Abstract
Studies have shown that angiotensin converting enzyme inhibitors (ACEIs) are superior in primary and secondary prevention for cardiac mortality and morbidity to angiotensin receptor blocker (ARBs). One of the common side effects from ACEI is dry cough. The aims of this systematic review, and network meta‐analysis are to rank the risk of cough induced by different ACEIs and between ACEI and placebo, ARB or calcium channel blockers (CCB). We performed a systematic review, and network meta‐analysis of randomized controlled trials to rank the risk of cough induced by each ACEI and between ACEI and placebo, ARB or CCB. A total of 135 RCTs with 45,420 patients treated with eleven ACEIs were included in the analyses. The pooled estimated relative risk (RR) between ACEI and placebo was 2.21 (95% CI: 2.05–2.39). ACEI had more incidences of cough than ARB (RR 3.2; 95% CI: 2.91, 3.51), and pooled estimated of RR between ACEI and CCB was 5.30 (95% CI: 4.32–6.50) Moexipril ranked as number one for inducing cough (SUCRA 80.4%) and spirapril ranked the least (SUCRA 12.3%). The order for the rest of the ACEIs are as follows: ramipril (SUCRA 76.4%), fosinopril (SUCRA 72.5%), lisinopril (SUCRA 64.7%), benazepril (SUCRA 58.6%), quinapril (SUCRA 56.5%), perindopril (SUCRA 54.1%), enalapril (SUCRA 49.7%), trandolapril (SUCRA 44.6%) and, captopril (SUCRA 13.7%). All ACEI has the similar risk of developing a cough. ACEI should be avoided in patients who have risk of developing cough, and an ARB or CCB is an alternative based on the patient's comorbidity.
Keywords: ACE inhibitors, angiotensin receptor blocker, calcium channel blockers, network meta‐analysis
1. INTRODUCTION
Angiotensin converting enzyme inhibitors (ACEIs) plays an essential road in the prevention and treatment of cardiovascular diseases such as hypertension, coronary heart disease, heart failure, and other vascular diseases such as stroke. 1 It is postulated that the activation of renin‐angiotensin‐aldosterone system (RAAS) leads to vasoconstriction, vascular smooth muscle and cardiac hypertrophy, and fibrosis. 2 The consequences of the actions result in detrimental cardiac effects such as hypertension, myocardial infarction, and heart failure. The blockade of the RAAS using ACEIs has shown to reduce cardiac mortality and morbidity. 3 , 4 , 5 , 6 One of the common side effects from ACEI is dry cough. 7 The incidence of cough associated with ACEI has been reported to be between 3.9% and 35%. 8 , 9 The exact mechanism of ACEI induced cough is unclear. It has been proposed that several mechanisms are involved. One study suggests that ACEI increase the sensitivity of the cough reflex. 10 The most common suggested mechanism is that ACEI break down bradykinin and other inflammatory peptide in the lungs. 10 , 11 Another possible mechanism for ACEI‐induced cough may be associated with a defect in the degradation of bradykinin, which elevates the level of bradykinin.12 Frequently, when patients develop a cough from ACEI, clinicians switch ACEIs with an angiotensin receptor blocker (ARB). The use of ACEI or ARB is similar in the prevention of cardiovascular outcomes with respect to acute myocardial infarction, stroke and heart failure or hospitalization. Nevertheless, the use of ACEs compared to ARB is more effective in the reduction of total deaths and cardiovascular deaths. 13 The objectives of this study are: (1) to complete a systematic review comparing ACEI with placebo, ARB, and calcium channel blockers (CCB) and cough; (2) to perform a network meta‐analysis to rank the risk of cough induced by different ACEIs (3) to perform a network meta‐analysis between placebo, ACEI, ARB and CCB to rank the risk of cough cause by each class of agents.
2. METHOD
The medical librarian (JYK) developed and executed comprehensive searches in Ovid MEDLINE, Ovid Embase, CINAHL, Scopus, and Cochrane Library (via Wiley) on March 21, 2022. To capture all relevant randomized controlled trials (RCTs) pertaining to ACE inhibitor induced cough in the general population, relevant keywords and controlled vocabulary were carefully selected. The search integrated a validated RCT filter for MEDLINE, which was subsequently adapted to other databases. Searches were limited to English language. Refer to appendix I for full‐text search strategies. The reporting of this network systematic review was guided by the standards of the Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA) Statement. 14 This network meta‐analysis was registered on the PROSPERO website (CRD42021274659).
2.1. Data extraction and quality assessment
The references were independently reviewed by two authors (YYH, HLB). Disagreements were resolved by a third author (SL). The data were independently extracted by two authors (YYH, HLB). The data extracted include subject demographic characteristics, first author, journal and the year of publication, population, intervention, comparator, sample size, maximum ACEI dose, and incidence of cough. The meta‐analysis and network meta‐analysis consisted of only randomized controlled trials with the following inclusion criteria: (1) ACE inhibitor use, (2) placebo, or ARB, or CCB use, (3) incidence of cough. The excluded criteria are: (1) occurrence of cough before the trial; (2) having the past medical history of asthma.
2.2. Statistical analysis
2.2.1. Network meta‐analysis
A network meta‐analysis was constructed to build the connective relationship within multi‐arms and between studies. The indirect evaluations of cough risk ratios (RRs) for different single ACEI treatments that had not been compared head‐to‐head directly were determined. By entering every event arm data and total numbers in the Stata software®, a network map of these connections and a network forest of estimated RRs were created. In addition, cough risks induced by different ACEIs were ranked according to the surface under the cumulative ranking curve (SUCRA). SUCRA values range from 0% to 100%. The higher the SUCRA value, and the closer to 100%, the higher the likelihood that ACEI is in the top rank inducing cough; the closer to 0 the SUCRA value, the more likely that ACEI is in the bottom rank inducing cough.
The process of network meta‐analysis includes using the global inconsistency test and node‐splitting approach to check for inconsistency to justify by using combination of direct and indirect evidence. Normally, the random model in the consistency test is used. If no heterogeneity was found in the inconsistency test, the fixed model was used to perform the consistency test. Publication bias was estimated by comparison‐adjusted funnel plots. A two‐tailed p‐value < .05 was considered statistically significant. All the statistical analyses were performed in Stata 14.1 (Stata Corp, College Station, TX).
3. RESULTS
The complete search strategies are summarized in appendix I. A total of 5822 results were retrieved and after removing duplicates, 3436 unique results remained for the initial title and abstract screening in Covidence, a web‐based tool (www.covidence.org). In addition to subscription databases, the research team reviewed the first 200 results from Google Scholar. Bibliographies from included studies were also reviewed. A total of 206 studies were identified. After screening the full text, 135 RCTs with 45,420 participants treated with eleven ACEIs were included. Figure 1. The age of the participants ranged from 7 to 78 years old. The studies included participants from a wide range of medical conditions including hypertension, transient ischemic attack, coronary artery disease, proteinuria, heart failure, and organ transplant. The basic characteristics of the studies are in Table 1. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 A total of 44 RCT compared ACEI with placebo, 68 RCT with ARB, and 35 RCT with CCB. Various doses of ACEIs were used in the RCTs.
FIGURE 1.
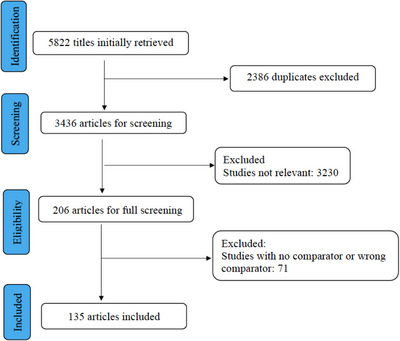
PRISMA diagram.
TABLE 1.
basic characteristics of included studies. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149
| Author year | Journal | Study design | Demographics | Acei | Comparator | Maximum acei daily dose |
|---|---|---|---|---|---|---|
| Abdul‐Rahim AH, 2016 | Eur Stroke J | Randomized, double blind |
Population: MI Mean age range (yr): 44–66 Patients: (G1 = 4697, G2 = 4668, G3 = 4684) |
G1: Captopril (236) G2: Captopril + Valsartan (217) |
G3: Valsartan 81 | 40 mg |
| Agabiti‐Rosei E, 1999 | Eur J Clin Pharmacol | Randomized, single blind |
Population: Postmenopausal women with HTN Mean age range (yr): 54–56 Patients (G1 = 45, G2 = 47) |
G1: Moexipril 4 | G2: Nitrendipine (0) | 15 mg |
| Akat PB, 2010 | Indian J Pharmacol | Randomized, open label |
Population: HTN Patients (G1 = 40, G2 = 40) |
G1: Enalapril 5 | G2: Telmisartan (0) | 10 mg |
| Amerena A, 2002 | Int J Med Res | Randomized, open label |
Population: HTN Mean age range (yr): 51–52 Patients (G1 = 255, G2 = 261) |
G1: Enalapril 23 | G2: Telmisartan 2 | 10 mg |
| Arashi H, 2020 | Am Heart J | Randomized, double blind |
Population: Heart transplant Mean age range (yr): 50–54 Patients (G1 = 45, G2 = 46) |
G1: Ramipril 20 , * | G2: Placebo (0) | 20 mg |
| Baptista LC, 2019 | Clin Med | Randomized, double blind (allocation concealment) |
Population: HTN Mean age range (yr): 67–72 Patients (G1 = 10, G2 = 13, G3 = 8) |
G1: Perindopril 2 |
G2: Losartan (0) G3: HCTZ (0) |
4 mg |
| Benz J, 1997 | Randomized, double blind |
Population: HTN Mean age range (yr): 52–56 Patients (G1 = 45, G2 = 42, G3 = 43) |
G1: Lisinopril 32 , * |
G2: Valsartan 9 G3: HCTZ 8 |
10 mg | |
| Bicknell CD, 2016 | Eur Heart J | Randomized, single blind |
Population: abdominal aortic aneurysms Mean age range (yr): 70–71 Patients (G1 = 73, G2 = 72, G3 = 79) |
G1: Perindopril 3 , * |
G2: Amlodipine 1 G3: Placebo (0) |
10 mg |
| Black HR, 1997 | J Hum Hypertens | Randomized, open label |
Population: Mean age range (yr): 53–54 Patients (G1 = 187, G2 = 364, G3: 183) |
G1: Lisinopril 16 |
G2: Valsartan (0) G3: Placebo (0) |
20 mg |
| Botero R, 2000 | Int J Cardiol | Randomized, open label |
Population: HTN Mean age range (yr): 53–57 Patients (G1 = 64, G2 = 64) |
G1: Enalapril 7 | G2: Valsartan 2 | 20 mg |
| Breeze E, 2001 | J Hum Hypertens | Randomized, double blind |
Population: Hypertension Patients: (G1 = 262, G2 = 261) |
G1: Enalapril 19 | G2: Eprosartan 8 | |
| Campo C, 2001 | J Clin Hypertens | Randomized, open label, parallel |
Population: HTN Mean age range (yr): 43–45 Patients: (G1 = 45, G2 = 40, G3 = 45, G4 = 46) |
G1: Lisinopril 5 |
G2: Atenolol (0) G3: Nisoldipine (0) G4: Losartan (0) |
40 mg |
| Chan P, 1997 | J Clin Pharmacol | Randomized, double blind |
Population: Confirmed ACEI cough HTN Mean age range (yr): 72–74 Patients: (G1 = 28, G2 = 28, G3 = 28) |
G1: Lisinopril 27 , * |
G2: Losartan 6 G3: Metolazone 5 |
10 mg |
| Chen JH, 2004 | J Clin Pract | Randomized, double blind |
Population: HTN Mean age range (yr): 49–53 Patients: (G1 = 76, G2 = 71) |
G1: Enalapril 3 , * | G2: Telmisartan (0) | 10 mg |
| Cheung BY, 1999 | Br J Clin Pharmacol | Randomized, double blind |
Population: LVH Mean age range (yr): 44–54 Patients: (G1 = 17, G2 = 16) |
G1: Fosinopril 4 | G2: Placebo (0) | 20 mg |
| Chockalingam A, 2004 | Am Heart J | Randomized, double blind |
Population: Aortic stenosis Mean age range (yr): 43–46 Patients: (G1 = 34, G2 = 18) |
G1: Enalapril 4 | G2: Placebo (0) | 20 mg |
| Chrysant SG, 1993 | Clin Pharmacol Ther | Randomized, double blind |
Population: HTN Mean age range (yr): 51–55 Patients: (G1 = 230, G2 = 59) |
G1: Perindopril 29 , * | G2: Placebo 2 | 16 mg |
| Cleland JG, 1995 | Brit Heart J | Randomized, double blind |
Population: HF Patients: (G1 = 20, G2 = 20) |
G1: Enalapril 1 | G2: Placebo (0) | 40 mg |
| Coca A, 2002 | Clin Ther | Randomized, double blind |
Population: HTN Mean age range (yr): 50–52 Patients: (G1 = 123, G2 = 115) |
G1: Enalapril 10 , * | G2: Irbesartan 1 | 20 mg |
| Cohen EP, 2008 | Int J Radiation Oncology Biol Phys | Randomized, double blind |
Population: BMT nephropathy Mean age range (yr): Patients (G1 = 28, G2 = 27) |
G1: Captopril (0) | G2: Placebo 1 | |
| Cushman WC, 1996 | Am J Heart | Randomized, double blind |
Population: HTN Mean age range (yr): 52 ‐ 55 Patients: (G1 = 439, G2 = 302, G3 = 150) |
G1: Enalapril 12 |
G2: Diltiazem SR 4 G3: Placebo (0) |
5 mg |
| Cuspidi C, 2002 | J Hypertens | Randomized, double blind |
Population: LVH Patients: (G1 = 105, G2 = 91) |
G1: Enalapril 9 | G2: Candesartan 3 | 10 mg |
| Dequattro V, 1997 | Clin Exp Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): 55 Patients: (G1 = 267, G2 = 378, G3 = 141) |
G1: Trandolapril 19 G2: Trandolapril + Verapamil 14 |
G2: Verapamil 5 | 8 mg |
| Derosa G, 2003 | Clin Ther | Randomized, double blind |
Population: T2DM + HTN Mean age range (yr): 53–55 Patients: (G1 = 49, G2 = 47) |
G1: Perindopril 2 | G2: Candesartan (0) | 4 mg |
| Dickstein K 1995 | J Am Coll Pharmacol | Randomized, double blind |
Population: HF Mean age range (yr): 52–65 Patients: (G1 = 58, G2 = 108) |
G1: Enalapril 4 | G2: Losartan 4 | 20 mg |
| Dickstein K, 2002 | Lancet | Randomized, double blind |
Population: MI Mean age range (yr): 67 Patients: (G1 = 2733, G2 = 2744) |
G1: Captopril 61 | G2: Losartan 47 | 45 mg |
| Dunselman PH, 2001 | Int J Cardiol | Randomized, double blind |
Population: HF Mean age range (yr): 63–65 Patients: (G1 = 77, G2 = 301) |
G1: Enalapril 4 | G2: Telmisartan 9 | 20 mg |
| Eguchi K, 2003 | Am J Cardiol | Randomized, double blind |
Population: HTN Mean age range (yr): 69 Patients: (G1 = 73, G2 = 73) |
G1: Lisinopril 9 | G2: Candesartan 2 | 20 mg |
| Eisner GM, 1991 | Am J Heart | Randomized, double blind |
Population: HTN Mean age range (yr): 24–74 Patients: (G1 = 82, G2 = 78) |
G1: Enalapril 4 | G2: Isradipine (0) | 20 mg |
| Elliott WJ, 1999 | J Hum Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): 55–56 Patients: (G1 = 264, G2 = 264) |
G1: Enalapril 14 , * | G2: Eprosartan 4 | 20 mg |
| EURopean trial, 2003 | Lancet | Randomized, Double blind |
Population: CHD Mean age range (yr): 60 Patients: (G1 = 6110, G2 = 6108) |
G1: Perindopril 161 , * | G2: Placebo 17 | 8 mg |
| Fan XH, 2008 | Ann Pharmacother | Randomized, double blind |
Population: HTN Mean age range (yr): 58–59 Patients: (G1 = 976, G2 = 594, G3 = 891, G4 = 947) |
G1: Captopril 139 |
G2: Atenolol (0) G3 HCTZ (0) G4: Nifedipine SR (0) |
50 mg |
| Fogari R, 2000 | Am J Hypertens | Randomized, open label |
Population: Microalbuiminuria Mean age range (yr): 61–63 Patients: (G1 = 102, G2 = 103, G3 = 104) |
G1: Fosinopril 2 G2: Fosinopril + Amlodipine 1 |
G3: Amlodipine (0) | 30 mg |
| Fogari R, 2005 | Eur J Clin Pharmacol | Randomized, open label |
Population: Microalbuiminuria Mean age range (yr): 59 ‐ 60 Patients: (G1 = 61, G2 = 60) |
G1: Lisinopril 2 | G2: Manidipine (0) | 10 mg |
| Gavras I, 1999 | Curr Med Res Opin | Randomized, double blinded |
Population: HTN Mean age range (yr): 55–56 Patients: (G1 = 264, G2 = 264) |
G1: Enalapril 59 , * | G2: Eprosartan 34 | 5 mg |
| Gradman AH, 1995 | Hypertension | Randomized, double blind |
Population: HTN Mean age range (yr): 52–56 Patients: (G1 = 83, G2 = 415, G3 = 78) |
G1: Enalapril 7 |
G2: Losartan 14 G3: Placebo 2 |
20 mg |
| Gross O, 2020 | Kid Int | Randomized, open label |
Population: Alport's syndrome Mean age range (yr): 7–9 Patients: (G1 = 53, G2 = 37) |
G1: Ramipril 2 | G2: Placebo (0) | 6 mg |
| Gueret P, 1990 | Drugs | Randomized, double blind |
Population: HTN Mean age range (yr): 55–58 Patients: (G1 = 68, G2 = 68) |
G1: Enalapril 4 | G2: Nifedipine (0) | 20 mg |
| Guitard C, 1997 | Cardio Drugs Ther | Randomized, double blind |
Population: HTN Mean age range (yr): 56–58 Patients (G1 = 100, G2 = 101, G3 = 50) |
G1: Spirapril (0) G2: Enalapril 1 |
G3: Placebo (0) |
G1: 6 mg G2: 20 mg |
| Hajjar I, 2020 | JAMA | Randomized, double blind |
Population: HTN Mean age range (yr): 65–66 Patients (G1 = 89, G2 = 87) |
G1: Lisinopril 24 , * | G2: Candesartan 7 | |
| Halimi JM, 2007 | Clin transplant | Randomized, open label |
Population: Renal transplant Mean age range (yr): 35–36 Patients (G1 = 70, G2 = 70, G3 = 58) |
G1: Enalapril 11 G2: Enalapril + amlodipine 6 |
G2: Amlodipine (0) | 20 mg |
| Hart W, 1993 | Postgrad Med J | Randomized, double blind |
Population: HTN Mean age range (yr): 52–56 Patients: (G1 = 63, G2 = 64) |
G1: Lisinopril 8 , * | G2: Nifedipine (0) | 40 mg |
| Himmelmann A, 2001 | Blood Press | Randomized, double blind |
Population: HTN Mean age range (yr): 54–55 Patients: (G1 = 194, G2 = 196) |
G1: Enalapril 15 , * | G2: Candesartan 7 | 20 mg |
| HOPE, 2000 | NEJM | Randomized, double blind |
Population: High risk CAD Mean age range (yr): 66 Patients: (G1 = 4645, G2 = 5652) |
G1: Ramipril (340)* | G2: Placebo 85 | 10 mg |
| Hou FF, 2006 | NEJM | Randomized, double blind |
Population: CRI Mean age range (yr): 44–45 Patients: (G1 = 112, G2 = 112) |
G1: Benazepril 1 | G2: Placebo (0) | 20 mg |
| Hou FF, 2007 | J Am Soc Nephrol | Randomized, open label |
Population: Proteinuria or CRI Mean age range (yr): 49–51 Patients: (G1 = 180, G2 = 180) |
G1: Benazepril 32 | G2: Losartan (0) | 40 mg |
| Ishimitsu T, 2007 | Nephr | Randomized, double blind |
Population: CRI Mean age range (yr): 53 Patients: (G1 = 15, G2 = 15) |
G1: Benazepril 2 | G2: Placebo (0) | 5 mg |
| Johnson BF, 1995 | Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): 54 Patients: (G1 = 82, G2 = 78) |
G1: Enalapril 12 | G2: Isradipine 6 | 40 mg |
| Juarez GF, 2013 | Am J Kidney Dis | Randomized, double blind, allocation concealment |
Population: diabetic nephropathy Mean age range (yr): 63–68 Patients: (G1 = 35, G2 = 28, G3 = 70) |
G2: Lisinopril + irbesartan 4 |
G3: Irbesartan (0) | 40 mg |
| Karlberg BE, 1999 | J Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): 57–61 Patients: (G1 = 139, G2 = 139) |
G1: Enalapril 22 | G2: Telmisartan 9 | 20 mg |
| Katoch N, 2019 | Asian J Pharm Clin Res | Randomized, open label |
Population: MI Mean age range (yr): 55–56 Patients: (G1 = 50, G2 = 50) |
G1: Ramipril 3 | G2: Losartan (0) | 2.5 mg |
| Ke YS, 2003 | Acta Pharmacol | Randomized, open label |
Population: HTN Mean age range (yr): 48–50 Patients: (G1 = 30, G2 = 30, G3 = 30) |
G1: Benazepril 3 G2: Benazepril + valsartan 2 |
G3: Valsartan (0) | 10 mg |
| Kereiakes DJ, 2007 | Am J Cardiovasc | Randomized, double blind |
Population: HTN Mean age range (yr): 54–56 Patients: (G1 = 96, G2 = 94) |
G1: Benazepril 11 | G2: Olmesartan 2 | 20 mg |
| Kitzman DW, 2010 | Circ Heart Fail | Randomized, double blind |
Population: HF Mean age range (yr): 69 ‐ 70 Patients: (G1 = 35, G2 = 36) |
G1: Enalapril 1 | G2: Placebo (0) | 20 mg |
| Ko GT, 2005 | Adv Ther | Randomized, double blind |
Population: T2DM with albuminuria Mean age range (yr): 59–62 Patients: (G1 = 20, G2 = 22) |
G1: Enalapril 7 | G2: Valsartan (0) | 10 mg |
| Kober LK 1995 | N Engl J Med | Randomized, double blind |
Population: LVD Mean age range (yr): 67 Patients: (G1 = 876, G2 = 873) |
G1: Trandolapril 39 , * | G2: Placebo 13 | 2 mg |
| Koch B, 1999 | J Hum Hypertens | Randomized, double blind |
Population: Post‐menopausal Mean age range (yr): 56–57 Patients: (G1 = 47, G2 = 48) |
G1: Moexipril 6 | G2: Placebo (0) | 15 mg |
| Kroll GA, 2016 | Lancet | Randomized, double blind, allocation concealment |
Population: Renal transplant Mean age range (yr): 52–54 Patients: (G1 = 104, G2 = 109) |
G1: Ramipril 4 | G2: Placebo (0) | 10 mg |
| Lacourciere Y, 2000 | Clin Ther | Randomized, double blind |
Population: HTN Mean age range (yr): 70–71 Patients: (G1 = 71, G2 = 70) |
G1: Enalapril 11 , * | G2: Irbesartan 3 | 20 mg |
| Lacourciere Y, 2006 | Am J Hypertens | Randomized, open label, blinded end point |
Population: HTN Mean age range (yr): 52 Patients: (G1 = 407, G2 = 405) |
G1: Ramipril 33 | G2: Telmisartan 1 | 10 mg |
| Larochelle P, 1997 | Am J Cardiol | Randomized, double blind |
Population: HTN Mean age range (yr): 52–53 Patients: (G1 = 61, G2 = 121) |
G1: Enalapril 8 | G2: Irbesartan 3 | 20 mg |
| Leonetti G, 2006 | Blood Press | Randomized, double blind |
Population: HTN Mean age range (yr): 51 Patients: (G1 = 114, G2 = 122) |
G1: Zofenopril 2 | G2: Candesartan (0) | 30 mg |
| Leu HB, 2004 | Jpn Heart J | Randomized, double blind |
Population: HTN Mean age range (yr): 57–59 Patients: (G1 = 20, G2 = 22) |
G1: Enalapril 5 | G2: Eprosartan 3 | 20 mg |
| Lohmann FW, 1999 | Clin Drug Invest | Randomized, open label |
Population: HTN Mean age range (yr): 67 Patients: (G1 = 293, G2 = 439, G3 = 309) |
G1: Ramipril 6 |
G2: Felodipine (0) G3: ISMN 9 |
5 mg |
| Lonn EM, 2009 | J Am Coll Cardiol | Randomized, double blind |
Population: IGT or IFG Mean age range (yr): 53–54 Patients: (G1 = 715, G2 = 710) |
G1: Ramipril 53 , * | G2: Placebo 11 | 15 mg |
| MacGregor MS, 2005 | Nephron Clin Pract | Randomized, open label |
Population: renal failure Mean age range (yr): 50 Patients: (G1 = 553, G2 = 549) |
G1: Ramipril 13 | G2: Olmesartan 2 | 10 mg |
| Malacco E, 2010 | J Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): 72 Patients: (G1 = 213, G2 = 222) |
G1: Zofinopril + HCTZ 6 , * | G2: Irbesartan + HCTZ (0) | 10 mg |
| Malacco E, 2004 | Clin Ther | Randomized, double blind |
Population: HTN Mean age range (yr): 54 Patients: (G1 = 609, G2 = 604) |
G1: Lisinopril 44 | G2: Valsartan 6 | 20 mg |
| Mallion JM, 2011 | Am J Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): 71–72 Patients: (G1 = 175, G2 = 170) |
G1: Ramipril 4 | G2: Olmesartan (0) | 10 mg |
| Malmqvist K, 2000 | J Hypertens | Randomized, double blind |
Population: HTN women Mean age range (yr): 57–58 Patients: (G1 = 146, G2 = 140, G3 = 143) |
G1: Enalapril 19 |
G2: Candesartan (0) G3: HCTZ 6 |
20 mg |
| Marketou ME, 2008 | J Hum Hypertens | Randomized, open label |
Population: DM normotensive Mean age range (yr): 63–64 Patients: (G1 = 32, G2 = 30) |
G1: Perindopril 2 | G2: Placebo (0) | 4 mg |
| Mauer M, 2009 | N Engl J Med | Randomized, double blind |
Population: T1DM Mean age range (yr): Patients: (G1 = 94, G2 = 96, G3 = 95) |
G1: Enalapril 12 |
G2: Losartan 6 G3: Placebo 4 |
20 mg |
| Menne J, 2008 | J Hypertens | Randomized, double blind |
Population: HTN with microalbuminuria Mean age range (yr): 57–59 Patients: (G1 = 47, G2 = 40, G3 = 42) |
G1: Lisinopril 2 G2: Lisinopril/ valsartan 1 |
G3: Valsartan (0) | 40 mg |
| Messerli F, 1998 | Am J Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): Patients: (G1 = 159, G2 = 163, G3 = 152, G4 = 157) |
G1: Trandolapril 12 G2: Trandolapril/ verapamil 9 |
G3: Placebo 4 G4: Verapamil 1 |
4 mg |
| Mimran A, 1998 | J Hum Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): 58 Patients: (G1 = 102, G2 = 98) |
G1: Enalapril 15 , * | G2: Irbesartan 7 | 40 mg |
| Morgan TO, 1992 | Am J Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): 67 Patients: (G1 = 10, G2 = 310, G3 = 10) |
G1: Enalapril 1 G2: Enalapril + felodipine 2 |
G3: Felodipine 2 | 10 mg |
| Nakamura T, 2009 | Int Heart J | Randomized, double blind |
Population: HTN Mean age range (yr): 63–66 Patients: (G1 = 27, G2 = 26) |
G1: Perindopril 2 | G2: Telmisartan (0) | 8 mg |
| Nalbantgil I, 2004 | Int J Clin Pract | Randomized, double blind |
Population: HTN Mean age range (yr): 50 ‐ 51 Patients: (G1 = 30, G2 = 30) |
G1: Perindopril 2 | G2: Telmisartan (0) | 4 mg |
| Neutel JM, 1999 | Am J Ther | Randomized, double blind |
Population: HTN Mean age range (yr): 53 Patients: (G1 = 193, G2 = 385) |
G1: Lisinopril 7 , * | G2: Telmisartan 3 | 40 mg |
| Niseen SE, 2004 | JAMA | Randomized, double blind, allocation concealment |
Population: CAD Mean age range (yr): 57–58 Patients: (G1 = 673, G2 = 663, G3 = 655) |
G1: Enalapril 84 |
G2: Amlodipine 34 G3: Placebo 38 |
20 mg |
| Northridge DB, 1993 | Eur Heart J | Randomized, double blind |
Population: HF Mean age range (yr): 57–62 Patients: (G1 = 60, G2 = 30) |
G1: Quinapril 6 |
G2: Placebo 2 |
20 mg |
| Omvik P, 1994 | Br J Clin Pract | Randomized, double blind |
Population: HTN Mean age range (yr): 54 Patients: (G1 = 230, G2 = 231) |
G1: Enalapril 29 | G2: Amlodipine 9 | 40 mg |
| ONTARGET, 2008 | NEJM | Randomized, double blind |
Population: Vascular disease Mean age range (yr): 66 Patients: (G1 = 8576, G2 = 8502, G3 = 8542) |
G1: Ramipril (360) G2: Ramipril + telmisartan (392) |
G3: Telmisartan 93 | 10 mg |
| Ormesher L, 2020 | Hypertens | Randomized, double blind |
Population: Preeclampsia Mean age range (yr): 30 ‐ 34 Patients: (G1 = 30, G2 = 30) |
G1: Ramipril 3 | G2: Placebo (0) | 20 mg |
| Ostergren J, 1996 | Am J Hypertens | Randomized, double blind |
Population: HTN Patients: (G1 = 119, G2 = 129) |
G1: Enalapril 30 , * | G2: Placebo (0) | 40 mg |
| Otero ML, 2005 | Clin Ther | Randomized, double blind |
Population: T2DM with HTN Mean age range (yr): 60 ‐ 64 Patients: (G1 = 58, G2 = 53) |
G1: Enalapril 6 , * | G2: Manidipine (0) | 10 mg |
| Perico N, 1998 | Clin Drug Invest | Randomized, double blind |
Population: HTN + renal insufficiency Mean age range (yr): 42–55 Patients: (G1 = 94, G2 = 94) |
G1: Lisinopril 1 | G2: Valsartan (0) | 10 mg |
| Pfeffer MA, 2003 | NEJM | Randomized, double blind, allocation concealment |
Population: LVD Mean age range (yr): 64–65 Patients: (G1 = 4909, G2 = 4885, G3 = 4909) |
G1: Captopril (245)* G2: Captopril + valsartan (225) |
G3: Valsartan 85 | 150 mg |
| Phakdeekitcharoen P, 2004 | Am J Kidney Dis | Randomized, open label |
Population: CAPD Mean age range (yr): 48–58 Patients: (G1 = 29, G2 = 29) |
G1: Enalapril 7 | G2: Candesartan (0) | 10 mg |
| Philipp T, 1997 | BMJ | Randomized, double blind |
Population: HTN Mean age range (yr): 53 Patients: (G1 = 220, G2 = 215, G3 = 218, G4 = 218) |
G1: Enalapril 6 , * |
G2: Atenolol (0) G3: Nitrendipine (0) G4: HCTZ (0) |
20 mg |
| Pitt B, 1997 | Lancet | Randomized, double blind |
Population: HF Mean age range (yr): 73–74 Patients: (G1 = 370, G2 = 352) |
G1: Captopril 14 , * | G2: Losartan (0) | 150 mg |
| Pitt B, 2001 | Am J Cardiol | Randomized, open label |
Population: Ischemic heart disease Mean age range (yr): 58 Patients: (G1 = 878, G2 = 872) |
G1: Quinapril 33 , * | G2: Placebo 2 | 20 mg |
| Prisant LM, 1995 | Am Heart J | Randomized, double blind |
Population: HTN Mean age range (yr): 53–55 Patients: (G1 = 71, G2 = 72, G3 = 75) |
G1: Enalapril 3 |
G2: Amlodipine 4 G3: Bisoprolol + HCTZ (0) |
20 mg |
| Prisant LM, 1998 | Am J Ther | Randomized, double blind |
Population: Patients: (G1 = 84, G2 = 82, G3 = 78, G4 = 74) |
G1: Enalapril 7 |
G2: Amlodipine 3 G3: Bisoprolol (0) G4: Placebo 3 |
40 mg |
| PROGRESS 2001 | Lancet | Randomized, open label |
Population: Stroke or TIA Mean age range (yr): 63–65 Patients: (G1 = 3051, G2 = 3054) |
G1: Perindopril 47 | G2: Placebo 69 | 4 mg |
| Radman S, 2007 | Eur J Clin Pharmacol | Randomized, double blind |
Population: T2DM or IGT Mean age range (yr): 46–48 Patients: (G1 = 11, G2 = 10, G3 = 10) |
G1: Ramipril 3 |
G2: Rosiglitazone (0) G3: Placebo (0) |
10 mg |
| Ragot S, 2002 | J Human Hypertens | Randomized, open label |
Population: HTN Mean age range (yr): 55 Patients: (G1 = 218, G2 = 217) |
G1: Perindopril 12 | G2: Telmisartan 2 | 4 mg |
| Ramsey LE, 1995 | J Hypertens | Randomized, double blind |
Population: HTN Patients: (G1 = 46, G2 = 48, G3 = 41) |
G1: Lisinopril 33 |
G2: Losartan 14 G3: HCTZ 14 |
20 mg |
| Reyes‐Marin, FA, 2012 | Rev Invest Clin | Randomized, double blind |
Population: Peritoneal dialysis Mean age range (yr): 42–49 Patients: (G1 = 30, G2 = 30) |
G1: Enalapril 2 | G2: Valsartan 3 | 10 mg |
| Rogstad B, 1994 | Eur J Pharmacol | Randomized, double blind |
Population: HTN Mean age range (yr): 49 ‐ 51 Patients: (G1 = 49, G2 = 53) |
G1: Lisinopril 9 | G2: Nifedipine 2 | 10 mg |
| Rosei EG, 2005 | Am J Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): 53–54 Patients: (G1 = 133, G2 = 134) |
G1: Enalapril 60 | G2: Nifedipine (0) | 20 mg |
| Rouleau JL, 2008 | Circulation |
Randomized, double blind, Allocation concealment |
Population: CABG Mean age range (yr): 61 Patients: (G1 = 1280, G2 = 1273) |
G1: Quinapril (269) | G2: Placebo 140 | 20 mg |
| Ruddy TD, 1997 | Cardiovasc Drugs Ther | Randomized, double blind |
Population: HTN Mean age range (yr): 51–53 Patients: (G1 = 140, G2 = 138) |
G1: Lisinopril 15 | G2: Nisoldipine 6 | 20 mg |
| Ruilope L, 2001 | Blood Pressure | Randomized, double blind |
Population: HTN Mean age range (yr): 73 Patients: (G1 = 163, G2 = 171) |
G1: Enalapril 10 | G2: Eposartan 1 | 20 mg |
| Sabharwal NK, 2005 | Clin Drug Invest | Randomized, double blind |
Population: HTN Mean age range (yr): 52–54 Patients: (G1 = 43, G2 = 43) |
G1: Imidapril 1 | G2: Nifedipine (0) | 10 mg |
| Sampaio RO, 2005 | Am J Cardiol |
Randomized, open label, blinded outcome, Allocation concealment |
Population: Mitral valve prolapse, rheumatic heart disease Mean age range (yr): 38–40 Patients: (G1 = 26, G2 = 21) |
G1: Enalapril 1 | G2: Placebo (0) | 40 mg |
| Schaefer F, 2011 | J Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): 13 Patients: (G1 = 149, G2 = 151) |
G1: Enalapril 10 | G2: Valsartan 9 | 20 mg |
| Schrader H, 2001 | BMJ | Randomized, double blind |
Population: Migraine Mean age range (yr): 41 Patients: (G1 = 60, G2 = 60) |
G1: Lisinopril 8 | G2: Placebo 3 | 20 mg |
| Sega R, 1999 | Am J Hypertens | Randomized, double blind |
Population: HTN Patients: (G1 = 59, G2 = 59) |
G1: Enalapril 2 | G2: Eprosartan 2 | 40 mg |
| Shionoiri I, 1999 | J Clin Pharmacol | Randomized, open label |
Population: HTN Mean age range (yr): 53 Patients: (G1 = 29, G2 = 31) |
G1: Imidapril 28 | G2: Amlodipine 2 | 5 mg |
| Silagy C, 1992 | Am J Cardiol | Randomized, double blind |
Population: HTN Mean age range (yr): 72 Patients: (G1 = 24, G2 = 23, G3 = 20, G4 = 23) |
G1: Enalapril 5 |
G2: HCTZ (0) G3: Atenolol 1 G4: Isradipine (0) |
10 mg |
| SOLVD, 1991 | N Engl J Med | Randomized, double blind |
Population: HF Mean age range (yr): 60–61 Patients: (G1 = 1285, G2 = 1284) |
G1: Enalapril (475) | G2: Placebo (398) | 20 mg |
| Sonbolestan S, 2013 | Int J Prev Med | Randomized, double blind |
Population: Migraine Mean age range (yr): 31–37 Patients: (G1 = 21, G2 = 19) |
G1: Enalapril 3 | G2: Placebo (0) | 10 mg |
| Song J, 2006 | Nephrol Dial Transplant | Randomized, double blind |
Population: T2DM with kidney disease Mean age range (yr): 49 Patients: (G1 = 8, G2 = 8, G3 = 9) |
G1: Ramipril (0) G2: Ramipril + candesartan (0) |
G3: Candesartan (0) | 10 mg |
| Spiner J, 2000 | Eur J Heart Fail | Randomized, single blind, double blind outcome |
Population: MI Mean age range (yr): 65 Patients: (G1 = 101, G2 = 100) |
G1: Captopril 26 | G2: Losartan 12 | 75 mg |
| Sumukadas D, 2018 | Age and Aging | Randomized, double blind |
Population: Postural instability elerly Mean age range (yr): 78 Patients: (G1 = 40, G2 = 40) |
G1: Perindopril 4 , * | G2: Placebo (0) | 4 mg |
| Tan F, 2010 | Singapore Med J | Randomized, open label |
Population: T2DM nephropathy Mean age range (yr): 57–58 Patients: (G1 = 16, G2 = 18) |
G1: Enalapril 4 , * | G2: Losartan (0) | 20 mg |
| Tanser P, 2000 | Am J Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): 60–61 Patients: (G1 = 66, G2 = 62, G3: 26) |
G1: Enalapril 20 |
G2: Candesartan 10 G3: Placebo 3 |
10 mg |
| Tikkamen I, 1995 | J Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): Patients: (G1 = 71, G2 = 80) |
G1: Enalapril 9 | G2: Losartan 1 | 20 mg |
| Tomlinson B, 1994 | Am J Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): 75–76 Patients: (G1 = 16, G2 = 18) |
G1: Spirapril 3 | G2: Isradipine 7 | 5 mg |
| Tomlinson B, 2004 | Clin Ther | Randomized, double blind |
Population: HTN Mean age range (yr): 59–61 Patients: (G1 = 40, G2 = 40) |
G1: Enalapril 13 , * | G2: Amlodipine 3 | 20 mg |
| Toto R, 1996 | Am J Kidney Dis | Randomized double blind |
Population: normotensive with proteinuria Patients: (G1 = 15, G2 = 15) |
G1: Ramipril 1 | G2: Placebo (0) | 5 mg |
| Townsend R, 1995 | Clin Ther | Randomized, double blind |
Population: HTN Mean age range (yr): 54 ‐ 55 Patients: (G1 = 136, G2 = 132) |
G1: Enalapril 13 | G2: Losartan 5 | 10 mg |
| Van Der Does R, 2001 | J Int Med Res | Randomized, double blind |
Population: HTN Mean age range (yr): 54 Patients: (G1 = 157, G2 = 162) |
G1: Imidapril 9 | G2: Nifedipine (0) | 10 mg |
| Velasco M, 1991 | J Cardiovasc Pharmacol | Randomized, double blind |
Population: HTN Mean age range (yr): 50–53 Patients: (G1 = 19, G2 = 21) |
G1: Captopril 2 | G2: Amlodipine 1 | 100 mg |
| Verkaaik R, 1991 | J Cardiovasc Pharmacol | Randomized, double blind |
Population: HTN Mean age range (yr): 53 Patients: (G1 = 44, G2 = 44) |
G1: Enalapril 3 | G2: Nitredipine (0) | 20 mg |
| Weber M, 2012 | J Clin Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): 47–50 Patients: (G1 = 189, G2 = 189, G3 = 188, G4 = 95) |
G1: Lisinopril 6 G2: Lisinopril + Nebivolol 3 |
G3: Nebivolol 4 G4: Placebo 1 |
40 mg |
| Wei F, 2011 | Heart | Randomized, open label, blinded outcomes |
Population: HTN Mean age range (yr): 57–58 Patients: (G1 = 255, G2 = 257) |
G1: Imidapril 8 | G2: Candesartan (0) | 5 mg |
| White M, 2002 | Am Heart J | Randomized, double blind |
Population: HTN Mean age range (yr): 54–56 Patients: (G1 = 99, G2 = 103, G3 = 109, G4 = 46) |
G1: Enalapril 12 |
G2: Losartan 11 G3: Verapamil 11 G4: Placebo (0) |
20 mg |
| White M, 2004 | Am Heart J | Randomized, double blind |
Population: HTN Mean age range (yr): 53–55 Patients: (G1 = 131, G2 = 128) |
G1: Ramipril 10 | G2: Diltiazem 1 | 20 mg |
| Widimsky J, 1995 | Eur J Clin Pharmacol | Randomized, double blind |
Population: HF Mean age range (yr): 57 Patients: (G1 = 152, G2 = 48, G3 = 48) |
G1: Spirapril 1 G2: Enalapril 3 |
G3: Placebo (0) |
6 mg 10 mg |
| Williams B, 2006 | J Hypertens | Randomized, open label, blinded outcomes |
Population: HTN Mean age range (yr): 53 Patients: (G1 = 404, G2 = 397) |
G1: Ramipril 23 | G2: Talmisartan 2 | 5 mg |
| Wu S, 2004 | Blood Vessels | Randomized, open label |
Population: HTN Mean age range (yr): 63–66 Patients: (G1 = 41, G2 = 40, G3 = 40) |
G1: Lisinopril 13 , * |
G2: Amlodipine (0) G3: Losartan 1 |
10 mg |
| Yokota T, 2010 | Heart Vessels | Randomized, open label |
Population: MI Mean age range (yr): 64–66 Patients: (G1 = 81, G2 = 82) |
G1: Enalapril 2 , * | G2: Telmisartan (0) | 10 mg |
| Zannad F, 1999 | J Hypertens | Randomized, double blind |
Population: HTN Mean age range (yr): Patients: (G1 = 49, G2 = 47) |
G1: Perindopril 7 | G2: Amlodipine 1 | 8 mg |
| Zi M, 2003 | Cardiovasc Drugs Ther |
Randomized, double blind |
Population: HF Mean age range (yr): 77–78 Patients: (G1 = 36, G2 = 38) |
G1: Quinapril 6 | G2: Placebo 1 | 40 mg |
Abbreviations: BMT, bone marrow transplant; CAD, coronary artery disease; CABG, coronary artery bypass grafting; CRI, chronic renal insufficiency; HCTZ, hydrochlorothiazide; HF, heart failure; HTN, hypertension; IGT, impaired glucose tolerance; LVD, left ventricular dysfunction; LVH, Left ventricular hypertrophy; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TIA, transient ischemic attack.
Patients required discontinuation due to cough.
The quality of eligibility studies is shown in Figure 2 and the overall quality has low risk of bias. Only four studies disclosed the allocation of concealment.
FIGURE 2.
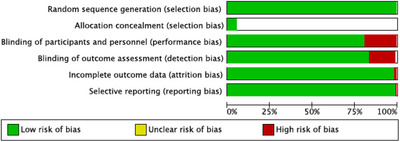
Bias risk assessment for the studies.
3.1. Network meta‐analysis
The network map illustrates the comparison of eleven different ACEIs based on the indirect evaluation of cough risk ratios (Figure 3A). The comparisons between the ACEIs group, ARBs group, CCBs group, and placebo are illustrated in the network map (Figure 3B) based on the combination data of cough risk ratios.
FIGURE 3.
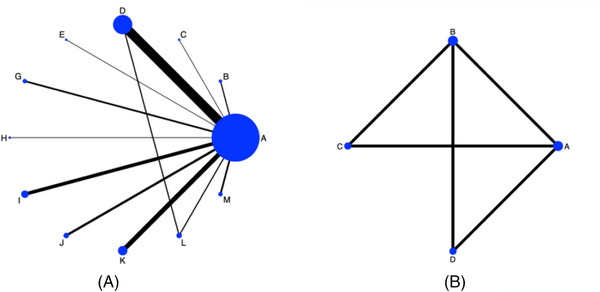
Network map. A: The network map of single ACEIs comparison for cough risk ratios. The width of the black line is positively proportional to the number of trials including every pair of treatments, whereas every circle size is positively proportional to the total number of participants for each treatment. A: Placebo; B: Benazepril; C: Captopril; D: Enalapril; E: Fosinopril; G: Lisinopril; H: Moexipril; I: perindopril; J: Quinapril; K: Ramipril; L: Spirapril; M: Trandolapril. Network map B: The network map of different groups comparisons for cough risk ratios. A: Placebo; B: ACEIs group; C: ARBs group; D: CCBs group.
3.1.1. Network meta‐analysis for cough induced by different ACEIs
The direct and indirect cough development comparisons of different single ACEIs were combined to perform the network meta‐analysis process. Based on the direct and indirect evidence extracted from the included RCTs, the comparisons between each ACEI and alternative ACEI or placebo were completed in the network forest. The ranking order from the maximal to the minimal cough risk was performed and demonstrated by the surface under the cumulative ranking curves (SUCRA). For each treatment, the ranking indicates which of the ACEI is more likely to cause cough and which one is less likely to cause cough. In Figure 4, moexipril ranked as number one for inducing cough (SUCRA 80.4%). The order for the rest of the ACEIs are as follows: ramipril (SUCRA 76.4%), fosinopril (SUCRA 72.5%), lisinopril (SUCRA 64.7%), benazepril (SUCRA 58.6%), quinapril (SUCRA 56.5%), perindopril (SUCRA 54.1%), enalapril (SUCRA 49.7%), trandolapril (SUCRA 44.6%), captopril (SUCRA 13.7%), and spirapril (SUCRA 12.3%) (Table 2).
FIGURE 4.

Single ACEI interventions network meta‐analysis for cough. League table showing results of the network meta‐analysis comparing cough of all treatments including RR and 95% credible intervals. RR > 1 means the top‐left treatment is better. The league table represents the relative risk with 95% confidence interval of single ACEIs compared with placebo. The probabilities beside the ACEIs are the treatment ranking based on SUCRA from left to right. The treatment drugs divide the figure into upper (blue colored) and lower (green colored) sections. For the lower section, the efficacy estimate is the ratio of the column defining treatment to the row defining treatment. For the upper part, the efficacy estimate was the ratio of the row defining treatment to the column defining treatment. The lower and the upper portions’ results are mutually reciprocal. The relative risk ratio in each treatment is compared to the treatment to the right in the same row.
TABLE 2.
Ranking of ACEI induced cough compared to placebo based on SUCRA.
| ACE inhibitor | SUCRA value |
|---|---|
| Ramipril | 76.4% |
| Fosinopril | 72.5% |
| Lisinopril | 64.7% |
| Benazepril | 58.6% |
| Quinapril | 56.5% |
| Perindopril | 54.1% |
| Enalapril | 49.7% |
| Trandolapril | 44.6% |
| Captopril | 13.7% |
| Spirapril | 12.3% |
With the exceptions of spirapril and captopril, other ACEIs resulted in higher risk rations (RRs) of cough compared with placebo. Spirapril ranked the least and captopril ranked next least probability for cough, but no statistical significance was observed (spirapril vs. placebo: RR = 1.8, 95% CI: 0.27–12.14; captopril vs. placebo: RR = 3.11, 95% CI: 0.10‐95.88). Ramipril ranked the second highest risk with RR = 5.79 (95% CI: 2.61–12.88) times risk of cough compared with placebo and 10.42 times risk compared with spirapril (95% CI: 1.32–82.16). Lisinopril has 4.39 times risk of cough compared with placebo (95% CI: 1.15‐16.81). Quinapril has 3.41 times of risk compared with placebo (95% CI: 1.36‐8.49). Perindopril and enalapril, two commonly used ACEIs, had 3.18, times and 2.9 times risk of developing cough respectively, and the RRs are statistically significant (perindopril vs. placebo: 95% CI: 1.42–7.13, enalapril vs. placebo: 95% CI: 1.63–5.17). Moexipril, fosinopril, benazepril, and trandolapril have higher risk of inducing cough compared with placebo, but no statistical significance was observed (Figure 4). The 95% CI of the inconsistency factors of the existing closed‐loops did not exclude zero implying that there was no observed inconsistency between direct and indirect evidence.
3.2. The different treatment comparisons for cough risk ratios
After applying the combination data of cough events from different classes of anti‐hypertension drugs, the risk ratios between ACEIs group, ARBs group, CCBs group, Sacubitril/valsartan and placebo are statistically significant with narrow confidence intervals. In Figure 5, the ACEI group ranked the top among five groups based on the SUCRA (99.9%). The next order was placebo (SUCRA, 50.7%), ARBs (SUCRA, 25%), and the CCBs ranked the least risk of inducing cough (SUCRA, 0%). ACEI have 2.24 times the risk of developing cough compared with placebo (95% CI: 2.06–2.3), 3.2 times compared with ARBs (95% CI: 2.9‐3.53), and 6.5 times compared with CCBs (95% CI: 5.07‐8.34). ARBs have 2.03 times the cough risk ratios compared with CCBs (95% CI: 1.56–2.66). Forest plots for the comparisons are presented in Figures 6, 7, 8 respectively. All comparisons were statistically significant. The 95% CI of the inconsistency factors of the existing closed‐loops did not exclude zero implying that there was no inconsistency observed between direct and indirect evidence.
FIGURE 5.
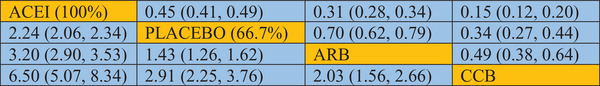
Five different types of anti‐hypertension drugs network meta‐analysis for cough. League table showing results of the network meta‐analysis comparing cough of five types of drugs including RR and 95% credible intervals. RR > 1 means the top‐left treatment is better. The league table represents the relative risk with 95% confidence interval of single ACEIs compared with placebo. The probabilities beside the ACEIs are the treatment ranking based on SUCRA from left to right. The treatment drugs divide the figure into upper (blue colored) and lower (green colored) sections. For the lower section, the efficacy estimate is the ratio of the column defining treatment to the row defining treatment. For the upper part, the efficacy estimate was the ratio of the row defining treatment to the column defining treatment. The lower and the upper portions’ results are mutually reciprocal. The relative risk ratio in each treatment is compared to the treatment to the right in the same row.
FIGURE 6.
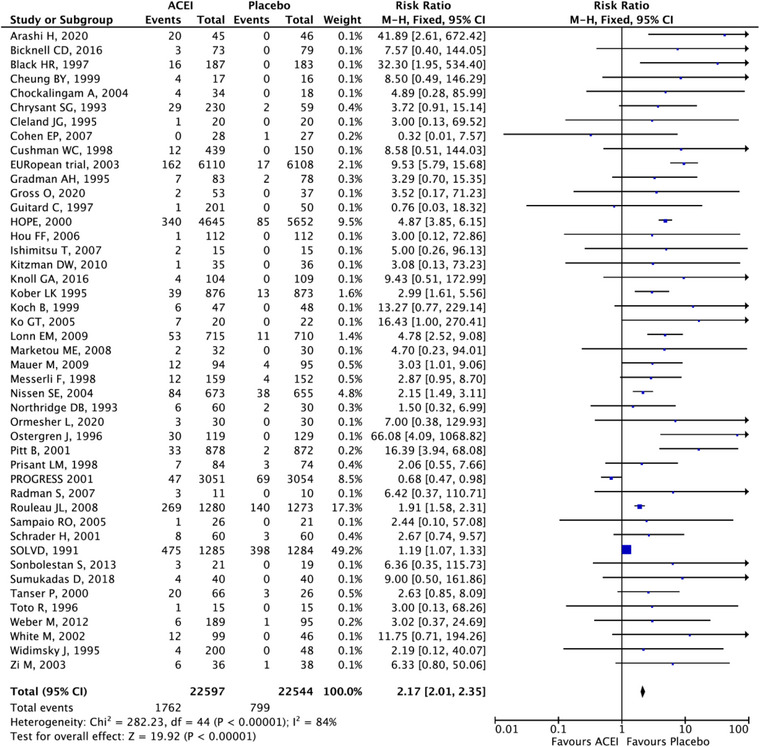
Forest plot comparing ACEI vs. placebo.
FIGURE 7.
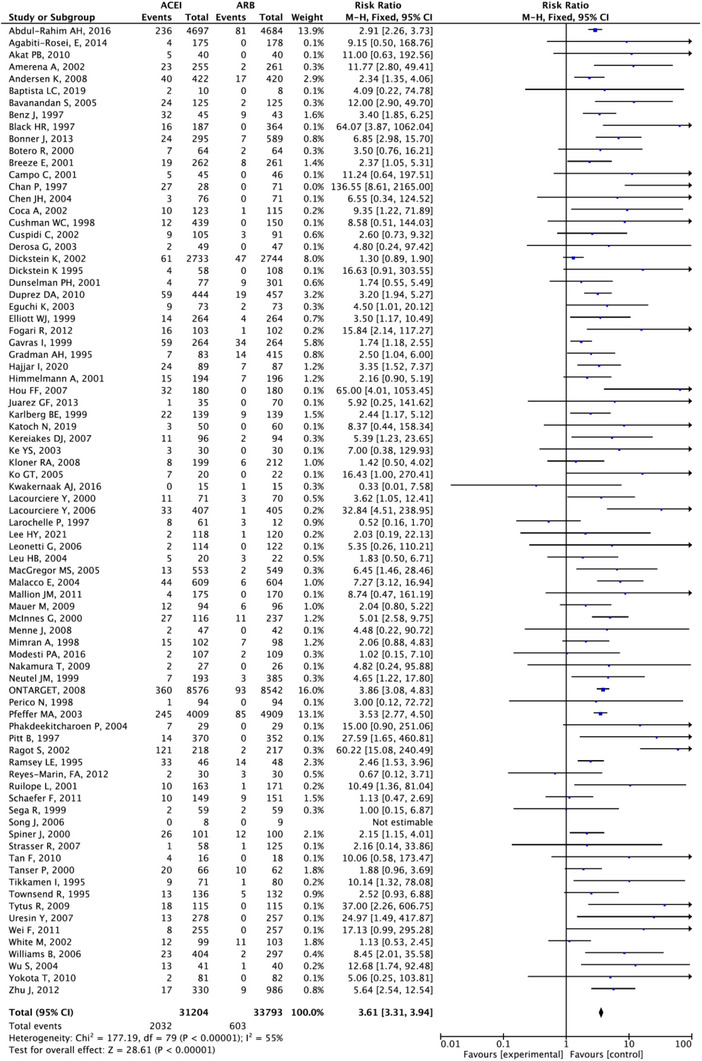
Forest plot comparing ACEI vs. ARB.
FIGURE 8.
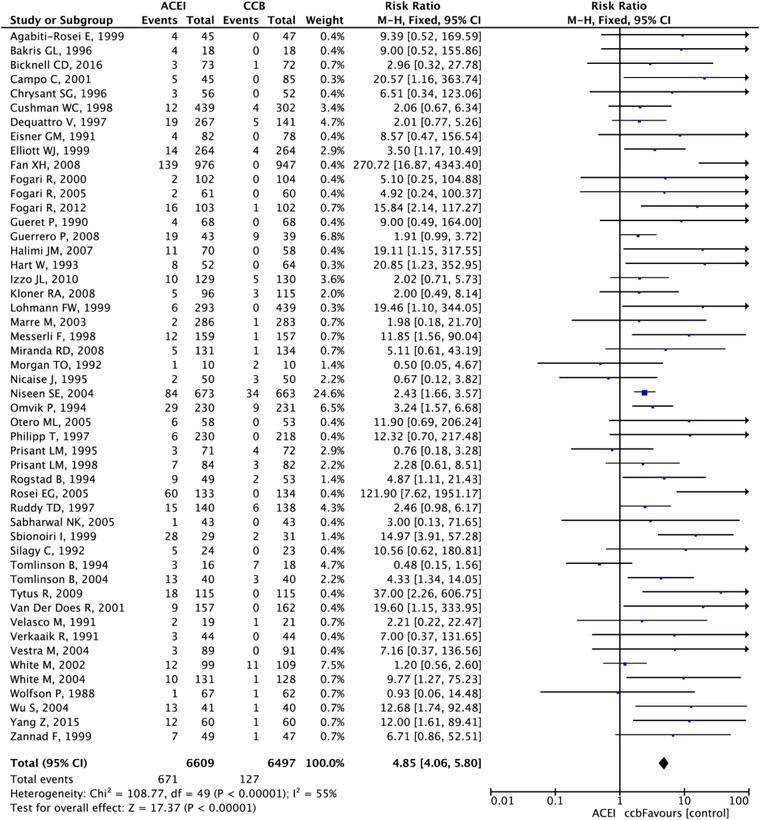
Forest plot comparing ACEI vs. CCB.
3.3. Publication bias
Publication bias was verified by using comparison‐adjusted funnel plot. The symmetrical funnel plots showed no obvious publication biases were detected in Figure 9.
FIGURE 9.
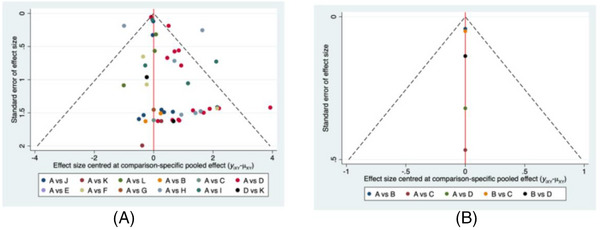
Comparison‐adjusted funnel plots of cough development. A: single ACEI versus placebo network meta‐analysis. A: Placebo; B: Benazepril; C: Captopril; D: Enalapril; E: Fosinopril; G: Lisinopril; H: Moexipril; I: Perindopril; J: Quinapril; K: Ramipril; L: Spirapril; M: Trandolapril. B: the five groups of anti‐hypertension drugs comparisons network meta‐analysis. A: placebo; B: ACEIs; C: ARBs; D: CCBs.
3.4. Withdrawal events related to cough between ACEIs and placebo
Ten studies reported participant discontinuation due to ACEI induced cough. Majority of participants who required discontinuation were from perindopril, ramipril, and enalapril groups. No severe outcome from cough was reported.
4. DISCUSSION
ACEIs are the cornerstone treatment of hypertension, heart failure, myocardial infarction, and cerebrovascular disease. 150 This is the first meta‐analysis and network meta‐analysis of ACEI induced cough compared to placebo, ARB, and CCB. A common reported side effect of ACEI is cough, and it does not appear to be dose dependent. 151 Ramipril is one of the most prescribed ACEIs and it ranked the second highest in causing cough among 11 ACEIs in this study. There is no significant difference of cough risk between each ACEI. Ramipril has six times higher risk of cough compared with placebo (95% CI: 2.61‐12.88) and 3.2 times (95% CI: 2.9‐3.53) with ARB. A large study, ONTARGET 152 with over 25,000 participants, showed that the ramipril group resulted in higher treatment discontinuation due to cough when compared to telmisartan, an ARB (4.2% vs. 1.1%, p < .001). Our meta‐analysis results on ACEI versus ARB are similar to the ONTARGET study.
While captopril ranked the second least risk of causing cough compared to placebo (RR 3.11, 95% CI: 0.10–95.88), it is not statistically significant, and the analysis consisted of only one study on captopril with a sample size of 55. As a result, the confidence interval was extremely wide. Captopril was the first ACEI approved for use in 1980. Unlike most ACEIs captopril is one of the few ACEIs that is not a prodrug 153 and it is well absorbed with a very short half‐life which requires administration three times a day. 154 Due to its quick onset of action, it causes postural hypotension, 155 captopril had been replaced by the newer ACEIs with longer half‐life, which requires once to twice a day administration. 155 , 156 Other commonly prescribed ACEIs such as perindopril and enalapril have 3.18 times, and 2.9 times risk of developing cough respectively, compared to placebo with statistical significance, but they are very similar to other ACEIs. Benazepril ranked top five in causing cough, however, it is not statistically significant compared with placebo or other ACEIs. The combined cough events caused by the ACEIs group ranked the highest when compared with other five groups. ACEIs performed 2.24 times versus placebo, 3.2 times versus ARBs and 6.5 times versus CCBs respectively. These risks are very similar to the risks in the individual meta‐analyses. This confirmed that the network meta‐analysis resulted in a good consistency and the network meta‐analysis was conducted satisfactorily.
The cough induced by ACEIs usually occurs within the first month of the first dose administration. 151 , 157 The symptoms resolve spontaneously after discontinuation of the ACEI within one to four weeks. 7 ACEI induced cough occurs more frequently in females and nonsmokers. 9 , 157 , 158 , 159 Recent studies showed that individuals with polymorphisms in gene coding the bradykinin receptors, ACE (insertion/deletion), and aminopeptidase P which is responsible for the degradation of bradykinin are more susceptible to ACEI induced cough. 160 , 161 , 162 , 163
Our network meta‐analysis included commonly used ACEIs and offered valuable evidence that the risk of developing a cough is very similar in all ACEI as a class of agents. In addition, the results suggest that if a patient develops a dry cough from ACEIs, the best alternative is to switch to an ARB or CCB based on the patient's comorbidity. Ramipril is the last choice for the patients who are at risk of developing dry cough such as evidence of the gene mutation or living in poor air quality environment. In patients with risk of cough and the use of an ACEI is absolutely necessary, enalapril would be an option.
4.1. LIMITATION
In this study, we were unable to analyze dose related cough due to numerous different dosages used in the RCTs. Many studies had a small sample size, resulting the wide confidence interval which is important to judge the significant difference.
5. CONCLUSIONS
All ACEI has the similar risk of developing a cough. ACEI should be avoided in patients who have risk of developing cough, and an ARB or CCB is an alternative based on the patient's comorbidity.
AUTHOR CONTRIBUTIONS
Yiyun Hu and Hoan Linh Banh conceived and conceptualized the research idea. Janice Y. Kung conducted comprehensive searches. Yiyun Hu and Hoan Linh Banh reviewed the search, performed the screening and full text assessment. Shuang Liu resolved any conflicts. Yiyun Hu and Hoan Linh Banh completed the quality assessment and data extraction. Ling Liang performed the data analyses, LL and Hoan Linh Banh interpreted the results. Ling Liang and Hoan Linh Banh contributed to the draft manuscript. All authors contributed to the final draft of the manuscript.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflicts of interest.
PATIENT CONSENT
This network meta‐analysis did not require patient recruitment. It does not require patient consent.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
All figures and tables are original and were created by the authors.
CLINICAL TRIAL REGISTRATION
Not applicable.
ACKNOWLEDGEMENTS
The authors have nothing to report.
SEARCH STRATEGIES.
| Database | Search Strategy | |
|---|---|---|
| MEDLINE Ovid MEDLINE(R) ALL 1946 to March 18, 2022 | 1. | exp Angiotensin‐Converting Enzyme Inhibitors/ |
| 2. | (ACE inhibitor* or angiotensin converting enzyme inhibitor*).mp. | |
| 3. | angiotensin converting enzyme antagonist*.mp. | |
| 4. | angiotensin converting enzyme blocker*.mp. | |
| 5. | dipeptidyl carboxypeptidase inhibitor*.mp. | |
| 6. | benazepril*.mp. | |
| 7. | Captopril/ or captopril.mp. | |
| 8. | cilazapril*.mp. or exp CILAZAPRIL/ | |
| 9. | exp Enalapril/ or enalapril*.mp. | |
| 10. | enalaprilat.mp. or exp ENALAPRILAT/ | |
| 11. | fosinopril*.mp. or exp FOSINOPRIL/ | |
| 12. | imidapril*.mp. | |
| 13. | exp LISINOPRIL/ or lisinopril.mp. | |
| 14. | moexipril*.mp. | |
| 15. | perindopril*.mp. or exp PERINDOPRIL/ | |
| 16. | quinapril*.mp. | |
| 17. | Ramipril/ or ramipril*.mp. | |
| 18. | saralasin.mp. or exp SARALASIN/ | |
| 19. | Teprotide.mp. or exp TEPROTIDE/ | |
| 20. | trandolapril*.mp. | |
| 21. | (alacepril or altiopril or ancovenin or ceranapril or ceronapril or deacetylalacepril or delapril or epicaptopril or fasidotril* or foroxymithine or gemopatrilat or idrapril or indolapril or libenzapril or moveltipril or omapatrilat or pentopril* or pivopril or rentiapril or s nitrosocaptopril or spirapril* or temocapril* or utibapril* or zabicipril* or zofenopril*).mp. | |
| 22. | or/1‐21 | |
| 23. | Cough/ | |
| 24. | cough*.mp. | |
| 25. | exp Bronchial Spasm/ or (bronchospasm* or bronchial spasm*).mp. | |
| 26. | 23 or 24 or 25 | |
| 27. | randomized controlled trial.pt. | |
| 28. | clinical trial.pt. | |
| 29. | randomi?ed.ti,ab. | |
| 30. | placebo.ti,ab. | |
| 31. | dt.fs. | |
| 32. | randomly.ti,ab. | |
| 33. | trial.ti,ab. | |
| 34. | groups.ti,ab. | |
| 35. | or/27‐34 | |
| 36. | animals/ | |
| 37. | humans/ | |
| 38. | 36 not 36 and 37 | |
| 39. | 35 not 38 | |
| 40. | 22 and 26 and 39 | |
| 41. | limit 40 to english language | |
| Embase Ovid Embase 1974 to 2022 March 16 | 1. | exp dipeptidyl carboxypeptidase inhibitor/ |
| 2. | (ACE inhibitor* or angiotensin converting enzyme inhibitor*).mp. | |
| 3. | angiotensin converting enzyme antagonist*.mp. | |
| 4. | angiotensin converting enzyme blocker*.mp. | |
| 5. | dipeptidyl carboxypeptidase inhibitor*.mp. | |
| 6. | benazepril*.mp. | |
| 7. | captopril.mp. | |
| 8. | cilazapril*.mp. | |
| 9. | enalapril*.mp. | |
| 10. | enalaprilat.mp. | |
| 11. | fosinopril*.mp. | |
| 12. | imidapril*.mp. | |
| 13. | lisinopril.mp. | |
| 14. | moexipril*.mp. | |
| 15. | perindopril*.mp. | |
| 16. | quinapril*.mp. | |
| 17. | ramipril*.mp. | |
| 18. | saralasin.mp. or exp saralasin/ | |
| 19. | Teprotide.mp. | |
| 20. | trandolapril*.mp. | |
| 21. | (alacepril or altiopril or ancovenin or ceranapril or ceronapril or deacetylalacepril or delapril or epicaptopril or fasidotril* or foroxymithine or gemopatrilat or idrapril or indolapril or libenzapril or moveltipril or omapatrilat or pentopril* or pivopril or rentiapril or s nitrosocaptopril or spirapril* or temocapril* or utibapril* or zabicipril* or zofenopril*).mp. | |
| 22. | or/1‐21 | |
| 23. | exp coughing/ | |
| 24. | cough*.mp. | |
| 25. | exp bronchospasm/ or (bronchospasm* or bronchial spasm*).mp. | |
| 26. | 23 or 24 or 25 | |
| 27. | Randomized controlled trial/ or Controlled clinical study/ or randomization/ or intermethod comparison/ or double blind procedure/ or human experiment/ | |
| 28. | (random$ or placebo or (open adj label) or ((double or single or doubly or singly) adj (blind or blinded or blindly)) or parallel group$1 or crossover or cross over or ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)) or assigned or allocated or (controlled adj7 (study or design or trial)) or volunteer or volunteers).ti,ab. | |
| 29. | (compare or compared or comparison or trial).ti. | |
| 30. | ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. | |
| 31. | or/27‐30 | |
| 32. | (random$ adj sampl$ adj7 (cross section$ or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) | |
| 33. | Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) | |
| 34. | (((case adj control$) and random$) not randomi?ed controlled).ti,ab. | |
| 35. | (Systematic review not (trial or study)).ti. | |
| 36. | (nonrandom$ not random$).ti,ab. | |
| 37. | Random field$.ti,ab. | |
| 38. | (random cluster adj3 sampl$).ti,ab. | |
| 39. | (review.ab. and review.pt.) not trial.ti. | |
| 40. | “we searched”.ab. and (review.ti. or review.pt.) | |
| 41. | update review.ab. | |
| 42. | (databases adj4 searched).ab. | |
| 43. | (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ | |
| 44. | Animal experiment/ not (human experiment/ or human/) | |
| 45. | or/32‐44 | |
| 46. | 31 not 45 | |
| 47. | 22 and 26 and 46 | |
| 48. | limit 47 to english language | |
| CINAHL | S1 | (MH “Angiotensin‐Converting Enzyme Inhibitors+”) |
| S2 | “ACE inhibitor*” or “angiotensin converting enzyme inhibitor*” | |
| S3 | “angiotensin converting enzyme antagonist*” | |
| S4 | “angiotensin converting enzyme blocker*” | |
| S5 | “dipeptidyl carboxypeptidase inhibitor*” | |
| S6 | “benazepril*” | |
| S7 | (MH “Captopril+”) or “captopril” | |
| S8 | “cilazapril*” | |
| S9 | (MH “Enalapril+”) or “enalapril*” | |
| S10 | (MH “Enalaprilat”) or enalaprilat | |
| S11 | (MH “Fosinopril”) or “fosinopril*” | |
| S12 | “imidapril*” | |
| S13 | (MH “Lisinopril+”) or “lisinopril” | |
| S14 | “moexipril*” | |
| S15 | (MH “Perindopril”) or “perindopril*” | |
| S16 | “quinapril*” | |
| S17 | “ramipril*” | |
| S18 | “saralasin” | |
| S19 | “Teprotide” | |
| S20 | (MH “Trandolapril+”) | |
| S21 | alacepril or altiopril or ancovenin or ceranapril or ceronapril or deacetylalacepril or delapril or epicaptopril or fasidotril* or foroxymithine or gemopatrilat or idrapril or indolapril or libenzapril or moveltipril or omapatrilat or pentopril* or pivopril or rentiapril or “s nitrosocaptopril” or spirapril* or temocapril* or utibapril* or zabicipril* or zofenopril* | |
| S22 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 | |
| S23 | (MH “Cough”) | |
| S24 | cough* | |
| S25 | (MH “Bronchial Spasm”) | |
| S26 | bronchospasm* or “bronchial spasm*” | |
| S27 | S23 OR S24 OR S25 OR S26 | |
| S28 | (MH (randomized controlled trials OR double‐blind studies OR single‐blind studies OR random assignment OR pretest‐posttest design OR cluster sample) OR TI (randomised OR randomized) OR AB random* OR TI trial OR ((MH (sample size) AND AB (assigned OR allocated OR control))) OR MH (placebos OR crossover design OR comparative studies) OR AB ((control W5 group) OR (cluster W3 RCT) OR PT (randomized controlled trial))) NOT ((MH animals+ OR MH (animal studies) OR TI (animal model*)) NOT MH (human)) | |
| S29 | S22 AND S27 AND S28 | |
| S30 | S22 AND S27 AND S28 (Limiters: English Language) | |
| Cochrane | #1 | [mh “Angiotensin‐Converting Enzyme Inhibitors”] |
| Library | #2 | ACE inhibitor* or angiotensin converting enzyme inhibitor* |
| (Wiley) | #3 | angiotensin converting enzyme antagonist* |
| Cochrane Reviews, Trials | #4 | angiotensin converting enzyme blocker* |
| #5 | dipeptidyl carboxypeptidase inhibitor* | |
| #6 | benazepril* | |
| #7 | [mh ^Captopril] or captopril | |
| #8 | [mh CILAZAPRIL] or cilazapril* | |
| #9 | [mh Enalapril] or enalapril* | |
| #10 | [mh ENALAPRILAT] or enalaprilat | |
| #11 | [mh FOSINOPRIL] or fosinopril* | |
| #12 | imidapril* | |
| #13 | [mh LISINOPRIL] or lisinopril | |
| #14 | moexipril* | |
| #15 | [mh PERINDOPRIL] or perindopril* | |
| #16 | quinapril* | |
| #17 | [mh ^Ramipril] or ramipril* | |
| #18 | [mh SARALASIN] or saralasin | |
| #19 | [mh TEPROTIDE] or Teprotide | |
| #20 | trandolapril* | |
| #21 | alacepril or altiopril or ancovenin or ceranapril or ceronapril or deacetylalacepril or delapril or epicaptopril or fasidotril* or foroxymithine or gemopatrilat or idrapril or indolapril or libenzapril or moveltipril or omapatrilat or pentopril* or pivopril or rentiapril or s nitrosocaptopril or spirapril* or temocapril* or utibapril* or zabicipril* or zofenopril* | |
| #22 | {OR #1‐#21} | |
| #23 | [mh ^Cough] | |
| #24 | cough* | |
| #25 | [mh “Bronchial Spasm”] or bronchospasm* or bronchial NEXT spasm* | |
| #26 | {OR #23‐#25} | |
| #27 | #22 AND #26 | |
| Scopus | TITLE‐ABS‐KEY ({ACE inhibitor*} OR {angiotensin converting enzyme inhibitor*} OR {angiotensin converting enzyme antagonist*} OR {angiotensin converting enzyme blocker*} OR {dipeptidyl carboxypeptidase inhibitor*} OR benazepril* OR captopril OR cilazapril* OR enalapril* OR enalaprilat OR fosinopril* OR imidapril* OR lisinopril OR moexipril* OR perindopril* OR quinapril* OR ramipril* OR saralasin OR teprotide OR trandolapril* OR alacepril OR altiopril OR ancovenin OR ceranapril OR ceronapril OR deacetylalacepril OR delapril OR epicaptopril OR fasidotril* OR foroxymithine OR gemopatrilat OR idrapril OR indolapril OR libenzapril OR moveltipril OR omapatrilat OR pentopril* OR pivopril OR rentiapril OR {s nitrosocaptopril} OR spirapril* OR temocapril* OR utibapril* OR zabicipril* OR zofenopril*) AND TITLE‐ABS‐KEY (cough* OR bronchospasm* OR {bronchial spasm*}) AND TITLE‐ABS‐KEY ({Clinical‐trial} OR {controlled‐trial} OR randomi* OR randomly OR (random W/4 (allocat* OR distribut* OR assign*)) OR {placebo} OR {trial} OR {groups} OR {subgroups}) OR TITLE (rct) AND (LIMIT‐TO (LANGUAGE, “English”)) AND (EXCLUDE (DOCTYPE, “no”) OR EXCLUDE (DOCTYPE, “sh”) OR EXCLUDE (DOCTYPE, “le”) OR EXCLUDE (DOCTYPE, “ed”) OR EXCLUDE (DOCTYPE, “ch”)) | |
| Excluded Document Type: Note, Short survey, Letter, Editorial, Book Chapter | ||
| Google Scholar | (ACE inhibitors OR “angiotensin converting enzyme”) AND cough |
Hu Y, Liang L, Liu S, Kung JY, Banh HL. Angiotensin‐converting enzyme inhibitor induced cough compared with placebo, and other antihypertensives: A systematic review, and network meta‐analysis. J Clin Hypertens. 2023;25:661–688. 10.1111/jch.14695
Contributor Information
Ling Liang, Email: ravennaliang@sina.com.
Hoan Linh Banh, Email: hoan@ualberta.ca.
DATA AVAILABILITY STATEMENT
Authors have no data availability to share.
REFERENCES
- 1. Nasution SA. The use of ACE inhibitor in cardiovascular disease. Acta Med Indones. 2006;38(1):60‐64. [PubMed] [Google Scholar]
- 2. Timmermans PB, Wong PC, Chiu AT, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmaco Rev. 1993;45(2):205‐251. [PubMed] [Google Scholar]
- 3. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887‐1898. [DOI] [PubMed] [Google Scholar]
- 4. Staessen JA, Fagard R, Thijs L, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350(9080):757‐764. [DOI] [PubMed] [Google Scholar]
- 5. SHEP . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the systolic hypertension in the Elderly Program (SHEP). JAMA. 1991;265(24):3255‐3264 [PubMed] [Google Scholar]
- 6. JATOS Study Group . Principal results of the japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hyertens Res. 2008;31(12):2115‐2127. [DOI] [PubMed] [Google Scholar]
- 7. Dicpinigaitis PV. Angiotensin‐converting enzyme inhibitor‐induced cough ACCP evidence‐based clinical practice guidelines. Chest. 2006;129(Suppl 1):169S‐173S. [DOI] [PubMed] [Google Scholar]
- 8. Israili ZH. Cough and Angioneurotic edema associated with angiotensin‐converting enzyme inhibitor therapy. Ann Intern Med. 1992;117(3):234‐242. [DOI] [PubMed] [Google Scholar]
- 9. Brugts JJ, Arima H, Remme W, et al. The incidence and clinical predictors of ACE‐inhibitor induced dry cough by perindopril in 27,492 patients with vascular disease. Int J Cardiol. 2014;176(3):718‐723. [DOI] [PubMed] [Google Scholar]
- 10. Fuller RW, Choudry NB. Increased cough reflex associated with angiotensin converting enzyme inhibitor cough. Br Med J. 1987;295(6605):1025‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skidgel RA, Erdös EG. The angiotensin I converting enzyme. Adv Exp Med Biol. 1989;247A:25‐28. [DOI] [PubMed] [Google Scholar]
- 12. Nikpoor B, Duan QL, Rouleau GA. Acute adverse reactions associated with angiotensin‐converting enzyme inhibitors: genetic factors and therapeutic implications. Expert Opin Pharmacother. 2005;6(11):1851‐1856. [DOI] [PubMed] [Google Scholar]
- 13. Salvador GL, Marmentini VM, Cosmo WR, Junior EL. Angiotensin‐converting enzyme inhibitors reduce mortality compared to angiotensin receptor blockers: systematic review and meta‐analysis. Eur J Prev Cardiol. 2017;24(18):1914‐1924. [DOI] [PubMed] [Google Scholar]
- 14. Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. J Med Libr Assoc. 2006;94(2):130‐136. [PMC free article] [PubMed] [Google Scholar]
- 15. Abdul‐Rahim AH, Docherty KF, Skali H, et al. Effect of single and dual renin‐angiotensin blockade on stroke in patients with and without diabetes in VALIANT. Eur Stroke J. 2016;1(2):93‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agabiti‐Rosei E, Ambrosioni E, Pirelli A, Stimpel M, Zanchetti A. Efficacy and tolerability of moexipril and nitrendipine in postmenopausal women with hypertension. Eur J Clin Pharmcol. 1999;55(3):185‐189. [DOI] [PubMed] [Google Scholar]
- 17. Akat PB, Bapat TR, Murthy MB, Karande VB, Burute SR. Comparison of the efficacy and tolerability of telmisartan and enalapril in patients of mild to moderate essential hypertension. Indian J Pharmacol. 2010;42(3):153‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amerena J, Pappas S, Ouellet JP, Williams L, O'shaughnessy D. ABPM comparison of the anti‐hypertensive profiles of telmisartan and enalapril in patients with mild‐to‐moderate essential hypertension. J Int Med Res. 2002;30(6):543‐552. [DOI] [PubMed] [Google Scholar]
- 19. Arashi H, Sato T, Kobashigawa J, et al. Long‐term clinical outcomes with use of an angiotensin‐converting enzyme inhibitor early after heart transplantation. Am Heart J. 2020;222:30‐37. [DOI] [PubMed] [Google Scholar]
- 20. Baptista LC, Jaeger BC, Anton SD, et al. Multimodal intervention to improve functional status in hypertensive older adults: a pilot randomized controlled trial. J Clin Med. 2019;8(2):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benz J, Oshrain C, Henry D, et al. Valsartan, a new angiotensin II receptor antagonist: a double‐blind study comparing the incidence of cough with lisinopril and hydrochlorothiazide. J Clin Pharmacol. 1997;37(2):101‐107. [DOI] [PubMed] [Google Scholar]
- 22. Bicknell CD, Kiru G, Falaschetti E, et al. An evaluation of the effect of an angiotensin converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomized placebo‐controlled trial (AARDVARK). Eur Heart J. 2016;37(42):3213‐3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Black HR, Graff A, Shute D, et al. Valsartan, a new angiotensin II antagonist for the treatment of essential hypertension: efficacy, tolerability and safety compared to an angiotensin‐converting enzyme inhibitor, lisinopril. J Hum Hypertens. 1997;11(8):483‐489. [DOI] [PubMed] [Google Scholar]
- 24. Botero R, Matiz H, Marıa E, et al. Efficacy and safety of valsartan compared with enalapril at different altitudes. Int J Cardiol. 2000;72(3):247‐254. [DOI] [PubMed] [Google Scholar]
- 25. Breeze E, Donoghue MD, Rake E, Fletcher A. Comparison of quality of life and cough on eprosartan and enalapril in people with moderate hypertension. J Hum Hypertens. 2001;15(12):857‐862. [DOI] [PubMed] [Google Scholar]
- 26. Campo C, Segura J, Fernández ML, et al. A prospective comparison of four antihypertensive agents in daily clinical practice. J Clin Hypertens. 2001;3(3):139‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan P, Tomlinson B, Huang TY, et al. Double‐blind comparison of losartan, lisinopril, and metolazone in elderly hypertensive patients with previous angiotensin‐converting enzyme inhibitor‐induced cough. J Clin Pharmacol. 1997;37:253‐257. [DOI] [PubMed] [Google Scholar]
- 28. Chen JH, Cheng JJ, Chen CY, et al. Comparison of the efficacy and tolerability of telmisartan 40 mg vs. enalapril 10 mg in the treatment of mild‐to‐moderate hypertension: a multicentre, double‐blind study in Taiwanese patients. Int J Clin Pract. 2004;145:S29‐S34. [DOI] [PubMed] [Google Scholar]
- 29. Cheung BM, Lau CP. Fosinopril reduces left ventricular mass in untreated hypertensive patients: a controlled trial reducing afterload. Br J Clin Pharmacol. 1999;47(2):178‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chockalingam A, Venkatesan S, Subramaniam T, et al. Safety and efficacy of angiotensin‐converting enzyme inhibitors in symptomatic severe aortic stenosis: symptomatic cardiac obstruction‐pilot study of enalapril in aortic stenosis (SCOPE‐AS). Am Heart J. 2004;147(4):E19. [DOI] [PubMed] [Google Scholar]
- 31. Chrysant SG, McDonald RH, Wright JT, Barden PL, Weiss RJ. Perindopril as monotherapy in hypertension: a multicenter comparison of two dosing regimens. Clin Pharmacol Ther. 1993;53(4):479‐484. [DOI] [PubMed] [Google Scholar]
- 32. Cleland JG, Dargie HJ, Ball SG, et al. Effects of enalapril in heart failure: a double blind study of effects on exercise performance, renal function, hormones, and metabolic state. Br Heart J. 1985;54(3):305‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coca A, Calvo C, García‐Puig J, et al. A multicenter, randomized, double‐blind comparison of the efficacy and safety of irbesartan and enalapril in adults with mild to moderate essential hypertension, as assessed by ambulatory blood pressure monitoring: the MAPAVEL study. Clin Ther. 2002;24(1):126‐138. [DOI] [PubMed] [Google Scholar]
- 34. Cohen EP, Irving AA, Drobyski WR, et al. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2008;70(5):1546‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cushman WC, Cohen JD, Jones RP, et al. Comparison of the fixed combination of enalapril/diltiazem ER and their monotherapies in stage 1 to 3 essential hypertension. Am J Hypertens. 1996;11(1 Pt 1):23‐30. [DOI] [PubMed] [Google Scholar]
- 36. Cuspidi C, Muiesan LM, Valagussa L, et al. Comparative effects of candesartan and enalapril on left ventricular hypertrophy in patients with essential hypertension: the candesartan assessment in the treatment of cardiac hypertrophy (CATCH) study. J Hypertens. 2002;20(11):2293‐2300. [DOI] [PubMed] [Google Scholar]
- 37. DeQuattro V, Lee D, Messerli F. Efficacy of combination therapy with trandolapril and verapamil SR in primary hypertension: a 4×4 trial design. Clin Exp Hypertens. 1997;19(3):373‐387. [DOI] [PubMed] [Google Scholar]
- 38. Derosa G, Cicero AF, Ciccarelli L, et al. A randomized, double‐blind, controlled, parallel‐group comparison of perindopril and candesartan in hypertensive patients with type 2 diabetes mellitus. Clin Ther. 2003;25(7):2006‐2021. [DOI] [PubMed] [Google Scholar]
- 39. Dickstein K, Chang P, Willenheimer R, et al. Comparison of the effects of losartan and enalapril on clinical status and exercise performance in patients with moderate or severe chronic heart failure. J Am Coll Cardiol. 1995;26(2):438‐445. [DOI] [PubMed] [Google Scholar]
- 40. Dickstein K, Kjekshus J, Steering OPTIMAAL. Committee of the OPTIMAAL Study group. Effects of losartan and captopril on mortality and morbidity in high‐risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal trial in myocardial infarction with angiotensin II antagonist losartan. Lancet. 2002;360(9335):752‐760. [DOI] [PubMed] [Google Scholar]
- 41. Dunselman PH. Effects of the replacement of the angiotensin converting enzyme inhibitor enalapril by the angiotensin II receptor blocker telmisartan in patients with congestive heart failure. Int J Cardiol. 2001;77(2‐3):131‐138. [DOI] [PubMed] [Google Scholar]
- 42. Eguchi K, Kario K, Shimada K. Comparison of candesartan with lisinopril on ambulatory blood pressure and morning surge in patients with systemic hypertension. Am J Cardiol. 2003;92(5):621‐624. [DOI] [PubMed] [Google Scholar]
- 43. Eisner GM, Johnson BF, Mcmahon FG, et al. A multicenter comparison of the safety and efficacy of isradipine and enalapril in the treatment of hypertension. Am J Hypertens. 1991;4(2 Pt 2):154S‐157S. [DOI] [PubMed] [Google Scholar]
- 44. Elliott WJ. Double‐blind comparison of eprosartan and enalapril on cough and blood pressure in unselected hypertensive patients. J Hum Hypertens. 1999;13(6):413‐417. [DOI] [PubMed] [Google Scholar]
- 45. Bots ML, Remme WJ, Lüscher TF, Grobbee DE. EUROPA‐PERFECT Investigators. PERindopril‐Function of the Endothelium in coronary artery disease Trial: pERFECT study–sub study of EUROPA: rationale and design. Cardiovasc Drugs Ther. 2003;16(3):227‐236. [DOI] [PubMed] [Google Scholar]
- 46. Fan X, Han Y, Sun K, et al. Hypertension sex differences in blood pressure response to antihypertensive therapy in Chinese patients with hypertension. Ann of Pharmacother. 2008;42(12):1772‐1781. [DOI] [PubMed] [Google Scholar]
- 47. Fogari R, Preti P, Zoppi A, et al. Effects of amlodipine fosinopril combination on microalbuminuria in hypertensive type 2 diabetic patients. Am J Hypertens. 2002;15(12):1042‐1049. [DOI] [PubMed] [Google Scholar]
- 48. Fogari R, Derosa G, Zoppi A, et al. Effects of manidipine/delapril versus olmesartan/hydrochlorothiazide combination therapy in elderly hypertensive patients with type 2 diabetes mellitus. Hypertens Res. 2008;31(1):43‐50. [DOI] [PubMed] [Google Scholar]
- 49. Gavras I, Gavras H. Effects of eprosartan versus enalapril in hypertensive patients on the renin‐angiotensin‐aldosterone system and safety parameters: results from a 26‐week, double‐blind, multicentre study. Curr Med Res Opin. 1999;15(1):15‐24. [DOI] [PubMed] [Google Scholar]
- 50. Gradman AH, Arcuri KE, Goldberg AI, et al. A randomized, placebo‐controlled, double‐blind, parallel study of various doses of losartan potassium compared with enalapril maleate in patients with essential hypertension. Hypertension. 1995;25(6):1345‐1350. [DOI] [PubMed] [Google Scholar]
- 51. Gross O, Tönshoff B, Weber LT, et al. A multicenter, randomized, placebo‐controlled, double‐blind phase 3 trial with open‐arm comparison indicates safety and efficacy of nephroprotective therapy with ramipril in children with Alport's syndrome. Kidney Int. 2020;97(6):1275‐1286. [DOI] [PubMed] [Google Scholar]
- 52. Gueret P, Artigou JY, Beniehou M, et al. Comparative efficacy and safety of enalapril and sustained‐release nifedipine in patients with mild to moderate hypertension. Drugs. 1990;39(Suppl 2):67‐72. [DOI] [PubMed] [Google Scholar]
- 53. Guitard C, Lohmann FW, Alfiero R, Ruina M, Alvisi V. Comparison of efficacy of spirapril and enalapril in control of mild‐to‐moderate hypertension Italy spirapril and enalapril in hypertension. Cardiovasc Drug Ther. 1997;11(3):449‐457. [DOI] [PubMed] [Google Scholar]
- 54. Hajjar I, Okafor M, McDaniel D, et al. Effects of candesartan vs lisinopril on neurocognitive function in older adults with executive mild cognitive impairment: a randomized clinical trial. JAMA Newt Open. 2020;3:e2012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Halimi JM, Giraudeau B, Buchler M, et al. Enalapril/amlodipine combination in cyclosporine‐treated renal transplant recipients: a prospective randomized trial. Clin Transplant. 2007;21(2):277‐284. [DOI] [PubMed] [Google Scholar]
- 56. Hart W, Clarke RJ. ACE inhibition versus calcium antagonism in the treatment of mild to moderate hypertension: a multicentre study. Postgrad Med J. 1993;69(2):450‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Himmelmann A, Keinänen‐Kiukaanniemi S, Wester A, et al. The effect duration of candesartan cilexetil once daily, in comparison with enalapril once daily, in patients with mild to moderate hypertension. Blood Press. 2001;10(1):43‐51. [DOI] [PubMed] [Google Scholar]
- 58. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med. 2000;342(3):145‐153. [DOI] [PubMed] [Google Scholar]
- 59. Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354(2):131‐140. [DOI] [PubMed] [Google Scholar]
- 60. Fan FH, Xie D, Zhang X, et al. Renoprotection of Optimal Antiproteinuric Doses (ROAD) study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol. 2007;18(6):1889‐1898. [DOI] [PubMed] [Google Scholar]
- 61. Ishimitsu T, Akashiba A, Kameda T, et al. Benazepril slows progression of renal dysfunction in patients with non‐diabetic renal disease. Nephrology. 2007;12(3):294‐298. [DOI] [PubMed] [Google Scholar]
- 62. Johnson BF, Eisner GM, Mcmahon GF, et al. A multicenter comparison of adverse reaction profiles of isradipine and enalapril at equipotent doses in patients with essential hypertension. J Clin Pharmacol. 1995;35(5):848‐892. [DOI] [PubMed] [Google Scholar]
- 63. Juarez GF, Luño J, Barrio V, et al. Effect of dual blockade of the renin‐angiotensin system on the progression of type 2 diabetic nephropathy: a randomized trial. Am J Kidney Dis. 2013;61(2):211‐218. [DOI] [PubMed] [Google Scholar]
- 64. Karlberg BE, Lins LE, Hermansson K. Efficacy and safety of telmisartan, a selective AT 1 receptor antagonist, compared with enalapril in elderly patients with primary hypertension. J Hypertens. 1999;17(2):293‐302. [DOI] [PubMed] [Google Scholar]
- 65. Katoch N, Kumar BD, Calton RK, Kumar N, Gulrez G. To compare the efficacy and safety of ramipril versus losartan in post myocardial infarction patients. Asian J Pharm Clin Res. 2019;12(6):115‐118. [Google Scholar]
- 66. Ke YS, Tao YY, Yang HY. Effects of valsartan with or without benazepril on blood pressure, angiotensin II, and endoxin in patients with essential hypertension. Acta Pharmacol Sin. 2003;24(4):337‐341. [PubMed] [Google Scholar]
- 67. Kereiakes DJ, Neutel JM, Punzi HA, et al. Efficacy and safety of olmesartan medoxomil and hydrochlorothiazide compared with benazepril and amlodipine besylate. Am J Cardiovasc Drugs. 2007;17(5):361‐372. [DOI] [PubMed] [Google Scholar]
- 68. Kitzman DW, Hundley WG, Brubaker PH, et al. A randomized double‐blind trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circ Heart Fail. 2010;3(4):477‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ko GT, Tsang CC, Chan KC. Stabilization and regression of albuminuria in Chinese patients with type 2 diabetes: a one‐year randomized study of valsartan versus enalapril. Adv Ther. 2005;22(2):155‐162. [DOI] [PubMed] [Google Scholar]
- 70. Løber L, Torp‐Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin converting‐enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1995;21(25):1670‐1676. [DOI] [PubMed] [Google Scholar]
- 71. Koch B, Oparil S, Stimpel M. Co‐administration of an ACE‐inhibitor (moexipril) and hormonal replacement therapy in postmenopausal women. J Hum Hypertens. 1999;13(5):337‐342. [DOI] [PubMed] [Google Scholar]
- 72. Knoll GA, Fergerson D, Chasse M, et al. Ramipril versus placebo in kidney transplant patients with proteinuria: a multicentre, double‐blind, randomised controlled trial. Lancet Diab Endocrinol. 2016;4(4):318‐326. [DOI] [PubMed] [Google Scholar]
- 73. Multicenter LacrouciereYA. Randomized, double‐blind study of the antihypertensive efficacy and tolerability of irbesartan in patients aged >or = 65 years with mild to moderate hypertension. Clin Ther. 2000;22(10):1213‐1224. [DOI] [PubMed] [Google Scholar]
- 74. Lacourcière Y, Neutel JM, Davidai G, Koval S. A multicenter, 14‐week study of telmisartan and ramipril in patients with mild‐to‐moderate hypertension using ambulatory blood pressure monitoring. Am J Hypertens. 2006;19(1):104‐112. [DOI] [PubMed] [Google Scholar]
- 75. Larochelle P, Flack JM, Marbury TC, et al. Effects and tolerability of irbesartan versus enalapril in patients with severe hypertension. Am J Cardiol. 1997;80(12):1613‐1615. [DOI] [PubMed] [Google Scholar]
- 76. Leonetti G, Rappelli A, Omboni S, et al. A similar 24‐h blood pressure control is obtained by zofenopril and candesartan in primary hypertensive patients. Blood Press; 2006:18‐26. [Google Scholar]
- 77. Leu HB, Charng MJ, Ding PY. A Double blind randomized trial to compare the effects of eprosartan and enalapril on blood pressure, platelets, and endothelium function in patients with essential hypertension. Jpn Heart J. 2004;45(4):623‐635. [DOI] [PubMed] [Google Scholar]
- 78. Lohmann FW. Treatment of isolated systolic hypertension: a comparative study of felodipine, ramipril and isosorbide mononitrate in 1041 patients. Clin Drug Invest. 1999;18(3):173‐181. [Google Scholar]
- 79. Lonn EM, Gerstein HC, Sheridan P, et al. Effect of ramipril and of rosiglitazone on carotid intima‐media thickness in people with impaired glucose tolerance or impaired fasting glucose. STARR (STudy of Atherosclerosis with Ramipril and Rosiglitazone). J Am Coll Cardiol. 2009;53(22):2028‐2035. [DOI] [PubMed] [Google Scholar]
- 80. MacGregor MS, Deighan CJ, Rodger RS, Boulton‐Jones JM. A prospective open‐label randomised trial of quinapril and/or amlodipine in progressive non‐diabetic renal failure. Nephron Clin Pract. 2005;10(3):139‐149. [DOI] [PubMed] [Google Scholar]
- 81. Malacco E, Omboni S, Volpe M, Auteri A, Zanchetti A. Antihypertensive efficacy and safety of olmesartan medoxomil and ramipril in elderly patients with mild to moderate essential hypertension: the ESPORT study. J Hypertens. 2010;28(11):2342‐2350. [DOI] [PubMed] [Google Scholar]
- 82. Malacco E, Santonastaso M, Vari NA, et al. Comparison of valsartan 160 mg with lisinopril 20 mg, given as monotherapy or in combination with a diuretic, for the treatment of hypertension: the blood pressure reduction and tolerability of valsartan in comparison with lisinopril (PREVAIL) Study. Clin Ther. 2004;26(66):855‐885. [DOI] [PubMed] [Google Scholar]
- 83. Mallion JM, Omboni S, Barton J, et al. Antihypertensive efficacy, and safety of olmesartan and ramipril in elderly patients with mild to moderate systolic and diastolic essential hypertension. Blood Press Suppl. 2011;20:3‐11. [DOI] [PubMed] [Google Scholar]
- 84. Malmqvist K, Kahan T, Dahl M. Angiotensin II type 1 (AT 1) receptor blockade in hypertensive women: benefits of candesartan cilexetil versus enalapril or hydrochlorothiazide. Am J Hypertens. 2000;13(5 Pt 1):504‐511. [DOI] [PubMed] [Google Scholar]
- 85. Marketou ME, Zacharis EA, Koukouraki S, et al. Effect of angiotensin‐converting enzyme inhibitors on systemic inflammation and myocardial sympathetic innervation in normotensive patients with type 2 diabetes mellitus. J Hum Hypertens. 2008;22(3):191‐196. [DOI] [PubMed] [Google Scholar]
- 86. Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361(1):40‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Menne J, Farsang C, Deák L, et al. Valsartan in combination with lisinopril versus the respective high dose monotherapies in hypertensive patients with microalbuminuria: the VALERIA trial. J Hypertens. 2008;26(9):1860‐1867. [DOI] [PubMed] [Google Scholar]
- 88. Messerli F, Frishman WH, Elliott WJ. Effects of verapamil and rrandolapril in the treatment of hypertension. Am J Hypertens. 1998;11(3 Pt 1):322‐327. [DOI] [PubMed] [Google Scholar]
- 89. Mimran A, Ruilope L, Kerwin L, et al. A randomised, double‐blind comparison of the angiotensin II receptor antagonist, irbesartan, with the full dose range of enalapril for the treatment of mild‐to‐moderate hypertension. J Hum Hypertens. 1998;12(3):203‐208. [DOI] [PubMed] [Google Scholar]
- 90. Morgan TO, Anderson A, Jones E. Comparison and interaction of low dose felodipine and enalapril in the treatment of essential hypertension in elderly subjects. Am J Hypertens. 1991;5(4 Pt 1):238‐243. [DOI] [PubMed] [Google Scholar]
- 91. Nakamura T, Kawachi K, Saito Y, et al. Effects of ARB or ACE‐Inhibitor administration on plasma levels of aldosterone and adiponectin in hypertension. Int Heart J. 2009;50(4):501‐512. [DOI] [PubMed] [Google Scholar]
- 92. Nalbantgil I, Nalbantgil S, Ozerkan F, et al. The efficacy of telmisartan compared with perindopril in patients with mild‐to‐moderate hypertension. Int J Clin Pract Suppl. 2004;145(140):50‐54. [PubMed] [Google Scholar]
- 93. Neutel JM, Fishman WH, Oparil S, Papdemitriou V, Guthrie G. Comparison of telmisartan with lisinopril in patients with mild‐to‐moderate hypertension. Am Ther. 1999;6(3):161‐166. [DOI] [PubMed] [Google Scholar]
- 94. Nissen SE, Tuzcu EM, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure The CAMELOT Study: a randomized controlled trial. JAMA. 2004;292(18):2217‐2226. [DOI] [PubMed] [Google Scholar]
- 95. Northridge DB, Rosef E, Rafteryt ED, et al. A multicentre, double‐blind, placebo‐controlled trial of quinapril in mild, chronic heart failure. Eur Heart J. 1993;14(3):403‐409. [DOI] [PubMed] [Google Scholar]
- 96. ONTARGET . Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547‐1559. [DOI] [PubMed] [Google Scholar]
- 97. Ormesher L, Higson S, Luckie M, et al. Postnatal enalapril to improve cardiovascular function following preterm preeclampsia (PICk‐UP): a randomized double‐Blind placebo‐controlled feasibility Trial. Hypertension. 2020;76(6):1828‐1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Östergren J, Storstein L, Karlberg BE, et al. Quality of life in hypertensive patients treated with either carvedilol or enalapril. Blood Press; 1996:41‐49. [DOI] [PubMed] [Google Scholar]
- 99. Otero ML, Claros NM. Manidipine Versus enalapril monotherapy in patients with hypertension and type 2 diabetes mellitus: a multicenter, randomized, double‐blind, 24‐week study. Clin Ther. 2005;27(2):166‐173. [DOI] [PubMed] [Google Scholar]
- 100. Perico N, Spormann D, Peruzzi E, et al. Efficacy and tolerability of valsartan compared with lisinopril in patients with hypertension and renal insufficiency. Clin Drug Invest. 1997;14(4):252‐259. [Google Scholar]
- 101. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart gailure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893‐1906. [DOI] [PubMed] [Google Scholar]
- 102. Phakdeekitcharoen B, Leelasa‐Nguan P. Effects of an ACE inhibitor or angiotensin receptor blocker on potassium in CAPD patients. Am J of Kidney Dis. 2004;44(4):738‐746. [PubMed] [Google Scholar]
- 103. Philipp T, Anlauf M, Distler A, et al. Randomised, double blind, multicentre comparison of hydrochlorothiazide, atenolol, nitrendipine, and enalapril in antihypertensive treatment: results of the HANE study. BMJ. 1997;315(7101):154‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pitt B, Segal R, Martinez FA, et al. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet. 1997;349(9054):747‐752. [DOI] [PubMed] [Google Scholar]
- 105. Pitt B, O'neill B, Feldman R, et al. The QUinapril Ischemic Event Trial (QUIET): evaluation of chronic ACE inhibitor therapy in patients with ischemic heart disease and preserved left ventricular function. Am J Cardiol. 2001;87(9):1058‐1064. [DOI] [PubMed] [Google Scholar]
- 106. Prisant ML, Weir MR, Papademetriou V, et al. Low‐dose drug combination therapy: an alternative first‐line approach to hypertension treatment. Am Heart J. 1995;130(2):359‐366. [DOI] [PubMed] [Google Scholar]
- 107. Prisant ML. Low‐dose combination treatment for hypertension versus single‐drug treatment ‐ bisoprolol/hydrochlorothiazide versus amlodipine, enalapril, and placebo: combined analysis of comparative studies. Am J Ther. 1998;5(5):313‐321. [DOI] [PubMed] [Google Scholar]
- 108. PROGRESS Collaborative Group . Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358(9287):1033‐1041. [DOI] [PubMed] [Google Scholar]
- 109. Rahman S, Ismail AA, Ismail SB, Naing NN, Rahman AR. Effect of rosiglitazone/ramipril on preclinical vasculopathy in newly diagnosed, untreated diabetes and IGT patients: 1‐Year randomised, double‐blind, placebo‐controlled study. Eur J Clin Pharmacol. 2007;63(8):733‐741. [DOI] [PubMed] [Google Scholar]
- 110. Ragot S, Ezzaher A, Meunier A, et al. Comparison of trough effect of telmisartan vs perindopril using self blood pressure measurement: eVERESTE study. J Hum Hypertens. 2002;16(12):865‐873. [DOI] [PubMed] [Google Scholar]
- 111. Ramsay LE, Yeo WW. The Losartan Cough Study Group. Double‐blind comparison of losartan, lisinopril and hydrochlorothiazide in hypertensive patients with a previous angiotensin converting enzyme inhibitor‐associated cough. J Hypertens Suppl. 1995;13(1):S73‐S76. [DOI] [PubMed] [Google Scholar]
- 112. Reyes‐Marín FA, Calzada C, Ballesteros A, Amato D. Comparative study of enalapril vs. losartan on residual renal function preservation in automated peritoneal dialysis. A randomized controlled study. Rev Invest Clin. 2012;64(4):315‐321. [PubMed] [Google Scholar]
- 113. Rogstad B. A comparison of lisinopril and nifedipine in the treatment of mild to moderate hypertension A muliticentre study. Eur J Clin Pharmacol. 1994;46(6):487‐489. [DOI] [PubMed] [Google Scholar]
- 114. Rosei E, Morelli P, Rizzoni D. Effects of nifedipine GITS 20 mg or enalapril 20 mg on blood pressure and inflammatory markers in patients with mild‐moderate hypertension. Blood Press Suppl. 2005;14:14‐22. [DOI] [PubMed] [Google Scholar]
- 115. Rouleau JL, Warnica WJ, Baillot R, et al. Effects of angiotensin‐converting enzyme inhibition in low‐risk patients early after coronary artery bypass surgery. Circulation. 2008;117(1):24‐31. [DOI] [PubMed] [Google Scholar]
- 116. Ruddy TD, Fodor JG. Nisoldipine CC and lisinopril alone or in combination for treatment of mild to moderate systemic hypertension nisoldipine CC therapy for hypertension. Cardiovasc Drugs Ther. 1997;11(4):581‐590. [DOI] [PubMed] [Google Scholar]
- 117. Ruilope L, Jäger B, Prichard B. Eprosartan versus enalapril in elderly patients with hypertension: a double‐blind, randomized trial. Blood Press. 2001;10(4):223‐229. [DOI] [PubMed] [Google Scholar]
- 118. Sabharwal NK, Swinburn J, Lahiri A, Senior R. Effect of imidapril and nifedipine on left ventricular hypertrophy in untreated hypertension. Clin Drug Invest. 2005;25(6):367‐375. [DOI] [PubMed] [Google Scholar]
- 119. Sampaio RO, Grinberg M, Leite JJ, et al. Effect of enalapril on left ventricular diameters and exercise capacity in asymptomatic or mildly symptomatic patients with regurgitation secondary to mitral valve prolapse or rheumatic heart disease. Am J of Cardiol. 2005;96(1):117‐121. [DOI] [PubMed] [Google Scholar]
- 120. Schaefer F, Litwin M, Zachwieja J, et al. Efficacy and safety of valsartan compared to enalapril in hypertensive children: a 12‐week, randomized, double‐blind, parallel‐group study. J Hypertens. 2011;29(12):2484‐2490. [DOI] [PubMed] [Google Scholar]
- 121. Schrader H, Stovner LJ, Helde G, Sand T, Bovim G. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomised, placebo controlled, crossover study. Br Med J. 2001;322(7277):19‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sega R. Efficacy and safety of eprosartan in severe hypertension. Blood Press. 1999;8(2):114‐121. [DOI] [PubMed] [Google Scholar]
- 123. Shionoiri H, Takasaki I, Minamisawa K, et al. Cough‐challenge trial with a new angiotensin‐converting enzyme inhibitor, imidapril. J Clin Pharmacol. 1998;38(5):442‐426. [DOI] [PubMed] [Google Scholar]
- 124. Silagy CA, Mcneil JJ, Mcgrath BP. Crossover comparison of atenolol, enalapril, hydrochlorothiazide and isradipine for isolated systolic systemic hypertension. Am J Cardiol. 1992;70(15):1299‐1305. [DOI] [PubMed] [Google Scholar]
- 125. The SOLVD Investigators , Yusuf S, Pitt B, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293‐302. [DOI] [PubMed] [Google Scholar]
- 126. Sonbolestan SA, Heshmat K, Javanmard SH, Saadatnia M. Efficacy of enalapril in migraine prophylaxis: a randomized, double‐blind, placebo‐controlled trial. Int J Prevent Med. 2013;4(1):72‐77. [PMC free article] [PubMed] [Google Scholar]
- 127. Song JH, Cha SH, Lee HJ, et al. Effect of low‐dose dual blockade of renin‐angiotensin system on urinary TGF‐β in type 2 diabetic patients with advanced kidney disease. Nephrol Dial Transplant. 2006;21(3):683‐689. [DOI] [PubMed] [Google Scholar]
- 128. Spinar J, Vitovec J, Spinarova L, et al. A comparison of intervention with losartan or captopril in acute myocardial infarction. Eur J Heart Fail. 2000;2(1):91‐100. [DOI] [PubMed] [Google Scholar]
- 129. Sumukadas D, Price R, Mcmurdo ME, et al. The effect of perindopril on postural instability in older people with a history of falls‐A randomised controlled trial. Age Ageing. 2018;47(1):75‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tan F, Mukherjee J, Lee K, Lim P, Liew C. Dual blockade of the renin‐angiotensin‐aldosterone system is safe and effective in reducing albuminuria in Asian type 2 diabetic patients with nephropathy. Singapore Med J. 2010;51(2):151‐156. [PubMed] [Google Scholar]
- 131. Tanser PH, Campbell LM, Carranza J, et al. Candesartan cilexetil is not associated with cough in hypertensive patients with enalapril‐induced cough. J Hypertens. 2000;123(2):214‐218. [DOI] [PubMed] [Google Scholar]
- 132. Tikkanen I, Omvik P, Jensen H, Group TSS. Comparison of the angiotensin II antagonist losartan with the angiotensin converting enzyme inhibitor enalapril in patients with essential hypertension. J Hypertens. 1995;13(11):1343‐1351. [DOI] [PubMed] [Google Scholar]
- 133. Tomlinson B, Woo J, Critchley JA, et al. Sustained‐release isradipine compared with spirapril in the treatment of elderly patients with isolated systolic hypertension. Am J Hypertens. 1994;7(7 Pt 2):35‐39. [DOI] [PubMed] [Google Scholar]
- 134. Tomlinson B, Woo J, Thomas GN, Chau YM, Critchley JA. Randomized, controlled, parallel‐group comparison of ambulatory and clinic blood pressure responses to amlodipine or enalapril during and after treatment in adult Chinese patients with hypertension. Clin Ther. 2004;26(8):1292‐1304. [DOI] [PubMed] [Google Scholar]
- 135. Toto RD, Adams‐Huet B, Fenves AZ, et al. Effect of ramipril on blood pressure and protein excretion rate in normotensive nondiabetic patients with proteinuria. Am J Kidney Dis. 1996;28(6):832‐840. [DOI] [PubMed] [Google Scholar]
- 136. Townsend R, Haggert B, Liss C, Edelman JM. Efficacy and tolerability of losartan versus enalapril alone or in combination with hydrochlorothiazide in patients with essential hypertension. Clin Ther. 1995;17(5):911‐923. [DOI] [PubMed] [Google Scholar]
- 137. van der Does R, Euler R. A randomized, double‐blind, parallel‐group study to compare the anti‐hypertensive effects of imidapril and nifedipine in the treatment of mild‐to‐ moderate essential hypertension. J Inter Med Rev. 2001;29(3):154‐162. [DOI] [PubMed] [Google Scholar]
- 138. Velasco M, Urbina A, Silva H, et al. A double‐blind, parallel, comparative evaluation of amlodipine vs captopril in the monotherapeutic treatment of mild and moderate essential hypertension. J Cardiovasc Pharmacol. 1991;17(Suppl 1):S19‐S21. [DOI] [PubMed] [Google Scholar]
- 139. Verkaaik R, Hogewind B, Woittiez A. A comparative study of the effects of nitrendipine and analapril in essential hypertension. J Cardiovasc Pharmacol. 1991;18(Suppl 1):S63‐S66. [PubMed] [Google Scholar]
- 140. Weber MA, Basile J, Stapff M, Khan B, Zhou D. Blood pressure effects of combined β‐blocker and angiotensin‐converting enzyme inhibitor therapy compared with the individual agents: a placebo‐controlled study with nebivolol and lisinopril. J Clin Hypertens (Greenwich). 2012;14(9):588‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wei F, Jia XJ, Yu SQ, et al. Candesartan versus imidapril in hypertension: a randomised study to assess effects of anti‐AT1 receptor autoantibodies. Heart. 2011;97(6):479‐484. [DOI] [PubMed] [Google Scholar]
- 142. White WB, Sica DA, Calhoun D, Mansoor GA, Anders RJ. Preventing increases in early‐morning blood pressure, heart rate, and the rate‐pressure product with controlled onset extended release verapamil at bedtime versus enalapril, losartan, and placebo on arising. Am Heart J. 2002;144(4):657‐665. [DOI] [PubMed] [Google Scholar]
- 143. White WB, Lacourciere Y, Gana T, et al. Effects of graded‐release diltiazem versus ramipril, dosed at bedtime, on early morning blood pressure, heart rate, and the rate‐pressure product. Am Heart J. 2004;148(4):628‐634. [DOI] [PubMed] [Google Scholar]
- 144. Widimsky J, Kremer HJ, Jerie P, Uhlir O. Czech and Slovak spirapril intervention study (CASSIS). A randomized, placebo and active‐controlled, double‐blind multicentre trial in patients with congestive heart failure. Eur J Clin Pharmacol. 1995;49(1):95‐102. [DOI] [PubMed] [Google Scholar]
- 145. Williams B, Gosse P, Lowe L, Harper R. The prospective, randomized investigation of the safety and efficacy of telmisartan versus ramipril using ambulatory blood pressure monitoring (PRISMA I). J Hypertens. 2006;24(1):193‐200. [DOI] [PubMed] [Google Scholar]
- 146. Wu SC, Liu CP, Chiang HT, Lin SL. Prospective and randomized study of the antihypertensive effect and tolerability of three antihypertensive agents, losartan, amlodipine, and lisinopril, in hypertensive patients. Heart Vessels. 2004;19(1):13‐18. [DOI] [PubMed] [Google Scholar]
- 147. Yokota T, Osanai T, Hanada K, et al. Effects of telmisartan on markers of ventricular remodeling in patients with acute myocardial infarction: comparison with enalapril. Heart Vessels. 2010;25(6):460‐468. [DOI] [PubMed] [Google Scholar]
- 148. Zannad F, Bernaud C, Fay R, Group GPI. Double‐blind, randomized, multicentre comparison of the effects of amlodipine and perindopril on 24 h therapeutic coverage and beyond in patients with mild to moderate hypertension. J Hypertens. 1999;17(1):137‐146. [DOI] [PubMed] [Google Scholar]
- 149. Zi M, Carmichael N, Lye M. The effect of quinapril on functional status of elderly patients with diastolic heart failure. Cardiovasc Drug Ther. 2003;17(2):133‐139. [DOI] [PubMed] [Google Scholar]
- 150. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from panel members appointed to the eighth joint national committee (JNC 8). JAMA. 2014;311(5):507‐520. [DOI] [PubMed] [Google Scholar]
- 151. Yilmaz I. Angiotensin‐converting enzyme inhibitors induce cough. Turk Thorac J. 2019 J;20(1):36‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. investigators ONTARGET, Yusuf S, Teo KK, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J of Med. 2008;358(15):1547‐1559. [DOI] [PubMed] [Google Scholar]
- 153. Brown NJ, Vaughan DE. Angiotensin‐converting enzyme inhibitors. Circulation. 1998;97(14):1411‐1420. [DOI] [PubMed] [Google Scholar]
- 154. Heel RC, Brogden RN, Speight TM, Avery GS. Captopril: a preliminary review of its pharmacological properties and therapeutics efficacy. Drugs. 1980;2(6):409‐452. [DOI] [PubMed] [Google Scholar]
- 155. Song JC, White CM. Clinical pharmacokinetics and selective pharmacodynamics of new angiotensin converting enzyme inhibitors. Clin Pharmacokinet. 2002;4(3):207‐224. [DOI] [PubMed] [Google Scholar]
- 156. RamC. Captopril. Arch Intern Med. 1982;142(5):914‐916. [PubMed] [Google Scholar]
- 157. Os I, Bratland B, Dahlöf B, et al. Female preponderance for lisinopril‐induced cough in hypertension. Am J Hypertens. 1994;7(11):1012‐1015. [DOI] [PubMed] [Google Scholar]
- 158. Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin‐converting enzyme inhibitors. J Eval Clin Pract. 2004;10(4):499‐509. [DOI] [PubMed] [Google Scholar]
- 159. Visser LE, Stricker BH, van der Velden J, Paes AH, Bakker A. Angiotensin converting enzyme inhibitor associated cough: a population‐based case‐control study. J Clin Epidemiol. 1995;48(6):851‐857. [DOI] [PubMed] [Google Scholar]
- 160. Lee YJ, Tsai JCR. Angiotensin‐converting enzyme gene insertion/deletion, not bradykinin B2 receptor ‐58T/C gene polymorphism, associated with angiotensin‐converting enzyme inhibitor‐related cough in Chinese female patients with non‐insulin‐dependent diabetes mellitus. Metabolism. 2001;50(11):1346‐1350. [DOI] [PubMed] [Google Scholar]
- 161. O'Connell F, Thomas VE, Pride NB, Fuller RW, Capsaicin cough sensitivity decreases with successful treatment of chronic cough. Am J Respir Crit Care Med. 1994;150(2):374‐380. [DOI] [PubMed] [Google Scholar]
- 162. Kaufman J, Schmitt S, Barnard J, Busse W. Angiotensin‐converting enzyme inhibitors in patients with bronchial responsiveness and asthma. Chest. 1992;101(4):922‐925. [DOI] [PubMed] [Google Scholar]
- 163. Kaufman J, Casanova JE, Riendl P, Schiuder DP. Bronchial hyperreactivity and cough due to angiotensin‐converting enzyme inhibitors. Chest. 1989;95(3):544‐548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Authors have no data availability to share.


