Abstract
The large form of the hepatitis delta virus (HDV) protein (L) can be isoprenylated near its C terminus, and this modification is considered essential for particle assembly. Using gel electrophoresis, we separated L into two species of similar mobilities. The slower species could be labeled by the incorporation of [14C]mevalonolactone and is interpreted to be isoprenylated L (Li). In serum particles, infected liver, transfected cells, and assembled particles, 25 to 85% of L was isoprenylated. Isoprenylation was also demonstrated by 14C incorporation in vitro with a rabbit reticulocyte coupled transcription-translation system. However, the species obtained migrated even slower than that detected by labeling in vivo. Next, in studies of HDV particle assembly in the presence of the surface proteins of human hepatitis B virus, we observed the following. (i) Relative to L, Li was preferentially assembled into virus-like particles. (ii) Li could coassemble the unmodified L and the small delta protein, S. (iii) In contrast, a form of L with a deletion in the dimerization domain was both isoprenylated and assembled, but it could not support the coassembly of S. Finally, to test the expectation that the isoprenylation of L would increase its hydrophobicity, we applied a phase separation strategy based on micelle formation with the nonionic detergent Triton X-114. We showed the following. (i) The unique C-terminal 19 amino acids present on L relative to S caused a significant increase in the hydrophobicity. (ii) This increase was independent of isoprenylation. (iii) In contrast, other, artificial modifications at either the N or C terminus of S did not increase the hydrophobicity. (iv) The increased hydrophobicity was not sufficient for particle assembly; nevertheless, we speculate that it might facilitate virion assembly.
Human hepatitis delta virus (HDV) is a subviral satellite of hepatitis B virus (HBV). A complete cycle of HDV replication is dependent on the envelope proteins of HBV and the expression of two related HDV proteins. The first is a 195-amino-acid (aa) species, known as the small delta protein, S, which is essential for replication of the RNA genome (32). The second, which arises as the consequence of a posttranscriptional RNA editing event (38), is 19 aa longer at the C terminus. This large delta protein, L, is a dominant negative inhibitor of genome replication, the ability of S to support (8). It is also essential for assembly of progeny virions (7).
Soon after L was found to be essential for particle assembly (7), it was shown that it could be isoprenylated at a unique cysteine located 4 aa from the C terminus (19). This study involved both delta protein expressed in mammalian cells and that translated in vitro, with rabbit reticulocyte extracts. This isoprenylation is exceptional; while various types of acylation are known for proteins of other viruses, this is the only known example of an isoprenylation (24). Such isoprenylation has also been demonstrated when L is expressed in insect cells from a recombinant baculovirus (26, 27) and has been inferred from assembly studies carried out in yeast (48). A recent report used an antibody specific for isoprenylated L (Li) in an immunofluorescence assay; in transfected cells, modified L was largely located within the nucleus (37).
Mutagenesis of this unique cysteine of L to serine blocks both the isoprenylation and the assembly of L. Such findings have been used to support the idea that the isoprenylation is essential for virus assembly (19). Recent data obtained with an isoprenylation inhibitor, BZA-5B, further support this interpretation (18). An earlier study confirms that isoprenylation is necessary for assembly but also shows that isoprenylation is not sufficient; the 15 aa located upstream of the isoprenylation site are also critical (36).
Two different kinds of isoprenylation of host proteins, involving addition of either a geranylgeranyl group or a farnesyl group, are known (6). For the cellular proteins, isoprenylation typically requires three enzymatic steps (6). First, a cysteine, located 4 aa from the C terminus, is covalently sulfhydryl linked to either a 15-carbon farnesyl group or a 20-carbon geranylgeranyl group; next, the 3 terminal aa are removed by an endopeptidase; finally, the newly created C terminus is methylated.
The precise nature of the HDV L modification has been considered in several studies. Initially a geranylgeranyl modification has been considered in several studies. Initially a geranylgeranyl modification was cited as an unpublished observation (16, 19). A more recent study showed that in vivo only farnesylation was detected, while in vitro, with purified transferases, both farnesylation and geranylgeranylation could be achieved (42). Most recently, from a study based on the specificity, in other contexts, of the isoprenyl transferase inhibitor BZA-5B, it has been inferred that L is farnesylated (16, 20). In the present study, we undertook to determine for the first time the fraction of the L species that is actually modified.
For host proteins, the three-step isoprenyl modification can sometimes result in a protein with a somewhat increased electrophoretic mobility (2, 21, 47). To determine whether isoprenylation of L involved a mobility change, we used high-resolution gel electrophoresis to separate Li from unmodified L. Contrary to expectation, the species Li that could be labeled in vivo by incorporation of [14C]mevalonolactone migrated somewhat less than L. We analyzed L from various sources and obtained quantitation of the extent of this modification.
We also report here the application of a phase fractionation procedure based on the ability of membrane proteins to associate with micelles of the nonionic detergent Triton X-114 (5, 44). Our intent was to clarify the hydrophobic properties of the various forms of delta protein and how they might be relevant to HDV assembly. These studies led us to conclude that even prior to isoprenylation L was relatively more hydrophobic than S, presumably because of the novel 19 aa at the C terminus.
MATERIALS AND METHODS
Plasmids.
pMEV (29), which expresses a mevalonic acid transporter, was used to enhance isoprenyl labeling. pSV45H, from Don Ganem, was used to express the surface proteins of HBV (sAg). pSVTVA (1), which expresses the simian virus 40 T antigen, was used to give a 16-fold enhancement of expression from pSVL-based constructs in transfected Huh7 human hepatoblastoma [41]) cells (3). For example, pDL444 and pDL445 are constructs in pSVL (Pharmacia) which express S and L, respectively (35). Similarly, pDL449 expresses L(Δ19–31), a species with a deletion of sequences necessary for dimerization (35), and pVB448 expresses L(C211A), with the cysteine at position 211 changed to alanine (3). Also used was a construct, pTW203, which expressed S with a histidine tag at the C terminus (9). Constructs pVB101, pVB102, and pVB108, in pcDNA3.1 (Invitrogen), use a cytomegalovirus immediate-early promoter to express forms of S, L, and L(C211A), respectively, with 36 extra aa at the N terminus, that include a histidine tag. Finally, pR5δV5 is a bacterial vector which expresses high levels of unmodified S, via codons which have been largely optimized for expression in bacteria (9).
Protein purification.
Harmon Zuccola and Jim Hogle provided the purified S protein expressed in bacteria by pR5δV5 (9). Various forms of S and L tagged with six histidines were expressed in transfected Huh7 cells and purified via nickel affinity procedures (9).
Transfection of mammalian cells.
Monolayers of Huh7 cells were transfected by using either Lipofectamine or Lipofectamine Plus (Life Technologies) according to the manufacturer’s instructions.
In vivo isoprenyl labeling.
The strategy was a modification of one previously described (42). Huh7 cells were cotransfected with pMEV (which increases the uptake of exogenous mevalonate) (29), pSVTVA, pDL444, and either pDL445 or pVB448, expressing L or L(C211A), respectively. After 2 days, Lovastatin (to inhibit the de novo synthesis of mevalonate by the cells [10]) was added to the medium to a final concentration of 30 μM. Following 1 h of incubation at 37°C, the medium was aspirated and replaced with medium containing 20 μM Lovastatin and 50 μCi of [14C]mevalonolactone (American Radiolabeled Chemicals) per ml. After 8 h of incubation at 37°C, the medium was removed, and the cells were washed twice with phosphate-buffered saline (PBS) and lysed in 300 μl of a buffer containing 100 mM NaCl, 20 mM Tris-HCl (pH 7.5), 2 mM MgCl2, and 1% Nonidet P-40. Then, samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to nitrocellulose, and radioactivity was detected and quantitated by direct contact with a bioimager screen (Fuji model BAS1000).
In vitro isoprenyl labeling.
A cDNA construct containing the coding region of L was expressed via a T7 promoter in a coupled transcription-translation system (Promega) as instructed by the manufacturer except that all the amino acids in the reaction mixture were unlabeled and [14C]mevalonolactone (1.25 mCi/ml) was added to the mixture.
Gel electrophoresis and immunoblot analysis.
We used the Laemmli procedure (34) with 12.5% acrylamide monomer and 0.72% bisacrylamide cross-linker. These changes, together with an increase in the electrophoresis time, made possible the resolution of L obtained from in vivo samples into two species. Only the slower species, Li, could be labeled by 14C incorporation, and it is deduced to be the isoprenylated form.
Assembly of HDV and into particles.
Huh7 cells were transfected as described previously (46) on 100-mm-diameter plates. Plasmid pSV45H was used for the expression of all three HBV sAg proteins, along with the appropriate plasmids expressing forms of S and L (46). After 8 days, cells were lysed in Laemmli buffer and 1/400 of the sample was subjected to SDS-PAGE. To assay for the release of HDV particles, tissue culture medium was collected at 4, 6, and 8 days after transfection, clarified by centrifugation for 10 min at 1,000 rpm, and stored at −80°C. Then viral particles were pelleted by ultracentrifugation through a 20% sucrose cushion containing 100 mM NaCl, 10 mM Tris-HCl (pH 7.5), and 1 mM EDTA for 18 h at 23,000 rpm in a Beckman SW28 rotor at 4°C. The pellet was resuspended in 80 μl of Laemmli buffer, and one-fourth of the sample was analyzed by SDS-PAGE. Results were quantitated as described above.
Triton X-114 phase separation.
Phase separation was carried out by a modification of the method described by Bordier (5). Samples were resuspended in Triton X-114 lysis buffer (Tris-buffered saline [TBS; 10 mM Tris-HCl, {pH 7.4} 0.15 M NaCl, 1 mM EDTA] containing 1% Triton X-114). After 1 h on ice with mild agitation, insoluble material was removed by centrifugation at 450 × g for 5 min. For the separation of the proteins, supernatants were overlaid on a sucrose cushion buffer (6% sucrose and 0.06% Triton X-114 in TBS), and tubes were incubated for 10 min in a 37°C water bath. Tubes were centrifuged for 3 min at 200 × g at room temperature, thereby separating the aqueous phase as the supernatant and the detergent phase as an oily drop in the bottom of the tube. This oily drop was resuspended in TBS, and the separation was repeated as before. Finally, the detergent and aqueous phases were precipitated by adding 9 volumes of ethanol and analyzed by SDS-PAGE. To eliminate protein-protein interactions during fractionation, aurintricarboxylic acid was added to the buffers at a final concentration of 100 μM.
RESULTS
Detection of Li by in vivo labeling and gel electrophoresis.
Previous studies by others working with host cell proteins have separated farnesylated from unfarnesylated species by gel electrophoresis (2, 21). Our initial objective was to obtain a similar separation of the unmodified form of L from the isoprenylated form, Li. Our strategy was first to obtain radioactive labeling of the modified protein and then determine whether this species could be separated from unmodified L, as detected by immunoblotting.
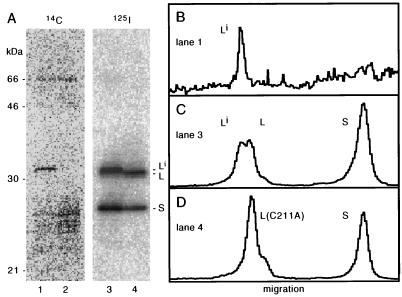
Others have been able to label L in vivo by means of the incorporation of radioactive mevalonolactone, a precursor to the isoprenyl group (19, 26, 27). As described in Materials and Methods, we used such a strategy, along with certain modifications which greatly increased the labeling efficiency. We tested the labeling of wild-type L and, as a negative control, the labeling of S. As an additional control, we also expressed a mutated form of L, L(C211A), that could no longer be isoprenylated. After electrophoresis, the proteins were electrotransferred to a nitrocellulose filter, and the 14C label was detected with a bioimager (Fig. 1A, lanes 1 and 2). There was significant labeling of a species of about the same electrophoretic mobility as Large (lane 1). It can be seen that this protein was significantly more highly labeled than any host protein, and it was not present in cells transfected with the L(C211A) mutant (lane 2).
FIG. 1.
Isoprenylation of L as detected by 14C labeling in vivo and immunoblotting. Huh7 cells were cotransfected and labeled with [14C]mevalonolactone, after which the total proteins were resolved by gel electrophoresis, followed by electrotransfer, all as described in Materials and Methods. (A) Lanes 1 and 2, detection of 14C, presumably incorporated into isoprenylated proteins; lanes 3 and 4, the same region of the filter after immunoblotting to detect HDV proteins with 125I-staphylococcal A protein. Lanes 1 and 3 contain S and L; lanes 2 and 4 contain S and the C211A mutant of L. (B to D) Radioactivity profiles for lanes 1, 3, and 4, respectively. The slower species of L, indicated as Li, was the only delta species labeled with 14C (A, lanes 1 and 3) and was not present for the mutated L (A, lanes 2 and 4); it is considered to be the isoprenylated form of L.
Next we took the same filter and carried out an immunoblot analysis with an antibody directed at all species of delta protein followed by detection with 125I-labeled staphylococcal A protein (Fig. 1A, lanes 3 and 4). We now detected two species of L and one species of S (lane 3). The slower species of L had the same mobility as that detected by 14C (lane 1), and it was not present in cells transfected with the L(C211A) mutant (lane 4). Therefore, we make the initial designation of this species as Li.
A quantitation of the radioactivity profiles corresponding to lanes 1, 3, and 4 of Fig. 1A, is shown in Fig. 1B to D, respectively. Figure 1C shows that about 50% of the total L migrated as Li.
With this gel electrophoretic separation and quantitation, we determined the fraction of the total L that migrated as Li for these samples and for a series of other HDV-related samples. The results are summarized in Table 1. We note that for all samples tested, the extent of modification was always less than 100%; in some cases it reached as high as 85%. As expected, for the L(C211A) mutant, no modification (<5%) was detected.
TABLE 1.
Extent of isoprenylation for HDV L protein
| Source of L | Isoprenylation (%)a |
|---|---|
| Serum virus | 75–83 |
| Infected liver | 85 |
| Cells transfected with L (in absence of HBV sAg) | 61 |
| Cells transfected with L (in presence of HBV sAg) | 27–34 |
| Virus-like particles from transfected cells | 72–74 |
| Cells transfected with L(C211A) | <5 |
Determined by gel electrophoresis and immunoblotting, with quantitation using a BioImager, as in Fig. 1.
Detection of Li by labeling in vitro in a rabbit reticulocyte lysate.
Previous studies show that during translation of L in a rabbit reticulocyte lysate the protein can become isoprenylated (19). Furthermore, the isoprenylated species was cited as migrating more slowly than unmodified L. As shown in Fig. 2, we used a coupled transcription-translation system, in the presence of [14C]mevalonolactone, to determine the electrophoretic mobility of the labeled L (lane 3) relative to that species of L labeled in vivo (lane 1). The in vitro sample was significantly slower, a finding confirmed by electrophoresis of both samples in the same well (lane 2). Our interpretation (see Discussion) is that in vitro, the rabbit reticulocyte lysate carries out only the first of the normal three steps of isoprenylation.
FIG. 2.
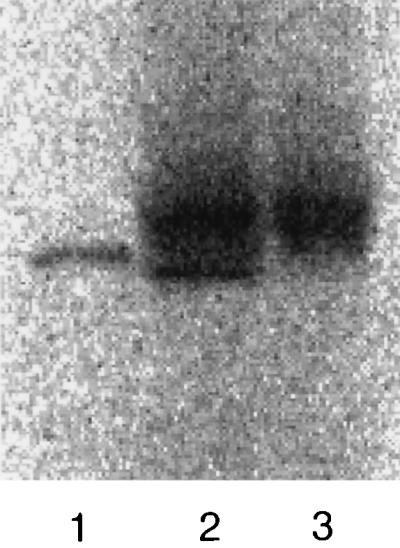
Comparison of isoprenylation of L as detected by 14C labeling in vivo and in vitro. Labeling was carried out in vivo, as for Fig. 1, or in vitro, using a coupled transcription-translation system. Samples were subject to electrophoresis as for Fig. 1, followed by electrotransfer and then direct quantitation of 14C with a bioimager. Lanes 1 and 3, the in vivo and in vitro samples, respectively; lane 2, a mixture of the two samples.
Assembly and coassembly.
Previous studies show that with the help of the envelope proteins of HBV, the HDV L protein is assembled into particles (7, 46). Also, studies with L mutants (19) and isoprenylation inhibitors (18) indicate that Li is essential for assembly. Furthermore, there are forms of the delta protein, such as S, which in the absence of Li cannot be assembled into particles yet in the presence of Li can be found in particles. This phenomenon, which we will call coassembly, depends on the ability of the coassembled proteins to form multimers with Li (35). Previous studies of HDV assembly and coassembly are deficient in that they included neither separation nor quantitation of L and Li. The following studies solve this deficiency for several different experimental situations.
In the first experiment, a comparison was made between Li in infected cultured cells and the particles released from those cells. Cells were cotransfected with plasmids expressing L and S, along with a plasmid that expressed the envelope proteins of HBV, HBV sAg. Gel analysis of the transfected cells showed that less than half of L was isoprenylated (Fig. 3, lane 2). In contrast, for the released particles, the majority of L was isoprenylated (Fig. 3, lane 3). In this respect, the released particles were very similar to those detected in serum particles (Fig. 3, lane 1) and infected liver (data not shown). A detailed quantitation of this assembly experiment is presented in Table 2, experiment 1. Around 7% of the intracellular species designated Li was released into particles. In contrast, only about 1.2% of either S or the unmodified L was released into particles. Our interpretation is that S and L were coassembled by Li. Note that in this situation the efficiency of coassembly was about six times less than the efficiency of assembly.
FIG. 3.
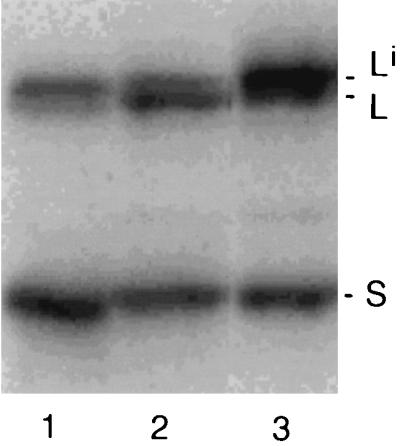
Assembly of L and S into virus-like particles in the presence of the envelope proteins of HBV. As described in Materials and Methods, following cotransfection of Huh7 cells, we used an immunoblot analysis to assay the cells (lane 2) and tissue culture medium (lane 3) for the presence of delta proteins. Lane 1 is a control of HDV particles in the serum of an infected woodchuck.
TABLE 2.
Efficiency of assembly and coassembly of HDV proteins
| Expta | HDV protein(s) expressed in transfected cells | Fraction of protein released into particles (%)b |
|---|---|---|
| 1 | Li | 7.0 |
| L | 1.20 | |
| S | 1.32 | |
| 2 | L(Δ19–31) and L(Δ19–31)i | 1.22 |
| S | 0.05 | |
| 3 | h6L and h6Li | 1.61 |
| L(C211A) | 1.37 | |
| 4 | S | 0.2 |
| 5 | L(C211A) | <0.1 |
Described in the text.
For each experiment, the fraction of protein assembled was determined by gel electrophoresis and immunoblotting, with quantitation using a bioimager, as in Fig. 1.
Previous studies have characterized a form of L with a deletion in the dimerization domain; this protein has either a significantly reduced ability or a total inability to form dimers (35). This mutant, designated L(Δ19–31), was expressed in cells along with HDV S and the HBV sAg. By immunoblotting we detected only a single, relatively broad band, which we interpret as containing both isoprenylated and unmodified forms of the mutant L (data not shown). As summarized in Table 2, experiment 2, 1.22% of the mutant protein was assembled into particles. In contrast, 24 times less S was found in particles. Our interpretation is that L(Δ19–31) could be isoprenylated to some extent and that the 1.22% assembled into particles was virtually all isoprenylated. We confirmed the modification by separate studies with [14C]mevalonolactone labeling, just as in Fig. 1 (data not shown). In contrast, we infer that unmodified L(Δ19–31) was, like S, coassembled at least 24 times less efficiently.
Others have shown that forms of L with the mutation C211S are unable to support particle assembly, presumably because of an inability to be isoprenylated (26). We tested a similar mutant, L(C211A), and observed <0.1% assembly (Table 2, experiment 5). However, when we expressed L(C211A) along with h6L, a form of L with a histidine tag at the N terminus, we observed 1.37% assembly (Table 2, experiment 3). We interpret this as coassembly. The histidine-tagged protein did not give electrophoretic separation of the isoprenylated and unmodified forms; however, in total, 1.61% was assembled into particles (Table 2, experiment 3). Separate studies with [14C]mevalonolactone, as in Fig. 1, showed that h6L could be labeled (data not shown), consistent with some of this protein being isoprenylated.
As an additional negative control, we examined assembly of S, in the absence of other forms of delta antigen, and detected only 0.2% (Table 2, experiment 4). We interpret this amount as a background level for our assembly assay.
Hydrophobicity.
It is known that acylation of host or viral proteins can make them markedly more hydrophobic and likely to make protein-protein and/or protein-lipid interactions (40). Extrapolation of these findings to HDV has been used to rationalize why the L protein becomes isoprenylated. In an attempt to directly test this extrapolation, we made use of an assay for protein hydrophobicity based on the temperature-sensitive micelle formation achieved with the nonionic detergent Triton X-114. On a shift from 4 to 37°C, this detergent will form micelles which trap hydrophobic proteins, especially ones known to be integral membrane proteins (5).
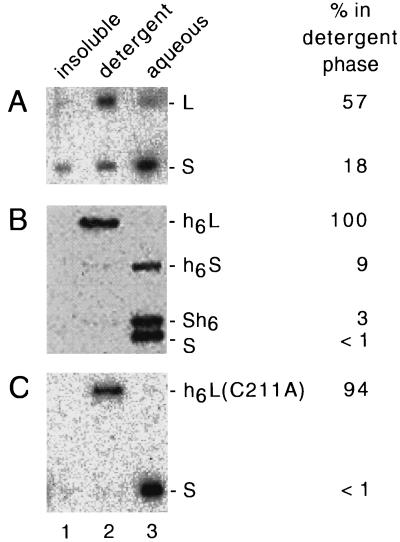
When we first applied this method to various forms of L and S, we found that protein-protein interactions perturbed the results. For example, fractionation of the RNP complexes present within serum virus, followed by immunoblot assays, gave results with ≥87% of both L and S in the detergent phase (data not shown). Thus, we modified the fractionation procedure by the addition throughout of aurintricarboxylic acid (an agent known to interfere with protein-protein interactions [22]) to 100 μM. We then found that most of the S left the detergent phase whereas most of the L remained (Fig. 4A). Using this modification, we assayed a series of additional forms of the delta proteins for their hydrophobicity, as shown in Fig. 4B and C. An unexpected finding was that each of the forms of L tested, regardless of whether it was isoprenylated, partitioned into the detergent phase. In contrast, each of the tested forms of S behaved as hydrophilic. The addition of a histidine tag to either the N or C terminus of S did not change its hydrophilicity. In contrast, addition to the C terminus of the 19-aa segment which converts S to L did make the protein hydrophobic.
FIG. 4.
Triton X-114 phase separation of delta proteins. As described in Materials and Methods, samples containing different forms of the delta proteins were separated into three fractions (insoluble, detergent, and aqueous; lanes 1 to 3, respectively) and then assayed by gel electrophoresis and immunoblotting. (A) Nonidet P-40-disrupted particles from the serum of an HDV-infected woodchuck. (B and C) Phase separations for different combinations of purified delta proteins, as indicated on the right. The immunoblots were quantitated to determine for each delta protein the fraction of the total soluble protein detected in the detergent phase; the results are indicated at the far right.
DISCUSSION
There are many examples of viral proteins that undergo posttranslational acylation, that is, modifications in which various fatty acid prosthetic groups are added; it is considered that these modifications make the viral proteins more hydrophobic and are equivalent to “greasing the wheels of assembly” (24). HDV L is the only example of an animal virus protein for which the acyl modification is an isoprenylation, even though there are speculations based on C-terminal sequences that there may be similar modifications for other animal virus proteins (16). We have provided here the first evidence that modified and unmodified forms of L are present both in infected liver and in released virions. We have obtained the first quantitation of the extent of this modification both during natural infections and in transfected cultured cells (Fig. 1; Table 1). For natural infections, the observed level of modification was high both in liver and in serum particles, and so we were unable to demonstrate whether isoprenylation was essential in such situations (Table 1). However, in experiments with transfected cells we were able to detect preferential assembly of Li into virus-like particles (Fig. 3; Table 2).
We observed that during electrophoresis Li migrated more slowly than L (Fig. 1C). This was in contrast to our expectation based on studies of other modified proteins, where farnesylated proteins migrate somewhat faster than the unmodified protein (2, 21, 47). It is possible that this discrepancy for Li is due to a difference in the levels of phosphorylation between L and Li. However, contrary to a previous study (3), we were unable to detect any difference by two-dimensional gel electrophoresis (data not shown).
We confirmed the result of others that the L protein can be isoprenylated in vitro in a rabbit reticulocyte lysate (16, 19, 42). However, we also showed that this protein migrated even more slowly than that labeled in vivo (Fig. 2). One interpretation of this mobility difference, based on published studies of isoprenylation using rabbit reticulocyte lysates (11), is that the species underwent only the first rather than all three steps of the isoprenylation. That is, it did not undergo either the tripeptide removal or the final carboxymethylation. Thus, we calculate that L modified in vitro would be 367 Da larger than L modified in vivo.
Previous studies indicate that the actual in vivo modification of L involves primarily a farnesyl rather than a geranylgeranyl group (42). We attempted to further clarify this issue by using mass spectrometry but were unsuccessful, for both the intact protein and C-terminal fragment released by proteolysis, in obtaining reproducible analyses (unpublished observations). Such difficulties are interpreted as due to the hydrophobicity of the modified C terminus because no difficulties were encountered for the unmodified protein, for a mutant unmodifiable protein, or for non-C-terminal proteolytic fragments.
HBV is the natural helper virus of HDV. Expression of the HBV envelope proteins, HBV sAg, leads to extensive particle formation (13). In cultured cells, expression of these HBV sAg can lead to the assembly of delta proteins. Previous studies have shown that expression of L is essential for assembly (7, 46) and provided indirect evidence that this is mediated via Li interacting with HBV sAg (19). We provide here direct evidence that Li is preferentially assembled (Fig. 3; Table 2). We observed up to 7% of the Li being assembled and released in an 8-day period (Table 2).
Our data and those of Glenn et al. (17, 18) show that it is the isoprenylation of L that is needed for assembly. Still unknown is whether this modification directly or indirectly mediates the interaction with the envelope protein(s) of the helper virus. We favor an indirect mechanism since HDV particles can be assembled not only by the envelope proteins of HBV but also by those of woodchuck hepatitis virus (43).
We have also found two mutated forms of L can be both isoprenylated and assembled. In one case, the N terminus was extended with a histidine tag (Table 2 and data not shown). In the other case, we used a deletion in the region near the N terminus that is essential for the dimerization and multimerization of the delta antigen (35). Our studies show that isoprenylation does not depend on multimerization.
For some time it has been known that certain forms of delta protein, which cannot be assembled by HBV sAg, can nevertheless be assembled if L and Li are present. To distinguish this latter process from assembly, we call it coassembly. As previously shown (35) and confirmed here (Table 2), coassembly of a delta protein depends on its ability to dimerize with L and/or Li. With our ability to separate and quantitate L and Li, we have been able to show that unmodified L is also coassembled. In this respect, L is no different from S or from mutant forms of L that cannot be isoprenylated. Others have studied C(211)S mutants of L (25), and we have obtained similar results with C(211)A (Table 2).
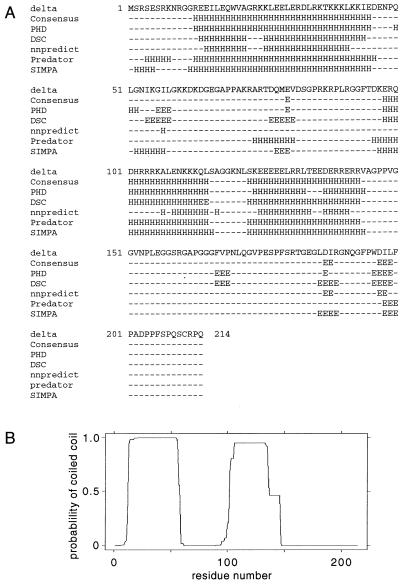
This brings us to the question of the hydrophobicity of delta proteins. We expected that Li might be more hydrophobic than either S or unmodified L. Therefore, we tested these and some related proteins in a phase separation assay (Fig. 4). S and two S-related proteins were all hydrophilic. However, we found that not only Li but also unmodified L, the mutant L(C211A), and an L-related protein were all more hydrophobic. A limitation of the assay was that we could not tell whether Li was more hydrophobic than L; other studies show that both addition of the isoprenyl group and the final methylation each dramatically increase the membrane affinity of a protein (15). However, our data do support the interpretation that the 19 aa unique to the C terminus of L, even prior to isoprenylation, confers a definite increase in hydrophobicity. We note that the sequence of this segment, WDILFPADPPFSPQSCRPQ, is more hydrophobic than most of the protein sequence, which is otherwise highly charged. It contains 11 hydrophobic aa, including five prolines. In Fig. 5A, we show the secondary structure predictions from a number of protein folding programs as well as a consensus prediction. The 19-aa C terminus is predicted to be mostly coil in structure because of the many prolines, although the first few amino acids may form the second strand of a β hairpin. If so, one face of this hairpin might be very hydrophobic (residues L and I in the first strand; W, I, and F in the second strand). In Fig. 5B, we show the prediction of two coiled-coil segments in the protein according to the COILS algorithm (39). The first segment of coiled coil is found in the crystal structure of Zuccola et al. (50). There is also clearly another coiled-coil domain in residues 100 to 150. There is evidence that this second region may also allow some dimerization in vitro (49); however, in the present study it was not sufficient to allow coassembly.
FIG. 5.
Predicted secondary structure of the large form of HDV antigen. (A) The protein sequence of Kuo et al. (33) was submitted to five different folding programs, PHD (45), DSC (30), nnpredict (31), predator (12), and SIMPA (4). The results and also a consensus folding are indicated. (B) Prediction of the coiled-coil segments by using the COILS algorithm (39).
An equally important questions is whether the detected isoprenylation-independent increase in hydrophobicity of L has biological relevance. We speculate that this increase might facilitate necessary interactions between this protein and the relevant isoprenyl transferase(s). In addition, our finding may help explain an earlier report of Lee et al. (36). These authors showed that the C-terminal 19 aa of L had a necessary role in virus assembly that was separate from the necessary role of acting as a site for isoprenylation. It is clear from our results that the increase in hydrophobicity, in the absence of isoprenylation per se, was not sufficient to direct assembly; it did not even directly facilitate coassembly, because S and L were coassembled with similar efficiencies (Table 2). One possible function is that the increased hydrophobicity enables L to reach a membrane site; it may be a second signal for membrane anchoring (23). Another possibility, not mutually exclusive of the first, is that the hydrophobicity is a facilitator of protein-protein associations, for example, at a membrane site (14). After all, we expect particle assembly to occur at the endoplasmic reticulum and to involve interactions between delta proteins and HB sAg (13, 28).
ACKNOWLEDGMENTS
J.T. was supported by grants AI-26522 and CA-06927 from NIH and by an appropriation from the Commonwealth of Pennsylvania. J.O. was funded by a fellowship from the Leukemia Society of America.
Harmon Zuccola and James Hogle provided purified recombinant S protein. Certain essential vectors were provided by Don Ganem and F. Asselbergs. Valuable help and advice was given by Said Sebti, Matt Bockol, Ting-Ting Wu, and Kate Dingle. Constructive comments on the manuscript were provided by William Mason.
REFERENCES
- 1.Asselbergs F A M, Grand P. A two-plasmid system for transient expression of cDNAs in primate cells. Anal Biochem. 1993;209:327–331. doi: 10.1006/abio.1993.1128. [DOI] [PubMed] [Google Scholar]
- 2.Bernard E J, Kao G, Cox A D, Sebti S M, Hamilton A D, Muschel R J. The farnesyltransferase inhibitor FTI-277 radiosensitizes H-ras-transformed rat embryo fibroblasts. Cancer Res. 1996;56:1727–1730. [PubMed] [Google Scholar]
- 3.Bichko V, Barik S, Taylor J. Phosphorylation of the hepatitis delta virus antigens. J Virol. 1997;71:512–518. doi: 10.1128/jvi.71.1.512-518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biou V, Gibrat J F, Levin J M, Robson J, Garnier J. Secondary structure prediction: combination of three different methods. Protein Eng. 1988;2:185–191. doi: 10.1093/protein/2.3.185. [DOI] [PubMed] [Google Scholar]
- 5.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 6.Casey P J, Seabra M C. Protein prenyltransferases. J Biol Chem. 1996;271:5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 7.Chang F L, Chen P J, Tu S J, Chiu M N, Wang C J, Chen D S. The large form of hepatitis δ antigen is crucial for the assembly of hepatitis δ virus. Proc Natl Acad Sci USA. 1991;88:8490–8494. doi: 10.1073/pnas.88.19.8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao M, Hsieh S-Y, Taylor J. Role of two forms of the hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J Virol. 1990;64:5066–5069. doi: 10.1128/jvi.64.10.5066-5069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dingle K, Bichko V, Zuccola H, Hogle J, Taylor J. Initiation of hepatitis delta virus genome replication. J Virol. 1998;72:4783–4788. doi: 10.1128/jvi.72.6.4783-4788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res. 1992;33:1569–1582. [PubMed] [Google Scholar]
- 11.Farh L, Mitchell D A, Deschenes R J. Farnesylation and proteolysis are sequential, but distinct steps in the CaaX box modification pathway. Arch Biochem Biophys. 1995;318:113–121. doi: 10.1006/abbi.1995.1211. [DOI] [PubMed] [Google Scholar]
- 12.Frishman D, Argos P. Seventy-five percent accuracy in protein secondary structure prediction. Proteins. 1997;27:329–335. doi: 10.1002/(sici)1097-0134(199703)27:3<329::aid-prot1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Ganem D. Assembly of hepadnaviral virions and subviral particles. Curr Top Microbiol Immunol. 1991;168:61–83. doi: 10.1007/978-3-642-76015-0_4. [DOI] [PubMed] [Google Scholar]
- 14.Garoff H, Hewson R, Opstelten D-J E. Virus maturation by budding. Microbiol Mol Biol Rev. 1998;62:1171–1190. doi: 10.1128/mmbr.62.4.1171-1190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghomashchi F, Zhang X, Liu L, Gelb M H. Binding of prenylated and polybasic peptides to membranes: affinities and intervesicle exchange. Biochemistry. 1995;34:11910–11918. doi: 10.1021/bi00037a032. [DOI] [PubMed] [Google Scholar]
- 16.Glenn J S. Prenylation and virion morphogenesis. In: Dinter-Gottlieb G, editor. The unique hepatitis delta virus. R. G. Austin, Tex: Landes, Co.; 1995. pp. 83–94. [Google Scholar]
- 17.Glenn J S. Shutting the door on hepatitis delta virus (HDV): sensitivity to prenylation inhibition prompts new therapeutic strategy. Viral Hepat Rev. 1999;5:13–26. [Google Scholar]
- 18.Glenn J S, Marsters J C, Jr, Greenberg H B. Use of a prenylation inhibitor as a novel antiviral agent. J Virol. 1998;72:9303–9306. doi: 10.1128/jvi.72.11.9303-9306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glenn J S, Watson J A, Havel C M, White J O. Identification of a prenylation site in the delta virus large antigen. Science. 1992;256:1331–1333. doi: 10.1126/science.1598578. [DOI] [PubMed] [Google Scholar]
- 20.Glenn J S, White J M. trans-dominant inhibition of human hepatitis delta virus genome replication. J Virol. 1991;65:2357–2361. doi: 10.1128/jvi.65.5.2357-2361.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez L, Magee A I, Marshall C J, Hancock J F. Post-translational processing of p21ras is two-step and involves carboxymethylation and carboxy-terminal proteolysis. EMBO J. 1989;8:1093–1099. doi: 10.1002/j.1460-2075.1989.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallick R B, Chelm B K, Gray P W, Orozco E M. Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977;4:3055–3063. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock J F, Paterson H, Marshall C J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- 24.Hruby D E, Franke C A. Viral acylproteins: greasing the wheels of assembly. Trends Microbiol. 1993;1:20–25. doi: 10.1016/0966-842x(93)90020-r. [DOI] [PubMed] [Google Scholar]
- 25.Hwang S B, Lai M M C. Isoprenylation masks a conformational epitope and enhances trans-dominant inhibitory function of the large hepatitis delta antigen. J Virol. 1994;68:2958–2964. doi: 10.1128/jvi.68.5.2958-2964.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang S B, Lai M M C. Isoprenylation mediates direct protein-protein interactions between hepatitis large delta antigen and hepatitis B virus surface antigen. J Virol. 1993;67:7659–7662. doi: 10.1128/jvi.67.12.7659-7662.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang S B, Lee C-Z, Lai M M C. Hepatitis delta antigen expressed by recombinant baculoviruses: comparison of biochemical properties and post-translational modifications between the large and small forms. Virology. 1992;190:413–422. doi: 10.1016/0042-6822(92)91227-l. [DOI] [PubMed] [Google Scholar]
- 28.Jenna S, Sureau C. Effect of mutations in the small envelope protein of hepatitis B virus on assembly and secretion of hepatitis delta virus. Virology. 1998;251:176–186. doi: 10.1006/viro.1998.9391. [DOI] [PubMed] [Google Scholar]
- 29.Kim C M, Goldstein J L, Brown M S. cDNA cloning of MEV, a mutant protein that facilitates cellular uptake of mevalonate, and identification of the point mutation responsible for its gain of function. J Biol Chem. 1992;267:23113–23121. [PubMed] [Google Scholar]
- 30.Kinh R D, Saqi M, Sayle R, Sternberg M J. DSC: public domain protein secondary structure prediction. Comput Appl Biosci. 1997;13:473–474. doi: 10.1093/bioinformatics/13.4.473. [DOI] [PubMed] [Google Scholar]
- 31.Kneller D G, Cohen F E, Langridge R. Improvements in secondary structure prediction by an enhanced neural network. J Mol Biol. 1990;214:171–182. doi: 10.1016/0022-2836(90)90154-E. [DOI] [PubMed] [Google Scholar]
- 32.Kuo M Y-P, Chao M, Taylor J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J Virol. 1989;63:1945–1950. doi: 10.1128/jvi.63.5.1945-1950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo M Y-P, Goldberg J, Coates L, Mason W, Gerin J, Taylor J. Molecular cloning of hepatitis delta virus RNA from an infected woodchuck liver: sequence, structure, and applications. J Virol. 1988;62:1855–1861. doi: 10.1128/jvi.62.6.1855-1861.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Lazinski D W, Taylor J M. Relating structure to function in the hepatitis delta virus antigen. J Virol. 1993;67:2672–2680. doi: 10.1128/jvi.67.5.2672-2680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee C-Z, Chen P-J, Lai M, Chen D-S. Isoprenylation of large hepatitis delta antigen is necessary but not sufficient for hepatitis delta virus assembly. Virology. 1994;199:169–175. doi: 10.1006/viro.1994.1109. [DOI] [PubMed] [Google Scholar]
- 37.Lin H-p, Hsu S-C, Wu J-C, Sheen I-J, Yan B-S, Syu W-J. Localization of isoprenylated antigen of hepatitis delta virus by anti-farnesyl antibodies. J Gen Virol. 1999;80:91–96. doi: 10.1099/0022-1317-80-1-91. [DOI] [PubMed] [Google Scholar]
- 38.Luo G, Chao M, Hsieh S-Y, Sureau C, Nishikura K, Taylor J. A specific base transition occurs on replicating hepatitis delta virus RNA. J Virol. 1990;64:1021–1027. doi: 10.1128/jvi.64.3.1021-1027.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lupas L, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 40.Marshall C J. Protein prenylation: a mediator of protein-protein interactions. Science. 1993;259:1865–1866. doi: 10.1126/science.8456312. [DOI] [PubMed] [Google Scholar]
- 41.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cell lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- 42.Otto J C, Casey P J. The hepatitis delta virus large antigen is farnesylated both in vitro and in animal cells. J Biol Chem. 1996;271:4569–4572. doi: 10.1074/jbc.271.9.4569. [DOI] [PubMed] [Google Scholar]
- 43.Ponzetto A, Cote P J, Popper H, Hoyer B H, London W T, Ford E C, Bonino F, Purcell R H, Gerin J L. Transmission of the hepatitis B virus-associated δ agent to the eastern woodchuck. Proc Natl Acad Sci USA. 1984;81:2208–2212. doi: 10.1073/pnas.81.7.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pryde J G. Triton X-114: a detergent that has come in from the cold. Trends Biochem Sci. 1986;11:160–163. [Google Scholar]
- 45.Rost B. PHD: predicting one-dimensional protein structure by profile based neural networks. Methods Enzymol. 1996;266:525–539. doi: 10.1016/s0076-6879(96)66033-9. [DOI] [PubMed] [Google Scholar]
- 46.Ryu W-S, Bayer M, Taylor J. Assembly of hepatitis delta virus particles. J Virol. 1992;66:2310–2315. doi: 10.1128/jvi.66.4.2310-2315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasseville A M-J, Raymond Y. Lamin A precursor is localized to intranuclear foci. J Cell Sci. 1995;108:273–285. doi: 10.1242/jcs.108.1.273. [DOI] [PubMed] [Google Scholar]
- 48.Wu H-L, Chen P-J, Mu J-J, Chi W-K, Kao T-L, Hwang L-H, Chen D-S. Assembly of hepatitis delta virus-like empty particles in yeast. Virology. 1997;236:374–381. doi: 10.1006/viro.1997.8743. [DOI] [PubMed] [Google Scholar]
- 49.Xia Y-P, Lai M M C. Oligomerization of hepatitis delta antigen is required for both the trans-activating and trans-dominant inhibitory activities of the delta antigen. J Virol. 1992;66:6641–6648. doi: 10.1128/jvi.66.11.6641-6648.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuccola H J, Rozzelle J E, Lemon S M, Erickson B W, Hogle J M. Structural basis of the oligomerization of hepatitis delta antigen. Structure. 1998;6:821–830. doi: 10.1016/s0969-2126(98)00084-7. [DOI] [PubMed] [Google Scholar]