Abstract
AIM:
Examine risk of Alzheimer’s disease and related dementia (ADRD) among adults with cerebral palsy (CP).
METHOD:
Using 2007-2017 administrative insurance claims data in the U.S., we identified adults (45+) with a CP diagnosis (n=5,176). Adults without a CP diagnosis were included as healthy controls (n=1,119,131). Using age, sex, race/ethnicity, other demographic variables, and a set of chronic morbidities, we propensity matched individuals with and without CP (n=5,038). Cox survival models were used to estimate ADRD risk within a 3-year follow up.
RESULTS:
Unadjusted incidence of ADRD was 9 and 2.4 times higher among adults 45-64 (1.8%) and 65 years and older (4.8%) cohorts with CP, respectively, than their respective unmatched controls without CP (0.2% and 2.0% among 45-64 and 65+ years old, respectively). Fully adjusted survival models indicated that adults with CP had a greater hazard for ADRD (among 45-64 years old: unmatched HR: 7.48 (95% CI: 6.05-9.25), matched HR: 4.73 (95% CI: 2.72-8.29); among 65+ years old: unmatched HR: 2.21 (95% CI: 1.95-2.51), matched HR: 1.73 (1.39-2.15)).
INTERPRETATION:
Clinical guidelines for early screening of cognitive function among individuals with CP need updating and preventative and/or therapeutic services should be used to reduce risk of ADRD.
Keywords: Alzheimer’s Disease, Dementia, Cerebral Palsy
Introduction
Cerebral palsy (CP) is a congenital illness that develops from injury or abnormal development of the brain during pregnancy or shortly after birth.(1-4) Despite improvements in obstetric and neonatal care, its prevalence has been consistent over the past 50 years, remaining at 2-2.5 incidences per 1,000 births.(2) Advancements in medicine have allowed the majority of individuals with CP to live into late adulthood. However, there is limited evidence on potential long-term complications associated with CP.(1, 5) Although CP is known to negatively affect coordination and contributes to muscle stiffness and tremors (6), emerging evidence suggests that people with CP may undergo accelerated brain aging.(1, 7-9) CP pathology involves injury to or defects of the central nervous system including the frontal and prefrontal cortex, cerebellum, and other sections of the brain that control executive function, working memory, and movement.(10-12) The association of infection and inflammation with CP may put adults with CP at a heightened risk for Alzheimer’s disease and related dementia (ADRD) later in life.(2, 3, 13, 14)
ADRD is an umbrella term for a progressive cognitive spectrum disorder and is the sixth leading cause of death in the U.S.(15) Approximately 5.0 million adults aged 65 years and older were diagnosed with ADRD in 2014. This number is expected to grow to 14 million by 2060.(15) Clinical descriptions of ADRD include cognitive degeneration, particularly severe memory deterioration; struggles with time, place, and personhood; changes in behavioral patterns; decline in language; and regression in daily living.(16) Although age is considered the main risk factor for ADRD, research has shown that certain medical conditions may also increase risk. (17)
Through inflammatory responses following infection at birth or shortly after, CP may lead to large increases in cytokine production, which could put a baby’s immature brain at risk of damage.(2, 3) Furthermore, cerebral white matter injury is a common issue in children with CP. Cerebral white matter regulates neuron transmission and cognition among the regions and hemispheres of the brain. (18) Cingulum are bundles of cerebral white matter fibers that are interconnected with multiple parts of the brain, controlling motor and cognitive functions.(6, 18) Injury to cerebral white matter has been linked with cognitive decline and may increase ADRD risk among adults with CP. (1) To date, no large-scale study in the U.S. has examined early and late onset ADRD hazard in adults with CP.
In this study, we examined a private national claims database to examine time to diagnosis and adjusted hazard ratio (HR) of incident ADRD, comparing adults with and without CP. Our study highlighted a gap in understanding ADRD risk among adults with CP. Such information is needed to develop more comprehensive and patient-centered clinical guidelines for this patient population.(19) Our main hypothesis is that adults with CP are at greater risk of ADRD and have shorter ADRD-free survival compared to adults without CP.
Methods
Data Source
We used national, private administrative claims from the Clinformatics Data Mart (OptumInsight, Eden Prairie, MN). OptumInsight is a de-identified claims database capturing all healthcare encounters and reimbursement for over 80 million privately insured people throughout their enrollment. Although clinical evaluation is a gold standard for assessing ADRD risk, claims data are used extensively in epidemiological analyses.(15, 20) A medical provider allocates a diagnosis code after a clinical encounter, which is consequently recorded in insurance claims for billing purposes. Although challenges exist in underdiagnosis and misdiagnosis of certain types of dementia,(21) sensitivity and specificity of Medicare claims data for diagnosis of ADRD have been reasonable, ranging between 0.85-0.89 and 0.64-0.95, respectively. (20)
Sample Selection
This study aimed to examine early- and late-onset ADRD.(22) All adults aged 45 years or older at the time of enrollment starting from 2007 to 2017 were deemed potentially eligible for analysis. We excluded individuals with less than 12 months of continuous enrollment so that there was a sufficient history of service utilization for comorbidity history. All medical claims, excluding laboratory and outpatient pharmacy, were considered to identify required diagnosis codes for conditions of interest. A schematic flow diagram is presented in Supplementary Figure S1.
Identification of Patients with CP
Since CP is a congenital disorder, all enrolled members with CP were considered to have the condition at the time of enrollment. To allow sufficient follow-up for individuals with CP, we retained those with four or more continuous years of enrollment following their starting date of enrollment on the plan (starting from 2007), with one year of enrollment as the lookback period and three years as the follow-up. All enrolled members with a diagnosis of CP were identified using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) (Supplementary Table S1). The U.S. switched to ICD-10 codes in October 2015.(23) To ensure enough follow-up years for analysis of ADRD risk, we only used ICD-9 codes for diagnosis of CP.
We identified a healthy control group without CP using the same inclusion criteria. The additional exclusion criteria for identifying the control cohort included removal of individuals with disabling neurological disorders such as multiple sclerosis and spinal cord injury during their enrollment. We removed these conditions because research indicates they may also increase ADRD risk.(24) Furthermore, our aim was to compare ADRD risk between adults with CP and the non-neurologically complex group (healthy controls). Among the remaining individuals without CP, a 20% simple random sample (representative of enrolled members)(25) was selected to represent the control group. Post-hoc analyses of demographic and baseline characteristics were compared between the 20% random sample of controls and all controls to ensure no unintentional sampling bias was introduced due to random selection.
Outcome
The primary outcome was days to incident ADRD following 12 months of enrollment on the patient’s plan. ADRD was identified in the follow-up period using ICD-9-CM or ICD-10-CM diagnosis codes on any single claim (Supplementary Table S2). If multiple claims on different service dates were identified, the first claim service date after CP was considered the incident date of ADRD.
Covariates and Comorbidities
Basic demographic and socioeconomic variables included age, sex, race/ethnicity, Elixhauser comorbidity index,(26) U.S. Census Division, educational attainment, and net worth. We divided age into two categories (45-64 and 65+) to assess the risk for both early- and late-onset ADRD. Additionally, we identified psychological, cardiometabolic, and musculoskeletal diseases that were prevalent in the one-year lookback period before index CP to risk adjust for relevant health conditions. Psychological, cardiometabolic, and musculoskeletal conditions were identified using a single claim with appropriate ICD-9 codes (Supplementary Table S3).
Statistical Analysis
Bivariate analyses of baseline characteristics between CP patients and population control were examined for meaningful differences between groups. For categorical variables, column percentages were compared between both groups using Cohen’s h effect size calculations.(27) For large sample studies, such as those using administrative claims, the Cohen’s h effect size calculation is used since these studies are typically statistically overpowered. For continuous variables, means and standard deviations were calculated, and Cohen’s d standardized mean differences were used to ascertain clinically meaningful differences between groups.
For individuals with CP, we captured a history of all documented comorbid conditions in the year of enrollment on their insurance plan prior to the index inclusion date. For randomly sampled controls, all patients with sufficient continuous enrollment within the study period of four years were randomly assigned a time zero to begin follow-up. The selection of the randomly assigned date required one year of enrollment to collect information about comorbid conditions, and three years of post-index date follow-up to measure incident ADRD. The random assignment of the dates was determined assuming a uniform distribution across a specified interval of candidate dates that meet pre- and post-index date. This approach was used to address potential bias of selecting patients who were systematically younger during their enrollment on the plan for the sampled control group.
To examine disease-free ADRD survival of CP patients compared to controls, patients with no evidence of ADRD during the one-year lookback period were graphed with Kaplan-Meier product limit survival curves for a three-year period post-index CP diagnosis. To establish incidence in claims, we used a one-year lookback period from the index date to obtain evidence of any service utilization with a diagnosis of ADRD. Patients with ADRD in the one-year lookback period were excluded from the product-limit survival curves and other subsequent analyses.
To estimate the risk of incident ADRD, we constructed parametric Weibull survival models to estimate HR for ADRD, comparing CP patients to the control group. The rationale for using these models is explained in Supplementary Table S4 and Figure S3. First, we used a bivariate regression to estimate the unadjusted HR for ADRD comparing the CP patients to the controls. Second, to estimate the adjusted HR, we performed multivariable regression to adjust for demographics, socioeconomic, and Elixhauser comorbidity index. To assess the association of CP and age on incident ADRD, we also interacted CP (binary variable) with our categorical age variable (45-64 and 65+) (Supplementary Table S5 and Figure S4; Area Under Curve (AUC=0.81; C-Statistic (standard error (SE)): 0.80 (0.00)).
Finally, to account for selection bias attributable to CP patients, we performed propensity-score matching. Specifically, we estimated the propensity to be in the CP group using multivariable logistic regression adjusting for demographics, individual comorbidities, and socioeconomic variables. We used a one-to-one caliper matching algorithm with caliper size of 0.0001 without replacement. To assess covariate balance, post-matched analyses of effect sizes were compared between cases (CP) and controls. All patients were right censored if they did not experience ADRD in the follow-up period or disenrolled from the plan (Supplementary Table S6 and Figure S5; AUC=0.81; C-Statistic (SE): 0.80 (0.01)).
This study was deemed exempt by the Institutional Review Board at the University of Michigan. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). All HRs included a calculation of the 95% confidence interval (CI). Statistical testing was two-tailed with a significance level of 0.05. Effect sizes used a 0.2 meaningful difference cutoff.
Results
Table 1 presents unadjusted characteristics of individuals with and without CP within our unmatched and matched cohorts during the 1-year lookback period. Average number of enrollment years for those with and without CP was 8.0 (standard deviation (SD)=3.5) and 7.7 (SD=3.4) years, respectively. Most individuals were in the 45–64-year-old age group [with CP: 62.9% (n=3,256); without CP: 55.0% (n=615,964)]. Females represent 50.4% (n=2,608) and 53.6% (n=599,699) of adults with and without CP, respectively. There were no substantial differences in race/ethnicity between adults with and without CP, with White being the majority in both groups [case: 60.9% (n=3,151); control: 63.4% (n=709,899)]. There were no large differences between case and control groups in their education. For example, about 53.4% (n=2,763) of people with CP vs. 52.7% (n=589,285) of people without had fewer than a 4-year college degree. People without CP had a higher net worth. For example, 16.9% (n=851) of people with CP vs. 26.5% (n=296,541) of those without had a net worth of more than $500K. There were no effect size differences between our post-matched case and control groups.
Table 1.
Descriptive Characteristics of Adults with (Case) and Without (Control) Cerebral Palsy
| Unmatched | Matched | |||||
|---|---|---|---|---|---|---|
| Cerebral Palsy | shows | Control | ES | Case | Control | ES |
| Overall | 5,176 | 1,119,131 | 5,038 | 5,038 | ||
| Full Enrollment Length (in years) | ||||||
| Mean (SD) | 8.0 (3.5) | 7.7 (3.4) | 8.1 (3.5) | 7.6 (3.4) | ||
| Median (Q1-Q3) | 7.0 (5.2-10.0) | 6.9 (5.0-9.7) | 7.0 (5.2-10.0) | 6.7 (5.0-9.2) | ||
| Years Post Eligibility Start Date (in years)† | ||||||
| Mean (SD) | 6.0 (2.3) | 5.6 (2.3) | 6.0 (2.3) | 5.5 (2.2) | ||
| Median (Q1-Q3) | 5.7 (4.0-7.8) | 5.0 (3.8-7.0) | 5.7 (4.0-7.8) | 5.0 (3.8-6.9) | ||
| Age Group | ||||||
| 45-64 years | 3256 (62.9%) | 615964 (55.0%) | 0.16 | 3154 (62.6%) | 3116(61.8%) | 0.02 |
| 65 years or Older | 1920 (37.1%) | 503167 (45.0%) | −0.16 | 1884 (37.4%) | 1922 (38.2%) | −0.02 |
| Gender | ||||||
| Female | 2608 (50.4%) | 599699 (53.6%) | −0.06 | 2529 (50.2%) | 2579 (51.2%) | −0.02 |
| Male | 2568 (49.6%) | 519432 (46.4%) | 0.06 | 2509 (49.8%) | 2459 (48.8%) | 0.02 |
| Race/Ethnicity | ||||||
| Asian | 97 (1.9%) | 33800 (3.0%) | −0.07 | 96 (1.9%) | 94 (1.9%) | 0.00 |
| Black | 539 (10.4%) | 91274 (8.2%) | 0.08 | 517 (10.3%) | 497 (9.9%) | 0.01 |
| Hispanic | 390 (7.5%) | 91832 (8.2%) | −0.03 | 383 (7.6%) | 371 (7.4%) | 0.01 |
| Unknown | 999 (19.3%) | 192326 (17.2%) | 0.05 | 953 (18.9%) | 949 (18.8%) | 0.00 |
| White | 3151 (60.9%) | 709899 (63.4%) | −0.05 | 3089 (61.3%) | 3127 (62.1%) | −0.02 |
| Education | ||||||
| Less than 12th Grade | 37 (0.7%) | 6380 (0.6%) | 0.01 | 37 (0.7%) | 39 (0.8%) | −0.01 |
| High School Diploma | 1616 (31.2%) | 286990 (25.6%) | 0.12 | 1568 (31.1%) | 1557 (30.9%) | 0.00 |
| Less than Bachelor | 2763 (53.4%) | 589285 (52.7%) | 0.01 | 2681 (53.2%) | 2703 (53.7%) | −0.01 |
| Bachelor Degree Plus | 603 (11.6%) | 184220 (16.5%) | −0.14 | 595 (11.8%) | 576 (11.4%) | 0.01 |
| Unknown | 157 (3.0%) | 52256 (4.7%) | −0.09 | 157 (3.1%) | 163 (3.2%) | −0.01 |
| Net Worth | ||||||
| Unknown | 1254 (24.2%) | 173784 (15.5%) | 0.22 | 1162 (23.1%) | 1144 (22.7%) | 0.01 |
| <$25K | 1057 (20.4%) | 149626 (13.4%) | 0.19 | 1033 (20.5%) | 1055 (20.9%) | −0.01 |
| $25K-$149K | 841 (16.2%) | 179914 (16.1%) | 0.00 | 831 (16.5%) | 817 (16.2%) | 0.01 |
| $150K-$249K | 476 (9.2%) | 116851 (10.4%) | −0.04 | 471 (9.3%) | 487 (9.7%) | −0.01 |
| $250K-$499K | 697 (13.5%) | 202415 (18.1%) | −0.13 | 694 (13.8%) | 690 (13.7%) | 0.00 |
| $500K+ | 851 (16.4%) | 296541 (26.5%) | −0.25 | 847 (16.8%) | 845 (16.8%) | 0.00 |
Source: The 2007-2017 OptumInsight
Abbreviations: SD: Standard Deviation; ES:Effect Size; Q1-Q3: Quartile 1 and Quartile 3.
Effect size equal to or greater than 0.20 considered significant.
In Supplementary Table S7, adults with and without CP were compared on prevalence of certain psychological, cardiometabolic, and musculoskeletal conditions. In our unmatched cohorts, prevalence of all conditions including any psychological (27.0% vs. 15.9%), any cardiometabolic (58.9% vs. 51.8%), and any musculoskeletal (46.7% vs. 32.5%) were higher among people with CP compared to those without. No significant difference in chronic conditions remained between post-matched case and control groups.
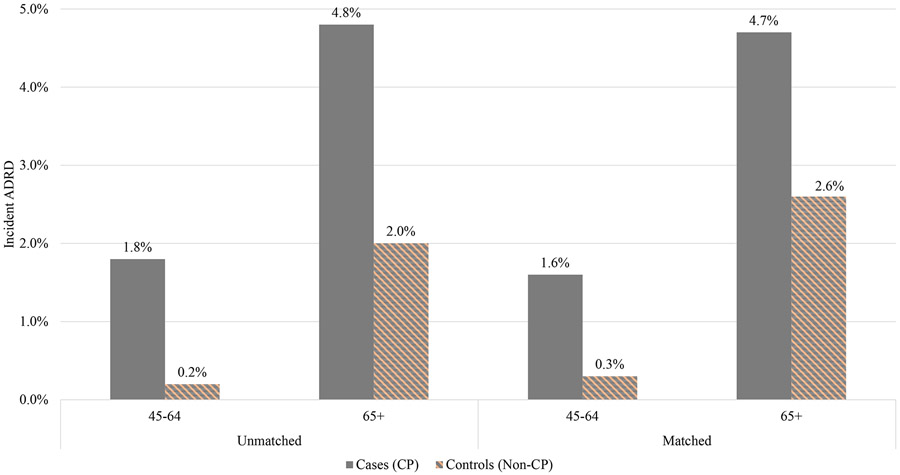
Figure 1 presents the incident ADRD among the 45-64 and 65-and-older unmatched and matched cohorts of adults with and without CP. Among the unmatched cohort, incidence rate of ADRD was 9 times higher among the 45-64 years old with CP (1.8%) compared to those without (0.2%). Among the 65-and-older group, 4.8% of people with CP vs. 2.0% of those without had incident ADRD. The results among the matched cohorts were similar.
Figure 1.
Unmatched and matched average incidences of ADRD among individuals with and without CP
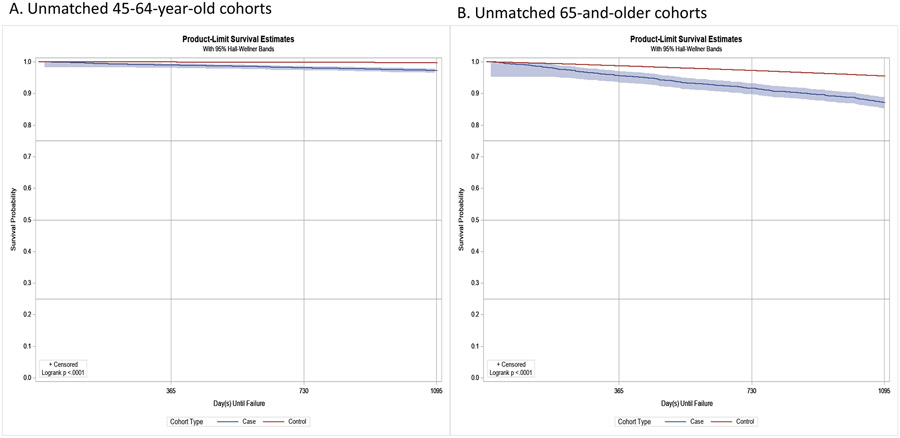
Kaplan-Meier curves in Figure 2 display the ratios of differences in ADRD-free survival probabilities between cases and controls. On average, within a three-year follow-up, ADRD-free survival probability was about 9 times higher among the 45-64-year-old controls than their corresponding cases (Figure 2A); and it was about two times higher among the 65-and-older controls compared with same age range cases (Figure 2B). The results for matched cohorts were similar (Supplementary Figure S2).
Figure 2.
Unmatched Kaplan-Meier product-limit survival curves (3-year) for adults with and without CP
Table 2 presents HRs for incident ADRD amid our two age groups. Within the younger cohorts (45-64 years old) adjusted unmatched and matched HRs for cases were 7.48 (95% CI: 6.05-9.25) and 4.73 (95% CI: 2.72-8.29), respectively. Among the older cohorts (65+ years old), adjusted unmatched and matched HRs were 2.21 (95% CI: 1.95-2.51) and 1.73 (95% CI: 1.39-2.15), respectively. Regression results are presented in Supplementary Tables S5 and S6.
Table 2.
Hazard Ratios of Early and Late Onset ADRD among Adults with Cerebral Palsy
| Unmatched Cohort | Matched Cohort | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Among those 45 to 64 years of age (early onset) | 10.22 (8.26-12.64) *** | 7.48 (6.05-9.25) *** | 5.18 (2.97-9.02) *** | 4.73 (2.72-8.29) *** |
| Among those 65+ years of age (late onset) | 2.99 (2.64-3.40) *** | 2.21 (1.95-2.51) *** | 1.88 (1.51-2.34) *** | 1.73 (1.39-2.15) *** |
Note: As with incidence estimates (Tables 1 and 2), all survival models used case (CP) and control cohorts, which required a one-year clean period with no evidence of the ADRD. To estimate the hazard ratio (HR) of CP among each age group, we examined the categorical age group (45-64 and 65+) with CP.
Regression models for fully adjusted unmatched and matched cohorts are reported in Supplementary Tables S5 and S6.
P-value < 0.001
Discussion
In this study, we examined the relative risk of incident ADRD in adults with CP compared to adults without CP. Three major findings developed. First, risk of incident ADRD is substantially higher among adults with CP compared to their counterparts without. Second, CP increases the risk of early-onset ADRD more than it increases the ADRD risk at an older age (65+). Finally, the difference in risk of incident ADRD between our unmatched and matched cohorts revealed that it is plausible that ADRD risk might be due to a higher prevalence of secondary chronic conditions among the CP population. Our findings revealed a critical need for updating clinical guidelines for adults with CP to preserve cognitive function.
Despite advances that have improved longevity among people with CP, the majority of research and clinical care has been focused on the pediatric period and/or challenges related to limits in range of motion and pain.(28) Research shows that adults with CP have poor muscle development, may experience gradual functional decline, and are at increased risk for secondary chronic conditions.(29) Our results were indicative that without intervention, adults living with CP may also be at a heightened risk of cognitive decline and incident ADRD. Consequently, the scope of care for people with CP needs to expand beyond the pediatric period and include monitoring for additional complications such as secondary chronic conditions and cognitive decline.
Furthermore, CP poses a substantially higher ADRD risk among middle-aged adults (early onset) than it does among older people (65+). Risk of incident ADRD increases with age for everyone. Thus, although people with CP are still at a greater risk for ADRD as they age, compared with other older adults, the late-onset ADRD risk is plausibly lower than early-onset ADRD because the probability increases by age for everyone.
Moreover, the greater risk for ADRD in our unmatched vs. matched cohorts might be explained by a higher prevalence of secondary comorbid conditions among adults with CP. Chronic psychological, cardiometabolic, or musculoskeletal conditions are associated with diagnosis of ADRD.(30, 31) Compared with the general adult population, people with CP have a more sedentary lifestyle with inadequate levels of physical and/or social activities.(29) Our own results and the work of others have shown a substantially higher prevalence of a broad range of preventable chronic conditions among adults with CP compared with healthy controls.(32, 33) Enabling a more active living environment (via physical or occupational therapy) and using preventative strategies in healthcare (such as early screening for preventable chronic illnesses) may not only reduce the risk of secondary chronic conditions but may also preserve cognitive function. More research on the association between high prevalence of certain chronic conditions and ADRD risk among adults with CP is warranted.
ADRD is a neurodegenerative condition, mainly defined by gradual cognitive decline and frailty causing extensive burden to patients, caregivers, and the healthcare system.(34) Our findings call for a careful examination of the types of care that may reduce the risk of cognitive decline among people with CP. Physical and therapeutic rehabilitative services may provide some neurologic preservation.(28) More research on how to lessen the risk of cognitive decline among people with CP is merited. For example, future research may focus on examining the efficacy of early cognitive screening, development of evidence-based preventative care, or greater use of physical or occupational therapy.
This study had several limitations. First, due to errors in administrative claims’ diagnostic codes, confirmatory identification of CP may not always be accurate. Although our estimates of CP and reported chronic conditions may not be 100% accurate, prior research indicates that claims-based estimates have high sensitivity and specificity. However, it is well known that certain conditions such as ADRD are underdiagnosed.(35, 36) It is therefore conceivable that our measures for incident ADRD diagnosis are underestimated. Second, without availability of the Gross Motor Function Classification System in claims data, it is hard to define CP severity. Development of a longitudinal national registry for this patient population will enable researchers and clinicians to conduct more granular examination of this topic. Finally, although we matched and risk-adjusted our models for a limited set of socioeconomic variables, we had no data on levels of physical activity, lifestyle choices, or degree and type of social and cognitive engagements, all of which have been shown to be associated with ADRD risk.(15) Finally, OptumInsight is not a nationally representative sample of the U.S. adult population, and thus the results of this study may not be generalizable.
Our study had several strengths. Based on our knowledge, this study was the first large-scale, longitudinal analysis of national claims data examining the risk of developing early- and late-onset ADRD among privately insured individuals with CP in the U.S. Conducting large observational studies has proven to be a feasible and efficient research approach, providing access to a large patient population data over time.(15, 37)
In short, we found that adults with CP are at greater risk of incident ADRD, particularly its early onset. Some of this increased risk might be explained by a sedentary lifestyle and a higher prevalence of preventable chronic conditions among this patient cohort. Future biomarker-based research may shed some light on potential etiological connections between CP and ADRD. To conclude, more research is necessary to develop clinical guidelines and recommendations for healthy brain aging in adults with CP.
Supplementary Material
What this paper adds:
Unadjusted hazard ratio (HR) of early onset ADRD was 10 times higher among adults 45-64 years old with CP compared to unmatched healthy controls within the same age group without CP.
Adjusted HR of early onset ADRD (before age 65) was 4.73 times higher among adults with CP compared with matched cohort of adults without CP.
Unadjusted HR of late onset ADRD (after age 65) was 2.99 times higher in adults 65 years and older with CP compared with older adults without CP.
Adjusted HR of late onset ADRD (after age 65) was 1.73 times higher in people with CP compared with matched cohort of older people without CP.
Funding Sources:
Funding/Support: This research was developed under grants from the Alzheimer’s Association (AARG-NTF-20-685960) and the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR #90RTHF0001-01-00).
Abbreviations:
- ADRD
Alzheimer’s Disease and Related Dementia
- AIC
Akaike Information Criterion
- CI
Confidence Interval
- CP
Cerebral Palsy
- CWM
Cerebral White Matter
- HR
Hazard Ratio
- ICD
International Classification of Diseases
- MRI
Magnetic Resonance Imaging
Footnotes
Conflict of Interest: We have no conflict of interest to declare.
References
- 1.Coq JO, Delcour M, Massicotte VS, Baud O, Barbe MF. Prenatal ischemia deteriorates white matter, brain organization, and function: implications for prematurity and cerebral palsy. Dev Med Child Neurol. 2016;58 Suppl 4:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacLennan AH, Thompson SC, Gecz J. Cerebral palsy: causes, pathways, and the role of genetic variants. Am J Obstet Gynecol. 2015;213(6):779–88. [DOI] [PubMed] [Google Scholar]
- 3.Wainwright MS. Pharmacogenomics of Alzheimer’s disease and cerebral palsy: implications of the apolipoprotein E genotype. Future Medicine. 2007;2(4):353–6. [Google Scholar]
- 4.Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017;171(9):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutton J, Pharoah P. Effects of cognitive, motor, and sensory disabilities on survival in cerebral palsy. Archives of Disease in Childhood. 2001;86(2):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haak P, Lenski M, Hidecker MJ, Li M, Paneth N. Cerebral palsy and aging. Dev Med Child Neurol. 2009;51 Suppl 4:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan JM, Allen E, Gormley J, Hurvitz EA, Peterson MD. The risk, burden, and management of non-communicable diseases in cerebral palsy: a scoping review. Developmental Medicine & Child Neurology. 2018;60(8):753–64. [DOI] [PubMed] [Google Scholar]
- 8.Whitney DG, Hurvitz EA, Ryan JM, Devlin MJ, Caird MS, French ZP, et al. Noncommunicable disease and multimorbidity in young adults with cerebral palsy. Clinical epidemiology. 2018; 10:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson MD, Kamdar N, Hurvitz EA. Age-related trends in cardiometabolic disease among adults with cerebral palsy. Developmental Medicine & Child Neurology. 2019;61(4):484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binks JA, Barden WS, Burke TA, Young NL. What do we really know about the transition to adult-centered health care? A focus on cerebral palsy and spina bifida. Archives of physical medicine and rehabilitation. 2007;88(8):1064–73. [DOI] [PubMed] [Google Scholar]
- 11.Jenks KM, De Moor J, Van Lieshout EC. Arithmetic difficulties in children with cerebral palsy are related to executive function and working memory. Journal of Child Psychology and Psychiatry. 2009;50(7):824–33. [DOI] [PubMed] [Google Scholar]
- 12.Bodimeade HL, Whittingham K, Lloyd O, Boyd RN. Executive function in children and adolescents with unilateral cerebral palsy. Developmental Medicine & Child Neurology. 2013;55(10):926–33. [DOI] [PubMed] [Google Scholar]
- 13.Muo R, Schindler A, Vernero I, Schindler O, Ferrario E, Frisoni GB. Alzheimer's disease-associated disability: an ICF approach. Disabil Rehabil. 2005;27(23):1405–13. [DOI] [PubMed] [Google Scholar]
- 14.Dunn N, Mullee M, Perry H, Holmes C. Association between Dementia and Infectious Disease. Alzheimer Disease & Associated Disorders. 2005;19(2):91–4. [DOI] [PubMed] [Google Scholar]
- 15.Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015–2060) in adults aged≥ 65 years. Alzheimer's & Dementia. 2019; 15(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muo R, Schindler A, Vernero I, Schindler O, Ferrario E, Frisoni GB. Alzheimer$s disease-associated disability: an ICF approach. Disability and rehabilitation. 2005;27(23):1405–13. [DOI] [PubMed] [Google Scholar]
- 17.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. Cardiac disease associated with increased risk of nonamnestic cognitive impairment: stronger effect on women. JAMA neurology. 2013;70(3):374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marks BL, Katz LM, Styner M, Smith JK. Aerobic fitness and obesity: relationship to cerebral white matter integrity in the brain of active and sedentary older adults. Br J Sports Med. 2011;45(15):1208–15. [DOI] [PubMed] [Google Scholar]
- 19.American Academy for Cerebral Palsy and Developmental Medicine (AACPDM). Fact Sheet. Care of Adults with Cerbral Palsy, [cited 2021. Available from: https://www.aacpdm.org/UserFiles/file/fact-sheet-pcp-doc-101415.pdf. [Google Scholar]
- 20.Taylor DH Jr, Østbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. Journal of Alzheimer's Disease. 2009;17(4):807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianattasio KZ, Prather C, Glymour MM, Ciarleglio A, Power MC. Racial disparities and temporal trends in dementia misdiagnosis risk in the United States. Alzheimer's & Dementia: Translational Research & Clinical Interventions. 2019;5:891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johns Hopkins Medicine. Health. Early-Onset Alzheimer’s Disease. [Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/alzheimers-disease/earlyonset-alzheimer-disease. [Google Scholar]
- 23.Khera R, Dorsey KB, Krumholz HM. Transition to the ICD-10 in the United States: an emerging data chasm. Jama. 2018;320(2):133–4. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoudi E, Lin P, Peterson MD, Meade MA, Tate DG, Kamdar N. Traumatic Spinal Cord Injury and Risk of Early and Late Onset Alzheimer’s Disease and Related Dementia: Large Longitudinal Study. Archives of physical medicine and rehabilitation. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waksberg J. Sampling methods for random digit dialing. Journal of the American Statistical Association. 1978;73(361):40–6. [DOI] [PubMed] [Google Scholar]
- 26.Li B, Evans D, Faris P, Dean S, Quan H. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC health services research. 2008;8(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bezeau S, Graves R. Statistical power and effect sizes of clinical neuropsychology research. Journal of clinical and experimental neuropsychology. 2001;23(3):399–406. [DOI] [PubMed] [Google Scholar]
- 28.Aisen ML, Kerkovich D, Mast J, Mulroy S, Wren TA, Kay RM, et al. Cerebral palsy: clinical care and neurological rehabilitation. The Lancet Neurology. 2011;10(9):844–52. [DOI] [PubMed] [Google Scholar]
- 29.Peterson M, Gordon P, Hurvitz E. Chronic disease risk among adults with cerebral palsy: the role of premature sarcopoenia, obesity and sedentary behaviour. Obesity reviews. 2013;14(2):171–82. [DOI] [PubMed] [Google Scholar]
- 30.Bhat NR. Linking cardiometabolic disorders to sporadic Alzheimer’s disease: a perspective on potential mechanisms and mediators. Journal of neurochemistry. 2010;115(3):551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143–53. [DOI] [PubMed] [Google Scholar]
- 32.Peterson MD, Ryan JM, Hurvitz EA, Mahmoudi E. Chronic conditions in adults with cerebral palsy. Jama. 2015;314(21):2303–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith KJ, Peterson MD, O’Connell NE, Victor C, Liverani S, Anokye N, et al. Risk of depression and anxiety in adults with cerebral palsy. JAMA neurology. 2019;76(3):294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnes DE, Yaffe KJTLN. The projected effect of risk factor reduction on Alzheimer's disease prevalence. 2011; 10(9):819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin P-J, Kaufer DI, Maciejewski ML, Ganguly R, Paul JE, Biddle AK. An examination of Alzheimer's disease case definitions using Medicare claims and survey data. Alzheimer's & Dementia. 2010;6(4):334–41. [DOI] [PubMed] [Google Scholar]
- 36.Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of dementia: an observational study of patterns in diagnosis and awareness in US older adults. Journal of general internal medicine. 2018;33(7):1131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. New England Journal of Medicine. 2000;342(25):1878–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.