Abstract
Patients with apparent treatment‐resistant hypertension (aTRH) are at increased risk of end‐organ damage and cardiovascular events. Little is known about the effects of blood pressure (BP) control in this population. Using a national claims database integrated with electronic medical records, the authors evaluated the relationships between uncontrolled BP (UBP; ≥130/80 mmHg) or controlled BP (CBP; <130/80 mmHg) and risk of major adverse cardiovascular events plus (MACE+; stroke, myocardial infarction, heart failure requiring hospitalization) and end‐stage renal disease (ESRD) in adult patients with aTRH (taking ≥3 antihypertensive medication classes concurrently within 30 days between January 1, 2015 and June 30, 2021). MACE+ components were also evaluated separately. Multivariable regression models were used to adjust for baseline differences in demographic and clinical characteristics, and sensitivity analyses using CBP <140/90 mmHg were conducted. Patients with UBP (n = 22 333) were younger and had fewer comorbidities at baseline than those with CBP (n = 11 427). In the primary analysis, which adjusted for these baseline differences, UBP versus CBP patients were at an 8% increased risk of MACE+ (driven by a 31% increased risk of stroke) and a 53% increased risk of ESRD after 2.7 years of follow‐up. Greater MACE+ (22%) and ESRD (98%) risk increases with UBP versus CBP were seen in the sensitivity analysis. These real‐world data showed an association between suboptimal BP control in patients with aTRH and higher incidence of MACE+ and ESRD linked with UBP despite the use of multidrug regimens. Thus, there remains a need for improved aTRH management.
Keywords: blood pressure control, cardiovascular disease, real‐world analysis, sensitivity analysis, treatment‐resistant hypertension
1. INTRODUCTION
Hypertension is a leading cause of cardiovascular (CV) disease, chronic kidney disease (CKD), and all‐cause mortality. 1 , 2 , 3 In 2019, >500 000 deaths in the United States included hypertension as a primary or contributing cause. 4 Nearly half of the US‐based adult population (∼120 million people) has hypertension. 5 According to the American College of Cardiology (ACC) and American Heart Association (AHA) 2017 guidelines, hypertension is defined as systolic blood pressure (SBP) >130 mmHg, diastolic blood pressure (DBP) >80 mmHg, or taking medication for hypertension. 6 Before the ACC/AHA updated their guidelines, target blood pressure (BP) in other guidelines was 140/90 mmHg. 7 , 8 Several guidelines maintain <140/90 mmHg as the primary BP target for the general population and a target <130/80 mmHg for patients at a higher risk for CV complications. 9 , 10
Despite the availability of multiple treatment options for hypertension, many patients face challenges to control their BP and may not respond to therapy. Among those who receive treatment for hypertension, 10% to 15% have apparent treatment‐resistant hypertension (aTRH). 6 , 7 , 8 In 2018, more than 10.3 million adults in the United States had aTRH. 9 The AHA defines aTRH as BP that remains uncontrolled despite concomitant use of 3 antihypertensive medications of different classes or BP that is controlled with the use of ≥4 antihypertensive medication classes. 1 These classes typically include a long‐acting calcium channel blocker (CCB), an angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), and a diuretic. 6 , 8 , 9 Current US‐based guidelines recommend mineralocorticoid antagonists (MRAs) as the fourth drug class for patients with resistant hypertension 6 ; however, MRA therapy is generally advised against in patients with hypertension and advanced stages of CKD. 11 , 12 Thus, new treatments with novel mechanisms of action could help these patients.
Limited studies have assessed the impact of BP control on these CV outcomes among aTRH patients, and there remains a wide knowledge gap with respect to a larger cohort study. This study evaluated clinical outcomes among patients with aTRH using real‐world data from 2 large US healthcare databases. The primary objective was to compare the risk of major adverse CV events plus (MACE+; stroke [ischemic or hemorrhagic], myocardial infarction [MI], and heart failure [HF] requiring hospitalization) between aTRH patients with uncontrolled BP (UBP) versus those with controlled BP (CBP). Individual MACE+ components and end‐stage renal disease (ESRD) were also evaluated separately.
2. METHODS
2.1. Data sources
This retrospective cohort study used linked data from 2 US‐based databases that have been collecting information since 2006. The IQVIA Ambulatory EMR—US database includes ∼90 million patient records provided by >100 000 physicians (∼40% primary care practitioners and ∼60% specialists) sourced from medium‐to‐large ambulatory practices. Patient‐level data are available for demographics, diagnoses, procedures, prescription drugs, vaccines, laboratory tests, and vital signs. The IQVIA PharMetrics Plus claims database includes >140 million enrollees (generally representative of those aged <65 years and commercially insured [90%]) from >70 contributing health plans and self‐insured employer groups. The database contains medical and pharmacy claims information (costs and descriptive services), patient‐level enrollment records, and patient demographics. All records in both databases are deidentified.
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical‐trials/transparency. These data were made available by IQVIA and used under license for the current study and are not publicly available. Other researchers should contact https://www.iqvia.com.
2.2. Study design and patients
This was a retrospective cohort study comparing aTRH patients with UBP versus those with CBP conducted between January 1, 2014 and June 30, 2021 (Figure 1). To be included, patients had to be ≥18 years of age and taking ≥3 antihypertensive medication classes concurrently within 30 days between January 1, 2015 and June 30, 2021. The index regimen date was defined as the date when the criterion for concurrent use of ≥3 antihypertensive medications was met. Patients were required to have 2 office‐based BP measurements (on different dates ≤90 days apart) between 30 days and 11 months after the index regimen date, and the date of the second measurement was defined as the index date. Patients had to have ≥365 days of continuous health plan enrollment before the index date, which served as the baseline period.
FIGURE 1.
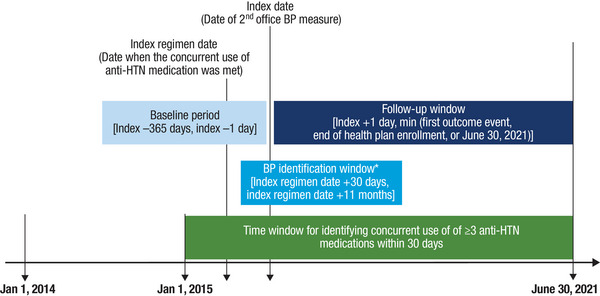
Study design. *The 2 office‐based BP measurements have to be on distinct dates and 1 to 90 days apart. Abbreviations: anti‐HTN, antihypertensive; BP, blood pressure.
Patients were excluded if they discontinued any antihypertensive medication included in the index regimen within 30 days following the index date. Discontinuation was defined as having a gap greater than 60 days between prescription fills. 13 , 14 Patients were excluded if they became pregnant at any time during the study period. Finally, patients were excluded if the mean of the 2 office BP measurements had SBP >300 mmHg.
2.3. Definitions and assessments
Baseline SBP and DBP were defined as the mean of the first 2 SBP and DBP measurements recorded during office visits on different dates ≤90 days apart between 30 days and 11 months after the index regimen date. This approach is consistent with clinical guidelines 6 recommending that hypertensive patients with above‐goal BP have repeat measures taken on separate occasions and the mean SBP and DBP measurements be used to evaluate BP control and guide treatment. When multiple SBP/DBP measurements were recorded on a single date, the mean of those SBP/DBP measurements was calculated and used for the SBP/DBP measurement.
In the primary analyses, UBP was defined as SBP ≥130 or DBP ≥80 mmHg, and CBP was defined as BP <130/80 mmHg, consistent with the 2021 ACC/AHA guideline recommendations. 15 We also conducted a sensitivity analysis using the BP <140/90 mmHg goal as CBP and BP ≥140/90 mmHg as UBP, recommended by the 2020 International Society of Hypertension (ISH) guidelines. 16
Eleven classes of antihypertensive medications were evaluated: diuretics (thiazide, potassium sparing, loop, MRAs, and combination); beta‐blockers; ACE inhibitors; ARBs, CCBs; alpha blockers; alpha‐2 receptor agonists; combined alpha‐ and beta‐blockers; central agonists; peripheral adrenergic inhibitors; and vasodilators. Patients taking fixed drug combination medications were considered as having been exposed to each constituent drug class.
The following patient demographic characteristics were evaluated on the index date: age, sex, race, type of health insurance coverage, and geographic US Census region. Patient clinical characteristics evaluated during the pre‐index period were identified based on the International Classification of Diseases, Ninth Revision, Clinical Modification or International Classification of Diseases, Tenth Revision diagnosis codes 17 for comorbidities, including possible causes of secondary hypertension (i.e., hyperaldosteronism, renal disease, sleep apnea, Cushing syndrome, renal artery stenosis, and alcohol use disorder); cardiometabolic risk factors and complications; and depression, anxiety, osteoarthritis, and anemia (Table 1, Supplemental Digital Content 1). Other clinical characteristics that were assessed included the Quan‐Charlson Comorbidity Index score and its components 18 (Table 2, Supplemental Digital Content 1), body mass index (BMI), and smoking status.
In addition to the use of antihypertensive medications, other medication classes were evaluated, including lipid‐lowering agents, platelet aggregation inhibitors, oral anticoagulants, antidiabetic agents, antiarrhythmic drugs, weight‐loss drugs, antimigraine drugs, stimulants, opioids, systemic corticosteroids, antineoplastic agents, antidepressants, anxiolytics, and chronic use of nonsteroidal anti‐inflammatory drugs (NSAIDs; >30‐day supply).
Clinical outcomes were assessed using an intent‐to‐treat approach and identified based on the International Classification of Diseases, Ninth Revision, Clinical Modification or International Classification of Diseases, Tenth Revision diagnosis and procedure codes 17 (Table 3, Supplemental Digital Content 1). The composite MACE+ endpoint included hospitalization with a primary diagnosis of stroke (ischemic or hemorrhagic), MI, or HF (identified by diagnosis code) during the follow‐up period. Patients were followed until the earliest of the first stroke, MI, or HF‐related hospitalization, end of health plan enrollment, or end of available data; CV death was not included in the MACE+ composite due to limited mortality data in the databases. ESRD was defined as being on dialysis for ≥90 days 19 or undergoing kidney transplantation during the follow‐up period. Patients were followed until the earliest of the first ESRD event, end of health plan enrollment, or end of available data.
2.4. Ethics
The use of the IQVIA databases was reviewed by the New England Institutional Review Board (IRB) and was determined to be exempt from broad IRB approval as this study did not involve human participants. Confidentiality of patient records was maintained throughout the study. Study reports contained aggregate data only and did not identify individual patients or physicians. The sponsor did not receive patient identifying information during the study.
2.5. Statistical analysis
For patient demographic and clinical characteristics, descriptive statistics, including means and standard deviations (SDs), were used for continuous variables, and relative frequencies and percentages were calculated for categorical variables. Between‐group differences were assessed using standardized differences. A standardized difference <10% was considered balanced.
For clinical outcomes, incidence rates were calculated as the total number of cases during the follow‐up over total follow‐up time (years) and reported per 1000 person‐years (PY). Multivariable regression models were used for between‐group comparisons. Conditional Cox proportional hazard models were used, adjusting for unbalanced covariates and calculating the hazard ratio (HR) and corresponding 95% confidence interval (CI). All analyses were performed using the Instant Health Data platform (Panalgo, LLC, Boston, MA) and SAS version 9.4 (SAS Institute, Cary, NC).
3. RESULTS
3.1. Patient disposition
Approximately 18 million patients were included in both databases, and ∼7 million patients (38.7%) had ≥1 diagnosis code for hypertension or ≥1 prescription for an antihypertensive medication. Of this total, nearly 900 000 patients (12.9%) met the criterion for concurrent use of ≥3 antihypertensive classes within a 30‐day period between January 1, 2015 and June 30, 2021. Twenty‐five percent of these patients had 2 BP measurements on distinct dates 1 to 90 days apart between 30 days and 11 months after the index regimen date. Nearly all of these patients were aged ≥18 years; fewer than 20% of adult patients had continuous health plan enrollment for ≥365 days before the index date. Of this total, 80.9% continued all of the antihypertensive medications included in their index regimen through 60 days following the index regimen date. When pregnant patients and those with mean SBP measurements >300 mmHg were excluded, the final analysis set included 33 760 patients: 22 333 (66.2%) in the UBP group and 11 427 (33.8%) in the CBP group (Figure 2). When the BP threshold was <140/90 mmHg in the sensitivity analysis, the percentages reversed; there were 11 384 (33.7%) in the UBP group and 22 376 (66.3%) patients in the CBP group. This is a consequence of many patients having baseline BP between the 130/80 and 140/90 mmHg thresholds.
FIGURE 2.
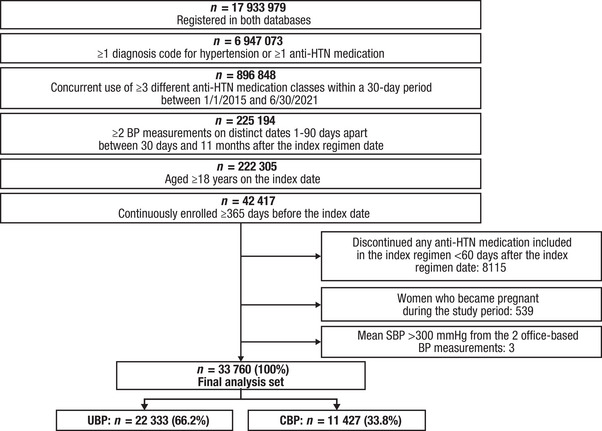
Patient flowchart for IQVIA Ambulatory EMR—US and PharMetrics Plus linked claims. Abbreviations: anti‐HTN, antihypertensive; BP, blood pressure; CBP, controlled blood pressure; EMR, electronic medical record; SBP, systolic blood pressure; UBP, uncontrolled blood pressure.
3.2. Patient demographic and clinical characteristics during baseline period
There were imbalances (standardized differences ≥10%) in demographic and clinical characteristics between patients with UBP and CBP (Tables 1 and 2). Patients in the UBP versus CBP group were younger (mean age 60.0 vs. 62.2 years). More patients in the UBP versus CBP group were African American (12.5% vs. 7.6%) and a lower percentage had Medicare insurance (14.0% vs. 19.1%). 53.3% of patients were male, and the highest proportion (48.2%) were from the South US Census region, with no imbalance between the UBP and CBP groups.
TABLE 1.
Patient baseline demographic characteristics.
| UBP (n = 22 333) | CBP (n = 11 427) | Standardized difference, a % | |
|---|---|---|---|
| Age, mean (SD) | 60.0 (12.1) | 62.2 (12.6) | 17.5 |
| Age, n (%) | |||
| 18−44 y | 2074 (9.3) | 843 (7.4) | 6.9 |
| 45−64 y | 13 408 (60.0) | 6272 (54.9) | 10.4 |
| 65−74 y | 4168 (18.7) | 2361 (20.7) | 5.0 |
| 75−79 y | 1193 (5.3) | 860 (7.5) | 8.9 |
| ≥80 y | 1490 (6.7) | 1091 (9.5) | 10.6 |
| Men, n (%) | 12 105 (54.2) | 5888 (51.5) | 5.4 |
| Race, n (%) | |||
| White | 15 987 (71.6) | 8899 (77.9) | 14.5 |
| Black or African American | 2790 (12.5) | 865 (7.6) | 16.4 |
| Asian | 601 (2.7) | 303 (2.7) | 0.2 |
| Other | 318 (1.4) | 153 (1.3) | 0.7 |
| Unknown | 2637 (11.8) | 1207 (10.6) | 4.0 |
| Insurance type, n (%) | |||
| Commercial | 11 526 (51.6) | 5589 (48.9) | 5.4 |
| Self‐insured | 6682 (29.9) | 3204 (28.0) | 4.2 |
| Medicare b | 3136 (14.0) | 2188 (19.1) | 13.8 |
| Medicaid | 976 (4.4) | 441 (3.9) | 2.6 |
| Unknown/missing | 13 (0.06) | 5 (0.04) | 0.6 |
| US Census region, n (%) | |||
| South | 11 016 (49.3) | 5258 (46.0) | 6.6 |
| Midwest | 4733 (21.2) | 2751 (24.1) | 6.9 |
| Northeast | 3667 (16.4) | 1915 (16.8) | 0.9 |
| West | 2903 (13.0) | 1491 (13.0) | 0.2 |
| Unknown | 14 (0.06) | 12 (0.1) | 1.5 |
Abbreviations: CBP, controlled blood pressure; SD, standard deviation; UBP, uncontrolled blood pressure.
Standardized difference with values ≥10% considered statistically significant.
Includes Medicare Part C, Medicare Advantage, and Medicare Supplemental Plans.
TABLE 2.
Patient baseline clinical characteristics, comorbid conditions, and concomitant medications.
| UBP (n = 22 333) | CBP (n = 11 427) | Standardized difference, a % | |
|---|---|---|---|
| SBP b | |||
| Mean (SD) | 141.5 (13.8) | 118.2 (8.2) | 204.6 |
| Median (IQR) | 139.0 (16.5) | 120.0 (10.5) | |
| DBP b | |||
| Mean (SD) | 82.2 (9.2) | 70.4 (6.4) | 148.8 |
| Median (IQR) | 82.0 (10.0) | 71.0 (9.0) | |
| QCI score | |||
| Mean (SD) | 1.52 (1.94) | 1.95 (2.17) | 20.9 |
| Comorbidities ≥10%, n (%) | |||
| Hyperlipidemia | 16 869 (75.5) | 8999 (78.8) | 7.7 |
| Type 2 diabetes mellitus | 9436 (42.3) | 4890 (42.8) | 1.1 |
| Chronic pulmonary disease | 5648 (25.3) | 3561 (31.2) | 13.1 |
| Congestive HF | 3534 (15.8) | 3087 (27.0) | 27.5 |
| Anemia | 4656 (20.8) | 2805 (24.5) | 8.8 |
| Sleep apnea | 4951 (22.2) | 2587 (22.6) | 1.1 |
| Depression | 4529 (20.3) | 2558 (22.4) | 5.1 |
| Anxiety | 4614 (20.7) | 2391 (20.9) | 0.7 |
| Atrial fibrillation | 2718 (12.2) | 2249 (19.7) | 20.6 |
| Renal disease | 3844 (17.2) | 2229 (19.5) | 5.9 |
| Peripheral vascular disease | 3068 (13.7) | 2215 (19.4) | 15.2 |
| Osteoarthritis | 3945 (17.7) | 2035 (17.8) | 0.4 |
| Cerebrovascular disease | 2921 (13.1) | 1870 (16.4) | 9.3 |
| Any malignancy c | 2377 (10.6) | 1471 (12.9) | 6.9 |
| MI | 1620 (7.3) | 1431 (12.5) | 17.7 |
| Possible causes of secondary hypertension, n (%) | |||
| Sleep apnea | 4951 (22.2) | 2587 (22.6) | 1.1 |
| Renal disease | 3784 (16.9) | 2180 (19.1) | 5.6 |
| Alcohol use disorder | 1268 (5.7) | 645 (5.6) | 0.1 |
| Renal artery stenosis | 147 (0.7) | 54 (0.5) | 2.5 |
| Hyperaldosteronism | 46 (0.2) | 15 (0.1) | 1.8 |
| Cushing syndrome | 12 (0.05) | 5 (0.04) | 0.5 |
| Specific diagnosis code for secondary hypertension | 170 (0.8) | 53 (0.5) | 3.8 |
| BMI, kg/m2 | |||
| Mean (SD) | 34.1 (10.4) | 31.9 (8.0) | 23.2 |
| Smoking status, n (%) | |||
| Smoker | 6852 (30.7) | 3881 (34.0) | 7.0 |
| Medication use | |||
| Antiarrhythmic drugs, d n (%) | 20 802 (93.1) | 10 725 (93.9) | 2.9 |
| Antiarrhythmic drugs excluding BBs/CCBs | 1853 (8.3) | 1367 (12.0) | 12.2 |
| Lipid‐lowering drugs, n (%) | |||
| Statins | 13 462 (60.3) | 7692 (67.3) | 14.7 |
| PCSK9 inhibitors | 18 (0.1) | 11 (0.1) | 0.5 |
| Other lipid‐lowering drugs | 3329 (14.9) | 1874 (16.4) | 4.1 |
| Opioids, n (%) | 11 185 (50.1) | 6121 (53.6) | 7.0 |
| Antidiabetic drugs, n (%) | 8538 (38.2) | 4417 (38.7) | 0.9 |
| Noninsulin antidiabetic drugs | 7593 (34.0) | 3938 (34.5) | 1.0 |
| Insulin | 3360 (15.0) | 1799 (15.7) | 1.9 |
| Antidepressants, n (%) | 7557 (33.8) | 4400 (38.5) | 9.7 |
| Systemic corticosteroids, n (%) | 7747 (34.7) | 4263 (37.3) | 5.5 |
| Anxiolytics, n (%) | 5062 (22.7) | 2791 (24.4) | 4.2 |
| NSAIDs, n (%) | 5343 (23.9) | 2480 (21.7) | 5.3 |
| Oral anticoagulants, n (%) | 2529 (11.3) | 2079 (18.2) | 19.5 |
| Direct oral anticoagulants | 1360 (6.1) | 1100 (9.6) | 13.2 |
| Warfarin/vitamin K antagonists | 1338 (6.0) | 1118 (9.8) | 14.1 |
| Platelet aggregation inhibitors, n (%) | 2616 (11.7) | 1955 (17.1) | 15.4 |
| Antineoplastic drugs, n (%) | 1047 (4.7) | 683 (6.0) | 5.7 |
| Antimigraine drugs, n (%) | 556 (2.5) | 289 (2.5) | 0.3 |
| Stimulants, e n (%) | 468 (2.1) | 260 (2.3) | 1.2 |
| Weight‐loss drugs, f n (%) | 371 (1.7) | 167 (1.5) | 1.6 |
Abbreviations: BB, beta‐blocker; BMI, body mass index; CBP, controlled blood pressure; CCB, calcium channel blocker; DBP, diastolic blood pressure; HF, heart failure; IQR, interquartile range; MI, myocardial infarction; NSAID, nonsteroidal anti‐inflammatory drug; PCSK9, proprotein convertase subtilisin/kexin type 9 serine protease; QCI, Quan‐Charlson Comorbidity Index; SBP, systolic blood pressure; SD, standard deviation; UBP, uncontrolled blood pressure.
Standardized difference with values ≥10% considered statistically significant.
Based on the mean of measurements obtained during 2 office visits on separate days 1 to 90 days apart.
Includes lymphoma and leukemia; excluded malignant neoplasm of the skin.
Includes sodium channel blockers (disopyramide, quinidine, and flecainide), all BBs, potassium channel blockers (amiodarone, dronedarone, and sotalol), and all CCBs.
Includes amphetamine, caffeine, armodafinil, atomoxetine, methylphenidate, methamphetamine, dextroamphetamine, dexmethylphenidate, doxapram, and modafinil.
Includes phentermine, phendimetrazine, orlistat, benzphetamine, bupropion/naltrexone, diethylpropion, and phentermine/topiramate.
Mean SBP/DBP was higher in patients with UBP (142/82 mmHg) versus those with CBP (118/70 mmHg; Table 2). The mean BMI was higher in the UBP versus CBP group (34.1 vs. 31.9 kg/m2). In both the UBP and CBP groups, the most frequently observed comorbidities were hyperlipidemia (75.5% and 78.8%) and type 2 diabetes (42.3% and 42.8%). The following comorbidities were less prevalent in patients with UBP versus those with CBP (all standardized differences ≥10%): chronic pulmonary disease (25.3% vs. 31.2%), congestive HF (15.8% vs. 27.0%), MI (7.3% vs. 12.5%), peripheral vascular disease (13.7% vs. 19.4%), and atrial fibrillation (12.2% vs. 19.7%).
The percentages of patients taking 3, 4, or ≥5 antihypertensive medication classes in the UBP versus CBP group were 79.1% versus 82.3%, 17.6% versus 15.4%, and 3.2% versus 2.2%, respectively. Baseline antihypertensive medication use is summarized in Table 4, Supplemental Digital Content 1. All standardized differences were <10%. The most common concomitant medications other than antihypertensives were antiarrhythmic drugs, statins, opioids, and antidiabetic drugs (Table 2).
3.3. Clinical outcomes
For MACE+, mean (SD) follow‐up time was 2.7 (2.1) years in both the UBP and CBP groups. Consistent with the younger age and lower incidence of comorbidities (i.e., congestive HF, peripheral vascular disease, atrial fibrillation, and chronic pulmonary disease) in the UBP versus CBP cohort at baseline, respectively, unadjusted rates of MACE+ were lower in this group; 9.7% and 12.0% of patients in the UBP and CBP groups experienced the MACE+ composite outcome, corresponding to incidence rates of 36.7 and 44.6 per 1000 PY. For the individual MACE+ components, 3.5% (13.1/1000 PY) and 3.4% (12.6/1000 PY) of patients in the UBP and CBP groups experienced stroke, 2.9% (11.0/1000 PY) and 3.6% (13.9/1000 PY) experienced MI, and 5.6% (21.9/1000 PY) and 7.8% (30.8/1000 PY) experienced HF‐related hospitalization. Less than 2% of patients in the UBP and CBP groups experienced ESRD (1.9% [7.1/1000 PY] and 1.5% [5.4/1000 PY]; Table 3).
TABLE 3.
Unadjusted clinical outcomes.
| UBP (n = 22 333) | CBP a (n = 11 427) | |||
|---|---|---|---|---|
| n (%) | Incidence (95% CI) b | n (%) | Incidence (95% CI) b | |
| MACE+ | 2173 (9.7) | 36.7 (35.2, 38.2) | 1369 (12.0) | 44.6 (42.4, 47.0) |
| Stroke | 776 (3.5) | 13.1 (12.2, 14.0) | 386 (3.4) | 12.6 (11.4, 13.9) |
| MI | 643 (2.9) | 11.0 (10.2, 11.9) | 414 (3.6) | 13.9 (12.6, 15.3) |
| HF hospitalization | 1241 (5.6) | 21.9 (20.7, 23.1) | 891 (7.8) | 30.8 (28.8, 32.8) |
| ESRD | 425 (1.9) | 7.1 (6.5, 7.9) | 166 (1.5) | 5.4 (4.6, 6.3) |
Abbreviations: CBP, controlled blood pressure; CI, confidence interval; ESRD, end‐stage renal disease; HF, heart failure; MACE+, major adverse cardiovascular events plus; MI, myocardial infarction; UBP, uncontrolled blood pressure.
CBP is the reference group.
Number is per 1000 PY at risk.
After adjusting for baseline differences in demographic and clinical characteristics, patients with UBP were at an 8% and 53% increased risk of developing MACE+ and ESRD compared with patients with CBP (Figure 3). MACE+ risk was mainly driven by a 31% increased risk of stroke.
FIGURE 3.
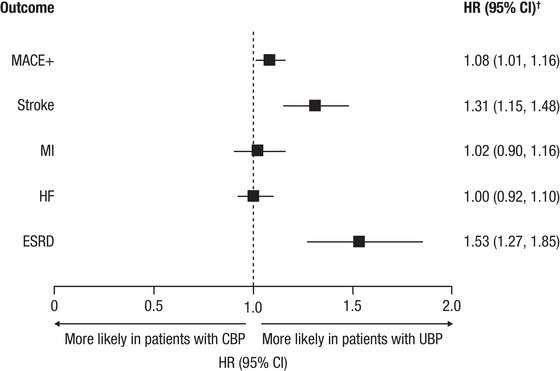
Adjusted clinical outcomes. *CBP is the reference group. †Model was adjusted for age cohort; gender; race; insurance type; US Census region; baseline comorbidities; BMI category; smoking status; baseline medication use; number of index anti‐HTN medication classes; and baseline healthcare resource utilization and costs. Abbreviations: anti‐HTN, antihypertensive; BMI, body mass index; CBP, controlled blood pressure; CI, confidence interval; ESRD, end‐stage renal disease; HF, heart failure; HR, hazard ratio; MACE+, major adverse cardiovascular event plus; MI, myocardial infarction; UBP, uncontrolled blood pressure.
3.4. Sensitivity analysis
In the sensitivity analysis of patients meeting the 140/90 mmHg threshold, the imbalances in age, gender, race, insurance type, and geographic region at baseline were comparable with the primary analyses. However, with a few exceptions, the magnitude of the standardized differences was generally smaller in the sensitivity analyses than in the primary analysis (Table 5, Supplemental Digital Content 1). Similar to the primary analysis, there were imbalances in baseline SBP/DBP, comorbidities, and BMI, and the most common concomitant medications were the same as those in the primary analysis (Table 6, Supplemental Digital Content 1). Approximately half of the UBP cohort in the primary analysis shifted to the CBP cohort in the sensitivity analysis, which is a consequence of many patients having baseline BP between the 130/80 mmHg and 140/90 mmHg thresholds.
In the sensitivity analysis using the BP 140/90 mmHg goal, mean (SD) follow‐up time for MACE+ was 2.7 (2.1) years in the UBP group and 2.6 (2.1) years in the CBP group. Based on unadjusted data, 10.4% (41.6/1000 PY) and 10.5% (41.2/1000 PY) of patients in the UBP and CBP groups experienced the MACE+ composite outcome. For the individual MACE+ components, 3.9% (14.7/1000 PY) and 3.2% (12.0/1000 PY) of patients in the UBP and CBP groups experienced stroke, 3.1% (11.6/1000 PY) and 3.2% (11.8/1000 PY) experienced MI, and 6.1% (23.4/1000) and 6.5% (24.5/1000 PY) experienced HF‐related hospitalization. In the UBP and CBP groups, 2.4% (9.3/1000 PY) and 1.4% (5.2/1000 PY) of patients experienced ESRD (Table 7, Supplemental Digital Content 1).
After adjusting for baseline differences in demographic and clinical characteristics, patients with UBP were at a 22% and 98% increased risk of developing MACE+ and ESRD compared with patients with CBP (Figure 1, Supplemental Digital Content 1). MACE+ risk was mainly driven by a 41% increased risk of stroke, although risk of MI and HF hospitalization were also significantly different in the sensitivity analysis.
4. DISCUSSION
aTRH is a growing public health problem and is associated with a substantial humanistic, societal, and economic burden due to treatment costs, disability, and early death. 20 , 21 , 22 In this retrospective cohort study, we used the IQVIA Ambulatory EMR—US and PharMetrics Plus claims databases to evaluate the risk of the composite MACE+ outcome, the individual MACE+ components, and ESRD in patients with aTRH. We found that hypertensive patients who are using 3 or more antihypertensive treatments from distinct classes possess higher risk of MACE+ and ESRD with elevated BP, and this risk increased with the higher BP threshold used in the sensitivity analyses.
At baseline, patients in the UBP cohort were younger and had lower incidence of comorbidities than patients in the CBP cohort (i.e., lower incidences of congestive HF, peripheral vascular disease, atrial fibrillation, and chronic pulmonary disease), so unadjusted rates of MACE+ were higher in the CBP group, which represents a sicker population. This sicker population may have had more controlled BP due to more frequent physician visits and better adherence resulting from management of comorbidities, which was confirmed by reviewing baseline healthcare resource utilization. In our primary analysis, after adjusting for differences in baseline demographic and clinical characteristics between the UBP and CBP groups, patients with UBP (≥130/80 mmHg) had an 8% increased risk of MACE+, driven by a 31% increased risk of stroke. In our sensitivity analysis, patients with UBP (≥140/90 mmHg) had a 22% increased risk of MACE+. Many patients who had UBP values between 130/80 and 140/90 mmHg impacted the overall risk of MACE+. The greater risk of MACE+ in the sensitivity analysis validates our hypothesis and other findings that elevated BP is associated with a higher risk of MACE+. 23 , 24 , 25 Thus, while the absolute number of patients meeting this less stringent criterion was reduced relative to the primary analysis, the risk for MACE+ was higher. This highlights the importance of earlier BP control using the more stringent 130/80 mmHg cutoff in potentially reducing the risk for CV events.
The risk for MACE+ in the current study was driven by a 31% and 41% increased risk of stroke in the primary and sensitivity analysis, respectively, in patients with UBP compared with CBP. In a retrospective longitudinal study by Sim and colleagues, using electronic health records from 2006 to 2010, of 60 237 aTRH patients from Southern California, there was a 23% higher risk of stroke/cerebrovascular events in patients with UBP versus CBP, respectively. 26 In this study, it should be noted that the definitions for UBP and CBP differ slightly from the current study, with UBP defined as BP ≥140/90 mmHg in patients on ≥3 medicines and CBP defined as BP < 140/90 mmHg in patients on ≥4 medicines. Similarly, a retrospective cohort study using data from 2830 patients with aTRH in the Maccabi Healthcare Services database found that patients with UBP had a 36% higher risk of stroke or transient ischemic attack than those with CBP. 27 However, in an analysis of data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study of Black and White US adults ≥45 years between 2003 and 2007, which assessed the association of aTRH with incident stroke, coronary artery disease, and all‐cause mortality in 2147 patients of different racial populations, there was no statistically significant difference found between patients with UBP versus CBP in the risk for stroke after full multivariable adjustment (HR, 1.05 [95% CI: 0.61, 1.81]). 28
In the current study, there were no significant between‐group differences in the risk of MI or HF‐related hospitalization in the primary analysis. In the sensitivity analysis, there was a 14% increased risk of MI and a 20% increased risk of HF‐related hospitalization. The risk of congestive HF and/or HF requiring hospitalization was not significantly increased in the Sim and colleagues study of aTRH patients with UBP versus CBP (HR, 1.06 [95% CI: 0.99, 1.12]). 26 In that study, like ours, the CBP population represented a sicker population with more comorbidities, and the low BP in this group could have reflected a weakened physiological state and/or potential overtreatment. 26
In our primary analysis, and after adjusting for baseline differences between the UBP and CBP groups in demographic and clinical characteristics, patients with UBP (≥130/80 mmHg) had a 53% increased risk of ESRD compared to those with CBP. In our sensitivity analysis, patients with UBP (≥140/90 mmHg) had a 98% increased risk of ESRD compared to those with CBP, showing an association between BP control and elevated incidence of comorbidities. Similar to the higher risk of MACE+ seen in patients with UBP (≥140/90 mmHg) versus CBP, the increased risk of ESRD compared with patients with CBP validates our hypothesis and other literature that UBP puts individuals at a higher risk of ESRD. 29 , 30 While the absolute number of patients meeting this less stringent criterion was reduced relative to the primary analysis, the risk for ESRD was nearly 2 times higher. Similar to the MACE+ results, this result highlights the potential for earlier and more stringent BP control in reducing the risk of ESRD. In addition, US‐based retrospective studies have reported comparable findings of an increased risk of ESRD in patients with UBP versus CBP. 26 , 31 The study by Sim and colleagues utilizing electronic health records of a large, ethnically diverse hypertension population found a 25% increased risk for ESRD in patients with UBP versus CBP. 26 In the REGARDS study, compared to patients with aTRH and CBP, the HR for ESRD was 2.7 (95% CI: 1.5, 4.9) for patients with aTRH and UBP after multivariable adjustment. 28 Thus, there is a need for innovations in patient management of aTRH to help a larger percentage of patients achieve CBP.
There are limitations in this study associated with the analysis of administrative claims data. As a retrospective analysis of observational data, we were limited by the information recorded and successfully translated into structured data elements. For example, data on CV‐related deaths were not reported in this analysis because they were not available in the dataset provided. Similarly, renal data were limited to ESRD, which was identified based on diagnosis codes, because laboratory values were not available to evaluate other renal effects. In addition, claims data are prone to coding errors and inconsistencies. Even after using statistical methods to balance the patient cohorts, residual confounding cannot be excluded. Due to the available data, results from this analysis are not generalizable to entire US population, and thus, some patient populations may not be well represented. Also, the existence of a claim for a medication does not indicate the medication was taken as prescribed. It was not possible to distinguish between guideline‐defined true TRH and pseudo‐resistant hypertension or to obtain information regarding individual agents, their intended daily dose, or medication adherence. Moreover, we could not determine the indication for which these antihypertensive medications were prescribed. It was not possible to determine the quality of the BP measurements taken. Because BP measurements were taken in office, some patients with elevated BP may have been experiencing white‐coat effect 1 ; the available data do not allow assessment of the prevalence of white‐coat effect or how the prevalence differs between patients with UBP versus CBP. If healthcare visits with another provider or hospital were not recorded in the database, data were incomplete for patients’ health status, prescriptions, other healthcare visits, and hospitalizations for adverse clinical outcomes. Despite these limitations, the results were derived from a large, population‐based sample of patients with treated hypertension that reflect recent, real‐world practice patterns. Electronic health record measures allowed for the assessment of SBP and DBP, and patients were followed longitudinally for changes in medication use.
5. CONCLUSIONS
Results from this real‐world study of US‐based patients demonstrated that there is suboptimal BP control in many patients with aTRH and multiple comorbid conditions despite the use of multidrug regimens. Patients with UBP had an increased risk of developing MACE+ and ESRD compared with patients with CBP. These findings suggest that physicians need to use appropriate guideline‐directed dosing of existing medications and possibly new medications that are complementary to the agents available.
AUTHOR CONTRIBUTIONS
All authors: Conceptualization, investigation, methodology, writing, and visualization. Cindy Chen: Data curation, formal analysis, and software. Mukul Singhal and Cindy Chen: Project administration and validation. Veronica Ashton and Mukul Singhal: Funding acquisition and supervision.
CONFLICT OF INTEREST STATEMENT
G.B. is supported by T32 NIH grant DK07011 and is a consultant to Bayer, KBP Biosciences, Ionis, Alnylam, AstraZeneca, Quantum Genomics, Janssen, Novo Nordisk, DiaMedica Therapeutics, and inRegen. M.S., C.C., A.K.C., and V.A. are employees of Janssen Scientific Affairs, LLC, and are Johnson & Johnson stockholders. L.H. is an employee of Janssen Research & Development, LLC, and is a Johnson & Johnson stockholder.
PATIENT CONSENT STATEMENT
Not applicable.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
CLINICAL TRIAL REGISTRATION
Not applicable.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Statistical programming for this manuscript was performed by Kalla Sun, PhD, of TechData Service Company, LLC. Medical writing support was provided by Laura Weber, PhD, of Lumanity Communications Inc., and was funded by Janssen Scientific Affairs, LLC.
Bakris G, Chen C, Campbell AK, Ashton V, Haskell L, Singhal M. Association of uncontrolled blood pressure in apparent treatment‐resistant hypertension with increased risk of major adverse cardiovascular events plus. J Clin Hypertens. 2023;25:737–747. 10.1111/jch.14701
DATA AVAILABILITY STATEMENT
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical‐trials/transparency. These data were made available by IQVIA and used under license for the current study and are not publicly available. Other researchers should contact https://www.iqvia.com.
REFERENCES
- 1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53‐e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165‐182. [DOI] [PubMed] [Google Scholar]
- 3. Mills KT, Xu Y, Zhang W, et al. A systematic analysis of worldwide population‐based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Center for Health Statistics . About Multiple Cause of Death, 1999–2019. In: CDC WONDER Online Database. Centers for Disease Control and Prevention; 2021. [Google Scholar]
- 5. Hypertension Cascade: Hypertension Prevalence, Treatment and Control Estimates Among US Adults Aged 18 Years and Older Applying the Criteria From the American College of Cardiology and American Heart Association's 2017 Hypertension GuidelineNHANES 2017‐2020 . Centers for Disease Control and Prevention (CDC). May 12, 2023. Accessed July 10, 2023. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html
- 6. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127‐e248. [DOI] [PubMed] [Google Scholar]
- 7. Armstrong C. Joint National Committee. JNC8 guidelines for the management of hypertension in adults. Am Fam Physician. 2014;90:503‐504. [PubMed] [Google Scholar]
- 8. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:14‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Diabetes Association. 9 . Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018 . Diabetes Care. 2018;41:S86‐S104. [DOI] [PubMed] [Google Scholar]
- 10. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953‐2041. [DOI] [PubMed] [Google Scholar]
- 11. Bakris G, Yang YF, Pitt B. Mineralocorticoid receptor antagonists for hypertension management in advanced chronic kidney disease: BLOCK‐CKD trial. Hypertension. 2020;76:144‐149. [DOI] [PubMed] [Google Scholar]
- 12. Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219‐2229. [DOI] [PubMed] [Google Scholar]
- 13. Halpern MT, Khan ZM, Schmier JK, et al. Recommendations for evaluating compliance and persistence with hypertension therapy using retrospective data. Hypertension. 2006;47:1039‐1048. [DOI] [PubMed] [Google Scholar]
- 14. Tajeu GS, Kent ST, Kronish IM, et al. Trends in antihypertensive medication discontinuation and low adherence among Medicare beneficiaries initiating treatment from 2007 to 2012. Hypertension. 2016;68:565‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones DW, Whelton PK, Allen N, et al. Management of stage 1 hypertension in adults with a low 10‐year risk for cardiovascular disease: filling a guidance gap: a scientific statement from the American Heart Association. Hypertension. 2021;77:e58‐e67. [DOI] [PubMed] [Google Scholar]
- 16. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334‐1357. [DOI] [PubMed] [Google Scholar]
- 17. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130‐1139. [DOI] [PubMed] [Google Scholar]
- 18. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676‐682. [DOI] [PubMed] [Google Scholar]
- 19. Gibertoni D, Voci C, Iommi M, et al. Developing and validating an algorithm to identify incident chronic dialysis patients using administrative data. BMC Med Inform Decis Mak. 2020;20:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carris NW, Ghushchyan V, Libby AM, Smith SM. Health‐related quality of life in persons with apparent treatment‐resistant hypertension on at least four antihypertensives. J Hum Hypertens. 2016;30:191‐196. [DOI] [PubMed] [Google Scholar]
- 21. Hajizadeh N, Assadi F. Resistant hypertension: current status, future challenges. Int J Prev Med. 2014;5:S21‐S24. [PMC free article] [PubMed] [Google Scholar]
- 22. Kaczmarski KR, Sozio SM, Chen J, Sang Y, Shafi T. Resistant hypertension and cardiovascular disease mortality in the US: results from the National Health and Nutrition Examination Survey (NHANES). BMC Nephrol. 2019;20:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwon CH, Kim W, Shin JH, et al. Office blood pressure range and cardiovascular events in patients with hypertension: a nationwide cohort study in South Korea. J Am Heart Assoc. 2021;10:e017890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim HL, Kim HM, Kwon CH, et al. Blood pressure levels and cardiovascular risk according to age in patients with diabetes mellitus: a nationwide population‐based cohort study. Cardiovasc Diabetol. 2020;19:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turnbull F. Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet. 2003;362:1527‐1535. [DOI] [PubMed] [Google Scholar]
- 26. Sim JJ, Bhandari SK, Shi J, et al. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int. 2015;88:622‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leiba A, Yekutiel N, Chodick G, et al. Resistant hypertension is associated with an increased cardiovascular risk compared to patients controlled on a similar multi‐drug regimen. J Hum Hypertens. 2023;37:542‐547. [DOI] [PubMed] [Google Scholar]
- 28. Irvin MR, Booth JN, Shimbo D, et al. Apparent treatment‐resistant hypertension and risk for stroke, coronary heart disease, and all‐cause mortality. J Am Soc Hypertens. 2014;8:405‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end‐stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165:923‐928. [DOI] [PubMed] [Google Scholar]
- 30. Bae EH, Lim SY, Han KD, et al. Association between systolic and diastolic blood pressure variability and the risk of end‐stage renal disease. Hypertension. 2019;74:880‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanner RM, Calhoun DA, Bell EK, et al. Incident ESRD and treatment‐resistant hypertension: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Kidney Dis. 2014;63:781‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical‐trials/transparency. These data were made available by IQVIA and used under license for the current study and are not publicly available. Other researchers should contact https://www.iqvia.com.


