Abstract
Membrane-less organelles (MLOs) formed through liquid-liquid phase separation (LLPS) are associated with numerous important biological functions, but the abnormal phase separation will also dysregulate the physiological processes. Emerging evidence points to the importance of LLPS in human health and diseases. Nevertheless, despite recent advancements, our knowledge of the molecular relationship between LLPS and diseases is frequently incomplete. In this review, we outline our current understanding about how aberrant LLPS affects developmental disorders, tandem repeat disorders, cancers and viral infection. We also examine disease mechanisms driven by aberrant condensates, and highlight potential treatment approaches. This study seeks to expand our understanding of LLPS by providing a valuable new paradigm for understanding phase separation and human disorders, as well as to further translate our current knowledge regarding LLPS into therapeutic discoveries.
Keywords: membrane-less organelles, developmental disorder, tandem repeat disorder, cancer, infectious diseases, abnormal phase separation
Introduction
Membrane-less organelles (MLOs) formed through liquid-liquid phase separation (LLPS), also known as biomolecular condensates, participate in various biological processes and are crucial for human health [ 1, 2] . These MLOs play vital roles in fundamental processes including heterochromatin formation [3], nucleocytoplasmic transport [ 4, 5] , nucleolus formation [6], transcription hub formation [ 7, 8] , innate immunity [9] and resistance to stresses [ 10‒ 12] . Nevertheless, the condensate may be disturbed or inclined to slowly transition into solid-like states due to various factors, including genetic mutations, diminished protein quality control, and impaired cellular transportation mechanisms. The aberrant transition of condensates is pathological and causatively associated with a variety of human diseases [ 13, 14] . This article mainly investigates a few different categories of diseases. In developmental disorders, the three major categories of LLPS-associated pathogenic processes are loss of function, gain of function, and gain of toxicity. Tandem repeat disorders (TRDs) can be classified based on the location of the repeating sequence and the production of aberrant RNA or proteins. In cancer, the aberrant phase separation can affect transcriptional regulation, signal transduction, or protein degradation. Furthermore, virus and host cells have a special connection in that cells use phase separation to resist viral infection, while viruses harness phase separation to complete infection. We will also explore the primary and possible LLPS mechanisms of pathogenesis under different conditions. This study aims to offer a thorough comprehension of the role of LLPS in various human diseases and to discuss potential points for therapeutic intervention.
Developmental Disorders with Aberrant Phase Separation
Developmental disorders are diseases that entail deviations from normal development and often manifest during early childhood, of which, intellectual disability (ID) and autism are two examples [15]. Recent research has shown that liquid-liquid phase separation (LLPS) is implicated in a wide range of developmental and differentiation processes [ 16, 17] . For instance, the protein FXR1 engages in LLPS to retain non-translating mRNA and recruits translational machinery to activate target translation, which is vital in the process of spermiogenesis [18]. Similarly, the phase separation of both transcription factor SOX9 and chromatin modulator CBX2 is essential for testicular development [ 19, 20] . In this part, we will review recent studies on human developmental diseases that are associated with LLPS. These LLPS-associated pathogenic mechanisms can generally be categorized into three main categories: loss of function, gain of function, and gain of toxicity ( Table 1).
Table 1 The pathological mutations of phase separated-protein on developmental disorders
|
Disease |
Associated protein |
Mutation |
Pathological type |
Mechanism |
Reference |
|
Congenital dilated cardiomyopathy |
RBM20 |
Arg636Ser |
Gain of function |
The mutation promotes RBM20 to transfer from the nucleus to cytoplasm and form liquid-like granules, which dock at myofibril Z-discs to disrupt the actin cytoskeleton of cardiomyocytes, further inducing congenital dilated cardiomyopathy. |
|
|
Developmental delay/intellectual disability |
DDX3X |
Leu556Ser |
Gain of toxicity |
The mutation results in DDX3X misfolding and self-aggregation, transferring LLPS to solid-like condensates, which sequestrates the healthy DDX3X and impairs cell viability. |
|
|
Rett syndrome |
MeCP2 |
Arg168Ter, Arg255Ter, Arg270Ter, Arg294Ter, Pro389Ter; Arg133Cys, Thr158Met, Pro225Arg, Arg306Cys, Pro322Leu |
Loss of function |
The mutations interfere with its capacity to form LLPS, which decreases MeCP2 mutant and its cofactor partitioning into heterochromatin condensates, causing altered chromatin architecture and other cellular abnormalities linked to Rett syndrome. |
|
|
Noonan syndrome |
SHP2 |
Asp61Gly, Glu76Ala, Glu76Lys |
Gain of function |
The SHP2 mutations trigger the closed to open conformation transition, which results in an electrostatic contact and promotes the formation of condensates, further recruiting WT SHP2 to encourage MAPK activation. |
|
|
Leopard syndrome |
SHP2 |
Tyr279Cys, Gly464Ala, Thr468Met, Arg498Leu, Gln506Pro |
Gain of function |
The SHP2 mutations trigger the closed to open conformation transition, which results in an electrostatic contact and promotes the formation of condensates, further recruiting WT SHP2 to encourage MAPK activation. |
|
|
Kabuki Syndrome |
MLL4 |
Gln4092Ter |
Loss of function |
The mutation impairs MLL4’s capacity for LLPS and reduces transcriptional condensate formation, which alters the balance between transcriptional and PcG condensates, further changing the nuclear architecture. |
|
|
Autism spectrum disorders |
CTTNBP2 |
Met120Ile, Arg533Ter |
Loss of function |
The CTTNBP2 mutant forms smaller and fewer condensates in dendritic spines than the WT, which impairs social interactions in mutant mice. |
|
|
Ulnar-mammary syndrome |
TBX3 |
Leu143Pro, Tyr149Ser, Ser190Arg, Gln475Ter |
Loss of function |
The mutation affects TBX3’s LLPS capacity to drive appropriate transcriptional regulation of important neuropeptides (TAC3 and KISS1) in KNDy neurons, which further impairs the identity of KNDy neurons and delays the beginning of puberty. |
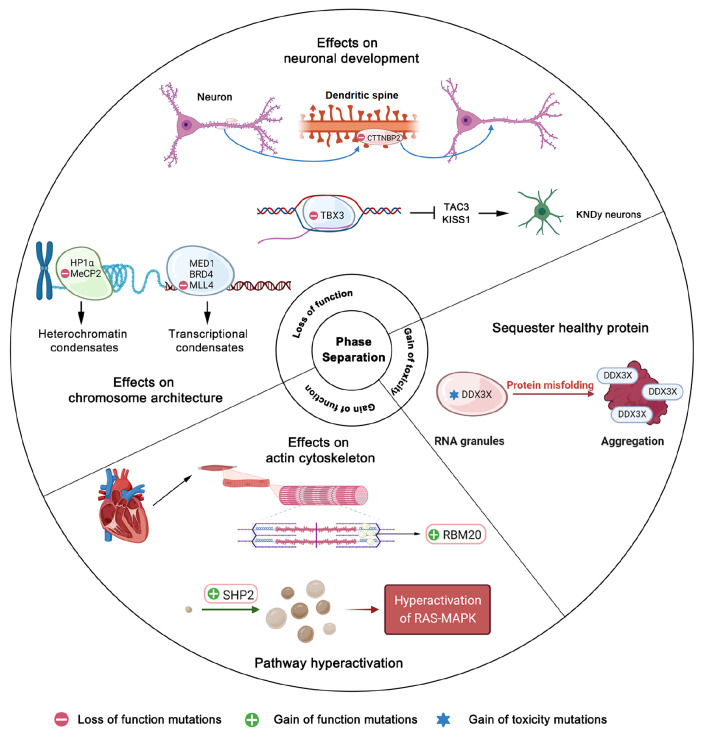
Loss of function
Mutations result in decreased or eliminated protein LLPS capacity, which in turn leads to biological processes that are defective [30]. Deficiencies in LLPS can result in various pathological characteristics, while this section primarily explores the effects of the loss of function on chromosomal architecture and neuronal development. Studies have shown that the disruption of biological condensates can alter the structure of chromosomes, further dysregulate gene expression and lead to diseases. For example, methyl CpG binding protein 2 (MeCP2) is a key component of constitutive heterochromatin, which is critical for chromosomal maintenance and transcriptional silence [31]. Many mutations in MeCP2 can impair its ability to undergo LLPS, which is commonly observed in patients with Rett syndrome, a progressive neurodevelopmental disorder associated with severe mental disability and autism-like symptoms that predominantly affect girls during early childhood [ 32, 23] . Among the pathological mutations, the R168X mutant protein exhibits a significant reduction in its ability to partition into heterochromatin condensates, leading to changes in chromatin architecture ( Figure 1 ). R168X mutant mouse embryonic stem cells also present evidence of Rett syndrome-associated cellular phenotypes [23]. Moreover, studies have reported that a minimal MeCP2 fragment (just containing methyl-DNA binding domain and NCoR-interaction domain) that retains condensate formation capability can partially prevent or reverse Rett syndrome phenotypes when introduced into MeCP2-deficient mice [ 23, 33] . These results further support the close correlation between the LLPS ability of MeCP2 and Rett syndrome. Another example of a protein involved in LLPS and associated with a developmental disorder is mixed lineage leukemia 4 (MLL4). MLL4 serves as a scaffold protein for transcriptional condensate nucleation, allowing for the recruitment of cofactors and activators through liquid-liquid phase separation [34]. Haploinsufficiency of KMT2D, which encodes MLL4, is mostly responsible for Kabuki syndrome, a rare multi-systemic disorder characterized by craniofacial anomalies, intellectual disability, and various organ malformations [35]. The LLPS ability of MLL4 depends on its intrinsically disordered region (IDR), which is conserved in multiple species and is specifically deleted in patients with Kabuki syndrome ( Figure 1) [ 36, 37] . Truncated mutations of MLL4’s IDR have been found to impair its LLPS ability and transcriptional condensates, altering the balance between transcriptional and Polycomb group (PcG) condensates, which are strong mediators of nuclear architecture [38]. This imbalance can lead to changes in the expression levels of genes that regulate chromatin architecture (such as TOP2A and TOP2B) [25], and it has been reported that restoring the expression levels of related genes can rescue the similar pathological features of Kabuki syndrome [ 25, 39] . These results suggest that the haploinsufficiency of MLL4 influences the expression levels of genes related to chromatin architecture, which could be the underlying cause of Kabuki syndrome.
Figure 1 .
Dysregulated phase separation by pathological mutations in developmental disorders
Loss of function (LoF), gain of function (GoF) and gain of toxicity (GoT) are three major categories of phase separation pathogenic mechanisms in developmental disorders. The dysregulation of phase separation can influence chromosome maintenance, neuronal development and normal signaling pathway, which induces craniofacial anomalies, autism, intellectual disability and other developmental abnormalities.
Furthermore, condensate disruption during neuronal development can potentially have an impact on synaptic distribution and neuronal identity. For example, the transcription factor Tbx3 plays a critical role in establishing and maintaining the identity of KNDy neurons, which trigger puberty [29]. Mutations in TBX3 are linked to delayed puberty onset and ulnar-mammary syndrome (UMS), an autosomal dominant disorder that causes developmental issues [ 40, 41] . TBX3 undergoes phase separation to maintain the expressions of TAC3 and KISS1 in humans, which are important for shaping the identity and regulating the activity of KNDy neurons [29]. Pathological mutations in TBX3 disrupt its ability to undergo LLPS and significantly attenuate the transcriptional activation of TAC3 and KISS1 ( Figure 1), further interfering with the onset of puberty in UMS patients [29]. Autism spectrum disorders (ASD) are a group of neurodevelopmental disorders characterized by enduring and impairing social communication [42]. Cortactin-binding protein 2 (CTTNBP2) is a strong candidate for ASD that controls dendritic spine formation and maintenance [26]. Several mutations in CTTNBP2 that are linked to ASD have been shown to affect its LLPS ability. Specifically, the R533* mutation reduce the number of condensates compared to wild-type CTTNBP2 and also impact the synaptic distribution of the R533* mutant protein ( Figure 1) [27]. Mice carrying these mutations exhibit impaired social interaction behaviors similar to those observed in individuals with ASD [ 26, 28] . Interestingly, the synaptic deficits and the social impairment of the mutant mice can be partially improved by stabilizing the condensates of CTTNBP2 and other synaptic proteins [27].
Gain of function
Gain of function denotes the possibility of activating or improving a protein’s capacity for phase separation as a result of mutations. Developmental diseases can be caused by the gain of LLPS, which result in disease-related pathways or reactions being hyperactivated or lead to the sequestration of RNAs, proteins, or both. The non-receptor protein tyrosine phosphatase (PTP) SHP2, encoded by PTPN11, plays a crucial role in normal development by mediating RAS-mitogen-activated protein kinase (MAPK) signaling [43]. Germline heterozygous mutations of PTPN11 are associated with Noonan syndrome (NS) in 50% of cases [44] and Leopard syndrome (NS-ML) in 90% of cases [45]. The activating mutations of PTPN11 are viewed as gain-of-function (GOF) mutations. While the wild-type SHP2 disperses throughout the cell, all SHP2 variants with disease-associated mutations form discrete puncta ( Figure 1) [24]. It was reported that the LLPS-promoting mutations of SHP2 lead to the hyperactivation of RAS-MAPK by increasing the phosphorylation levels of both MEK1/2 and ERK1/2, which may explain the pathogenesis of the NS and NS-ML [ 46, 47] . Another example is the pathogenic R636S mutation of the human RNA-binding motif protein-20 (RBM20), which is associated with dilated cardiomyopathy (DCM) and heart failure [48]. Unlike the wild-type SHP2 that diffuses throughout the cell, RBM20 originally and primarily displays prominent splicing speckles in the nucleus ( Figure 1) [ 49, 21] . In patients with DCM, the RBM20 R636S granules significantly accumulate in the sarcoplasm and distribute on the myofibril Z-discs [21]. The RBM20 R636S granule may result in the sequestration of actin alpha cardiac muscle 1, further disrupting the actin cytoskeleton of cardiomyocytes [ 21, 50] .
Gain of toxicity
Different from the gain of function, protein gain of toxicity represents the mutation of the gene results in the acquisition of the aggregation propensity that can interrupt the normal function of the wild-type protein, which may lead to abnormal aggregation and cell toxicity. Similar to that in neurodegenerative diseases, the aggregation state of associated proteins is correlated with the dysregulation of physiological function [51]. For example, DDX3X is a prominent component of cytosolic RNA granules and participates in all facets of RNA metabolism [52]. DDX3X-related developmental delay/intellectual disability (ID) typically occurs in females and very rarely in males. The L556S missense mutation in DDX3X’s core helicase domain induces its conformational changes, exposing hydrophobic residues to the solvent and resulting in a high propensity for self-aggregation and production of amyloid-like assemblies ( Figure 1) [22]. These aggregates can sequester wild-type DDX3X protein and lead to cell toxicity, because even a 25% reduction in DDX3X levels can strongly deregulate neurogenesis [53]. In addition, more cases of neurodegenerative diseases that are caused by gain-of-toxicity will be shown in the next session.
Phase Separation with Tandem Repeat Disorders
In addition to developmental disorders, phase separation also plays a critical role in tandem repeat disorders (TRDs). TRDs are caused by the abnormal expansions of short tandem repeats (STRs) that are 2‒12 base pairs long DNA repeating tracts and locate in both coding and noncoding regions. More than 50 human disorders are now known to be caused by STR expansion [ 54, 55] . These diseasecausing repeat expansions can range from a few to thousands of repeats and reside within gene 5′ untranslated regions (UTRs), coding exons, 3′ UTRs or introns [56]. STR expansions induce a series of changes in molecular and cellular processes through either loss of function or gain of toxicity mechanisms at the DNA, RNA or protein levels ( Table 2). Loss-of-function mechanism includes expansion within non-coding region that induces transcription silencing, and expansion within coding region generates nonfunctional protein. However, there is little evidence that the mechanism involves phase separation.
Table 2 Phase separation and nucleotide expansion disease
|
Gene |
Pathological type |
Disease |
Repeat sequence |
Mechanism |
Reference |
|
FMR1 |
RNA gain of function |
Fragile X-associated tremor/ataxia syndrome (FXTAS) |
CGG |
RNA-mediated recruitment of proteins attracted by CGG repeats in FMR1 RNA foci. |
|
|
DMPK |
RBP sequestration and RAN translation |
Myotonic dystrophy type 1 (DM1) |
CTG |
The expanded DMPK RNA and MBNL1 are regulators of the formation and turnover of cytoplasmic SGs in DM1. |
|
|
CNBP |
RBP sequestration and RAN translation |
Myotonic dystrophy type 2 (DM2) |
CCTG |
The expanded CNBP RNA and MBNL1 are regulators of the formation and turnover of cytoplasmic SGs in DM1. |
|
|
C9orf72 |
RBP sequestration and RAN translation |
Amyotrophic lateral sclerosis and frontotemporal dementia (ALS and FTD) |
GGGCCC |
Through multivalent base pairing alone, (GGGGCC)5 RNAs can form RNA droplets through phase separation in vitro. |
|
|
HTT |
polyglutamine gain of function |
Huntington disease (HD) |
CAG |
The huntingtin exon1 proteins can form reversible liquid-like assemblies, which are converted to solid-like assemblies when poly Q abnormal expansion. |
|
|
ATXN1 |
polyglutamine gain of function |
spinocerebellar ataxias 1 (SCA1) |
CAG |
The aggregation of ATXN1 with expanded poly Q in neuronal processes can disrupt crucial cargo trafficking and trap other proteins. |
|
|
ATXN2 |
polyglutamine gain of function |
spinocerebellar ataxias 2 (SCA2) |
CAG |
ATXN2 has a C-terminal low-complexity domain (LCD), which regulates ATXN2 liquid-liquid phase separation. Expanded poly Q in ATXN2 alters stress granule dynamics and induces protein aggregation. |
|
|
ATXN3 |
polyglutamine gain of function |
spinocerebellar ataxias 3 (SCA3), also known as Machado-Joseph Disease (MJD) |
CAG |
Poly Q expansion promoted ATXN3 self-assembly into insoluble SDS-resistant aggregates. |
|
|
CACNA1A |
polyglutamine gain of function |
spinocerebellar ataxias 6 (SCA6) |
CAG |
A C-terminal fragment of CACNA1A containing the polyQ tract remains soluble in normal brains, but becomes insoluble mainly in the cytoplasm of human SCA6 Purkinje cells. |
|
|
ATXN7 |
polyglutamine gain of function |
spinocerebellar ataxias 7 (SCA7) |
CAG |
Polyglutamine expansion in ATXN7 causes its misfolding and intranuclear accumulation, leading to changes in interaction proteins, resulting insoluble nuclear inclusions. |
|
|
TBP |
polyglutamine gain of function |
spinocerebellar ataxias 17 (SCA17) |
CAG |
PolyQ expansions within TBP alter its cellular distribution and transcriptional activity. TBP becomes progressively insoluble as polyQ repeat length increases. |
|
|
AR |
polyglutamine gain of function |
Spinal and bulbar muscular atrophy (SBMA) |
CAG |
The expanded polyQ tract severely affects AR transcriptional activity and increases AR aggregation. |
|
|
PABPN1 |
Polyalanine gain of function |
Oculopharyngeal muscular dystrophy (OPMD) |
GCG |
The RNA binding protein PABPN1 promotes the formation of NPAD through its N-terminal disordered domain and RNA-recognized motif by liquid phase separation. |
|
|
HOXD13 |
Polyalanine gain of function |
Type II synpolydactyly (SPD II) |
GCG |
I Synpolydactyly-associated repeat expansions enhance HOXD13 IDR phase separation and alter the transcriptional co-activators in HOXD13-containing condensates. |
|
|
HOXA13 |
Polyalanine gain of function |
Hand-foot-genital syndrome (HFGS) |
GCG |
The HOXA13 IDR facilitated phase separation and HOXA13 IDR droplets exhibited a liquid-like FRAP rate. The HOXA13 IDR containing a short (+7A) HFGS-linked expansion tended to form aggregates with negligible FRAP rate. |
|
|
RUNX2 |
Polyalanine gain of function |
Cleidocranial dysplasia (CCD) |
GCG |
The RUNX2 IDR containing a CCD-associated alanine expansion a (+10A) tended to form solid aggregates, while RUNX2 IDR droplets exhibited a liquid-like FRAP rate. |
|
|
NOTCH2NLC |
Polyglycine gain of function |
Neuronal intranuclear inclusion disease (NIID) |
GGC |
GGC repeats embed into the open reading frame of a small protein (uN2C) and is translated into a uN2C polyglycine-containing protein (uN2CpolyG) in NIID. |
A growing number of studies have discovered that aberrant phase separation is associated with gain-of-toxicity in STRs. For example, at RNA level, the premutation range of CGG repeat expansions in the 5′ UTR of FMR1 gene can generate toxic RNA foci that are regulated by multivalent interactions, leading to intranuclear inclusion formation in fragile X-associated tremor and ataxia syndrome (FXTAS) [76]. At protein level, CAG repeat expansions in the exon of huntingtin ( HTT) gene promote a solid-like state and cause intracellular aggregation of HTT protein in Huntington’s disease (HD) [62]. In this section, we will briefly review the expanded repeat toxicity resulting in aberrant phase separation in TRDs.
Formation of aberrant RNA foci
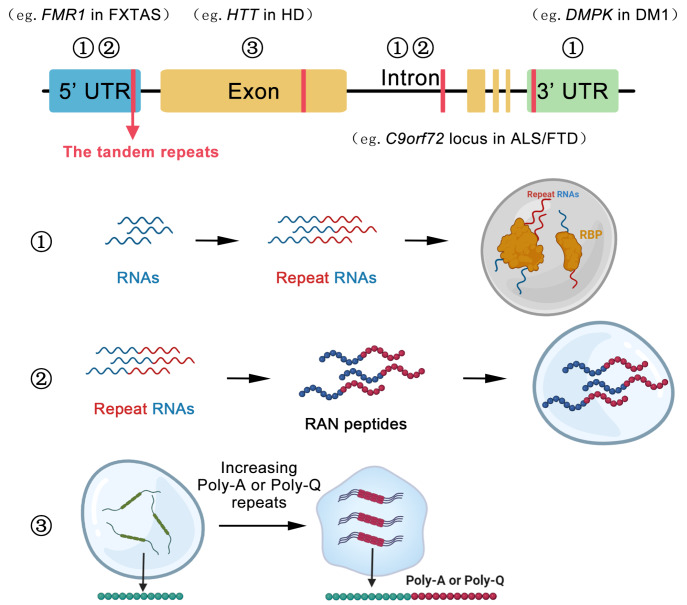
The formation of RNA foci is a classic example of an RNA-mediated gain of toxicity mechanism. This process is intricate and involves the formation of unusual secondary structures by repeat RNA products. Through Watson-Crick and noncanonical base pairing, repeat RNAs can form imperfect hairpin structures and G-quadruplexes. The GGGGCC expansion in the intron of C9orf72 locus is the most frequently known cause of amyotrophic lateral sclerosis and frontotemporal dementia (ALS/FTD) ( Figure 2). GGGGCC expansions have been found to form G-quadruplexes both in vitro and in vivo [ 77‒ 79] . Repeat hairpins and G-quadruplexes are thought to facilitate RNA-RNA interactions. Through multivalent base pairing alone, 47×(CUG) and 5×(GGGGCC) RNAs can form RNA droplets through phase separation in vitro. The longer length of these expanded RNA repeats promotes the formation of gel-like or solid-like droplets [61]. As another example, Myotonic dystrophy type 1 (DM1) is a complex neuromuscular disorder caused by CTG repeat expansions in the 3′ UTR of DM1 Protein Kinas ( DMPK) gene [ 80‒ 82] ( Figure 2). Healthy individuals carry repeat tracts below 37 repeats, whereas DM1 patients usually carry more than 50 repeats. Expanded CUG RNAs likely form highly stable hairpin structures [ 83, 84] .
Figure 2 .
Molecular mechanisms of tandem repeat disorder pathogenesis
(1) The tandem repeats can locate in UTR and intron, the tandem repeat RNAs may recruit RNA-binding proteins and cause the formation of RNA foci, such as the CGG repeats in the 5' UTR of FMR1, CTG repeats in the 3' UTR of DMPK and GGGGCC repeats in the intron of C9orf72 locus, which cause FXTAS, DM1 and ALS/FTD, respectively. (2) The repeat-associated non-AUG (RAN) translation of toxic peptides contributes to the pathogenesis of various TRDs, such as FMRpolyG encoded by FMR1 mRNA containing expanded CGG repeats and the PRn-poly-dipeptide encoded by C9orf27 repeat expansions. (3) Also, the CAG and GCG repeat expansions in the exon result in the formation of poly-Q and poly-A tracts in related proteins, respectively, that promote the formation of solid aggregates and cause neuronal dysfunction. For example, the CAG repeats in the exon of HTT cause the expression of polyglutamine (poly-Q) and cause Huntington disease (HD)
Besides repeat RNAs, RNA-binding proteins (RBPs) attracted by expanded repeat RNAs can also impair the dynamics of condensates and stabilize the formation of RNA foci [56]. In DM1, the expanded DMPK RNA foci in the cell nucleus recruit a multi-functional RBP—muscleblind-like splicing regulator 1 (MBNL1). The expanded DMPK RNA and MBNL1 are regulators of the formation and turnover of cytoplasmic SGs in DM1 [59]. Similarly, myotonic dystrophy type 2 (DM2) is caused by unstable CCTG repeat expansions in the intron of the CHC-type zinc finger nucleic acid binding protein ( CNBP) gene. The expanded CNBP RNAs and multi-functional RBP MBNL1 promote the assembly of ribonucleoprotein (RNP) granules or RNA foci in DM2 [60]. The formation of RNA foci is also the typical hallmark of FXTAS which is a progressive neurodegenerative disorder [76]. FXTAS is caused by the premutation range (55‒200×CGG) repeats of FMR1 [ 85‒ 88] ( Figure 2). One of the main mechanisms to explain the onset and development of FXTAS is the RNA-mediated recruitment of RBPs complex attracted by CGG repeats in FMR1 RNA. The RBPs complex includes DiGeorge syndrome critical region gene 8 (DGCR8), heterogeneous nuclear ribonucleoprotein (hnRNP) A2/B1 and src-associated substrate during mitosis of 68 kDa (Sam68). The RNA foci behave as ribonucleoprotein (RNP) condensates that phase separate in the nucleus, forming large ubiquitylated inclusions [89].
The toxic RNA foci in repeat expansion diseases are involved in the transition from soluble RNA to RNA-protein phase separation. This process is defined by the sum of RNA-RNA, RNA-protein and protein-protein interactions. New discoveries are expected to follow, which will further broaden our understanding of how phase separation is regulated and the detailed mechanisms of RNA toxicity in TRDs.
RAN-translation generates toxic peptides
Moreover, it was found that repeat-associated non-AUG (RAN) translation of toxic peptides contributes to the pathogenesis of various TRDs. RAN translation represents the translation of tandem repeats into peptides consisting of repeating amino acid sequences that do not require AUG initiation [55]. Apart from RNA foci formation, repeat RNAs can recruit translation machinery and produce toxic peptides by RAN-translation [90]. Expanded C9orf72 RNA aberrantly recruits translation machinery and expresses putatively toxic RAN translation products [ 91‒ 93] . Recent studies have shown that PR n-poly-dipeptide encoded by C9orf72 repeat expansions can bind to different low-complexity domain (LCD)-containing proteins, which in turn results in impaired functions of multiple membrane-less organelles [94] ( Figure 2). In FXTAS, another main mechanism is the aggregation of repeat-associated RAN polyglycine peptides [ 57, 58] . FMR1 mRNA containing expanded CGG repeats initiates RAN translation and produces a polyglycine-containing protein, FMRpolyG ( Figure 2). The polyglycine region of FMRpolyG has low-complexity disordered domains with RNA binding ability. FMRpolyG can directly interact with CGG repeat-derived RNAs and undergo the liquid-to-solid transition, leading to FMR polyG aggregates [95]. In DM2, the role of RNA toxicity is well established, in which the RAN-translated peptides polyLPAC and polyQAGR are expressed in various brain regions [96]. Because RAN translation can produce peptides in a variety of reading frames [97], toxic peptides may contribute to dysfunction in various tandem repeat disorders.
Expansion within coding regions generates toxic proteins
When the tandem repeats locate within an exon coding region, the resulting TRDs typically exhibit gain-of-function phenotypes, characterized by abnormal protein aggregation and phase separation [ 98, 99] . In repeat expansion diseases, aggregated proteins play a direct role in pathogenesis.
Polyglutamine tracts-mediated gain of toxicity
At least nine disorders, including Huntington disease (HD), several spinocerebellar ataxias (SCAs), and spinal and bulbar muscular atrophy (SBMA), are caused by CAG repeat expansions in coding sequences that result in the expressions of polyglutamine-containing proteins [100]. As follows, we will introduce several TRDs caused by CAG repeat expansions.
HD is an extensively studied polyglutamine TRD that is characterized by the expansion of a translated CAG repeat located in the N-terminus of the huntingtin (HTT) protein. Wild-type individuals contain 6–34 CAG repeats in the HTT gene, while HD patients contain 36–121 repeats ( Figure 2). The expanded CAG repeats disrupt the normal splicing of the HTT gene, leading to the production of huntingtin exon 1 protein that is encoded by the first exon of HTT gene and contains an abnormally polyQ region. Huntingtin exon 1 protein can form reversible liquid-like assemblies, a process driven by huntingtin’s polyglutamine tract and a proline-rich region. However, the aberrantly expanded polyglutamine promotes the liquid-like to solid-like assemblies with a fibrillar structure in neurons, leading to neuron death, especially neuronal dysfunction in the striatum [62].
Another main type of TRD with tandem repeated polyglutamine tracts in an exon is Spinocerebellar ataxias (SCAs). The clinical hallmark of all SCAs is progressive atrophy of the cerebellum, brainstem, and spinal cord [101]. Most SCAs (including SCA1, 2, 3, 6, 7, and 17) are caused by the expansion of a translated CAG repeat. For instance, spinocerebellar ataxia type 2 (SCA2) is attributed to the abnormal CAG expansion in ATXN2, an RBP which could regulate stress granule assembly and translation [102]. ATXN2 has a C-terminal LCD, which contributes to liquid-liquid phase separation. ATXN2 normally contains 22‒23 CAG repeats on the N-terminus. Intermediate-length (27‒33) CAG repeat expansions in ATXN2 act as risk alleles for ALS [103] and larger expansions (34 or more repeats), trigger protein aggregation, and cause SCA2 [ 64‒ 66] . Various cellular functions and cellular homeostasis can be compromised by the aggregation of polyglutamine disorder proteins in neural processes, which can interfere with important cargo trafficking [63] and trap other proteins [104]. In addition to RNA-binding proteins, transcription factors can also harbor polyglutamine expansion. The CAG repeat expansions in the androgen receptor (AR), a transcription factor that controls the development of the prostate, result in spinal and bulbar muscular atrophy (SBMA), an X-linked, adult-onset neuromuscular illness [105]. The N-terminal domain (NTD) is critical for efficient condensate formation [106]. The AR transcriptional activity is significantly impacted by the enlarged polyQ tract. SBMA’s pathophysiology has been connected to the accumulation of motor neuron-toxic AR-polyQ in the nucleus [72].
Polyalanine tracts-mediated gain of function
The repeat expansions in an exon that encodes polyalanine tracts instead of the polyglutamine tracts outlined above are frequently linked to severe developmental abnormalities like synpolydactyly, X-linked mental retardation, and muscular dystrophy [107]. Many of these repeat expansions in human disorders occur in IDRs of transcription factors (TFs). Disease-associated repeat expansions in TFs such as HOXA13, RUNX2 and HOXD13 have been found to alter their phase separation properties [74]. These diseases are associated with the propensity of the protein to form solid aggregates and to alter its subcellular localization [ 108, 109] .
The polyalanine tracts in IDR of these three typical TFs (HOXA13, RUNX2 and HOXD13) show varied effects on their phase separation ability, including both impairment and enhancement. Firstly, HOXA13 is a homeobox TF. The polyalanine repeat expansion from 18 to 24–26 in the N-terminal IDR of HOXA13 results in hand-foot-genital syndrome (HFGS) [110], a rare, dominantly inherited condition characterized by distal limb malformations and genitourinary tract defects [111]. While the wild-type HOXA13 IDR can undergo phase separation and form liquid-like droplets, the HOXA13 IDR with a short (+7A) HFGS-linked expansion tends to aggregate [74]. Secondly, RUNX2 is a RUNT family TF that controls bone morphogenesis and expansions of a short alanine and glutamine repeat in the RUNX2 IDR. GCG repeats expansion in RUNX2 IDR is associated with cleidocranial dysplasia (CCD), a rare autosomal dominant disorder of severe skeletal defects [ 112, 113] . The RUNX2 IDR containing a CCD-associated alanine expansion a (+10A) tends to form solid aggregates, while RUNX2 IDR droplets exhibit liquid-like dynamics [74]. The pathological alanine repeat expansion alters its phase separation capacity, co-condensation with the MED1 IDR, and transcriptional activity. Another similar example is the alanine repeat expansions in the IDR of HOXD13, which cause type II synpolydactyly (SPD II) in humans [114].
In addition to transcription factors (TFs), alanine repeat expansions also occur in polyA binding proteins. PABPN1 is an abundant nuclear protein that binds with high affinity to nascent polyA tails. The RNA binding protein PABPN1 promotes the formation of nuclear polyA domains (NPADs) through its N-terminal disordered domain and RNA-recognized motif by liquid phase separation [73]. Expansion of GCG repeat from the normal 6 copies to 8‒13 copies leads to autosomal dominant oculopharyngeal muscular dystrophy (OPMD) disease [115]. In OPMD muscle models, alanine-expanded PABPN1 accumulates in insoluble intranuclear inclusions (INIs) and also abnormally accumulates in the cytoplasm [116].
Polyglycine tracts-mediated gain of function
In addition to polyglutamine and polyalanine disease, polyglycine (polyG) disease is defined as a novel class of TRDs recently. Expansion of GGC repeats in the 5′ UTR of the NOTCH2NLC ( N2C) gene causes neuronal intranuclear inclusion disease (NIID), which is a neurodegenerative disease characterized by the presence of intranuclear inclusions [75]. GGC repeats embed into the open reading frame of a small protein (uN2C) and is translated into a uN2C polyglycine-containing protein (uN2CpolyG) in NIID [ 75, 117] .
Altogether, short tandem repeats may alter the structure and function of proteins, differentially engage with their interacting partners and result in abnormal phase separation in the form of intracellular inclusions, and finally lead to neurological and muscular disorders cord [101].
Aberrant Phase Separation and Cancer
In addition to the tandem repeat disorders and developmental disorders, aberrant MLOs have also been linked to a range of cancers by disrupting tumor suppression and normal signal transduction pathways, hyperactivating oncogenic genes or affecting protein quality control machinery. In this part, we will discuss tumorigenesis in relation to abnormal phase separation ( Table 3).
Table 3 Protein phase separation and cancer
|
Protein |
Pathological type |
Cancer |
Mechanism |
Reference |
|
DnaJB1-PKAcat |
Gain of function |
Oncocytic pancreatic and biliary neoplasms, fibrolamellar hepatocellular carcinoma |
DnaJB1-PKAcat suppresses the phase separation of RIα and leads to signal transduction disorder. |
|
|
EWS-FLI1 |
Gain of function |
Ewing Sarcoma |
EWS-FLI1 condensate recruits BRG1–BRM-associated factor (BAF) chromatin remodeling complex to upregulate the cancer-associated gene expression. |
|
|
NUP98-HOXA9 |
Gain of function |
Myelodysplastic syndromes and acute myeloid leukemia |
NUP98-HOXA9 condensate promotes its chromatin occupancy and upregulates leukemogenic genes. |
|
|
EML4–ALK |
Gain of function |
Non-small cell lung cancer |
EML4-ALK condensate enriches with RAS-activating factors (GRB2/SOS1/GAB1) and excludes the RAS activity negative regulators (GTPase-activating protein) to hyperactivate the oncogenic RTK/RAS signaling. |
|
|
CCDC6-RET |
Gain of function |
Lung adenocarcinoma, thyroid gland papillary carcinoma, poorly differentiated thyroid gland carcinoma, breast invasive ductal carcinoma, and thyroid gland undifferentiated (anaplastic) carcinoma |
CCDC6-RET condensate increases RAS signaling and MAPK signaling. |
|
|
PML- RARα |
Gain of function |
Acute promyelocytic leukaemia |
PML-RARα disturbs the formation of PML bodies which function as tumor suppressors and triggers the formation of dispersed microspeckles and promote cancer development. |
|
|
SHP2 |
Gain of function |
Juvenile myelomonocytic leukemias |
Activating SHP2 mutants’ condensate triggers Ras-MAPK pathway hyperactivation. Inactivating SHP2 mutants’ condensate recruits the wildtype SHP2 to trigger Ras-MAPK pathway hyperactivation. |
|
|
ENL |
Gain of function |
Wilms tumor and acute myelocytic leukemia |
ENL mutants phase separates native target genes and drives oncogenic gene hyperactivation. |
|
|
Androgen receptor (AR) |
Gain of function |
Prostate cancer |
Antiandrogen treatment will promote the formation of transcriptional condensates formed by antiandrogen-resistant androgen receptor mutants. |
|
|
KDM6A/UTX |
Loss of function |
Acute myeloid leukemia, bladder carcinoma, breast cancer, chronic myeloid leukemia, colorectal adenocarcinoma, endometrial adenocarcinoma, and glioblastoma |
UTX mutants lose phase separation capability which was associated with the cancer-suppressive properties. |
|
|
SPOP |
Loss of function |
Prostate cancer |
SPOP mutants lose phase separation capability and have decreased ubiquitination activity, leading to substrate accumulation. |
|
|
AKAP95 |
Loss of function |
Triple negative breast cancer |
AKAP95 mutants’ condensate was less dynamic and had decreased splicing and transcription regulation activity. |
|
|
Axin and APC |
Loss of function |
Colorectal cancer |
APC mutants lose phase separation capability and release β-catenin protein, leading to the hyperactivation of the Wnt pathway. |
|
|
Gα protein i2 (Gαi2) |
Loss of function |
Colorectal cancer |
Gαi2 mutant can not induce the formation of axin2 condensate which promotes the degradation of β-catenin. |
|
|
KAT8 |
None |
Lung cancer |
KAT8 undergoes phase separation and forms condensate with IRF1, enriching the transcription apparatus to promote tumor immune evasion. |
Abnormal condensates impair tumor-suppressive function
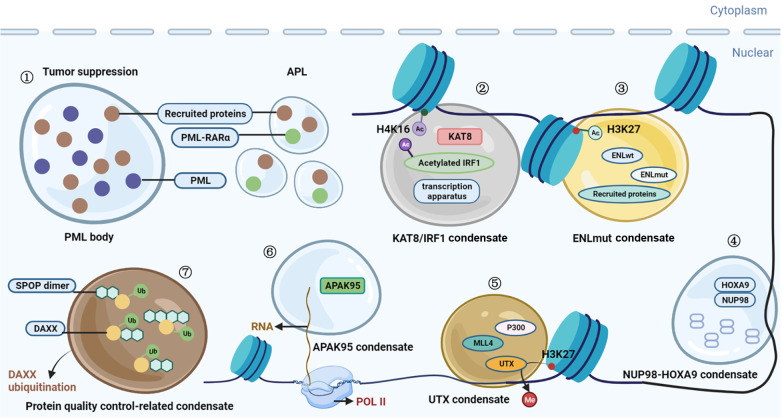
Condensates are essential in preventing and restraining cancer development. They achieve this by assembling protein complexes involved in tumor suppression, and enhancing the expression of the related gene to suppress antitumor immune surveillance. However, the abnormal condensates will impair these tumor-suppressive functions. For instance, promyelocytic leukemia (PML) bodies are submicron-scale nuclear membrane-less organelles composed of proteins including the PML protein which function as tumor suppressors [ 136, 137] . PML protein contains a conserved RING finger/B-box/coiled-coil (RBCC) domain and a SUMO-interacting motif (SIM) [138]. The RBCC domain promotes the assembly of PML bodies, while the SIM interacts with sumoylated proteins, enhancing the assembly of PML bodies [138]. In acute promyelocytic leukemia (APL), a subtype of acute myeloid leukemia, the N-terminal of PML fuses with retinoic acid receptor alpha (RARα), resulting in the absence of the C-terminal SIM [124]. This fusion protein, PML-RARα, disturbs the PML bodies and triggers the formation of dispersed microspeckles, leading to the dysfunction of nuclear receptor-induced differentiation and PML-triggered apoptosis, which may aid the development of cancer [ 124‒ 126] ( Figure 3). Additionally, LLPS works to promote tumor immune evasion. In tumor cells, the histone acetyltransferase KAT8 undergoes phase separation and forms condensate with IRF1 upon induction by interferon-γ. KAT8/IRF1 condensation promotes IRF acetylation by binding to the promoter of PD-L1, which further enriches the transcription apparatus to upregulate PD-L1 [135] ( Figure 3). PD-L1 has been demonstrated to be a dominant suppressor of antitumor immune surveillance [ 139, 140] . Notably, based on the mechanism of KAT8/IRF1 condensate formation, a constructed competitive peptide disrupts condensate formation and consequently inhibits PD-L1 expression to enhance antitumor immune responses [135].
Figure 3 .
Phase separation and cancer
Aberrant MLOs are linked to a range of cancers. (1) The disruption of promyelocytic leukemia (PML) nuclear bodies impairs the tumor suppressor function. (2) KAT8 acetylates IRF1 and forms condensate with PD-L1 and other transcription apparatus to increase PD-L1 expression, allowing cancer cells to evade immune surveillance. (3) ENL mutants form functional condensates with wild-type ENL to activate oncogenic gene. (4) NUP98–HOXA9 condensates promote its chromatin occupancy, contributing to the progression of leukemia. (5) Mutations in the UTX gene can disrupt the formation of tumor-suppressor condensates to dysregulated gene expression and promote cancer development. (6) AKAP95 forms phase-separated, liquid-like condensates in the nucleus and plays a crucial role in tumorigenesis. (7) Disruption the condensates of SPOP and DAXX could affect protein regulation, contributing to the development or progression of cancer.
Abnormal condensates impacting transcriptional functions
Abnormal condensates exert a significant influence on transcriptional processes, thereby driving the development and progression of cancer. These aberrant biomolecular assemblies can upregulate the expression of oncogenes, leading to cancer cell growth and survival. For example, ENL is a histone acetylation reader that uses its YEATS (Yes-associated protein) domain to recognize acetylated lysine residues [141]. Gain-of-function ENL YEATS mutations have been linked to acute myeloid leukemia (AML) and Wilms tumor, as they enhance transcriptional activation [ 142, 143] ( Figure 3). Recent research has shown that these mutations can trigger condensate formation at native target genes, even though the mutations are located in the structured YEATS domain. This, in turn, drives the hyperactivation of oncogenic genes [127]. EWS-FLI1 is a fusion protein that is generated from chromosomal rearrangement that is found in Ewing′s sarcoma, a rare type of cancer [144]. Through its low-complexity prion-like domain, EWS-FLI1 can undergo phase separation and interact with other proteins and DNA [ 145, 146] . Unlike the wild-type FLI1, the EWS-FLI1 droplet tightly binds GGAA microsatellites and recruits the BAF chromatin remodeling complex, leading to the upregulation of cancer-associated genes and the development of cancer [146]. NUP98-HOXA9 is another fusion protein commonly found in AML [147]. Due to the FG repeats from the N-terminus of NUP98, NUP98-HOXA9 can undergo phase separation and form condensates, which promotes chromatin occupancy and upregulates leukemogenic genes through a super-enhancer-like binding pattern [ 121, 122] ( Figure 3). In more detail, NUP98-HOXA9 condensate induces the formation of aberrant chromatin loops at protooncogenes-rich regions often without the assistance of CTCF [122]. In addition, transcription-related proteins can form condensates to upregulate cancer-suppressive genes, and certain site mutations may disrupt the formation of condensates, resulting in transcriptional repression and ultimately leading to cancer. KDM6A, also known as UTX, is an H3K27 demethylase [148]. Recent studies have demonstrated that UTX phase separation is linked to tumor-suppressive properties [17]. UTX phase-separated condensate recruits KMT2D/MLL4 and p300, thereby increasing their enzymatic activity at enhancers and upregulating tumor-suppressive genes [17] ( Figure 3). Specific mutations in the IDR of UTX may impair its phase separation capacity and cancer-suppressive function and replacing the IDR by another IDR can restore the phase separation capacity and tumor-suppressive function [17]. Furthermore, the dynamics of mRNA splicing and transcription-related condensates may also be important for cancer development. AKAP95 (A-kinase anchor protein 95) can regulate mRNA splicing and transcription. When overexpressed, AKAP95 can form condensates and is consistently linked to ovarian, rectal, and breast cancers [131]. However, tyrosine-to-phenylalanine mutations in the 101–210 region of AKAP95 result in the formation of more solid condensates with reduced biochemical reaction kinetics. Those mutations also impair the tumor-supporting abilities of AKAP95 while inhibiting the suppression of oncogene-induced senescence [131] ( Figure 3).
Abnormal condensates disrupt signal transduction
In addition to dysregulated gene expression, abnormal MLOs can also affect signal transduction related to cancer. Recent papers have shown that mutant protein-formed condensates result in abnormal signal transduction and contribute to tumorigenesis. One such example is the non-receptor protein tyrosine phosphatase SHP2, which was mentioned above in Noonan syndrome and Leopard syndrome. Certain mutations result in hyperactivated RAS-MAPK pathway by activating SHP2 enzymatic activity or by recruiting and activating wild-type SHP2 protein, and ultimately cause malignancies [ 46, 47] . Additionally, fusion proteins may also form abnormal condensates that disrupt normal signal transduction pathways and contribute to cancer. One example is the EML4-ALK (echinoderm microtubule-associated protein-like 4–anaplastic lymphoma kinase) fusion protein, which is resulted from chromosomal rearrangements and is frequently detected in lung cancer [ 149, 150] . ALK is a receptor tyrosine kinase (RTK) that, in the chimeric protein, retains its intracellular kinase domain but loses its native transmembrane domain, while EML4 retains its trimerization domain and truncated TAPE domain [123]. EML4-ALK can undergo phase separation to form condensates in the cytoplasm, which are enriched with RAS-activating factors (GRB2/SOS1/GAB1) and exclude RAS activity negative regulators (GTPase-activating protein). This process is critical for the hyperactivation of oncogenic RTK/RAS signaling, which can contribute to the development of cancer [123]. Another example is the CCDC6-RET (coiled-coil domain containing 6- rearranged during transfection), another chimeric RTK oncoprotein which also promotes cancer development [ 151, 152] . Like EML4-ALK, CCDC6-RET can undergo phase separation driven by a coiled-coil domain and form cytoplasmic condensates that increase RAS signaling and downstream MAPK signaling [123]. Furthermore, apart from the hyperactivation of oncogenic signaling, disruption of normal condensates-mediated signal transduction by other proteins can also lead to cancer. RIα, a regulatory subunit of protein kinase A (PKA), forms functional condensates that determine signaling specificity [118]. In fibrolamellar carcinoma, the phase separation of RIα is suppressed by the fusion protein DnaJB1-PKAcat, leading to signal transduction disorder and uncontrolled hepatocyte growth [118]. Aside from proteins and nucleic acids, glycogen is another biomacromolecule that can undergo phase separation, as recently discovered [153]. Glycogen has been found to accumulate in liver tumor cells [154]. The condensate formed by this glycogen accumulation is enriched with the Laforin-Mst1/2 complex, which strongly inhibits the kinase activity of Mst1/2 and disrupts the formation of the WW45-Mst1/2 complex. This ultimately leads to the activation of oncogenic YAP signaling, which promotes cancer cell survival and transformation [153].
Abnormal condensates interfere with protein degradation
Apart from influencing transcription and signal transduction, mutations in proteins may impact the formation of condensates that are associated with protein degradation, potentially contributing to the development of cancer. SPOP (speckle-type POZ protein) is a protein that belongs to a family of enzymes called E3 ubiquitin ligases [155]. Its mutant is always associated with solid tumors [156]. SPOP could undergo phase separation and form condensate with its substrate DAXX (death-domain-associated protein), which functions as active ubiquitination compartments to ubiquitinate DAXX [130]. However, certain cancer-associated mutants have lost their ability to bind with substrates, and consequently fail to undergo phase separation, leading to a significant reduction in ubiquitination activity, which results in substrate accumulation and contributes to cancer development [130] ( Figure 3).
Phase Separation and Infectious Diseases
Abnormal protein phase separation is closely associated with several non-communicable diseases. Recent studies, however, have shown that protein phase separation also plays a significant role in the emergence of some infectious diseases, particularly viral disorders. For most viruses, phase separation is involved in the majority of viral lifecycles, including protein synthesis, genome assembly, virus assembly, budding and release. For example, the 52-KDa protein of human adenovirus regulates viral assembly by phase separation, and failure to form condensates results in failed packaging and assembly of only non-infectious particles. In contrast to non-communicable diseases, LLPS in viral diseases occurs in two distinct ways. It can either be employed by the host as a defense mechanism against invading pathogens, or it can be utilized by the pathogen to aid in their invasion [ 157‒ 159] .
Phase separation promotes innate immunity to combat infection
Innate immunity is a non-specific immune response and is also the first line of defense against pathogens. Upon recognizing the pathogen, host cells initiate a cascade of events to activate the cells to attack and kill the pathogens. The cyclic GMP-AMP synthase (cGAS, cGAMP synthase) is an enzyme that initiates the innate immune response after detecting double-stranded DNA (dsDNA), either from the pathogen or self, in the cytoplasm [ 160, 161] . It converts GTP and ATP to cGAMP, a second messenger that activates the protein STING [ 160, 162] . This induces the synthesis of type I interferon and activates the NF-κB pathway [163]. cGAS directly recognizes dsDNA to undergo phase separation and forms a condensate in the cell, which is important for cGAS activation [ 9, 164] . However, pathogens have also evolved strategies to limit the formation of cGAS condensates. For example, ORF52 from gamma-herpesvirinae and VP22 from alpha-herpesvirinae, both belonging to the viral tegument protein family, can disrupt DNA-induced cGAS condensate formation in the early stages of infection [ 165‒ 167] . Another research group identified a spherical ER membranous condensate formed by ER-resident STING in the cell infected by a DNA virus or treated with cGAMP. This condensate recruits TBK1 (TANK binding protein 1), similar to the “STING-TBK1-cGAMP sponge”, to prevent innate immunity from overactivation [168].
Viruses infect by impairing or utilizing stress granules (SGs)
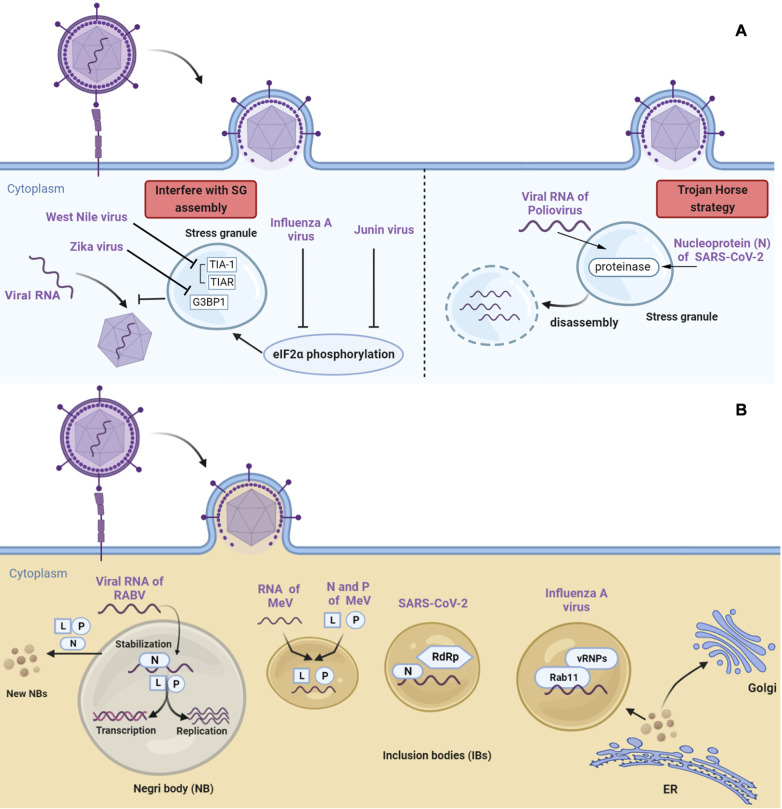
SGs are considered as a defense mechanism against various stresses, which can sequester certain transcripts and proteins from the soluble portion of cytoplasm during physiological stress [ 12, 169] . In the context of viral infections, the formation of SG is also considered an antiviral strategy [170], but viruses have also developed ways to interfere with SG assembly [ 10, 170] ( Table 4). For example, the West Nile Virus can successfully infect cells by suppressing SG formation [ 178, 179] . The virus does this by blocking the TIA-1 and TIAR proteins, which are the scaffold proteins of SG, through its negative strand 3′ terminal stem-loop structure, disrupting SG [171] ( Figure 4A). Host cells that lack TIAR show compromised virus replication. Zika virus also limits SG formation by interacting with SG core proteins, and it even hijacks G3BP1 to facilitate viral RNA synthesis [177] ( Figure 4A). Junin virus uses an alternative mechanism to prevent SG assembly by inhibiting eIF2α phosphorylation, which is necessary for SG formation [173]. Moreover, Junin virus nucleoprotein and glycoprotein precursor can also interact with SG components to interfere with SG formation [173]. Influenza A virus follows a similar strategy, with its nonstructural proteins directly interacting with protein kinase R to block its kinase activity, which prevents eIF2α phosphorylation and suppresses SG formation [177]. In addition to interfering with SG formation, viruses can also use the Trojan Horse strategy to evade host immunity. For example, during the initial infection of Poliovirus, SGs enriched with viral RNA are formed, excluding G3BP-1, PABP, and eIF4G. These proteins are then cleaved by a proteinase expressed by the viral genome, named 3C proteinase, leading to the disassembly of SG [172]. Similarly, the SARS-CoV-2 nucleoprotein (N) can be recruited into SGs to block the interaction between G3BP1 and other core SG components, leading to SG disassembly [ 174‒ 176] ( Figure 4A). N protein-formed condensates can also inhibit the formation of MAVS (mitochondrial antiviral-signaling protein) condensates, downregulating the cytosolic IKK and TBK1 kinase activity, failing to activate the transcription factors IRF3 and NF-κB. This chain of events hampers the necessary upregulation of IFN1, thus impairing the initiation of innate immunity, which is key for fighting infections [180].
Table 4 Viruses fight with host phase separation-mediated antiinfection
|
Virus |
Targeted MLOs |
Mechanism |
Reference |
|
West Nile virus |
Stress granule |
Viral RNA binds with the host’s TIA-1 and TIAR to disrupt the assembly of stress granules. |
|
|
Poliovius |
Stress granule |
Viral RNA enters into stress granules to exclude G3BP-1, PABP, and eIF4G. |
|
|
Junin virus |
Stress granule |
Its protein inhibits eIF2α phosphorylation to disrupt the assembly of stress granules. |
|
|
SARS-CoV-2 |
Stress granule |
SARS-CoV-2 nucleoprotein interacts with G3BP1 and causes the disassembly of stress granules. |
|
|
Zika virus |
Stress granule |
Its protein interacts with SG core proteins to limit the formation of stress granules. |
|
|
Influenza A virus |
Stress granule |
Its nonstructural proteins block kinase R activity, causing the dephosphorylation of eIF2α, leading to the disassembly of stress granules. |
Figure 4 .
Phase separation in infectious diseases
(A) Stress granule (SG) and Viral infection. The virus can suppress the formation of SGs to promote viral infection (left), also can evade host immunity using the Trojan Horse strategy (right). (B) Phase separation was exploited by the virus. The virus form liquid inclusion bodies (IBs) via LLPS and contribute to viral replication and even viral packaging.
Phase separation exploited by the virus for replication and assembly
Phase separation is a process that has been exploited by viruses. In addition to countering host condensate-based antiviral cell signaling, viruses have developed various strategies to take advantage of condensates enriched with both host and viral proteins for their replication, assembly and trafficking ( Table 5). During viral infection, viral liquid inclusion bodies (IBs) form inside cells, which are composed of viral proteins, nucleic acids, and other biomolecules that are critical for viral replication. For example, rabies virus (RABV) belongs to the order of Mononegavirales and possesses a single-stranded negative RNA genome [197]. Recent papers have shown that RABV is present in the cytoplasm of the infected neuron as liquid IBs, also known as Negri bodies (NBs), enriched with all the ribonucleoparticle (RNP) components, including nucleoprotein (N) protein, large (L) protein, and the phosphoprotein (P) [ 198‒ 201] . The N protein binds to viral RNA to stabilize it, while L and P proteins are responsible for the viral RNA transcription and replication [ 181‒ 183] ( Figure 4B). Researchers have also found that the RNPs of RABV could be excluded from NBs and transported via the microtubule network, with free RNPs able to assemble new virions and form new NBs [ 183, 198, 201] . Measles virus (MeV) also uses a similar replication mechanism, with N and P proteins undergoing phase separation and forming liquid-like condensates (or IBs) enriched with viral RNA [ 184, 185] . The formation rate of nucleocapsid correlates with the phase separation of N and P proteins, suggesting that viral replication is closely linked to viral protein phase separation [184] ( Figure 4B). Human respiratory syncytial virus (RSV) utilizes intracellular bodies (IBs) for RNA replication, which compartmentalize the N, P, L, and M2-1 proteins responsible for RNA replication [ 186, 187] . Researchers have identified two small molecules, the steroidal alkaloid cyclopamine and its analog A3E, which can disrupt the dynamics of IBs and suppress RSV replication [188]. Besides conventional IBs, another type of condensate called IB-associated granules (IBAGs) have been found in RSV-infected cells, which are enriched with phosphorylated M2-1, viral mRNA, and host phosphatase PP1 [ 189, 190] . In the cytosol, M2-1 is phosphorylated to unload its cargo, the mature polyadenylated mRNA [190]. The phosphorylated M2-1 is then recruited into IBAGs and dephosphorylated by PP1. The dephosphorylated form has a high affinity with newly synthesized viral mRNAs, protecting them and facilitating their polyadenylation [190]. In addition, phase separation is not only used by viruses to form IBs for viral RNA replication and maturation, but also for assembly and trafficking purposes. For example, Influenza A virus (IAV) forms liquid IBs with the host protein Rab11, concentrating the viral ribonucleoprotein (vRNP) complex and RNA close to the endoplasmic reticulum (ER) exit sites, promoting virion assembly and trafficking between the ER and Golgi [191] ( Figure 4B). Similarly, human immunodeficiency virus 1 (HIV-1) utilizes phase separation of its nucleocapsid protein (NC) to package the viral genome into the capsid and facilitate RNA trafficking. Disrupted nucleocytoplasmic transportation was observed when phase separation was impeded by chelating zinc ions [ 192‒ 194] . In SARS-CoV-2, the phase separation of N protein not only helps the virus evade host innate immunity but also potentially facilitates viral RNA transcription and replication through the recruitment of viral RNA-dependent RNA polymerase (RdRp) and viral mimic RNA [ 195, 196] ( Figure 4B). Additionally, the phase separation of N protein may play a role in viral packaging [ 202, 203] , with the characteristic of phase separation determined by the binding RNA and structure [203]. Changing the ratio of N protein and viral RNA can determine whether the mixture undergoes phase separation or dissolves, potentially affecting the organization of the long genome RNA or facilitating packaging into virions [203].
Table 5 Viruses exploit phase separation
|
Virus |
Targeted MLOs |
Mechanism |
Reference |
|
Rabies virus |
Negri bodies (NBs) |
NBs enriched with and stabilize all the ribonucleoparticle components responsible for viral replication. |
|
|
Measles virus |
Inclusion bodies (IBs) and N and P proteins phase separation |
IBs enrich with viral RNA. Viral replication depends on viral N and P proteins phase separation. |
|
|
Human respiratory syncytial virus |
Inclusion bodies (IBs) and IB associated granules (IBAGs) |
IBs compartmentalize N, P, L and M2-1 for RNA replication. IBAGs recruit and dephosphorylate M2-1 protein to protect and polyadenylate viral RNA. |
|
|
Influenza A |
Inclusion bodies (IBs) |
The IBs concentrate viral ribonucleoprotein and RNA and promote the assembly of virion. |
|
|
Human immunodeficiency virus 1 |
nucleocapsid protein phase separation |
Facilitating viral RNA trafficking. |
|
|
SARS-CoV-2 |
N protein phase separation |
N protein condensate recruits viral RNA-dependent RNA polymerase and RNA to promote viral RNA transcription and replication. N protein condensate may also play a role in viral packaging. |
Phase separation: dual role in virus infections
Occasionally, phase separation plays a dual role during viral infections, serving both advantageous and disadvantageous functions depending on the specific situations and interactions at play. On one hand, phase separation is beneficial for the virus infection process. The p26 movement protein from the Pea enation mosaic virus 2 (PEMV2) undergoes phase separation and forms droplets within the host cell [204]. This concentration of proteins and other viral components facilitates systemic virus movement within the plant. Interaction with a host protein, fibrillarin (Fib2), appears essential for this process, suggesting that the virus exploits cellular processes for its replication. On the other hand, phase separation can be detrimental to the virus. The host plant can upregulate the expression of the RNA-binding protein G3BP under stress, leading to the formation of stress granules. This upregulation of G3BP, and the subsequent phase separation, restricts PEMV2 RNA accumulation, demonstrating an antiviral response. This highlights that host cells can use phase separation as a defense mechanism to limit viral replication. In summary, phase separation can be both beneficial and detrimental to viral infection, depending on the specific viral and host factors involved, reflecting the complex nature of virus-host interactions.
The Potential LLPS-Associated Therapeutic Strategies
In recent years, it has been discovered that concentrating only on individual molecules may not be able to fully explain the complex illness phenotypes, and that it is also challenging to make a beneficial contribution to disease treatment methods by changing the structure or function of a single protein or nucleic acid. The investigation of phase separation may provide complex disease mechanisms, and even open up exciting new avenues for therapeutic intervention. We have discussed in detail how abnormal phase separation can lead to developmental abnormalities, neurodegenerative diseases, cancers, and viral infection. However, in most occasions, the relationships between phase separation and pathogenesis are currently simply correlative rather than causative, and warrant further investigation. In the meantime, finding effective therapies for diseases caused by abnormal phase separation remain challenging. Here, we list several potential therapeutic strategies. (1) Develop small molecules that can change the conformation of mutant proteins to inhibit their abnormal phase separation. For example, the allosteric inhibitors of SHP2 attenuate the phase separation of mutant SHP2 by locking it in a closed conformation [24]. (2) Using small molecule drugs to dissolve abnormally phase-separated condensates. For example, cisplatin selectively changes super-enhancer DNA, where MED1 is concentrated and forms condensate, and exerts its anti-neoplastic effect by dissolving these condensates [ 205, 206] . (3) Specific degradation of abnormally phase-separated proteins using PROTAC, AUTAC, and ATTEC, to dissolve the aberrant condensates [ 207‒ 209] . Recently, a BRD4-targeting PROTAC molecule was shown to significantly reduce the BRD4 condensates [210]. (4) Using specific competitive peptides to disrupt condensates. A 2142-R8 blocking peptide could competitively bind with KAT8 to disrupt KAT8-IRF1 condensates, further enhancing antitumor immune responses [135].
Conclusions
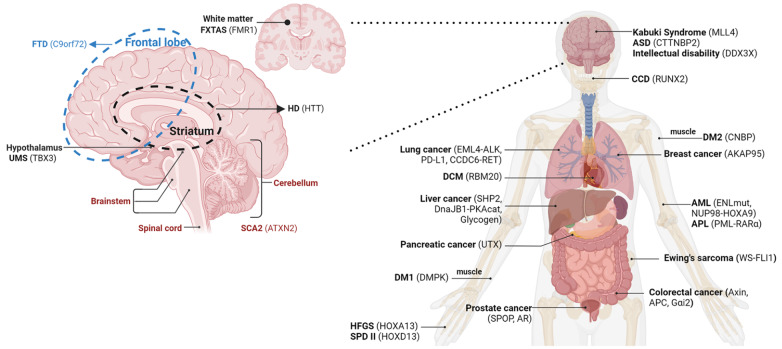
In this review, we have laid out that phase separation is significantly correlated with various diseases and showed that both normal and abnormal phase separation can be related to disease onset. Aberrantly disrupted phase separation causes transcription dysregulation, chromatin architecture changes, low ubiquitination activity or impaired innate immunity. The mutants that gain LLPS ability can promote the condensate formation and lead to hyperactivating the disease-associated pathway or overexpressing the oncogenic genes. In addition, the fibrillar structure or the liquid-to-solid transition, which allows for the conversion into solid-like states, is the primary cause of many neurodegenerative illnesses. Further evidence that phase separation may be the primary cause of diseases is provided by the fact that LLPS-associated diseases are broadly spread across the entire human body, as illustrated in Figure 5. Therefore, phase separation is not only macromolecular membrane-less organelles with biological functions but also plays an important role in understanding and investigating the essence behind the disease, and even finding effective treatment strategies. As a result, phase-separated macromolecular organelles without a membrane play a crucial role in comprehending a variety of biological processes as well as the underlying causes of disease and even the development of effective therapeutic strategies.
Figure 5 .
Onset location and causative protein of phase separation-associated non-infectious diseases
Schematic diagram shows the location of the main disease-related proteins at the onset site.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 32170684 to Y.L., 32150023 and 32125010 to P.L.), the Ministry of Science and Technology of the People’s Republic of China (Nos. 2022ZD0213900 and 2022ZD0204900 to Y.L.), and the National Key Research and Development Program of China (No. 2019YFA0508403 to P.L.)
References
- 1.Yang P, Mathieu C, Kolaitis RM, Zhang P, Messing J, Yurtsever U, Yang Z, et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell. . 2020;181:325–345.e28. doi: 10.1016/j.cell.2020.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Decker CJ, Parker R. P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb Perspect Biol. . 2012;4:a012286. doi: 10.1101/cshperspect.a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larson AG, Elnatan D, Keenen MM, Trnka MJ, Johnston JB, Burlingame AL, Agard DA, et al. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature. . 2017;547:236–240. doi: 10.1038/nature22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frey S, Görlich D. A saturated FG-Repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell. . 2007;130:512–523. doi: 10.1016/j.cell.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Hülsmann BB, Labokha AA, Görlich D. The permeability of reconstituted nuclear pores provides direct evidence for the selective phase model. Cell. . 2012;150:738–751. doi: 10.1016/j.cell.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, et al. Coexisting liquid phases underlie nucleolar subcompartments. Cell. . 2016;165:1686–1697. doi: 10.1016/j.cell.2016.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plys AJ, Kingston RE. Dynamic condensates activate transcription. Science. . 2018;361:329–330. doi: 10.1126/science.aau4795. [DOI] [PubMed] [Google Scholar]
- 8.Hnisz D, Shrinivas K, Young RA, Chakraborty AK, Sharp PA. A phase separation model for transcriptional control. Cell. . 2017;169:13–23. doi: 10.1016/j.cell.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du M, Chen ZJ. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. . 2018;361:704–709. doi: 10.1126/science.aat1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller CL. Stress granules and virus replication. Future Virol. . 2011;6:1329–1338. doi: 10.2217/fvl.11.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riggs CL, Ivanov P. Stress, membraneless organelles, and liquid–liquid phase separation. Droplets of Life. 2023: 505–529
- 12.Wolozin B, Ivanov P. Stress granules and neurodegeneration. Nat Rev Neurosci. . 2019;20:649–666. doi: 10.1038/s41583-019-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrone L, Drexler HCA, Wang J, Tripathi P, Distler T, Heisterkamp P, Anderson EN, et al. FUS pathology in ALS is linked to alterations in multiple ALS-associated proteins and rescued by drugs stimulating autophagy. Acta Neuropathol. . 2019;138:67–84. doi: 10.1007/s00401-019-01998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spannl S, Tereshchenko M, Mastromarco GJ, Ihn SJ, Lee HO. Biomolecular condensates in neurodegeneration and cancer. Traffic. . 2019;20:890–911. doi: 10.1111/tra.12704. [DOI] [PubMed] [Google Scholar]
- 15.Vorstman JAS, Ophoff RA. Genetic causes of developmental disorders. Curr Opin Neurol. . 2013;26:128–136. doi: 10.1097/WCO.0b013e32835f1a30. [DOI] [PubMed] [Google Scholar]
- 16.West JA, Mito M, Kurosaka S, Takumi T, Tanegashima C, Chujo T, Yanaka K, et al. Structural, super-resolution microscopy analysis of paraspeckle nuclear body organization. J Cell Biol. . 2016;214:817–830. doi: 10.1083/jcb.201601071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi B, Li W, Song Y, Wang Z, Ju R, Ulman A, Hu J, et al. UTX condensation underlies its tumour-suppressive activity. Nature. . 2021;597:726–731. doi: 10.1038/s41586-021-03903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang JY, Wen Z, Pan D, Zhang Y, Li Q, Zhong A, Yu X, et al. LLPS of FXR1 drives spermiogenesis by activating translation of stored mRNAs. Science. . 2022;377:eabj6647. doi: 10.1126/science.abj6647. [DOI] [PubMed] [Google Scholar]
- 19.Tatavosian R, Kent S, Brown K, Yao T, Duc HN, Huynh TN, Zhen CY, et al. Nuclear condensates of the Polycomb protein chromobox 2 (CBX2) assemble through phase separation. J Biol Chem. . 2019;294:1451–1463. doi: 10.1074/jbc.RA118.006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiba K, Katoh-Fukui Y, Yoshida K, Narumi S, Miyado M, Hasegawa Y, Fukami M. Role of liquid-liquid separation in endocrine and living cells. J Endocrine Soc. . 2021;5:bvab126. doi: 10.1210/jendso/bvab126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider JW, Oommen S, Qureshi MY, Goetsch SC, Pease DR, Sundsbak RS, Guo W, et al. Dysregulated ribonucleoprotein granules promote cardiomyopathy in RBM20 gene-edited pigs. Nat Med. . 2020;26:1788–1800. doi: 10.1038/s41591-020-1087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Castro Fonseca M, de Oliveira JF, Araujo BHS, Canateli C, do Prado PFV, Amorim Neto DP, Bosque BP, et al. Molecular and cellular basis of hyperassembly and protein aggregation driven by a rare pathogenic mutation in DDX3X. iScience. . 2021;24:102841. doi: 10.1016/j.isci.2021.102841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li CH, Coffey EL, Dall′Agnese A, Hannett NM, Tang X, Henninger JE, Platt JM, et al. MeCP2 links heterochromatin condensates and neurodevelopmental disease. Nature. . 2020;586:440–444. doi: 10.1038/s41586-020-2574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu G, Xie J, Kong W, Xie J, Li Y, Du L, Zheng Q, et al. Phase separation of disease-associated SHP2 mutants underlies MAPK hyperactivation. Cell. . 2020;183:490–502.e18. doi: 10.1016/j.cell.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fasciani A, D′Annunzio S, Poli V, Fagnocchi L, Beyes S, Michelatti D, Corazza F, et al. MLL4-associated condensates counterbalance Polycomb-mediated nuclear mechanical stress in Kabuki syndrome. Nat Genet. . 2020;52:1397–1411. doi: 10.1038/s41588-020-00724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shih PY, Hsieh BY, Tsai CY, Lo CA, Chen BE, Hsueh YP. Autism-linked mutations of CTTNBP2 reduce social interaction and impair dendritic spine formation via diverse mechanisms. Acta Neuropathol Commun. . 2020;8:1–9. doi: 10.1186/s40478-020-01053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shih PY, Fang YL, Shankar S, Lee SP, Hu HT, Chen H, Wang TF, et al. Phase separation and zinc-induced transition modulate synaptic distribution and association of autism-linked CTTNBP2 and SHANK3. Nat Commun. . 2022;13:2664. doi: 10.1038/s41467-022-30353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shih PY, Hsieh BY, Lin MH, Huang TN, Tsai CY, Pong WL, Lee SP, et al. CTTNBP2 controls synaptic expression of zinc-related autism-associated proteins and regulates synapse formation and autism-like behaviors. Cell Rep. . 2020;31:107700. doi: 10.1016/j.celrep.2020.107700. [DOI] [PubMed] [Google Scholar]
- 29.Shi X, Zhuang Y, Chen Z, Xu M, Kuang J, Sun XL, Gao L, et al. Hierarchical deployment of Tbx3 dictates the identity of hypothalamic KNDy neurons to control puberty onset. Sci Adv. . 2022;8:eabq2987. doi: 10.1126/sciadv.abq2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun M, Jia M, Ren H, Yang B, Chi W, Xin G, Jiang Q, et al. NuMA regulates mitotic spindle assembly, structural dynamics and function via phase separation. Nat Commun. . 2021;12:7157. doi: 10.1038/s41467-021-27528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janssen A, Colmenares SU, Karpen GH. Heterochromatin: guardian of the genome. Annu Rev Cell Dev Biol. . 2018;34:265–288. doi: 10.1146/annurev-cellbio-100617-062653. [DOI] [PubMed] [Google Scholar]
- 32.Ip JPK, Mellios N, Sur M. Rett syndrome: insights into genetic, molecular and circuit mechanisms. Nat Rev Neurosci. . 2018;19:368–382. doi: 10.1038/s41583-018-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tillotson R, Selfridge J, Koerner MV, Gadalla KKE, Guy J, De Sousa D, Hector RD, et al. Radically truncated MeCP2 rescues Rett syndrome-like neurological defects. Nature. . 2017;550:398–401. doi: 10.1038/nature24058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho WK, Spille JH, Hecht M, Lee C, Li C, Grube V, Cisse II. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science. . 2018;361:412–415. doi: 10.1126/science.aar4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng SB, Bigham AW, Buckingham KJ, Hannibal MC, McMillin MJ, Gildersleeve HI, Beck AE, et al. Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat Genet. . 2010;42:790–793. doi: 10.1038/ng.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cocciadiferro D, Augello B, De Nittis P, Zhang J, Mandriani B, Malerba N, Squeo GM, et al. Dissecting KMT2D missense mutations in Kabuki syndrome patients. Hum Mol Genet. . 2018;27:3651–3668. doi: 10.1093/hmg/ddy241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehman N, Mazery AC, Visier A, Baumann C, Lachesnais D, Capri Y, Toutain A, et al. Molecular, clinical and neuropsychological study in 31 patients with Kabuki syndrome and KMT2D mutations . Clin Genet. . 2017;92:298–305. doi: 10.1111/cge.13010. [DOI] [PubMed] [Google Scholar]
- 38.Rada‐Iglesias A, Grosveld FG, Papantonis A. Forces driving the three‐dimensional folding of eukaryotic genomes. Mol Syst Biol. . 2018;14:e8214. doi: 10.15252/msb.20188214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Laarhoven PM, Neitzel LR, Quintana AM, Geiger EA, Zackai EH, Clouthier DE, Artinger KB, et al. Kabuki syndrome genes KMT2D and KDM6A: functional analyses demonstrate critical roles in craniofacial, heart and brain development . Hum Mol Genet. . 2015;24:4443–4453. doi: 10.1093/hmg/ddv180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bamshad M, Lin RC, Law DJ, Watkins WS, Krakowiak PA, Moore ME, Franceschini P, et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat Genet. . 1997;16:311–315. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- 41.Bamshad M, Le T, Watkins WS, Dixon ME, Kramer BE, Roeder AD, Carey JC, et al. The spectrum of mutations in TBX3: genotype/phenotype relationship in ulnar-mammary syndrome. Am J Hum Genet. . 1999;64:1550–1562. doi: 10.1086/302417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antshel KM, Russo N. Autism spectrum disorders and adhd: overlapping phenomenology, diagnostic issues, and treatment considerations. Curr Psychiatry Rep. . 2019;21:34. doi: 10.1007/s11920-019-1020-5. [DOI] [PubMed] [Google Scholar]
- 43.Tajan M, de Rocca Serra A, Valet P, Edouard T, Yart A. SHP2 sails from physiology to pathology. Eur J Med Genet. . 2015;58:509–525. doi: 10.1016/j.ejmg.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Tartaglia M, Gelb BD. Noonan syndrome and related disorders: genetics and pathogenesis. Annu Rev Genom Hum Genet. . 2005;6:45–68. doi: 10.1146/annurev.genom.6.080604.162305. [DOI] [PubMed] [Google Scholar]
- 45.Digilio MC, Conti E, Sarkozy A, Mingarelli R, Dottorini T, Marino B, Pizzuti A, et al. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet. . 2002;71:389–394. doi: 10.1086/341528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carvajal-Vergara X, Sevilla A, D′Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. . 2010;465:808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oishi K, Zhang H, Gault WJ, Wang CJ, Tan CC, Kim IK, Ying H, et al. Phosphatase-defective LEOPARD syndrome mutations in PTPN11 gene have gain-of-function effects during Drosophila development. Hum Mol Genet. . 2009;18:193–201. doi: 10.1093/hmg/ddn336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brauch KM, Karst ML, Herron KJ, de Andrade M, Pellikka PA, Rodeheffer RJ, Michels VV, et al. Mutations in ribonucleic acid binding protein gene cause familial dilated cardiomyopathy. J Am Coll Cardiol. . 2009;54:930–941. doi: 10.1016/j.jacc.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li S, Guo W, Dewey CN, Greaser ML. Rbm20 regulates titin alternative splicing as a splicing repressor. Nucleic Acids Res. . 2013;41:2659–2672. doi: 10.1093/nar/gks1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Protter DSW, Parker R. Principles and properties of stress granules. Trends Cell Biol. . 2016;26:668–679. doi: 10.1016/j.tcb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Candelise N, Scaricamazza S, Salvatori I, Ferri A, Valle C, Manganelli V, Garofalo T, et al. Protein aggregation landscape in neurodegenerative diseases: clinical relevance and future applications. Int J Mol Sci. . 2021;22:6016. doi: 10.3390/ijms22116016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linder P, Jankowsky E. From unwinding to clamping — the DEAD box RNA helicase family. Nat Rev Mol Cell Biol. . 2011;12:505–516. doi: 10.1038/nrm3154. [DOI] [PubMed] [Google Scholar]
- 53.Lennox AL, Hoye ML, Jiang R, Johnson-Kerner BL, Suit LA, Venkataramanan S, Sheehan CJ, et al. Pathogenic DDX3X mutations impair RNA metabolism and neurogenesis during fetal cortical development. Neuron. . 2020;106:404–420.e8. doi: 10.1016/j.neuron.2020.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gall-Duncan T, Sato N, Yuen RKC, Pearson CE. Advancing genomic technologies and clinical awareness accelerates discovery of disease-associated tandem repeat sequences. Genome Res. . 2022;32:1–27. doi: 10.1101/gr.269530.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hannan AJ. Tandem repeats mediating genetic plasticity in health and disease. Nat Rev Genet. . 2018;19:286–298. doi: 10.1038/nrg.2017.115. [DOI] [PubMed] [Google Scholar]
- 56.Malik I, Kelley CP, Wang ET, Todd PK. Molecular mechanisms underlying nucleotide repeat expansion disorders. Nat Rev Mol Cell Biol. . 2021;22:589–607. doi: 10.1038/s41580-021-00382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Botta-Orfila T, Tartaglia GG, Michalon A. Molecular pathophysiology of fragile X-Associated tremor/ataxia syndrome and perspectives for drug development. Cerebellum. . 2016;15:599–610. doi: 10.1007/s12311-016-0800-2. [DOI] [PubMed] [Google Scholar]
- 58.Usdin K, Kumari D. Repeat-mediated epigenetic dysregulation of the FMR1 gene in the fragile X-related disorders. Front Genet. . 2015;6:192. doi: 10.3389/fgene.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gulyurtlu S, Magon MS, Guest P, Papavasiliou PP, Morrison KD, Prescott AR, Sleeman JE. Condensation properties of stress granules and processing bodies are compromised in myotonic dystrophy type 1. Dis Model Mech. . 2022;15:dmm049294. doi: 10.1242/dmm.049294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sznajder ŁJ, Swanson MS. Short tandem repeat expansions and RNA-mediated pathogenesis in myotonic dystrophy. Int J Mol Sci. . 2019;20:3365. doi: 10.3390/ijms20133365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jain A, Vale RD. RNA phase transitions in repeat expansion disorders. Nature. . 2017;546:243–247. doi: 10.1038/nature22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peskett TR, Rau F, O′Driscoll J, Patani R, Lowe AR, Saibil HR. A liquid to solid phase transition underlying pathological huntingtin exon1 aggregation. Mol Cell. . 2018;70:588–601.e6. doi: 10.1016/j.molcel.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gunawardena S, Her LS, Brusch RG, Laymon RA, Niesman IR, Gordesky-Gold B, Sintasath L, et al. Disruption of axonal transport by loss of huntingtin or expression of pathogenic PolyQ proteins in drosophila. Neuron. . 2003;40:25–40. doi: 10.1016/S0896-6273(03)00594-4. [DOI] [PubMed] [Google Scholar]
- 64.Sanpei K, Takano H, Igarashi S, Sato T, Oyake M, Sasaki H, Wakisaka A, et al. Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique, DIRECT. Nat Genet. . 1996;14:277–284. doi: 10.1038/ng1196-277. [DOI] [PubMed] [Google Scholar]
- 65.Imbert G, Saudou F, Yvert G, Devys D, Trottier Y, Garnier JM, Weber C, et al. Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet. . 1996;14:285–291. doi: 10.1038/ng1196-285. [DOI] [PubMed] [Google Scholar]
- 66.Pulst SM, Nechiporuk A, Nechiporuk T, Gispert S, Chen XN, Lopes-Cendes I, Pearlman S, et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet. . 1996;14:269–276. doi: 10.1038/ng1196-269. [DOI] [PubMed] [Google Scholar]
- 67.Bevivino AE, Loll PJ. An expanded glutamine repeat destabilizes native ataxin-3 structure and mediates formation of parallel β-fibrils. Proc Natl Acad Sci USA. . 2001;98:11955–11960. doi: 10.1073/pnas.211305198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chow MKM, Ellisdon AM, Cabrita LD, Bottomley SP. Polyglutamine expansion in ataxin-3 does not affect protein stability. J Biol Chem. . 2004;279:47643–47651. doi: 10.1074/jbc.M405799200. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi M, Obayashi M, Ishiguro T, Sato N, Niimi Y, Ozaki K, Mogushi K, et al. Cyoplasmic location of α1A voltage-gated calcium channel C-terminal fragment (Cav2.1-CTF) aggregate is sufficient to cause cell death. PLoS One. 2013, 8: e50121 . [DOI] [PMC free article] [PubMed]
- 70.Niewiadomska-Cimicka A, Trottier Y. Molecular targets and therapeutic strategies in spinocerebellar ataxia type 7. Neurotherapeutics. . 2019;16:1074–1096. doi: 10.1007/s13311-019-00778-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reid SJ, Rees MI, van Roon-Mom WMC, Jones AL, MacDonald ME, Sutherland G, During MJ, et al. Molecular investigation of TBP allele length. Neurobiol Dis. . 2003;13:37–45. doi: 10.1016/S0969-9961(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 72.McCampbell A, Fischbeck KH. Polyglutamine and CBP: fatal attraction? Nat Med. . 2001;7:528–530. doi: 10.1038/87842. [DOI] [PubMed] [Google Scholar]
- 73.Dai XX, Pi SB, Zhao LW, Wu YW, Shen JL, Zhang SY, Sha QQ, et al. PABPN1 functions as a hub in the assembly of nuclear poly(A) domains that are essential for mouse oocyte development. Sci Adv. . 2022;8:eabn9016. doi: 10.1126/sciadv.abn9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Basu S, Mackowiak SD, Niskanen H, Knezevic D, Asimi V, Grosswendt S, Geertsema H, et al. Unblending of transcriptional condensates in human repeat expansion disease. Cell. . 2020;181:1062–1079.e30. doi: 10.1016/j.cell.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boivin M, Deng J, Pfister V, Grandgirard E, Oulad-Abdelghani M, Morlet B, Ruffenach F, et al. Translation of GGC repeat expansions into a toxic polyglycine protein in NIID defines a novel class of human genetic disorders: the polyG diseases. Neuron. . 2021;109:1825–1835.e5. doi: 10.1016/j.neuron.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Todd PK, Oh SY, Krans A, He F, Sellier C, Frazer M, Renoux AJ, et al. CGG repeat-associated translation mediates neurodegeneration in Fragile X tremor ataxia syndrome. Neuron. . 2013;78:440–455. doi: 10.1016/j.neuron.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conlon EG, Lu L, Sharma A, Yamazaki T, Tang T, Shneider NA, Manley JL. The C9ORF72 GGGGCC expansion forms RNA G-quadruplex inclusions and sequesters hnRNP H to disrupt splicing in ALS brains. Elife. . 2016;5:e17820. doi: 10.7554/eLife.17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reddy K, Zamiri B, Stanley SYR, Macgregor Jr. RB, Pearson CE. The disease-associated r(GGGGCC) repeat from the C9orf72 gene forms tract length-dependent uni- and multimolecular RNA G-quadruplex structures. J Biol Chem. . 2013;288:9860–9866. doi: 10.1074/jbc.C113.452532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fratta P, Mizielinska S, Nicoll AJ, Zloh M, Fisher EMC, Parkinson G, Isaacs AM. C9orf72 hexanucleotide repeat associated with amyotrophic lateral sclerosis and frontotemporal dementia forms RNA G-quadruplexes. Sci Rep. . 2012;2:1016. doi: 10.1038/srep01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harley HG, Brook JD, Rundle SA, Crow S, Reardon W, Buckler AJ, Harper PS, et al. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature. . 1992;355:545–546. doi: 10.1038/355545a0. [DOI] [PubMed] [Google Scholar]
- 81.Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, Hunter K, et al. Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. . 1992;68:799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- 82.Aslanidis C, Jansen G, Amemiya C, Shutler G, Mahadevan M, Tsilfidis C, Chen C, et al. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature. . 1992;355:548–551. doi: 10.1038/355548a0. [DOI] [PubMed] [Google Scholar]
- 83.van Cruchten RTP, Wieringa B, Wansink DG. Expanded CUG repeats in DMPK transcripts adopt diverse hairpin conformations without influencing the structure of the flanking sequences . RNA. . 2019;25:481–495. doi: 10.1261/rna.068940.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian B, White RJ, Xia T, Welle S, Turner DH, Mathews MB, Thornton CA. Expanded CUG repeat RNAs form hairpins that activate the double-stranded RNA-dependent protein kinase PKR. RNA. . 2000;6:79–87. doi: 10.1017/S1355838200991544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kremer EJ, Pritchard M, Lynch M, Yu S, Holman K, Baker E, Warren ST, et al. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science. . 1991;252:1711–1714. doi: 10.1126/science.1675488. [DOI] [PubMed] [Google Scholar]
- 86.Verkerk AJMH, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DPA, Pizzuti A, Reiner O, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. . 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-H. [DOI] [PubMed] [Google Scholar]
- 87.Oberlé I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boué J, et al. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. . 1991;252:1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- 88.Fu YH, Kuhl DPA, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkert AJMH, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: Resolution of the Sherman paradox. Cell. . 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 89.Tassone F, Iwahashi C, Hagerman PJ. FMR1 RNA within the intranuclear inclusions of Fragile X-Associated tremor/ataxia syndrome (FXTAS) RNA Biol. . 2004;1:103–105. doi: 10.4161/rna.1.2.1035. [DOI] [PubMed] [Google Scholar]
- 90.Krans A, Skariah G, Zhang Y, Bayly B, Todd PK. Neuropathology of RAN translation proteins in fragile X-associated tremor/ataxia syndrome. Acta Neuropathol Commun. . 2019;7:152. doi: 10.1186/s40478-019-0782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zu T, Liu Y, Bañez-Coronel M, Reid T, Pletnikova O, Lewis J, Miller TM, et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia . Proc Natl Acad Sci USA. . 2013;110:E4968–77. doi: 10.1073/pnas.1315438110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS . Science. . 2013;339:1335–1338. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- 93.Ash PEA, Bieniek KF, Gendron TF, Caulfield T, Lin WL, DeJesus-Hernandez M, van Blitterswijk MM, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. . 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin Y, Mori E, Kato M, Xiang S, Wu L, Kwon I, McKnight SL. Toxic PR Poly-Dipeptides encoded by the C9orf72 repeat expansion target LC domain polymers. Cell. . 2016;167:789–802.e12. doi: 10.1016/j.cell.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asamitsu S, Yabuki Y, Ikenoshita S, Kawakubo K, Kawasaki M, Usuki S, Nakayama Y, et al. CGG repeat RNA G-quadruplexes interact with FMRpolyG to cause neuronal dysfunction in fragile X-related tremor/ataxia syndrome. Sci Adv. . 2021;7:eabd9440. doi: 10.1126/sciadv.abd9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zu T, Cleary JD, Liu Y, Bañez-Coronel M, Bubenik JL, Ayhan F, Ashizawa T, et al. RAN translation regulated by muscleblind proteins in myotonic dystrophy type 2. Neuron. . 2017;95:1292–1305.e5. doi: 10.1016/j.neuron.2017.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cleary JD, Pattamatta A, Ranum LPW. Repeat-associated non-ATG (RAN) translation. J Biol Chem. . 2018;293:16127–16141. doi: 10.1074/jbc.R118.003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Paulson, H. Repeat expansion diseases. Handb Clin Neurol. , 2018, 147: 105–123 . [DOI] [PMC free article] [PubMed]
- 99.La Spada AR, Taylor JP. Repeat expansion disease: progress and puzzles in disease pathogenesis. Nat Rev Genet. . 2010;11:247–258. doi: 10.1038/nrg2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bäuerlein FJB, Saha I, Mishra A, Kalemanov M, Martínez-Sánchez A, Klein R, Dudanova I, et al. In situ architecture and cellular interactions of PolyQ inclusions. Cell. . 2017;171:179–187.e10. doi: 10.1016/j.cell.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 101.Klockgether T, Mariotti C, Paulson HL. Spinocerebellar ataxia. Nat Rev Dis Primers. . 2019;5:24. doi: 10.1038/s41572-019-0074-3. [DOI] [PubMed] [Google Scholar]
- 102.Becker LA, Huang B, Bieri G, Ma R, Knowles DA, Jafar-Nejad P, Messing J, et al. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature. . 2017;544:367–371. doi: 10.1038/nature22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. . 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gruber A, Hornburg D, Antonin M, Krahmer N, Collado J, Schaffer M, Zubaite G, et al. Molecular and structural architecture of polyQ aggregates in yeast. Proc Natl Acad Sci USA. . 2018;115:E3446. doi: 10.1073/pnas.1717978115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, et al. The nuclear receptor superfamily: the second decade. Cell. . 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xie J, He H, Kong W, Li Z, Gao Z, Xie D, Sun L, et al. Targeting androgen receptor phase separation to overcome antiandrogen resistance. Nat Chem Biol. . 2022;18:1341–1350. doi: 10.1038/s41589-022-01151-y. [DOI] [PubMed] [Google Scholar]
- 107.Albrecht A, Mundlos S. The other trinucleotide repeat: polyalanine expansion disorders. Curr Opin Genet Dev. . 2005;15:285–293. doi: 10.1016/j.gde.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 108.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. . 2004;10:S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 109.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. . 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 110.Utsch B, Becker K, Brock D, Lentze MJ, Bidlingmaier F, Ludwig M. A novel stable polyalanine [poly(A)] expansion in the HOXA13 gene associated with hand-foot-genital syndrome: proper function of poly(A)-harbouring transcription factors depends on a critical repeat length? Hum Genet. . 2002;110:488–494. doi: 10.1007/s00439-002-0712-8. [DOI] [PubMed] [Google Scholar]
- 111.Goodman FR, Bacchelli C, Brady AF, Brueton LA, Fryns JP, Mortlock DP, Innis JW, et al. Novel HOXA13 mutations and the phenotypic spectrum of hand-foot-genital syndrome. Am J Hum Genet. . 2000;67:197–202. doi: 10.1086/302961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shibata A, Machida J, Yamaguchi S, Kimura M, Tatematsu T, Miyachi H, Matsushita M, et al. Characterisation of novel RUNX2 mutation with alanine tract expansion from Japanese cleidocranial dysplasia patient. Mutagenesis. 2016. 31: 61–67 . [DOI] [PubMed]
- 113.Mastushita M, Kitoh H, Subasioglu A, Kurt Colak F, Dundar M, Mishima K, Nishida Y, et al. A glutamine repeat variant of the RUNX2 gene causes cleidocranial dysplasia. Mol Syndromol. . 2015;6:50–53. doi: 10.1159/000370337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuss P, Villavicencio-Lorini P, Witte F, Klose J, Albrecht AN, Seemann P, Hecht J, et al. Mutant Hoxd13 induces extra digits in a mouse model of synpolydactyly directly and by decreasing retinoic acid synthesis. J Clin Invest. 2009, 119: 146–156 . [DOI] [PMC free article] [PubMed]
- 115.Richard P, Trollet C, Stojkovic T, de Becdelievre A, Perie S, Pouget J, Eymard B. Correlation between PABPN1 genotype and disease severity in oculopharyngeal muscular dystrophy . Neurology. . 2017;88:359–365. doi: 10.1212/WNL.0000000000003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klein AF, Ebihara M, Alexander C, Dicaire MJ, Sasseville AMJ, Langelier Y, Rouleau GA, et al. PABPN1 polyalanine tract deletion and long expansions modify its aggregation pattern and expression. Exp Cell Res. . 2008;314:1652–1666. doi: 10.1016/j.yexcr.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 117.Liufu T, Zheng Y, Yu J, Yuan Y, Wang Z, Deng J, Hong D. The polyG diseases: a new disease entity. Acta Neuropathol Commun. . 2022;10:79. doi: 10.1186/s40478-022-01383-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang JZ, Lu TW, Stolerman LM, Tenner B, Yang JR, Zhang JF, Falcke M, et al. Phase separation of a PKA regulatory subunit controls cAMP compartmentation and oncogenic signaling. Cell. . 2020;182:1531–1544.e15. doi: 10.1016/j.cell.2020.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vyas M, Hechtman JF, Zhang Y, Benayed R, Yavas A, Askan G, Shia J, et al. DNAJB1-PRKACA fusions occur in oncocytic pancreatic and biliary neoplasms and are not specific for fibrolamellar hepatocellular carcinoma. Modern Pathol. . 2020;33:648–656. doi: 10.1038/s41379-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.AACR Project GENIE Consortium. AACR project GENIE: powering precision medicine through an international consortium. Cancer Discov. . 2017;7:818–831. doi: 10.1158/2159-8290.CD-17-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chandra B, Michmerhuizen NL, Shirnekhi HK, Tripathi S, Pioso BJ, Baggett DW, Mitrea DM, et al. Phase separation mediates NUP98 fusion oncoprotein leukemic transformation. Cancer Discov. . 2022;12:1152–1169. doi: 10.1158/2159-8290.CD-21-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ahn JH, Davis ES, Daugird TA, Zhao S, Quiroga IY, Uryu H, Li J, et al. Phase separation drives aberrant chromatin looping and cancer development. Nature. . 2021;595:591–595. doi: 10.1038/s41586-021-03662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tulpule A, Guan J, Neel DS, Allegakoen HR, Lin YP, Brown D, Chou YT, et al. Kinase-mediated RAS signaling via membraneless cytoplasmic protein granules. Cell. . 2021;184:2649–2664.e18. doi: 10.1016/j.cell.2021.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taniue K, Akimitsu N. Aberrant phase separation and cancer. FEBS J. . 2022;289:17–39. doi: 10.1111/febs.15765. [DOI] [PubMed] [Google Scholar]
- 125.di Masi A, Cilli D, Berardinelli F, Talarico A, Pallavicini I, Pennisi R, Leone S, et al. PML nuclear body disruption impairs DNA double-strand break sensing and repair in APL. Cell Death Dis. . 2016;7:e2308. doi: 10.1038/cddis.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nason-Burchenal K, Takle G, Pace U, Flynn S, Allopenna J, Martin P, George ST, et al. Targeting the PML/RARα translocation product triggers apoptosis in promyelocytic leukemia cells. Oncogene. . 1998;17:1759–1768. doi: 10.1038/sj.onc.1202075. [DOI] [PubMed] [Google Scholar]
- 127.Song L, Yao X, Li H, Peng B, Boka AP, Liu Y, Chen G, et al. Hotspot mutations in the structured ENL YEATS domain link aberrant transcriptional condensates and cancer. Mol Cell. . 2022;82:4080–4098.e12. doi: 10.1016/j.molcel.2022.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang F, Biswas M, Massah S, Lee J, Lingadahalli S, Wong S, Wells C, et al. Dynamic phase separation of the androgen receptor and its coactivators key to regulate gene expression. Nucleic Acids Res. . 2023;51:99–116. doi: 10.1093/nar/gkac1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Haaften G, Dalgliesh GL, Davies H, Chen L, Bignell G, Greenman C, Edkins S, et al. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet. . 2009;41:521–523. doi: 10.1038/ng.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bouchard JJ, Otero JH, Scott DC, Szulc E, Martin EW, Sabri N, Granata D, et al. Cancer mutations of the tumor suppressor SPOP disrupt the formation of active, phase-separated compartments. Mol Cell. . 2018;72:19–36.e8. doi: 10.1016/j.molcel.2018.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li W, Hu J, Shi B, Palomba F, Digman MA, Gratton E, Jiang H. Biophysical properties of AKAP95 protein condensates regulate splicing and tumorigenesis. Nat Cell Biol. . 2020;22:960–972. doi: 10.1038/s41556-020-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhong ZA, Michalski MN, Stevens PD, Sall EA, Williams BO. Regulation of Wnt receptor activity: Implications for therapeutic development in colon cancer. J Biol Chem. . 2021;296:100782. doi: 10.1016/j.jbc.2021.100782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nong J, Kang K, Shi Q, Zhu X, Tao Q, Chen YG. Phase separation of Axin organizes the β-catenin destruction complex. J Cell Biol. . 2021;220:e202012112. doi: 10.1083/jcb.202012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miete C, Solis GP, Koval A, Brückner M, Katanaev VL, Behrens J, Bernkopf DB. Gαi2-induced conductin/axin2 condensates inhibit Wnt/β-catenin signaling and suppress cancer growth. Nat Commun. . 2022;13:674. doi: 10.1038/s41467-022-28286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu Y, Zhou L, Zou Y, Zhang Y, Zhang M, Xu L, Zheng L, et al. Disrupting the phase separation of KAT8–IRF1 diminishes PD-L1 expression and promotes antitumor immunity. Nat Cancer. . 2023;4:382–400. doi: 10.1038/s43018-023-00522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mediani L, Guillén-Boixet J, Alberti S, Carra S. Nucleoli and promyelocytic leukemia protein (PML) bodies are phase separated nuclear protein quality control compartments for misfolded proteins. Mol Cell Oncol. . 2019;6:e1415624. doi: 10.1080/23723556.2019.1652519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Corpet A, Kleijwegt C, Roubille S, Juillard F, Jacquet K, Texier P, Lomonte P. PML nuclear bodies and chromatin dynamics: catch me if you can! Nucleic Acids Res. . 2020;48:11890–11912. doi: 10.1093/nar/gkaa828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Van Damme E, Laukens K, Dang TH, Van Ostade X. A manually curated network of the PML nuclear body interactome reveals an important role for PML-NBs in SUMOylation dynamics. Int J Biol Sci. . 2010;6:51–67. doi: 10.7150/ijbs.6.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. . 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. . 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li Y, Wen H, Xi Y, Tanaka K, Wang H, Peng D, Ren Y, et al. AF9 YEATS domain links histone acetylation to DOT1L-mediated H3K79 methylation. Cell. . 2014;159:558–571. doi: 10.1016/j.cell.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wan L, Chong S, Xuan F, Liang A, Cui X, Gates L, Carroll TS, et al. Impaired cell fate through gain-of-function mutations in a chromatin reader. Nature. . 2020;577:121–126. doi: 10.1038/s41586-019-1842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Erb MA, Scott TG, Li BE, Xie H, Paulk J, Seo HS, Souza A, et al. Transcription control by the ENL YEATS domain in acute leukaemia. Nature. . 2017;543:270–274. doi: 10.1038/nature21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.May WA, Lessnick SL, Braun BS, Klemsz M, Lewis BC, Lunsford LB, Hromas R, et al. The ewing’s sarcoma EWS/FLI-1 fusion gene encodes a more potent transcriptional activator and is a more powerful transforming gene than FLI-1 . Mol Cell Biol. . 1993;13:7393–7398. doi: 10.1128/mcb.13.12.7393-7398.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zuo L, Zhang G, Massett M, Cheng J, Guo Z, Wang L, Gao Y, et al. Loci-specific phase separation of FET fusion oncoproteins promotes gene transcription. Nat Commun. . 2021;12:1491. doi: 10.1038/s41467-021-21690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boulay G, Sandoval GJ, Riggi N, Iyer S, Buisson R, Naigles B, Awad ME, et al. Cancer-specific retargeting of BAF complexes by a prion-like domain. Cell. . 2017;171:163–178.e19. doi: 10.1016/j.cell.2017.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bisio V, Zampini M, Tregnago C, Manara E, Salsi V, Di Meglio A, Masetti R, et al. NUP98-fusion transcripts characterize different biological entities within acute myeloid leukemia: a report from the AIEOP-AML group. Leukemia. . 2017;31:974–977. doi: 10.1038/leu.2016.361. [DOI] [PubMed] [Google Scholar]
- 148.Lan F, Bayliss PE, Rinn JL, Whetstine JR, Wang JK, Chen S, Iwase S, et al. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature. . 2007;449:689–694. doi: 10.1038/nature06192. [DOI] [PubMed] [Google Scholar]
- 149.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. . 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 150.Mano H. Non-solid oncogenes in solid tumors: EML4-ALK fusion genes in lung cancer . Cancer Sci. . 2008;99:2349–2355. doi: 10.1111/j.1349-7006.2008.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Matsubara D, Kanai Y, Ishikawa S, Ohara S, Yoshimoto T, Sakatani T, Oguni S, et al. Identification of CCDC6-RET fusion in the human lung adenocarcinoma cell line, LC-2/ad. J Thoracic Oncol. . 2012;7:1872–1876. doi: 10.1097/JTO.0b013e3182721ed1. [DOI] [PubMed] [Google Scholar]
- 152.Cerrato A, Visconti R, Celetti A. The rationale for druggability of CCDC6-tyrosine kinase fusions in lung cancer. Mol Cancer. . 2018;17:46. doi: 10.1186/s12943-018-0799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu Q, Li J, Zhang W, Xiao C, Zhang S, Nian C, Li J, et al. Glycogen accumulation and phase separation drives liver tumor initiation. Cell. . 2021;184:5559–5576.e19. doi: 10.1016/j.cell.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 154.Xie H, Song J, Godfrey J, Riscal R, Skuli N, Nissim I, Simon MC. Glycogen metabolism is dispensable for tumour progression in clear cell renal cell carcinoma. Nat Metab. . 2021;3:327–336. doi: 10.1038/s42255-021-00367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hernández-Muñoz I, Lund AH, van der Stoop P, Boutsma E, Muijrers I, Verhoeven E, Nusinow DA, et al. Stable X chromosome inactivation involves the PRC1 Polycomb complex and requires histone MACROH2A1 and the CULLIN3/SPOP ubiquitin E3 ligase. Proc Natl Acad Sci USA. . 2005;102:7635–7640. doi: 10.1073/pnas.0408918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim MS, Je EM, Oh JE, Yoo NJ, Lee SH. Mutational and expressional analyses of SPOP, a candidate tumor suppressor gene, in prostate, gastric and colorectal cancers . APMIS. . 2013;121:626–633. doi: 10.1111/apm.12030. [DOI] [PubMed] [Google Scholar]
- 157.Uversky VN. Phase separation and infectious diseases. Droplets of Life. 2023: p. 681-698
- 158.Li H, Ernst C, Kolonko-Adamska M, Greb-Markiewicz B, Man J, Parissi V, Ng BWL. Phase separation in viral infections. Trends Microbiol. . 2022;30:1217–1231. doi: 10.1016/j.tim.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 159.Wang B, Zhang L, Dai T, Qin Z, Lu H, Zhang L, Zhou F. Liquid–liquid phase separation in human health and diseases. Sig Transduct Target Ther. . 2021;6:290. doi: 10.1038/s41392-021-00678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li X, Shu C, Yi G, Chaton CT, Shelton CL, Diao J, Zuo X, et al. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity. . 2013;39:1019–1031. doi: 10.1016/j.immuni.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type i interferon pathway. Science. . 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Barber GN. STING-dependent cytosolic DNA sensing pathways. Trends Immunol. . 2014;35:88–93. doi: 10.1016/j.it.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 163.Balka KR, Louis C, Saunders TL, Smith AM, Calleja DJ, D′Silva DB, Moghaddas F, et al. TBK1 and IKKε act redundantly to mediate STING-induced NF-κB responses in myeloid cells. Cell Rep. . 2020;31:107492. doi: 10.1016/j.celrep.2020.03.056. [DOI] [PubMed] [Google Scholar]
- 164.Zhou W, Mohr L, Maciejowski J, Kranzusch PJ. cGAS phase separation inhibits TREX1-mediated DNA degradation and enhances cytosolic DNA sensing. Mol Cell. . 2021;81:739–755.e7. doi: 10.1016/j.molcel.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu J, Li W, Shao Y, Avey D, Fu B, Gillen J, Hand T, et al. Inhibition of cGAS DNA sensing by a herpesvirus virion protein. Cell Host Microbe. . 2015;18:333–344. doi: 10.1016/j.chom.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu G, Liu C, Zhou S, Li Q, Feng Y, Sun P, Feng H, et al. Viral tegument proteins restrict cGAS-DNA phase separation to mediate immune evasion. Mol Cell. . 2021;81:2823–2837.e9. doi: 10.1016/j.molcel.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 167.Hertzog J, Zhou W, Fowler G, Rigby RE, Bridgeman A, Blest HT, Cursi C, et al. Varicella‐Zoster virus ORF9 is an antagonist of the DNA sensor cGAS. EMBO J. . 2022;41:e109217. doi: 10.15252/embj.2021109217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yu X, Zhang L, Shen J, Zhai Y, Jiang Q, Yi M, Deng X, et al. The STING phase-separator suppresses innate immune signalling. Nat Cell Biol. . 2021;23:330–340. doi: 10.1038/s41556-021-00659-0. [DOI] [PubMed] [Google Scholar]
- 169.Youn JY, Dyakov BJA, Zhang J, Knight JDR, Vernon RM, Forman-Kay JD, Gingras AC. Properties of stress granule and p-body proteomes. Mol Cell. . 2019;76:286–294. doi: 10.1016/j.molcel.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 170.Zhang Q, Sharma NR, Zheng ZM, Chen M. Viral regulation of RNA granules in infected cells. Virol Sin. . 2019;34:175–191. doi: 10.1007/s12250-019-00122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Emara MM, Brinton MA. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc Natl Acad Sci USA. . 2007;104:9041–9046. doi: 10.1073/pnas.0703348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dougherty J, Tsai WC, Lloyd R. Multiple poliovirus proteins repress cytoplasmic RNA granules. Viruses. . 2015;7:6127–6140. doi: 10.3390/v7122922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Linero FN, Thomas MG, Boccaccio GL, Scolaro LA. Junín virus infection impairs stress-granule formation in Vero cells treated with arsenite via inhibition of eIF2α phosphorylation. J Gen Virol. . 2011;92:2889–2899. doi: 10.1099/vir.0.033407-0. [DOI] [PubMed] [Google Scholar]
- 174.Luo L, Li Z, Zhao T, Ju X, Ma P, Jin B, Zhou Y, et al. SARS-CoV-2 nucleocapsid protein phase separates with G3BPs to disassemble stress granules and facilitate viral production. Sci Bull. . 2021;66:1194–1204. doi: 10.1016/j.scib.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lu S, Ye Q, Singh D, Cao Y, Diedrich JK, Yates Iii JR, Villa E, et al. The SARS-CoV-2 nucleocapsid phosphoprotein forms mutually exclusive condensates with RNA and the membrane-associated M protein. Nat Commun. . 2021;12:502. doi: 10.1038/s41467-020-20768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pizzato M, Baraldi C, Sopetto GB, Finozzi D, Gentile C, Gentile MD, Marconi R, et al. SARS-CoV-2 and the host cell: a tale of interactions. Front Virol. 2022, 1: https://doi.org/10.3389/fviro.2021.815388
- 177.Hou S, Kumar A, Xu Z, Airo AM, Stryapunina I, Wong CP, Branton W, et al. Zika virus hijacks stress granule proteins and modulates the host stress response. J Virol. 2017, 91: e00474-17 . [DOI] [PMC free article] [PubMed]
- 178.Courtney SC, Scherbik SV, Stockman BM, Brinton MA. West nile virus infections suppress early viral RNA synthesis and avoid inducing the cell stress granule response. J Virol. . 2012;86:3647–3657. doi: 10.1128/JVI.06549-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Quicke K, Suthar M. The innate immune playbook for restricting west nile virus infection. Viruses. . 2013;5:2643–2658. doi: 10.3390/v5112643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zotta A, Hooftman A, O′Neill LAJ. SARS-CoV-2 targets MAVS for immune evasion. Nat Cell Biol. . 2021;23:682–683. doi: 10.1038/s41556-021-00712-y. [DOI] [PubMed] [Google Scholar]
- 181.Nakagawa K, Kobayashi Y, Ito N, Suzuki Y, Okada K, Makino M, Goto H, et al. Molecular function analysis of rabies virus RNA polymerase L protein by using an L gene-deficient virus. J Virol. 2017, 91: e00826-17 . [DOI] [PMC free article] [PubMed]
- 182.Albertini ÁAV, Wernimont AK, Muziol T, Ravelli RBG, Clapier CR, Schoehn G, Weissenhorn W, et al. Crystal structure of the rabies virus nucleoprotein-RNA complex. Science. . 2006;313:360–363. doi: 10.1126/science.1125280. [DOI] [PubMed] [Google Scholar]
- 183.Liu G, Chen C, Xu R, Yang M, Han Q, Wang B, Song Y, et al. Function of host protein staufen1 in rabies virus replication. Viruses. . 2021;13:1426. doi: 10.3390/v13081426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Guseva S, Milles S, Jensen MR, Salvi N, Kleman JP, Maurin D, Ruigrok RWH, et al. Measles virus nucleo- and phosphoproteins form liquid-like phase-separated compartments that promote nucleocapsid assembly. Sci Adv. . 2020;6:eaaz7095. doi: 10.1126/sciadv.aaz7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhou Y, Su JM, Samuel CE, Ma D. Measles virus forms inclusion bodies with properties of liquid organelles. J Virol. 2019, 93: e00948-19 . [DOI] [PMC free article] [PubMed]
- 186.Galloux M, Risso-Ballester J, Richard CA, Fix J, Rameix-Welti MA, Eléouët JF. Minimal elements required for the formation of respiratory syncytial virus cytoplasmic inclusion bodies in vivo and in vitro. mBio. 2020, 11: e01202–20 . [DOI] [PMC free article] [PubMed]
- 187.Selvaraj M, Yegambaram K, Todd EJAA, Richard CA, Dods RL, Pangratiou GM, Trinh CH, et al. The structure of the human respiratory syncytial virus M2-1 protein bound to the interaction domain of the phosphoprotein P defines the orientation of the complex. mBio. 2018, 9: e01554–18 . [DOI] [PMC free article] [PubMed]
- 188.Risso-Ballester J, Galloux M, Cao J, Le Goffic R, Hontonnou F, Jobart-Malfait A, Desquesnes A, et al. A condensate-hardening drug blocks RSV replication in vivo . Nature. . 2021;595:596–599. doi: 10.1038/s41586-021-03703-z. [DOI] [PubMed] [Google Scholar]
- 189.Rincheval V, Lelek M, Gault E, Bouillier C, Sitterlin D, Blouquit-Laye S, Galloux M, et al. Functional organization of cytoplasmic inclusion bodies in cells infected by respiratory syncytial virus. Nat Commun. . 2017;8:563. doi: 10.1038/s41467-017-00655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Richard CA, Rincheval V, Lassoued S, Fix J, Cardone C, Esneau C, Nekhai S, et al. RSV hijacks cellular protein phosphatase 1 to regulate M2-1 phosphorylation and viral transcription. PLoS Pathog. 2018, 14: e1006920 . [DOI] [PMC free article] [PubMed]
- 191.Alenquer M, Vale-Costa S, Etibor TA, Ferreira F, Sousa AL, Amorim MJ. Influenza A virus ribonucleoproteins form liquid organelles at endoplasmic reticulum exit sites. Nat Commun. . 2019;10:1629. doi: 10.1038/s41467-019-09549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Scoca V, Di Nunzio F. Membraneless organelles restructured and built by pandemic viruses: HIV-1 and SARS-CoV-2. J Mol Cell Biol. 2021, 13: 259–268 . [DOI] [PMC free article] [PubMed]
- 193.Monette A, Niu M, Nijhoff Asser M, Gorelick RJ, Mouland AJ. Scaffolding viral protein NC nucleates phase separation of the HIV-1 biomolecular condensate. Cell Rep. . 2022;40:111251. doi: 10.1016/j.celrep.2022.111251. [DOI] [PubMed] [Google Scholar]
- 194.Monette A, Niu M, Chen L, Rao S, Gorelick RJ, Mouland AJ. Pan-retroviral nucleocapsid-mediated phase separation regulates genomic RNA positioning and trafficking. Cell Rep. . 2020;31:107520. doi: 10.1016/j.celrep.2020.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Savastano A, Ibáñez de Opakua A, Rankovic M, Zweckstetter M. Nucleocapsid protein of SARS-CoV-2 phase separates into RNA-rich polymerase-containing condensates. Nat Commun. . 2020;11:6041. doi: 10.1038/s41467-020-19843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhao D, Xu W, Zhang X, Wang X, Ge Y, Yuan E, Xiong Y, et al. Understanding the phase separation characteristics of nucleocapsid protein provides a new therapeutic opportunity against SARS-CoV-2. Protein Cell. . 2021;12:734–740. doi: 10.1007/s13238-021-00832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Luo Y, Zhang Y, Liu X, Yang Y, Yang X, Zhang D, Deng X, et al. Complete genome sequence of a highly virulent rabies virus isolated from a rabid pig in south china. J Virol. . 2012;86:12454–12455. doi: 10.1128/JVI.02234-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lahaye X, Vidy A, Pomier C, Obiang L, Harper F, Gaudin Y, Blondel D. Functional characterization of negri bodies (NBs) in rabies virus-infected cells: evidence that NBs are sites of viral transcription and replication. J Virol. . 2009;83:7948–7958. doi: 10.1128/JVI.00554-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Brocca S, Grandori R, Longhi S, Uversky V. Liquid-liquid phase separation by intrinsically disordered protein regions of viruses: roles in viral life cycle and control of virus-host interactions. Int J Mol Sci. 2020, 21: 9045 . [DOI] [PMC free article] [PubMed]
- 200.Nevers Q, Scrima N, Glon D, Le Bars R, Decombe A, Garnier N, Ouldali M, et al. Properties of rabies virus phosphoprotein and nucleoprotein biocondensates formed in vitro and in cellulo. PLoS Pathog. 2022, 18: e1011022 . [DOI] [PMC free article] [PubMed]
- 201.Nikolic J, Le Bars R, Lama Z, Scrima N, Lagaudrière-Gesbert C, Gaudin Y, Blondel D. Negri bodies are viral factories with properties of liquid organelles. Nat Commun. . 2017;8:58. doi: 10.1038/s41467-017-00102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jack A, Ferro LS, Trnka MJ, Wehri E, Nadgir A, Nguyenla X, Fox D, et al. SARS-CoV-2 nucleocapsid protein forms condensates with viral genomic RNA. PLOS Biology. 2021, 19: e3001425 . [DOI] [PMC free article] [PubMed]
- 203.Iserman C, Roden CA, Boerneke MA, Sealfon RSG, McLaughlin GA, Jungreis I, Fritch EJ, et al. Genomic RNA elements drive phase separation of the SARS-CoV-2 nucleocapsid. Mol Cell. . 2020;80:1078–1091.e6. doi: 10.1016/j.molcel.2020.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Brown SL, Garrison DJ, May JP. Phase separation of a plant virus movement protein and cellular factors support virus-host interactions. PLOS Pathogens. 2021, 17: e1009622 . [DOI] [PMC free article] [PubMed]
- 205.Strzyz P. Drugs enter a liquid phase. Nat Rev Mol Cell Biol. . 2020;21:419. doi: 10.1038/s41580-020-0268-2. [DOI] [PubMed] [Google Scholar]
- 206.Klein IA, Boija A, Afeyan LK, Hawken SW, Fan M, Dall′Agnese A, Oksuz O, et al. Partitioning of cancer therapeutics in nuclear condensates. Science. . 2020;368:1386–1392. doi: 10.1126/science.aaz4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li Z, Wang C, Wang Z, Zhu C, Li J, Sha T, Ma L, et al. Allele-selective lowering of mutant HTT protein by HTT–LC3 linker compounds. Nature. . 2019;575:203–209. doi: 10.1038/s41586-019-1722-1. [DOI] [PubMed] [Google Scholar]
- 208.Takahashi D, Moriyama J, Nakamura T, Miki E, Takahashi E, Sato A, Akaike T, et al. AUTACs: cargo-specific degraders using selective autophagy. Mol Cell. . 2019;76:797–810.e10. doi: 10.1016/j.molcel.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 209.Békés M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. . 2022;21:181–200. doi: 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shi Y, Liao Y, Liu Q, Ni Z, Zhang Z, Shi M, Li P, et al. BRD4-targeting PROTAC as a unique tool to study biomolecular condensates. Cell Discov. . 2023;9:1–13. doi: 10.1038/s41421-023-00544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]