Abstract
The recent expansion of the habitat of several wildlife species, comprising anthropized areas, is a relevant risk factor for many zoonotic diseases and should be considered in national and regional sanitary monitoring systems. We evaluated adult intestinal Taenia spp. parasites isolated from wild carnivores and cystic larval forms isolated from wild mammals analysed at the Istituto Zooprofilattico Sperimentale della Lombardia ed Emilia-Romagna (IZSLER) as part of the regional wildlife sanitary surveillance plan. Then, we assessed parasite species through molecular analysis (multiplex PCR followed by ribosomal 12S subunit gene sequencing) in order to update the epidemiological situation on Taeniids infection in the Emilia-Romagna wildlife, reporting the prevalence of each isolated species. The most commonly isolated species was Taenia serialis, which we detected in both wolves and foxes as definitive hosts and in roe deer as intermediate host. More attention on the distribution of Taeniids in wildlife should be paid, considering their potential zoonotic role: several Taenia spp. (Taenia solium, Taenia multiceps, Taenia serialis, Taenia brauni, Taenia glomerulatus) are known for causing coenurosis in humans, with possible severe or fatal outcomes.
Keywords: Wildlife parasitology, Cestodes, Phylogenetic analysis
Graphical abstract
Highlights
-
•
The most common species in the Emilia-Romagna region are T. serialis and T. hydatigena.
-
•
The wolf is the main definitive host for Taenia serialis, while roe deer is the main intermediate host.
-
•
Potentially zoonotic Taenia spp. are present in Northern-Italian wildlife populations.
1. Introduction
Taeniidae are a family of cestodes whose life cycle requires two hosts: the adult form of the parasite resides in the intestinal tract of a definitive host (which can be either a strict mammalian carnivore or a human), while larvae are fluid-filled cysts which develop in the peritoneal cavity, visceral organs, skeletal muscle or central nervous system of an intermediate host (Taylor et al., 2015). Infection is trophic-related in both sylvatic and domestic cycles: in the first case, transmission occurs from preys to predators and vice versa, while in the second case humans and domestic animals are involved (Fig. 1 is a schematic representation of Taenia spp. life cycle). For the species that involve humans as definitive hosts (Taenia solium, Taenia saginata and Taenia asiatica) the intermediate hosts are specific: for T. solium and T. asiatica it is the domestic pig (Dixon et al., 2021; Galán-Puchades and Fuentes, 2013), while for T. saginata is cattle (Braae et al., 2018). The definitive host species in European wildlife include the red fox (Vulpes Vulpes), the wolf (Canis lupus), the golden jackal (Canis aureus), the European lynx (Lynx lynx), the raccoon (Procyon lotor), the beech marten (Martes foina) and the bear (Ursus arctos). A wider range of mammal species, including ruminants (Filip et al., 2019), wild boars (Sgroi et al., 2020), rodents (Bajer et al., 2020) and lagomorphs (Remesar et al., 2021) can act as intermediate hosts.
Fig. 1.
Schematic representation of Taenia spp. life cycle in wildlife with accidental involvement of humans as intermediate hosts. Animals images taken from www.phylopic.org (Authors: Tracy Heath, Ferran Sayol, Anthony Caravaggi, Katy Lawler). The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Many of the potential definitive hosts of Taenia spp. have been increasing in number in the last decades. In particular, there has been a relevant increase in wolf populations, with a direct impact on parasitic richness and diversity, as demonstrated by Lesniak et al. (2017): this could lead to an increase, at the same time, of the prevalence and abundance of different parasitic species, including Taeniidae. A similar upward trend for wolfs happened in Italy, after a period of scarce number of packs in the country, as observed in the last census carried out by the Italian Institute for Protection and Research on Environment -ISPRA- (La Morgia et al., 2022); according to the last report available, Italian wolves are estimated to be between 2945 and 3608 (95% confidence interval), occupying 41600 km2 in the Alps and 108534 km2 in the Apennines. Another noteworthy element is the ability to occupy anthropized areas, as recently described by Macchioni et al. (2021) and Coppola et al. (2022).
Red foxes are more abundant than wolves across Europe: they can establish in different habitats and have a flexible diet, so that they do not have any conservation issues (Spagnesi and De Marinis, 2002). Another relevant aspect of fox distribution is that, unlike other wildlife carnivore species, they occupy also anthropized environments (Torretta et al., 2021), increasing conversely the risk for animal-to-human transmission of zoonotic pathogens (Hassell et al., 2017).
The upward trend of wildlife populations in Europe has involved not only carnivores, but also several other mammals that can potentially act as intermediate hosts for cestodes: the wild boar (Sus scrofa), for instance, has increased dramatically throughout Europe (Apollonio et al., 2010). Moreover, all the different wild ruminant species present in Europe noticeably increased in density, with the only exception of the European fallow deer (Dama dama), whose populations remained numerically stable over time (Morellet et al., 2011). In particular, the species identified as the most overabundant in forestry settings is the roe deer (Capreolus capreolus), while red deer (Cervus elaphus) is pointed out as the most overabundant in protected areas and hunting areas across Europe (Carpio et al., 2021).
If not properly managed by regional veterinary services, uncontrolled wild animal populations can represent a threat for public health, as many wildlife species are involved in cycles of zoonotic diseases. In particular, humans can act as accidental intermediate hosts of Taenia spp, developing larval forms after ingestion of material contaminated by eggs. Human teniosis (the disease related to adult parasites in the intestine) or cysticercosis (the disease related to larval forms in internal viscera or central nervous system) have been included by the World Health Organization in the list of neglected tropical diseases. Neglected tropical diseases are a group of conditions mainly present in tropical areas, affecting more than 1 billion people who live mostly in impoverished communities. Their epidemiology is often related to environmental conditions. Despite their relevant socioeconomic impact on affected populations, they are almost absent from global health agenda, hence their definition by the term “neglected” (World Health Organization, 2017).
The most relevant form of infection for public health is the so called neurocoenurosis, characterized by the development of parasitic cysts in the central nervous system and subsequent manifestation of neurological clinical signs (headache, seizures, hemiparesis and hydrocephalus) due to an increase in intracranial pressure. Such condition has a noticeable impact on public health as surgery is generally the most effective treatment. The only species known for causing such disease are Taenia multiceps (Lescano and Zunt, 2013) and, as recently discovered, Taenia serialis (Yamazawa et al., 2020).
When evaluating the epidemiological situation about Taeniids infection in one particular area, the identification of the exact parasitic species is a pivotal piece of information, but this data can be difficult to evaluate: morphological identification of Taenia species can be made on adult parasites through injection of staining substances, such as china ink, through the genital pore of mature proglottids in order to evaluate the number of uterine branches or measuring the length of hooks in the scolex after clarification of specimen with lactophenol or acetic acid (Taylor et al., 2015). Morphological identification methods require time and expert personnel for interpretation. Moreover, such methods are reliable only if well preserved samples are obtained, and this is not always feasible with wildlife species. In veterinary medicine, available methods based on PCR for the identification of Taenia spp. include a PCR and Restriction Fragments of Length Polymorphisms (RFLP) of rDNA internal transcribed spacers 2 (ITS 2), described by Gasser and Chilton (1995), a multiplex PCR from the HDP2 DNA sequence specific for T. solium and T. saginata (González et al., 2000), a multiplex PCR for the distinction between Echinococcus granulosus, Echinococcus multilocularis and Taenia spp. (Trachsel et al., 2007), a multiplex PCR targeting the 18S rDNA and the ITS2 for species recognition within the genus Taenia (Al-Sabi and Kapel, 2011) Overall, exact species identification within the genus Taenia is possible through PCR followed by either RFLP or genome sequencing. In alternative, the multiplex PCR proposed by Al-Sabi and Kapel (2011) permits the distinction between 7 Taenia species (Taenia parva, Taenia mustelae, Taenia taeniaeformis T. multiceps, T. crassiceps, T. serialis, T. pisiformis).
We performed 12S ribosomal RNA sequencing of the samples resulted positive for Taenia spp. by PCR according to the protocol proposed by Trachsel et al. (2007) to evaluate the circulation of Taeniids in the Emilia-Romagna wildlife and providing an update on the epidemiological data about Taeniids in the region.
2. Materials and methods
2.1. Anatomopathological evaluation and sample collection
This study has considered the data obtained from 2738 carcasses of wild animals found in the Emilia-Romagna region in the period from January 2017 to December 2022.
Wild mammals analysed in this study were collected as part of the regional surveillance plan in force since 2017 (Deliberation of Emilia-Romagna Regional Council n. 1763, November 29, 2017). This plan comprises a targeted search for various infectious agents in wild animals, such as parasites (Trichinella spp., Leishmania infantum, Toxoplasma gondii), bacteria (Brucella spp., Mycobacterium spp., Francisella tularensis) and viruses (Aujeszky Disease Virus, West Nile and Usutu Virus, Influenza Virus, Newcastle Disease Virus, Classical and African Swine Fever, Blue Tongue Virus, Rabies lyssavirus), with analyses carried out on animals found dead in the natural environment, deceased at wildlife rescue centres, or culled according to control plans of certain species. The examinations include anatomopathological evaluation and laboratory analysis.
Carcasses or samples were accompanied by a form reporting relevant information such as geolocalization data, sex and age of animals. Forms are reported in the supplementary materials section (Fig. 1, Fig. 2, Fig. 3, in Italian).
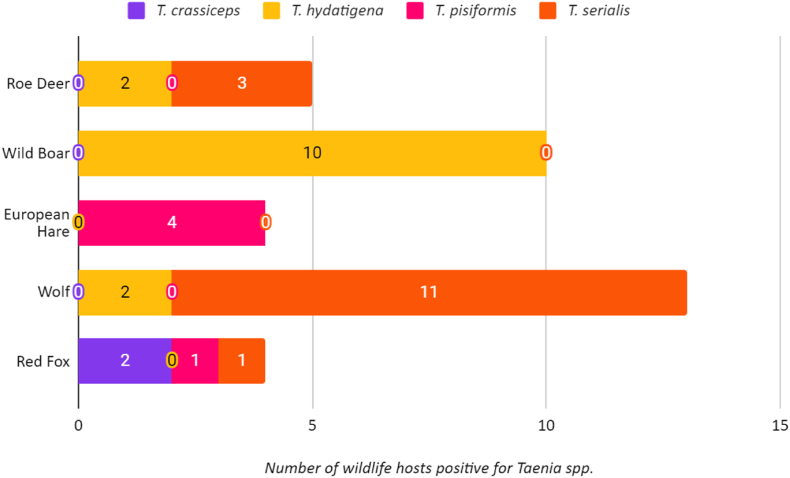
Fig. 2.
– Distribution of different Taenia species in each host species considered.
Fig. 3.
Distribution of wildlife species in which each Taenia spp. were detected.
Necropsies were performed by veterinarians working at the IZSLER (Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia-Romagna) following a common protocol, derived from the method proposed by King et al. (2014). When cestodes were found at necropsy, the adult stages from definitive hosts or larval forms from intermediate hosts were carefully collected with forceps, paying attention not to disrupt them, and then transferred in plastic containers stored at −20 °C for PCR identification.
2.2. Molecular analysis
For PCR analysis, 59 samples were diluted in sterile PBS at a 10% weight/volume ratio and then homogenised. DNA was extracted from samples (either adult cestodes or cystic forms) through a commercial kit (DNeasy Blood & Tissue Kit, QIAGEN) and amplified through a multiplex polymerase chain reaction, following the protocol described by Trachsel et al. (2007): for E. multilocularis identification, primers Cest 1 and Cest 2 were used to amplify a 395 bp sequence of the mitochondrial gene Nad1 (NADH dehydrogenase subunit 1); for E. granulosus and Taenia spp., a common reverse primer (Cest 5) and two specific reverse primers (Cest 3 and Cest 4, for E. granulosus and Taenia sp., respectively) were used to amplify a 267 bp sequence of 12S ribosomal RNA gene (rrnS) in Taenia sp. and a 117 bp sequence in E. granulosus.
For 100 μL of primer mix, 20 μL of Cest5 10 μL of Cest4, 10 μL of Cest3, 10 μL of Cest1 and 10 μL of Cest2 were added to 40 μL of DNAse-free water. A detail on each primer sequence can be found in Table 1.
Table 1.
Detailed description of primers used for multiple PCR.
| Primer | Target species | Sequence (5’ → 3′) | Forward (F)/Reverse (R) | Size (bp) | Reference |
|---|---|---|---|---|---|
| Cest1 | E. multilocularis | TGC TGA TTT GTT AAA GTT AGT GAT | F | 25 | Trachsel et al. (2007) |
| Cest2 | E. multilocularis | CAT AAA TCA ATG GAA ACA ACA ACA AG | R | 26 | Trachsel et al. (2007) |
| Cest3 | Taenia spp. | YGA YTC TTT TTA GGG GAA GGT GTG | F | 24 | Trachsel et al. (2007) |
| Cest4 | E. granulosus | GTT TTT GTG TGT TAC ATT AAT AAG GGT G | F | 28 | Trachsel et al. (2007) |
| Cest5 | Taenia spp. | GCG GTG TGT ACM TGA GCT AAA C | R | 22 | Trachsel et al. (2007) |
For each primer, a working concentration of 100 pmol/μl was obtained, while the concentration of the DNA templates was 2.5 mM for each dNTP.
The thermal profile of PCR is the following:
-
•
1 cycle of denaturation (5 min at 95 °C)
-
•
40 cycles of denaturation (30 s at 95 °C) + annealing (30 s at 52 °C) + extension (45 s at 72 °C)
-
•
1 cycle of final extension (8 min at 72 °C).
Samples testing positive for Taenia spp. were then further analysed to identify the species, by automatic Sanger sequencing of the amplified fragments of PCR through the Applied Biosystems platform (Genetic Analyzer 3500xl, Life Technologies). For sequencing, the Big dye terminator ready reaction v1.1 kit (Life Technologies) was used, while purification of sequencing products was obtained through the BigDye Xterminator Purification kit (Life Technologies).
The software BEAST2.6 (Bouckaert et al., 2019), which perform Bayesian phylogenetic analysis of molecular sequences, was used to infer the phylogenetic tree. The HKJ + G substitution model was chosen according to the model selection function of MEGAX software (Tamura et al., 2021): the number of iterations was selected to ensure an ESS Effective Sampling Size >200, as observed in Tracer. With the coalescent constant population as prior, the chain length was established at 10 million of iterations. The phylogenetic tree was then created using FigTree software v. 1.4.4 (available at http://tree.bio.ed.ac.uk/software/figtree/). Our sequences were compared to other publicly available on GenBank, the details of which are reported in Table 2.
Table 2.
- GenBank sequences used for molecular analysis.
| Species | GenBank submission code | Host | Geographical origin of samples |
|---|---|---|---|
| Taenia serialis | MF495483 | Golden Jackal (Canis aureus) | Croatia |
| MF495484 | Golden Jackal (Canis aureus) | Croatia | |
| MF495485 | Golden Jackal (Canis aureus) | Croatia | |
| DQ104238 | Coyote (Canis latrans) | USA (California) | |
| EU219546 | Dog (Canis lupus familiaris) | Germany | |
| KP965913 | Arctic fox (Vulpes lagopus) | Greenland | |
| Taenia pisiformis | AB031353 | n/d | Japan |
| AB329716 | Japanese hare (Lepus brachyurus angustidens) | Japan | |
| AB704403 | Dog (Canis lupus familiaris) | Japan | |
| DQ104227 | European rabbit (Oryctolagus cuniculus) | USA (California) | |
| DQ104229 | Coyote (Canis latrans) | USA (California) | |
| DQ104233 | Coyote (Canis latrans) | USA (California) | |
| GU569096 | Dog (Canis lupus familiaris) | China | |
| NC_013844 | Dog (Canis lupus familiaris) | China | |
| Taenia crassiceps | AB031358 | n/d | Japan |
| AF216699 | n/d | USA | |
| KP965908 | Arctic fox (Vulpes lagopus) | Greenland | |
| KP965910 | Arctic fox (Vulpes lagopus) | Greenland | |
| KP965911 | Arctic fox (Vulpes lagopus) | Greenland | |
| MN505206 | Red Fox (Vulpes Vulpes) | Poland | |
| NC_002547 | n/d | USA | |
| Taenia hydatigena | KX094340 | Sheep (Ovis aries) | Iran |
| LC672187 | Goat (Capra aegagrus hircus) | Bangladesh | |
| LC672189 | Goat (Capra aegagrus hircus) | Bangladesh | |
| LC749827 | Sheep (Ovis aries) | Iraq | |
| MK858250 | Sheep (Ovis aries) | Iraq | |
| MT784876 | Sheep (Ovis aries) | China |
2.3. Map creation
We obtained the coordinates of the centroids of each municipality in which wild animals were found from the Geonames database. Such coordinates were then inserted in QGIS software v. 3.30.1: the basemap was obtained from ©MapTiler service.
3. Results
3.1. Prevalence of Taenia spp. findings
Among all the carcasses of wildlife animals we evaluated in the period of time considered, we found tapeworms macroscopically compatible with Taeniids in 31 carnivores, and 16 of them (12 wolves and four red foxes) were confirmed as Taenia spp. by PCR. 3 distinct adult cestodes were extracted from 1 wolf, so we analysed a total of 33 adult Taeniids from 31 animals.
Conversely, cystic formations macroscopically compatible with Taeniids larvae were found in 26 mammals, and PCR revealed positivity for Taenia spp. in 19 samples (10 wild boars, five roe deer, four hares).
Taenia spp. was confirmed in 35 animals (1.28% of animals examined at the Institute under the wildlife sanitary surveillance plan, as detailed in Table 3).
Table 3.
Positivity for Taenia spp. in different species. Animals tested for Taenia spp. by PCR were the animals in which either adult cestodes or cystic larval forms were found at necropsy.
| Species tested | Number of carcasses analysed | Animals tested for Taenia spp. by PCR | Animals positive for Taenia spp. (PCR) | % of animals positive for Taenia spp. |
|---|---|---|---|---|
| Roe Deer (Capreolus capreolus) | 1227 | 8 | 5 | 0.41% (CI 95%: 0.05–0.76%) |
| Red Fox (Vulpes vulpes) | 575 | 5 | 4 | 0.70% (CI 95%: 0.02–1.38%) |
| Brown hare (Lepus europaeus) | 446 | 7 | 4 | 0.90% (CI 95%: 0.02–1.77%) |
| Wild boar (Sus scrofa) | 238 | 11 | 10 | 4.20% (CI 95%: 1.65–6.75%) |
| European hedgehog (Erinaceus europaeus) | 153 | 1 | 0 | 0% |
| Wolf (Canis lupus lupus) | 90 | 23 | 13 | 14.44% (CI 95%: 7.18–21.71%) |
| Beech marten (Martes foina) | 6 | 1 | 0 | 0% |
| Golden jackal (Canis aureus) | 2 | 2 | 0 | 0% |
| European dormouse (Glis glis) | 1 | 1 | 0 | 0% |
| TOTAL | 2738 | 59 | 36 | 1.28% (CI 95%: 0.89–1.74%) |
According to our data, the most frequently isolated species in the Emilia-Romagna region in the period considered was T. serialis, found in 11 wolves, one fox and three roe deer, followed by T. hydatigena, which was identified in two wolves, 10 wild boars and two roe deer. Other less frequently isolated species were T. pisiformis (isolated from four hares and one fox) and T. crassiceps (isolated from two foxes). In this calculation is included one wolf, which resulted infested by both T. serialis and T. hydatigena.
The percentage of isolation of each Taenia species is represented in the graph reported in Fig. 4.
Fig. 4.
Percentage of isolation of different Taenia species.
The locations of the carcasses resulted positive for Taenia spp. are reported in the map in Fig. 5.
Fig. 5.
Geographical location of animals resulted positive for Taenia spp. Icons placed around circles are located in the same position, represented by the yellow point. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Phylogenetic analysis
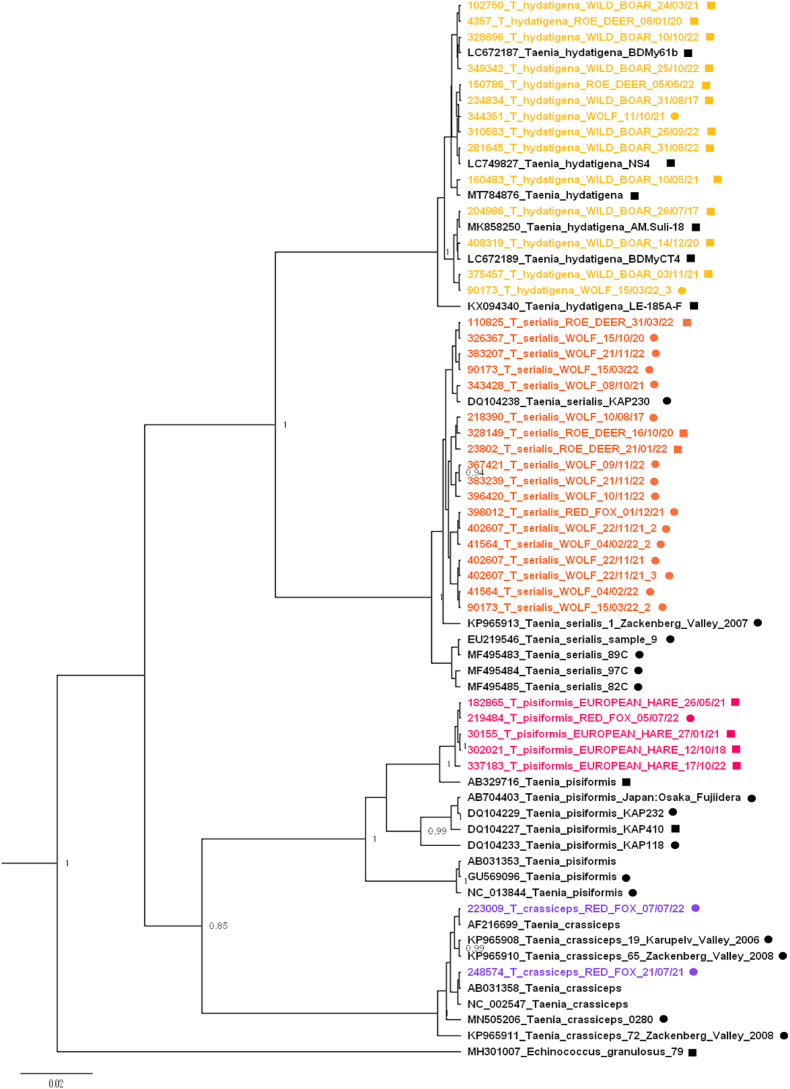
According to the results of phylogenetic analysis, different Taenia species form homogeneous clades, with well-supported nodes as can be seen in the phylogenetic tree in Fig. 6. In particular, the sequences highlighted with different colours are the ones we analysed at the IZSLER (one colour per species), while the sequences highlighted in black text are the publicly deposited sequences we extracted from GenBank. A detailed list of the GenBank accession codes for the sequences analysed at IZSLER is reported in Tables 1–4 in the supplementary material section.
Fig. 6.
Phylogenetic tree with the sequences of small ribosomal RNA subunit (rrnS) of T.hydatigena (yellow), T. serialis (orange), T. pisiformis (magenta), T. crassiceps (purple) sequenced at the IZSLER, compared with public sequences deposited in GenBank (black). The tree was obtained using the HKJ + G substitution model. Chain length = 10 million iterations. Node labels are posterior probabilities ≥0.9. Squares = sequence from intermediate host; circles = sequence from definitive host. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
According to our results, different species of cestodes belonging to the genus Taenia circulate in the wildlife of northern Italy. In particular, our data suggest that the main cycle of T. serialis (the most frequently isolated species) in the Emilia-Romagna region mainly involves wolves as definitive hosts and roe deer as intermediate hosts. Such a sylvatic cycle has already been hypothesized in the article presented by Morandi et al. (2021), who studied a case of neurocenurosis in a roe deer from central Italy, in an area bordering the Emilia-Romagna region, with similar geographical characteristics. The trophic transmission from roe deer to wolves is supported by studies that question the common feeding behaviour of wolves in Europe. In particular, Sin et al. (2019) and Guimarães et al. (2022) agreed that wild ungulates are the main components of their diet. In addition to wolves, we also identified such parasite species in a red fox, which can act as a definitive host as previously reported in northeastern Italy by Citterio et al. (2021).
To our knowledge, the only other reported case of T. serialis infection in Italian wolves was reported by Crotti et al. (2023), while the prevalence of the same parasite in foxes was estimated to be relatively low (0.03% according to the study published by Citterio et al. in 2021). In Europe, T. serialis has been reported in wolves from Portugal (Pereira et al., 2023) with a prevalence of 5.9% and from Serbia (Ćirović et al., 2015) with a prevalence of 1.0%. Our data on the prevalence of T. serialis (12.23% ± 0.07 95% CI), although lower than the prevalence reported by Crotti et al. (2023), 21.6%, is still the most common Taenia spp. isolated from wolves in our study. These data differ from previous literature on T. serialis in wildlife, as the most commonly reported intermediate hosts were lagomorphs, such as hares and rabbits (Pfaffenberger and Valencia, 1988; Zhang et al., 2018).
We identified adult forms of T. hydatigena mainly in wolves and larval stages of the same parasite species in the wild boar, a common prey of wolf packs in Italy. This parasite species has already been reported in wolves from Italy (Gori et al., 2015, Poglayen et al., 2017; Poglayen et al., 2017) and in wild boar from Estonia (Järvis et al., 2007), Slovakia (Jarošová et al., 2022), Poland (Filip et al., 2019), Germany (Barutzki et al., 1991) and Spain (De La Muela et al., 2001). T. hydatigena has been frequently reported in Italian wild boars from central Italy by Di Nicola et al. (2015) and by Paoletti et al. (2019), and in southern Italy by Sgroi et al. (2020). According to our data, T. hydatigena was detected in 4.2% of the analysed boars, and the prevalence is within the range reported in the Italian literature (3.4%–15%).
T. hydatigena was found in almost all provinces of Emilia-Romagna, probably due to the wide spatial distribution of wild boar populations in the region. Conversely, T. serialis was mostly restricted to the contiguous provinces of Piacenza, Parma and Reggio Emilia, as in the hilly and mountainous areas of these provinces more wolves are reported (La Morgia et al., 2022).
Another wild carnivore, the red fox, is the main definitive host for T. pisiformis, another parasite species we have detected. In Europe, this species has already been reported in Slovenia (Vergles Rataj et al., 2013), Germany (Loos-Frank and Zeyhle, 1982), France (Petavy and Deblock, 1980) and Spain (Sanchis-Monsonís et al., 2020). We also identified larval stages of the same parasite species in a common prey of red foxes, the European hare, as investigated in the review conducted by Soe et al. (2017). In particular, predation on lagomorphs is more common in areas with a higher human footprint index.
One of the main limitations of our study is that the finding of adult cestodes in the intestine of definitive hosts, as well as larval stages in the muscle or visceral organs of intermediate hosts, was only incidental. This is because the regional wildlife surveillance plan does not require the opening of the intestines of carnivores, and low levels of infection in intermediate hosts may be challenging to detect, particularly with muscular localisation of larval stages. Since we only sampled cestodes from obvious cases of infection, the calculated prevalence of Taenia spp. is likely to be underestimated. We have, however, proved that different Taenia spp. are circulating in wildlife from the geographical area we investigated, even though additional research is needed to evaluate the exact prevalence of each species.
Another constraint of the study is dependent on molecular techniques: we adopted the PCR protocol that is normally used in diagnostics to determine the presence of Taenia spp. in samples, but such analysis can have limited efficiency if multiple targets are amplified. Furthermore, despite the sequences we used for species recognition are highly conserved, they are short in size and provide less information compared to whole genome sequencing.
5. Conclusion
Implementing a parasitological examination of the animals in the plan for sanitary assessment of wildlife species is crucial to gather reliable epidemiological data on the prevalence of parasitic infections in a geographical area. At present, wildlife surveillance in Italy is carried out and organized at regional level, so that the prioritized diseases searched in animals are not the same across different regions. Identification of Taenia spp. is not a requested analysis, so it represents an accidental finding most of the times.
We demonstrated that the life cycle of T. serialis can be maintained in this area of Italy, as the parasite has been found in both intermediate and definitive hosts. Wolves are capable of preying on roe deer, so transmission through a prey-predator cycle is likely. The recent increase in density of both species (La Morgia et al., 2022) sets a favourable condition for T. serialis and Taeniids diffusion (Lesniak et al., 2017). Moreover, the impact of urbanization on increasing the probability of spillover from sylvatic to domestic cycles of many pathogens should be considered (Hassell et al., 2017).
This finding is particularly important for parasitic species, such as T. serialis itself, which could be transmitted to humans by ingestion of material contaminated by faeces of definitive hosts. The recent occupation by different wildlife carnivores of anthropized areas is a relevant risk factor for transmission to humans. Another factor to consider is the possibility of infection by domestic dogs, which living in strict contact with humans noticeably increase the risk of transmission (Mutwiri et al., 2023). The zoonotic potential of T. serialis has been only recently demonstrated (Yamazawa et al., 2020), but previous clinical reports in non-human primates had already been published in past years (Schneider-Crease et al., 2017; Deplazes et al., 2019). Therefore, a systematic and more detailed search for parasites of wildlife with relevance for public health is required and desirable in the future planning for sanitary monitoring of wildlife in Italy.
Declaration of competing interest
The authors declare no conflict of interest.
Funding
This work was supported by the Regional Institutes of Health of the Emilia-Romagna region: the analyses were executed under the regional surveillance plan in force (Deliberation of Emilia-Romagna Regional Council n. 1763, November 29, 2017).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2023.08.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Al-Sabi M.N.S., Kapel C.M.O. Multiplex PCR identification of Taenia spp. in rodents and carnivores. Parasitol. Res. 2011;109:1293–1298. doi: 10.1007/s00436-011-2373-9. [DOI] [PubMed] [Google Scholar]
- Apollonio M., Andersen R., Putman R. Cambridge University Press; 2010. European Ungulates and Their Management in the 21st Century. [Google Scholar]
- Bajer A., Alsarraf Mohammed, Dwużnik D., Mierzejewska E.J., Kołodziej-Sobocińska M., Behnke-Borowczyk J., Banasiak Ł., Grzybek M., Tołkacz K., Kartawik N., Stańczak Ł., Opalińska P., Krokowska-Paluszak M., Górecki G., Alsarraf Mustafa, Behnke J.M. Rodents as intermediate hosts of cestode parasites of mammalian carnivores and birds of prey in Poland, with the first data on the life-cycle of Mesocestoides melesi. Parasites Vectors. 2020;13:95. doi: 10.1186/s13071-020-3961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barutzki D., Schoierer R., Gothe R. Helminth infections in wild boars kept in enclosures in southern Germany: severity of infections and fecal intensity. Tierarztl. Prax. 1991;19:644–648. [PubMed] [Google Scholar]
- Bouckaert R., Vaughan T.G., Barido-Sottani J., Duchêne S., Fourment M., Gavryushkina A., Heled J., Jones G., Kühnert D., De Maio N., Matschiner M., Mendes F.K., Müller N.F., Ogilvie H.A., du Plessis L., Popinga A., Rambaut A., Rasmussen D., Siveroni I., Suchard M.A., Wu C.-H., Xie D., Zhang C., Stadler T., Drummond A.J. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput Biol. 2019;15 doi: 10.1371/journal.pcbi.1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braae U.C., Thomas L.F., Robertson L.J., Dermauw V., Dorny P., Willingham A.L., Saratsis A., Devleesschauwer B. Epidemiology of Taenia saginata taeniosis/cysticercosis: a systematic review of the distribution in the Americas. Parasites Vectors. 2018;11:518. doi: 10.1186/s13071-018-3079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpio A.J., Apollonio M., Acevedo P. Wild ungulate overabundance in Europe: contexts, causes, monitoring and management recommendations. Mamm Rev. 2021;51:95–108. [Google Scholar]
- Ćirović D., Pavlović I., Penezić A. Intestinal helminth parasites of the grey wolf (Canis lupus L.) in Serbia. Acta Vet. Hung. 2015;63:189–198. doi: 10.1556/AVet.2015.016. [DOI] [PubMed] [Google Scholar]
- Citterio C.V., Obber F., Trevisiol K., Dellamaria D., Celva R., Bregoli M., Ormelli S., Sgubin S., Bonato P., Da Rold G., Danesi P., Ravagnan S., Vendrami S., Righetti D., Agreiter A., Asson D., Cadamuro A., Ianniello M., Capelli G. Echinococcus multilocularis and other cestodes in red foxes (Vulpes vulpes) of northeast Italy, 2012-2018. Parasites Vectors. 2021;14:29. doi: 10.1186/s13071-020-04520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola F., Baldanti S., Di Rosso A., Vecchio G., Casini L., Russo C., Lucchini V., Boni C.B., Malasoma M., Gabbani C., Felicioli A. Settlement of a stable wolf pack in a highly anthropic area of Pisan hills: relationship with animal husbandry and hunting in a human-wolf coexistence perspective. Anim. Sci. J. 2022;93 doi: 10.1111/asj.13799. [DOI] [PubMed] [Google Scholar]
- Crotti S., Spina S., Cruciani D., Bonelli P., Felici A., Gavaudan S., Gobbi M., Morandi F., Piseddu T., Torricelli M., Morandi B. Tapeworms detected in wolf populations in Central Italy (Umbria and Marche regions): a long-term study. Int. J. Parasitol. Parasites Wildl. 2023;21:11–16. doi: 10.1016/j.ijppaw.2023.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Muela N., Hernández-de-Luján S., Ferre I. Helminths of wild boar in Spain. J. Wildl. Dis. 2001;37:840–843. doi: 10.7589/0090-3558-37.4.840. [DOI] [PubMed] [Google Scholar]
- Deplazes P., Eichenberger R.M., Grimm F. Wildlife-transmitted Taenia and Versteria cysticercosis and coenurosis in humans and other primates. Int. J. Parasitol. Parasites Wildl. 2019;9:342–358. doi: 10.1016/j.ijppaw.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nicola U., Scacchia M., Marruchella G. Pathological and serological findings in wild boars (Sus scrofa) from gran Sasso and Monti della Laga national Park (Central Italy) Large Anim. Rev. 2015;21:167–171. [Google Scholar]
- Dixon M.A., Winskill P., Harrison W.E., Basáñez M.-G. In: Adv Parasitol. Rollinson D., Stothard R., editors. Academic Press; 2021. Chapter Four - Taenia solium taeniasis/cysticercosis: from parasite biology and immunology to diagnosis and control; pp. 133–217. [DOI] [PubMed] [Google Scholar]
- Filip K.J., Pyziel A.M., Jeżewski W., Myczka A.W., Demiaszkiewicz A.W., Laskowski Z. First molecular identification of Taenia hydatigena in wild ungulates in Poland. EcoHealth. 2019;16:161–170. doi: 10.1007/s10393-019-01392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán-Puchades M.T., Fuentes M.V. Taenia asiatica: the most neglected human Taenia and the possibility of cysticercosis. Kor. J. Parasitol. 2013;51:51–54. doi: 10.3347/kjp.2013.51.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser R.B., Chilton N.B. Characterisation of taeniid cestode species by PCR-RFLP of ITS2 ribosomal DNA. Acta Trop. 1995;59:31–40. doi: 10.1016/0001-706x(94)00085-f. [DOI] [PubMed] [Google Scholar]
- González L.M., Montero E., Harrison L.J.S., Parkhouse R.M.E., Garate T. Differential diagnosis of Taenia saginata and Taenia solium infection by PCR. J. Clin. Microbiol. 2000;38:737–744. doi: 10.1128/jcm.38.2.737-744.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori F., Armua-Fernandez M.T., Milanesi P., Serafini M., Magi M., Deplazes P., Macchioni F. The occurrence of taeniids of wolves in Liguria (northern Italy) Int. J. Parasitol. Parasites Wildl. 2015;4:252–255. doi: 10.1016/j.ijppaw.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães N.F., Álvares F., Ďurová J., Urban P., Bučko J., Iľko T., Brndiar J., Štofik J., Pataky T., Barančeková M., Kropil R., Smolko P. What drives wolf preference towards wild ungulates? Insights from a multi-prey system in the Slovak Carpathians. PLoS One. 2022;17 doi: 10.1371/journal.pone.0265386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J.M., Begon M., Ward M.J., Fèvre E.M. Urbanization and disease emergence: dynamics at the wildlife–livestock–human interface. Trends Ecol. Evol. 2017;32:55–67. doi: 10.1016/j.tree.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarošová J., Antolová D., Iglodyová A., Königová A., Dolinská M.U., Víchová B. Molecular identification of Taenia hydatigena from domestic and free-living animals in Slovakia, Central Europe. Parasitol. Res. 2022;121:1345–1354. doi: 10.1007/s00436-022-07481-z. [DOI] [PubMed] [Google Scholar]
- Järvis T., Kapel C., Moks E., Talvik H., Mägi E. Helminths of wild boar in the isolated population close to the northern border of its habitat area. Vet. Parasitol. 2007;150:366–369. doi: 10.1016/j.vetpar.2007.09.015. [DOI] [PubMed] [Google Scholar]
- King J.M., Roth-Johnson L., Dodd D.C., Newsom M.E. The Internet-First University Press; 2014. The Necropsy Book: A Guide for Veterinary Students, Residents, Clinicians, Pathologists, and Biological Researchers. [Google Scholar]
- La Morgia V., Marucco F., Aragno P., Salvatori V., Gervasi V., De Angelis D., Fabbri E., Caniglia R., Velli E., Avanzinelli E., Boiani M.V., Genovesi P. 2022. Stima della distribuzione e consistenza del lupo a scala nazionale 2020/2021. [Google Scholar]
- Lescano A.G., Zunt J. In: Handbook of Clinical Neurology, Neuroparasitology and Tropical Neurology. Garcia H.H., Tanowitz H.B., Del Brutto O.H., editors. Elsevier; 2013. Chapter 27 - other cestodes: sparganosis, coenurosis and Taenia crassiceps cysticercosis; pp. 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak I., Heckmann I., Heitlinger E., Szentiks C.A., Nowak C., Harms V., Jarausch A., Reinhardt I., Kluth G., Hofer H., Krone O. Population expansion and individual age affect endoparasite richness and diversity in a recolonising large carnivore population. Sci. Rep. 2017;7 doi: 10.1038/srep41730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos-Frank B., Zeyhle E. The intestinal helminths of the red fox and some other carnivores in southwest Germany. Z. Parasitenkd. 1982;67:99–113. doi: 10.1007/BF00929518. [DOI] [PubMed] [Google Scholar]
- Macchioni F., Coppola F., Furzi F., Gabrielli S., Baldanti S., Boni C.B., Felicioli A. Taeniid cestodes in a wolf pack living in a highly anthropic hilly agro-ecosystem. Parasite. 2021;28:10. doi: 10.1051/parasite/2021008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi B., Bazzucchi A., Gambini S., Crotti S., Cruciani D., Morandi F., Napoleoni M., Piseddu T., Di Donato A., Gavaudan S. A novel intermediate host for Taenia serialis (Gervais, 1847): the European roe deer (Capreolus capreolus L. 1758) from the Monti Sibillini National Park (MSNP), Italy. Int. J. Parasitol. Parasites Wildl. 2021;17:110–113. doi: 10.1016/j.ijppaw.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morellet N., Klein F., Solberg E., Andersen R. In: Ungulate Management in Europe: Problems and Practices. Apollonio M., Andersen R., Putman R., editors. Cambridge University Press; Cambridge: 2011. The census and management of populations of ungulates in Europe; pp. 106–143. [Google Scholar]
- Mutwiri T., Muigai A.W.T., Magambo J., Mulinge E., Gitau L., Muinde P., Bettridge J.M., Rogan M., Fèvre E.M., Falzon L.C. The potential role of roaming dogs in establishing a geographically novel life cycle of taeniids (Echinococcus spp. and Taenia spp.) in a non-endemic area. Vet. Parasitol. Reg. Stud. Rep. 2023;38 doi: 10.1016/j.vprsr.2022.100829. [DOI] [PubMed] [Google Scholar]
- Paoletti B., Della Salda L., Di Cesare A., Iorio R., Vergara A., Fava C., Olivastri A., Dessì G., Scala A., Varcasia A. Epidemiological survey on cystic echinococcosis in wild boar from Central Italy. Parasitol. Res. 2019;118:43–46. doi: 10.1007/s00436-018-6112-3. [DOI] [PubMed] [Google Scholar]
- Pereira A.L., Mateus T.L., Llaneza L., Vieira-Pinto M.M., Madeira de Carvalho L.M. Gastrointestinal parasites in Iberian wolf (Canis lupus signatus) from the Iberian Peninsula. Parasitologia. 2023;3:15–32. [Google Scholar]
- Petavy A.F., Deblock S. [Helminths of the common fox (Vulpes vulpes L.) from the massif central (France) (author's transl)] Ann. Parasitol. Hum. Comp. 1980;55:379–391. [PubMed] [Google Scholar]
- Pfaffenberger G.S., Valencia V.B. Helminths of sympatric black-tailed Jack rabbits (Lepus californicus) and desert Cottontails (Sylvilagus audubonii) from the high Plains of eastern New Mexico. J. Wildl. Dis. 1988;24:375–377. doi: 10.7589/0090-3558-24.2.375. [DOI] [PubMed] [Google Scholar]
- Poglayen G., Gori F., Morandi B., Galuppi R., Fabbri E., Caniglia R., Milanesi P., Galaverni M., Randi E., Marchesi B., Deplazes P. Italian wolves (Canis lupus italicus Altobello, 1921) and molecular detection of taeniids in the foreste Casentinesi national Park, northern Italian Apennines. Int. J. Parasitol. Parasites Wildl. 2017;6:1–7. doi: 10.1016/j.ijppaw.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remesar S., Castro-Scholten S., Jiménez-Martín D., Camacho-Sillero L., Morrondo P., Rouco C., Gómez-Guillamón F., Cano-Terriza D., García-Bocanegra I. Spatiotemporal monitoring of Cysticercus pisiformis in European wild rabbit (Oryctolagus cuniculus) in Mediterranean ecosystems in southern Spain. Prev. Vet. Med. 2021;197 doi: 10.1016/j.prevetmed.2021.105508. [DOI] [PubMed] [Google Scholar]
- Sanchis-Monsonís G., Fanelli A., Martínez-Carrasco C., Tizzani P. The typical cestodes of the red fox in eastern areas of the Iberian Peninsula have a grouped distribution. Vet. Parasitol. 2020;283 doi: 10.1016/j.vetpar.2020.109168. [DOI] [PubMed] [Google Scholar]
- Schneider-Crease I., Griffin R.H., Gomery M.A., Dorny P., Noh J.C., Handali S., Chastain H.M., Wilkins P.P., Nunn C.L., Snyder-Mackler N., Beehner J.C., Bergman T.J. Identifying wildlife reservoirs of neglected taeniid tapeworms: non-invasive diagnosis of endemic Taenia serialis infection in a wild primate population. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgroi G., Varcasia A., D'Alessio N., Varuzza P., Buono F., Amoroso M.G., Boufana B., Otranto D., Fioretti A., Veneziano V. Taenia hydatigena cysticercosis in wild boar (Sus scrofa) from southern Italy: an epidemiological and molecular survey. Parasitology. 2020;147:1636–1642. doi: 10.1017/S0031182020001559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin T., Gazzola A., Chiriac S., Rîșnoveanu G. Wolf diet and prey selection in the South-eastern Carpathian Mountains, Romania. PLoS One. 2019;14 doi: 10.1371/journal.pone.0225424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe E., Davison J., Süld K., Valdmann H., Laurimaa L., Saarma U. Europe-wide biogeographical patterns in the diet of an ecologically and epidemiologically important mesopredator, the red fox Vulpes vulpes: a quantitative review. Mamm Rev. 2017;47:198–211. [Google Scholar]
- Spagnesi M., De Marinis A.M. 2002. Mammiferi d'Italia. Ministero dell’ambiente e della tutela del territorio, Direzione conservazione della natura. [Google Scholar]
- Tamura K., Stecher G., Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.A., Coop R.L., Richard L.W. Veterinary Parasitology. Wiley-Blackwell; 2015. Chapter 12: parasites of dogs and cats. [Google Scholar]
- Torretta E., Riboldi L., Costa E., Delfoco C., Frignani E., Meriggi A. Niche partitioning between sympatric wild canids: the case of the golden jackal (Canis aureus) and the red fox (Vulpes vulpes) in north-eastern Italy. BMC Ecol. Evol. 2021;21:129. doi: 10.1186/s12862-021-01860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel D., Deplazes P., Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134:911–920. doi: 10.1017/S0031182007002235. [DOI] [PubMed] [Google Scholar]
- Vergles Rataj A., Posedi J., Zele D., Vengušt G. Intestinal parasites of the red fox (Vulpes vulpes) in Slovenia. Acta Vet. Hung. 2013;61:454–462. doi: 10.1556/AVet.2013.029. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2017. Integrating Neglected Tropical Diseases into Global Health and Development: Fourth WHO Report on Neglected Tropical Diseases. [Google Scholar]
- Yamazawa E., Ohno M., Satomi K., Yoshida A., Miyakita Y., Takahashi M., Satomi N., Asanome T., Maeshima A., Shiotsuka M., Iwata S., Yamasaki H., Morishima Y., Sugiyama H., Narita Y. First case of human neurocoenurosis caused by Taenia serialis: a case report. Int. J. Infect. Dis. 2020;92:171–174. doi: 10.1016/j.ijid.2020.01.004. [DOI] [PubMed] [Google Scholar]
- Zhang X.-Y., Jian Y.-N., Ma L.-Q., Li X.-P., Karanis P. A case of coenurosis in a wild rabbit (Lepus sinensis) caused by Taenia serialis Metacestode in Qinghai Tibetan plateau area, China. Kor. J. Parasitol. 2018;56:195–198. doi: 10.3347/kjp.2018.56.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.