Abstract
The development of collateral circulation is not that rare in patients with congenital heart defects. These collaterals can affect cardiovascular hemodynamics and cause systemic arterial desaturation, which arises the question whether these should be closed. To date, few if any reports have been published on the therapeutic management of collaterals in adult patients with congenital heart disease in heart failure (HF). The focus of this article is to provide a pragmatic approach in the assessment of collateral circulation of the patient with HF. By considering the underlying hemodynamics and overall effects of the collateral circulation, we aim to provide a practical tool useful in clinical decision making. The paper highlights mainly the systemic venous to systemic venous collaterals, systemic venous to pulmonary venous (or pulmonary venous atrium) collaterals, and pulmonary arterio-venous malformations. Systemic venous anomalies are frequent and reported in 20% to 40% of patients who underwent Glenn or Fontan procedure. A reduction in effective pulmonary blood flow, coupled with increasing oxygen demands with growth, as well as a pressure difference between the higher pressure caval venous system and lower pressure atria (so called decompressing collaterals) are potential causes of collateral formation. Whether angiogenesis de novo or reappearance of embryological venous channels is responsible for collateral formation remains to be elucidated.
Keywords: Collateral, Fontan, Glenn, single ventricle, closure
Introduction
The development of collateral circulation is not that rare in patients with congenital heart defects. These collaterals can affect cardiovascular hemodynamics and cause systemic arterial desaturation, which arises the question whether these should be closed.
To date, few if any reports have been published on the therapeutic management of collaterals in adult patients with congenital heart disease in heart failure (HF). The focus of this article is to provide a pragmatic approach in the assessment of collateral circulation of the patient with HF. By considering the underlying hemodynamics and overall effects of the collateral circulation, we aim to provide a practical tool useful in clinical decision making. The paper highlights mainly the systemic venous to systemic venous collaterals, systemic venous to pulmonary venous (or pulmonary venous atrium) collaterals, and pulmonary arterio-venous malformations.
Systemic venous anomalies are frequent and reported in 20% to 40% of patients who underwent Glenn or Fontan procedure (1-3). A reduction in effective pulmonary blood flow, coupled with increasing oxygen demands with growth, as well as a pressure difference between the higher pressure caval venous system and lower pressure atria (so called decompressing collaterals) are potential causes of collateral formation. Whether angiogenesis de novo or reappearance of embryological venous channels is responsible for collateral formation remains to be elucidated (1).
Collaterals in patients with a Glenn and Fontan circulation
The article aims to focus specifically on collaterals that develop in a Glenn and Fontan circulation. The question that needs answering is whether it is useful to close a collateral in the setting of signs and symptoms of HF, here defined as systolic and/or diastolic dysfunction of the systemic ventricle requiring a medical intervention.
Systemic venous to systemic venous collaterals
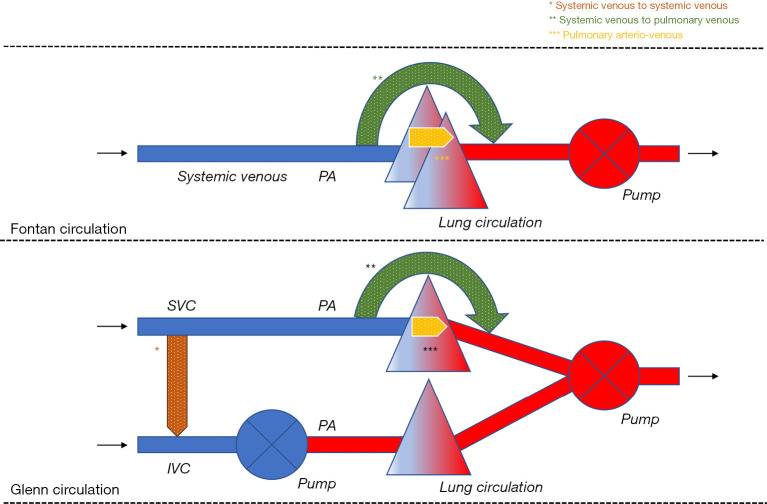
Systemic venous to systemic venous collaterals are found in the chest and the abdominal cavity. They usually develop in a Glenn circulation where higher pressure in the superior vena cava (SVC) causes a collateral network that finds its way towards a low-pressure area (4). In a Glenn circulation, this mainly includes the system venous vascular bed connected to the inferior van cava (IVC), either directly or indirectly. This way, a systemic venous to systemic venous collateral is formed and there is run-off of blood from the SVC to the IVC. Deoxygenated blood mixes with deoxygenated blood so that no direct effect on the systemic venous and arterial circulation is expected. Nevertheless, if effective pulmonary blood flow is decreased as deoxygenated blood flows preferentially to the IVC, systemic arterial desaturation will occur making the Glenn circulation less efficient. The effect on the preload of the systemic heart is negligible because enough blood is still offered through the IVC. Any conditions that cause high pressures in the SVC can give rise to the development of these collaterals. Obstructions in the hemi-Fontan or Glenn circuit, insufficient banding of the truncus pulmonalis, increased pulmonary vascular resistance, or an immature pulmonary vascular bed can be considered the main causes. Figure 1.
Figure 1.
Schematic representation of systemic venous to systemic venous collaterals, systemic venous to pulmonary venous collaterals, and pulmonary arterio-venous collaterals. IVC, inferior vena cava; PA, pulmonary artery; SVC, superior vena cava.
Systemic venous to pulmonary venous collaterals
Most of the systemic venous to pulmonary venous collaterals are found in the chest, but they can also develop in the abdominal cavity or occur in both. One or more collaterals might originate from any part of the systemic venous circulation (IVC, SVC, the innominate vein, and the azygos and hemiazygos system) and end in the pulmonary venous circulation or the pulmonary venous atrium (5). Multiple collaterals are not uncommon in a single ventricle circulation. In the context of a Glenn (1) or Fontan circulation, the direction of blood flow is from deoxygenated blood to oxygenated blood. If a relatively large vessel is involved, this can cause increased preload of the systemic ventricle as well as progressive systemic arterial oxygen desaturation. Systemic arterial desaturation has functional repercussions. Exercise capacity is reduced, and central cyanosis may give rise to long-term organ damage.
It is believed that this type of collaterals arises from increased systemic venous pressures and that blood is seeking for a low-pressure circulation. The collateral then functions like a bypass. From a theoretical standpoint, increased pressures might be caused by an obstruction in the pulmonary arterial circuit, an immature pulmonary circulation (6), or increased pulmonary vascular resistance. Moreover, the presence of such collaterals also appears to be associated with more common hepatic fibrosis (7). In case of large systemic venous to pulmonary venous collaterals the risk to develop secondary erythrocytosis and the paradoxical embolism is increased (8,9) (Figure 1).
Pulmonary arterio-venous malformations
These collaterals, or rather called malformations in this case, occur in the lung parenchyma. These malformations bridge the pulmonary arterial and the pulmonary venous circulation. There are usually multiple malformations. In the context of a Glenn or Fontan circulation, blood flow is from deoxygenated blood to oxygenated blood. The hemodynamic effect is similar to the systemic venous to pulmonary venous collaterals. A relatively large blood vessel may cause increased preload of the systemic ventricle and progressive systemic desaturation. Also, high output has been documented in the presence of these collaterals (10). In case of a more restrictive ventricle, ventricular filling pressures of the systemic ventricle will increase and negatively impact over Fontan physiology. Moreover, the resultant systemic arterial desaturation can also have functional repercussions. Exercise capacity is reduced, and central cyanosis may give rise to organ damage in the longer term. In 84% of patients undergoing catheterization in preparation for a Fontan procedure these collaterals are found (11). Some collaterals represent vessels that pre-exist, while others consist of de novo vessels. The most suggested stimuli are cyanosis and decreased pulmonary flow volume, lower velocities, and decreased pulsatility (12). These collaterals usually develop in a lung that does not adequately participate in gas exchange in the attempt to improve oxygenation (9). Another explanation is that the occurrence of these collaterals has probably its origin in the absence of the hepatic factor “X” in the pulmonary circulation (13). It has been shown that in the lung receiving less blood that has passed through the liver circulation, these malformations develop more quickly. There is a lot of speculation about this hepatic factor ‘X’ and several molecules have already been put forward as explanations (13). It is almost certain that factor ‘X’ prevents the growth of new blood vessels. As such, these malformations appear to develop independently of an obstructed pulmonary circulation; consequently, pulmonary vascular resistance may be normal or increased in these patients. The preload in the systemic ventricle increases and the systemic saturation progressively decreases. In case of a large pulmonary arterio-venous malformation the risk to develop secondary erythrocytosis and the paradoxical embolism is increased (Figure 1).
Aortopulmonary collaterals
Aortopulmonary collaterals occur frequently in congenital heart defects. When the pulmonary vascular bed does not develop adequately, blood vessels are stimulated to remain open or to develop from the aorta intending to support the pulmonary circulation (14). These collaterals may connect with the native pulmonary arteries, thus causing a volume overload in the pulmonary circulation and the systemic ventricle. This occurs most often in a Glenn or Fontan circulation (15-17), tetralogy of Fallot (18) and pulmonary atresia with ventricular septal defect. In the latter these collaterals might be quite large and are described as major aortopulmonary collateral arteries (MAPCAs). A volume load in the pulmonary circulation can also lead to an additional pressure load. Therefore, this left-to-right shunt might be responsible for systemic ventricular failure (volume related), subpulmonary ventricular failure (pressure related) in case of the presence of a subpulmonary pump or increased systemic venous pressures in a Glenn or Fontan circulation.
Assessment of collaterals
If there is suspicion of the presence of collaterals, there are a few questions that require consideration. Location, size, direction of blood flow along with the type of vessels that are connected, and the hemodynamic consequences will determine if percutaneous closure has a beneficial effect in that specific patient.
What is the location of the collateral vessel?
Collateral blood vessels are found in the extremities, in the abdominal cavity, or in the chest. In congenital heart defects, most collaterals are found within the chest: systemic venous to systemic venous collaterals, systemic venous to pulmonary venous (or pulmonary venous atrium) connections, and pulmonary arterio-venous malformations. Arterio-venous fistula are mainly found in the peripheral extremities whereas aorto-to-pulmonary collaterals are found in the chest. It is important to realize that in some congenital heart defect patients, there may be more than 1 collateral or a combination of types of collaterals.
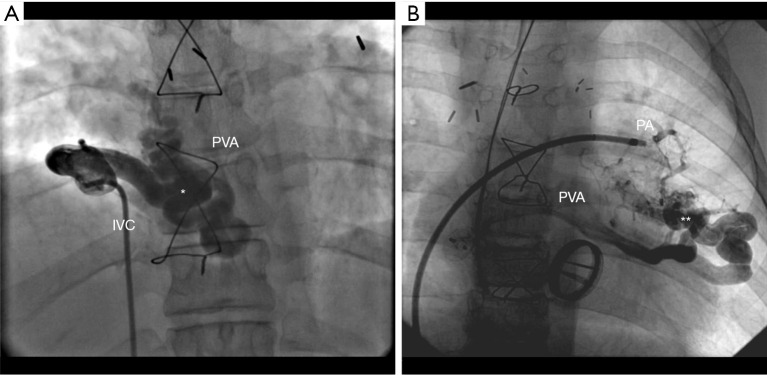
Although collaterals can be directly visualized using axial imaging, such as cardiac magnetic resonance (CMR) (10,19) or using cardiac computed tomography (CCT) (19,20), careful angiographic evaluation during cardiac catheterization is still the gold standard to assess collateral blood vessels, especially determining their origin and course (Figure 2). Saline contrast echocardiography is less invasive and can be used to detect the presence of right-to-left shunts, more specifically when searched for systemic venous to pulmonary venous collaterals or pulmonary arterio-venous malformations (21). Agitated saline which is injected in a brachial vein, is almost immediately visualizable in the pulmonary venous atrium and suspect the presence of collaterals with a right-to-left shunt.
Figure 2.
Example of (A) systemic venous to PVA collateral (*); (B) pulmonary arterio-venous malformation (**). IVC, inferior vena cava; PVA, pulmonary venous atrium; PA, pulmonary artery.
Which blood vessels connect to each other?
Basically, a vein can connect to a vein, an artery can connect to a vein, and finally a high-pressure artery can connect to a low-pressure artery. As such, each collateral has an afferent and efferent part. Prior to Fontan repair, systemic venous to systemic venous collateralization is often to the lower-pressure IVC, either directly or through intermediaries of the azygos-hemiazygos system, pericardial veins, paravertebral veins, internal mammary veins, hepatic veins, or mediastinal veins. Other collaterals connect to the coronary sinus. Another typical veno-venous connection that is commonly observed is systemic venous to pulmonary venous (or pulmonary venous atrium). In terms of arterio-venous connections, for the purpose of this manuscript, we will focus on pulmonary arterio-venous malformations which typically occur in patients who have a classic Glenn shunt. These patients have non-confluent pulmonary arteries with the SVC connected to the right pulmonary artery. Table 1 provides a summary.
Table 1. Collaterals in congenital heart disease.
| Veno-venous |
| Systemic venous to systemic venous |
| Systemic venous to pulmonary venous |
| Arterio-venous |
| Systemic arterial to systemic venous |
| Pulmonary arterial to pulmonary venous |
| Arterio-arterial |
| Systemic arterial to pulmonary arterial |
What is the direction of blood flow in the collateral?
Blood flows from a high pressure to a low-pressure environment, representing the “run-off” of contrast seen on cardiac catheterization (Figure 2). Various imaging techniques are helpful to determine the direction of blood flow within the collateral blood vessel. The direction of blood flow in the collateral in conjunction with the type of blood vessels that are connected (and their respective position in the circulation) will determine the effect of the collateral on the systemic arterial saturation. Only when there is blood running from the systemic venous circulation (i.e., deoxygenated blood) to the arterial circulation (i.e., oxygenated blood) there will be systemic desaturation. Collateral blood flow can cause volume overload to the efferent part of the circulation, independent of the direction of blood flow, but directly related to the amount of blood flow shunted.
What is the size of the collateral?
In clinical practice, the size of collateral blood vessels is extremely variable, ranging from tiny to relatively large blood vessels. This is an important consideration given that large collaterals can be readily appreciated using various imaging techniques (CMR, CCT, angiography), whereas others are very small, but in combination they can cause systemic arterial desaturation. In general, those small vessels have a limited hemodynamic impact, whereas the impact of a large vessel is usually greater. Still, although large vessels have a larger hemodynamic potential, it is the degree of flow through that collateral that will determine its impact. Finally, in the presence of multiple collaterals, it is the combined effect of number of collaterals, size of the collaterals and flow through the collaterals that will determine the overall impact. In addition, the size and shape of the collateral vessel are important determinants for which devices and what sizes of devices will be used for closure.
What is the hemodynamic effect of the collaterals?
In principle, collaterals between systemic veins have little hemodynamic implications. There are some exceptions (1) where the drainage of the SVC and the drainage of the IVC must be kept separate (mainly in hemi-Fontan patients and patients with a Glenn—either prior to Fontan completion or as a definite palliation). The systemic venous to pulmonary venous (and pulmonary venous atrium) connections are usually responsible for increased preload of the systemic ventricle and for systemic arterial desaturation. However, if reversed, the shunt through the collateral acts as a decompressing blood vessel for the subaortic pump with left right shunt and consequent volume and pressure overload of the sub-pulmonary circulation (22). Collaterals from systemic arterial to systemic venous blood vessels cause increased preload of both the sub-pulmonary and subaortic circulation with a potential risk for high output failure. Intrapulmonary collaterals from pulmonary arterial to pulmonary venous blood vessels cause increased preload of the systemic ventricle and induce systemic arterial desaturation. Finally, systemic arterial to pulmonary arterial collaterals may be responsible for an additional volume load of the pulmonary circulation and an additional pressure load for the sub-pulmonary ventricle and leaving systemic arterial saturation unaffected. Increased preload of (mainly restrictive) ventricles can cause increased filling pressures (shifting the pressure-volume loop to the right) and eventually induce or aggravate HF. The hemodynamic consequences are briefly summarized in Table 2.
Table 2. Collaterals and the effect on circulation.
| Type | Shunt | Effect on circulation |
|---|---|---|
| Systemic venous > systemic venous | No | Decreased flow via Glenn |
| Systemic venous > pulmonary venous | Deoxy > Oxy | Increased preload SV; (high output state); systemic desaturation |
| Oxy > Deoxy | Increased preload SPV | |
| Systemic arterial > systemic venous | Oxy > Deoxy | Increased preload SV and SPV |
| Pulmonary arterial > pulmonary venous | Deoxy > Oxy | Increased preload SV; high output state; systemic desaturation |
| Systemic arterial > pulmonary arterial | Oxy > Deoxy | Increased afterload SPV |
Deoxy, deoxygenated; Oxy, oxygenated; SV, system ventricle; SPV, subpulmonary ventricle.
How and why did the collateral develop?
It is important to find out and to understand how the collateral occurred. Some collateral vessels are present from birth, whereas others develop de novo (through angiogenesis, or by reopening of previously closed venous channels). If collaterals develop after birth, there is usually a specific cause, often a specific hemodynamic situation. Finally, a collateral/fistula may also have an iatrogenic etiology. The reason why the abnormal blood vessel arose is helpful in determining the most appropriate therapeutic decision.
What happens when the collateral is closed?
The effect of collateral closure is a crucial consideration in the therapeutic management of any collateral and is directly related to all the considerations made above. If the collateral has been present from birth or was iatrogenic, closure will usually have a favorable hemodynamic effect. On the other hand, if the collateral is due to unfavorable hemodynamics, closure is questionable. If the effect of closure is unclear, a trial occlusion with a balloon is helpful in determining the immediate hemodynamic effect: does the systemic arterial saturation improve or not; does the closure effect preload and hemodynamics of the heart; does the closure have any effect on afterload. Even though the immediate effect of a closure can be estimated, the long-term effect will remain somewhat unclear.
In hemi-Fontan or Glenn patients, the closure of the systemic venous to systemic venous collateral may increase systemic arterial oxygen saturation indirectly by increasing effective pulmonary blood flow (4). The flip side being that pressure in the supplying system venous circulation (SVC) may increase, significantly impacting hemodynamics and causing symptoms for the patient. In the case of HF, closing the collaterals is not going to provide a hemodynamic benefit.
Progressive desaturation and/or increased preload of the systemic ventricle in a symptomatic patient may raise the question whether systemic venous to pulmonary venous collaterals should be closed. Data in the literature looking at outcome are rather limited. Closure can improve the systemic oxygen saturation (5,23). However, if the collateral originated as a bypass because of obstructive flow, closure is expected to have a potentially pernicious effect (24). After closure of the collateral, pressure in the systemic venous circulation will increase, preload will decrease, and eventually cardiac output may become compromised. For system venous pressures higher than 18 mmHg, closure of collaterals seems to lead to higher mortality rates (25) and confirms that if the collateral arose because of obstruction, closure needs to be avoided. Only at lower systemic venous pressures closure can have a net positive effect on symptoms and hemodynamics. In the case of HF, closure of such collaterals is not going to provide a hemodynamic benefit, quite the contrary.
The effects of the pulmonary arterio-venous collaterals on the systemic ventricle and the systemic saturation raise the question of whether these collaterals must be closed. Data in the literature looking at outcome are rather limited. With low systemic venous pressures and a low pulmonary resistance, closure can have a net positive effect on the hemodynamics. Saturation increases and preload decreases so that filling pressures in the systemic ventricle decrease. Especially in a restrictive ventricle, the effect of closure will be beneficial. The pressure volume loop shifts to the left and allows the systemic circulation to operate at lower filling pressures. Lower filling pressures favor a Glenn and Fontan circuit. With high systemic venous pressures and high pulmonary vascular resistance, closure is probably less favorable. System saturation does increase, and preload will decrease. But because of high pulmonary vascular resistance, system venous pressures will also increase. In case of an additional restrictive ventricle, the effect of closure is more likely to be unfavorable (26). The pressure volume loop might shift too much to the left and compromises the effective stroke volume. In this view, it is better to leave the collateral unaffected. Living a with lower systemic oxygen saturation is better than having a low cardiac output. As such, in the case of HF, collateral closure is only going to be of benefit if pulmonary vascular resistance is sufficiently low to reduce cardiac preload.
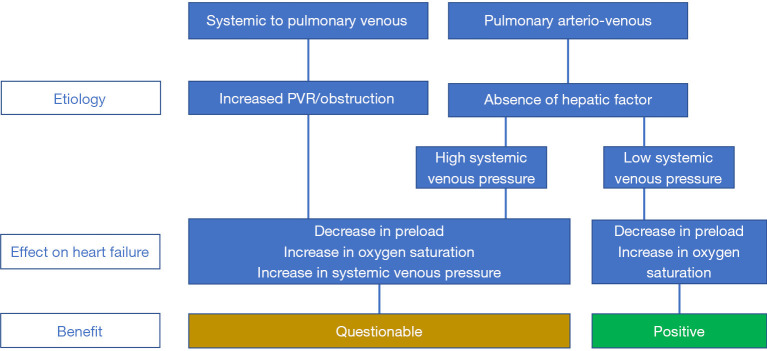
Figure 3 summarizes the potential benefit of the closure of the systemic venous to pulmonary venous collaterals and the closure of the pulmonary arterio-venous collaterals.
Figure 3.
Potential benefit of the closure of the systemic venous to pulmonary venous collaterals and the closure of the pulmonary arterio-venous collaterals.
Closure of collaterals
Preferably, collaterals are closed percutaneously (9). A surgical approach can be complex, and a responsible collateral can be difficult to identify or access during a surgical procedure.
Nowadays there are many percutaneous devices available to close collaterals (Figure 4). A commonly used device is the vascular plug (27,28). This device is easy to implant and has a high probability of closure. Ventricular septal defect plugs might also be applied in case of larger vessels (29). Besides the plugs, coiling (3,30,31) is also preferred. This device is easy to place and has a high probability of closure. The collaterals can be closed on both their inlet and outlet sides. It is suggested to occlude from the distal to the proximal part of the collateral in order to avoid recanalization (32). Especially in adults, temporary treatment with oral anticoagulation may be indicated after closure to counteract mobilizing clots in the distal trajectory of the collateral. The latter could give rise to systemic embolization and cerebral infarction. Overall, the closure of the collaterals is a low-risk intervention and the long-term complications related to the devices used are low (or even not reported). However, percutaneous closure remains an invasive procedure. Literature reports complications such as the development of back pain (33) and the occurrence of hemoptysis (34), especially when fairly large collaterals are closed.
Figure 4.
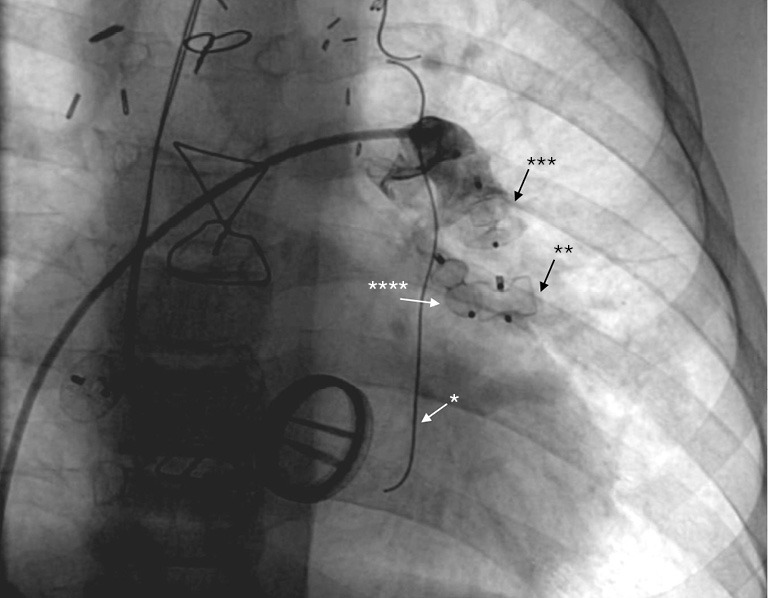
Example of closure systemic venous to pulmonary venous collateral with coil (*), pulmonary arterio-venous malformation with vascular plugs [Amplatzer type I (**), II (***), and IV (****)].
Finally, it should be kept in mind that if the trigger of the occurrence of the collateral does not disappear or isn’t treated, re-occurrence may occur so that repeated interventions emerge.
Conclusions
Data on management of collaterals in typical systemic ventricular failure are scarce (or even non-existing). Collaterals in Fontan or Glenn circulation are common and are mainly closed for systemic desaturation. The long-term effect of closure of collaterals in a single ventricle physiology is unknown. Increased systemic venous pressures is considered as a contraindication for closure of collaterals. In case of low systemic venous pressures, occluding collaterals might be useful to decrease the preload of the systemic ventricle.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yskert von Kodolitsch, Harald Kaemmerer, Koichiro Niwa) for the series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-10/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-10/coif). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Magee AG, McCrindle BW, Mawson J, et al. Systemic venous collateral development after the bidirectional cavopulmonary anastomosis. Prevalence and predictors. J Am Coll Cardiol 1998;32:502-8. 10.1016/S0735-1097(98)00246-0 [DOI] [PubMed] [Google Scholar]
- 2.Heinemann M, Breuer J, Steger V, et al. Incidence and impact of systemic venous collateral development after Glenn and Fontan procedures. Thorac Cardiovasc Surg 2001;49:172-8. 10.1055/s-2001-14339 [DOI] [PubMed] [Google Scholar]
- 3.Weber HS. Incidence and predictors for the development of significant supradiaphragmatic decompressing venous collateral channels following creation of Fontan physiology. Cardiol Young 2001;11:289-94. 10.1017/S1047951101000312 [DOI] [PubMed] [Google Scholar]
- 4.Karur S, Mahima J, Nanjappa MC. Systemic venous collateral channel causing desaturation after bidirectional cavopulmonary anastomosis: percutaneous closure. Cardiol Young 2011;21:107-9. 10.1017/S104795111000140X [DOI] [PubMed] [Google Scholar]
- 5.Lluri G, Levi DS, Aboulhosn J. Systemic to pulmonary venous collaterals in adults with single ventricle physiology after cavopulmonary palliation. Int J Cardiol 2015;189:159-63. 10.1016/j.ijcard.2015.04.065 [DOI] [PubMed] [Google Scholar]
- 6.Kodama Y, Ishikawa Y, Kuraoka A, et al. Systemic-to-Pulmonary Collateral Flow Correlates with Clinical Condition Late After the Fontan Procedure. Pediatr Cardiol 2020;41:1800-6. 10.1007/s00246-020-02450-8 [DOI] [PubMed] [Google Scholar]
- 7.Evans WN, Acherman RJ, Mayman GA, et al. Fontan venovenous collaterals and hepatic fibrosis. J Card Surg 2020;35:2974-8. 10.1111/jocs.14951 [DOI] [PubMed] [Google Scholar]
- 8.Runkel BG, Drake WB, Raghuveer G. Brain Abscess and the Nonfenestrated Fontan Circulation. JACC Case Rep 2020;2:1029-32. 10.1016/j.jaccas.2020.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalal Z, Gewillig M, Boudjemline Y, et al. Transcatheter interventions in patients with a Fontan circulation: Current practice and future developments. Front Pediatr 2022;10:965989. 10.3389/fped.2022.965989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raimondi F, Martins D, Coenen R, et al. Prevalence of Venovenous Shunting and High-Output State Quantified with 4D Flow MRI in Patients with Fontan Circulation. Radiol Cardiothorac Imaging 2021;3:e210161. 10.1148/ryct.210161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spicer RL, Uzark KC, Moore JW, et al. Aortopulmonary collateral vessels and prolonged pleural effusions after modified Fontan procedures. Am Heart J 1996;131:1164-8. 10.1016/S0002-8703(96)90092-7 [DOI] [PubMed] [Google Scholar]
- 12.Ferns SJ, El Zein C, Multani K, et al. Is additional pulsatile pulmonary blood flow beneficial to patients with bidirectional Glenn? J Thorac Cardiovasc Surg 2013;145:451-4. 10.1016/j.jtcvs.2012.11.027 [DOI] [PubMed] [Google Scholar]
- 13.Kavarana MN, Jones JA, Stroud RE, et al. Pulmonary arteriovenous malformations after the superior cavopulmonary shunt: mechanisms and clinical implications. Expert Rev Cardiovasc Ther 2014;12:703-13. 10.1586/14779072.2014.912132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alex A, Ayyappan A, Valakkada J, et al. Major Aortopulmonary Collateral Arteries. Radiol Cardiothorac Imaging 2022;4:e210157. 10.1148/ryct.210157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latus H, Gummel K, Diederichs T, et al. Aortopulmonary collateral flow is related to pulmonary artery size and affects ventricular dimensions in patients after the fontan procedure. PLoS One 2013;8:e81684. 10.1371/journal.pone.0081684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latus H, Kruppa P, Hofmann L, et al. Impact of aortopulmonary collateral flow and single ventricle morphology on longitudinal hemodynamics in Fontan patients: A serial CMR study. Int J Cardiol 2020;311:28-34. 10.1016/j.ijcard.2020.01.065 [DOI] [PubMed] [Google Scholar]
- 17.Schmiel M, Kido T, Georgiev S, et al. Aortopulmonary collaterals in single ventricle: incidence, associated factors and clinical significance. Interact Cardiovasc Thorac Surg 2022;35:ivac190. 10.1093/icvts/ivac190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaikunth SS, Bauser-Heaton H, Lui GK, et al. Repair of Untreated Older Patients With Tetralogy of Fallot With Major Aortopulmonary Collaterals. Ann Thorac Surg 2019;107:1218-24. 10.1016/j.athoracsur.2018.11.016 [DOI] [PubMed] [Google Scholar]
- 19.Zentner D, Celermajer DS, Gentles T, et al. Management of People With a Fontan Circulation: a Cardiac Society of Australia and New Zealand Position statement. Heart Lung Circ 2020;29:5-39. 10.1016/j.hlc.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 20.Kumar P, Bhatia M. Computed Tomography in the Evaluation of Fontan Circulation. J Cardiovasc Imaging 2021;29:108-22. 10.4250/jcvi.2020.0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaidi SJ, Adhikari RR, Patel DR, et al. Saline Contrast Transesophageal Echocardiography in Fontan Patients: Assessment of the Presence, Type, and Size of Right to Left Shunts. Pediatr Cardiol 2019;40:1199-207. 10.1007/s00246-019-02132-0 [DOI] [PubMed] [Google Scholar]
- 22.Van Mieghem N, Daenen W, Budts W. How a left-to-right shunt may protect against haemodynamic deterioration in restrictive cardiomyopathy. Acta Cardiol 2005;60:337-40. 10.2143/AC.60.3.2005014 [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama H, Yoo SJ, Williams W, et al. Characterization and treatment of systemic venous to pulmonary venous collaterals seen after the Fontan operation. Cardiol Young 2003;13:424-30. 10.1017/S1047951103000908 [DOI] [PubMed] [Google Scholar]
- 24.Kanter KR, Vincent RN, Raviele AA. Importance of acquired systemic-to-pulmonary collaterals in the Fontan operation. Ann Thorac Surg 1999;68:969-74; discussion 974-5. 10.1016/S0003-4975(99)00782-1 [DOI] [PubMed] [Google Scholar]
- 25.Poterucha JT, Johnson JN, Taggart NW, et al. Embolization of Veno-venous Collaterals after the Fontan Operation Is Associated with Decreased Survival. Congenit Heart Dis 2015;10:E230-6. 10.1111/chd.12276 [DOI] [PubMed] [Google Scholar]
- 26.Budts W, Ravekes WJ, Danford DA, et al. Diastolic Heart Failure in Patients With the Fontan Circulation: A Review. JAMA Cardiol 2020;5:590-7. 10.1001/jamacardio.2019.5459 [DOI] [PubMed] [Google Scholar]
- 27.Witzke C, Bhatt A, Inglessis I. Percutaneous embolization of a giant collateral vessel originating from the azygos vein via the inferior vena cava. Catheter Cardiovasc Interv 2013;82:E798-802. 10.1002/ccd.24641 [DOI] [PubMed] [Google Scholar]
- 28.Kuo JA. Percutaneous device occlusion of hepatocardiac venous collateral via left transhepatic access in a patient with heterotaxy syndrome following Fontan procedure. Catheter Cardiovasc Interv 2015;85:E140-3. 10.1002/ccd.25679 [DOI] [PubMed] [Google Scholar]
- 29.Girisch M, Sieverding L, Rauch R, et al. Recanalisation of bilateral superior vena cava after total cavopulmonary connection. Interventional occlusion with the Amplatzer VSD Occluder. Z Kardiol 2005;94:469-73. 10.1007/s00392-005-0250-1 [DOI] [PubMed] [Google Scholar]
- 30.Kaulitz R, Ziemer G, Paul T, et al. Fontan-type procedures: residual lesions and late interventions. Ann Thorac Surg 2002;74:778-85. 10.1016/S0003-4975(02)03756-6 [DOI] [PubMed] [Google Scholar]
- 31.Sonomura T, Ikoma A, Kawai N, et al. Usefulness of the Guglielmi detachable coil for embolization of a systemic venous collateral after Fontan operation: A case report. World J Radiol 2012;4:418-20. 10.4329/wjr.v4.i9.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jalal Z, Iriart X, De Lédinghen V, et al. Liver stiffness measurements for evaluation of central venous pressure in congenital heart diseases. Heart 2015;101:1499-504. 10.1136/heartjnl-2014-307385 [DOI] [PubMed] [Google Scholar]
- 33.Okada S, Kamada M, Nakagawa N, et al. Intractable Back Pain After Coil Embolization of Giant Veno-Venous Collaterals in a Patient With Fontan Circulation. Int Heart J 2017;58:298-301. 10.1536/ihj.16-194 [DOI] [PubMed] [Google Scholar]
- 34.Pastuszko P, Rome JJ, Gleason MM, et al. Hemoptysis after CardioSEAL device embolization of a venous collateral after the Fontan operation. J Thorac Cardiovasc Surg 2005;129:1447-8. 10.1016/j.jtcvs.2004.10.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as