positive | 2439 (76.1)¶ | 456 (78.8) | 232 (73.9) | 1312 (76.5)¶ | 249 (77.8) | 115 (71.0) | 521 (80.3) | 141 (79.7) |
| ACPA positive | 2505 (78.2)** | 455 (78.6) | 236 (75.2) | 1318 (76.9)** | 247 (77.2) | 116 (71.6) | 521 (80.4) | 138 (78.0) |
| Prior bDMARD use | 952 (29.7) | 55 (9.5) | 0 | 544 (31.7) | 30 (9.4) | 0 | 54 (8.3) | 17 (9.6) |
| Prior TNFi therapy | 819 (25.5) | 37 (6.4) | 0 | 478 (27.8) | 22 (6.9) | 0 | 42 (6.5) | 12 (6.8) |
| Other bDMARD therapy | 304 (9.5) | 20 (3.5) | 0 | 168 (9.8) | 8 (2.5) | 0 | 14 (2.2) | 5 (2.8) |
| Concomitant csDMARD use | ||||||||
| MTX alone | 2182 (68.0) | 579 (100) | 0 | 1181 (68.8) | 320 (100) | 0 | 647 (99.7) | 177 (100) |
| MTX and other csDMARD | 169 (5.3) | 0 | 0 | 88 (5.1) | 0 | 0 | 2 (0.3) | 0 |
| csDMARDs other than MTX | 196 (6.1) | 0 | 0 | 112 (6.5) | 0 | 0 | 0 | 0 |
| None | 662 (20.6) | 0 | 314 (100) | 336 (19.6) | 0 | 162 (100) | 0 | 0 |
| Other concomitant treatments | ||||||||
| Glucocorticoid | 1763 (54.9) | 350 (60.4) | 164 (52.2) | 900 (52.4) | 195 (60.9) | 85 (52.5) | 379 (58.4) | 114 (64.4) |
| Aspirin | 270 (8.4) | 36 (6.2) | 24 (7.6) | 250 (14.6) | 34 (10.6) | 19 (11.7) | 92 (14.2) | 18 (10.2) |
| Statin | 369 (11.5) | 55 (9.5) | 26 (8.3) | 322 (18.8) | 49 (15.3) | 24 (14.8) | 108 (16.6) | 19 (10.7) |
| Antithrombotic agent | 316 (9.8) | 42 (7.3) | 26 (8.3) | 290 (16.9) | 38 (11.9) | 21 (13.0) | 102 (15.7) | 20 (11.3) |
| Smoking status†† | ||||||||
| Never smoked | 1986 (61.9) | 378 (65.5) | 194 (61.8) | 800 (46.6) | 162 (50.9) | 69 (42.6) | 342 (52.7) | 78 (44.6) |
| Ever smoked | 1221 (38.1) | 199 (34.5) | 120 (38.2) | 915 (53.4) | 156 (49.1) | 93 (57.4) | 307 (47.3) | 97 (55.4) |
| History of hypertension | 1277 (39.8) | 252 (43.5) | 112 (35.7) | 1106 (64.4) | 225 (70.3) | 96 (59.3) | 429 (66.1) | 123 (69.5) |
| History of diabetes mellitus | 383 (11.9) | 61 (10.5) | 31 (9.9) | 327 (19.0) | 54 (16.9) | 28 (17.3) | 119 (18.3) | 27 (15.3) |
| History of VTE | 53 (1.7) | 9 (1.6) | 3 (1.0) | 38 (2.2) | 9 (2.8) | 0 | 14 (2.2) | 6 (3.4) |
| History of CV event | 385 (12.0) | 63 (10.9) | 27 (8.6) | 346 (20.2) | 56 (17.5) | 24 (14.8) | 141 (21.7) | 20 (11.3) |
| HDL-C <40 mg/dL | 354 (11.0) | 53 (9.2) | 39 (12.4) | 224 (13.0) | 33 (10.3) | 26 (16.0) | 87 (13.4) | 19 (10.7) |
*Overall population, n=3205. Overall higher-risk population, n=1715.
†n = 3208.
‡Overall population: upadacitinib 15 mg QD, n=3040; adalimumab 40 mg EOW, n=546; MTX, n=299. Overall higher-risk population: upadacitinib 15 mg QD, n=1632; adalimumab 40 mg EOW, n=306; MTX, n=153. SELECT-COMPARE higher-risk population: upadacitinib 15 mg QD, n=618; adalimumab 40 mg EOW, n=170.
§Overall population: upadacitinib 15 mg QD: n=3192; adalimumab 40 mg EOW: n=575; MTX: n=314. Overall higher-risk population: upadacitinib 15 mg QD, n=1709; adalimumab 40 mg EOW, n=318; MTX, n=162. SELECT-COMPARE higher-risk population: upadacitinib 15 mg QD, n=646; adalimumab 40 mg EOW, n=176.
¶Overall population: n=3207. Overall higher-risk population: n=1716.
**Overall population: n=3203. Overall higher-risk population: n=1714.
††Overall population: upadacitinib 15 mg QD: n=3207; adalimumab 40 mg EOW: n=577; MTX: n=314. Overall higher-risk population: upadacitinib 15 mg QD: n=1715; adalimumab 40 mg EOW: n=318; MTX: n=162. SELECT-COMPARE higher-risk population: upadacitinib 15 mg QD: n=649; adalimumab 40 mg EOW: n=175.
ACPA, anti-cyclic citrullinated peptide antibody; bDMARD, biological disease-modifying antirheumatic drug; BMI, body mass index; CDAI, Clinical Disease Activity Index; CRP, C reactive protein; csDMARD, conventional synthetic disease-modifying antirheumatic drug; CV, cardiovascular; DAS28(CRP), 28-Joint Disease Activity Score Based on C Reactive Protein; EOW, every other week; HDL-C, high-density lipoprotein cholesterol; MTX, methotrexate; QD, once daily; RF, rheumatoid factor; TNFi, tumour necrosis factor inhibitor; VTE, venous thromboembolism.
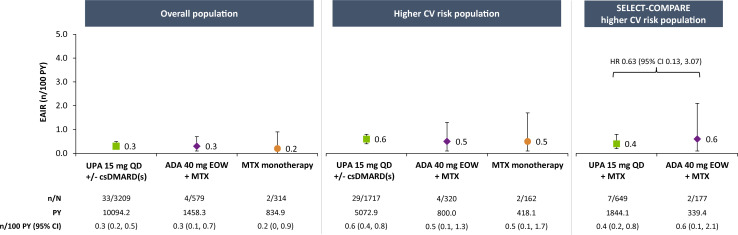
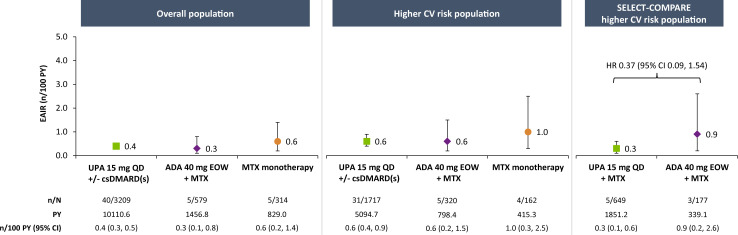
Major adverse cardiovascular events
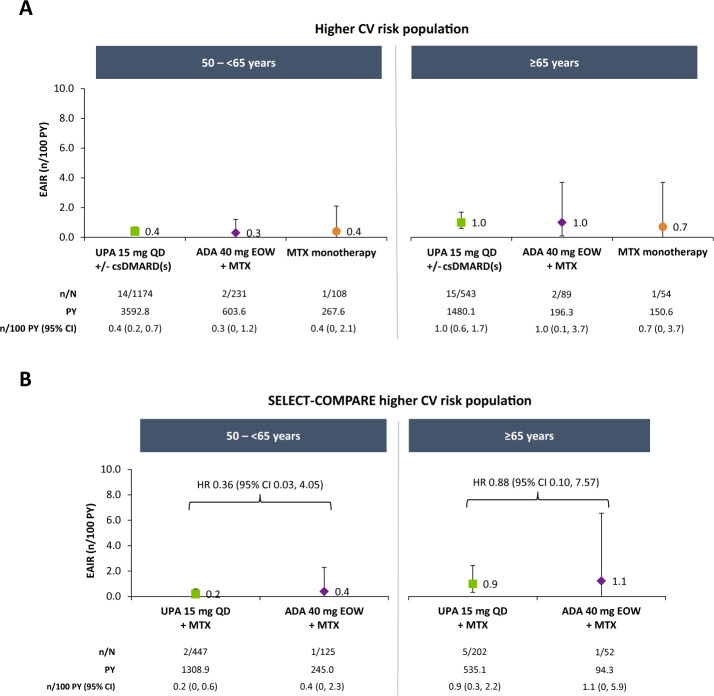
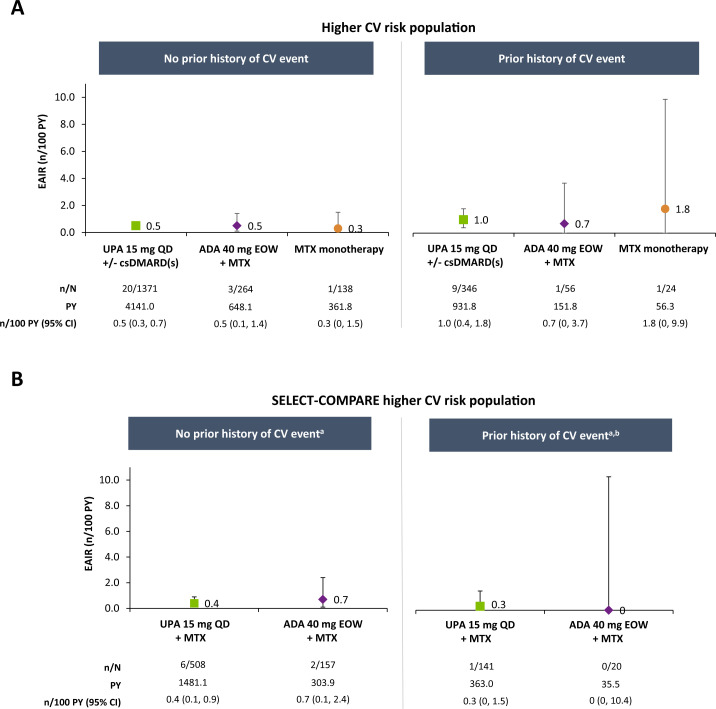
EAIRs of adjudicated MACE were 0.3, 0.3 and 0.2 n/100 PY with upadacitinib 15 mg, adalimumab and MTX monotherapy, respectively, in the overall population (figure 1). Among patients at higher CV risk, rates were numerically higher than in the overall population but comparable between therapies (0.6, 0.5 and 0.5 n/100 PY with upadacitinib 15 mg, adalimumab and MTX monotherapy, respectively). In subgroup analyses of this higher-risk population, numerically higher EAIRs of MACE were observed in patients aged ≥65 years compared with those aged 50–<65 years and among patients aged ≥65 years or who ever smoked versus those aged 50–65 years who never smoked, with no apparent differences between treatment groups (figure 2 and online supplemental figure 2). Medical history of a CV event appeared to be associated with risk of MACE occurrence, irrespective of treatment (figure 3).
Figure 1.
Exposure-adjusted incidence of adjudicated MACE. MACE defined as CV death (includes acute myocardial infarction, sudden cardiac death, heart failure, CV procedure-related death, death due to CV haemorrhage, fatal stroke, pulmonary embolism and other CV causes), non-fatal myocardial infarction and non-fatal stroke. ADA, adalimumab; csDMARD, conventional synthetic disease-modifying antirheumatic drug; CV, cardiovascular; EAIR, exposure-adjusted incidence rate; EOW, every other week; MACE, major adverse cardiovascular event; MTX, methotrexate; PY, patient-years; QD, once daily; UPA, upadacitinib.
Figure 2.
Exposure-adjusted incidence of adjudicated MACE in higher CV risk populations by age. MACE defined as CV death (includes acute myocardial infarction, sudden cardiac death, heart failure, CV procedure-related death, death due to CV haemorrhage, fatal stroke, pulmonary embolism and other CV causes), non-fatal myocardial infarction and non-fatal stroke. ADA, adalimumab; csDMARD, conventional synthetic disease-modifying antirheumatic drug; CV, cardiovascular; EAIR, exposure-adjusted incidence rate; EOW, every other week; MACE, major adverse cardiovascular event; MTX, methotrexate; PY, patient-years; QD, once daily; UPA, upadacitinib.
Figure 3.
Exposure-adjusted incidence of MACE in higher CV risk populations by medical history of a CV event. aDue to the small number of events in these subgroups, HRs for upadacitinib versus adalimumab were not calculated. bNo events occurred in patients treated with adalimumab who had a history of a CV event. MACE defined as CV death (includes acute myocardial infarction, sudden cardiac death, heart failure, CV procedure-related death, death due to CV haemorrhage, fatal stroke, pulmonary embolism and other CV causes), non-fatal myocardial infarction and non-fatal stroke. ADA, adalimumab; csDMARD, conventional synthetic disease-modifying antirheumatic drug; CV, cardiovascular; EAIR, exposure-adjusted incidence rate; EOW, every other week; MACE, major adverse cardiovascular event; MTX, methotrexate; PY, patient-years; QD, once daily; UPA, upadacitinib.
Among the patients with higher CV risk from SELECT-COMPARE, incidence of MACE was 0.4/100 PY for upadacitinib 15 mg and 0.6/100 PY for adalimumab (figure 1). Cox regression analysis did not suggest an elevated risk with upadacitinib versus adalimumab treatment (HR 0.63, 95% CI 0.13 to 3.07). Subgroup analyses of the SELECT-COMPARE higher-risk population were generally consistent with those from the overall higher-risk population (figures 2 and 3 and online supplemental figure 2).
Most occurrences of adjudicated MACE were non-fatal. In the overall higher-risk population, 19 of 29 (66%) and 3 of 4 (75%) cases were non-fatal on upadacitinib 15 mg and adalimumab, respectively (online supplemental materials). Approximately 86% of patients who experienced MACE interrupted or discontinued upadacitinib after the event.
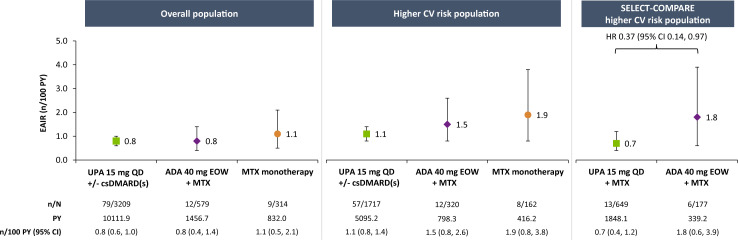
Malignancy
EAIRs of malignancy excluding NMSC were 0.8, 0.8 and 1.1 n/100 PY with upadacitinib 15 mg, adalimumab and MTX monotherapy, respectively, in the overall population (figure 4). While the rates were numerically higher in the overall higher-risk population, they were comparable between treatments (1.1, 1.5 and 1.9 n/100 PY with upadacitinib 15 mg, adalimumab and MTX monotherapy, respectively). In subgroup analyses of the overall higher-risk population stratified by age, rates were numerically higher among patients ≥65 years of age than those aged 50–<65 years, with comparable rates between upadacitinib 15 mg and adalimumab (online supplemental figure 3). Similar results were observed after stratifying by age and smoking status (online supplemental figure 4).
Figure 4.
Exposure-adjusted incidence of malignancies (excluding NMSC). Data are presented as treatment-emergent malignancy rates, with a data cut-off of no more than 30 days after the last dose of study drug for upadacitinib or MTX and up to 70 days for adalimumab if patients discontinued prematurely from the study. ADA, adalimumab; csDMARD, conventional synthetic disease-modifying antirheumatic drug; CV, cardiovascular; EAIR, exposure-adjusted incidence rate; EOW, every other week; MTX, methotrexate; NMSC, non-melanoma skin cancer; PY, patient-years; QD, once daily; UPA, upadacitinib.
In the SELECT-COMPARE higher-risk population, the incidence of malignancy (excluding NMSC) was 0.7 and 1.8 n/100 PY with upadacitinib 15 mg and adalimumab, respectively, and Cox regression analysis showed no elevation of risk with upadacitinib 15 mg relative to adalimumab (HR 0.37, 95% CI 0.14 to 0.97) (figure 4). Similarly, no apparent elevated risk was observed with upadacitinib 15 mg versus adalimumab in higher-risk subgroups stratified by age or smoking status (online supplemental figures 3 and 4), although no events were reported among adalimumab-treated patients aged 50–<65 years and never smoked.
Lung cancer was the most common type of malignancy (excluding NMSC) reported among upadacitinib-treated patients in the overall higher-risk population, occurring in a similar proportion of patients who received either upadacitinib 15 mg (0.3 n/100 PY) or adalimumab (0.2 n/100 PY) (online supplemental table 2). All patients with lung cancer were current or former smokers. Colorectal cancer and lymphoma were the most common cancer types reported with adalimumab treatment.
The incidence of NMSC was higher in patients treated with upadacitinib 15 mg versus adalimumab or MTX. In the overall population, the EAIR of NMSC was 0.4 n/100 PY with upadacitinib 15 mg and <0.1 n/100 PY with adalimumab; no NMSC events were reported with MTX. Rates of NMSC were also higher in the overall higher-risk population (0.7 for upadacitinib 15 mg versus 0.1 n/100 PY for adalimumab) and SELECT-COMPARE higher-risk population (0.7 for upadacitinib 15 mg versus 0.3 n/100 PY for adalimumab). Cox regression analysis indicated an elevated risk with upadacitinib 15 mg versus adalimumab (HR 2.26, 95% CI 0.29 to 17.39).
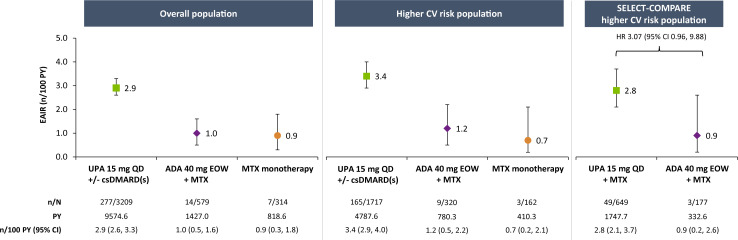
Venous thromboembolism
EAIRs of adjudicated VTE events were 0.4, 0.3 and 0.6 n/100 PY with upadacitinib 15 mg, adalimumab and MTX monotherapy, respectively, in the overall population (figure 5). A numerically higher EAIR was observed in the overall higher-risk population, but the rates remained comparable between treatments (0.6, 0.6 and 1.0 n/100 PY in the upadacitinib 15 mg, adalimumab or MTX monotherapy groups, respectively). In subgroup analyses of the overall higher-risk population, rates were generally similar between upadacitinib 15 mg and adalimumab in patients aged ≥65 years and those aged 50–<65 years, as well as in patients aged ≥65 years or who ever smoked versus those aged 50-<65 years and never smoked (online supplemental figures 5 and 6). Higher rates of VTE were observed among upadacitinib-treated patients with a medical history of a VTE event (online supplemental figure 7). No VTE events were reported in adalimumab-treated patients with a history of VTE.
Figure 5.
Exposure-adjusted incidence of adjudicated VTE. VTE events include deep vein thrombosis and pulmonary embolism. ADA, adalimumab; csDMARD, conventional synthetic disease-modifying antirheumatic drug; CV, cardiovascular; EAIR, exposure-adjusted incidence rate; EOW, every other week; MTX, methotrexate; PY, patient-years; QD, once daily; UPA, upadacitinib; VTE, venous thromboembolism.
In the SELECT-COMPARE higher-risk population, the EAIR for VTE was 0.3 n/100 PY on upadacitinib 15 mg versus 0.9 n/100 PY on adalimumab, with no elevated risk identified for upadacitinib by Cox regression analysis (HR 0.37, 95% CI 0.09 to 1.54) (figure 5) or in subgroup analyses by age and smoking status (online supplemental figures 5 and 6).
Of the 31 adjudicated VTE events reported among patients who received upadacitinib 15 mg in the overall higher-risk population, 29 events were non-fatal; all VTE events (5/5) reported with adalimumab were non-fatal (online supplemental materials).
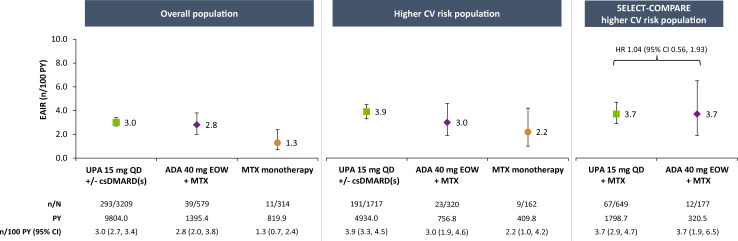
Infections
EAIRs of SIE were 3.0, 2.8 and 1.3 n/100 PY with upadacitinib 15 mg, adalimumab and MTX monotherapy, respectively, in the overall population (figure 6). While the incidences were marginally elevated in the overall higher-risk population across all therapies, numerically higher rates of SIE were observed with upadacitinib 15 mg (3.9 n/100 PY) than adalimumab or MTX (3.0 and 2.2 n/100 PY, respectively). Among higher-risk patients, rates were numerically higher in those aged ≥65 years versus 50–<65 years (online supplemental figure 8), with numerically higher rates observed with upadacitinib 15 mg than adalimumab or MTX. Across treatment groups, pneumonia was the most frequently reported type of SIE in the overall higher-risk population (online supplemental table 3).
Figure 6.
Exposure-adjusted incidence of serious infection. ADA, adalimumab; csDMARD, conventional synthetic disease-modifying antirheumatic drug; CV, cardiovascular; EAIR, exposure-adjusted incidence rate; EOW, every other week; MTX, methotrexate; PY, patient-years; QD, once daily; UPA, upadacitinib.
In the SELECT-COMPARE higher-risk population, EAIRs were comparable between upadacitinib 15 mg and adalimumab (3.7 n/100 PY for either treatment), with no apparent elevated risk with upadacitinib 15 mg (HR 1.04, 95% CI 0.56 to 1.93) (figure 6). In patients ≥65 years of age, however, a numerically elevated risk of SIE was associated with upadacitinib 15 mg versus adalimumab (HR 1.53, 95% CI 0.46 to 5.06) (online supplemental figure 8). No increased risk of SIE for upadacitinib 15 mg compared with adalimumab was detected among patients aged 50–<65 years.
Consistent with previous upadacitinib safety analyses,8 14 15 higher incidence of HZ was observed in patients receiving upadacitinib 15 mg versus adalimumab or MTX monotherapy in the overall population and across higher-risk patient populations (figure 7). An elevated risk of HZ was observed for upadacitinib 15 mg versus adalimumab in the SELECT-COMPARE higher-risk population (HR 3.07, 95% CI 0.96 to 9.88).
Figure 7.
Exposure-adjusted incidence of herpes zoster. ADA, adalimumab; csDMARD, conventional synthetic disease-modifying antirheumatic drug; EAIR, exposure-adjusted incidence rate; EOW, every other week; MTX, methotrexate; PY, patient-years; QD, once daily; UPA, upadacitinib.
Mortality
EAIRs of death were 0.8, 1.0 and 0.8 n/100 PY with upadacitinib 15 mg, adalimumab and MTX monotherapy, respectively, in the overall population. Although numerically higher in the overall higher-risk population, the mortality rates were similar between treatment groups (1.3, 1.2 and 1.4 n/100 PY with upadacitinib 15 mg, adalimumab or MTX, respectively). Excluding COVID-19-related deaths, the most common AE leading to death among upadacitinib-treated patients in the overall higher-risk population was CV disorders, followed by malignancies and non-COVID-19 infections. For adalimumab-treated patients, the most common AEs leading to death were malignancies, followed by CV disorders and non-COVID-19 infections. Rates of death due to infections (excluding COVID-19) were similar between patients treated with upadacitinib 15 mg or adalimumab in the overall higher-risk population. All 15 COVID-19-related deaths were reported in upadacitinib-treated patients, most of whom (87%) discontinued their treatment upon COVID-19 diagnosis. There was no evidence suggesting an increased risk of deaths for patients in the overall higher-risk population who received upadacitinib 15 mg versus patients in the general population (SMR 0.46, 95% CI 0.31 to 0.66, excluding COVID-19 deaths). In the SELECT-COMPARE higher-risk population, the rate of death was 1.4 n/100 PY for patients treated with upadacitinib 15 mg versus 1.5 n/100 PY with adalimumab (HR 0.91, 95% CI 0.35 to 2.38).
Safety outcomes in patients receiving upadacitinib 30 mg
Safety events were also evaluated in patients who received upadacitinib 30 mg treatment, which has not been approved for RA but was included in four of the SELECT trials. A total of 1204 patients in the overall population received upadacitinib 30 mg, with a median duration of exposure of 3.2 (max 5.5) years. More than one-half of patients from the overall population of patients treated with upadacitinib 30 mg were identified as being at higher CV risk (677 (56%)). Consistent with upadacitinib 15 mg analyses, the rates of AEs of interest were numerically higher in upadacitinib 30mg-treated patients at higher CV risk compared with those in the overall population (online supplemental table 4). Though a direct comparison cannot be made between upadacitinib 15 mg and 30 mg because of differences in the datasets, rates of malignancy excluding NMSC, VTE and death were similar between both upadacitinib dosages, but numerically higher rates were observed for MACE, NMSC, SIE and HZ in the 30 mg group.
Disease activity and AE occurrence
In the overall higher-risk population, upadacitinib 15 mg-treated patients who experienced MACE or VTE had significantly less improvement in the time-weighted changes in disease activity as measured by CDAI or 28-Joint Disease Activity Score Based on C Reactive Protein (DAS28(CRP)) (CDAI −24.1 and −24.2, DAS28-CRP −2.4 and −2.3 for MACE and VTE, respectively) compared with patients who did not experience MACE or VTE (CDAI −28.4 and −28.5 (p=0.015 and p=0.015); DAS28-CRP −2.8 and −2.8 (p=0.037 and p=0.010) for MACE and VTE, respectively). A similar difference was not observed for malignancies excluding NMSC (upadacitinib 15 mg-treated patients with malignancy versus no event: CDAI −29.2 versus –28.4, DAS28-CRP −3.0 versus –2.8); specific malignancy types could not be assessed, given the low number of events. Consistent results were observed in the overall population.
Discussion
The publication of the findings from ORAL Surveillance has engendered discussion regarding the safety of JAKi as a class relative to TNFi, particularly for the occurrence of MACE and malignancy but also VTE, SIE and mortality.3 Importantly, it should be noted that these AEs of special interest were also observed in patients treated with TNFi but numerically less, underscoring the need to evaluate the potential risk in all patients when prescribing any therapy for RA.
In this integrated post hoc analysis from six upadacitinib phase III trials, similar incidences for MACE, malignancy (excluding NMSC), VTE and mortality were observed for upadacitinib 15 mg, adalimumab and MTX in the overall population. Higher rates of HZ, a known AE with JAK inhibition,14 16 17 and NMSC were observed with upadacitinib across populations. Our analysis of patient subgroups with risk factors mimicking the ORAL Surveillance patient population similarly demonstrated increased rates of MACE, malignancy (excluding NMSC) and VTE in the higher-risk population; however, the rates appeared comparable between upadacitinib 15 mg, adalimumab and MTX. Assessment of the higher-risk patients in SELECT-COMPARE showed comparable risk of MACE, malignancy excluding NMSC and VTE between upadacitinib 15 mg and adalimumab. Numerically higher rates of SIE were observed with upadacitinib 15 mg versus adalimumab in the overall higher-risk population.
In ORAL Surveillance, the elevated risk of MACE and malignancy for tofacitinib versus TNFi therapy was most apparent in patients aged ≥65 years (versus 50–<65 years), among current and former smokers (>90% had a duration of smoking >10 years), and in patients who had a previous cardiac event or history of coronary artery disease/atherosclerotic CV disease.3 18–20 Our analysis is consistent with ORAL Surveillance in the higher CV risk population, which demonstrated generally greater risk of MACE, malignancy (excluding NMSC) and VTE among older patients (≥65 years versus 50–<65 years) and those aged ≥65 years or who were current or former smokers (smoking duration not available). This is unsurprising, given the known risk of these factors for the development of the aforementioned events.3 21 In contrast to ORAL Surveillance, in SELECT-COMPARE, a much smaller study not powered for safety, HRs for these TEAEs were similar for upadacitinib 15 mg versus adalimumab therapy stratified by age and smoking status. Due to the limited number of patients and reported events, these findings should be interpreted with caution and need replication in a larger population.
COVID-19 deaths were among the most common causes of death observed in the upadacitinib treatment group, whereas none were reported with adalimumab, possibly due to the much larger number of patients treated with upadacitinib than adalimumab. These global clinical trials also varied in how much they preceded the onset of the pandemic, further complicating the assessment of mortality data. The effect of JAKi treatment on COVID-19 outcomes is complex. In the initial analysis of the Global Alliance registry data, it was reported that treatment with a JAKi at the time of diagnosis with COVID-19 was associated with worse clinical outcomes.22 A subsequent analysis of the same registry revealed that patients who discontinued JAKi upon diagnosis had worse COVID-19 outcomes. In contrast, patients who continued JAKi therapy during the course of their infection fared better, suggesting that continuation of JAKi could potentially have a protective effect, depending on the stage of infection.23 24 A systematic review of randomised clinical trial data also suggests that JAKi treatment may decrease the worsening of clinical status and all-cause mortality in patients hospitalised with COVID-19.25 26 Of note, the majority of patients (13 out of 15) who died of COVID-19-related causes in our upadacitinib 15 mg dataset stopped treatment following COVID-19 diagnosis, consistent with current guidelines to discontinue upadacitinib treatment in the case of an SIE until the infection is under control.27 28 Considered more broadly, COVID-19 vaccination plays an essential role in risk reduction, and effective vaccination strategies are possible in patients receiving JAKi therapy.29
In a separate analysis of upadacitinib 30 mg data, rates of AEs of interest were consistently higher in patients at increased CV risk compared with those in the overall population. Rates of malignancy excluding NMSC, VTE and death were similar between both upadacitinib dosages, but numerically higher rates were observed for MACE, NMSC, SIE and HZ in the 30 mg group. However, a direct comparison of the event rates between upadacitinib 15 mg and 30 mg should be made with caution, given that the integrated analyses for the two treatment groups were not based on the same trials (SELECT-COMPARE and SELECT-CHOICE did not include a matched upadacitinib 30 mg treatment arm). Moreover, there are differences between the patient populations for upadacitinib 15 mg and 30 mg, including background csDMARD use, that could affect the rates of some AEs like SIE.
Although we show results from a upadacitinib clinical trial population with similar baseline CV risks and population characteristics described in ORAL Surveillance, a direct comparison of the safety of these two molecules is not possible, given the fundamental differences in the study design and the lack of any head-to-head safety trial of tofacitinib compared with upadacitinib. ORAL Surveillance was a large prospective, randomised safety study, whereas this report is a post hoc analysis of upadacitinib phase III trials of patients not enriched for CV risks, nor were these trials powered to show differences in safety outcomes between upadacitinib and adalimumab. Despite these limitations, the apparent lack of elevated risks for AEs of interest provides additional information for the healthcare community.
Of note, the possible safety signals raised in ORAL Surveillance with respect to malignancies and CV disease were observed in a high-risk population of patients with RA with a relatively large number needed to harm for tofacitinib versus TNFi.3 30 Similar results have not been reported in an unselected RA population with any JAKi. A recent post hoc analysis of tofacitinib clinical trial datasets reported a lower incidence of these AEs than that observed in ORAL Surveillance.31 In another post hoc analysis of tofacitinib clinical trial data across indications, VTE rates were generally higher in patients with baseline CV or VTE risk factors compared with patients without those risks.32 In keeping with our findings, VTE rates in higher-risk patients with RA from that dataset appeared similar between tofacitinib 5 mg, tofacitinib 10 mg and TNFi therapy, but interpretation was limited due to the low number of events. A systemic review of JAKi safety and efficacy data across indications also suggests a favourable clinical profile in patients with RA and other rheumatic diseases.33 Our findings are generally consistent with the known safety profile of upadacitinib,14 34 including an analysis of the long-term safety and efficacy of versusupadacitinib versus adalimumab through 3 years in SELECT-COMPARE.15 However, this is the first upadacitinib study to evaluate safety (post hoc) in a population of patients at higher CV risk. Published reports from real-world evidence assessments of higher-risk patients have not consistently supported a significantly different risk of MACE and VTE with JAKi versus TNFi.35 36 However, while the STAR-RA study did not find an elevated risk of CV outcomes for tofacitinib versus TNFi in a real-world setting, tofacitinib was associated with an increased (although statistically insignificant) risk in patients with CV risk factors compared with TNFi.36 Recently, study BO23, a meta-analysis across 14 postmarketing data sources, showed a significantly increased risk of only VTE with baricitinib relative to TNFi.37 A Swedish cohort study also reported an elevated risk of VTE in patients who primarily received tofacitinib or baricitinib.38
RA disease activity and control play a key role in reducing the risk of AEs associated with inflammation. Patients with RA are known to be at an increased risk of MACE, certain malignancies, VTEs and serious infections, while management of RA is associated with a reduction in these risks.39–42 Consistently, we observed here that patients on upadacitinib 15 mg who experienced MACE or VTE showed less improvement in their disease activity than those who did not have such an event. However, a similar relationship between disease activity and malignancy was not detected. Of note, upadacitinib has previously demonstrated greater efficacy than adalimumab or MTX, with higher attainment of CDAI remission and low disease activity observed in patients receiving upadacitinib.8 12 15 Thus, the ability of upadacitinib-treated patients to achieve remission could potentially lead to reduced incidences of at least some RA comorbidities. Additionally, given that several of the upadacitinib trials included in this analysis were conducted in harder-to-treat patient populations, comprising many individuals who failed or only partially responded to previous bDMARD treatment, these more refractory patients could have higher risks associated with their underlying inflammatory condition.
Major limitations include the post hoc nature of the analysis, which was not based on prespecified endpoints, in addition to the low sample size, limited PY of exposure in some patient groups, and that some inclusion criteria assessed in ORAL Surveillance are not available within the SELECT RA clinical trial programme (eg, family history of heart disease) and could not be used to identify patients at increased CV risk. Additionally, more patients discontinued adalimumab earlier than upadacitinib 15 mg in the long-term extension of SELECT-COMPARE; this lower duration of follow-up for the adalimumab treatment group may have restricted detection of differences between upadacitinib and adalimumab therapy, particularly given that the cumulative incidence curves did not start to diverge until after 2–3 years in ORAL Surveillance.3 Moreover, because a higher proportion of patients switched from adalimumab to upadacitinib 15 mg than vice versa in SELECT-COMPARE, this could overestimate the risk for upadacitinib, given the potential for more difficult-to-treat patients in the upadacitinib 15 mg group versus the adalimumab group. Many of the TEAEs evaluated were rare events (eg, n<10), limiting the precision of the EAIRs and comparisons with adalimumab or MTX. Furthermore, while inclusion of MTX monotherapy data provides a safety reference point in the absence of a placebo control, patients in the MTX treatment group were mostly MTX-naïve and had a shorter duration since diagnosis of RA than those in the upadacitinib or adalimumab groups. As a general limitation, our data pooling approach across trial phases (double-blind, placebo-controlled and long-term extension) and the inclusion of different clinical trials having various treatment contexts could potentially impact the results. Short-term, double-blind, placebo-controlled safety data have been previously reported.7–12 Notably, the safety profile of upadacitinib has remained largely consistent over time, and safety events were observed to be generally stable over time of follow-up.34 Indeed, while rates of many events will vary across studies, one of the advantages of a pooling strategy is to minimise this by increasing the overall robustness of the data for evaluation. Finally, the analysis of malignancy events is limited by the relatively short duration of treatment exposure from the upadacitinib clinical trials, given the fact that it takes years or decades for most solid cancers to develop for clinical diagnosis.
In summary, the incidence of MACE, malignancy (excluding NMSC), VTE and mortality was typically higher in patients at increased CV risk compared with the overall RA population, but the rates remained generally similar between upadacitinib 15 mg and adalimumab. Higher rates of HZ and NMSC in all populations and SIE in the higher CV risk population were observed with upadacitinib versus comparators. In the SELECT-COMPARE higher-risk population, upadacitinib 15 mg did not appear to be associated with increased risk of any examined TEAEs, except for HZ, NMSC and SIE in patients aged ≥65 years. These findings in patients with RA at risk of potential CV events may help to clinically contextualise the overall risk profile of upadacitinib.
Acknowledgments
AbbVie and the authors thank the patients, trial sites and investigators who participated in this clinical trial. AbbVie was the trial sponsor, contributed to the trial design, data collection, analysis and interpretation, and to the writing, reviewing and approval of final version. No honoraria or payments were made for authorship. Medical writing support was provided by Matthew Eckwahl, PhD, of AbbVie. Editorial support was provided by Angela T Hadsell of AbbVie.
Footnotes
Handling editor: Josef S Smolen
Contributors: RF, GRB, and SS contributed to the study conception and design. All authors participated in the analysis and interpretation of the data. All authors also contributed to the critical revision of the manuscript and approved the final version. RF is responsible for the overall content as the guarantor.
Funding: AbbVie funded the study and had a role in the study design, data collection, data analysis, data interpretation and writing of the report.
Competing interests: RF: research grants and consulting fees from AbbVie, Amgen, AstraZeneca, Biogen, BMS, Boehringer-Ingleheim, Flexion, Galapagos, Galvani, Genentech, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi-Aventis and UCB. JRC: consulting fees and research support from AbbVie, Amgen, Bristol Myers Squibb, Janssen, CorEvitas, Lilly, Novartis, Myriad, Sanofi, Pfizer and UCB. CC-S: research grants from AbbVie, BMS, CSL Behring and Pfizer; consultancy for AbbVie, Priovant Therapeutics, Octapharma, BMS, Pfizer, Gilead and Regeneron-Sanofi. EFM: research grants and consulting fees from AbbVie, Amgen, Astra Zeneca, Novartis, Lilly, Pfizer, Roche, BMS, Sandoz, GSK, Janssen and Sanofi. KY: consultancy fees from AbbVie, Pfizer, Gilead G.K., Asahi Kasei Pharma, Astellas Pharma, Eli Lilly Japan and Japan Tobacco; member of the speaker’s bureau at Astellas, Bristol Myers Squibb, Chugai, Eisai, Eli Lilly Japan, GlaxoSmithKline, Janssen, Mitsubishi-Tanabe Pharma, Pfizer and Takeda; and research funding from Bristol Myers Squibb, Chugai, GlaxoSmithKline and Mitsubishi-Tanabe Pharma. CR: consultancy and speaking fees from Amgen, AstraZeneca, Roche, BMS, Galapagos, GSK, Lilly, Hospira, Biogen, Sandoz, Mylan, Novartis and Pfizer. GRB: speaking or consulting fees from AbbVie, BMS, Lilly, Galapagos, Janssen, MSD, Pfizer, Roche, Sanofi and UCB. JL, HP, DD and SS: AbbVie employees and may own stocks or options.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymised, individual and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time after approval in the USA and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.html.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. Studies were conducted per the International Conference on Harmonisation guidelines, applicable regulations and the Declaration of Helsinki. Study-related documents were approved by independent ethics committees and institutional review boards. All patients provided written, informed consent. Participants gave informed consent to participate in the study before taking part.
References
- 1. Liu C, Kieltyka J, Fleischmann R, et al. A decade of JAK inhibitors: what have we learned and what may be the future Arthritis Rheumatol 2021;73:2166–78. 10.1002/art.41906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis 2021;80:71–87. 10.1136/annrheumdis-2020-218398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:316–26. 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
- 4. US Food and Drug Administration . FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. 2021. Available: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death [Accessed 19 Dec 2022].
- 5. Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis 2023;82:3–18. 10.1136/ard-2022-223356 [DOI] [PubMed] [Google Scholar]
- 6. European Medicines Agency . EMA confirms measures to minimise risk of serious side effects with Janus kinase inhibitors for chronic inflammatory disorders. 2022. Available: https://www.ema.europa.eu/en/news/ema-confirms-measures-minimise-risk-serious-side-effects-janus-kinase-inhibitors-chronic [Accessed 19 Dec 2022].
- 7. Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018;391:2503–12. 10.1016/S0140-6736(18)31115-2 [DOI] [PubMed] [Google Scholar]
- 8. Fleischmann R, Pangan AL, Song I-H, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol 2019;71:1788–800. 10.1002/art.41032 [DOI] [PubMed] [Google Scholar]
- 9. Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet 2018;391:2513–24. 10.1016/S0140-6736(18)31116-4 [DOI] [PubMed] [Google Scholar]
- 10. Rubbert-Roth A, Enejosa J, Pangan AL, et al. Trial of upadacitinib or Abatacept in rheumatoid arthritis. N Engl J Med 2020;383:1511–21. 10.1056/NEJMoa2008250 [DOI] [PubMed] [Google Scholar]
- 11. Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet 2019;393:2303–11. 10.1016/S0140-6736(19)30419-2 [DOI] [PubMed] [Google Scholar]
- 12. van Vollenhoven R, Takeuchi T, Pangan AL, et al. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately-to-severely active rheumatoid arthritis (SELECT-EARLY): a multicenter, multi-country, randomized, double-blind, active comparator-controlled trial. Arthritis Rheumatol 2020;72:1607–20. 10.1002/art.41384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanaka Y. A review of upadacitinib in rheumatoid arthritis. Modern Rheumatology 2020;30:779–87. 10.1080/14397595.2020.1782049 [DOI] [PubMed] [Google Scholar]
- 14. Cohen SB, van Vollenhoven RF, Winthrop KL, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis 2021;80:304–11. 10.1136/annrheumdis-2020-218510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fleischmann R, Mysler E, Bessette L, et al. Long-term safety and efficacy of upadacitinib or adalimumab in patients with rheumatoid arthritis: results through 3 years from the SELECT-COMPARE study. RMD Open 2022;8:e002012. 10.1136/rmdopen-2021-002012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. 10.1136/annrheumdis-2016-210457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smolen JS, Genovese MC, Takeuchi T, et al. Safety profile of Baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol 2019;46:7–18. 10.3899/jrheum.171361 [DOI] [PubMed] [Google Scholar]
- 18. Buch MH, Charles-Schoeman C, Curtis J, et al. POS0237 major adverse cardiovascular events, Malignancie, and venous thromboembolism by baseline cardiovascular risk: A post hoc analysis of oral surveillance [Abstract]. Ann Rheum Dis 2022;81:356–7. 10.1136/annrheumdis-2022-eular.1182 [DOI] [Google Scholar]
- 19. European Medicines Agency [Internet] . [Xeljanz] product information as approved by the CHMP on 10 November 2022, pending endorsement by the European Commission. 2022. Available: https://www.ema.europa.eu/en/documents/referral/xeljanz-epar-product-information-approved-chmp-10-november-2022-pending-endorsement-european_en.pdf [Accessed 19 Dec 2022].
- 20. Charles-Schoeman C, Buch MH, Dougados M, et al. Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: a post hoc analysis from ORAL surveillance. Ann Rheum Dis 2023;82:119–29. 10.1136/ard-2022-222259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodgers JL, Jones J, Bolleddu SI, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis 2019;6:19. 10.3390/jcdd6020019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sparks JA, Wallace ZS, Seet AM. Associations of baseline use of biologic or targeted synthetic Dmards with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 global rheumatology alliance physician registry. Ann Rheum Dis 2021;80:1137–46. 10.1136/annrheumdis-2021-220418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Vollenhoven RF, Tas SW, Nurmohamed MT. “Correspondence on "associations of baseline use of biologic or targeted synthetic Dmards with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 global rheumatology alliance physician Registry"” Ann Rheum Dis 2023;82:e177. 10.1136/annrheumdis-2021-221146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sparks JA, Wallace ZS, Seet AM, et al. Response to: correspondence on "associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis" by sparks et al. Ann Rheum Dis 2021. 10.1136/annrheumdis-2021-221157 [Epub ahead of print 13 Aug 2021]. [DOI] [PubMed] [Google Scholar]
- 25. Lan S-H, Wang C-K, Chang S-P, et al. Janus kinase inhibitors for hospitalized patients with COVID-19: a meta-analysis of randomized controlled trials. Expert Rev Anti Infect Ther 2022;20:773–9. 10.1080/14787210.2022.2004120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kramer A, Prinz C, Fichtner F, et al. Janus kinase inhibitors for the treatment of COVID-19. Cochrane Database Syst Rev 2022;6:CD015209. 10.1002/14651858.CD015209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. AbbVie [Internet] . Summary of product characteristics [upadacitinib]. Available: https://www.ema.europa.eu/en/documents/product-information/rinvoq-epar-product-information_en.pdf [Accessed 25 Mar 2023].
- 28. AbbVie [Internet . Highlights of Prescribing information [upadacitinib]. Available: https://www.rxabbvie.com/pdf/rinvoq_pi.pdf [Accessed 25 Mar 2023].
- 29. Tran AP, Tassone DF, Ding NS, et al. Antibody response to the COVID-19 ChAdOx1nCov-19 and Bnt162B vaccines after temporary suspension of DMARD therapy in immune-mediated inflammatory disease: an extension study [RESCUE 2]. RMD Open 2023;9:e002871. 10.1136/rmdopen-2022-002871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winthrop KL, Cohen SB. Oral surveillance and JAK inhibitor safety: the theory of relativity. Nat Rev Rheumatol 2022;18:301–4. 10.1038/s41584-022-00767-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dougados M, Charles-Schoeman C, Szekanecz Z, et al. OP0264 impact of baseline cardiovascular risk on the incidence of major adverse cardiovascular events in the tofacitinib rheumatoid arthritis clinical programme [Abstract]. Ann Rheum Dis 2022;81:175–6. 10.1136/annrheumdis-2022-eular.879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mease P, Charles-Schoeman C, Cohen S, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis 2020;79:1400–13. 10.1136/annrheumdis-2019-216761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kerschbaumer A, Smolen JS, Nash P, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a systematic literature research. RMD Open 2020;6:e001374. 10.1136/rmdopen-2020-001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burmester GR, Cohen SB, Winthrop KL, et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, Ankylosing spondylitis and atopic dermatitis. RMD Open 2023;9:e002735. 10.1136/rmdopen-2022-002735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoisnard L, Pina Vegas L, Dray-Spira R, et al. Risk of major adverse cardiovascular and venous thromboembolism events in patients with rheumatoid arthritis exposed to JAK inhibitors versus adalimumab: a nationwide cohort study. Ann Rheum Dis 2023;82:182–8. 10.1136/ard-2022-222824 [DOI] [PubMed] [Google Scholar]
- 36. Khosrow-Khavar F, Kim SC, Lee H, et al. Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine Care Patients with Rheumatoid Arthritis (STAR-RA) study. Ann Rheum Dis 2022;81:798–804. 10.1136/annrheumdis-2021-221915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salinas CA, Louder A, Polinski J, et al. Evaluation of VTE, MACE, and serious infections among patients with RA treated with baricitinib compared to TNFi: a multi-database study of patients in routine care using disease registries and claims databases. Rheumatol Ther 2023;10:201–23. 10.1007/s40744-022-00505-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Molander V, Bower H, Frisell T, et al. Venous thromboembolism with JAK inhibitors and other immune-modulatory drugs: a Swedish comparative safety study among patients with rheumatoid arthritis. Ann Rheum Dis 2023;82:189–97. 10.1136/ard-2022-223050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Molander V, Bower H, Frisell T, et al. Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: a nationwide cohort study from Sweden. Ann Rheum Dis 2021;80:169–75. 10.1136/annrheumdis-2020-218419 [DOI] [PubMed] [Google Scholar]
- 40. Cho S-K, Lee J, Han M, et al. The risk of malignancy and its incidence in early rheumatoid arthritis patients treated with biologic DMARDs. Arthritis Res Ther 2017;19:277. 10.1186/s13075-017-1482-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arts EEA, Fransen J, den Broeder AA, et al. The effect of disease duration and disease activity on the risk of cardiovascular disease in rheumatoid arthritis patients. Ann Rheum Dis 2015;74:998–1003. 10.1136/annrheumdis-2013-204531 [DOI] [PubMed] [Google Scholar]
- 42. Myasoedova E, Chandran A, Ilhan B, et al. The role of rheumatoid arthritis (RA) flare and cumulative burden of RA severity in the risk of cardiovascular disease. Ann Rheum Dis 2016;75:560–5. 10.1136/annrheumdis-2014-206411 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2023-223916supp001.pdf (1.3MB, pdf)
Data Availability Statement
Data are available upon reasonable request. AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymised, individual and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time after approval in the USA and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.html.