Abstract
The major structural viral protein, VP1, of the human polyomavirus JC virus (JCV), the causative agent of progressive multifocal leukoencephalopathy (PML), was expressed by using recombinant baculoviruses. Recombinant VP1 formed virus-like particles (VLP) with the typical morphology of empty JCV capsids. Purified VP1 VLP bind to SVG, B, and T cells, as well as to monkey kidney cells. After binding, VP1 VLP were also internalized with high efficiency and transported to the nucleus. Immunization studies revealed these particles as highly immunogenic when administered with adjuvant, while immunization without adjuvant induced no immune response. VP1 VLP hyperimmune serum inhibits binding to SVG cells and neutralizes natural JCV. Furthermore, the potential of VP1 VLP as an efficient transporter system for gene therapy was demonstrated. Exogenous DNA could be efficiently packaged into VP1 VLP, and the packaged DNA was transferred into COS-7 cells as shown by the expression of a marker gene. Thus, VP1 VLP are useful for PML vaccine development and represent a potential new transporter system for human gene therapy.
The human polyomavirus JC virus (JCV) is the etiological agent of progressive multifocal leukoencephalopathy (PML), the only known viral demyelinating disease of humans (14). Owing to the worldwide AIDS pandemic, PML is becoming increasingly more frequent (3). Immunodeficiency in AIDS patients leads to reactivation of JCV, causing PML (14). About 7% of all patients with AIDS ultimately develop PML, and at least 85% of all PML cases occur in these patients (4). PML can be specifically correlated to the appearance of JCV DNA in the cerebrospinal fluid (22, 23). Moreover, the immune response to JCV in the course of infection and the development of PML has been recently investigated, and elevated VP1-specific antibody synthesis can be quite reliably used to diagnose PML (24). Clinically, no attempts are currently being made to use gene therapy or boost the immunological state of patients, especially of human immunodeficiency virus type 1-infected but asymptomatic individuals, to protect against JCV reactivation and the development of PML.
For the development of such new approaches, we describe in the present study the expression of the major structural protein VP1 of JCV as recombinant virus-like particles (VLP) and their usefulness for PML-specific vaccine development. Furthermore, the potential of VP1 VLP as a new transporter system for gene therapy was demonstrated.
Expression, purification, and characterization of VP1 VLP.
With the help of recombinant baculoviruses, the major structural protein VP1 of JCV could be successfully expressed in the insect cell line Sf158. VP1 was detected in the cell extract and the cell culture supernatant, and the highest expression was obtained 5 days after infection. Therefore, recombinant VP1 VLP were purified from cell culture supernatants and cell extracts 5 days after infection by a simple and rapid two-step procedure including sedimentation through a 40% sucrose cushion, followed by a second centrifugation through a step gradient of 40% sucrose and 50% metrizamide. VP1 VLP were obtained in the pellet after the final centrifugation step and consisted of two major proteins with molecular masses of 42 and 40 kDa and a third minor protein of about 38 kDa. By Western blot (WB) analysis with anti-simian virus 40 (SV40) hyperimmune serum (23), the VP1-specific 42-kDa protein was identified as the major immunoreactive component. Minor reactivities were also obtained with the 40- and 38-kDa proteins, as well as with a protein of about 27 kDa.
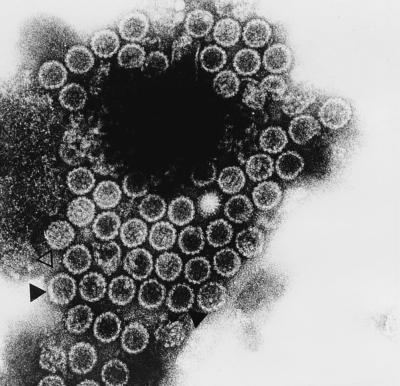
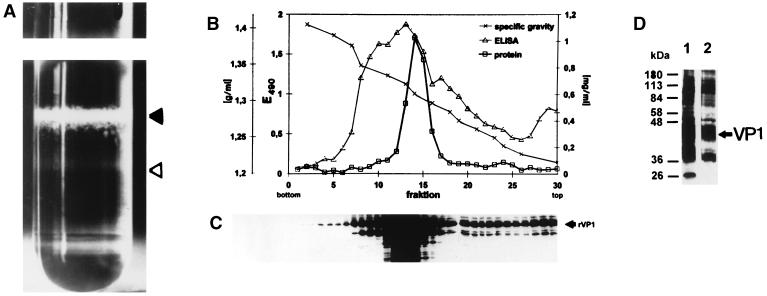
For further characterization of the purified VP1 VLP, electron microscopy was performed. The electron micrograph (Fig. 1) demonstrated that most of the VP1 VLP exhibits the typical morphology of empty JCV capsids having a mean diameter of 55 to 60 nm (closed triangle). A very small minority of full particles was also identified (open triangle). This result was confirmed by further analysis of the VP1 VLP in a CsCl density gradient. As shown in Fig. 2A, two bands were visible: a major band in the middle of the gradient with a density of about 1.32 g/ml (closed triangle; empty particles) and a minor band at a density of about 1.34 g/ml (open triangle; full particles). Analysis of the gradient fractions by enzyme-linked immunosorbent assay revealed a major immunoreactive peak in the density range of 1.31 to 1.33 g/ml (Fig. 2B, fractions 11 to 16; empty particles) and a minor immunoreactive shoulder in the density range of 1.33 to 1.35 g/ml (Fig. 2B, fractions 7 to 10; full particles). WB analysis of each gradient fraction with VP1-specific hyperimmune serum (see below) defined the VP1-specific 42-kDa protein as the major reactive component in the density range of empty particles (1.31 to 1.33 g/ml; fractions 11 to 16) (Fig. 2C). A similar strong VP1-specific reactivity was obtained with the 40-kDa protein, whereas the reactivity with the 27-kDa protein was somewhat reduced. In contrast, in the density range of the full particles (1.33 to 1.35 g/ml; fractions 7 to 10), the major and only reactive components were identified as the VP1-specific 42- and 40-kDa proteins. These results were confirmed by WB analysis of identical amounts of empty and full particles. Again, in empty particles, the VP1-specific 42- and 40-kDa proteins were identified as the major reactive components, whereas the reactivity with the 27-kDa protein was reduced (Fig. 2D, lane 1). The major reactive components comprised by the full particles were identified as the VP1-specific 42-kDa protein and, at a somewhat lower intensity, the 40-kDa protein (Fig. 2D, lane 2).
FIG. 1.
Electron micrograph of purified VP1 VLP. Empty particles are marked with a closed triangle, and a full particle is marked with an open triangle.
FIG. 2.
Distribution of VP1 VLP in a CsCl density gradient. (A) Picture of the tube after gradient centrifugation. The band representing empty particles is marked with a closed triangle, and the band representing full particles is marked with an open triangle. After gradient fractionation, immunoreactive fractions were identified by enzyme-linked immunosorbent assay (B) and WB (C) with VP1-specific hyperimmune serum. (D) WB analysis of empty (lane 1) and full (lane 2) particles using identical amounts of protein with VP1-specific hyperimmune serum.
Immunological characterization and reactivity of VP1 VLP.
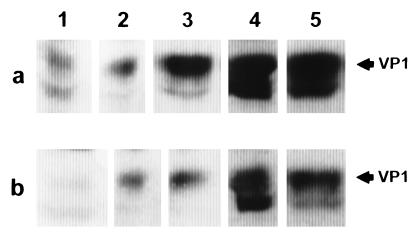
To characterize the immunogenicity of VP1 VLP, immunization studies were performed with rabbits by using different application routes. To produce VP1-specific hyperimmune serum, purified VP1 VLP were dialyzed overnight against reassociation buffer. For immunization, 100 μg of VP1 VLP was mixed with 300 μg of keyhole limpet hemocyanin and emulsified in complete Freund’s adjuvant (CFA) and a rabbit was immunized intramuscularly. One booster immunization was given 4 weeks later with incomplete Freund’s adjuvant (ICFA). Alternatively, VP1 VLP were administered intravenously without CFA or ICFA. Whereas the intravenous administration induced no VP1-specific immune response, intramuscular administration with CFA or ICFA resulted in strong immune reactivity. Twenty-five weeks after the last booster immunization, the VP1-specific hyperimmune serum exhibited an endpoint titer of more than 105 and the major reactive component was the VP1-specific 42-kDa protein as shown by WB analysis. Strong VP1-specific reactivity was also obtained with the 40-kDa protein, whereas only minor reactivity was shown with the 27-kDa protein. The hyperimmune serum cross-reacts with the VP1 present in natural JCV and SV40 particles. Furthermore, the VP1-specific hyperimmune serum was able to neutralize the infection of SVG cells (14) with natural JCV (Fig. 3). SVG-cell binding inhibition experiments with this immune serum revealed that the binding of 125I-labelled VP1 VLP, which were iodinated as previously described (17), was inhibited to about 90% at serum dilutions of 1:10 and 1:20. At serum dilutions of 1:40 and 1:80, binding inhibition levels of about 70 and 35% were obtained, whereas at serum dilutions of 1:160 and 1:320, no binding inhibition was measured. No binding inhibition was also obtained with preimmune serum at a dilution of 1:20 or without serum.
FIG. 3.
Neutralization of JCV by VP1 specific hyperimmune serum. For neutralization, the JCV infection stock was diluted 1:80 (row a) or 1:160 (row b) and incubated for 1 h with VP1-specific hyperimmune serum at endpoint dilutions of 1:40 (lane 1), 1:80 (lane 2), 1:160 (lane 3), and 1:320 (lane 4) and with preimmune serum at a dilution of 1:40 (lane 5). Thereafter, JCV-antibody complexes were incubated with SVG cells for 3 days and intracellular VP1 was detected by WB analysis with anti-SV40 hyperimmune serum.
To investigate if the 40- and 27-kDa proteins represent VP1-specific proteolytic cleavage products, the 42-kDa VP1-specific protein was purified by three rounds of sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The finally electroeluted protein exhibited a molecular mass of 42 kDa, and immunization studies were performed as described for intact VP1 VLP with CFA and ICFA. WB analysis demonstrated that the resulting immune serum recognized only the 42-kDa protein in the electroeluted fraction with high specificity. However, when purified VP1 VLP were used as the antigen, the immune serum also detected, in addition to the 42-kDa protein, the 40- and 27-kDa components. These results suggested that the 40- and 27-kDa proteins are proteolytic-cleavage products of the VP1-specific 42-kDa protein.
VP1 VLP as a transporter system for gene therapy.
For this reason, the capacity of VP1 VLP to bind to different cell lines was investigated. Saturating SVG-cell-binding conditions were obtained with 2 × 106 cpm of 125I-labelled VP1 VLP (specific activity, 5 × 106 cpm of VP1 VLP per μg) and 105 SVG cells within 80 min. The binding component was identified as the VP1-specific 42-kDa protein. In a competition assay with natural JCV, VP1 VLP binding could be competed in a concentration-dependent manner (Table 1). Under these conditions, VP1 VLP were able to bind to the monkey kidney cell lines COS-7 (10) and TC7 (19), showing more than 50% better binding than to SVG cells. Binding to the human B-cell line Raji (4) was 25% more efficient, whereas equal binding efficiency was obtained with the human T-cell line CEM (5). VP1 VLP not only bound to these cell lines but were also internalized and transported to the nucleus as demonstrated by immunofluorescence analysis.
TABLE 1.
Competition of VP1 VLP binding to SVG cells by natural JCV
| JCV (μg) | VP1 VLP (μg) | Competition (%) |
|---|---|---|
| 0.00 | 0.5 | 0 |
| 0.25 | 0.5 | 10 |
| 0.50 | 0.5 | 17 |
| 2.00 | 0.5 | 25 |
| 4.00 | 0.5 | 44 |
| 8.00 | 0.5 | 60 |
| 20.00 | 0.5 | 84 |
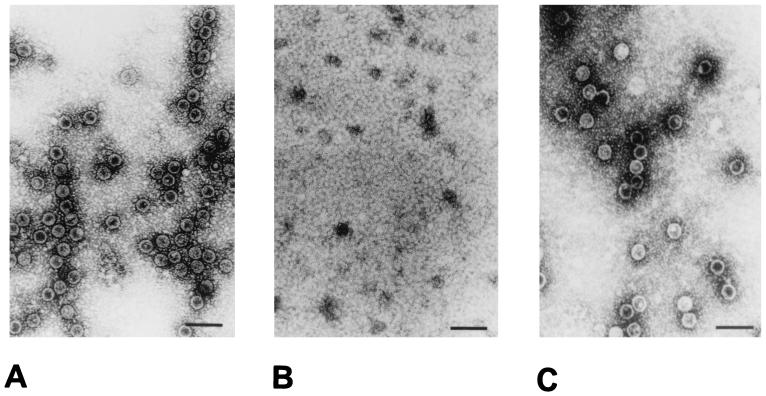
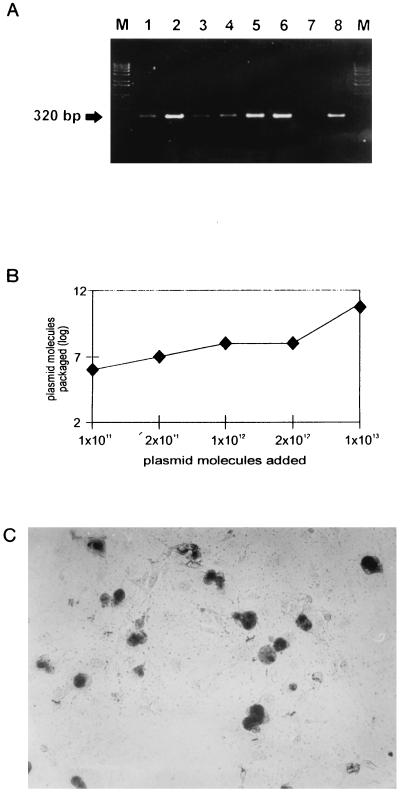
To explore the potential of VP1 VLP for packaging of foreign DNA, we investigated if VP1 VLP are dissociable like natural JCV (6) and if a reassociation reaction can be initiated to reconstitute intact VP1 VLP. The principle of the dissociation reaction is removal of Ca2+ ions and reduction of disulfide bonds. Optimal dissociation conditions were examined in pilot experiments. Compared to VP1 VLP before dissociation (Fig. 4A) with a specific dissociation buffer (10 mM Tris/HCl [pH 8.5], 150 mM NaCl, 10 mM EGTA, 5 mM dithiothreitol), VP1 VLP were completely dissociable to VP1 pentamers during incubation for 1 h at 4°C (Fig. 4B). Intact VP1 VLP could then be reconstituted during a reassociation reaction, started by the addition of 1 mM CaCl2, followed by dialysis overnight against reassociation buffer (10 mM Tris/HCl [pH 7.5], 150 mM NaCl2, 1 mM CaCl2). The reassociation resulted in the formation of VP1 VLP having nearly the same morphology as the VP1 VLP before dissociation (Fig. 4C). Thereafter, we investigated if foreign DNA can be packaged into VP1 VLP during this dissociation-reassociation cycle. For comparison with the dissociation-reassociation cycle, two more packaging procedures were tested, namely, osmotic shock (2) and dissociation of VP1 VLP in double-distilled H2O before reassociation. Packaging data are given in Fig. 5. As can be seen, all of the packaging procedures result in VP1 VLP association of the pCMV-β-Gal plasmid (4.5 kb), where the expression of the reporter gene is driven by a cytomegalovirus promotor. The packaged plasmid DNA was demonstrated to be resistant to DNase I digestion, indicating uptake into the VP1 VLP. From the results, it could be assumed that a package procedure using the dissociation-reassociation cycle (Fig. 5A, lanes 2, 5, and 6; e.g., suspension of VP1 VLP in dissociation buffer or double-distilled H2O) was superior. As quantitated by competitive PCR, a plateau was reached around 1012 added plasmid molecules, resulting in about 108 to 109 packaged plasmid molecules (Fig. 5B). The calculated packaging efficiency was 1:3. This means, for example, that about 3 × 106 VP1 VLP are able to package 106 plasmid molecules.
FIG. 4.
Electron micrographs of purified, intact VP1 VLP before (A) and after (B) dissociation and intact VP1 VLP after reassociation (C). Bars, 50 nm.
FIG. 5.
Packaging of foreign plasmid DNA by VP1 VLP. (A) Packaging of foreign plasmid DNA was performed with ratios of DNA to VP1 VLP of 1:10 (lanes 1 to 3) and 1:20 (lanes 4 to 6) by osmotic shock (lanes 1 and 4), as well as particle dissociation in dissociation buffer (lanes 2 and 5) and double-distilled H2O (lanes 3 and 6). After reassociation and DNase I digestion, particles were pelleted through a 40% sucrose cushion and packaged DNA was extracted and analyzed. As a control, unpackaged DNA was digested with DNase I (lane 7) or not digested (lane 8). (B) Quantification of packaged DNA by quantitative competitive PCR. VP1 VLP (3 × 1011) were dissociated and incubated with increasing amounts of plasmid molecules for 1 h at 37°C and then subjected to dialysis overnight against reassociation buffer. Packaged VP1 VLP were digested with DNase I and pelleted through a 40% sucrose cushion. DNA was extracted and quantified by quantitative competitive PCR. (C) Analysis of β-galactosidase expression. The reporter plasmid was packaged in VP1 VLP, and COS-7 cells were exposed for 2 days. Cells were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and then examined microscopically and photographed.
Transfer efficiency was investigated in COS-7 cells. As is shown in Fig. 5C, VP1 VLP can efficiently transduce packaged plasmid DNA into COS-7 cells, resulting in the expression of a functional β-galactosidase, as demonstrated by the presence of blue cells. Transfer efficiency was clearly reduced when an equal amount of plasmid molecules was transfected using liposomes. If a multiplicity of infection (MOI) in relation to the packaged plasmid molecules of 50 was selected (MOI of VP1 VLP, 150) and 500,000 cells were used for transduction, about 100,000 COS-7 cells efficiently expressed the β-galactosidase (Table 2). In comparison to transfection experiments with identical plasmid molecules packaged in liposomes, transduction with VP1 VLP was about 200 times more efficient.
TABLE 2.
Transduction efficiency of VP1 VLP
| Transduction system | Plasmid molecules (MOI) | No. of COS-7 cells used for transduction | β-Galactosidase expression (no. of blue cells) |
|---|---|---|---|
| Liposomes | 50 | 500,000 | 500 |
| VP1 VLP | 50 | 500,000 | 100,000 |
VP1 VLP as a tool for vaccine development and gene therapy.
Antiviral therapy to combat JCV reactivation and the development of PML has been attempted, as has enhancement of cellular immunity (for a review, see reference 15). However, the efficacy of these treatments remains inconclusive owing to the fact that only case reports and small controlled trials have been reported. Furthermore, new strategies for the development of a vaccine or gene therapy have not been reported.
Here we show the successful expression of the major structural protein, VP1, of JCV, resulting in the formation of VLP with a predominant morphology of empty JCV capsids. Purified VP1 VLP exhibited high immunogenicity when administered intramuscularly with an adjuvant, whereas no immune response was obtained after intravenous application without an adjuvant. VP1-specific hyperimmune serum neutralized VP1 VLP binding to and JCV infection of SVG cells. Therefore, VP1 VLP appear to exhibit neutralizing and cell-binding epitopes like natural JCV and offer an opportunity to develop prophylactic or therapeutic candidate vaccines to prevent PML by interfering with primary infection or reactivation of JCV. Similar recombinant VLP has been described for human papillomaviruses, and such HPV VLP will now be applied as a prophylactic or therapeutic vaccine to combat, for example, cervical cancer (for reviews, see references 11, 13, 20 and 21).
VP1 VLP could also be demonstrated as a potential new transporter system for gene therapy. They not only showed efficient binding to B and T cells, as well as to kidney cells, but were also internalized and transported to the nucleus. All of these cell lines are derived from body compartments which either represent a major site of JCV persistence, such as the kidneys (16), or may function as a ferry for JCV to enter the brain, such as B cells (1, 18). Recently, we have shown that T cells may also be a site of JCV latency (unpublished observation). Furthermore, a number of problems and drawbacks of other gene transfer systems (for a review, see reference 12) could be circumvented with VP1 VLP. For example, adenovirus-derived systems induce a strong immune response irrespective of the application route, not allowing repeated administration (7–9). However, when VP1 VLP were administered intravenously, no immune response was obtained. Furthermore, gene therapeutic approaches based on viral vectors suffer from the drawback of introducing unwanted additional viral genetic information into the recipient host alongside the gene of interest, and a packaging cell line is needed (for a review, see reference 12). VP1 VLP, however, were able to package foreign DNA in a manner that protected it from the action of an external nuclease during a dissociation-reassociation cycle. Unwanted additional viral information is not necessary alongside the gene of interest, and a packaging cell line is also not needed. It could further be shown that the transfer of the packaged DNA by VP1 VLP was clearly superior to DNA transfer by liposomes. However, quantitative comparisons to other gene transfer systems could not be performed. Titers are commonly not given as MOIs, which is, to our knowledge, a basic prerequisite for estimation of the potential of any gene therapy transporter system. VP1 VLP may now open up ways not only to combat JCV reactivation and the development of PML but also to develop new human gene therapy approaches.
Acknowledgments
We thank E. O. Major, Laboratory of Molecular Medicine and Neuroscience, NINDS, Bethesda, Md., for generously providing SVG cells.
T.W. and S.F. were supported by grants from the Graduiertenkolleg Molekularbiologische Analyse pathophysiologischer Prozesse. This work was further supported by a grant from the Stiftung zur Bekämpfung neuroviraler Erkrankungen to T.W. (MSC/2738.rhe 27 33641). W.L. was supported by a grant of the Verbundantrag HIV/SIV Immunpathogenese und Immunprävention. Furthermore, W.L. and T.W. were supported by a grant from the Deutsche Forschungsgemeinschaft (Lu 397/5-1, We 1297/3-1).
REFERENCES
- 1.Atwood W, Ameyami K, Traub R, Harms J, Major E. Interaction of the human polyomavirus JCV with human B-lymphocytes. Virology. 1992;190:716–723. doi: 10.1016/0042-6822(92)90909-9. [DOI] [PubMed] [Google Scholar]
- 2.Barr M, Keck K, Aposhian H. Cell-free assembly of a polyoma-like particle from empty capsids and DNA. Virology. 1979;96:656–659. doi: 10.1016/0042-6822(79)90124-7. [DOI] [PubMed] [Google Scholar]
- 3.Berger J, Concha M. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J Neurovirol. 1995;1:5–18. doi: 10.3109/13550289509111006. [DOI] [PubMed] [Google Scholar]
- 4.Epstein M, Achong Y, Barr Y, Zajac B, Henle G, Henle W. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji) J Natl Cancer Inst. 1966;37:547–559. [PubMed] [Google Scholar]
- 5.Foley G, Lazarus H, Farber S, Uzman G, Boone B, McCarty R. Continuous culture of human lymphoblasts from peripheral blood of a child with acute leukemia. Cancer. 1965;18:522–528. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Frye S, Trebst C, Dittmer U, Petry H, Bodemer M, Hunsmann G, Weber T, Lüke W. Efficient production of JCV in SVG cells and the use of purified viral antigens for analysis of specific humoral and cellular immune response. J Virol Methods. 1997;63:81–92. doi: 10.1016/s0166-0934(96)02117-9. [DOI] [PubMed] [Google Scholar]
- 7.Gahéry-Ségard H, Juillard V, Gaston J, Lengagne R, Pavirani A, Boulanger P, Guillet J-G. Humoral immune response to the capsid components of recombinant adenoviruses: routes of immunization modulate virus-induced Ig subclass shifts. Eur J Immunol. 1997;27:653–659. doi: 10.1002/eji.1830270312. [DOI] [PubMed] [Google Scholar]
- 8.Gahéry-Ségard H, Molinier-Frenkel V, LeBoulaire C, Saulnier P, Opolon P, Lengagne R, Gautier E, LeCesne A, Zitvogel L, Venet A, Schatz C, Courtney M, LeChevalier T, Tursz T, Guillet J-G, Farace F. Phase I trial of recombinant adenovirus gene transfer in lung cancer: longitudinal study of the immune responses to transgene and viral products. J Clin Investig. 1997;100:2218–2226. doi: 10.1172/JCI119759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gahéry-Ségard H, Farace F, Godfrin D, Gaston J, Lengagne R, Tursz T, Boulanger P, Guillet J-G. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J Virol. 1998;72:2388–2397. doi: 10.1128/jvi.72.3.2388-2397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 11.Jarrett W, Smith K, O’Neil B, Gaukroger J, Chandrachud L, Grindlay G, McGarvie G, Campo M. Studies on vaccination against papillomaviruses: prophylactic and therapeutic vaccination with recombinant structural proteins. Virology. 1991;184:33–42. doi: 10.1016/0042-6822(91)90819-w. [DOI] [PubMed] [Google Scholar]
- 12.Jolly D. Viral vector systems for gene therapy. Cancer Gene Ther. 1994;1:51–64. [PubMed] [Google Scholar]
- 13.Kirnbauer R. Papillomavirus-like particles for serology and vaccine development. Intervirology. 1996;39:54–61. doi: 10.1159/000150475. [DOI] [PubMed] [Google Scholar]
- 14.Major E, Miller A, Mourrain P, Traub R, DeWidt E, Sever J. Establishment of a line of human fetal glial cells that supports JCV multiplication. Proc Natl Acad Sci USA. 1985;82:1257–1261. doi: 10.1073/pnas.82.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Major E O, Amemiya K, Tornatore C S, Houff S A, Berger J R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992;5:49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCance D. Persistence of animal and human papovavirus in renal and nervous tissues. Prog Clin Biol Res. 1983;105:343–357. [PubMed] [Google Scholar]
- 17.Polzien F, Scharf J, Lüke W, Hunsmann G. CD4-binding of gp130 micelles isolated from SIVagmTYO-7. AIDS Res Hum Retroviruses. 1992;8:1171–1177. doi: 10.1089/aid.1992.8.1171. [DOI] [PubMed] [Google Scholar]
- 18.Rieckmann P, Michel U, Kehrl J H. Regulation of JC virus expression in B lymphocytes. J Virol. 1994;68:217–222. doi: 10.1128/jvi.68.1.217-222.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robb J, Huebner K. Effect of cell chromosome number on simian virus 40 replication. Exp Cell Res. 1973;81:120–126. doi: 10.1016/0014-4827(73)90118-3. [DOI] [PubMed] [Google Scholar]
- 20.Sapp M, Volpers C, Streeck R. Synthesis, properties and applications of papillomavirus-like particles. Intervirology. 1996;39:49–53. doi: 10.1159/000150474. [DOI] [PubMed] [Google Scholar]
- 21.Van Driel W, Ressing M, Brandt R, Toes R, Fleuren G, Trimbos J, Kast W, Melief C. The current status of therapeutic HPV vaccine. Ann Med. 1996;28:471–477. doi: 10.3109/07853899608999110. [DOI] [PubMed] [Google Scholar]
- 22.Weber T, Turner R, Frye S, Ruf B, Haas J, Schielke E, Pohle H, Lüke W, Lüer W, Felgenhauer K, Hunsmann G. Specific diagnosis of progressive multifocal leukoencephalopathy by polymerase chain reaction. J Infect Dis. 1994;169:1138–1141. doi: 10.1093/infdis/169.5.1138. [DOI] [PubMed] [Google Scholar]
- 23.Weber T, Turner R, Frye S, Lüke W, Kretzschmar H, Lüer W, Hunsmann G. Progressive multifocal leukoencephalopathy diagnosed by amplification of JC virus-specific DNA from cerebrospinal fluid. AIDS. 1994;8:49–57. doi: 10.1097/00002030-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Weber T, Trebst C, Frye S, Cinque P, Vago L, Sindic C, Schulz-Schaeffer W, Kretzschmar H, Enzensberger W, Hunsmann G, Lüke W. Analysis of the systemic and intrathecal humoral immune response in progressive multifocal leukoencephalopathy. J Infect Dis. 1997;176:250–254. doi: 10.1086/514032. [DOI] [PubMed] [Google Scholar]