Abstract
Objective
The objective of this study was to utilize machine learning techniques to analyze perioperative factors and identify blood glucose levels that can predict the occurrence of surgical site infection following posterior lumbar spinal surgery.
Methods
A total of 4019 patients receiving lumbar internal fixation surgery from an institute were enrolled between June 2012 and February 2021. First, the filtered data were randomized into the test and verification groups. Second, in the test group, specific variables were screened using logistic regression analysis, Lasso regression analysis, support vector machine, and random forest. Specific variables obtained using the four methods were intersected, and a dynamic model was constructed. ROC and calibration curves were constructed to assess model performance. Finally, internal model performance was verified in the verification group using ROC and calibration curves.
Results
The data from 4019 patients were collected. In total, 1327 eligible cases were selected. By combining logistic regression analysis with three machine learning algorithms, this study identified four predictors associated with SSI, namely Modic changes, sebum thickness, hemoglobin, and glucose. Using this information, a prediction model was developed and visually represented. Then, we constructed ROC and calibration curves using the test group; the area under the ROC curve was 0.988. Further, calibration curve analysis revealed favorable consistency of nomogram-predicted values compared with real measurements. The C-index of our model was 0.986 (95% CI 0.981–0.994). Finally, we used the validation group to validate the model internally; the AUC was 0.987. Calibration curve analysis revealed favorable consistency of nomogram-predicted values compared with real measurements. The C-index was 0.982 (95% CI 0.974–0.999).
Conclusion
Logistic regression analysis and machine learning were employed to select four risk factors: Modic changes, sebum thickness, hemoglobin, and glucose. Then, a dynamic prediction model was constructed to help clinicians simplify the monitoring and prevention of SSI.
Keywords: surgical site infection, lumbar spine surgery, machine learning, blood glucose, dynamic prediction model
Introduction
Surgical site infection (SSI)1 frequently develops postoperatively; this condition can be fatal for both surgeons and patients. Many factors are responsible for the infection of surgical incisions, including smoking status, diabetes, advanced age, hypoproteinemia, and internal fixation.2,3 In spinal surgery,4 SSI is associated with prominent morbidity, healthcare expenses owing to readmission and reoperation, and poor prognosis.5,6 Artificial intelligence is widely used in medical research, and the predictive effectiveness of machine learning is widely recognized. After achieving great success in various predictions, machine learning has attracted the attention of clinicians and medical researchers.7,8 In our previous studies, we constructed a machine learning prediction model and demonstrated good prediction ability.9,10
In this study, a machine learning model and a web-based prediction tool were developed to predict SSI in patients undergoing lumbar spinal surgery. Various machine learning algorithms were compared to identify the most effective approach.11,12 As a super data processing and calculation method, machine learning has considerable reliability in screening variables.13,14 However, the current prediction models based on machine learning mostly compare the effectiveness of different algorithms to select the best one.
Therefore, we aimed to select the ideal clinical variables using various machine learning algorithms and their intersection to build an ideal prediction model and perform internal verification. This prediction model might guide clinical diagnosis and prevention.
Methods
Dataset Preprocessing
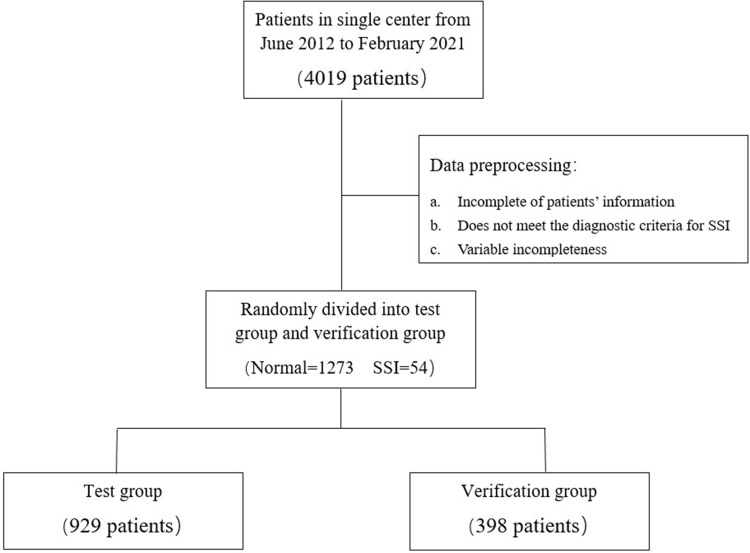
We obtained ethical approval from the Institutional Review Board of our institute (Approval No. 2022-E398-01). This retrospective study adheres to the principles outlined in the Declaration of Helsinki. A total of 4019 patients who underwent lumbar internal fixation surgery at our institute from June 2012 to February 2021 were included in the study. Clinical data such as age, sex, diabetes, Modic changes, anesthesia score, operation status, and serological and imaging indexes of patients were collected for statistical analysis. Operation status included the following parameters: use of antibiotics during the operation, operation time, anesthesia time, vertebral body number spanned, screw number, and intraoperative blood transfusion. The serological parameters were glucose, WBC, hemoglobin (Hb), PLT, ESR, and albumin. Imaging indexes were skin-to-lamina thickness and sebum thickness (In this study, sebum thickness at three distinct locations of lumbar surgical incisions was measured using CT examination as the measurement method. The average value derived from these measurements was considered as the value included in our study). Patients with incomplete information or those that did not meet the diagnostic criteria15 were excluded. Finally, 54 and 1273 patients were grouped into the SSI and normal lumbar fixation groups (Figure 1). Through random grouping, the data were classified into the test and verification groups (Table 1).
Figure 1.
Data filtering and grouping.
Table 1.
The Distribution of Each Variable That Meets the Screening Condition
| Name | Levels | Normal | SSI | P |
|---|---|---|---|---|
| (N=1273) | (N=54) | |||
| Gender | Female | 685 (53.8%) | 38 (70.4%) | 0.024 |
| Male | 588 (46.2%) | 16 (29.6%) | ||
| Age | Mean ±SD | 56.4 ±11.1 | 65.6 ±6.6 | <0.001 |
| Diabetes | No | 1155 (90.7%) | 28 (51.9%) | <0.001 |
| Yes | 118 (9.3%) | 26 (48.1%) | ||
| Modic | I | 1086 (85.3%) | 0 (0%) | <0.001 |
| II | 135 (10.6%) | 18 (33.3%) | ||
| III | 52 (4.1%) | 36 (66.7%) | ||
| ASA | 1 | 65 (5.1%) | 5 (9.3%) | 0.225 |
| 2 | 1010 (79.3%) | 38 (70.4%) | ||
| 3 | 198 (15.6%) | 11 (20.4%) | ||
| Antibiotics | Mean ±SD | 0.3 ±0.5 | 0.4 ±0.5 | 0.012 |
| OP times | Mean ±SD | 95.8 ±48.7 | 99.8 ±62.4 | 0.641 |
| Number of vertebral bodies spanned | Mean ±SD | 1.9 ±0.9 | 2.4 ±2.2 | 0.066 |
| Number of screws | Mean ±SD | 5.1 ±1.6 | 6.3 ±3.3 | 0.012 |
| AT | Mean ±SD | 114.5 ±54.0 | 94.4 ±66.2 | 0.032 |
| Glucose | Mean ±SD | 5.6 ±2.0 | 8.3 ±3.0 | <0.001 |
| WBC | Mean ±SD | 8.4 ±3.4 | 9.7 ±3.8 | 0.011 |
| Hb | Mean ±SD | 126.5 ±19.8 | 106.7 ±19.7 | <0.001 |
| PLT | Mean ±SD | 257.7 ±98.4 | 252.4 ±112.3 | 0.699 |
| Albumin | Mean ±SD | 40.1 ±5.0 | 36.5 ±6.4 | <0.001 |
| ESR | Mean ±SD | 18.2 ±17.7 | 32.0 ±26.2 | <0.001 |
| Blood transfusion | No | 1166 (91.6%) | 19 (35.2%) | <0.001 |
| Yes | 107 (8.4%) | 35 (64.8%) | ||
| Sebum thickness | Mean ±SD | 7.8 ±4.0 | 12.2 ±8.2 | <0.001 |
| Skin to lamina thickness | Mean ±SD | 47.4 ±9.7 | 48.7 ±12.0 | 0.426 |
Abbreviations: ASA, American Society of Anesthesiologists; OP-time, Operation time; AT, anesthesia time; WBC, white blood cell; Hb, hemoglobin; PLT, platelet; ESR, erythrocyte sedimentation rate.
Data Statistics
R software (version 4.2.1; https://www.R-project.org) was used for statistical analyses. First, the filtered data were randomized into the test and verification groups. Second, in the test group, specific variables were screened via logistic regression analysis, Lasso regression analysis, support vector machine (SVM), and random forest. Specific variables acquired using these four methods were intersected, and a dynamic model was constructed. ROC and calibration curves were constructed to assess model performance. Finally, using the verification group, model performance was verified internally using ROC and calibration curves.
Logistic Regression Analysis
Single-factor logistic regression analysis was performed to select variables with p < 0.05. Then, multi-factor logistic regression analysis was performed; p < 0.05 was set as the threshold to select the predictive variables of this method.
Lasso Regression Analysis
Lasso regression analysis was performed, and a model was developed as a contraction approach to select risk factors from various variables as well as optimal predicting features based on SSI case data. LASSO regression and visualized analyses were conducted using the R “glmnet” package.
SVM
SVM recursive feature elimination (SVM-RFE) has been developed as an efficient approach under machine learning. To predict SSI, we developed an SVM-RFE model using the “rms” package. Data were analyzed via tenfold cross-validation, followed by the acquisition of an output vector feature index and variable sorting in the descending order of usefulness.
Random Forest
To construct the random forest model, the R “random forest” package was used to select variables, perform calculations, and visualize relative variable importance. “%IncMSE” indicates an increase in mean squared error. Random values were assigned to each variable to assess the importance of predicting variables. The model’s prediction error increased when a predicting variable of greater importance had its value randomly replaced. Consequently, a higher value indicated a higher level of variable importance. “IncNodePurity” indicates an increase in node purity and can be calculated as the sum of squares of residual errors; it indicates how one variable affects observed value heterogeneity in every node within the classification tree, with a higher value indicating higher variable importance.
We selected “IncNodePurity” as the indicating factors to judge whether a predictive variable was important. We identified the value with the highest importance to be the optimal predictive variable via tenfold cross-validation under five iterations.
Intersection Variable Selection
The abovementioned methods were used to screen the predictive variables. The same variables were obtained using a Wayne diagram. After constructing a dynamic prediction model with common variables, ROC and calibration curves were constructed to evaluate model prediction performance; its effectiveness was verified using the verification group.
Results
Data Characteristics
In total, the data of 1327 patients meeting the inclusion criteria were collected: age, sex, diabetes, Modic changes, anesthesia score, antibiotic use during the operation, operation time, anesthesia time, vertebral body number spanned, screw number, intraoperative blood transfusion, WBC, glucose, PLT, Hb, ESR, albumin, skin-to-lamina thickness, and sebum thickness. These variables were randomly divided into the test and verification groups. The distribution characteristics between the two groups are presented in Table 1. Additionally, Supplementary Figure 1 illustrates the correlations among the different variables in the test group.
Logistic Regression Analysis
Univariate logistic regression analysis revealed a statistically significant difference between p-values < 0.05, and the screened variables were age, diabetes, Modic changes, anesthesia time, vertebral body number spanned, screw number, blood transfusion, WBC, glucose, albumin, ESR, Hb, and sebum thickness. Multivariate logistic regression analysis revealed a statistically significant difference between p-values < 0.05; the screened variables were blood transfusion, glucose, Modic changes, Hb, vertebral body number spanned, and sebum thickness. Table 2 displays the results of the logistic regression analysis.
Table 2.
Results After Logistic Regression Analysis
| Variables | Single Factor Logistic | Multi-Factor Logistic | ||
|---|---|---|---|---|
| OR | P | OR | P | |
| Age | 1.098 | <0.001* | 0.999 | 0.332 |
| Albumin | 0.855 | <0.001* | 0.999 | 0.644 |
| Antibiotics | 1.827 | 0.064 | ||
| ASA | 0.987 | 0.970 | ||
| AT | 0.991 | 0.017 | 0.999 | 0.117 |
| Blood transfusion | 14.265 | <0.001* | 1.121 | <0.001* |
| Diabetes | 8.604 | <0.001* | 1.033 | 0.168 |
| ESR | 1.027 | <0.001* | 1.000 | 0.136 |
| Gender | 0.526 | 0.061 | ||
| Glucose | 1.304 | <0.001* | 1.016 | <0.001* |
| Hb | 0.951 | <0.001* | 0.998 | <0.001* |
| Modic | 16.042 | <0.001* | 1.182 | <0.001* |
| Number of screws | 1.405 | <0.001* | 1.008 | 0.152 |
| Number of vertebral bodies spanned | 1.459 | 0.003 | 0.974 | 0.022 |
| OP times | 1.001 | 0.557 | ||
| PLT | 0.997 | 0.284 | ||
| Sebum thickness | 1.110 | <0.001* | 1.004 | <0.001* |
| Skin to lamina thickness | 1.025 | 0.118 | ||
| WBC | 1.094 | 0.021 | 0.998 | 0.254 |
Notes: “P<0.05”: The representation was statistically significant; “*”: means the statistical significance is more obvious.
Abbreviations: ASA, American Society of Anesthesiologists; AT, anesthesia time; ESR, erythrocyte sedimentation rate; Hb, hemoglobin; OP-time, Operation time; PLT, platelet; WBC, white blood cell.
Machine Learning
Lasso Regression Analysis
Results for Lasso regression analysis of dependent variables are shown in Supplementary Figure 2A; 12 significant variables in patients with SSI were compared with those in patients with SSI (Supplementary Figure 2B).
SVM-RFE and Random Forest
Following SVM-RFE analysis, Supplementary Figure 3A illustrates that ten variables with the lowest error rate were selected as predictive factors. Each of these factors was found to be statistically significant. Variables with the highest importance were determined using the random forest algorithm “IncNodePurity.” Supplementary Figure 3B shows that the best regression effect was obtained by leaving the 10 variables with the highest importance after tenfold cross-validation.
Model Development
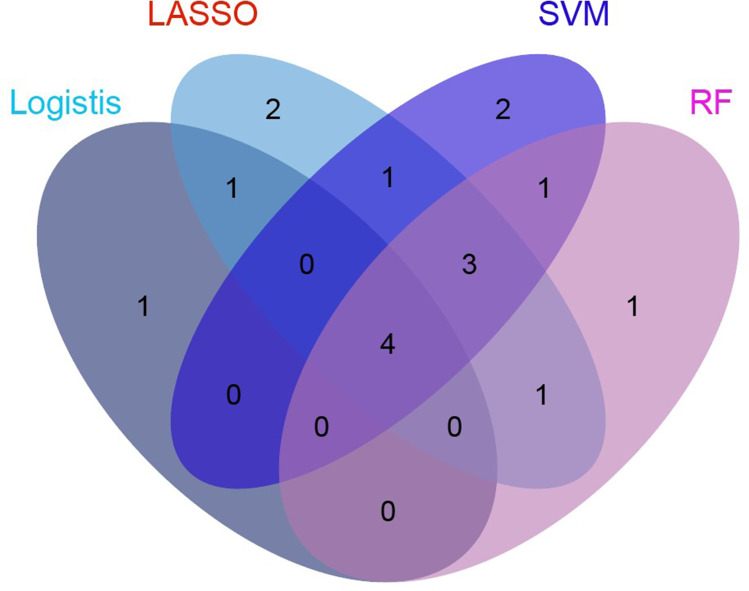
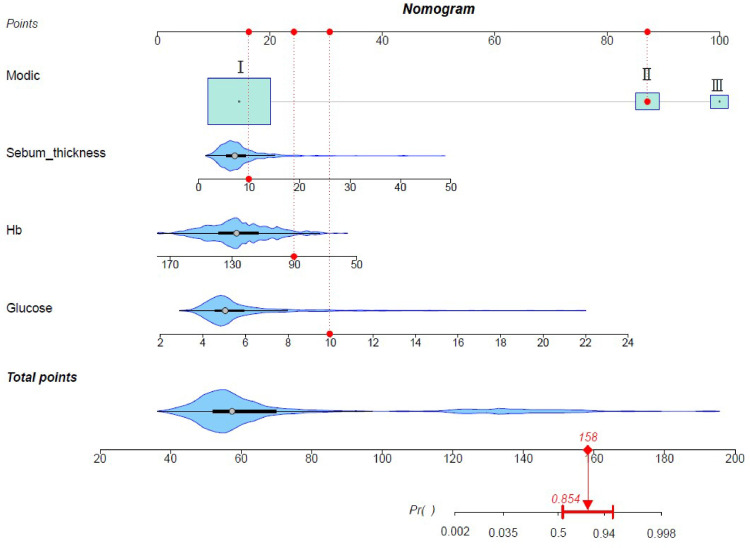
Table 3 displays the variables with the highest importance selected via Lasso regression analysis, SVM-RFE, and random forest. The intersection of the results obtained using the four methods was determined using a Venn diagram (Figure 2). Four predictors were obtained: Hb, glucose, Modic change, and sebum thickness. We used these four predictors to build a prediction model (Figure 3).
Table 3.
Risk Factors Screened by Three Machine Learning Algorithms
| LASSO | SVM-RFE | RF |
|---|---|---|
| Sebum thickness | Sebum thickness | Modic |
| Modic | PLT | Glucose |
| Age | WBC | Albumin |
| AT | Hb | Diabetes |
| Hb | Glucose | WBC |
| Diabetes | Albumin | Hb |
| Glucose | Skin to lamina thickness | ESR |
| Blood transfusion | OP times | Sebum thickness |
| Gender | ESR | Blood transfusion |
| ESR | Modic | AT |
| OP times | ||
| WBC |
Abbreviations: OP-time, Operation time; AT, anesthesia time; WBC, white blood cell; Hb, hemoglobin; PLT, platelet; ESR, erythrocyte sedimentation rate.
Figure 2.
The intersection of variables screened by using logistic regression analysis, LASSO, random forest, and SVM-RFE.
Figure 3.
Four independent risk factors were identified, including Modic change, sebum thickness, Hb, glucose, and a dynamic model was constructed. Categorical variables were visually represented using block plots, while the distribution of continuous variables was depicted through violin plots. Larger plots accommodated more variables for comprehensive visualization. The red marker on the graph indicates that the probability of postoperative surgical site infection (SSI) was found to be 85.4% when all four independent risk factors were at their respective values.
Model Performance
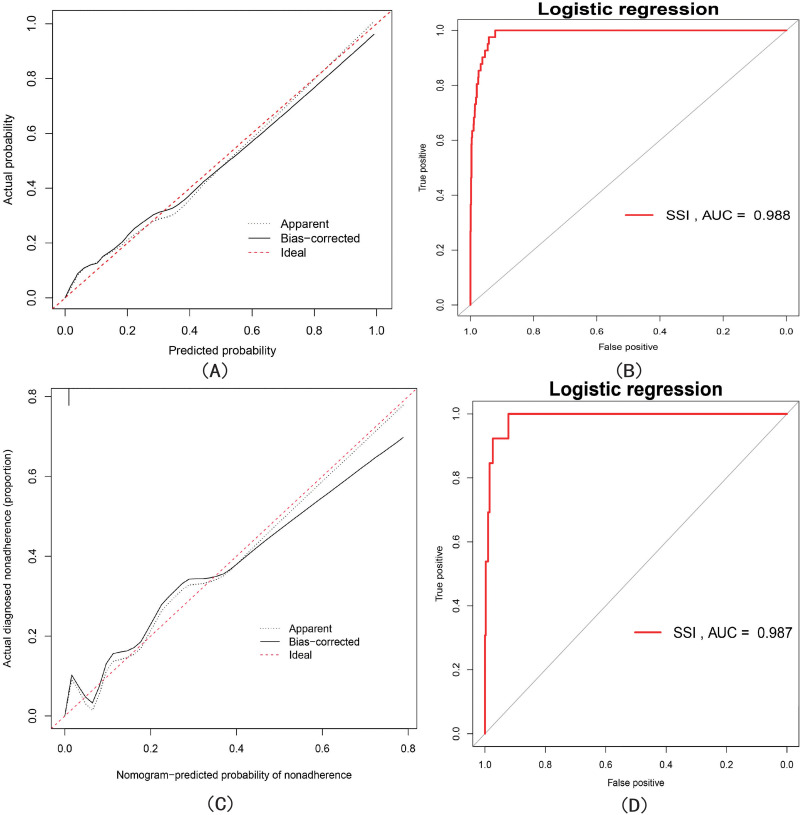
To verify model efficiency, ROC (Figure 4B) and calibration (Figure 4A) curves were constructed using the test group; the area under the ROC curve (AUC) was 0.988. Calibration curve analysis revealed favorable consistency of the nomogram-predicted values compared with real measurements. In addition, the C-index of the model was 0.9861 (95% CI 0.981–0.994). Finally, we used the validation group for internal validation; the ROC and calibration curves are shown in Figures 4D and C, respectively. The AUC was 0.987, and calibration curve analysis revealed favorable consistency of the nomogram-predicted values compared with real measurements. The C-index was 0.982 (95% CI 0.974–0.999).
Figure 4.
(A and B) represent the calibration curve and ROC curve of the training group, respectively, where the area under the curve (AUC) is 0.988. (C and D) represent the calibration curve and ROC curve of the validation group, respectively, where the area under the curve (AUC) is 0.987.
Discussion
In this study, we used machine learning algorithms and related data to develop an SSI prediction model according to various predictions. Three machine learning models were employed to filter variables, and their validity was assessed using the verification group. This strategy based on artificial intelligence has been adopted to help clinicians select early diagnostic approaches.13,16,17 The relationship between machine learning and medicine is extensive, involving the diagnosis and treatment of cancer, surgery, and internal diseases.18–20 Machine learning includes imaging, metabolomics, proteomics, etc.;21,22 random forest, SVM, CNN, GBX, and other algorithms are a very small part of machine learning.23 We can diagnose and predict various diseases, including tumors, specific diseases, and inflammatory diseases, via machine learning.9,10,24
Many studies have assessed SSI risk factors after spinal surgery,25–28 including the establishment of predictive models based on machine learning.12,29 In our study, we utilized a combination of logistic regression analysis and machine learning to identify common risk factors and develop a prediction model, which has not been accomplished in previous studies. Further, we identified four risk factors that were closely related to the occurrence of SSI: Modic change, sebum thickness, Hb, and glucose. The constructed prediction model has good predictive efficacy and visualization, further simplifying the clinician’s judgment and intervention on SSI.
Modic Changes
Modic changes cause the degeneration of the lumbar spine on imaging and are probably involved in the body’s immune response.30,31 Pradip et al found that Modic changes were chronic subclinical infection foci rather than degeneration markers alone.32 Ohtori et al reported that endplate abnormality is associated with TNF-induced axonal development and inflammation. This conclusion is drawn from the observation that patients with Modic Type 1 or 2 endplate changes on MRI exhibited a significantly higher presence of TNF immunoreactive cells and PGP 9.5 immunoreactive nerve fibers in the affected vertebral endplates compared to patients without any endplate abnormalities on MRI.33 In our study, we also determined Modic changes as a risk factor for SSI following lumbar surgery. Therefore, Modic changes are not only a manifestation of lumbar disc degeneration but also that of chronic inflammation and should hence receive added attention from clinicians.
Sebum Thickness
Studies have shown that obesity is positively correlated with postoperative SSI occurrence.34,35 We found that sebum thickness, a critical factor for predicting the risk of postoperative SSI, was positively correlated with SSI occurrence. We obtained this result despite insufficient direct pathophysiological evidence for sebum thickness and SSI. As sebum thickness and obesity are often positively correlated, we believe the pathophysiological mechanism between sebum thickness and SSI is equivalent to the relationship between obesity and SSI.36,37 Preoperative fat reduction is instructive for SSI prevention.38
Hb and Glucose
Hb content is often negatively correlated with SSI occurrence;39 we also confirmed this finding. Tissue growth at the incision site after surgery is inseparable from energy perfusion. Insufficient tissue blood perfusion is not conducive to tissue recovery and even leads to tissue necrosis.40,41 Anemia is closely associated with SSI development; however, it is worth noting that perioperative blood transfusion may also be an independent factor for predicting postoperative SSI.42 Moreover, glucose has been a focal point in research related to SSIs.43–45 We found that preoperative blood glucose levels were positively correlated with SSI occurrence. Liu et al identified high preoperative serum glucose as an independent factor predicting SSI risk following posterior lumbar spinal surgery.46 Thus, spinal surgeons should pay attention to patients’ preoperative blood glucose levels and intervene in time to prevent SSI.
Given the strong predictive efficacy of the model developed in our study, spine doctors can anticipate the potential occurrence of SSIs in patients prior to surgery by considering factors such as Modic changes, sebum thickness, hemoglobin levels, and preoperative blood glucose. In cases where a high risk is identified, appropriate intervention measures can be implemented before surgery, such as stabilizing blood glucose, administering blood transfusions, and prophylactic antibiotic use. The goal is to mitigate the risk of postoperative SSIs, facilitate patients’ speedy recovery, and alleviate unnecessary financial burdens. Additionally, we identified intraoperative blood transfusion as a risk factor for outcomes using logistic regression analysis, Lasso regression analysis, and random forest techniques. This finding is noteworthy and warrants attention from healthcare providers and patients alike.
Limitations
Although we used various screening methods and constructed a prediction model with good performance, our study has some limitations. First, there might be selection and subjective bias owing to the retrospective nature of the study. Second, we constructed the machine learning algorithm model based on data from a single center; as a result, this model might not be applicable to other centers and requires external verification. Third, additional data are warranted, which might improve the diagnostic effectiveness of our model.
Conclusions
In our study, we employed logistic regression analysis and machine learning to create a dynamic model with strong predictive capabilities for SSIs. This dynamic model can be a valuable tool for healthcare professionals and patients in clinical practice.
Acknowledgments
We would like to thank Dr. Xinli Zhan and Dr. Chong Liu for their efforts in this work.
Abbreviations
SSI, surgery site infection; CI, Confidence interval; AUC, Area under the curve; BMI, body mass index; ASA, American Society of Anesthesiologists; OP-time, Operation time; AT, anesthesia time; WBC, white blood cell; Hb, hemoglobin; PLT, platelet; ESR, erythrocyte sedimentation rate.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
We confirm that all subjects and/or their legal guardians provided written informed consent for participation in this study. Prior approval of the study was obtained from the institutional ethical review board of The First Affiliated Hospital of Guangxi Medical University (Approval No. 2022-E398–01). The study complies with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Seidelman J, Anderson DJ. Surgical site infections. Infect Dis Clin North Am. 2021;35(4):901–929. [DOI] [PubMed] [Google Scholar]
- 2.Ma T, Lu K, Song L, et al. Modifiable factors as current smoking, hypoalbumin, and elevated fasting blood glucose level increased the SSI risk following elderly hip fracture surgery. J Invest Surg. 2020;33(8):750–758. [DOI] [PubMed] [Google Scholar]
- 3.Skeie E, Koch AM, Harthug S, et al. A positive association between nutritional risk and the incidence of surgical site infections: a hospital-based register study. PLoS One. 2018;13(5):e0197344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Wang R, Huo X, Xiong W, Kang L, Xue Y. Incidence of surgical site infection after spine surgery: a systematic review and meta-analysis. Spine. 2020;45(3):208–216. [DOI] [PubMed] [Google Scholar]
- 5.Strobel RM, Leonhardt M, Förster F, et al. The impact of surgical site infection-a cost analysis. Langenbeck’s Arch Surgery. 2022;407(2):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFarland A, Reilly J, Manoukian S, Mason H. The economic benefits of surgical site infection prevention in adults: a systematic review. J Hosp Infect. 2020;106(1):76–101. [DOI] [PubMed] [Google Scholar]
- 7.Sultan AS, Elgharib MA, Tavares T, Jessri M, Basile JR. The use of artificial intelligence, machine learning and deep learning in oncologic histopathology. J Oral Pathol Med. 2020;49(9):849–856. [DOI] [PubMed] [Google Scholar]
- 8.Sidey-Gibbons JAM, Sidey-Gibbons CJ. Machine learning in medicine: a practical introduction. BMC Med Res Methodol. 2019;19(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J, Lu Q, Liang T, et al. Development and validation of a machine learning-based nomogram for prediction of ankylosing spondylitis. Rheumatol Therapy. 2022;9(5):1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou C, Huang S, Liang T, et al. Machine learning-based clustering in cervical spondylotic myelopathy patients to identify heterogeneous clinical characteristics. Front Surgery. 2022;9:935656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu WC, Ying H, Liao WJ, et al. Using preoperative and intraoperative factors to predict the risk of surgical site infections after lumbar spinal surgery: a machine learning-based study. World Neurosurg. 2022;162:e553–e60. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Fan T, Yang B, Lin Q, Li W, Yang M. Development and internal validation of supervised machine learning algorithms for predicting the risk of surgical site infection following minimally invasive transforaminal lumbar interbody fusion. Front med. 2021;8:771608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handelman GS, Kok HK, Chandra RV, Razavi AH, Lee MJ, Asadi H. eDoctor: machine learning and the future of medicine. J Intern Med. 2018;284(6):603–619. [DOI] [PubMed] [Google Scholar]
- 14.Cote MP, Lubowitz JH, Brand JC, Rossi MJ. Artificial intelligence, machine learning, and medicine: a little background goes a long way toward understanding. Arthroscopy. 2021;37(6):1699–1702. [DOI] [PubMed] [Google Scholar]
- 15.Borchardt RA, Tzizik D. Update on surgical site infections: the new CDC guidelines. JAAPA. 2018;31(4):52–54. [DOI] [PubMed] [Google Scholar]
- 16.Lee D, Yoon SN. Application of Artificial Intelligence-Based Technologies in the Healthcare Industry: opportunities and Challenges. Int J Environ Res Public Health. 2021;18(1):567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsting M. Machine learning will change medicine. J Nucl Med. 2017;58(3):357–358. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto DA, Witkowski E, Gao L, Meireles O, Rosman G. Artificial Intelligence in Anesthesiology: current Techniques, Clinical Applications, and Limitations. Anesthesiology. 2020;132(2):379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong J, Ha D, Lee J, et al. Network-based machine learning approach to predict immunotherapy response in cancer patients. Nat Commun. 2022;13(1):3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groot OQ, Ogink PT, Lans A, et al. Machine learning prediction models in orthopedic surgery: a systematic review in transparent reporting. J Orthop Res. 2022;40(2):475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison JH, Gilbertson JR, Hanna MG, et al. Introduction to artificial intelligence and machine learning for pathology. Arch Pathol Lab Med. 2021;145(10):1228–1254. [DOI] [PubMed] [Google Scholar]
- 22.Staziaki PV, Wu D, Rayan JC, et al. Machine learning combining CT findings and clinical parameters improves prediction of length of stay and ICU admission in torso trauma. Eur Radiol. 2021;31(7):5434–5441. [DOI] [PubMed] [Google Scholar]
- 23.Nayarisseri A, Khandelwal R, Tanwar P, et al. Artificial intelligence, big data and machine learning approaches in precision medicine & drug discovery. Curr Drug Targets. 2021;22(6):631–655. [DOI] [PubMed] [Google Scholar]
- 24.Tran KA, Kondrashova O, Bradley A, Williams ED, Pearson JV, Waddell N. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021;13(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Gan Z, Huang S, et al. Blood transfusion risk prediction in spinal tuberculosis surgery: development and assessment of a novel predictive nomogram. BMC Musculoskelet Disord. 2022;23(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Namba T, Ueno M, Inoue G, et al. Prediction tool for high risk of surgical site infection in spinal surgery. Infect Control Hospital Epidemiol. 2020;41(7):799–804. [DOI] [PubMed] [Google Scholar]
- 27.Haddad S, Millhouse PW, Maltenfort M, Restrepo C, Kepler CK, Vaccaro AR. Diagnosis and neurologic status as predictors of surgical site infection in primary cervical spinal surgery. Spine J. 2016;16(5):632–642. [DOI] [PubMed] [Google Scholar]
- 28.Bohl DD, Shen MR, Mayo BC, et al. Malnutrition predicts infectious and wound complications following posterior lumbar spinal fusion. Spine. 2016;41(21):1693–1699. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins BS, Mazmudar A, Driscoll C, et al. Using artificial intelligence (AI) to predict postoperative surgical site infection: a retrospective cohort of 4046 posterior spinal fusions. Clin Neurol Neurosurg. 2020;192:105718. [DOI] [PubMed] [Google Scholar]
- 30.Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC. Pathobiology of Modic changes. Eur Spine J. 2016;25(11):3723–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vigeland MD, Flåm ST, Vigeland MD, et al. Correlation between gene expression and MRI STIR signals in patients with chronic low back pain and Modic changes indicates immune involvement. Sci Rep. 2022;12(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pradip IA, Dilip Chand Raja S, Rajasekaran S, et al. Presence of preoperative Modic changes and severity of endplate damage score are independent risk factors for developing postoperative surgical site infection: a retrospective case-control study of 1124 patients. Eur Spine J. 2021;30(6):1732–1743. [DOI] [PubMed] [Google Scholar]
- 33.Ohtori S, Inoue G, Ito T, et al. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back Pain and Modic Type 1 or Type 2 changes on MRI. Spine. 2006;31(9):1026–1031. [DOI] [PubMed] [Google Scholar]
- 34.Yuan K, Chen HL. Obesity and surgical site infections risk in orthopedics: a meta-analysis. Int j Surgery. 2013;11(5):383–388. [DOI] [PubMed] [Google Scholar]
- 35.Lynch RJ, Ranney DN, Shijie C, Lee DS, Samala N, Englesbe MJ. Obesity, surgical site infection, and outcome following renal transplantation. Ann Surg. 2009;250(6):1014–1020. [DOI] [PubMed] [Google Scholar]
- 36.Onyekwelu I, Glassman SD, Asher AL, Shaffrey CI, Mummaneni PV, Carreon LY. Impact of obesity on complications and outcomes: a comparison of fusion and nonfusion lumbar spine surgery. J Neurosurg Spine. 2017;26(2):158–162. [DOI] [PubMed] [Google Scholar]
- 37.Lee JS, Terjimanian MN, Tishberg LM, et al. Surgical site infection and analytic morphometric assessment of body composition in patients undergoing midline laparotomy. J Am Coll Surg. 2011;213(2):236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inacio MC, Kritz-Silverstein D, Raman R, et al. The risk of surgical site infection and re-admission in obese patients undergoing total joint replacement who lose weight before surgery and keep it off postoperatively. Bone Joint J. 2014;96-b(5):629–635. [DOI] [PubMed] [Google Scholar]
- 39.Kim BD, Smith TR, Lim S, Cybulski GR, Kim JY. Predictors of unplanned readmission in patients undergoing lumbar decompression: multi-institutional analysis of 7016 patients. J Neurosurg Spine. 2014;20(6):606–616. [DOI] [PubMed] [Google Scholar]
- 40.Rammell J, Perre D, Boylan L, et al. The adverse impact of preoperative anaemia on survival following major lower limb amputation. Vascular. 2022;17085381211065622. [DOI] [PubMed] [Google Scholar]
- 41.Lasocki S, Krauspe R, von Heymann C, Mezzacasa A, Chainey S, Spahn DR. PREPARE: the prevalence of perioperative anaemia and need for patient blood management in elective orthopaedic surgery: a multicentre, observational study. Eur J Anaesthesiol. 2015;32(3):160–167. [DOI] [PubMed] [Google Scholar]
- 42.Higgins RM, Helm MC, Kindel TL, Gould JC. Perioperative blood transfusion increases risk of surgical site infection after bariatric surgery. Surg Obesity Related Dis. 2019;15(4):582–587. [DOI] [PubMed] [Google Scholar]
- 43.Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017;152(8):784–791. [DOI] [PubMed] [Google Scholar]
- 44.Hagedorn JM, Bendel MA, Hoelzer BC, Aiyer R, Caraway D. Preoperative hemoglobin A1c and perioperative blood glucose in patients with diabetes mellitus undergoing spinal cord stimulation surgery: a literature review of surgical site infection risk. Pain Pract. 2022:76. [DOI] [PubMed] [Google Scholar]
- 45.Pennington Z, Lubelski D, Westbroek EM, Ahmed AK, Passias PG, Sciubba DM. Persistent Postoperative Hyperglycemia as a Risk Factor for Operative Treatment of Deep Wound Infection After Spine Surgery. Neurosurgery. 2020;87(2):211–219. [DOI] [PubMed] [Google Scholar]
- 46.Liu JM, Deng HL, Chen XY, et al. Risk Factors for Surgical Site Infection After Posterior Lumbar Spinal Surgery. Spine. 2018;43(10):732–737. [DOI] [PubMed] [Google Scholar]