Abstract
BACKGROUND
In the asymptomatic “preclinical” phase of Alzheimer's disease (AD), abnormal biomarkers indicate risk for developing cognitive impairment. Biomarker information is increasingly being disclosed to participants in research settings, and biomarker testing and results disclosure will be implemented in clinical settings in the future. Biomarker disclosure has potential psychosocial benefits and harms, impacting affected individuals and their support person(s). Limited data are available about with whom research participants share their results, information that will be necessary to develop disclosure protocols and post‐disclosure resources. Additionally, existing research has been conducted in largely White cohorts, limiting applicability to future clinical populations.
METHODS
We enrolled a diverse cohort of 329 adults (184 non‐Hispanic White and 145 Black/African American individuals) who previously participated in AD research. After reviewing a vignette describing a hypothetical biomarker research study, participants indicated their anticipated willingness to share biomarker results with loved ones, and what reactions they anticipated from others. Using mixed‐methods analysis, we identified responses related to willingness to share results.
RESULTS
A majority (78.7%) were willing to share their results with support persons. Many (59.6%) felt it would not be difficult to share, and most (90.6%) believed their loved ones would be supportive. The most common reasons for sharing were to prepare for possible future AD (41.0% of respondents), while the most common reason for not sharing was to avoid worrying loved ones (4.8% of respondents). A total of 7.3% of respondents related reasons regarding being unsure about sharing.
DISCUSSION
Participants’ interest in sharing results supports integrating support persons into AD biomarker research, and may help maximize potential benefits for participants. Communicating with this "dyad" of research participant and support person(s) may improve involvement in research, and help prepare for implementation of clinical biomarker testing by clarifying communication preferences and the influence of support persons on psychosocial outcomes.
Keywords: Alzheimer's disease, biomarkers, disclosure, ethics, preclinical diagnosis
1. INTRODUCTION
Biomarker changes underlying Alzheimer's disease (AD) accumulate in the brain in the “preclinical” phase of AD, 1 , 2 many years before cognitive impairment. Amyloid (Aβ 40 and 42) and phospho‐tau are currently the most widely used biomarkers, and abnormal levels indicating AD pathological changes 3 can accurately be detected using cerebrospinal fluid (CSF) or positron emission tomography (PET) imaging. 4 , 5 These biomarkers are routinely collected in longitudinal cohort studies and clinical trials, and are increasingly communicated to research participants in a process typically referred to as “biomarker disclosure”. 6 , 7 Reasons for disclosing results include respecting participants’ autonomy, increasing involvement in research, and improving transparency. All of these may in turn positively impact participant trust and engagement, improving recruitment and retention in the research process. 6 , 8
Individuals often want to learn if they are at risk for AD, for instance because of benefits such as having more information about their health or to motivate lifestyle changes. 9 , 10 Other reasons include sharing their results with family members or friends, or what can broadly be termed “support persons” to plan for the future or receive emotional support. 11 , 12 , 13 At the same time, possible stigma and/or discrimination because of others’ negative perceptions of AD, 14 or potential psychological distress for the individual and their support person are reported reasons not to share results. 15
Despite the wide‐ranging potential benefits and harms of disclosure, very little is known about whether and why individuals want to share results with others, particularly among those without cognitive symptoms. The existing data are limited to a qualitative study of research participants enrolled in secondary amyloid prevention trials who reported they shared their biomarker results with family members, for reasons such as having a good relationship with the other person or feeling the information was relevant to them. 16 Another study found that participants wanted their study partner(s) to be present during biomarker disclosure, suggesting that participants want their results to be shared with at least some support persons. 17
While biomarker testing and disclosure is currently mostly limited to research settings, there has been rapid progress toward validation of reliable and non‐invasive amyloid and tau blood‐based biomarker testing. 18 , 19 The availability of these tools will likely lead to widespread disclosure in clinical settings in the future, particularly when disease‐modifying treatments are more widely accessible. 20 , 21 In light of the potentially profound psychosocial impacts of biomarker testing and disclosure, a more robust evidence base about how and with whom to effectively communicate biomarker results will be needed to inform high‐quality care for diverse groups of individuals and their loved ones affected by AD. 22 There is a particular need to understand whether individuals want to share their biomarker results with others, and why. This information will be essential to counsel individuals about their decision to learn and share their biomarker results, inform disclosure protocols, and identify areas in which additional support may be needed after disclosure, for instance in communicating prognosis or advanced planning. Because existing studies have been conducted in majority white, non‐Hispanic populations, there is further a critical need for data from more representative cohorts.
Using a mixed‐method analyses, we examined data from the Alzheimer's Biomarker Survey (ABS), which assessed hypothetical willingness to share results in a diverse, racially balanced cohort of participants who identified as either Black/African American or White. This study contributes to understanding the factors influencing individuals’ willingness to share biomarker results with their support person(s), providing insight into what has been termed a participant “dyad”. 23 These analyses also help to establish a scientific and ethical rationale for more consistently including support persons in AD biomarker disclosure research.
2. METHODS
2.1. Participants
Participants were recruited into the ABS from two longitudinal study cohorts: the Wisconsin Registry for Alzheimer's Prevention (WRAP) 24 and the Wisconsin Alzheimer's Disease Research Center Clinical Core (Wisconsin ADRC). Participants were required to be aged 45–89 and cognitively unimpaired. Eligible participants were mailed a recruitment letter and then contacted by phone. If interested, participants were asked to review an information sheet and provide oral consent. As participants could select multiple racial identities, all participants selecting Black or African American as a self‐identification were categorized as such, even when more than one racial identity was selected.
2.2. Survey
The ABS is a validated 30‐min telephone survey about willingness to enroll in hypothetical biomarker studies and anticipated reactions when communicating AD biomarker results to research participants. 25 The study team developed the ABS using feedback from the University of Wisconsin Survey Center (UWSC) in an iterative process. Several drafts were reviewed by the study team, Survey Center, and external content expert consultants. The ABS has been found to be a reliable and validated measure of anticipated reactions when communicating AD biomarker results to research participants, 25 and results about the willingness to enroll in biomarker studies have been previously described. 26
To assess willingness to share biomarker results, we used a vignette describing a hypothetical AD biomarker study (see Table 1). The vignette depicts the biomarker detection method in general, non‐technical terms. After the vignette, participants were asked to rank their willingness to share their biomarker results, followed by an open‐ended question asking participants to describe why they chose their response and two questions about their perspectives on sharing results.
TABLE 1.
Survey vignette and follow‐up questions.
| Vignette | ||
|---|---|---|
| Let's say you are asked to join a study that would measure a marker in your brain that shows if you are at a higher risk of developing Alzheimer's. The brain marker does not show if you currently have Alzheimer's or predict if you actually will develop Alzheimer's in the future. Although in this study you would learn your results, there are currently no medications to cure Alzheimer's or to reduce your brain marker. | ||
| Follow‐up questions | Response options | |
| 1) | If you learned that your brain marker results were high and you were at higher risk of developing Alzheimer's, how likely would you be to tell a loved one your results? |
(1) Not at all willing (2) A little willing (3) Somewhat willing (4) Very willing (5) Extremely willing |
| 2) | Tell me why you would be [not at all/ a little/ somewhat/ very/ extremely] likely to tell a loved one your results? | Open‐ended |
| 3) | If you learned that your brain marker results were high and you were at higher risk of developing Alzheimer's, how difficult would it be to talk about your results with family members? |
(1) Not at all difficult (2) A little difficult (3) Somewhat difficult (4) Very difficult (5) Extremely difficult |
| 4) | If you learned that your brain marker results were high and you were at higher risk of developing Alzheimer's, how supportive do you feel that your family would be? |
(1) Not at all supportive (2) A little supportive (3) Somewhat supportive (4) Very supportive (5) Extremely supportive |
RESEARCH IN CONTEXT
Systematic review: We conducted a cohort study with a diverse group of participants currently enrolled in longitudinal Alzheimer's Disease (AD) research, to determine participants’ anticipated willingness to share their individual biomarker results. As AD biomarker testing moves toward clinical use, more information is needed about if and why individuals want to share their individual AD biomarker information with others to inform evidence‐based protocols for safely and effectively communicating biomarker results.
Interpretation: The majority of individuals were very willing to share their biomarker results and expected positive outcomes as a result of sharing. This is the first study in a diverse cohort with a large proportion of Black/African‐American participants.
Future directions: We propose expanding disclosure research to be inclusive both of the individual undergoing biomarker testing, as well as their support person(s) with whom those results will be shared.
2.3. Data collection and analysis
This study was approved by the University of Wisconsin Institutional Review Board. Data were collected from January through March 2020. The survey was conducted by trained interviewers using a computer‐assisted telephone interviewing system (CATI), employing CASES 5.6 software provided by the Computer‐Assisted Survey Methods Program at the University of California‐Berkeley.
2.3.1. Survey response patterns
Descriptive statistics (e.g., means, percentages) were calculated to provide information on participant characteristics and evaluate responses to survey questions 1, 3, and 4, asking how likely participants would be to share their results with loved ones, how difficult they thought it would be to share, and how supportive they anticipated their loved ones would be. In a second step, we applied mixed‐methods to link each participant's willingness to share their biomarker results Likert‐scale response (question 1) to their qualitative response (question 2). The frequency of each response category was calculated for each of the five possible willingness to share results Likert‐scale responses (which ranged from “not at all willing” to “extremely willing” to share).
2.3.2. Qualitative analysis
Question 2 was open ended, asking participants to describe why they chose their level of willingness to share results. Responses were analyzed using qualitative content analysis. 27 Qualitative data were first coded by UWSC coders, who used a combination of inductive and deducing coding (see Coding Manual for Qualitative Researchers 28 ) using NVivo (Version 12). Responses were grouped into categories generated from initial coding of responses themselves rather than defined a priori. This process continued until no new categories were identified from responses. A coding framework was then created by two UWSC coders, and discrepancies between coders were resolved through discussion with members of the research team. Using that coding framework, responses were reviewed again several times to ensure each response was accurately categorized. Responses could be assigned to multiple response categories. To establish trustworthiness in analysis, 28 both initial coding categories and further refinements of categories were reviewed with several members of the team, and questions regarding coding categories were resolved in discussion. Team members represented different fields of expertise, including qualitative research, psychology, neurology, and/or AD disclosure research, and we noted any potential bias among researchers.
3. RESULTS
3.1. Participant characteristics
The sample was recruited from ongoing Alzheimer's research cohorts. We contacted 400 cognitively unimpaired participants. The overall response rate for the survey was 84% (81.3% for Black/African American respondents; 85.3% for White respondents). Of the 66 participants who were mailed recruitment letters but did not enroll, 13 declined participation, 5 were unable to participate due to physical or cognitive limitations, and 48 were unable to be reached. The final sample included 329 participants who had complete data for the survey items in this analysis (see Table 2). Participants were on average 65 years old (range: 45–85), and most were women (74.2%). One hundred and forty‐five participants (44.1%) identified as non‐Hispanic Black or African American, and 184 (55.9%) participants identified as non‐Hispanic White. They were more likely to be highly educated (59.3% with a Bachelor's degree or higher), and a majority had a family history of dementia (62.9%).
TABLE 2.
Participant characteristics.
| No. of participants | 329 |
| Age (years), mean (range) | 65 (45–85) |
| Gender | |
| No. of women (%) | 244 (74.2%) |
| No. of men (%) | 85 (25.8%) |
| Race | |
| Non‐Hispanic White (%) | 184 (55.9%) |
| Non‐Hispanic Black or African‐American (%) | 145 (44.1%) |
| Education, N ≥ bachelor's degree (%) | 195 (59.3%) |
| Family history of dementia (%) | 207 (62.9%) |
3.2. Participants’ willingness to share results and anticipated reactions among loved ones
Respondents generally were very willing to share their results with loved ones (see Table 3). Of 329 respondents, 259 (78.7%) were very or extremely interested in sharing their results, with 44 (13.4%) being somewhat, and 26 (7.9%) not at all or a little interested in sharing.
TABLE 3.
Responses to vignette follow‐up questions (329 total respondents).
| Question | Not at all (1) | A little (2) | Somewhat (3) | Very (4) | Extremely (5) | Mean (range 1–5) | Standard deviation |
|---|---|---|---|---|---|---|---|
| How likely would you be to tell a loved one your results? (n, %) | 11 (3.3%) | 15 (4.6%) | 44 (13.4%) | 128 (38.9%) | 131 (39.8%) | 4.08 | 0.98 |
| How difficult would it be to talk about your results with family members? (n, %) | 140 (42.6%) | 56 (17.0%) | 79 (24.0%) | 44 (13.4%) | 9 (2.7%) | 2.17 | 1.20 |
| How supportive do you feel that your family would be? (n, %) | 2 (0.6%) | 4 (1.22%) | 23 (7.0%) | 138 (42.0%) | 160 (48.6%) | 4.38 | 0.72 |
When asked how hard they thought it would be to share results, 196 (59.5%) felt it would be not at all or only a little difficult, while 79 (24.0%) felt it would be somewhat difficult, and 53 (16.1%) felt it would be difficult to share. Regarding how supportive they thought their loved ones would be, 298 (90.5%) felt they would be very or extremely supportive, with 23 (5.8%) feeling loved ones would be somewhat supportive, and only 6 (2.0%) feeling they would not be supportive or only somewhat supportive.
3.3. Response categories related to sharing biomarker results with loved ones
Participants described several responses that were related to being willing to share, unwilling to share, or unsure about sharing their biomarker results (see Table 4 and Figure 1). Among responses related to being willing to share, the most common reason was “so my loved ones could support me or we could prepare for my possible future AD” (n = 135, 41.0%). The second most frequently mentioned reason was “because I am in trusting and honest relationships with my loved ones” (n = 57, 17.3%). Other reasons were “so loved ones could monitor for or understand potential behavior changes” (n = 47, 14.3%) and “because I want my loved ones to know”, cited by 47 (11.9%). The least frequently mentioned reason among those willing to share was among participants who wanted their family members to understand their family history or risk for AD (n = 27, 8.2%), and who wanted to encourage loved ones to participate in research (n = 3, 0.9%). See Table 5 for examples of participant quotes.
TABLE 4.
Frequency of response categories related to sharing biomarker results with loved ones (329 total respondents).
| Response categories related to sharing results | Willingness to share results % of total (n) | ||||||
|---|---|---|---|---|---|---|---|
| Not at all | A little | Somewhat | Very | Extremely | Totals | ||
| Willing to share results | So my loved ones could support me or we could prepare for my possible future AD | 0.3% (1) | 0% (0) | 2.7% (9) | 17.0% (56) | 21.0% (69) | 41.0% (135) |
| Because I am in a trusting and honest relationship with my loved ones | 0% (0) | 0.3% (1) | 1.2% (4) | 7.6% (25) | 8.2% (27) | 17.3% (57) | |
| So loved ones could monitor for or understand potential behavior changes | 0.3% (1) | 0% (0) | 1.5% (5) | 6.4% (21) | 6.1% (20) | 14.3% (47) | |
| Because my loved ones may be impacted by the results, or I feel they should know | 0% (0) | 1.2% (4) | 0% (0) | 7.0% (23) | 6.1% (20) | 11.9% (47) | |
| So family members understand their family history or risk for AD | 0% (0) | 0.3% (1) | 0.6% (2) | 3.3% (11) | 4.0% (13) | 8.2% (27) | |
| To encourage research or so that loved ones could participate in research | 0% (0) | 0% (0) | 0% (0) | 0.3% (1) | 0.6% (2) | 0.9% (3) | |
| Somewhat willing or uncertain about sharing results | I would be selective in whom or what to share | 0.6% (2) | 0.6% (2) | 2.7% (9) | 0.9% (3) | 0.9% (4) | 6.1% (20) |
| Because I would want more information before sharing | 0% (0) | 0% (0) | 0.6% (2) | 0% (0) | 0% (0) | 0.6% (2) | |
| Because of uncertainty about prognosis | 0% (0) | 0.3% (1) | 0.3% (1) | 0% (0) | 0% (0) | 0.6% (2) | |
| Unwilling to share results | To not worry or upset loved ones | 1.2% (4) | 1.2% (4) | 2.4% (8) | 0% (0) | 0% (0) | 4.8% (16) |
| Because I am worried about stigma | 0.3% (1) | 0.9% (3) | 2.4% (8) | 0% (0) | 0% (0) | 3.7% (12) | |
| To preserve privacy of my health information | 0.6% (2) | 0.3% (1) | 0.6% (2) | 0% (0) | 0% (0) | 1.5% (5) | |
FIGURE 1.
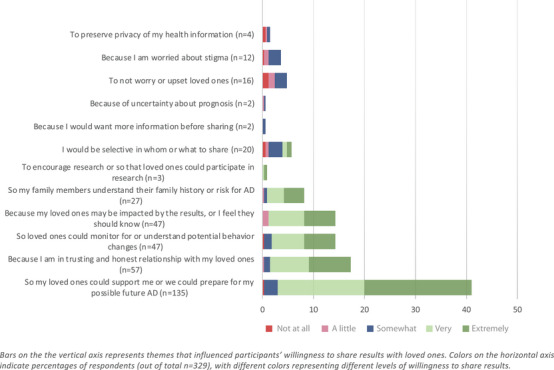
Relationship between response categories and willingness to share biomarker results.
TABLE 5.
Quotes illustrating participant responses about sharing results.
| Responses related to being willing to share results | |
|---|---|
| So my loved ones could support me or we could prepare for my possible future AD |
So I don't have to carry the burden by myself. (Participant 395, 63yo White female) Because I would want them to know so we could set up some kind of a plan or routine so we would kind of have an idea what is going on…would try to plan out whatever I could in terms of finance and health and arrangements so they would know what I wanted. (Participant 061, 65yo Black/African American Female) |
| Because I am in a trusting and honest relationship with my loved ones |
I would see that as the same as any other medical condition, I'd share it with my husband and children. (Participant 191, 72year old (yo) Black/African American female) I don't think it would affect our relationship, it wouldn't be any different than it is now. (Participant 334, 68yo White female) |
| So loved ones could monitor for or understand potential behavior changes |
So they could watch for signs and symptoms. (Participant 052, 59yo White Female) I see no reason to keep it a secret, it is what it is and those are the cards that have been dealt, this is the reason I might be acting differently instead of them wondering why I am acting differently. (Participant 207, 62yo White male) |
| Because my loved ones may be impacted by the results, or I feel they should know |
Because they have the right to know, because it will affect them as well, being around me and knowing what to expect. (197, 58yo Black/African American Female) For their benefit, not mine, so that they can deal with it, for what's coming. (Participant 238, 63yo White female) |
| So family members understand their family history or risk for AD |
So those who are biologically related can also make a decision to learn about their risk. (083, 59yo Black/African American female) I would like them to then do things that might prevent them from getting the disease. (Participant 110, 65yo White female) |
| Responses related to being somewhat willing or uncertain about sharing results | |
| I would be selective in whom or what to share |
To answer that question, if my wife is the loved one, I'd tell my wife, but other loved ones, might not. Loved ones is a broad term. I don't know who falls under that term. I have kids, grandkids, they're all loved ones, but I don't know that I'd tell everybody. (Participant 360, 66yo White male) Particularly my husband. He'd be a caregiver. He needs to know and wants to know. I don't know about my kids. I would tell them but maybe not in as great a detail as him. (Participant 152, 67yo Black/African American female) |
| Because I would want more information before sharing | Just until there would be further clarification or understanding of what the explanation would be, whether they would be able to know what I am telling them, that it is a serious diagnosis or whether it's not as much a serious concern. (Participant 392, 73yo White female) |
| Because of uncertainty about prognosis | Because it's not really definitive as to whether or not I would get it. It means I'm at a higher risk, but that doesn't mean I would develop it. (Participant 106, 60yo White female) |
| Responses related to being unwilling to share results | |
| To not worry or upset loved ones |
I would not want them to worry and to be looking for symptoms. (Participant 298, 73yo White female) I would feel that I couldn't offer any solutions in terms of getting better. There is no medicine and I have this terrible disease and I'd have no way to escape, no treatment, nothing positive. (Participant 007, 84yo Black/African American female) |
| Because I am worried about stigma |
I wouldn't want them to know and them to treat me differently. (Participant 073, 68yo Black/African American male) I would want to try live my life as normal as possible. Once they find out they would begin to treat me differently. (189, 63yo Black/African American female) |
| To preserve privacy of my health information | It isn't any of their business. (Participant 286, 61yo Black/African American male) |
Among responses related to being somewhat willing or uncertain about sharing, several reasons related to sharing selectively (n = 20, 6.1%), while others wanted more information before sharing (n = 2, 0.6%) or were uncertain about the prognostic implications of results (n = 2, 0.6%). Among responses related to being unwilling to share results, the most common reasons were not wanting to worry or upset loved ones (n = 16, 4.8%), worry about stigma (n = 12, 3.7%), or worry about the privacy of their health information (n = 5, 1.5%).
4. DISCUSSION
Among prospective participants in AD biomarker research, a majority felt they would be very or extremely likely to share their results, thought it would be easy to share, and expected to benefit from sharing. These findings are congruent with limited data showing that research participants are interested in sharing their biomarker results. 16 , 17 and is the first study to examine this question in a diverse, racially balanced cohort of participants who identified as either Black/African American or White. These findings have both scientific and ethical implications for the design of clinical AD biomarker research.
Existing disclosure research has focused on communicating with research participants. 7 , 13 , 29 Yet, participants’ interest in sharing results suggests it may be productive to conceptualize disclosure as involving not just research participants but also their support person(s), or dyad. Specifically for biomarker clinical trials, it has been suggested that there are benefits to more fully incorporating dyads (i.e., support persons of participants) into clinical trial designs. 23 Much current AD biomarker and clinical trials research already involves dyads in the form of a “study partner”, who is often a family member or close friend providing information about the participant's cognitive and functional status. 24 , 30 More comprehensively involving support persons in disclosure may help address the pressing need for data to inform clinical implementation of biomarkers. 21 Given the high willingness among participants to share results, research could at a minimum incorporate support persons in the disclosure components of preclinical AD research, or even encourage their participation. If conducted in diverse patient populations, such data could expand the evidence base for disclosure best‐practices by providing opportunities to evaluate various communication strategies; and by assessing participant and support person perspectives on key elements of disclosure such as comprehension, planning, and discussions about lifestyle changes in different groups. 23 , 31
Incorporating dyads may also allow a more robust determination of what influences psychosocial outcomes after disclosure. While outcomes in clinical trial populations are safe, surveys in registry and general public populations suggest that around 10% of individuals would consider suicide if they learned they were at increased risk for AD. 9 , 32 , 33 The risk of planning for suicide was associated with being single and feeling less supported. 34 Participants in our study largely expected they would be supported if they shared their results, which may portend a safer outcome after disclosure. Yet, the precise factors contributing to positive psychosocial outcomes remain unknown; for instance, robust safety outcomes may be due to selecting participants who do not have significant mental health co‐morbidities, 35 or to the presence of a supportive study partner. More closely approximating the likely real‐world conditions under which individuals will share their results will be important to understand psychosocial outcomes of biomarker disclosure in clinically representative populations.
Research in real‐world populations will also be necessary to leverage biomarker testing and disclosure to address disparities in AD research. 36 Diagnosis is often delayed in underrepresented and minoritized populations, at the same time as cognitive impairment is expected to increase as this growing population ages. 37 The National Institute of Health's Minority Health and Health Disparities (NIHMD) research framework describes disparities as health differences that adversely affect the health outcomes among disadvantaged populations, and specifies domains that interact to shape health outcomes by influencing individual, interpersonal, community, and societal strata through biological, behavioral, environmental, social‐cultural, and health system effects. 38 While our results reflect the perspective of a diverse cohort on disclosure, future research to understand the impact of behavioral, social‐cultural, and health systems domains (e.g., on how results are shared within dyads, how decisions about testing are made, outcomes, and even whether "dyad" best reflects the interpersonal unit within which support is provided 39 ), will be essential to support the implementation of biomarkers in a way that reduces rather than exacerbates disparities.
Biomarker disclosure will likely impact support persons, who may be asked to provide emotional help, caregiving, and engage in future planning or lifestyle changes. 15 Participants in our study expected their loved ones to be supportive, but this may be more difficult than participants anticipate. Overall very little is known about psychosocial outcomes among support persons, but a qualitative study among study partners of individuals who had learned their AD risk and amyloid PET scan result showed an expected pattern — that support persons had positive reactions to favorable risk assessments, and negative reactions to learning of the research participant's increased AD risk. 40 Literature from conditions in which risk is assessed through genetic tests, such as breast cancer, or Huntington's disease (a neurodegenerative disorder causing dementia in middle‐aged adults) shows that that reactions between members of the dyad differ: patients may be less distressed than partners and underestimate the negative effects the diagnosis has on the relationship, 41 , 42 and partners can experience the prodromal state as burdensome. 43 While there are differences between biomarker and genetic risk, 16 these findings indicate that there may be different reactions to risk assessments, and these reactions may differ between those affected and their support persons. Understanding the outcomes among support persons is an additional area in which data are needed to prepare for clinical implementation, with the ultimate goal of developing evidence‐based approaches to counseling and support after disclosure. 22 , 23
From the perspective of AD biomarker research design, our findings support ethical rationales to consider including dyads. According to widely accepted criteria, research should seek to minimize risks and maximize benefits for participants. 44 A substantial potential benefit of disclosure is that it allows participants to understand their individual risk, and make informed decisions about lifestyle changes and future preparation (e.g., medical, legal, financial, or personal planning). 11 , 12 The reasons our study participants gave for sharing results with others imply that that sharing results is an essential part of realizing certain benefits of disclosure. For instance, effectively planning for someone's care needs and medical decision‐making or receiving emotional support depends on support persons being aware of that person's diagnosis.
If one goal of biomarker disclosure is to minimize negative outcomes and maximize positive outcomes for research participants (and in the future, patients) who learn their AD biomarker results, 45 it may be important to consider how support persons can or should be involved in research involving biomarker testing and results disclosure. While AD biomarker results have increasingly been disclosed to research participants in recent years, the extent of disclosure varies. A survey of federally funded Alzheimer's Disease Research Centers (ADRCs) in 2021 showed that, while neuropsychological test results are disclosed over 70% of the time, amyloid imaging results are disclosed only 43% of the time, with tau imaging and genetic results being disclosed in only 7%–10% of cases. 46 Researchers have called for more consistent sharing of biomarker results with research participants, 7 which would potentially increase the benefits available to participants. While disclosure is not appropriate in all research protocols or for all participants, 8 if participants in research studies in which biomarker results are disclosed want to receive their results together with someone else, ideally researchers will be in a position to effectively communicate to both the participant and their support person, or be able to provide materials outlining the results that participants can share later.
Incorporating participant preferences in returning research results would have the effect of acknowledging participants’ autonomy, and more robustly involving participants in the conduct of research. Both of these aspects are avenues to improve research quality and engagement. 8 , 44 Further, efforts to increase the benefits of research through integrating support persons more or disclosing biomarker results because individuals feel they are useful to them may offer opportunities for increasing enrollment, and may improve retention among cohorts already enrolled in research. 47
Finally, it is important to note that, though most participants wanted to share their results, some did not. In line with the ethical principle of autonomy, individuals’ wishes around disclosure with others should be ascertained. Protocols must be able to accommodate the option of not disclosing at all, or only disclosing to a participant without a study partner. 48 Even though this may only apply to a small minority of participants, understanding how a desire not to share results impacts research participation or safety may provide another opportunity to improve enrollment by designing studies that can accommodate different preferences toward disclosure. Also, some participants indicated a concern about stigma or their results being in an electronic medical record. While only mentioned by a few participants, addressing anxiety about privacy may be a way to reduce psychological risks to participants. These considerations may be less applicable to many cohorts already involved in research, who often have high levels of education and trust in research. 49 , 50 However, the issue of selective disclosure is likely to arise frequently in clinical practice, and disclosure research provides a unique opportunity to develop and implement disclosure protocols that can be tested in clinical settings in the future. Understanding why individuals do not want to share their results may offer opportunities to address gaps in education, or identify potential self‐stigma 51 or concerns about discrimination.
This study has several limitations. Methodological limitations include the necessity of using a vignette in which participants responded to a hypothetical question, which might not accurately predict their behavior. The qualitative data are derived from a single free‐response item, which limits their explanatory power because we were not able to include follow‐up questions. These data can provide initial information about motivations and identify some important aspects, but we likely only captured a portion of those factors influencing individuals’ decisions to share. Follow‐up questions would be essential to provide a more complete understanding, and also to provide more detailed information about participants’ reasoning and would be particularly valuable for those reasons against sharing (e.g., privacy, uncertainty).
Enrollment of samples into AD research has been influenced by recruitment strategies, and these research samples may not reflect the composition of future clinical populations. 52 , 53 Our sample was drawn from highly educated participants who are already involved with AD research and may hold views different from more general populations. Given the high levels of family history of AD, which has been associated with a higher interest in participating in AD research, this may have motivated increased levels of interest in sharing results than in populations with lower levels of AD family history. While we enrolled a diverse cohort with nearly half of participants being Black/African‐American individuals (2 of whom additionally identified as Native American), we did not enroll individuals identifying as members of other racially and ethnically minoritized groups. It will be essential for future studies to enroll participants from multiple racially and ethnically minoritized groups. Further, the studies from which we drew our participants required a study partner to enroll. Since these individuals already had someone involved in research with them, participants may have been more willing to share results than if they did not already have such a person available. Thus, our study may have overestimated the willingness to share biomarker results. Nonetheless, the size of our sample and range of responses that are concordant with existing research suggests that the responses identified would plausibly be represented in other, non‐research populations as well. Finally, our population was primarily composed of women (74%) and was too small to discern differences between self‐identified genders. These limitations suggest the significance of making research populations more inclusive not only in terms of racial and ethnically minoritized status, but also regarding gender and family history. Arguably most important, however, is future work that expands to populations who are not already involved in AD research.
5. CONCLUSION
This study examined the hypothetical willingness of research participants to share AD biomarker results with support persons, and identified responses associated with willingness to share. Most participants were very interested in sharing their results, and did so because they wanted to plan for the future, feel supported, or share information that impacted their loved ones. These data suggest that it would be advantageous to conceptualize AD biomarker disclosure as involving the “dyad” of both participant and support person(s), rather than focusing exclusively on the research participant. This may generate a critically needed evidence base about disclosure of AD biomarkers that can inform both research and clinical practice in the future. Further, incorporating support persons into disclosure research may optimize and support the benefits available to participants, and provide an opportunity to increase study enrollment and/or retention. Though AD biomarkers pertain to individual brains, information about a person's biomarker results affects many others in their social and familia network. 15 Research that is inclusive of all those affected by AD, beginning with the dyad of participant and support persons, offers a chance to develop effective means to communicate about AD as the field expands biomarker research and moves toward clinical use of AD biomarkers.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to report. Author disclosures are available in the supporting information.
CONSENT STATEMENT
Verbal informed consent was obtained from all participants in this study.
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors extend our deepest thanks to the WRAP and WADRC participants and staff for their invaluable contributions to the study. The authors acknowledge the University of Wisconsin Survey Center for their assistance with survey development, data collection, and coding of open‐ended responses in coordination with the authors. The authors also gratefully acknowledge the assistance of Kristin Harkins, Shana Stites, and Jason Karlawish for aiding in the development of the Alzheimer's Biomarker Survey. This publication was supported by funding from the National Institute on Aging (R03 AG062975 (Clark), R01 AG054059 (Gleason), R01 AG027161, P30 AG062715). Other authors (Ketchum, Erickson, Chin, Basche, Lambrou) have no disclosures.
Ketchum FB, Chin NA, Erickson C, et al. The importance of the dyad: Participant perspectives on sharing biomarker results in Alzheimer's disease research. Alzheimer's Dement. 2023;9:e12416. 10.1002/trc2.12416
REFERENCES
- 1. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement J Alzheimers Assoc 2018;14:535‐562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jack CR, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016;87:539‐547. 10.1212/WNL.0000000000002923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alawode DOT, Heslegrave AJ, Ashton NJ, et al. Transitioning from cerebrospinal fluid to blood tests to facilitate diagnosis and disease monitoring in Alzheimer's disease. J Intern Med 2021;290:583‐601. 10.1111/joim.13332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frisoni GB, Boccardi M, Barkhof F, et al. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. Lancet Neurol 2017;16:661‐676. 10.1016/S1474-4422(17)30159-X [DOI] [PubMed] [Google Scholar]
- 5. Teipel S, Drzezga A, Grothe MJ, et al. Multimodal imaging in Alzheimer's disease: validity and usefulness for early detection. Lancet Neurol 2015;14:1037‐1053. 10.1016/S1474-4422(15)00093-9 [DOI] [PubMed] [Google Scholar]
- 6. Erickson CM, Chin NA, Johnson SC, Gleason CE, Clark LR. Disclosure of preclinical Alzheimer's disease biomarker results in research and clinical settings: why, how, and what we still need to know. Alzheimers Dement Diagn Assess Dis Monit 2021;13:e12150. 10.1002/dad2.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grill JD, Karlawish J. Disclosing Alzheimer Disease Biomarker Results to Research Participants. JAMA Neurol 2022;79:645‐646. 10.1001/jamaneurol.2022.1307 [DOI] [PubMed] [Google Scholar]
- 8. National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division , Board on Health Sciences Policy, Committee on the Return of Individual‐Specific Research Results Generated in Research Laboratories. Returning Individual Research Results to Participants: Guidance for a New Research Paradigm. Washington (DC): National Academies Press (US); 2018. [PubMed] [Google Scholar]
- 9. Caselli RJ, Langbaum J, Marchant GE, et al. Public perceptions of presymptomatic testing for Alzheimer disease. Mayo Clin Proc 2014;89:1389‐1396. 10.1016/j.mayocp.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wikler EM, Blendon RJ, Benson JM. Would you want to know? Public attitudes on early diagnostic testing for Alzheimer's disease. Alzheimers Res Ther 2013;5:43. 10.1186/alzrt206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Milne R, Bunnik E, Diaz A, et al. Perspectives on communicating biomarker‐based assessments of Alzheimer's disease to cognitively healthy individuals. J Alzheimers Dis 2018;62:487‐98. 10.3233/JAD-170813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Largent EA, Harkins K, van DCH, Hachey S, Sankar P, Karlawish J. Cognitively unimpaired adults’ reactions to disclosure of amyloid PET scan results. PLOS ONE 2020;15:e0229137. 10.1371/journal.pone.0229137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Wilde A, van Buchem MM, Otten RHJ, et al. Disclosure of amyloid positron emission tomography results to individuals without dementia: a systematic review. Alzheimers Res Ther 2018;10. 10.1186/s13195-018-0398-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stites SD, Rubright JD, Karlawish J. What features of stigma do the public most commonly attribute to Alzheimer's disease dementia? Results of a survey of the U.S. general public. Alzheimers Dement 2018;14:925‐32. 10.1016/j.jalz.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Largent EA, Karlawish J. Preclinical Alzheimer disease and the dawn of the pre‐caregiver. JAMA Neurol 2019;76:631‐632. 10.1001/jamaneurol.2019.0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Largent EA, Stites SD, Harkins K, Karlawish J. ‘That would be dreadful’: the ethical, legal, and social challenges of sharing your Alzheimer's disease biomarker and genetic testing results with others. J Law Biosci 2021;8. 10.1093/jlb/lsab004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cox CG, Ryan MM, Gillen DL, Grill JD. Is reluctance to share Alzheimer's disease biomarker status with a study partner a barrier to preclinical trial recruitment? J Prev Alzheimers Dis 2021;8:52‐58. 10.14283/jpad.2020.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cullen NC, Leuzy A, Janelidze S, et al. Plasma biomarkers of Alzheimer's disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat Commun 2021;12:3555. 10.1038/s41467-021-23746-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 2020;19:422‐433. 10.1016/S1474-4422(20)30071-5 [DOI] [PubMed] [Google Scholar]
- 20. Hampel H, O'Bryant SE, Molinuevo JL, et al. Blood‐based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol 2018;14:639‐652. 10.1038/s41582-018-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood‐based biomarkers for Alzheimer's disease: towards clinical implementation. Lancet Neurol 2022;21:66‐77. 10.1016/S1474-4422(21)00361-6 [DOI] [PubMed] [Google Scholar]
- 22. Ketchum FB, Chin NA, Grill J, et al. Moving beyond disclosure: stages of care in preclinical Alzheimer's disease biomarker testing. Alzheimers Dement 2022;18:1969‐1979. 10.1002/alz.12620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grill JD, Karlawish J. Study partners should be required in preclinical Alzheimer's disease trials. Alzheimers Res Ther 2017;9:93. 10.1186/s13195-017-0327-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson SC, Koscik RL, Jonaitis EM, et al. The Wisconsin Registry for Alzheimer's Prevention: a review of findings and current directions. Alzheimers Dement Diagn Assess Dis Monit 2018;10:130‐142. 10.1016/j.dadm.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clark LR, Erickson CM, Jonaitis EM, et al. Anticipated reactions to learning Alzheimer's disease biomarker results. Alzheimers Res Ther 2022;14:85. 10.1186/s13195-022-01027-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Erickson CM, Chin NA, Ketchum FB, et al. Predictors of willingness to enroll in hypothetical Alzheimer disease biomarker studies that disclose personal results. Alzheimer Dis Assoc Disord 2022;36:125‐132. 10.1097/WAD.0000000000000490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mayring P. Qualitative Content Analysis: Theoretical Foundation, Basic Procedures and Software Solution. Klagenfurt: 2014. [Google Scholar]
- 28. Saldana J. The Coding Manual for Qualitative Researchers. Los Angeles, Calif: ornia: Sage; 2009. [Google Scholar]
- 29. Burns JM, Johnson DK, Liebmann E, Bothwell R, Morris JK, Vidoni ED. Safety of disclosing amyloid status in cognitively normal older adults. Alzheimers Dement J Alzheimers Assoc 2017;13:1024‐1030. 10.1016/j.jalz.2017.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grill JD, Raman R, Ernstrom K, et al. Short‐term psychological outcomes of disclosing amyloid imaging results to research participants who do not have cognitive impairment. JAMA Neurol 2020;77:1504‐1513. 10.1001/jamaneurol.2020.2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosen AC. Communicating and using dementia risk evidence. J Alzheimers Dis JAD 2022. 10.3233/JAD-220722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Largent EA, Terrasse M, Harkins K, Sisti DA, Sankar P, Karlawish J. Attitudes toward physician‐assisted death from individuals who learn they have an Alzheimer disease biomarker. JAMA Neurol 2019;76:864‐866. 10.1001/jamaneurol.2019.0797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ott BR, Pelosi MA, Tremont G, Snyder PJ. A survey of knowledge and views concerning genetic and amyloid positron emission tomography status disclosure. Alzheimers Dement Transl Res Clin Interv 2016;2:23‐29. 10.1016/j.trci.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Caselli RJ, Marchant GE, Hunt KS, et al. Predictive testing for Alzheimer's disease: suicidal ideation in healthy participants. Alzheimer Dis Assoc Disord 2015;29:252‐254. 10.1097/WAD.0000000000000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim H, Lingler JH. Chapter 8 ‐ Disclosure of amyloid PET scan results: A systematic review. In: Becker JT, Cohen AD, editors. Prog. Mol. Biol. Transl. Sci. 165, Academic Press; 2019:401‐14. 10.1016/bs.pmbts.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 36. Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: update and areas of immediate need. Alzheimers Dement 2019;15:292‐312. 10.1016/j.jalz.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gillis C, Montenigro P, Nejati M, Maserejian N. Estimating prevalence of early Alzheimer's disease in the United States, accounting for racial and ethnic diversity. Alzheimers Dement 2023;19:1841‐1848. 10.1002/alz.12822 [DOI] [PubMed] [Google Scholar]
- 38. Alvidrez J, Castille D, Laude‐Sharp M, Rosario A, Tabor D. The national institute on minority health and health disparities research framework. Am J Public Health 2019;109:S16‐S20. 10.2105/AJPH.2018.304883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manly JJ, Gilmore‐Bykovskyi A, Deters KD. Inclusion of underrepresented groups in preclinical alzheimer disease trials—opportunities abound. JAMA Netw Open 2021;4:e2114606. 10.1001/jamanetworkopen.2021.14606 [DOI] [PubMed] [Google Scholar]
- 40. Largent EA, Abera M, Harkins K, Feldman SJ, Uhlmann WR, Roberts JS, et al. Family members’ perspectives on learning cognitively unimpaired older adults’ amyloid‐β PET scan results. J Am Geriatr Soc 2021;n/a. 10.1111/jgs.17362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sherman KA, Kasparian NA, Mireskandari S. Psychological adjustment among male partners in response to women's breast/ovarian cancer risk: a theoretical review of the literature. Psychooncology 2010;19:1‐11. 10.1002/pon.1582 [DOI] [PubMed] [Google Scholar]
- 42. Decruyenaere M, Evers‐Kiebooms G, Boogaerts A, Demyttenaere K, Dom R, Fryns J‐P. Partners of mutation‐carriers for Huntington's disease: forgotten persons? Eur J Hum Genet 2005;13:1077‐1085. 10.1038/sj.ejhg.5201462 [DOI] [PubMed] [Google Scholar]
- 43. Halpin MA. Into the prodrome: diagnosis, disadvantage, and biomedical ambiguity. Soc Ment Health 2021;11:38‐53. 10.1177/2156869320912456 [DOI] [Google Scholar]
- 44. Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA 2000;283:2701‐2711. 10.1001/jama.283.20.2701 [DOI] [PubMed] [Google Scholar]
- 45. Mozersky J, Hartz S, Linnenbringer E, Levin L, Streitz M, Stock K, et al. Communicating 5‐year risk of Alzheimer's disease dementia: development and evaluation of materials that incorporate multiple genetic and biomarker research results. J Alzheimers Dis JAD 2021;79:559‐572. 10.3233/JAD-200993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roberts JS, Ferber R, Blacker D, Rumbaugh M, Grill JD, for the AGREED Group . Disclosure of individual research results at federally funded Alzheimer's Disease Research Centers. Alzheimers Dement Transl Res Clin Interv 2021;7:e12213. 10.1002/trc2.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ketchum FB, Erickson CM, Chin NA, et al. What influences the willingness of blacks and african americans to enroll in preclinical Alzheimer's disease biomarker research? A qualitative vignette analysis. J Alzheimers Dis 2022;87:1167‐1179. 10.3233/JAD-215521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vanderschaeghe G, Schaeverbeke J, Bruffaerts R, Vandenberghe R, Dierickx K. From information to follow‐up: ethical recommendations to facilitate the disclosure of amyloid PET scan results in a research setting. Alzheimers Dement Transl Res Clin Interv 2018;4:243‐251. 10.1016/j.trci.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Advani AS, Atkeson B, Brown CL, et al. Barriers to the participation of African‐American patients with cancer in clinical trials: a pilot study. Cancer 2003;97:1499‐1506. 10.1002/cncr.11213 [DOI] [PubMed] [Google Scholar]
- 50. Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol 2002;12:248‐256. 10.1016/S1047-2797(01)00265-4 [DOI] [PubMed] [Google Scholar]
- 51. Nguyen T, Li X. Understanding public‐stigma and self‐stigma in the context of dementia: a systematic review of the global literature. Dementia 2020;19:148‐181. 10.1177/1471301218800122 [DOI] [PubMed] [Google Scholar]
- 52. Raman R, Quiroz YT, Langford O, et al. Disparities by race and ethnicity among adults recruited for a preclinical Alzheimer disease trial. JAMA Netw Open 2021;4:e2114364. 10.1001/jamanetworkopen.2021.14364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gleason CE, Norton D, Zuelsdorff M, et al. Association between enrollment factors and incident cognitive impairment in Blacks and Whites: data from the Alzheimer's Disease Center. Alzheimers Dement 2019;15:1533‐1545. 10.1016/j.jalz.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


