Abstract
Background
Pancreatic exocrine insufficiency is a cause of malabsorption. It is generally diagnosed if faecal elastase-1 (FE-1) levels are below 200 µg/g. Pancreatic function is assumed to be normal when faecal elastase levels are >500 µg/g. The significance of faecal elastase levels above 200 µg/g but less than 500 µg/g is unclear.
Methods
This retrospective study reports the response to treatment in patients who had an FE-1 level between 200 and 500 µg/g.
Results
Of these 82 patients, 28 were offered pancreatic enzyme replacement therapy (PERT). A clinical response, defined as an improvement in their initial symptoms after commencing PERT, was seen in 20 patients (71%), 7 with potentially predisposing conditions and 13 with functional diarrhoea. PERT particularly abolished or improved diarrhoea, steatorrhoea and flatulence.
Conclusion
Clinicians should, therefore, be aware that a trial of PERT given to patients with FE-1 levels between 200 and 500 µg/g may lead to improvement in gastrointestinal symptoms.
Keywords: malabsorption, diarrhoea, pancreatic elastase-1, pancreatic enzymes
WHAT IS ALREADY KNOWN ON THIS TOPIC
The diagnosis of pancreatic exocrine insufficiency (PEI) is often delayed in patients without an obvious predisposing cause. National Institute for Health and Care Excellence guidance recommends that this should not be considered in patients with functional diarrhoea or IBS-like symptoms. Recent UK guidance on the management of PEI is very comprehensive but does not address what to do with patients with a pancreatic faecal elastase level which lies between 200 and 500 ug/g.
WHAT THIS STUDY ADDS
We show that three-quarters of patients with a faecal elastase level between 200 and 500 ug/g trialled with pancreatic enzyme replacement therapy (PERT) appear to have a beneficial response, the majority of whom had no obvious predisposing cause. It is also of note that the majority of patients who had a faecal elastase-1 (FE-1) in the range of 200–500 ug/g but were not offered PERT by their managing clinician, continued to remain symptomatic despite other interventions. Therefore, clinicians should be aware that a trial of PERT in these cases can lead to a rapid improvement in symptoms.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study suggests that there may not be distinct cut-off level for FE-1 levels to represent PEI and that instead, this is a graded and gradual decline. Future randomised controlled trials should, therefore, seek to gather more information about the use of PERT in patients with an FE-1 level between 200 and 500 ug/g to see if this response is clinically significant.
Introduction
The pancreas plays an essential role in the digestion, absorption and metabolism of carbohydrates, fats and proteins.1 Pancreatic exocrine insufficiency (PEI) is a condition whereby reduced pancreatic enzyme activity (mainly pancreatic lipase) in the intestinal lumen leads to impaired digestion.1 Impaired digestion potentially results in nutrient malabsorption and malnutrition, as well as disturbed regulation of gastrointestinal (GI) motor and secretory functions.2 PEI may present with symptoms such as bloating, abdominal discomfort, steatorrhoea (clay-coloured, loose, greasy, foul smelling stool), diarrhoea, excess flatulence and weight loss and may be difficult to distinguish from many other GI conditions.3 Symptoms of PEI are thought to develop when secretion of lipase and other pancreatic enzymes are reduced to <10% of normal values. However, symptomatic PEI may depend not only on pancreatic enzyme levels but also on underlying conditions (table 1); intrinsic pancreatic disease, as a result of reduced cholecystokinin (CCK) secretion or as a result of congenital conditions or secondary to other conditions4 5 and potentially is better measured by a graded response in faecal elastase-1 (FE-1) levels rather than a precise cut-off.6
Table 1.
Conditions/therapies associated with pancreatic exocrine insufficiency
| Type of issue | Specific conditions | Prevalence |
| Intrinsic pancreatic disease | Chronic pancreatitis neoplasia |
94% within 10 years of onset of chronic pancreatitis.18
66%–94% of patients with unresectable pancreatic cancer.19 20 |
| Reduced CCK secretion | Coeliac disease | 4%–80% in untreated coeliac disease (measured by FE-1).21–26
12% in patients with chronic diarrhoea on a gluten-free diet (based on pancreatic testing or trial of PERT) 18% (based on steatorrhoea and trial of PERT).27 28 |
| Congenital disease | Pancreaticum divisum cystic fibrosis |
48% based on faecal fat excretion.29
85% before the age of 1 year.14 |
| Inflammatory bowel disease | Crohn’s disease | 14%–30%.11 30 |
| Ulcerative colitis | Up to 22% using FE.11
Up to 50% using a secretin-cerulein test and 74% using para-aminobenzoic acid test.31 32 |
|
| Bile acid malabsorption | May coexist27 | No data but should be considered.13 |
| Small intestinal bacterial overgrowth | May coexist27 | Consider antibiotic therapy in those not responding to/not tolerating PERT.33 |
| HIV disease | Due to disease34 35 or secondary to antiretroviral medication particularly Didanosine31 36 | Up to 54% with improvements in faecal fat loss following institution of PERT.37 38 |
| Diabetes mellitus | Type 1 diabetes Type 2 diabetes |
Up to 6% in type 1 diabetics with diarrhoea39 and 26–44% otherwise.40–43
12%–20%40–43. Inadequate data whether there is any symptom improvement with PERT in those without diarrhoea.44 |
| Oncological therapies | Tyrosine kinase inhibitors Checkpoint inhibitors Somatostatin analogues |
7% in those treated with sorafenib.45
1% after nivolumab46 47 and reported after pembrolizumab.48 49 Chronic use can affect up to 38%.50 |
CCK, cholecystokinin; FE-1, faecal elastase-1; PERT, pancreatic enzyme replacement therapy.
The most accurate tests for diagnosing pancreatic insufficiency are either unpleasant (prolonged faecal fat collection and quantification) or cumbersome (radiolabelled studies or endoscopically obtained pancreatic secretion analysis) and in clinical practice have been largely superseded by measurement of pancreatic FE-1 testing. FE‐1 is an enzyme produced and released by the pancreas and remains intact during intestinal transit.7 Vagal innervation to the pancreas stimulates FE-1 secretion in response to the sense, smell and taste of food and as a result of stomach wall distension. Acidic chyme entering the duodenum also leads to CCK release which stimulates secretion of pancreatic enzymes. As it is not degraded, stool concentration of FE-1 is a measure of the exocrine function of the pancreas. It is highly sensitive and specific for detecting advanced PEI but may be less reliable in milder cases, in patients following pancreatic resection or in patients with looser stools.3 7–9 Increased water content in stools may have a dilutional effect on FE-1 concentration, thereby giving falsely low results. However, this limitation can be addressed by lyophilisation of the stool sample or centrifugation to reduce the water content.10 In addition, a diurnal variation in FE-1 levels may affect interpretation of results.11
Patients who are diagnosed with PEI and treated with pancreatic enzyme replacement therapy (PERT) have increased survival and improvement in quality of life (QoL).12 However, recent UK guidelines do not discuss the management of patients with potential PEI but who have FE-1 levels above the frequently quoted cut-off level of 200 µg/g,.12 13 Therefore, the aim of this study was to assess the significance of an FE-1 level between 200 and 500 µg/g.
Methods
This was a retrospective study which was approved by the United Lincolnshire Hospitals Trust audit committee (L0448) and deemed not to require the consent of the patient.
In our hospital laboratory, FE-1 concentration is determined using an ELISA. The polyclonal antibodies used are specific to human FE-1 and not affected by PERT.14 A list of all patients who had an FE-1 sample measured at United Lincolnshire Hospitals NHS Trust were identified by the pathology laboratory. Those with results of <200 µg/g or >500 µg/g were excluded.
Notes and medical records of those remaining were retrospectively reviewed. For those seen as outpatients, clinical symptoms were recorded before PERT was trialled and subsequent clinic letters were reviewed and used to assess response to treatment. A response to PERT was defined for this study if the patient reported that their presenting symptoms had responded to treatment, if PERT improved their QoL and if they continued to take PERT following an initial trial.
Results
Eighty-two patients who were tested between April 2019 and March 2021 were were found to have an FE-1 level of 200–500 µg/g, of whom 78 were seen in a clinic. Patient characteristics are detailed in table 2. Symptoms that prompted FE-1 testing were diarrhoea, steatorrhoea, bloating, flatulence and abdominal pain.
Table 2.
Patient characteristics of those with an FE-1 result between 200 and 500 µg/g
| Male | Female | Total | |
| Number | 28 | 50 | 78 |
| Age in years (median and range) |
68 (30–90) | 60 (27–86) | 63 (27–90) |
| Abdominal symptoms | |||
| Bloating | 8 | 16 | 24 |
| Diarrhoea | 21 | 34 | 55 |
| Flatulence | 5 | 10 | 15 |
| Pain | 5 | 17 | 22 |
| Steatorrhoea | 2 | 6 | 8 |
| Other GI conditions | |||
| Coeliac disease | 1 | 1 | 2 |
| IBD | 8 | 0 | 8 |
| Pancreatic surgery | 0 | 1 | 1 |
| History of colorectal cancer | 2 | 3 | 5 |
| Other | 6 | 9 | 15 |
| No other GI condition | 11 | 36 | 47 |
| Numbers with FE level: | |||
| 200–300 | 6 | 13 | 19 |
| 300–400 | 12 | 16 | 28 |
| 400–500 | 10 | 21 | 31 |
| Numbers offered PERT | 10 | 18 | 28 |
FE-1, faecal elastase-1; GI, gastrointestinal; IBD, inflammatory bowel disease; PERT, pancreatic enzyme replacement therapy.
FE-1 testing for 68 patients was carried out specifically to exclude PEI as a diagnosis due to the presenting symptoms, in 5 patients to assess for a change in levels following treatment with an antibiotic, in 2 patients for symptoms of steatorrhoea, in 2 patients it was tested alongside a faecal calprotectin, with the reason for this being unclear, and in one patient for diarrhoeal episodes but not specifically to exclude PEI.
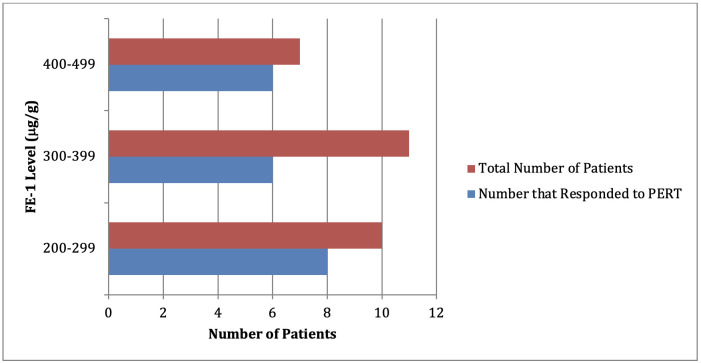
Six patients were told explicitly that the FE-1 level of between 200–500 µg/g was a normal result and Creon was not trialled. A further 44 were not given a trial of PERT, but no reason was stated for this in the notes. Of the 28 (36%) patients given a trial of Creon, 20 (71%) reported a beneficial response to treatment (figure 1). Adjuvant treatment was also used in some of these patients with eight prescribed multivitamins and 3 offered proton pump inhibitors. Although other forms of PERT are available, within our cohort, only Creon was prescribed. The dosing regime varied, with the majority being trialled on two capsules (50 000 units) with meals and one capsule (25 000 units) with snacks. Others were offered a total daily dose between 150 000–200 000 units daily. 3 patients did not tolerate Creon, developing nausea and vomiting.
Figure 1.
The number of patients with FE-1 of between 200–500 µg/g that responded to a trial of PERT.
From the 20 patients who responded to PERT, thereby suggesting a diagnosis of PEI, only 7 (35%) had risk factors associated with the condition, 4 had diabetes mellitus, 1 had coeliac disease, 1 had Crohn’s disease, 1 had undergone pancreatic surgery. The remaining 21 (65%) had symptoms labelled as irritable bowel syndrome (IBS) or functional diarrhoea.
Our data suggest the main symptoms that responded to PERT were diarrhoea, steatorrhoea, bloating and abdominal pain. All 20 patients who showed improvement stated that their diarrhoea was either no longer present or improved considerably following commencement of PERT (table 3).
Table 3.
Showing the response of symptoms for patients when given a trial of PERT
| Symptoms | Improvement | No change | Worse | Not stated |
| Bloating | 1 | 0 | 0 | 3 |
| Diarrhoea | 20 | 0 | 0 | 0 |
| Flatulence | 2 | 0 | 0 | 3 |
| Pain | 2 | 0 | 0 | 1 |
| Steatorrhoea | 2 | 0 | 0 | 1 |
PERT, pancreatic enzyme replacement therapy.
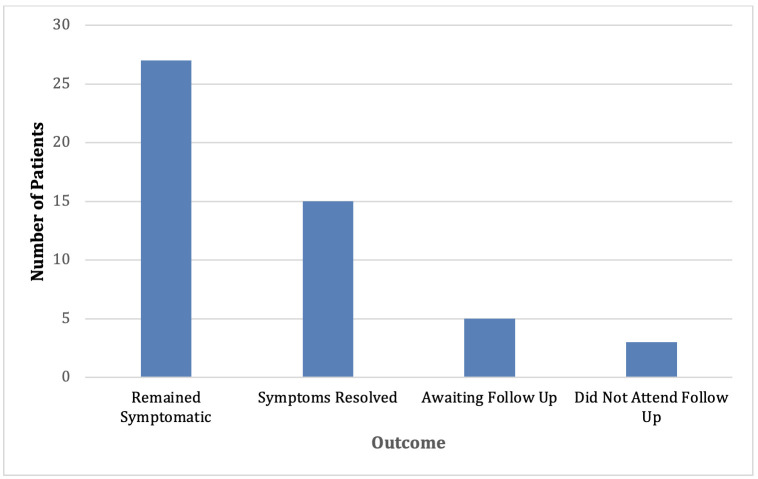
Of those not given a trial of PERT (figure 2), over 50% of those remained symptomatic. However, 30% reported that their symptoms resolved. In this retrospective study, the data were not available to comment definitively on the reasons for the resolution of symptoms.
Figure 2.
Illustrates the outcomes of patients who were not trialled on PERT.
Discussion
Our results show that three-quarters of patients with an FE-1 level of between 200 and 500 µg/g trialled with PERT report a significant clinical response to treatment.
Although it is widely believed patients with PEI will only display symptoms when pancreatic function has dropped to <10%13 it has been suggested that these patients may instead demonstrate a graded response to reduced exocrine function and so might benefit from having a trial of treatment at higher levels of faecal elastase than those which identify severe loss of function.6 Our findings support this suggestion.
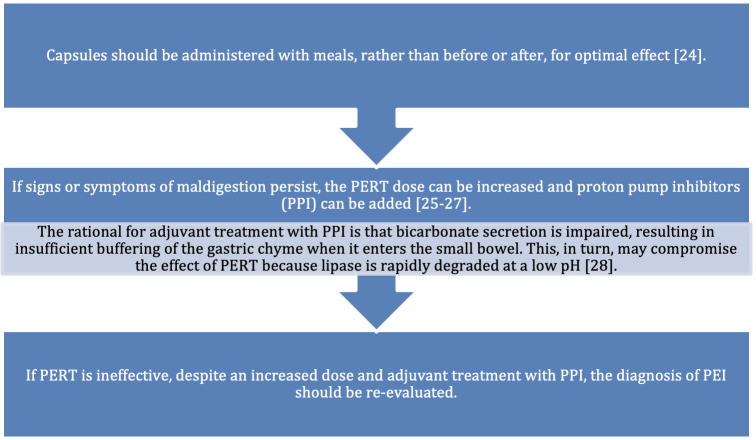
The prompt diagnosis and treatment of PEI has significant benefits to patients. There is substantial evidence that PERT allows patients with PEI to improve body weight by preventing malnutrition, a known risk factor for decreased survival.15 Furthermore, treatment also reduces the occurrence of GI symptoms and abdominal pain commonly associated with PEI, thereby improving patients’ QoL.16 17 Patients are most likely to benefit if they are properly educated on how to take PERT correctly (figure 3).
Figure 3.
Explaining how PERT should be administered.
Our results show that while coexisting GI conditions may be present many patients have no clear cause for PEI. In our experience, a trial of 10 days of PERT is adequate in most patients to be clear whether PERT is beneficial.
There were limitations associated with our study. First, this is a retrospective notes review and the patient cohort was heterogeneous. As data were also not recorded specifically for this study, specific questions or information may not have been noted. A variety of clinicians with differing ways of interpreting what the patient tells them and possibly differing priorities saw these patients. Our sample size is also small and based in the rural area of Lincolnshire and our results might not be generalisable to the overall population. No formal and potentially more robust patient-reported outcome measure (PROMs) was used to assess objectively patients’ symptoms before and after treatment and it is impossible to say unequivocally that a placebo response was not responsible for improvement in some patients. Indeed, we saw that in patients not treated with PERT a minority reported spontaneous resolution of their symptoms. Finally, patient follow-up was often limited so we cannot comment whether reported improvements were sustained.
Conclusion
We have shown in our retrospective study an association between FE-1 levels between 200 and 500 µg/g and an improvement in GI symptoms following initiation of PERT. It is for this reason that clinicians should remain open to the possibility that PEI may occur in a graded manner and that there are a group of patients who may benefit from treatment. In addition, for those who do not fully respond to PERT, other GI diagnoses should also be sought. It is therefore hoped that this retrospective data can be used in helping with the formation of new, prospectively collected cohort studies which would assess the use of PERT in patients with FE-1 levels between 200 and 500 µg/g and that responses are measured during a standardised follow-up period using validated PROMs to reach a definitive conclusion.
Footnotes
Contributors: DF and HJNA came up with the concept for the study. AM and DF performed data collection and analysis. DF, AM and HJNA were all involved in writing and reviewing the final manuscript. DF is the guarantor of the study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This was a retrospective study which was approved by the United Lincolnshire Hospitals Trust audit committee (L0448) and deemed not to require the consent of the patient.
References
- 1. Alhassan O G, Samy A A. Pancreatic insufficiency. Treasure Island, FL: StatPearls Publishing, 2021. [Google Scholar]
- 2. Layer P, Kashirskaya N, Gubergrits N. Contribution of pancreatic enzyme replacement therapy to survival and quality of life in patients with pancreatic exocrine insufficiency. World J Gastroenterol 2019;25:2430–41. 10.3748/wjg.v25.i20.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Othman MO, Harb D, Barkin JA. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. Int J Clin Pract 2018;72:e13066. 10.1111/ijcp.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frulloni L, Falconi M, Gabbrielli A, et al. Italian consensus guidelines for chronic pancreatitis. Dig Liver Dis 2010;42 Suppl 6:S381–406. 10.1016/S1590-8658(10)60682-2 [DOI] [PubMed] [Google Scholar]
- 5. Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut 2005;54 Suppl 6(Suppl 6):vi1–28. 10.1136/gut.2005.065946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Struyvenberg MR, Martin CR, Freedman SD. Practical guide to exocrine pancreatic insufficiency-breaking the myths. BMC Med 2017;15:29.:29. 10.1186/s12916-017-0783-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lindkvist B. Diagnosis and treatment of pancreatic exocrine insufficiency. World J Gastroenterol 2013;19:7258–66. 10.3748/wjg.v19.i42.7258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leeds JS, Oppong K, Sanders DS. The role of fecal elastase-1 in detecting exocrine pancreatic disease. Nat Rev Gastroenterol Hepatol 2011;8:405–15. 10.1038/nrgastro.2011.91 [DOI] [PubMed] [Google Scholar]
- 9. Brydon WG, Kingstone K, Ghosh S. Limitations of faecal elastase-1 and chymotrypsin as tests of exocrine pancreatic disease in adults. Ann Clin Biochem 2004;41(Pt 1):78–81. 10.1258/000456304322664753 [DOI] [PubMed] [Google Scholar]
- 10. Fischer B, Hoh S, Wehler M, et al. Faecal elastase-1: lyophilization of stool samples prevents false low results in diarrhoea. Scand J Gastroenterol 2001;36:771–4. 10.1080/003655201300192058 [DOI] [PubMed] [Google Scholar]
- 11. Maconi G, Dominici R, Molteni M, et al. Prevalence of pancreatic insufficiency in inflammatory bowel diseases. assessment by fecal elastase-1. Dig Dis Sci 2008;53:262–70. 10.1007/s10620-007-9852-y [DOI] [PubMed] [Google Scholar]
- 12. Phillips ME, Hopper AD, Leeds JS, et al. Consensus for the management of pancreatic exocrine insufficiency: UK practical guidelines. BMJ Open Gastroenterol 2021;8:e000643. 10.1136/bmjgast-2021-000643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arasaradnam RP, Brown S, Forbes A, et al. Guidelines for the investigation of chronic diarrhoea in adults: British Society of gastroenterology, 3rd edition. Gut 2018;67:1380–99. 10.1136/gutjnl-2017-315909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilschanski M, Durie PR. Pathology of pancreatic and intestinal disorders in cystic fibrosis. J R Soc Med 1998;91 Suppl 34(Suppl 34):40–9. 10.1177/014107689809134S07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakajima K, Oshida H, Muneyuki T, et al. Pancrelipase: an evidence-based review of its use for treating pancreatic exocrine insufficiency. Core Evid 2012;7:77–91. 10.2147/CE.S26705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Czakó L, Takács T, Hegyi P, et al. Quality of life assessment after pancreatic enzyme replacement therapy in chronic pancreatitis. Can J Gastroenterol 2003;17:597–603. 10.1155/2003/515848 [DOI] [PubMed] [Google Scholar]
- 17. D’Haese JG, Ceyhan GO, Demir IE, et al. Pancreatic enzyme replacement therapy in patients with exocrine pancreatic insufficiency due to chronic pancreatitis: a 1-year disease management study on symptom control and quality of life. Pancreas 2014;43:834–41. 10.1097/MPA.0000000000000131 [DOI] [PubMed] [Google Scholar]
- 18. Dumasy V, Delhaye M, Cotton F, et al. Fat malabsorption screening in chronic pancreatitis. Am J Gastroenterol 2004;99:1350–4. 10.1111/j.1572-0241.2004.30661.x [DOI] [PubMed] [Google Scholar]
- 19. Saito T, Hirano K, Isayama H, et al. The role of pancreatic enzyme replacement therapy in unresectable pancreatic cancer: a prospective cohort study. Pancreas 2017;46:341–6. 10.1097/MPA.0000000000000767 [DOI] [PubMed] [Google Scholar]
- 20. Sikkens ECM, Cahen DL, de Wit J, et al. A prospective assessment of the natural course of the exocrine pancreatic function in patients with a pancreatic head tumor. J Clin Gastroenterol 2014;48:e43–6. 10.1097/MCG.0b013e31829f56e7 [DOI] [PubMed] [Google Scholar]
- 21. Nousia-Arvanitakis S, Fotoulaki M, Tendzidou K, et al. Subclinical exocrine pancreatic dysfunction resulting from decreased cholecystokinin secretion in the presence of intestinal villous atrophy. J Pediatr Gastroenterol Nutr 2006;43:307–12. 10.1097/01.mpg.0000228098.66583.85 [DOI] [PubMed] [Google Scholar]
- 22. Vujasinovic M, Tepes B, Volfand J, et al. Exocrine pancreatic insufficiency, MRI of the pancreas and serum nutritional markers in patients with coeliac disease. Postgrad Med J 2015;91:497–500. 10.1136/postgradmedj-2015-133262 [DOI] [PubMed] [Google Scholar]
- 23. Leeds JS, Hopper AD, Hurlstone DP, et al. Is exocrine pancreatic insufficiency in adult coeliac disease a cause of persisting symptoms? Aliment Pharmacol Ther 2007;25:265–71. 10.1111/j.1365-2036.2006.03206.x [DOI] [PubMed] [Google Scholar]
- 24. Licul V, Cizmarević NS, Ristić S, et al. Ctla-4 +49 and TNF-alpha-308 gene polymorphisms in celiac patients with exocrine pancreatic insufficiency. Coll Antropol 2013;37:1191–4. [PubMed] [Google Scholar]
- 25. Walkowiak J, Herzig KH. Fecal elastase-1 is decreased in villous atrophy regardless of the underlying disease. Eur J Clin Invest 2001;31:425–30. 10.1046/j.1365-2362.2001.00822.x [DOI] [PubMed] [Google Scholar]
- 26. Gomez JC, Morán CE, Mauriño EC, et al. Exocrine pancreatic insufficiency in celiac disease. Gastroenterology 1998;114:621–3. 10.1016/s0016-5085(98)70562-1 [DOI] [PubMed] [Google Scholar]
- 27. Fine KD, Meyer RL, Lee EL. The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology 1997;112:1830–8. 10.1053/gast.1997.v112.pm9178673 [DOI] [PubMed] [Google Scholar]
- 28. Abdulkarim AS, Burgart LJ, See J, et al. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol 2002;97:2016–21. 10.1111/j.1572-0241.2002.05917.x [DOI] [PubMed] [Google Scholar]
- 29. Lindström E, von Schenck H, Ihse I. Pancreatic exocrine and endocrine function in patients with pancreas divisum and abdominal pain. Int J Pancreatol 1990;6:17–24. 10.1007/BF02924340 [DOI] [PubMed] [Google Scholar]
- 30. Barthet M, Lesavre N, Desplats S, et al. Frequency and characteristics of pancreatitis in patients with inflammatory bowel disease. Pancreatology 2006;6:464–71. 10.1159/000094564 [DOI] [PubMed] [Google Scholar]
- 31. Heikius B, Niemelä S, Lehtola J, et al. Pancreatic duct abnormalities and pancreatic function in patients with chronic inflammatory bowel disease. Scand J Gastroenterol 1996;31:517–23. 10.3109/00365529609006775 [DOI] [PubMed] [Google Scholar]
- 32. Angelini G, Cavallini G, Bovo P, et al. Pancreatic function in chronic inflammatory bowel disease. Int J Pancreatol 1988;3:185–93. 10.1007/BF02798930 [DOI] [PubMed] [Google Scholar]
- 33. El Kurdi B, Babar S, El Iskandarani M, et al. Factors that affect prevalence of small intestinal bacterial overgrowth in chronic pancreatitis: a systematic review, meta-analysis, and meta-regression. Clin Transl Gastroenterol 2019;10:e00072. 10.14309/ctg.0000000000000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kotler DP, Gaetz HP, Lange M, et al. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med 1984;101:421–8. 10.7326/0003-4819-101-4-421 [DOI] [PubMed] [Google Scholar]
- 35. Carroccio A, Di Prima L, Di Grigoli C, et al. Exocrine pancreatic function and fat malabsorption in human immunodeficiency virus-infected patients. Scand J Gastroenterol 1999;34:729–34. 10.1080/003655299750025958 [DOI] [PubMed] [Google Scholar]
- 36. Lam KW, Leeds J. How to manage: patient with a low faecal elastase. Frontline Gastroenterol 2021;12:67–73. 10.1136/flgastro-2018-101171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carroccio A, Guarino A, Zuin G, et al. Efficacy of oral pancreatic enzyme therapy for the treatment of fat malabsorption in HIV-infected patients. Aliment Pharmacol Ther 2001;15:1619–25. 10.1046/j.1365-2036.2001.01070.x [DOI] [PubMed] [Google Scholar]
- 38. Price DA, Schmid ML, Ong ELC, et al. Pancreatic exocrine insufficiency in HIV-positive patients. HIV Med 2005;6:33–6. 10.1111/j.1468-1293.2005.00263.x [DOI] [PubMed] [Google Scholar]
- 39. Leeds JS, Hadjivassiliou M, Tesfaye S, et al. Lower gastrointestinal symptoms are associated with worse glycemic control and quality of life in type 1 diabetes mellitus. BMJ Open Diabetes Res Care 2018;6:e000514. 10.1136/bmjdrc-2018-000514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hardt PD, Hauenschild A, Nalop J, et al. High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. A multicenter study screening fecal elastase 1 concentrations in 1,021 diabetic patients. Pancreatology 2003;3:395–402. 10.1159/000073655 [DOI] [PubMed] [Google Scholar]
- 41. Nunes ACR, Pontes JM, Rosa A, et al. Screening for pancreatic exocrine insufficiency in patients with diabetes mellitus. Am J Gastroenterol 2003;98:2672–5. 10.1111/j.1572-0241.2003.08730.x [DOI] [PubMed] [Google Scholar]
- 42. Icks A, Haastert B, Giani G, et al. Low fecal elastase-1 in type I diabetes mellitus. Z Gastroenterol 2001;39:823–30. 10.1055/s-2001-17867 [DOI] [PubMed] [Google Scholar]
- 43. Rathmann W, Haastert B, Icks A, et al. Low faecal elastase 1 concentrations in type 2 diabetes mellitus. Scand J Gastroenterol 2001;36:1056–61. 10.1080/003655201750422657 [DOI] [PubMed] [Google Scholar]
- 44. Ewald N, Bretzel RG, Fantus IG, et al. Pancreatin therapy in patients with insulin-treated diabetes mellitus and exocrine pancreatic insufficiency according to low fecal elastase 1 concentrations. Results of a prospective multi-centre trial. Diabetes Metab Res Rev 2007;23:386–91. 10.1002/dmrr.708 [DOI] [PubMed] [Google Scholar]
- 45. Díaz-González Á, Belmonte E, Sapena V, et al. Pancreatic insufficiency in patients under sorafenib treatment for hepatocellular carcinoma. J Clin Gastroenterol 2021;55:263–70. 10.1097/MCG.0000000000001366 [DOI] [PubMed] [Google Scholar]
- 46. Sweep B, Wilgenhof S, Anten S. Nivolumab-induced exocrine pancreatic insufficiency. Case Rep Oncol 2021;14:1627–31. 10.1159/000519588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jones A, Rodgers K, Jeffrey D, et al. Nivolumab-induced exocrine pancreatic insufficiency. Frontline Gastroenterol 2023;14:167–70. 10.1136/flgastro-2021-102013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prasanna T, McNeil CM, Nielsen T, et al. Isolated immune-related pancreatic exocrine insufficiency associated with pembrolizumab therapy. Immunotherapy 2018;10:171–5. 10.2217/imt-2017-0126 [DOI] [PubMed] [Google Scholar]
- 49. Liu Y, Zhang H, Zhou L, et al. Immunotherapy-associated pancreatic adverse events: current understanding of their mechanism, diagnosis, and management. Front Oncol 2021;11:627612.:627612. 10.3389/fonc.2021.627612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saif MW, Romano A, Smith MH, et al. Chronic use of long-acting somatostatin analogues (ssas) and exocrine pancreatic insufficiency (EPI) in patients with gastroenteropancreatic neuroendocrine tumors (GEP-nets): an under-recognized adverse effect. Cancer Med J 2020;3:75–84. 10.46619/Cmj.2020.3-1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article.