Abstract
Epidemiologists face a unique challenge in measuring risk relationships involving time-varying exposures in early pregnancy. Each week in early pregnancy is distinct in its contribution to fetal development, and this period is commonly characterized by shifts in maternal behavior and, consequently, exposures. In this simulation study, we used alcohol as an example of an exposure that often changes during early pregnancy and miscarriage as an outcome affected by early exposures. Data on alcohol consumption patterns from more than 5,000 women in the Right From the Start cohort study (United States, 2000–2012) informed measures of the prevalence of alcohol exposure, the distribution of gestational age at cessation of alcohol use, and the likelihood of miscarriage by week of gestation. We then compared the bias and precision of effect estimates and statistical power from 5 different modeling approaches in distinct simulated relationships. We demonstrate how the accuracy and precision of effect estimates depended on alignment between model assumptions and the underlying simulated relationship. Approaches that incorporated data about patterns of exposure were more powerful and less biased than simpler models when risk depended on timing or duration of exposure. To uncover risk relationships in early pregnancy, it is critical to carefully define the role of exposure timing in the underlying causal hypothesis.
Keywords: alcohol consumption, bias, data analysis, maternal exposure, pregnancy, spontaneous abortion, statistical models
Selecting a statistical model that accurately specifies the role of exposure timing is critical for quantifying risk relationships involving time-varying exposures. However, the temporal pattern of exposure is often overlooked or simplified in analyses, potentially obscuring true associations. The importance of accounting for temporal pattern of exposure is amplified when studying pregnancy, since exposures occur in the context of a specific developmental timeline (1).
Although 1 in 5 pregnancies ends in loss (2), known modifiable risk factors for miscarriage are particularly scarce. Limitations in the operationalization and methods used for modeling risk relationships for time-varying behaviors in early pregnancy probably contribute to the challenge of identifying risk factors for pregnancy loss. Many women alter their health behaviors at the time of a positive pregnancy test, which occurs across a range of gestational ages (3–5). Gestational age at pregnancy recognition is nonrandom and is linked to maternal characteristics that also inform miscarriage risk (6). Further, a pregnancy’s susceptibility to exposures evolves throughout early gestation. Still, many studies of miscarriage operationalize time-varying exposures as the woman’s status after pregnancy recognition or in a way that neglects gestational age at exposure or duration of exposure. Use of more sophisticated methods to account for the time-varying aspect of modifiable behaviors may uncover determinants of miscarriage that were previously undetected.
In this study, we compared 5 approaches for modeling the association between a time-varying exposure and miscarriage in data sets simulated to reflect different hypothetical mechanisms by which exposure could confer risk. We used alcohol consumption as an example of a behavior that often changes during the first trimester. We tested model performance in data sets simulated such that miscarriage risk related to any exposure, cumulative exposure, exposure during the preceding week, or exposure in a specific week of gestation. Since the most appropriate modeling approach depends on assumptions about how exposure timing dictates risk, our primary aim was to assess how assumptions implicit in different models affect the ability to detect and measure an effect in distinct temporal relationships.
METHODS
Empirical data
Right From the Start is a community-based, prospective cohort study of women from North Carolina, Tennessee, and Texas who were pregnant between 2000 and 2012 (7). To be eligible, women had to be at least 18 years of age, English- or Spanish-speaking, and not using reproductive technologies to conceive. In our simulation study, we used observations from 5,424 pregnancies to inform assignment of outcome timing, prevalence of alcohol use in early and late pregnancy, and gestational age at change in alcohol consumption.
With institutional review board approval, Right From the Start used several methods to optimize recruitment of women in early gestation who were representative of the general obstetrics population (for participant characteristics, see Web Table 1, available at https://doi.org/10.1093/aje/kwad021). The study was advertised through direct mailing, e-mail messages, bus advertisements, flyers in the community, and pregnancy test coupons in pharmacies. Private and public prenatal care providers distributed information about the study to patients. Women were required to be less than 12 weeks pregnant to enroll, and women planning a pregnancy could enter the study before fully enrolling at the time of a positive pregnancy test. Informed consent was obtained at enrollment. In this sample, median gestational age at enrollment was 47 days (interquartile range, 38–58 days), and 25.8% of participants (1,401/5,424) entered the study prior to conceiving.
At a first-trimester interview, participants were asked about current alcohol consumption and whether a change in alcohol use had occurred during the past 4 months (see Web Appendix 1 for questionnaire). If a participant reported a change in alcohol use, she was asked about the timing of the change and her alcohol consumption prior to the change. If a participant had already experienced a miscarriage prior to interview, she received an interview with modified language that acknowledge the pregnancy had ended and asked about behavior prior to the loss. Alcohol use near conception and in early gestation was common (>50%) in both women with intended pregnancies and women with unintended pregnancies, and 91% of participants who used alcohol modified their behavior during the first trimester (median gestational age at change, 30 days; interquartile range, 21–36 days) (3). Similarly, alcohol consumption was common in both women who enrolled prior to conception and those who enrolled after conceiving (47% and 50%, respectively). For women who reported a change in alcohol exposure during pregnancy, over half reported the timing of the change as being within 3 days of the first positive pregnancy test. Information on pregnancy outcome was obtained through maternal self-report and validated by medical or vital records.
Simulation parameters
We conducted a series of simulation studies to investigate the performance of 5 regression approaches for quantifying the effect of a time-varying exposure following one of the 5 risk relationships described below. Each scenario was replicated 1,000 times in data sets of 1,500 individuals.
In simulation studies, we assumed that 55% of subjects were exposed at baseline (t = 0). Among those exposed at baseline, 6% continued exposure through 140 days (t = 140). The distribution of timing of alcohol cessation was based on observations from Right From the Start. We used binomial distributions to assign exposure status. Exposure status only changed from exposed to unexposed, since we did not observe any instances of participants who initiated alcohol use during pregnancy.
Simulation parameters were designed so the expected proportion of pregnancies to end in miscarriage in the population was 12% for all scenarios to reflect the outcome prevalence observed in Right From the Start. The distribution of outcome timing for pregnancies ending in miscarriage reflected the distribution of gestational age at loss observed in Right From the Start. Subjects without pregnancies ending in miscarriage were censored at 140 days’ gestation. Pregnancy outcome was assigned using conditional probabilities to reflect each of the 5 relationships described below.
Relationships modeled
We used alcohol consumption as an example of a time-varying exposure in pregnancy that may relate to pregnancy loss. We designed 5 hypothetical relationships between exposure and miscarriage risk in our simulated data sets based on plausible mechanisms by which alcohol may confer risk (Table 1). These relationships describe 5 “truths” generated in exposure-outcome associations in different sets of simulated data. While keeping 1) the proportion of participants exposed to alcohol at pregnancy onset, 2) the distribution of timing of change in alcohol use, 3) the timing of miscarriage, and 4) the proportion of pregnancies to end in miscarriage consistent in the simulated data sets, we used different probability distributions to assign exposure status conditioned on outcome to generate data that would reflect the intended relationships (see Web Table 2 for simulation parameters).
Table 1.
The 5 Hypothetical Relationships Between Alcohol Consumption During Pregnancy and Risk of Miscarriage Simulated in Data Setsa
| Simulated Relationship | Assumption | Comments |
|---|---|---|
| Relationship 1 | Alcohol does not increase risk of miscarriage. | Biologically plausible if alcohol exposure does not threaten normal pregnancy development. Allows for assessment of model performance under the null. |
| Relationship 2 | Any alcohol exposure increases risk of miscarriage independent of timing or duration of use. | This mechanism does not seem biologically likely, but it is the implicit assumption most frequently reflected in the literature when exposure is modeled as a dichotomous variable. |
| Relationship 3 | Exposure in a critical window increases risk (week 5 selected as critical window in simulated data). | Biologically plausible, since developmental windows exist when a pregnancy is particularly vulnerable to insult (1, 30). We know that alcohol exposure can increase oxidative stress and that oxidative stress from exposure incurred at 5–8 weeks’ gestation (when normal pregnancy develops in an anaerobic state) may be more detrimental than alcohol exposure after maternal-fetal circulation has been fully established (31). |
| Relationship 4 | Cumulative duration of exposure to alcohol increases risk of miscarriage in a dose-response fashion. | Biologically plausible, since alcoholic beverages contain congeners that may accumulate in developing placental tissue and be toxic in pregnancy development (32). |
| Relationship 5 | Alcohol increases risk of miscarriage during the week following exposure. | Biologically plausible, since alcohol can increase oxidative stress, alter placental profusion, and reduce retinoic acid signaling in a transient manner (33). |
a The simulations used data on alcohol consumption patterns from the Right From the Start cohort study, United States, 2000–2012.
Modeling approaches
We selected the 5 approaches below as examples of statistical models an analyst might use for estimating the association between an exposure and an outcome when the true nature of the underlying relationship is unknown. Selection of models was informed by a panel of experts comprised of 2 epidemiologists, 2 biostatisticians, and 2 translational scientists independent from the study. The models were chosen with consideration of different ways in which timing of exposure may relate to pregnancy outcome (approaches 2–5), versus what is most common in the literature (approach 1). Each approach was used to estimate effects in sets of data sets simulated for the 5 relationships described in Table 1, with the goal of understanding how the alignment between the truth and model assumptions affects the bias and error of estimates.
Approach 1: simple Cox proportional hazards model.
In approach 1, exposure enters the model as a dichotomous variable (exposed/not exposed) without incorporating information about the timing of exposure, where X1 is constant and 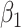 is the log hazard attributable to any exposure during pregnancy.
is the log hazard attributable to any exposure during pregnancy.
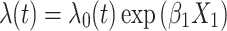 |
Approach 2: Cox proportional hazards model with a lag term.
The approach 2 model incorporates information about timing of exposure cessation, where Xt is exposure status at time t and  is the log hazard of exposure status at t − x. In our simulation, we set x to 7 days to indicate that exposure anytime in the past week could influence hazard at time t.
is the log hazard of exposure status at t − x. In our simulation, we set x to 7 days to indicate that exposure anytime in the past week could influence hazard at time t.
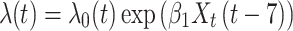 |
Approach 3: sequential logistic model.
Approach 3 quantifies the risk associated with exposure during each week of gestation in separate models, where 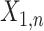 is exposure status in week n and
is exposure status in week n and 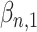 is the log odds of miscarriage given alcohol exposure in week n. Individuals who had not had an event by the first day of week n were included in the equation for week n.
is the log odds of miscarriage given alcohol exposure in week n. Individuals who had not had an event by the first day of week n were included in the equation for week n.
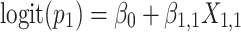 |
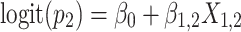 |
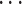 |
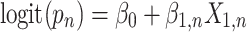 |
Approach 4: Poisson regression with time interaction.
In the approach 4 model, time t in number of days’ gestation is modeled using a fractional polynomial determined by the simulated data and t′ and t″ are the first and second fractional polynomial terms, respectively. Xt denotes exposure status at time t. Terms for the interaction between exposure and time allow the effect of alcohol exposure to be time-dependent in this model.
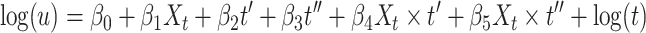 |
Approach 5: cumulative Cox regression.
In the approach 5 model, Xt(t) denotes the cumulative number of days in which a participant was exposed to alcohol at time t and 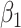 represents the incremental risk associated with each additional day of exposure.
represents the incremental risk associated with each additional day of exposure.
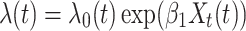 |
In the survival model approaches (1, 2, 4, and 5), women accrue time in the model until pregnancy loss at a particular gestational age or 140 days (20 weeks), whichever comes first.
Performance measures
We evaluated the performance of the 5 modeling approaches under each simulated relationship (see Web Appendix 2 for simulation software code). For each iteration, we collected data on the point estimate, standard error, and significance of effect estimates resulting from each approach (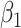 for approaches 1 and 2,
for approaches 1 and 2, 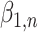 for approach 3, and a linear combination of
for approach 3, and a linear combination of 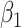 ,
, 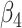 , and
, and 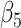 for approach 4). For modeling approaches that allowed effect estimates to vary with time (approaches 2 and 4), estimates were stored for the first day of weeks 4–8. We report the mean value and bias of simulated log effect estimates and the root mean square error of the effect estimate, calculated as the square root of the average of squared standard errors. We report coverage as the proportion of simulations in which the 95% confidence intervals for the effect estimate included the true effect and statistical power as the proportion of confidence intervals not including the null value. Analyses were performed in Stata, version 14.2 (StataCorp LLC, College Station, Texas).
for approach 4). For modeling approaches that allowed effect estimates to vary with time (approaches 2 and 4), estimates were stored for the first day of weeks 4–8. We report the mean value and bias of simulated log effect estimates and the root mean square error of the effect estimate, calculated as the square root of the average of squared standard errors. We report coverage as the proportion of simulations in which the 95% confidence intervals for the effect estimate included the true effect and statistical power as the proportion of confidence intervals not including the null value. Analyses were performed in Stata, version 14.2 (StataCorp LLC, College Station, Texas).
RESULTS
All models performed well under the null simulation setting in terms of bias, nominal confidence interval coverage rate, and type I error (Figure 1) (see Web Table 3 for model performance under the null).
Figure 1.
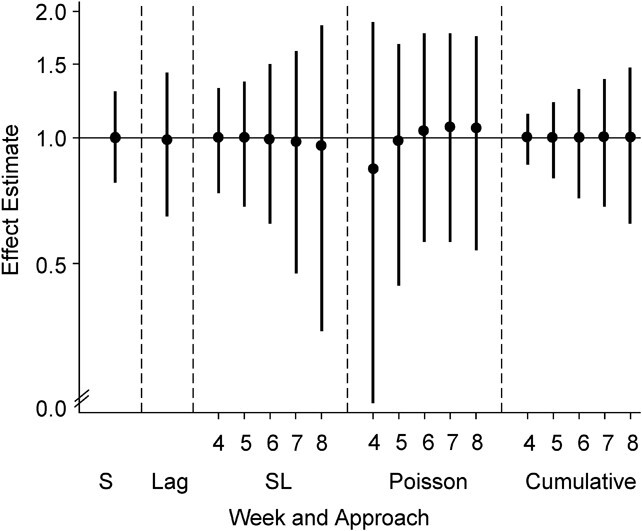
Distribution of effect estimates from 5 modeling approaches in 1,000 simulation trials when exposure is not related to miscarriage risk (relationship 1). Black circles represent the 50th percentile, and the intervals (vertical bars) span the 5th–95th percentile range of simulated effect estimates (hazard ratios from Cox models, odds ratios from logistic regression models, and incidence rate ratios from Poisson models). The solid line indicates the true effect in the data set. The simulations used data on alcohol consumption patterns from the Right From the Start cohort study, United States, 2000–2012. S, simple; SL, sequential.
For relationship 2, where any exposure uniformly increased risk of miscarriage, the simple Cox regression model performed best in terms of bias, coverage, and power (Table 2) (see Web Table 4 for confidence interval coverage). The sequential logistic model and the Poisson regression with time as a fractional polynomial consistently underestimated the strength of association (Table 3). Estimates from the sequential model became attenuated towards the null as the number of exposed individuals to experience the outcome decreased with increasing gestational age, whereas for the Poisson model, estimates calculated in earlier weeks of gestation were more biased (Figure 2A). The Cox model with a lag term underestimated risk associated with exposure, had low nominal coverage (88%), and was underpowered in comparison with the simple Cox model (17% vs. 83%).
Table 2.
Bias of Effect Estimates for Gestational Weeks 4–8 From 5 Modeling Approaches and Model Power Across 4 Simulated Relationships Between Exposure and Outcomea
| Bias | ||||||
|---|---|---|---|---|---|---|
| Scenario | True Effect | Simple | Lag | Sequential | Poisson | Cumulative |
| Bias b | ||||||
| Relationship 2: any exposure increases risk | ||||||
| Week 4 | 0.405 | 0.027 | −0.205 | −0.120 | −0.384 | −0.247 |
| Week 5 | 0.405 | 0.027 | −0.205 | −0.161 | −0.237 | −0.168 |
| Week 6 | 0.405 | 0.027 | −0.205 | −0.198 | −0.167 | −0.089 |
| Week 7 | 0.405 | 0.027 | −0.205 | −0.228 | −0.150 | −0.010 |
| Week 8 | 0.405 | 0.027 | −0.205 | −0.290 | −0.177 | 0.069 |
| Relationship 3: exposure in week 5 increases risk | ||||||
| Week 4 | 0 | 0.165 | 0.231 | 0.292 | 0.095 | 0.164 |
| Week 5 | 0.405 | −0.240 | −0.174 | −0.010 | −0.190 | −0.159 |
| Week 6 | 0 | 0.165 | 0.231 | 0.293 | 0.262 | 0.329 |
| Week 7 | 0 | 0.165 | 0.231 | 0.175 | 0.254 | 0.411 |
| Week 8 | 0 | 0.165 | 0.231 | 0.047 | 0.198 | 0.493 |
| Relationship 4: cumulative exposure is associated with risk | ||||||
| Week 4 | 0.190 | 0.036 | 0.025 | 0.129 | 0.251 | −0.013 |
| Week 5 | 0.286 | −0.060 | −0.071 | 0.041 | −0.153 | −0.020 |
| Week 6 | 0.381 | −0.155 | −0.166 | −0.066 | −0.156 | −0.027 |
| Week 7 | 0.476 | −0.250 | −0.261 | −0.242 | −0.231 | −0.033 |
| Week 8 | 0.572 | −0.346 | −0.357 | −0.489 | −0.365 | −0.040 |
| Relationship 5: exposure increases risk during the following week | ||||||
| Week 4 | 0.405 | −0.282 | −0.034 | −0.222 | −0.153 | −0.294 |
| Week 5 | 0.405 | −0.282 | −0.034 | −0.212 | −0.046 | −0.239 |
| Week 6 | 0.405 | −0.282 | −0.034 | −0.234 | −0.035 | −0.183 |
| Week 7 | 0.405 | −0.282 | −0.034 | −0.326 | −0.073 | −0.128 |
| Week 8 | 0.405 | −0.282 | −0.034 | −0.468 | −0.184 | −0.072 |
| Power c | ||||||
| Relationship 2: any exposure increases risk | ||||||
| Week 4 | 0.825 | 0.172 | 0.409 | 0.030 | 0.544 | |
| Week 5 | 0.825 | 0.172 | 0.258 | 0.089 | 0.544 | |
| Week 6 | 0.825 | 0.172 | 0.174 | 0.151 | 0.544 | |
| Week 7 | 0.825 | 0.172 | 0.106 | 0.158 | 0.544 | |
| Week 8 | 0.825 | 0.172 | 0.080 | 0.136 | 0.544 | |
| Relationship 3: exposure in week 5 increases risk | ||||||
| Week 4 | 0.182 | 0.236 | 0.437 | 0.057 | 0.558 | |
| Week 5 | 0.182 | 0.236 | 0.572 | 0.131 | 0.558 | |
| Week 6 | 0.182 | 0.236 | 0.281 | 0.181 | 0.558 | |
| Week 7 | 0.182 | 0.236 | 0.118 | 0.172 | 0.558 | |
| Week 8 | 0.182 | 0.236 | 0.051 | 0.119 | 0.558 | |
| Relationship 4: cumulative exposure is associated with risk | ||||||
| Week 4 | 0.312 | 0.195 | 0.488 | 0.038 | 0.615 | |
| Week 5 | 0.312 | 0.195 | 0.422 | 0.091 | 0.615 | |
| Week 6 | 0.312 | 0.195 | 0.305 | 0.151 | 0.615 | |
| Week 7 | 0.312 | 0.195 | 0.165 | 0.160 | 0.615 | |
| Week 8 | 0.312 | 0.195 | 0.091 | 0.133 | 0.615 | |
| Relationship 5: exposure increases risk during the following week | ||||||
| Week 4 | 0.125 | 0.461 | 0.209 | 0.098 | 0.314 | |
| Week 5 | 0.125 | 0.461 | 0.174 | 0.252 | 0.314 | |
| Week 6 | 0.125 | 0.461 | 0.128 | 0.312 | 0.314 | |
| Week 7 | 0.125 | 0.461 | 0.065 | 0.312 | 0.314 | |
| Week 8 | 0.125 | 0.461 | 0.047 | 0.135 | 0.314 | |
a The simulations used data on alcohol consumption patterns from the Right From the Start cohort study, United States, 2000–2012.
b Mean ln(effect estimate) − ln(effect estimate).
c Proportion of 95% confidence intervals including the null value.
Table 3.
Mean Effect and Root Mean Square Error of Effect Estimates for Gestational Weeks 4–8 From 5 Modeling Approaches Across 4 Simulated Relationships Between Exposure and Outcomea
| Mean Effectb (RMSEc) | ||||||
|---|---|---|---|---|---|---|
| Scenario | TrueEffect | Simple | Lag | Sequential | Poisson | Cumulative |
| Relationship 2: any exposure increases risk | ||||||
| Week 4 | 0.405 | 0.432 (0.154) | 0.201 (0.222) | 0.285 (0.166) | 0.022 (0.598) | 0.158 (0.080) |
| Week 5 | 0.405 | 0.432 (0.154) | 0.201 (0.222) | 0.244 (0.189) | 0.168 (0.353) | 0.237 (0.120) |
| Week 6 | 0.405 | 0.432 (0.154) | 0.201 (0.222) | 0.207 (0.243) | 0.239 (0.300) | 0.316 (0.160) |
| Week 7 | 0.405 | 0.432 (0.154) | 0.201 (0.222) | 0.177 (0.340) | 0.255 (0.308) | 0.396 (0.200) |
| Week 8 | 0.405 | 0.432 (0.154) | 0.201 (0.222) | 0.115 (0.486) | 0.228 (0.325) | 0.475 (0.240) |
| Relationship 3: exposure in week 5 increases risk | ||||||
| Week 4 | 0 | 0.165 (0.150) | 0.231 (0.220) | 0.292 (0.166) | 0.095 (0.592) | 0.164 (0.080) |
| Week 5 | 0.405 | 0.165 (0.150) | 0.231 (0.220) | 0.396 (0.185) | 0.216 (0.350) | 0.246 (0.120) |
| Week 6 | 0 | 0.165 (0.150) | 0.231 (0.220) | 0.293 (0.237) | 0.262 (0.300) | 0.329 (0.160) |
| Week 7 | 0 | 0.165 (0.150) | 0.231 (0.220) | 0.175 (0.340) | 0.254 (0.311) | 0.411 (0.200) |
| Week 8 | 0 | 0.165 (0.150) | 0.231 (0.220) | 0.047 (0.500) | 0.198 (0.335) | 0.493 (0.240) |
| Relationship 4: cumulative exposure is associated with risk | ||||||
| Week 4 | 0.190 | 0.226 (0.154) | 0.215 (0.225) | 0.320 (0.168) | −0.060 (0.650) | 0.177 (0.081) |
| Week 5 | 0.286 | 0.226 (0.154) | 0.215 (0.225) | 0.327 (0.190) | 0.133 (0.374) | 0.266 (0.121) |
| Week 6 | 0.381 | 0.226 (0.154) | 0.215 (0.225) | 0.315 (0.239) | 0.225 (0.310) | 0.354 (0.162) |
| Week 7 | 0.476 | 0.226 (0.154) | 0.215 (0.225) | 0.234 (0.337) | 0.245 (0.318) | 0.443 (0.202) |
| Week 8 | 0.572 | 0.226 (0.154) | 0.215 (0.225) | 0.082 (0.501) | 0.206 (0.340) | 0.532 (0.243) |
| Relationship 5: exposure increases risk during the following week | ||||||
| Week 4 | 0.405 | 0.123 (0.152) | 0.371 (0.210) | 0.183 (0.170) | 0.252 (0.519) | 0.111 (0.082) |
| Week 5 | 0.405 | 0.123 (0.152) | 0.371 (0.210) | 0.193 (0.196) | 0.360 (0.320) | 0.167 (0.123) |
| Week 6 | 0.405 | 0.123 (0.152) | 0.371 (0.210) | 0.172 (0.252) | 0.380 (0.290) | 0.222 (0.165) |
| Week 7 | 0.405 | 0.123 (0.152) | 0.371 (0.210) | 0.080 (0.365) | 0.332 (0.303) | 0.278 (0.206) |
| Week 8 | 0.405 | 0.123 (0.152) | 0.371 (0.210) | −0.062 (0.551) | 0.221 (0.339) | 0.333 (0.247) |
Abbreviation: RMSE, root mean square error.
a The simulations used data on alcohol consumption patterns from the Right From the Start cohort study, United States, 2000–2012.
b Natural log of the mean estimated effect.
c The RMSE was calculated as the square root of the average of squared standard errors.
Figure 2.
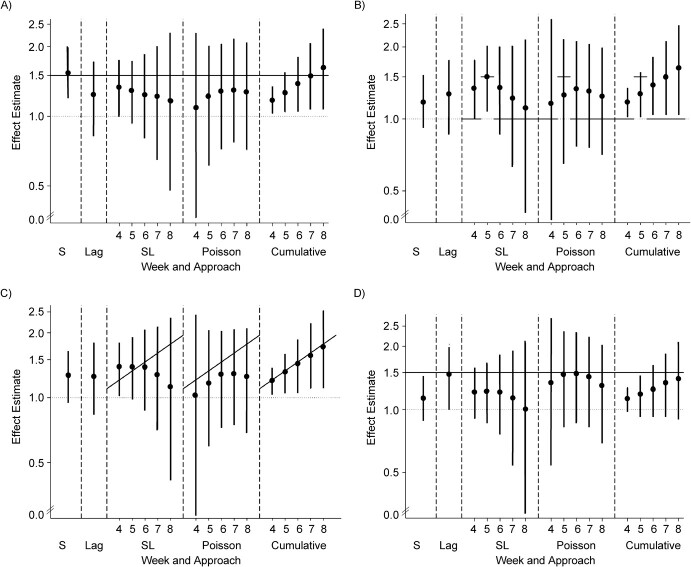
Distribution of effect estimates from 5 modeling approaches in 1,000 simulation trials when A) any exposure incurred during pregnancy increases risk (relationship 2); B) exposure during gestational week 5 increases risk (relationship 3); C) duration of exposure has a dose-response effect on risk (relationship 4); or D) exposure increases risk during the following week (relationship 5). Black circles represent the 50th percentile, and the intervals (vertical bars) span the 5th–95th percentile range of simulated effect estimates (hazard ratios from Cox models, odds ratios from logistic regression models, and incidence rate ratios from Poisson models). The solid line indicates the true effect in the data set. The simulations used data on alcohol consumption patterns from the Right From the Start cohort study, United States, 2000–2012. S, simple; SL, sequential.
In relationship 3, where exposure in gestational week 5 increased risk, the sequential logistic approach was the only model to correctly identify week 5 as the critical exposure window (Figure 2B). At week 5 in the sequential logistic approach, bias was minimal (−0.010) and nominal confidence interval coverage was satisfactory (95%), but power was low (57%). While week 5 was the only window designed to associate with risk, we observed effects in adjacent weeks due to correlation in exposure status between weeks (i.e., the tendency for a woman exposed in week n to also be exposed in weeks n − 1 and n + 1). The Poisson model misspecified weeks 6 and 7 as critical weeks of exposure and had less precise estimates throughout in comparison with the sequential model. The simple Cox model and the Cox model with the lag term could not capture interaction between exposure and gestational age, and both underestimated the magnitude of the association.
For relationship 4, where cumulative duration of exposure was associated with risk, the cumulative Cox modeled performed best. The sequential and Poisson models inappropriately measured a decrease in risk for exposure in later weeks of gestation (Figure 2C; Table 3). In simulations of relationship 5 (exposure increases risk during the following week), the Cox model with a lag term provided the most accurate estimate and other approaches underestimated the true association (Figure 2D; Table 2).
Overall, the simple Cox model performed well when the simulated relationship between exposure and outcome was not time-dependent (relationships 1 and 2). In other scenarios, it tended to underestimate the true association, and it could not detect time-varying relationships if present (relationships 3–5). The Cox model with a lag term had poor nominal confidence interval coverage and power throughout, but it provided the best estimate in relationship 5. Likewise, this approach could not detect or characterize complex interactions between exposure and gestational age. The sequential logistic approach accurately identified a critical window of exposure (relationship 3), but the precision of its estimates suffered across simulation scenarios in later weeks of gestation (>7 weeks), where there were few individuals in the population who both were exposed and went on to experience the outcome. The Poisson regression approach detected exposure × time interactions but did not correctly approximate the shape of the relationship across weeks 4–8 in most scenarios. Parameter estimates were less precise than the sequential logistic model throughout and were underpowered in comparison with other approaches. The cumulative model forced a dose-response effect by week of gestation in all scenarios, but it was the only model to correctly approximate the scenario in which duration of exposure was related to risk (relationship 4).
DISCUSSION
In this simulation based on observations from the Right From the Start pregnancy cohort study, the performance of modeling approaches for measuring the effect of a time-varying exposure depended on the relationship defined in the underlying simulated data set. Conventional methods for estimating risk associated with behaviors in pregnancy often involve a gross simplification of the temporal pattern of exposure. Many studies use self-reported behavior after pregnancy recognition to assign exposure status, even though many women alter their habits after pregnancy detection (3–5). As a result, effects of behaviors occurring early in pregnancy frequently go unmeasured. Leveraging longitudinal data for time-varying exposures captures more information than using simpler methods, but model selection depends on assumptions about how exposure, outcome, and timing interrelate. In our study, the degree to which estimates from a given approach approximated the true effect varied considerably across simulated relationships, underlining the importance of defining beliefs about the mechanism of effect when developing an analytical plan.
We selected alcohol use as an example of a behavior that changes in the first trimester to illustrate how simplification of exposure operationalization may affect the evidence about known risk. During pregnancy, exposures occur in the context of gestational age, and behavioral exposures tend to change near the time of pregnancy recognition. Using the example of alcohol exposure, more than 50% of women used alcohol near conception, regardless of pregnancy intention, in the Right From the Start pregnancy cohort, and 6% reported continued alcohol use through the first trimester. In a review of 19 studies that measured the relationship between alcohol exposure and miscarriage, 45% assessed only alcohol use after pregnancy recognition (8–15). This approach misses information about early-pregnancy alcohol use and misrepresents exposure status for 90% of women who consume alcohol during pregnancy. The decision to use only data about behaviors measured after pregnancy detection assumes that exposures incurred very early in gestation do not influence miscarriage risk. Risk is unlikely to operate in this way biologically, since critical milestones in development occur during the first weeks of gestation, when women who use alcohol tend to be exposed, and duration of alcohol exposure varies between women. In our review, 11 studies assessed change in alcohol use during the first trimester (16–26). In most of the studies, the investigators included alcohol exposure before and after pregnancy in separate models or operationalized alcohol use as an across-pregnancy average amount, even though exposure is ubiquitously heavier and more prevalent in early gestation. Each of these approaches neglects temporal patterns of exposure.
If an investigator resolves to collect data about exposure timing, the question remains how to incorporate that information into analysis, especially when there may be several valid hypotheses concerning how timing of exposure may affect risk. We designed this simulation study to assess how assumptions about the relationship between exposure timing and outcome inherent to different modeling approaches influence estimates of association. The prevalence of exposure and the gestational age distributions for alcohol cessation and miscarriage were the same in all simulated data sets. Despite these constants, altering how risk of the outcome was related to timing of exposure in the simulated data sets drastically altered the performance of the modeling approaches. For example, when exposure status was set to increase miscarriage risk in the following week, the Cox model with a lag term performed best (modeling approach 2). However, this approach cannot accurately specify any scenarios in which risk varies across gestational ages (i.e., when exposure in a given gestational week or duration of exposure determines risk). Similarly, when exposure in week 5 of gestation was set to drive risk, the sequential logistic modeling approach (approach 3) accurately identified the critical window of exposure and the effect magnitude. Yet when risk was not tied to exposure in a specific week of gestation, this approach systematically underestimated risk. With longer durations of pregnancy, women had the opportunity to have longer durations of alcohol exposure, and approach 5 was the only model that incorporated information about total duration of exposure. This model performed well when cumulative exposure was set to determine risk, but it forced an artificially linear relationship between risk and duration of exposure when other risk relationships were specified, which introduced bias. Thus, model performance heavily depended on the relationship in the simulated data set. These findings emphasize that arriving at accurate estimates requires correctly specified assumptions about the underlying causal relationship. We used the lessons learned from this simulation to inform the analysis of the relationship between alcohol and miscarriage in the primary data (27).
Considerations
Observations from more than 5,000 pregnancies informed assignment of exposure prevalence and cessation timing in the simulation. In our study, exposure was common at the onset of pregnancy (55%). The proportion exposed and timing of exposure cessation was constant for all simulated data sets. We did not explore how varying levels of baseline exposure or different distributions in the timing of exposure cessation affect model performance. These simulations did not seek to represent behaviors that stop and start over the course of early pregnancy or fluctuations in exposure dose. Instead, our simulated relationships demonstrated the danger of simplifying temporal data for exposure characterized as nonreversible cessation with the understanding that greater granularity in data about exposure characteristics must be met with increasing thoughtfulness concerning underlying mechanisms of risk and intentionality in modeling.
To observe how different relationships between timing of exposure and outcome affect measures of association in isolation, we designed the simulation to be free of other sources of bias that often affect epidemiologic studies. For instance, the exposure-outcome associations were not confounded by other variables, which is rarely true of organic relationships. We also assumed that pregnancies were observed from onset to outcome, which is logistically difficult in reproductive studies. Challenges in determining timing of outcome in pregnancies that end in miscarriage can additionally bias studies of miscarriage and time-varying exposures (28). Additionally, we did not attempt to simulate or quantify other potential sources of bias or error that studies of miscarriage face, including selection bias that results from necessitating enrollment sufficiently early in pregnancy to capture miscarriage events, recall bias or reporting bias resulting from the stigma of exposure, or how the timing of pregnancy detection influences the ability to determine enrollment eligibility and pattern of exposure for modifiable behaviors. While our objective in this paper was to highlight the importance of forming hypotheses about the role of exposure timing, other sources of bias or imprecision also warrant careful attention during study design and analysis.
Conclusion
All models are wrong, but some are useful (29). This simulation study highlights how a model’s usefulness is fettered to its alignment with nuances of the relationship it measures. Epidemiologists must carefully consider the biological mechanism by which an exposure is thought to increase risk to determine which characteristics of the exposure to measure and model. In studies of pregnancy, a greater emphasis should be placed on timing of exposure, since behavioral changes occur in the context of a distinct developmental timeline. Failure to do so could contribute to the accumulation of falsely negative studies, leading to inappropriate confidence in an exposure’s safety. Careful consideration of how to best model exposure timing in studies of pregnancy health may unmask risk factors and provide insight into which characteristics of exposure dictate risk.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Vanderbilt Epidemiology Center, Institute of Medicine and Public Health, Vanderbilt University Medical Center, Nashville, Tennessee, United States (Alexandra C. Sundermann, Digna R. Velez Edwards, Katherine E. Hartmann); Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, United States (James C. Slaughter); Department of Obstetrics and Gynecology, Vanderbilt University Medical Center, Nashville, Tennessee, United States (Digna R. Velez Edwards, Katherine E. Hartmann); and Department of Bioinformatics, Vanderbilt University Medical Center, Nashville, Tennessee, United States (Digna R. Velez Edwards).
The results reported herein correspond to the specific aim of National Institutes of Health grant F30HD094345 awarded to A.C.S. by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). This work was also supported by grants R01HD043883 and R01HD049675 from the NICHD, award 2579 from the American Water Works Association (Denver, Colorado) Water Research Foundation, grant T32GM07347 from the National Institute of General Medical Sciences, and grant UL1TR000445 from the National Center for Advancing Translational Sciences.
Software code for the simulation study is provided in Web Appendix 2.
The views expressed in this article are those of the authors and do not reflect those of the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1. Weinberg CR, Wilcox AJ. Methodological issues in reproductive epidemiology. In: Rothman KJ, Greenland S, Lash TL, eds. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:620–640. [Google Scholar]
- 2. Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–194. [DOI] [PubMed] [Google Scholar]
- 3. Pryor J, Patrick SW, Sundermann AC, et al. Pregnancy intention and maternal alcohol consumption. Obstet Gynecol. 2017;129(4):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tough S, Tofflemire K, Clarke M, et al. Do women change their drinking behaviors while trying to conceive? An opportunity for preconception counseling. Clin Med Res. 2006;4(2):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCormack C, Hutchinson D, Burns L, et al. Prenatal alcohol consumption between conception and recognition of pregnancy. Alcohol Clin Exp Res. 2017;41(2):369–378. [DOI] [PubMed] [Google Scholar]
- 6. Branum AM, Ahrens KA. Trends in timing of pregnancy awareness among US women. Matern Child Health J. 2017;21(4):715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Promislow JH, Makarushka CM, Gorman JR, et al. Recruitment for a community-based study of early pregnancy: the Right From The Start study. Paediatr Perinat Epidemiol. 2004;18(2):143–152. [DOI] [PubMed] [Google Scholar]
- 8. Armstrong BG, McDonald AD, Sloan M. Cigarette, alcohol, and coffee consumption and spontaneous abortion. Am J Public Health. 1992;82(1):85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borges G, Tapia-Conyer R, Lopez-Cervantes M, et al. Alcohol consumption and pregnancy in the Mexican National Addiction Survey. Cad Saude Publica. 1997;13(2):205–211. [DOI] [PubMed] [Google Scholar]
- 10. Feodor Nilsson S, Andersen PK, Strandberg-Larsen K, et al. Risk factors for miscarriage from a prevention perspective: a nationwide follow-up study. BJOG. 2014;121(11):1375–1385. [DOI] [PubMed] [Google Scholar]
- 11. Harlap S, Shiono PH. Alcohol, smoking, and incidence of spontaneous abortions in the first and second trimester. Lancet. 1980;2(8187):173–176. [DOI] [PubMed] [Google Scholar]
- 12. Kesmodel U, Wisborg K, Olsen SF, et al. Moderate alcohol intake in pregnancy and the risk of spontaneous abortion. Alcohol Alcohol. 2002;37(1):87–92. [DOI] [PubMed] [Google Scholar]
- 13. Long MG, Waterson EJ, MacRae KD, et al. Alcohol consumption and the risk of first trimester miscarriage. J Obstet Gynaecol. 1994;14(2):69–70. [Google Scholar]
- 14. Paszkowski M, Czuczwar P, Wozniak S, et al. Selected non-somatic risk factors for pregnancy loss in patients with abnormal early pregnancy. Ann Agric Environ Med. 2016;23(1):153–156. [DOI] [PubMed] [Google Scholar]
- 15. Rasch V. Cigarette, alcohol, and caffeine consumption: risk factors for spontaneous abortion. Acta Obstet Gynecol Scand. 2003;82(2):182–188. [DOI] [PubMed] [Google Scholar]
- 16. Buck Louis GM, Sapra KJ, Schisterman EF, et al. Lifestyle and pregnancy loss in a contemporary cohort of women recruited before conception: the LIFE study. Fertil Steril. 2016;106(1):180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyles SH, Ness RB, Grisso JA, et al. Life event stress and the association with spontaneous abortion in gravid women at an urban emergency department. Health Psychol. 2000;19(6):510–514. [DOI] [PubMed] [Google Scholar]
- 18. Cavallo F, Russo R, Zotti C, et al. Moderate alcohol consumption and spontaneous abortion. Alcohol Alcohol. 1995;30(2):195–201. [PubMed] [Google Scholar]
- 19. Chiodo LM, Bailey BA, Sokol RJ, et al. Recognized spontaneous abortion in mid-pregnancy and patterns of pregnancy alcohol use. Alcohol. 2012;46(3):261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Halmesmaki E, Valimaki M, Roine R, et al. Maternal and paternal alcohol consumption and miscarriage. Br J Obstet Gynaecol. 1989;96(2):188–191. [DOI] [PubMed] [Google Scholar]
- 21. Han JY, Choi JS, Ahn HK, et al. Foetal and neonatal outcomes in women reporting ingestion of low or very low alcohol intake during pregnancy. J Matern Fetal Neonatal Med. 2012;25(11):2186–2189. [DOI] [PubMed] [Google Scholar]
- 22. Kline J, Levin B, Stein Z, et al. Epidemiologic detection of low dose effects on the developing fetus. Environ Health Perspect. 1981;42:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maconochie N, Doyle P, Prior S, et al. Risk factors for first trimester miscarriage—results from a UK-population-based case–control study. BJOG. 2007;114(2):170–186. [DOI] [PubMed] [Google Scholar]
- 24. Parazzini F, Tozzi L, Chatenoud L, et al. Alcohol and risk of spontaneous abortion. Hum Reprod. 1994;9(10):1950–1953. [DOI] [PubMed] [Google Scholar]
- 25. Windham GC, Fenster L, Swan SH. Moderate maternal and paternal alcohol consumption and the risk of spontaneous abortion. Epidemiology. 1992;3(4):364–370. [DOI] [PubMed] [Google Scholar]
- 26. Windham GC, Von Behren J, Fenster L, et al. Moderate maternal alcohol consumption and risk of spontaneous abortion. Epidemiology. 1997;8(5):509–514. [DOI] [PubMed] [Google Scholar]
- 27. Sundermann AC, Velez Edwards DR, Slaughter JC, et al. Week-by-week alcohol consumption in early pregnancy and spontaneous abortion risk: a prospective cohort study. Am J Obstet Gynecol. 2021;224(1):97.e91–97.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sundermann AC, Mukherjee S, Wu P, et al. Gestational age at arrest of development: an alternative approach for assigning time at risk in studies of time-varying exposures and miscarriage. Am J Epidemiol. 2019;188(3):570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Box GEP. Science and statistics. J Am Stat Assoc. 1976;71(356):791–799. [Google Scholar]
- 30. Moore K, Persaud TVN, Torchia M. The Developing Human: Clinically Oriented Embryology. 10th ed. Philadelphia, PA: Elsevier B.V.; 2015. [Google Scholar]
- 31. Kay HH, Grindle KM, Magness RR. Ethanol exposure induces oxidative stress and impairs nitric oxide availability in the human placental villi: a possible mechanism of toxicity. Am J Obstet Gynecol. 2000;182(3):682–688. [DOI] [PubMed] [Google Scholar]
- 32. Avalos LA, Roberts SC, Kaskutas LA, et al. Volume and type of alcohol during early pregnancy and the risk of miscarriage. Subst Use Misuse. 2014;49(11):1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med. 2005;230(6):394–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.