Abstract
Background
This study evaluated the risk factors of long-term mortality in patients with multidrug/rifampicin-resistant tuberculosis (MDR/RR-TB) in South Korea who were lost to follow-up (LTFU).
Methods
This was a retrospective longitudinal follow-up study using an integrated database constructed by data linkage of the three national databases, which included 7226 cases of MDR/RR-TB notified between 2011 and 2017 in South Korea. Post-treatment outcomes of patients who were LTFU were compared with those of patients who achieved treatment success.
Results
Of the 7226 MDR/RR-TB cases, 730 (10.1%) were LTFU. During a median follow-up period of 4.2 years, 101 (13.8%) of the LTFU patients died: 25 deaths (3.4%) were TB related and 76 (10.4%) were non-TB related. In the LTFU group, the adjusted hazard ratio (aHR) of all-cause mortality (aHR 2.50, 95% CI 1.99–3.15, p<0.001), TB-related mortality (aHR 5.38, 95% CI 3.19–9.09, p<0.001) and non-TB-related mortality (HR 2.21, 95% CI 1.70–2.87, p<0.001) was significantly higher than that in the treatment success group. Independent risk factors for all-cause mortality in the LTFU group were age >55 years, fluoroquinolone resistance, cancer and no retreatment. In the LTFU patients who did not receive retreatment, the risk of non-TB-related mortality (aHR 5.00, 95% CI 1.53–16.37, p=0.008) and consequent all-cause mortality (aHR 2.18, 95% CI 1.08–4.40, p=0.030) was significantly higher than that of patients who received retreatment.
Conclusion
Non-TB-related mortality was the main cause of death and might be reduced by retreatment in LTFU patients with MDR/RR-TB.
Tweetable abstract
Retreatment after loss to follow-up may reduce mortality in patients with multidrug/rifampicin-resistant tuberculosis https://bit.ly/45Hi3AZ
Introduction
Traditional treatment for multidrug/rifampicin-resistant tuberculosis (MDR/RR-TB) is lengthy, associated with severe adverse events and complicated. This often leads to non-adherence, frequent treatment interruption and eventual loss to follow-up. Loss to follow-up has long been a major obstacle to TB control.
The frequency of loss to follow-up among patients with MDR/RR-TB varies by country [1–3]. The World Health Organization (WHO) estimates that 14.3% of patients with MDR/RR-TB worldwide are lost to follow-up (LTFU) [4]. In Korea, the high LTFU rate severely hinders MDR/RR-TB management. In large cohort studies in Korea, the LTFU rate was 39.0% for MDR-TB patients treated in 1988–1996 [5], 32.2% in 2000–2002 [6] and 11.4% in 2011–2014 [7].
Loss to follow-up can cause serious public health problems. These patients have an increased risk of TB-related death [8–12]. Furthermore, patients in whom the disease does not cause morbidity or death may continue to spread resistant bacilli and amplify drug resistance within the community [10]. Many studies have focused on the prevalence, timing and predictors of loss to follow-up to develop strategies for preventing this in patients with MDR/RR-TB [1]. However, there are few population-based studies to trace long-term outcomes after loss to follow-up in patients with MDR/RR-TB, e.g. how many patients were retreated, survival rates and which factors determine these outcomes. Although some studies have evaluated post-treatment outcomes [8–12], they were limited in terms of sample size, population coverage, time coverage and considerable missing cases.
Determining the burden and outcome of LTFU patients is essential in the management of MDR/RR-TB. Prospective follow-up studies are ideal for estimating the LTFU burden but are often not feasible because they are costly and time-consuming. Data linkage has emerged as a promising tool to complement the limitations of prospective studies [13]. Data linkage research can trace long-term outcomes without losing patients while evaluating various risk factors, including comorbidities and social determinants.
We developed an integrated TB database by linking three national databases [14] and reported treatment outcomes of patients with MDR/RR-TB in South Korea [15]. Using this database, we conducted a retrospective longitudinal follow-up study from the end of treatment in patients with MDR/RR-TB. The primary objective of this study was to estimate post-treatment mortality of patients with MDR/RR-TB who were LTFU compared with those who had achieved treatment success. The secondary objectives were to evaluate the risk factors for all-cause mortality, proportion of retreatment and outcomes of retreated cases among patients with MDR/RR-TB who were LTFU.
Methods
Data sources and collection
The Korean Tuberculosis and Post-Tuberculosis (TB-POST) cohort was constructed by linking the data in the following national databases: 1) the Korean Tuberculosis Surveillance System (KTBS) between 2011 and 2018, 2) the National Health Insurance Database (NHID) between 2006 and 2018 and 3) the Causes of Death Statistics databases between 2011 and 2018 [14].
Each data source is a nationwide database that contains information on all notified TB patients, all claims data in the NHID and all death causes during the study period in South Korea. The KTBS is a web-based notification system established in 2000. TB notification is mandatory in Korea and its completeness was reaching 94% in 2014 [16]. Information on patients with TB is continuously registered in the KTBS from notification to the end of treatment. The KTBS contains patient demographics, clinical characteristics, diagnostic test results, treatment modalities and final treatment outcomes.
The NHID is a public database on healthcare utilisation, health screening, sociodemographic variables and mortality, formed by the National Health Insurance Service [17]. South Korea has a universal healthcare insurance system. As of 2014, National Health Insurance covers almost 98% of the total population in Korea [17]. The NHID can provide additional information on post-treatment outcomes, comorbidities and socioeconomic variables that are not identified in the KTBS.
The Causes of Death Statistics databases were developed based on death certificates and cover almost the entire population. Causes of death are obtained from the International Statistical Classification of Disease and Related Health Problems, 10th revision (ICD-10).
Study design and population
This was a retrospective, longitudinal follow-up study of patients with MDR/RR-TB. The study population included MDR/RR-TB cases notified between 1 January 2011 and 31 December 2017 [15], which were extracted from the Korean TB-POST cohort [14]. After excluding patients who died during treatment, all patients were classified according to the treatment outcomes and were traced from the end-of-treatment date to 30 July 2020. Post-treatment outcomes of the LTFU group were compared with those of the treatment success group.
TB case management
In South Korea, >90% of patients with TB are treated in the private sector. To improve TB case management in the private sector, a public–private mix (PPM) cooperation project has been conducted since 2011 [18]. The government dispatches trained TB nurses to private medical institutions. They are responsible for counselling, education and monitoring of patients with TB in the private sector. Approximately 70% of patients with TB are treated at PPM institutions [18]. If a patient is LTFU, a TB nurse calls the patient and persuades them to visit the hospital. If this is not successful, the patient is reported to the local health centre as a noncompliant patient. Public health staff make telephone calls and home visits to persuade the patient to resume treatment. If the patient refuses treatment, the patient can be lawfully forced into hospitalisation in isolation. No additional incentives are offered to patients to resume treatment. In South Korea, that direct medical cost of TB management was partially covered by the government until 2015 and has been fully covered since 2016.
Definition and measurement
MDR-TB is defined as TB resistant to at least isoniazid (INH) and rifampicin (RIF) [19]. Extensively drug-resistant TB (XDR-TB) is defined as TB resistant to at least INH and RIF plus any fluoroquinolones and at least one of the injectable second-line drugs (amikacin, kanamycin or capreomycin). Pre-XDR-TB is defined as TB with resistance to INH and RIF and either a fluroquinolone or a second-line injectable agent but not both. RR is defined as RIF-resistance without evidence of INH resistance.
Treatment outcomes were defined as that of the first treatment episode registered in the KTBS. These were assigned by the attending physicians according to the criteria suggested by the WHO [19]. The sum of the cure and treatment completion was designated as treatment success. A patient who was LTFU was defined as a TB patient whose treatment was interrupted for two consecutive months or more. A treatment episode was defined as a set of consecutive events without treatment interruption for >2 months. If a patient experienced multiple treatment episodes, the treatment outcome was defined as that from the first treatment episode. For example, if a patient was transferred and subsequently registered in a new institution within 2 months, this was considered a continuous treatment episode. Conversely, if the transferred patient was not registered with another institution within 2 months, this case was designated as not evaluated.
Post-treatment outcomes such as retreatment, death or survival were traced in the integrated database. Retreatment was defined as the occurrence of a new treatment episode in a patient who already had a treatment outcome. This included both bacteriologically confirmed and clinically diagnosed cases. Death cases were classified into TB-related and non-TB-related deaths according to the ICD-10 code in the Cause of Death Statistics database. The post-treatment follow-up period was from the end of the first treatment episode to 30 July 2020.
Household income was classified into quintiles (1=lowest, 5=highest) according to the National Health Insurance premium. Medical aid beneficiaries were classified as group 0. Finally, variables that may influence post-treatment outcomes were measured as covariates. These included age, gender, nationality, residential region, type of notifying health institution, previous TB treatment history, lesion site, sputum smear results, sputum culture results and comorbidities (diabetes mellitus and cancer).
Statistical analysis
Continuous variables are presented as mean±sd if the variable was normally distributed or as median (IQR), and categorical variables are expressed as n (%). A t-test was used if the variable was normally distributed or the Mann–Whitney test was used if the variable was not normally distributed to compare continuous variables. A chi-square test was used to compare categorical variables. A Cox proportional hazards model was used to establish the hazard ratio of the risk factors associated with mortality. Variables with p-values <0.2 in the univariate analysis were entered into the multivariate models. The Kaplan–Meier method and log-rank test were used to compare survival times between two or more groups. All p-values were two-tailed, and p<0.05 was considered statistically significant. All statistical analyses were performed using STATA/MP version 17 (StataCorp LLC, College Station, TX, USA).
Ethical statement
The study protocol was reviewed and approved by the institutional review board of the National Evidence-based Healthcare Collaborating Agency (NECAIRB19–008-1). The requirement for informed consent was waived owing to the retrospective nature of the study using public de-identified data.
Results
Baseline characteristics
A total of 7226 cases of MDR/RR-TB notified between 2011 and 2017 were included in the integrated TB database. After excluding patients who died during treatment (n=699), 6527 cases were followed from the end-of-treatment date to 30 July 2020. Their median follow-up time was 4.2 years (IQR 2.5–6.1 years). Finally, after excluding the failure group and the not evaluated group, the LTFU group and the treatment success group were included in the study (figure 1).
FIGURE 1.
Flow diagram of the study population. Data are presented as n (%), unless otherwise indicated. MDR: multidrug resistant; RR: rifampicin resistant; TB: tuberculosis.
Of the total 7226 cases, 730 (10.1%) were classified as the LTFU group and 5308 (73.5%) as the treatment success group. The baseline characteristics of the LTFU group compared to the treatment success group are shown in table 1. The mean age of the LTFU group was 47.5 years. Compared to the treatment success group, the proportion of male, metropolitan resident, immigrant, low-income class and previously treated cases was higher in the LTFU group. None of the LTFU group was HIV-positive.
TABLE 1.
Baseline characteristics of patients with MDR/RR-TB according to treatment outcomes
| Lost to follow-up | Treatment success | p-value | |
| Subjects, n | 730 | 5308 | |
| Age (years) # | 47.5±15.8 | 47.9±17.4 | 0.556 |
| Sex | <0.001 | ||
| Female | 158 (21.6) | 1809 (35.2) | |
| Male | 572 (78.4) | 3499 (64.8) | |
| Resident region | 0.001 | ||
| Metropolitan | 387 (53.0) | 2469 (46.5) | |
| Others | 343 (47.0) | 2839 (53.5) | |
| Nationality | <0.001 | ||
| Korean | 603 (82.6) | 4968 (93.6) | |
| Immigrant | 127 (17.4) | 340 (6.4) | |
| Household income | <0.001 | ||
| 0 (lowest) | 109 (14.9) | 439 (8.3) | |
| 1 | 152 (20.8) | 934 (17.6) | |
| 2 | 183 (25.1) | 1007 (19.0) | |
| 3 | 149 (20.4) | 1081 (20.4) | |
| 4 | 76 (10.4) | 948 (17.9) | |
| 5 (highest) | 61 (8.4) | 899 (16.9) | |
| Treatment history | <0.001 | ||
| New | 344 (47.1) | 3213 (60.5) | |
| Previously treated | 386 (52.9) | 2095 (39.5) | |
| Lesion site | 0.090 | ||
| Pulmonary | 713 (97.7) | 5119 (96.4) | |
| Extrapulmonary | 17 (2.3) | 189 (3.6) | |
| Notifying institution 1 | 0.003 | ||
| Non-PPM | 213 (29.2) | 1278 (24.1) | |
| PPM | 517 (70.8) | 4030 (75.9) | |
| Notifying institution 2 | 0.035 | ||
| Health centre | 84 (11.5) | 482 (9.1) | |
| Private institution | 646 (88.5) | 4826 (90.9) | |
| Smear | 0.020 | ||
| Positive | 407 (55.8) | 2680 (50.5) | |
| Negative | 284 (38.9) | 2354 (44.4) | |
| ND/unknown | 39 (5.3) | 274 (5.2) | |
| Culture | <0.001 | ||
| Positive | 524 (71.8) | 4057 (76.4) | |
| Negative | 77 (10.6) | 618 (11.6) | |
| ND/unknown | 129 (17.7) | 633 (11.9) | |
| Resistance pattern | 0.002 | ||
| RR | 58 (8.0) | 632 (11.9) | |
| MDR | 576 (78.9) | 3839 (72.3) | |
| pre-XDR (SLID) | 20 (2.7) | 139 (2.6) | |
| pre-XDR (FQ) | 23 (3.2) | 219 (4.1) | |
| XDR | 53 (7.3) | 479 (9.0) | |
| Comorbidity | |||
| Diabetes mellitus | 136 (18.6) | 810 (15.3) | 0.019 |
| Cancer | 14 (1.9) | 70 (1.3) | 0.212 |
| HIV | 0 | 11 (0.2) | 0.218 |
Data are presented as n (%), unless otherwise indicated. MDR: multidrug resistant; RR: rifampicin resistant; TB: tuberculosis; PPM: public–private mix; ND: no data; XDR: extensively drug resistant; SLID: second-line injectable drug; FQ: fluoroquinolone. #: mean±sd.
Mortality after loss to follow-up
Loss to follow-up occurred at a median of 319 days (IQR 188–518 days) from the start of MDR-TB treatment. Of the 730 LTFU cases, 101 (13.8%) died at a median of 443 days (IQR 158–1157 days) after being LTFU: 25 (3.4%) deaths were TB-related (median 280 days, IQR 151–649 days) and 76 (10.4%) were non-TB-related (median 606 days, IQR 189–1258 days). During the follow-up period, 162 (22.2%) returned to treatment at a median of 398 days (IQR 195–872 days) after being LTFU and 629 (86.2%) remained alive.
Table 2 shows the number of deaths and hazard ratios in the LTFU group with the treatment success group as a reference. The data were adjusted for potential confounders and other variables (age, gender, nationality, income, TB treatment history, lesion site, health institution, sputum smear result, drug susceptibility pattern, diabetes and cancer). In the LTFU group, the adjusted HR (aHR) of all-cause mortality (aHR 2.50, 95% CI 1.99–3.15, p<0.001), TB-related mortality (aHR 5.38, 95% CI 3.19–9.09, p<0.001) and non-TB-related mortality (aHR 2.21, 95% CI 1.70–2.87, p<0.001) was significantly higher than that in the treatment success group.
TABLE 2.
Post-treatment mortality among patients with MDR/RR-TB
| Success | Lost to follow-up | p-value | |
| Subjects, n | 5308 | 730 | |
| All-cause death | |||
| Death, n (%) | 313 (5.9) | 101 (13.8) | |
| HR (95% CI) | 1 | 2.37 (1.89–2.96) | <0.001 |
| aHR (95% CI) | 1 | 2.50 (1.99–3.15) | <0.001 |
| TB-related death | |||
| Death, n (%) | 37 (0.7) | 25 (3.4) | |
| HR (95% CI) | 1 | 5.21 (3.14–8.66) | <0.001 |
| aHR (95% CI) | 1 | 5.38 (3.19–9.09) | <0.001 |
| Non-TB-related death | |||
| Death, n (%) | 276 (5.2) | 76 (10.4) | |
| HR (95% CI) | 1 | 2.03 (1.58–2.62) | <0.001 |
| aHR (95% CI) | 1 | 2.21 (1.70–2.87) | <0.001 |
Adjusted for age, gender, nationality, income, TB treatment history, lesion site, health institution, sputum smear result, drug susceptibility pattern, diabetes and cancer. MDR: multidrug resistant; RR: rifampicin resistant; TB: tuberculosis; HR: hazard ratio; aHR: adjusted hazard ratio.
Risk of non-TB-related mortality was also significantly higher in the treatment failure group (aHR 1.99, 95% CI 1.17–3.40, p=0.011) and the not evaluated group (aHR 1.87, 95% CI 1.32–2.66, p<0.001) than in the success group (supplementary table S1).
Risk factors for mortality
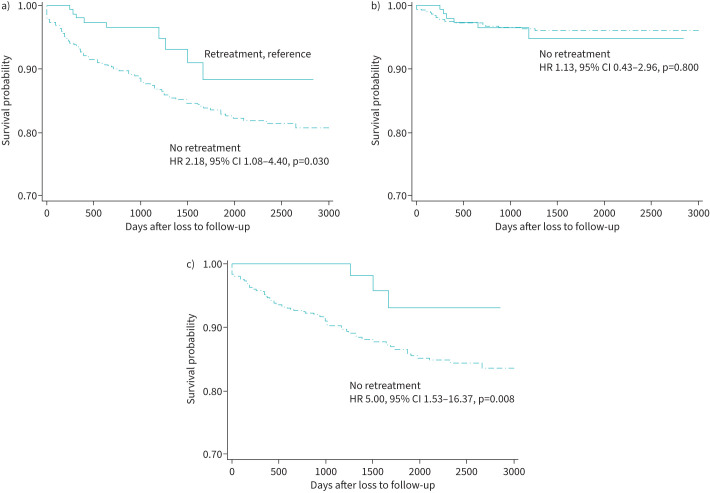
The risk factors of all-cause mortality in the LTFU group are shown in table 3. In the LTFU group, the independent risk factors for all-cause mortality were age >55 years, fluoroquinolone resistance, cancer and no retreatment. After adjusting for potential confounders and other variables (age, gender, nationality, income, TB treatment history, lesion site, health institution, sputum smear result, drug susceptibility pattern, diabetes and cancer), the mortality hazard according to retreatment was compared in the LTFU group. In patients who did not receive retreatment, the hazard of all-cause mortality (aHR 2.18, 95% CI 1.08–4.40, p=0.030) and non-TB-related mortality (aHR 5.00, 95% CI 1.53–16.37, p=0.008) was significantly higher than that in those who received retreatment, although this was not the case for TB-related mortality (aHR 1.13, 95% CI 0.43–2.96, p=0.800) (figure 2).
TABLE 3.
Risk factors for all-cause mortality among patients with MDR/RR-TB who were LTFU
| Survivor | Death | HR (95% CI) | p-value | aHR (95% CI) | p-value | |
| Subjects, n | 629 | 101 | ||||
| Age group (years) | ||||||
| ≤24 | 55 (8.7) | 0.0 | ||||
| 25–34 | 110 (17.5) | 4 (4.0) | 1 | 1 | ||
| 35–44 | 132 (21.0) | 9 (8.9) | 1.92 (0.59–6.22) | 0.279 | 1.18 (0.35–3.95) | 0.787 |
| 45–54 | 174 (27.7) | 28 (27.7) | 4.32 (1.52–12.32) | 0.006 | 2.67 (0.90–7.88) | 0.076 |
| 55–64 | 96 (15.3) | 24 (23.8) | 6.69 (2.32–19.30) | <0.001 | 3.92 (1.31–11.75) | 0.015 |
| 65–74 | 33 (5.3) | 13 (12.9) | 8.79 (2.87–26.97) | <0.001 | 4.22 (1.30–13.73) | 0.017 |
| ≥75 | 29 (4.6) | 23 (22.8) | 15.52 (5.36–44.91) | <0.001 | 9.49 (3.08–29.25) | <0.001 |
| Sex | ||||||
| Female | 140 (32.3) | 18 (17.8) | 1 | 1 | ||
| Male | 489 (77.7) | 83 (82.2) | 1.37 (0.82–2.27) | 0.231 | 1.54 (0.87–2.73) | 0.135 |
| Resident region | ||||||
| Metropolitan | 332 (52.8) | 55 (54.5) | 1 | |||
| Others | 297 (47.2) | 46 (45.5) | 0.96 (0.65–1.42) | 0.838 | ||
| Nationality | ||||||
| Korean | 502 (79.8) | 101 (100) | ||||
| Immigrant | 127 (20.2) | 0 | ||||
| Household income | ||||||
| 0 (lowest) | 80 (12.7) | 29 (28.7) | 1.63 (0.82–3.27) | 0.166 | 1.52 (0.72–3.19) | 0.269 |
| 1 | 136 (21.6) | 16 (15.8) | 0.62 (0.29–1.33) | 0.216 | 0.69 (0.30–1.57) | 0.374 |
| 2 | 166 (26.4) | 17 (16.8) | 0.54 (0.25–1.14) | 0.107 | 0.72 (0.33–1.61) | 0.426 |
| 3 | 138 (21.9) | 11 (10.9) | 0.41 (0.18–0.95) | 0.036 | 0.64 (0.27–1.54) | 0.320 |
| 4 | 59 (9.4) | 17 (16.8) | 1.35 (0.63–2.87) | 0.443 | 1.28 (0.59–2.80) | 0.529 |
| 5 (highest) | 50 (8.0) | 11 (10.9) | 1 | 1 | ||
| Treatment history | ||||||
| New | 305 (48.5) | 39 (38.6) | 1 | 1 | ||
| Previously treated | 324 (51.5) | 62 (61.4) | 1.39 (0.93–2.08) | 0.104 | 1.49 (0.95–2.33) | 0.080 |
| Lesion site | ||||||
| Pulmonary | 613 (97.5) | 100 (99.0) | 1 | |||
| Extrapulmonary | 16 (2.5) | 1 (1.0) | 0.41 (0.06–2.93) | 0.373 | ||
| Notifying institution 1 | ||||||
| PPM | 434 (69.0) | 83 (82.2) | 2.06 (1.24–3.43) | 0.005 | 1.10 (0.62–1.96) | 0.737 |
| Non-PPM | 195 (31.0) | 18 (17.8) | 1 | 1 | ||
| Notifying institution 2 | ||||||
| Health centre | 81 (12.9) | 3 (3.0) | 1 | 1 | ||
| Private institution | 548 (87.1) | 98 (97.0) | 4.64 (1.47–14.62) | 0.009 | 3.30 (0.94–11.53) | 0.062 |
| Smear | ||||||
| Positive | 351 (55.8) | 56 (55.5) | 1 | 1 | ||
| Negative | 241 (38.3) | 43 (42.6) | 1.09 (0.73–1.62) | 0.679 | 0.78 (0.50–1.22) | 0.282 |
| ND/unknown | 37 (5.9) | 2 (2.0) | 0.33 (0.08–1.34) | 0.121 | 0.30 (0.07–1.25) | 0.099 |
| Culture | ||||||
| Positive | 454 (72.2) | 70 (69.3) | 1 | 1 | ||
| Negative | 66 (10.5) | 11 (10.9) | 1.06 (0.56–2.00) | 0.866 | 1.06 (0.56–2.00) | 0.866 |
| ND/unknown | 109 (17.3) | 20 (19.8) | 1.04 (0.63–1.71) | 0.874 | 1.04 (0.63–1.71) | 0.874 |
| Resistance pattern | ||||||
| RR | 55 (8.7) | 3 (3.0) | 1 | 1 | ||
| MDR | 499 (79.3) | 77 (76.2) | 2.43 (0.77–7.71) | 0.131 | 2.29 (0.71–7.40) | 0.166 |
| pre-XDR (SLID) | 18 (2.9) | 2 (2.0) | 1.89 (0.32–11.30) | 0.486 | 2.03 (0.32–12.83) | 0.450 |
| pre-XDR (FQ) | 18 (2.9) | 5 (5.0) | 5.02 (1.20–21.03) | 0.027 | 4.50 (1.02–19.80) | 0.047 |
| XDR | 39 (6.2) | 14 (13.9) | 5.49 (1.58–19.12) | 0.007 | 3.33 (0.93–11.94) | 0.065 |
| Comorbidity | ||||||
| Diabetes mellitus | 105 (16.7) | 31 (30.7) | 2.18 (1.43–3.33) | <0.001 | 1.35 (0.86–2.12) | 0.198 |
| Cancer | 6 (1.0) | 8 (7.9) | 7.13 (3.45–14.72) | <0.001 | 6.67 (2.88–15.44) | <0.001 |
| Time to LTFU (days) | ||||||
| 0–269 | 237 (37.7) | 47 (46.5) | 1 | 1 | ||
| 270–539 | 236 (37.5) | 37 (36.6) | 0.80 (0.62–1.23) | 0.311 | 0.73 (0.46–1.15) | 0.175 |
| 540 | 156 (24.8) | 17 (16.8) | 0.67 (0.38–1.16) | 0.153 | 0.63 (0.35–1.12) | 0.114 |
| Retreatment | 153 (24.3) | 9 (8.9) | 0.44 (0.22–0.88) | 0.020 | 0.46 (0.22–0.93) | 0.031 |
Data are presented as n (%), unless otherwise indicated. MDR: multidrug resistant; RR: rifampicin resistant; TB: tuberculosis; LTFU: lost to follow-up; HR: hazard ratio; aHR: adjusted hazard ratio; PPM: public–private mix; ND: no data; XDR: extensively drug resistant; SLID: second-line injectable drug; FQ: fluoroquinolone.
FIGURE 2.
Kaplan–Meier survival curves comparing a) all-cause mortality, b) tuberculosis (TB)-related mortality and c) non-TB-related mortality according to retreatment in patients with multidrug/rifampicin-resistant TB who were lost to follow-up. Adjusted for age, gender, nationality, income, TB treatment history, lesion site, health institution, sputum smear result, drug susceptibility pattern, diabetes and cancer. HR: hazard ratio.
Retreatment outcomes
Of the 730 LTFU patients, 162 (22.2%) received retreatment at a median of 398 days (IQR 195–872 days) after being LTFU. Of them, 71 (43.8%) were successfully treated, 62 (38.3%) were LTFU again, five (3.1%) were treatment failures and nine (5.6%) died during treatment. The retreatment outcomes for other groups are shown in supplementary table S2.
Discussion
This study evaluated the post-treatment outcomes of patients with MDR/RR-TB by linking three representative national databases, focusing on the LTFU group. During the median follow-up period of 4.2 years, all-cause mortality and retreatment rates in the LTFU group were 13.8% and 22.2%, respectively. Retreatment was an independent risk factor for survival in the LTFU group. After adjusting for potential confounders and other variables, the hazard of all-cause mortality was 2.18-times higher in the LTFU patients who did not receive retreatment than in those who received retreatment. To the best of our knowledge, this is the first population-based study to demonstrate that retreatment may reduce mortality in patients with MDR/RR-TB who were LFTU.
The mortality rate of LTFU patients with MDR/RR-TB was higher in previous studies (53.2% in Peru [8], 27% in South Africa [9], 23% and 32.5% in Georgia [10, 11] and 29.4% in Estonia [12]) than in this study (18%). This may be due to differences in the study populations and follow-up periods. Moreover, we found that non-TB-related mortality was the main cause of death in the LTFU group and was significantly higher than that in the treatment success group. In our study, non-TB-related mortality accounted for ∼75% of all the deaths in the LTFU group. After adjusting for potential confounders and other variables, the hazard ratio of non-TB-related mortality in the LTFU group was approximately two times higher than that in the treatment success group.
Non-TB-related mortality has already been reported as the main cause of death during TB treatment in certain countries [20–22]. Moreover, post-treatment mortality was also higher in patients who had achieved treatment success than in the general population [23–25]. The potential mechanism of high mortality after microbiological cure is that residual lung injuries after TB treatment might increase chronic respiratory diseases, such as chronic obstructive pulmonary disease and bronchiectasis [26]. In addition, TB is associated with an increased risk of cardiovascular disease [27] and cancer [28]. In this study, the adjusted hazard of non-TB-related mortality was consistently increased in the treatment failure, LTFU and not evaluated groups compared to the treatment success group (supplementary table S1). This finding suggests that if TB persists without treatment completion, the risk of non-TB-related mortality persistently increases. Conversely, the successful completion of treatment may reduce long-term mortality in patients with TB.
In this study, there was no difference in TB-related mortality between the retreatment and no retreatment groups. While it is difficult to explain this based on the results of the study, two possible explanations are as follows: first, the retreatment group might have included patients with more severe disease who had to visit hospital because the TB worsened and became life-threatening. LTFU patients had a high mortality rate, even if retreated [8], because they were likely to have increased disease severity and acquired additional drug resistance compared to initial presentation. Conventional longer regimens might not be effective enough to reduce mortality in these deteriorating patients. Second, there might have been misclassification of cause of death in the no retreatment group. During the TB treatment period, TB-related death may have been captured appropriately. However, clinicians were likely to have paid more attention to ongoing diseases other than TB when they judged the cause of death during periods of no TB retreatment [29]. A few TB-related deaths were not confirmed and the relationship with TB was not intensively assessed by clinicians [30]. As a result, misclassification of cause of death may have occurred more frequently among the no retreatment group.
In addition, 37 of the 313 deaths of successfully treated patients were classified as TB-related deaths. Among them, six patients had TB-related deaths during retreatment. It is difficult to determine why the remaining 31 deaths were classified as TB-related. Considering the clinical practice patterns in South Korea, patients who died of TB sequelae (e.g. massive haemoptysis or respiratory failure caused by destroyed lung) were likely to have their death registered as TB related.
Our study found that retreatment in the LTFU group reduced the risk of non-TB-related mortality. Many factors are associated with high mortality from MDR-TB, including demographic factors (age and gender), behavioural factors (smoking, alcohol use and substance addiction) and clinical factors (comorbidities, HIV infection, malnutrition, clinical complications, adverse effects and type of drug resistance) [31]. Returning to a medical institution for retreatment might provide the patient with the opportunity to receive comprehensive management of TB as well as other medical conditions. This includes management of comorbidities such as diabetes, timely response to superimposed acute illness and lifestyle modifications. Retreatment may reduce non-TB-related mortality by reducing the risk of modifiable mortality factors. Therefore, intensified efforts are needed to trace patients who are LTFU and resume comprehensive management.
This study showed that data linkage was an effective tool for tracing the post-treatment outcomes of patients with MDR/RR-TB. Several studies have attempted to link registration and mortality data [6, 11]. This study was more representative than others in terms of database size, population coverage and time coverage. In addition, we linked the National Health Insurance claim data, which enabled the analysis of more relevant covariates, such as comorbidities and socioeconomic status.
Our study had several limitations, mainly due to its retrospective nature using routinely collected health data. First, the follow-up period of the included patients was different, which may have introduced a significant bias in the long-term mortality estimates. There was no change on the survival curve from 1500 to 3000 days, because few deaths were observed with lots of censored (follow-up completion) cases. Second, the cause of death relied on the Cause of Death Statistics data based on the death certificate. The accuracy of these data has not been verified by autopsies or other methods. Therefore, a reporting bias may have occurred if there were inaccurate death certificates. Third, patients who were diagnosed with MDR/RR-TB but did not start treatment were not included in the LTFU group, because the KTBS currently does not identify these patients. Fourth, regimen-related factors were not included in the assessment of the risk factors for mortality in the LTFU group, because those were largely missing in the KTBS. Fifth, variables that could affect the occurrence and outcome of loss to follow-up, such as substance use and psychiatric comorbidities, were not included. Sixth, because this study was conducted in a country with low HIV prevalence, this should be considered when interpreting the study results.
In conclusion, patients with MDR/RR-TB who are LTFU have an increased risk of TB-related as well as non-TB-related mortality compared to those who achieve treatment success. Non-TB-related mortality was the main cause of death and might be reduced by retreatment in patients with MDR/RR-TB who were LTFU. Therefore, a stronger strategy for tracing LTFU patients is urgently needed so that they can resume comprehensive management.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00135-2023.SUPPLEMENT (113.3KB, pdf)
Acknowledgments
This study used the National Health Information Database (NHIS-2019-1-662) of the National Health Insurance Service.
Provenance: Submitted article, peer reviewed.
Author contributions: H. Choi contributed to interpretation, funding acquisition, initial drafting of the manuscript, manuscript review and revision, and final approval of the version to be submitted. Y.A. Kang participated in the design of the study, performed the data analysis and interpretation, and revised the manuscript. J. Mok, D. Jeong, H-Y. Kang, H.J. Kim and H-S. Kim contributed to interpretation and critically reviewed the manuscript for important intellectual content. D. Jeon conceptualised and designed the study, acquired and interpreted the data, reviewed and revised the manuscript, and gave final approval of the version to be submitted.
Support statement: This study was financially supported by the National Evidence-based Healthcare Collaborating Agency, funded by the Ministry of Health and Welfare (grant numbers NC19-002, NC20-003 and NC21-001). Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: All authors have no potential conflicts of interest to disclose.
References
- 1.Walker IF, Shi O, Hicks JP, et al. Analysis of loss to follow-up in 4099 multidrug-resistant pulmonary tuberculosis patients. Eur Respir J 2019; 54: 1800353. doi: 10.1183/13993003.00353-2018 [DOI] [PubMed] [Google Scholar]
- 2.Andargie A, Molla A, Tadese F, et al. Lost to follow-up and associated factors among patients with drug resistant tuberculosis in Ethiopia: a systematic review and meta-analysis. PLoS One 2021; 16: e0248687. doi: 10.1371/journal.pone.0248687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law S, Daftary A, O'Donnell M, et al. Interventions to improve retention-in-care and treatment adherence among patients with drug-resistant tuberculosis: a systematic review. Eur Respir J 2019; 53: 1801030. doi: 10.1183/13993003.01030-2018 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Global Tuberculosis Report 2021. 2021. www.who.int/publications/i/item/9789240037021. Date last accessed: 25 February 2023.
- 5.Kim HJ, Hong YP, Kim SJ, et al. Ambulatory treatment of multidrug-resistant pulmonary tuberculosis patients at a chest clinic. Int J Tuberc Lung Dis 2001; 5: 1129–1136. [PubMed] [Google Scholar]
- 6.Kim DH, Kim HJ, Park SK, et al. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med 2008; 178: 1075–1082. doi: 10.1164/rccm.200801-132OC [DOI] [PubMed] [Google Scholar]
- 7.Lee M, Han J, Kim YR, et al. Multidrug-resistant tuberculosis in South Korea: a retrospective analysis of national registry data in 2011–2015. Int J Tuberc Lung Dis 2019; 23: 850–857. doi: 10.5588/ijtld.18.0658 [DOI] [PubMed] [Google Scholar]
- 8.Franke MF, Appleton SC, Bayona J, et al. Risk factors and mortality associated with default from multidrug-resistant tuberculosis treatment. Clin Infect Dis 2008; 46: 1844–1851. doi: 10.1086/588292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holtz TH, Lancaster J, Laserson KF, et al. Risk factors associated with default from multidrug-resistant tuberculosis treatment, South Africa, 1999–2001. Int J Tuberc Lung Dis 2006; 10: 649–655. [PubMed] [Google Scholar]
- 10.Kuchukhidze G, Baliashvili D, Adamashvili N, et al. Long-term mortality and active tuberculosis disease among patients who were lost to follow-up during second-line tuberculosis treatment in 2011–2014: population-based study in the country of Georgia. Open Forum Infect Dis 2021; 8: ofab127. doi: 10.1093/ofid/ofab127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank M, Adamashvili N, Lomtadze N, et al. Long-term follow-up reveals high posttreatment mortality rate among patients with extensively drug-resistant tuberculosis in the country of Georgia. Open Forum Infect Dis 2019; 6: ofz152. doi: 10.1093/ofid/ofz152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kliiman K, Altraja A. Predictors and mortality associated with treatment default in pulmonary tuberculosis. Int J Tuberc Lung Dis 2010; 14: 454–463. [PubMed] [Google Scholar]
- 13.Bohensky MA, Jolley D, Sundararajan V, et al. Data linkage: a powerful research tool with potential problems. BMC Health Serv Res 2010; 10: 346. doi: 10.1186/1472-6963-10-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong D, Kang HY, Kim J, et al. Cohort profile: Korean Tuberculosis and Post-Tuberculosis Cohort constructed by linking the Korean National Tuberculosis Surveillance System and National Health Information Database. J Prev Med Public Health 2022; 55: 253–262. doi: 10.3961/jpmph.21.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi H, Mok J, Kang YA, et al. Nationwide treatment outcomes of patients with multidrug/rifampin-resistant tuberculosis in Korea, 2011–2017: a retrospective cohort study (Korean TB-POST). J Korean Med Sci 2023; 38: e33. doi: 10.3346/jkms.2023.38.e33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang HY, Yoo H, Park W, et al. Tuberculosis notification completeness and timeliness in the Republic of Korea during 2012–2014. Osong Public Health Res Perspect 2016; 7: 320–326. doi: 10.1016/j.phrp.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JA, Yoon S, Kim LY, et al. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci 2017; 32: 718–728. doi: 10.3346/jkms.2017.32.5.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Min J, Kim HW, Ko Y, et al. Tuberculosis surveillance and monitoring under the national public–private mix tuberculosis control project in South Korea 2016–2017. Tuberc Respir Dis (Seoul) 2020; 83: 218–227. doi: 10.4046/trd.2020.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . Definitions and Reporting Framework for Tuberculosis – 2013 Revision: Updated December 2014 and January 2020. https://apps.who.int/iris/handle/10665/79199 Date last accessed: 25 February 2023.
- 20.Sterling TR, Zhao Z, Khan A, et al. Mortality in a large tuberculosis treatment trial: modifiable and non-modifiable risk factors. Int J Tuberc Lung Dis 2006; 10: 542–549. [PubMed] [Google Scholar]
- 21.Lin CH, Lin CJ, Kuo YW, et al. Tuberculosis mortality: patient characteristics and causes. BMC Infect Dis 2014; 14: 5. doi: 10.1186/1471-2334-14-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min J, Kim JS, Kim HW, et al. Clinical profiles of early and tuberculosis-related mortality in South Korea between 2015 and 2017: a cross-sectional study. BMC Infect Dis 2019; 19: 735. doi: 10.1186/s12879-019-4365-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranzani OT, Rodrigues LC, Bombarda S, et al. Long-term survival and cause-specific mortality of patients newly diagnosed with tuberculosis in São Paulo state, Brazil, 2010–15: a population-based, longitudinal study. Lancet Infect Dis 2020; 20: 123–132. doi: 10.1016/S1473-3099(19)30518-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi H, Han K, Jung JH, et al. Long-term mortality of tuberculosis survivors in Korea: a population-based longitudinal study. Clin Infect Dis 2022; 25: ciac411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romanowski K, Baumann B, Basham CA, et al. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2019; 19: 1129–1137. doi: 10.1016/S1473-3099(19)30309-3 [DOI] [PubMed] [Google Scholar]
- 26.Byrne AL, Marais BJ, Mitnick CD, et al. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis 2015; 32: 138–146. doi: 10.1016/j.ijid.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 27.Chung WS, Lin CL, Hung CT, et al. Tuberculosis increases the subsequent risk of acute coronary syndrome: a nationwide population-based cohort study. Int J Tuberc Lung Dis 2014; 18: 79–83. doi: 10.5588/ijtld.13.0288 [DOI] [PubMed] [Google Scholar]
- 28.Simonsen DF, Farkas DK, Søgaard M, et al. Tuberculosis and risk of cancer: a Danish nationwide cohort study. Int J Tuberc Lung Dis 2014; 18: 1211–1219. doi: 10.5588/ijtld.14.0161 [DOI] [PubMed] [Google Scholar]
- 29.Jensen HH, Godtfredsen NS, Lange P, et al. Potential misclassification of causes of death from COPD. Eur Respir J 2006; 28: 781–785. doi: 10.1183/09031936.06.00152205 [DOI] [PubMed] [Google Scholar]
- 30.Reeve BWP, Centis R, Theron G. Still dying in plain sight: missed and misclassified deaths due to tuberculosis in hospitals. Eur Respir J 2019; 54: 1901578. doi: 10.1183/13993003.01578-2019 [DOI] [PubMed] [Google Scholar]
- 31.Alemu A, Bitew ZW, Worku T, et al. Predictors of mortality in patients with drug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One 2021; 16: e0253848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00135-2023.SUPPLEMENT (113.3KB, pdf)