Abstract
Cytokine release syndrome (CRS) is a severe and life‐threatening toxicity typically reported in chimeric antigen receptor T cell therapy and is rarely reported in immune checkpoint inhibitor (ICI) therapy. This study reports the case of a 75‐year‐old Japanese woman who received nivolumab plus ipilimumab therapy for the postoperative recurrence of non‐small cell lung cancer. She was admitted to our hospital with fever, hypotension, hepatic disorder, and thrombocytopenia. We observed slight skin rashes on her neck on admission, which spread rapidly across her body within a few days. We diagnosed CRS complicated by severe rashes. CRS symptoms were resolved with corticosteroid therapy, and did not recur thereafter. CRS is a rare, but important, immune‐related adverse event associated with ICI therapy.
Keywords: cytokine release syndrome, immune checkpoint inhibitors, immune‐related adverse events
ICI‐induced CRS is a rare adverse event, however, ICI combination therapy induced CRS in this case. CRS with severe dermatological disorders improved by steroid therapy.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) are important agents in cancer treatment. Anti‐programmed cell death 1 (PD‐1) plus anticytotoxic T lymphocyte‐associated antigen 4 (CTLA‐4) combination therapy can be performed in lung cancer treatment. 1 ICIs can induce various immune‐related adverse events (irAEs) in multiple organs and occasionally become life‐threatening. Furthermore, anti‐PD‐1 and anti‐CTLA‐4 combination therapy increases the risk of irAEs compared to anti‐PD‐1 monotherapy. 2 , 3
Cytokine release syndrome (CRS) is a cytokine‐associated toxicity typically reported in T cell‐engaging immune therapies, such as chimeric antigen receptor T cell (CAR‐T) therapy. 4 , 5 It is a systemic inflammatory disease that presents with various symptoms including fever, fatigue, nausea, vomiting, rash, arthralgia, and myalgia. 6 Hypotension and hypoxemia can be fatal complications in severe cases. ICI‐induced CRS has rarely been described in case reports.
Herein, we report a case of CRS complicated with severe rashes during nivolumab plus ipilimumab therapy for the postoperative recurrence of non‐small cell lung cancer (NSCLC).
CASE REPORT
A 75‐year‐old Japanese woman who had no history of smoking visited our hospital in September 2020 with an abnormal shadow in the chest. Chest computed tomography (CT) demonstrated a mass in the left lower lobe of the lung without lymphadenopathy. Surgical lung resection was performed because of a strong suspicion of lung cancer, and the patient was diagnosed with stage IIB lung adenocarcinoma. She received four cycles of postoperative adjuvant chemotherapy with cisplatin and vinorelbine.
In January 2022, new pulmonary nodules and mediastinal lymphadenopathy were identified on a follow‐up chest CT. Fluorodeoxyglucose (FDG)‐positron emission tomography (PET)/CT revealed newly emerging lesions with high FDG uptake; therefore, the patient was diagnosed with recurrence. Tumor cell programmed death‐ligand 1 (PD‐L1) expression was <1% and no driver oncogene was detected using next‐generation sequencing. We introduced nivolumab plus ipilimumab therapy for the postoperative recurrence. The pulmonary and mediastinal lesions responded to combination ICI therapy; however, the patient complained of fever, fatigue, and appetite loss at 9 months after the first infusion of nivolumab plus ipilimumab. She had a fever of 38.5°C and a blood pressure of 95/65 mmHg on arrival at the hospital. Blood tests revealed a marked elevation of transaminase levels, mild renal impairment, high levels of inflammatory markers and thrombocytopenia (Table 1). There were no abnormal liver findings and infectious foci on the abdominal CT. Furthermore, slight skin rashes were observed on her neck. We diagnosed CRS complicated by hypotension and severe liver disorder and administered 250 mg intravenous methylprednisolone. Hypotension improved with hydration and the liver disorder also improved immediately. However, the rash spread across her body and oral mucosal eruptions developed on day 3 of admission (Figure 1). Additionally, the thrombocytopenia worsened, and disseminated intravascular coagulation (DIC) induced by CRS was considered. Skin biopsy revealed keratinocyte necrosis and inflammatory cell infiltration (Figure 2). The patient was diagnosed with severe dermatological disorders with toxic epidermal necrosis (TEN).
TABLE 1.
Laboratory data on admission.
| <Hematology> | <Biochemistry> | ||
|---|---|---|---|
| WBC | 7500/μL | Alb | 3.2 g/dL |
| Neutrophils | 31.6% | BUN | 19.6 mg/dL |
| Lymphocytes | 41.2% | Cre | 1.07 mg/dL |
| Monocytes | 14.6% | T‐Bil | 2.07 mg/dL |
| Eosinophils | 10.8% | AST | 641 IU/L |
| Basophils | 1.8% | ALT | 382 IU/L |
| Hb | 14.5 g/dL | γ‐GTP | 239 IU/L |
| Plt | 9.1 × 104/μL | LDH | 694 IU/L |
| AMY | 35 IU/L | ||
| <Serology> | CPK | 21 IU/L | |
| CRP | 10.61 mg/dL | Na | 132.3 mEq/L |
| PCT | 1.65 pg/mL | K | 4.1 mEq/L |
| IL‐6 | 374 pg/mL | Glu | 129 mg/dL |
Abbreviations: Alb, albumin; AST, aspartate aminotransferase; ALT, alanine transaminase; AMY, amylase; BUN, blood urea nitrogen; CRP, C‐reactive protein; Cre, creatinine; CPK, creatine phosphokinase; Glu, glucose; γ‐GTP, gamma glutamyl transpeptidase; Hb, hemoglobin; IL‐6, interleukin‐6; K, potassium; LDH, lactate dehydrogenase; Na, sodium; Plt, platelets; PCT, procalcitonin; T‐Bil, total bilirubin; WBC, white blood cells.
FIGURE 1.
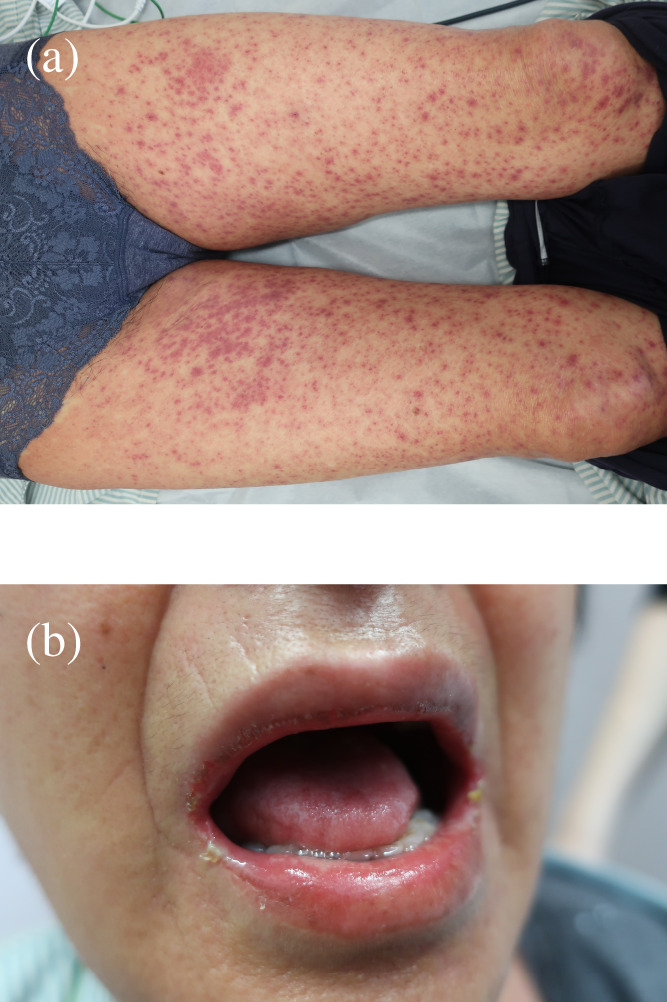
Skin rashes on (a) the lower limbs and (b) the mucous membrane of the lip.
FIGURE 2.
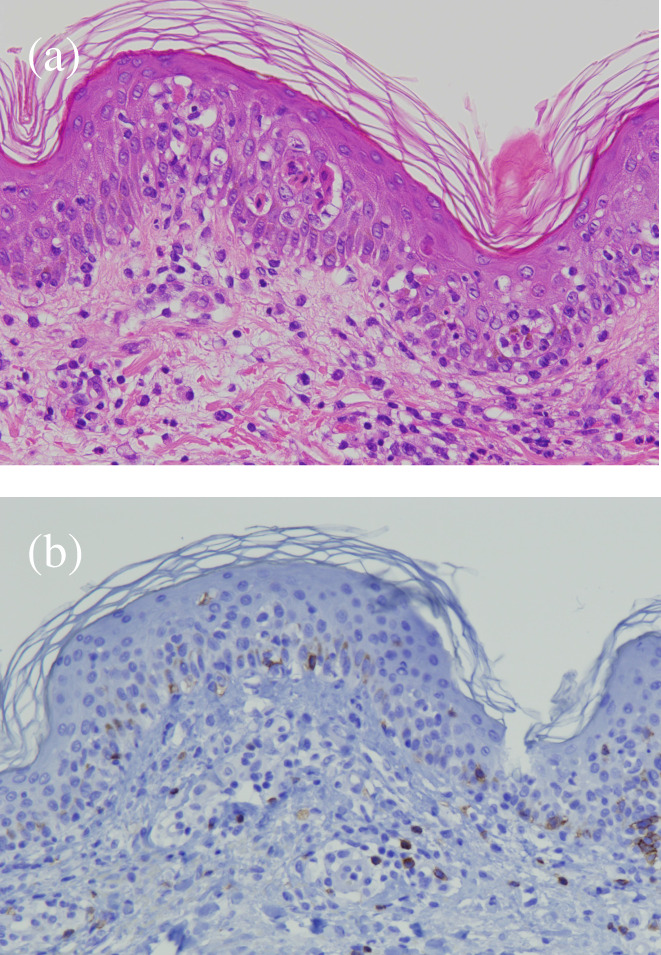
(a) Hematoxylin and eosin staining showed keratinocyte necrosis and inflammatory cell infiltration, with mild lymphocytes infiltration from the epidermal layer to the shallow dermis. (b) Immunohistochemical staining showed CD8‐positive T cells infiltration from the epidermal layer to the shallow dermis.
Steroid pulse therapy (methylprednisolone 1000 mg) and recombinant human soluble thrombomodulin were administered on day 3 of admission. Consequently, the dermatological disorders, DIC, liver disorder, and inflammatory findings gradually improved. Prednisolone 50 mg (1 mg/kg) was administered after steroid pulse therapy, and all symptoms of CRS were improved (Figure 3). The CRS symptoms did not recur thereafter, and the patient was discharged on day 31 after admission.
FIGURE 3.
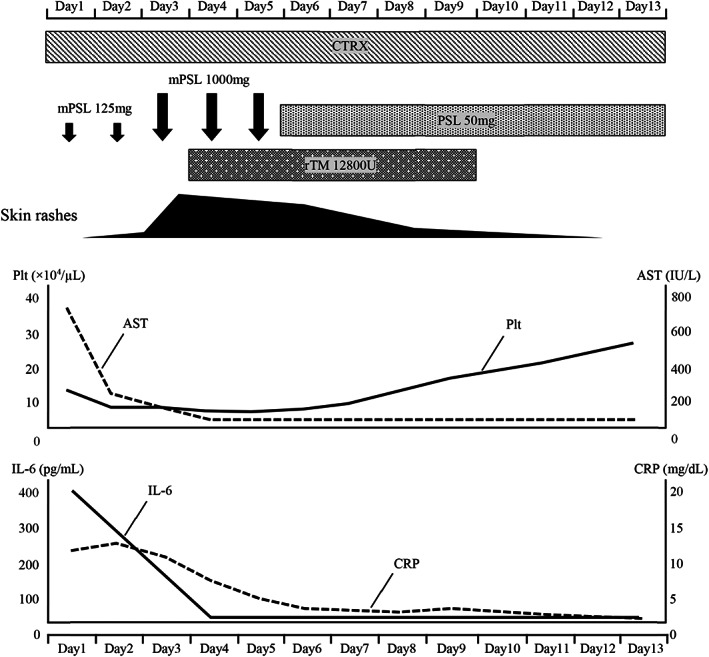
Clinical course of the patient from first day of admission until day 13. AST, aspartate aminotransferase; CRP, C‐reactive protein; CTRX, ceftriaxone; IL‐6, interleukin‐6; mPSL, methylprednisolone; PSL, prednisolone; Plt, platelets; rTM, recombinant thrombomodulin.
DISCUSSION
Herein, we present a case of CRS complicated with TEN induced by ICI combination therapy. ICI‐induced CRS has not been sufficiently recognized because of the rarity of irAEs. Based on an analysis of the World Health Organization global pharmacovigilance database, the incidence of ICI‐induced CRS is ~0.07%. 7 In contrast, Tay et al. reported that the prevalence of ICI‐induced CRS is 4.6% based on their study. 8 An irAE that has been managed as a single organ disorder may be a part of CRS. Asselin described that CRS comprises a subset of infusion reactions associated with the use of immune therapies. 9 We did not recognize CRS as a common irAE.
CRS is a reported ICI‐induced adverse effect mediated by multiple cytokines, including IL‐6, interferon‐γ, tumor necrosis factor‐α, IL‐2, IL‐6, IL‐8, IL‐10, and granulocyte‐macrophage colony‐stimulating factor, among which IL‐6 has been suggested as the key mediator. 6 IL‐6 may be a potential biomarker reflecting disease behavior in CRS. The IL‐6 inhibitor tocilizumab is a known therapeutic agent for CAR‐T cell‐induced CRS. 10 The patient recovered after receiving corticosteroid monotherapy. The addition of tocilizumab may be considered if corticosteroid therapy cannot control the disease.
Stevens‐Johnson syndrome (SJS) and TEN are life‐threatening adverse skin reactions characterized by severe mucosal eruptions, blisters, erosions, and rapidly progressive necrosis. Liver disorder and multiple organ failure can occur as the symptoms of TEN; however, multiple organ failure preceded the skin rashes in this case. Therefore, we decided that these symptoms were not caused by TEN. Yamamoto described that blockade of the PD‐1/PD‐L1 signaling pathway allows autoreactive CD8‐positive T‐cells targeting keratinocytes to proliferate and be activated. 11 Histopathology of the patient was compatible with skin toxicities induced by ICIs. Approximately 30%–40% of patients receiving ICI therapy may develop dermatological disorders; however, SJS/TEN has been reported infrequently. 12 Based on an analysis of clinical trials and the Food and Drug Administration pharmacovigilance database, ICIs are associated with an increased risk of SJS/TEN. 13 The anti‐CTLA‐4 antibodies result in a significantly higher incidence of severe enteritis and skin toxicity than anti‐PD‐1/PD‐L1 antibodies. 14 Therefore, it is necessary to identify SJS/TEN in its early stages to prevent dermatological disorders from becoming severe in patients receiving combination therapy with anti‐ CTLA‐4 antibodies.
In conclusion, this informative case report reminds us of the importance of the identification of rare irAEs. Owing to the possible risk of severe CRS, careful management may be necessary, especially in patients receiving ICI combination therapy.
AUTHOR CONTRIBUTIONS
All authors (1) made substantial contributions to the study concept; (2) drafted the manuscript or revised it critically for important intellectual content; (3) approved the final version of the manuscript to be published; and (4) agreed to be accountable for all aspects of the work.
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest.
Tsutsui T, Hata K, Kawaguchi M, Kobayashi H, Kakizaki Y, Miyashita Y. Cytokine release syndrome complicated with severe rashes induced by nivolumab plus ipilimumab therapy in a patient with non‐small cell lung cancer: A case report. Thorac Cancer. 2023;14(23):2310–2313. 10.1111/1759-7714.15015
REFERENCES
- 1. Hellmann MD, Paz‐Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non‐small‐cell lung cancer. N Engl J Med. 2019;381:2020–31. [DOI] [PubMed] [Google Scholar]
- 2. Xu H, Tan P, Ai J, Zhang S, Zheng X, Liao X, et al. Antitumor activity and treatment‐related toxicity associated with nivolumab plus ipilimumab in advanced malignancies: a systematic review and meta‐analysis. Front Pharmacol. 2019;10:1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamat S, Patel J, Brown BR, Vyas A. Adverse events induced by nivolumab plus ipilimumab vs. nivolumab monotherapy among cancer patients: a systematic review and meta‐analysis. Cancer Invest. 2022;40:777–88. [DOI] [PubMed] [Google Scholar]
- 4. Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller‐Tidow C, et al. Side‐effect management of chimeric antigen receptor (CAR) T‐cell therapy. Ann Oncol. 2021;32:34–48. [DOI] [PubMed] [Google Scholar]
- 5. Brudno JN, Kochenderfer JN. Recent advances in CAR T‐cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ceschi A, Noseda R, Palin K, Verhamme K. Immune checkpoint inhibitor‐related cytokine release syndrome: analysis of WHO global pharmacovigilance database. Front Pharmacol. 2020;11:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tay SH, Toh MMX, Thian YL, Vellayappan BA, Fairhurst AM, Chan YH, et al. Cytokine release syndrome in cancer patients receiving immune checkpoint inhibitors: a case series of 25 patients and review of the literature. Front Immunol. 2022;13:807050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asselin B. Immunology of infusion reactions in the treatment of patients with acute lymphoblastic leukemia. Future Oncol. 2016;12:1609–21. [DOI] [PubMed] [Google Scholar]
- 10. Hay KA. Cytokine release syndrome and neurotoxicity after CD 19 chimeric antigen receptor‐ modified (CAR‐) T cell therapy. Br J Haematol. 2018;183:364–74. [DOI] [PubMed] [Google Scholar]
- 11. Yamamoto T. Skin manifestation induced by immune checkpoint inhibitors. Clin Cosmet Investig Dermatol. 2022;15:829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti‐PD‐1 and anti‐PD‐L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu J, Chen G, He Z, Zheng Y, Gao S, Li J, et al. Stevens‐Johnson syndrome/toxic epidermal necrolysis in patients treated with immune checkpoint inhibitors: a safety analysis of clinical trials and FDA pharmacovigilance database. EClinicalMedicine. 2021;37:100951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Velasco G, Je Y, Bossé D, Awad MM, Ott PA, Moreira RB, et al. Comprehensive meta‐analysis of key immune‐related adverse events from CTLA‐4 and PD‐1/PD‐L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5:312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


