Abstract
Introduction
We investigated public interest in shopping and point-of-sales (POS) of JUUL and Puff Bar products in the context of five regulatory, company sales policy and other events of interest that may have influenced the trajectory of these products during 2019–2021.
Methods
Outcome variables included relative search volume (RSV) from Google search queries indicative of shopping interest in and aggregate dollar sales from Nielsen POS for JUUL and Puff Bar in the USA from March 2019 to May 2021. Adjusted autoregressive integrated moving average assessed the observed and predicted trends and adjusted linear regression analysis measured the relative rate of change in the outcome variables for each time period of interest.
Results
After the Trump administration announced its plans to ban flavoured e-cigarettes and JUUL Labs, Inc.’s decided to suspend the sales of its sweet and fruity flavoured products, JUUL’s shopping interest RSV and sales declined while Puff Bar’s shopping interest RSV peaked, and its sales increased. From the period following FDA’s announcement of its enforcement guidance policy on unauthorised flavoured cartridge-based e-cigarettes until May 2021, JUUL’s shopping interest RSV and sales continued to decline. Puff Bar’s shopping interest RSV increased, and its sales peaked until the House approved the flavoured e-cigarette ban bill. Puff Bar’s sales steeply declined following suspension of its sales in February 2020. The decline, however, slowed after Puff Bar products were relaunched as ‘synthetic nicotine’ e-cigarettes.
Conclusions
Puff Bar’s unprecedented peak in the shopping interest and sales of Puff Bar warrants continued surveillance.
Keywords: Electronic nicotine delivery devices, Nicotine, Advertising and Promotion, Addiction, Surveillance and monitoring
Introduction
The electronic cigarette (e-cigarette) marketplace in the USA is characterised by unprecedented growth, rapidly evolving product characteristics and a dynamic regulatory landscape.1 JUUL, a rechargeable, cartridge-based pod mod and addictive product,2 3 has been the most popular device overall and particularly among adolescents.3 4 This is concerning since nicotine has an adverse effect on adolescent brain development5 and early exposure increases the risk of subsequent initiation to combustible cigarettes.6
Amidst rising pressure from advocacy and regulatory organisations, JUUL suspended the online and point-of-sales (POS) of its sweet and fruity flavoured products (except menthol) in October 2019.7 In the meantime, disposable (non-rechargeable, non-cartridge-based) e-cigarettes are an emerging public health concern. These products are the fastest growing category of e-cigarette products in the USA and have risen in popularity among high school and middle school students,8 9 and are potentially more harmful than rechargeable look-alike e-cigarettes.10
Puff Bar, initially introduced as a disposable, single-use and self-contained vaping device, has been gaining wide currency in the e-cigarette market since early 2019. It is available in a variety of flavours and priced significantly lower than JUUL pod mods. Recent evidence suggests that Puff Bar has replaced JUUL as the most popular e-cigarette brand among youth. In 2020, 26.1% of high school students and 303 .% of middle school students reported that their usual brand was Puff Bar, and 5.7% of high school students and 12.5% middle school students reported using JUUL.11 Increasing adoption of Puff Bar in the USA may pose threats to public health and potentially exacerbate the youth vaping epidemic in the USA.
From a regulatory perspective, in July 2020, the FDA issued a warning letter to Puff Bar for selling or distributing unauthorised tobacco products introduced or modified after 8 August 2016—the effective date of the deeming rule, and for marketing their products as modified risk tobacco products without FDA authorisation.12 Puff Bar suspended sales of its products in the USA market shortly after.13 In March 2021, Puff Bar repositioned its products as synthetic nicotine products and resumed sales while claiming that these were no longer under FDA’s regulatory purview which is limited to electronic nicotine delivery products derived from tobacco. Since then, the FDA’s enforcement policy on nationwide ban of unauthorised cartridge-based flavoured e-cigarettes finalised in January 2020, and Congress’s amendment to the Family Smoking Prevention and Tobacco Control Act, raising the federal minimum legal sales age from 18 to 21 effective December 2019, do not apply to the purchase of Puff Bar products.14
Retrospective and event-contextual monitoring of public interest in shopping and sales of JUUL and Puff Bar can highlight their potential role in the ongoing e-cigarette public health crisis in the United States and inform future health interventions. Digital health surveillance offers opportunities to passively collect organic data about public interest in tobacco products.15 Google search queries can be a valuable monitoring tool to track public interest in, information seeking and searching about tobacco products in response to changes in tobacco product regulations,15–17 launch of new tobacco products 18 19 and other events.20 21 Google search queries are also known to be a reliable tool to forecast consumer behaviour and consumption.22 23 More recently, Leas et al investigated Google search queries indicative of shopping interest in JUUL and IQOS during the e-cigarette, or vaping, product use associated lung injury (EVALI) outbreak in the USA.24 The Nielsen POS (NPOS) data have been used to capture national-level sales trends of tobacco products including JUUL as well.25 However, to date, no prior work has evaluated JUUL and Puff Bar aggregate POS trends in the USA.
This study leveraged Google search queries and NPOS to investigate public interest in shopping JUUL and Puff Bar products, and sales of these products, in the context of major events of interest including regulatory, and company sales policy and other events (see below) that potentially shaped the trajectory of public interest in and use of these products during 2019–2021.
Time period 1 (T1): Both JUUL and Puff Bar sold and marketed as tobacco-derived nicotine-containing products (24/3/19–10/9/19).
Time period 2 (T2): Trump Administration announced plans to ban flavours26; JUUL Labs’ voluntary announcement to halt online sales of its sweet and fruity products27 (11/9/19–1/1/20).
Time period 3 (T3): FDA published enforcement guidance policy on unauthorised flavoured cartridge-based e-cigarettes;28 House approved flavoured e-cigarette ban bill29 (2/1/20–14/6/20).
Time period 4 (T4): Puff Bar battled counterfeit sales, received FDA warning letter and suspended sale13 30 (15/6/20–1/3/21).
Time period 5 (T5): Puff Bar resumed sales as a synthetic nicotine-containing product31 (2/3/21–22/5/21).
Findings are expected to advance future investigations examining the impact of disposable and synthetic nicotine-containing e-cigarettes on overall e-cigarette use in the USA and demonstrate the utility of using online and offline e-cigarette surveillance.
Methods
Data and procedures
Data comprised of national-level aggregate POS sales data from Nielsen and Google search queries indicative of public interest in shopping JUUL and Puff Bar in the USA from March 2019 to May 2021.
Nielsen point-of-sales (NPOS) data
NPOS data comprised of JUUL’s and Puff Bar’s national-level aggregate US$ sales (referred to as sales from hereon) from 24 March 2019 to 22 May 2021. Previous studies32–34 have used aggregate US$ e-cigarette sales in the past. NPOS data contains state-level, 4-week sales of e-cigarettes products (referred to as monthly sales from hereon) available in dollar value and total units sold, identified by Universal Product Code, collected from participating retailers including convenience stores, food, drug and mass stores, located in 33 states in the USA. The analytic sample comprised of 28 months of sales data for JUUL and Puff Bar.
Google search queries indicative of shopping interest
Google search queries comprised of weekly relative search volume (RSV) indicative of shopping interest in JUUL and Puff Bar during the same time period—24 March 2019 to 22 May 2021 (referred to as JUUL’s shopping interest RSV and Puff Bar’s shopping interest RSV, from hereon). RSV is defined as a user’s likelihood of Google search for a particular keyword depending on their location and time. RSV is scaled between 0 and 100, where 100 represents the highest search volume. In line with previous research, weekly RSVs related to Google search queries comprising of a combination of at least one of the shopping-related keywords (‘buy’, ‘order’, ‘shop(s)’, ‘retailer(s)’ or ‘sale(s)’) and one of the product-related keywords (‘JUUL’ or ‘Puff Bar’) were retrieved. The analytic sample comprised of 113 weeks of JUUL-shopping and Puff Bar-shopping interest RSV.
Mapping events time points of interest to data
Extending prior work, weekly RSV and monthly NPOS data were mapped approximately to the events time points of interest (see online supplemental table 1 for more details on timeline mapping where calendar dates of each event of interest were mapped as close to corresponding NPOS and RSV data available for that period).
tobaccocontrol-2021-056953supp001.pdf (289.3KB, pdf)
Measures
JUUL’s and Puff Bar’s shopping interest RSV were operationalised as separate continuous variables, representing search queries indicative of shopping interests in these products individually during the study period.
Sales of JUUL and Puff Bar products was derived from the total dollars sold at POS. Average price per item for JUUL’s kits, JUUL’s refills and Puff Bar’s disposable products was calculated by dividing aggregate sales ($) by standardised number of units sold (determined by multiplying pack size, total size and the units sold) and adjusted for inflation using the Consumer Price Index35 by converting them to the 2021 May price. Note that JUUL’s kits were inclusive of assorted form, e-cigarette liquid pod and electronic vapour device, JUUL’s refills comprised of e-cigarette cartridge and e-cigarette liquid pod. E-cigarette, or vaping, product use-associated lung injury (EVALI) was defined in terms of the total number of EVALI cases per million individuals during each time period of interest in the USA, based on data the Centers for Disease Control and Prevention’s daily volume of EVALI cases36 and U.S. population census monthly estimates.37 The COVID-19 pandemic was conceptualised as the total new COVID-19 cases per million individuals during each time period in the USA based on data repository maintained by The New York Times38 Seasonality included separate dummy variables (0/1) for each calendar month in a year.
Separate event time period variables for each dataset were based on the timeline mapping (see online supplemental table 1). The event time periods were operationalised in terms of a multicategorical variable where each category represented a specific time period of interest.
Analytic approach
Preliminary analysis involved exploring the distribution of the Google search queries and NPOS data for JUUL and Puff Bar products separately. Please refer to online supplemental note 1 for corresponding skewness and kurtosis test results and online supplemental table 2 for more information on preliminary analysis related to findings from parametric and non-parametric regression analysis for non-normal distribution.
Based on prior work based on Google search queries,17 adjusted autoregressive integrated moving average (ARIMA) models were trained on baseline data from the time period (T1-T2) to predict the expected values of JUUL’s and Puff Bar’s shopping interest RSV in the subsequent time periods (assuming no event impacts) (see online supplemental note 2 for more details). Figure 1A,B illustrate trends in shopping interest RSV and figure 2A,B illustrate trends in sales. We controlled for EVALI cases, the average price of Puff Bar’s disposable products and JUUL’s kits and refills to predict JUUL and Puff Bar sales, for the baseline period T1 and T2. The ARIMA analysis was not adjusted for the COVID-19 pandemic because the baseline period occurred prior to the pandemic, and sensitivity analysis suggested that there were very minor differences between the observed sales and RSV shopping interest trends for both products with and without the impact of the COVID-19 pandemic (please see online supplemental figures 1, 2 for more details). Seasonality was not included as a covariate due to multi-collinearity issues.
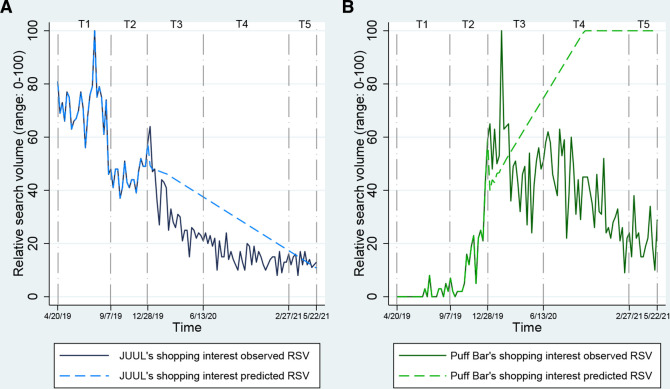
Figure 1.
Trends in Google search queries indicative of JUUL’s and Puff Bar’s shopping interest RSV and predicted RSV in relation to regulatory, company sales policy and other events during 2019–2021. RSV, relative search volume.
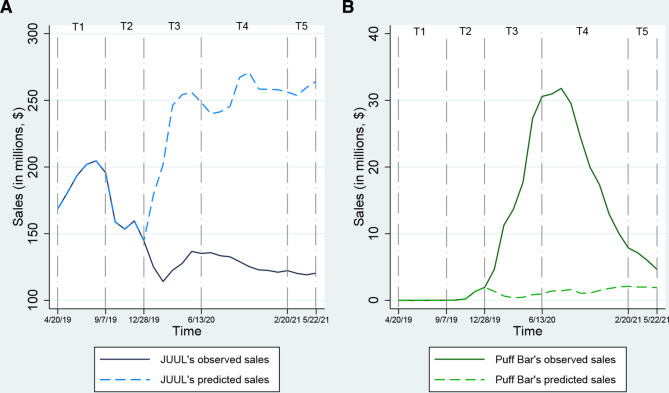
Figure 2.
Observed and predicted point-of-sales trends for JUUL and Puff Bar in relation to regulatory, company sales policy and other events during 2019–2021.
Next, linear regression analysis was used to assess the rate of change in the outcome variables at each time period of interest in reference to the baseline time period (T1) (see table 1). Separate outcome variables comprised of JUUL’s sales, Puff Bar’s sales, JUUL’s shopping interest RSV and Puff Bar’s shopping interest RSV. We controlled for EVALI cases, the COVID-19 cases, seasonality, the average price per item sold for JUUL’s kits and refills and Puff Bar products given youth appeal for disposable electronic cigarettes may be higher than that of JUUL, due to their low cost among other factors.
Table 1.
Adjusted rate of change in point-on-sales and Google queries indicative of shopping interest for JUUL and Puff Bar regulatory, company sales policy and other events during 2019–2021
| Point-of-sales | Shopping interest relative search volume | |||
| JUUL (n=28 cycles) |
Puff Bar (n=28 cycles) |
JUUL (n=113 weeks) |
Puff Bar (n=113 weeks) |
|
| B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | |
| T2: Trump Administration announced plans to ban flavours; JUUL Labs announced to halt online sales of its sweet and fruity e-cigarette products | −24.93* (−42.67 to –7.20) |
9.68 (−3.01 to 22.38) |
−23.77*** (−31.56 to –15.98) |
10.42 (−2.07 to 22.90) |
| T3: FDA published enforcement guidance policy on unauthorised flavoured cartridge-based e-cigarettes; House approved flavour e-cigarette ban bill | −75.39*** (−91.05 to –59.74) |
32.51*** (21.31 to 43.71) |
−42.06*** (−46.77 to –37.35) |
50.94*** (43.39 to 58.49) |
| T4: Puff Bar battled counterfeit sales and suspended sale | −70.61*** (−87.01 to –54.21) |
31.96*** (20.22 to 43.69) |
−54.46*** (−62.17 to –46.74) |
43.35*** (30.99 to 55.72) |
| T5: Puff Bar back on the market | −87.25** (−129.89 to –44.60) |
40.31* (9.80 to 70.83) |
−53.38*** (−59.54 to –47.21) |
32.61*** (22.73 to 42.48) |
| Puff Bar average price per unit | −8.99 (−23.63 to 5.64) |
3.19 (−7.29 to 13.66) |
– | – |
| JUUL Kit average price per unit | 2.63** (0.87 to 4.39) |
−0.63 (−1.89 to 0.63) |
– | – |
| JUUL Refill average price per unit | −74.23 (−184.65 to 36.20) |
43.83 (−35.20 to 122.85) |
– | – |
| EVALI cases | −1.91 (−14.63 to 10.82) |
−1.64 (−10.75 to 7.47) |
−17.65 (−35.78 to 0.49) |
−20.48 (−49.55 to 8.58) |
| COVID-19 cases | −0.00 (−0.00 to 0.00) |
0.00 (−0.00 to 0.00) |
−0.00* (−0.01 to –0.00) |
−0.01*** (−0.01 to –0.00) |
| Intercept | 618.06* (13.03 to 1223.09) |
−260.34 (−693.30 to 172.62) |
84.68*** (78.03 to 91.32) |
10.03 (−0.62 to 20.68) |
*P<0.05, **p<0.01, ***p<0.001.
Reference time period: T1 (both JUUL and Puff Bar sold and marketed as tobacco-derived nicotine-containing products).
Seasonality was also controlled for.
EVALI is conceptualised as total EVALI cases per million individuals during each time period of the dataset.
COVID-19 is conceptualised as the total new COVID-19 cases per million individuals during each time period of the dataset.
EVALI, e-cigarette, or vaping, product use associated lung injury.
Stata 15.1 was used to conduct the ARIMA and linear regression analyses. Python 3.7 was used to retrieve Google search queries data. Statistical significance was defined as p<0.05.
Results
Trends in Google search queries indicative of shopping interest in JUUL and Puff Bar
Figure 1 demonstrates the observed and predicted trends related to JUUL’s and Puff Bar’s weekly shopping interest RSV from March 2019 to May 2021. During T1, when both JUUL and Puff Bar were on the market as tobacco-derived nicotine-containing products, JUUL’s shopping interest RSV varied and declined sharply towards the end (range 46–100, median 72), and the Puff Bar’s shopping interest RSV remained relatively stable (range 0–8, median 0). Subsequently during T2, when the Trump administration announced its plans to ban unauthorised flavoured e-cigarettes on 11 September 2019, and JUUL Labs decided to voluntarily halt online sales of its sweet and fruity e-cigarette products on 17 October 2019, there was a decrease in JUUL’s shopping interest RSV (range 37–58, median 45.5), while the Puff Bar’s shopping interest RSV increased (range 0–59, median 14). At T3, JUUL’s observed shopping interest RSV declined (range 15–65, median 26.5) but was in the same direction as its predicted RSV (blue dotted line); Puff Bar’s shopping interest RSV rose steeply and trended much higher than the predicted levels (green dotted line) initially after the FDA’s announcement of its enforcement guidance policy on unauthorised flavoured cartridge-based e-cigarettes on January 2, 2020 and the House’s approval of flavoured e-cigarette ban bill on 28 February 2020 and declined sharply thereafter (range 24–100, median 50.5). JUUL’s and Puff Bar’s declining shopping interest RSV trends continued (JUUL: range 8–24, median 15; Puff Bar: range 9–63, median 38) during T4 when Puff Bar battled counterfeit sales, received FDA’s warning letter and announced its withdrawal from the market. Last, during T5, when Puff Bar resumed sales in the USA as a synthetic nicotine-containing product, JUUL’s shopping interest RSV maintained similar levels as T5 and was slightly above the predicted levels (range 8–17, median 12.5), and Puff Bar’s shopping interest RSV remained below the predicted levels.
Table 1 highlights the rate of change in JUUL’s and Puff bar’s shopping interest RSV during each time period of interest compared with T1 (when both JUUL and Puff Bar were on the market as tobacco-derived nicotine-containing products). JUUL’s shopping interest RSV was significantly lower during T2 (B=−16.36 per week; 95% CI –24.24 to –8.49) when the Trump administration announced plans to ban flavoured e-cigarettes and when JUUL withdrew its fruity and sweet flavoured products from the market. In contrast, Puff Bar’s shopping interest RSV was significantly higher (B=17.29; 95% CI 3.82 to 30.755) during T2. JUUL’s shopping interest RSV continued to be significantly lower in the subsequent time periods (T3: B=−42.06; 95% CI –46.77 to –37.35; T4: B=−54.46; 95% CI –62.17 to –46.74; T5: B=−53.38; 95% CI –59.54 to –47.21). Compared with T1, Puff Bar’s shopping interest RSV was also higher during T3, the period after JUUL announced plans to discontinue the sale of its sweet and fruity flavoured products (B=50.94; 95% CI 43.39 to 58.49). In the subsequent time periods, Puff Bar’s shopping interest RSV remained significantly higher than in T1 although not as high as those in T3 (T4: B=43.35 95% CI 30.99 to 55.72; T5: B=32.61; 95% CI 22.73 to 42.48).
Trends in JUUL’s and Puff Bar’s point-of-sales
Figure 2 demonstrates the observed and predicted trends related to JUUL’s and Puff Bar’s monthly sales from March 2019 to May 2021. During T1 (when both products were on the market as tobacco-derived nicotine-containing products), JUUL’s sales initially increased and then started declining towards the end of T1 (range $168.73–$204.88 million, median $194.60 million), while Puff Bar’s sales was on an increasing trend (range $9.99–$15 203.51, median $1844.8). As JUUL’s sales continued to decline during T2 (range $144.65–$159.57 million, median $156.12 million), Puff Bar’s sales (range $46 729.8–$ 1.95 million, median $0.78 million) increased to $1.95 million by the end of T2. After the Trump administration’s announcement of its plan to ban flavoured e-cigarettes on 11 September 2019 and JUUL Labs’ voluntary decision to halt the online sales of its sweet and fruity e-cigarette products on 17 October 2019 (T2), JUUL’s sales (range $114.12–$136.68 million, median $126.35 million) continued to decline and reached its lowest point of $114.12 million and lower than its predicted levels. At the same time, Puff Bar’s sales (range $4.66–$30.59 million, median $15.69 million) increased to $15.69 million by the end of T3. During T4, when Puff Bar suspended the sales of its products, JUUL’s sales started to decline and continued to fall below its predicted levels (range $121.09–$135.75 million, median $125.31 million) while Puff Bar’s sales started declining but continued to be above its predicted levels (range $7.87–$31.79 million, median $19.96 million). When Puff Bar became available again as a synthetic nicotine-containing product in the market during T5, JUUL’s sales remained relatively stable (range $119.14–$120.47 million, median $120.22 million) while decline in Puff Bar’s sales slowed such that the gap between the observed and the predicted sales was at the lowest level (range $4.63–$7.11 million, median $5.98 million).
Table 1 also reports the rate of changes in JUUL’s and Puff Bar’s sales during each time period of interest compared with T1. JUUL’s sales were significantly lower during T2 compared with the sales during T1 (B=−24.70 per month; 95% CI –48.85 to –0.54-4) when the Trump administration announced its plan for flavoured e-cigarettes ban and when JUUL halted the sales of its sweet and fruity flavoured products. JUUL’s sales continued to be lower in the subsequent time periods (T3: B=−75.39; 95% CI –91.05 to –59.74; T4: B=−70.61; 95% CI –87.01 to –54.21; T5 B=−87.25; 95% CI –129.89 to –44.06). Puff Bar’s sales were significantly higher than T1 during T3 until T6 (T3: B=32.51; 95% CI 21.31 to 43.71; T4: B=31.96; 95% CI 20.22 to 43.69; T5: B=40.31; 9.80 to 70.83).
Discussion
This study assessed public interest in shopping and sales of JUUL and Puff Bar during 2019–2021. Peaks in Puff Bar’s shopping interest RSV and sales intersected with declines in JUUL’s shopping interest RSV and sales in early 2020 suggesting that uptake of disposable products may warrant continued surveillance. The declining trend in Puff Bar’s sales has slowed since March 2021 when it resumed sales and relaunched its products as ‘synthetic nicotine’ e-cigarettes. Moving forward, the sales trends of JUUL and Puff Bar are likely to be determined by future regulatory, advocacy and product availability factors affecting both JUUL and Puff Bar sales.
The contemporaneous trends in the shopping interest of these potentially close alternative products are notable. Recent surveillance of Twitter discourse referencing both JUUL and Puff Bar products found that conversations pertaining to switching from JUUL to Puff Bar were predominant.39 As such, ensuring FDA’s purview includes synthetic nicotine e-cigarette products and can address gaps in the Family Smoking Prevention and Tobacco Control Act for e-cigarettes and adverse public health outcomes.
Puff Bar’s and JUUL’s sales are on a declining trend since mid-2020. The effects of Puff Bar’s recent repositioning of its products and continued sales in the market may result in a spike in the uptake of these products. Federal, tribal, state and local laws that restrict the sale of tobacco products may encourage consumers to switch to Puff Bar products that may be potentially exempt. Additionally, the unprecedented peak in the shopping interest and actual sales of Puff Bar in the past is indicative of its potential impact on the vaping crisis. Given these considerations, federal action should be taken to regulate synthetic nicotine products including Puff Bar. More specially, as discussed previously, the FDA CDER could potentially regulate Puff Bar and other similar products as drug products and permit their sales in the U.S. market only on approval of a new drug application.40 Taken together, enactment of proposed comprehensive regulations pertaining to flavoured e-cigarettes, introduction of new flavours, regional and local flavour bans, and the impact of menthol bans on e-cigarette consumption may help sustain the declining trend.
Notably, RSV shopping interest and POStrends were similar for each product during the study period, suggesting that Google Trends may offer valuable means to monitor immediate effects of policies on public interest. Additionally, multimodal surveillance leveraging different data sources including cohort studies, social media data and electronic health records can play a significant role in capturing and validating early warnings.
This study is limited to an aggregate national-level analysis given the nature of Google search queries and POS data. As such, we are unable to determine the demographics of individuals who search on Google or purchase these products, if Google queries resulted in purchases, and whether purchases occurred online or offline. NPOS data also do not offer information about whether individuals purchasing these products from retail stores consumed these products, are limited to participating convenience stores and do not capture internet sales or other in-person sales transactions. While every effort was made to map the starting dates of Nielsen’s monthly sales periods and weekly RSV periods data with the event dates for each timepoint, these did not align perfectly. Findings also cannot be generalised to other products or time periods. The RSV measure is also relative to the total number of Google searches executed at a certain timepoint. As such, it is possible that a potential steep increase in the total number of Google searches indicative of shopping interest in JUUL and Puff Bar at the onset of the pandemic may not be discernible in the trends. Additionally, we were unable to control for seasonality in the ARIMA analysis due to collinearity for the following calendar months: February, March, April, June, July, September and December. We were unable to incorporate separate time periods representing the emergence of EVALI and the COVID-19 pandemic because there was only one single observation representing the retail sales of these products during each of the above-mentioned time periods. We controlled for EVALI hospitalizations and COVID-19 pandemic infections to account for their effects on the outcomes; findings should be interpreted cautiously as related media coverage may have impacted sales and Google search trends. Controlling for the EVALI hospitalizations and COVID-19 pandemic infections per 1000 individuals in the USA does not account for the effects of media coverage of these major health issues on sales and public interest in shopping these products. Future work may consider investigating the interaction effect between the volume of EVALI and COVID-19 media coverage and the regulatory events on the sales and public interest in shopping these products.
Taken together, this study conducted event-contextual digital and traditional surveillance to offer insights about the trajectory of Puff Bar’s and JUUL’s popularity. Armed with the insights from this study, public health practitioners can plan strategic efforts including public health education interventions and campaigns to sustain the declining trend in JUUL and Puff Bar in the future. Additionally, unlike more traditional forms of research such as surveys and focus groups, Google search queries make it possible to map organic changes in public shopping interest retrospectively at low cost and in real-time. At the same time, trajectories in POS validate these changes in the e-cigarette marketplace and evolving landscape.
Conclusion
This study mapped trends in Google shopping queries indicative of shopping interest in JUUL and Puff products and their POS from March 2019 until May 2021 in relation to regulatory, company sales policy announcements and other events. Peaks in Puff Bar’s shopping interest RSV and sales intersected with declines in JUUL’s shopping interest RSV and sales. Puff Bar’s declining sales trends in 2021 has slowed, after it relaunched its products as ‘synthetic nicotine’ e-cigarettes. These trends may change in the near future as Puff Bar continues to expand sales in the U.S. market.
Key messages.
What is already known on this topic
Puff Bar has replaced JUUL as the most popular e-cigarette brand among youth. Currently, Puff Bar is positioned as synthetic nicotine-containing product and is beyond the FDA’s purview.
What this study adds
Peaks in Puff Bar’s shopping interest relative search volume (RSV) and sales intersected with declines in JUUL’s shopping interest RSV and sales.
Puff Bar’s declining trend of its sales has slowed since March 2021.
How this study might affect research, practice and/or policy
Armed with the insights from this study, public health practitioners can plan strategic efforts including public health education interventions and campaigns to sustain the declining trend in JUUL and Puff Bar in the future.
Footnotes
Twitter: @AnujaMajmundar
Contributors: AM: conceptualisation, data collection, data analysis, first draft of the manuscript, guarantor. AM, ZX: data analysis. AM, ZX, SA, NN: revision of manuscript. NN: supervision.
Funding: Supported by the Department of Surveillance & Health Equity Science of the American Cancer Society.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: The authors’ own analyses and calculations were based in part on data reported by Nielsen through its Scantrack Service for e-cigarettes for the 4-week period ending July 2021 for the stateline market and convenience and all other retail channels. Copyright©2021, The Nielsen Company. The conclusions drawn from the Nielsen data are those of authors and do not reflect the views of Nielsen. Nielsen is not responsible for and had no role in analysing and preparing the results reported herein.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Google trends data are available on request. We are unable to share Nielsen Point-Of-Sales data due to licensing restrictions.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Dai H, Leventhal AM. Prevalence of e-cigarette use among adults in the United States, 2014-2018. JAMA 1824;2019;322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sidani JE, Colditz JB, Barrett EL, et al. I wake up and hit the JUUL: analyzing Twitter for JUUL nicotine effects and dependence. Drug Alcohol Depend 2019;204:107500. 10.1016/j.drugalcdep.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miech R, Leventhal A, Johnston L, et al. Trends in use and perceptions of nicotine Vaping among US youth from 2017 to 2020. JAMA Pediatr 2021;175:185–90. 10.1001/jamapediatrics.2020.5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Craver R. Juul ends 2018 with 76 percent market share, 2019. Winston-Salem Journal. Available: https://journalnow.com/business/juul-ends-2018-with-76-percent-market-share/article_6f50f427-19ec-50be-8b0c-d3df18d08759.html [Accessed 28 Jun 2021].
- 5. Yuan M, Cross SJ, Loughlin SE, et al. Nicotine and the adolescent brain. J Physiol 2015;593:3397–412. 10.1113/JP270492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults. JAMA Pediatr 2017;171:788–97. 10.1001/jamapediatrics.2017.1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aubrey A. Juul suspends sales of flavored Vapes and signs settlement to stop marketing to youth, 2019. NPR.org. Available: https://www.npr.org/sections/health-shots/2019/10/17/771098368/juul-suspends-sales-of-flavored-vapes-and-signs-settlement-to-stop-marketing-to- [Accessed 28 Jun 2021].
- 8. Wang TW, Gentzke AS, Neff LJ, et al. Disposable E-Cigarette Use among U.S. Youth - An Emerging Public Health Challenge. N Engl J Med 2021;384:1573–6. 10.1056/NEJMc2033943 [DOI] [PubMed] [Google Scholar]
- 9. FRM A. E-Cigarette unit sales, by product and flavor type — United States, 2014–202. MMWR Morb Mortal Wkly Rep 0;2020:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Talih S, Salman R, Soule E, et al. Electrical features, liquid composition and toxicant emissions from 'pod-Mod'-like disposable electronic cigarettes. Tob Control 2022;31:667–70. 10.1136/tobaccocontrol-2020-056362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Food & Drug Administration . Youth e-cigarette use remains serious public health concern amid COVID-19 pandemic, 2021. Food & Drug Administration, Center for Tobacco Products. Available: https://www.fda.gov/news-events/press-announcements/youth-e-cigarette-use-remains-serious-public-health-concern-amid-covid-19-pandemic [Accessed 29 Oct 2021].
- 12. Food & Drug Administration . FDA Notifies companies, including puff bar, to remove flavored disposable e-cigarettes and Youth-Appealing E-Liquids from market for not having required authorization, 2020. FDA. Available: https://www.fda.gov/news-events/press-announcements/fda-notifies-companies-including-puff-bar-remove-flavored-disposable-e-cigarettes-and-youth [Accessed 29 Oct 2021].
- 13. Wolfe E. Controversial E-Cigarette Company Puff Bar Says It’s Suspending US Sales, 2020. FairWarning. Available: https://www.fairwarning.org/2020/07/e-cigarette-company-suspends-sales/ [Accessed 29 Jun 2021].
- 14. American Academy of Pediatrics, American Cancer Society Cancer Action Network, American Heart Association . Partners letters to FDA acting commissioner re puff bar and synthetic nicotine, 2021. Available: https://www.lung.org/getmedia/4748ba27-73ba-4271-997d-a363edba78ba/fda-letter-re-puff-bar-synthetic-nicotine-final.pdf
- 15. AbadZ SH, Kline A, Sultana M. Digital public health surveillance: a systematic scoping review. Npj Digit Med 2021;4:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ayers JW, Althouse BM, Ribisl KM, et al. Digital detection for tobacco control: online reactions to the 2009 U.S. cigarette excise tax increase. Nicotine & Tobacco Research 2014;16:576–83. 10.1093/ntr/ntt186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dai H, Hao J. Online popularity of JUUL and puff bars in the USA: 2019–2020. Tob Control 2022;31:7–10. 10.1136/tobaccocontrol-2020-055727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tabuchi T, Gallus S, Shinozaki T, et al. Heat-not-burn tobacco product use in Japan: its prevalence, predictors and perceived symptoms from exposure to secondhand heat-not-burn tobacco aerosol. Tob Control 2018;27:e25–33. 10.1136/tobaccocontrol-2017-053947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caputi TL, Leas E, Dredze M, et al. They’re heating up: Internet search query trends reveal significant public interest in heat-not-burn tobacco products. PLoS One 2017;12:e0185735. 10.1371/journal.pone.0185735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tabuchi T, Fukui K, Gallus S. Tobacco price increases and population interest in smoking cessation in Japan between 2004 and 2016: a Google trends analysis. Nicotine & Tobacco Research 2019;21:475–80. 10.1093/ntr/nty020 [DOI] [PubMed] [Google Scholar]
- 21. Ayers JW, Althouse BM, Allem J-P, et al. A novel evaluation of World no tobacco day in Latin America. J Med Internet Res 2012;14:e77. 10.2196/jmir.2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vosen S, Schmidt T. Forecasting private consumption: survey‐based indicators vs. Google trends - Vosen - 2011 - Journal of Forecasting - Wiley Online Library. J Forecast 2011;30:565–78. [Google Scholar]
- 23. Goel S, Hofman JM, Lahaie S, et al. Predicting consumer behavior with web search. Proc Natl Acad Sci U S A 2010;107:17486–90. 10.1073/pnas.1005962107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leas EC, Moy NH, Nobles AL, et al. Google shopping queries for vaping products, JUUL and IQOS during the e-cigarette, or Vaping, product use associated lung injury (EVALI) outbreak. Tob Control 2022;31:e74–7. 10.1136/tobaccocontrol-2021-056481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019;28:146–51. 10.1136/tobaccocontrol-2018-054382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson H, Welker K, Griffith J. Trump administration plans to ban sale of flavored electronic cigarettes, 2019. NBC News. Available: https://www.nbcnews.com/politics/politics-news/trump-administration-meeting-address-vaping-crisis-n1052396 [Accessed 29 Jun 2021].
- 27. JUUL Labs suspends sale of non-tobacco, non-menthol-based flavors in the US, 2019. Available: https://www.juullabs.com/juul-labs-suspends-sale-of-non-tobacco-non-menthol-based-flavors-in-the-u-s
- 28. Office of the Commissioner . Fda finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint, 2020. FDA. Available: https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children [Accessed 29 Jun 2021].
- 29. DeBonis M. House votes to ban flavored tobacco to curb youth vaping epidemic, 2020. The Washington Post. Available: https://www.washingtonpost.com/powerpost/house-votes-to-ban-flavored-tobacco-to-curb-youth-vaping-epidemic/2020/02/28/4cb4c15a-5a38-11ea-9b35-def5a027d470_story.html [Accessed 29 Jun 2021].
- 30. Products C for T, Cool Clouds Distribution, Inc. d/b/a Puff Bar - 608526 - 07/20/2020 . Center for tobacco products. Available: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/cool-clouds-distribution-inc-dba-puff-bar-608526-07202020 [Accessed 29 Oct 2021].
- 31. Maloney J. Puff bar defies FDA Crackdown on fruity e-cigarettes by Ditching the tobacco, 2021. The Wall Street Journal. Available: https://www.wsj.com/articles/puff-bar-defies-fda-crackdown-on-fruity-e-cigarettes-by-ditching-the-tobacco-11614681003 [Accessed 29 Jun 2021].
- 32. Liber A, Cahn Z, Larsen A, et al. Flavored e-cigarette sales in the United States under self-regulation from January 2015 through October 2019. Am J Public Health 2020;110:785–7. 10.2105/AJPH.2020.305667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Day HR, Ambrose BK, Schroeder MJ, et al. Point of sale scanner data for rapid surveillance of the e-cigarette market. Tob Regul Sci 2017;3:325–32. 10.18001/TRS.3.3.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diaz MC, Donovan EM, Schillo BA, et al. Menthol e-cigarette sales rise following 2020 FDA guidance. Tob Control 2021;30:700–3. 10.1136/tobaccocontrol-2020-056053 [DOI] [PubMed] [Google Scholar]
- 35. U.S. Bureau of Labor Statistics . Consumer Price Index U.S. Bureau of Labor Statistics. Available: https://www.bls.gov/cpi/ [Accessed 29 Jun 2021]. [Google Scholar]
- 36. Health CO on S and. smoking and tobacco use; electronic cigarettes, 2021. Centers for Disease Control and Prevention. Available: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html [Accessed 29 Oct 2021].
- 37. U.S. Bureau of Economic Analysis . Population, 1959. FRED, Federal Reserve Bank of St. Louis. Available: https://fred.stlouisfed.org/series/POPTHM [Accessed 29 Oct 2021].
- 38. New York Times . COVID in the U.S.: latest map and case count, 2021. The new York times. Available: https://www.nytimes.com/interactive/2021/us/covid-cases.html [Accessed 29 Oct 2021].
- 39. Allem J-P, Dormanesh A, Majmundar A, et al. Leading topics in Twitter discourse on JUUL and puff bar products: content analysis. J Med Internet Res 2021;23:e26510. 10.2196/26510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zettler PJ, Hemmerich N, Berman ML. Closing the regulatory gap for synthetic nicotine products. Boston Coll Law Rev 2018;59:1933–82 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6329380/ [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
tobaccocontrol-2021-056953supp001.pdf (289.3KB, pdf)
Data Availability Statement
Google trends data are available on request. We are unable to share Nielsen Point-Of-Sales data due to licensing restrictions.