Abstract
Background:
Regular self-monitoring of blood glucose (SMBG) remains the mainstay method for diabetes monitoring. The major limitation of SMBG is poor compliance and it only provides a snapshot of glucose values at that point of time. Continuous glucose monitors (CGMs) are non-invasive devices which measure subcutaneous interstitial glucose for every five minutes and provide glucose variability throughout the day.
Aim and Objective:
To assess the effectiveness of intermittent continuous blood glucose monitoring in comparison with SMBG on the percentage reduction in HbA1c level in children with type 1 diabetes mellitus (DM).
Methods:
Children diagnosed with type 1 DM of age group 3–18 years were enlisted into the study. Participants were randomised to the study arm (CGMs+SMBG) or the control arm (SMBG alone). Subjects in the study group were given CGM along with regular SMBG for 14 days. The control group was asked to perform SMBG. HbA1c levels were measured in both groups after three months of intervention.
Results:
There were 62 children in each group. After three months, in the intervention group HbA1c level dropped from 11.23% ± 1.53% (Mean ± SD) to 10.14% ± 1.99%, in control group HbA1c level dropped from 11.62% ± 1.62% to 11.32% ± 1.57%. The fall in HbA1c level in intervention group is significant (p value –0.01).
Conclusion:
In a resource-limited setting, intermittent use of CGMs atleast once every two to three months will help in understanding the factors influencing glucose variation throughout the day and, with appropriate therapeutic modifications, will aid in achieving optimal glycaemic control.
Keywords: Continuous glucose monitoring, glycaemic variability, HbA1c, time in range, type I diabetes mellitus
BACKGROUND
In India, the incidence of type 1 diabetes mellitus (T1DM) is 10.5/100,000 per year, with a peak age of incidence of 10–12 years.[1] International society for paediatrics and adolescent diabetes (ISPAD) recommends a target HbA1c level of less than 7% in children and adolescent.[2] This is a challenging goal to achieve, especially in children considering their food fad and traditional food habits of the Indian sub-continent and the carbohydrate-rich diet, especially in South India. Regular self-monitored blood glucose measurement (SMBG) remains the mainstay method in diabetes monitoring. For strict glycaemic control, finger stick blood glucose tests should be performed 6–10 times per day.[2] As a result, multiple painful pricks are a major limitation with SMBG, particularly in children. Furthermore, it only provides a snapshot of glucose values at that point in time and does not provide glucose trends over the day, even if the SMBG is performed on a regular basis, target glycaemic control is achieved, many hypoglycaemic (nocturnal and asymptomatic), hyperglycaemic episodes go undetected.
HbA1c remains the best parameter in assessing long term glycaemic control, several studies have shown that HbA1c has significant limitation when used in isolation to assess individual glycaemic control as it takes average of glucose range over 2–3 months. Recent studies have found out that glycaemic variability is responsible for long term complications of diabetes mellitus, and it cannot be detected by regular self-monitored blood glucose (SMBG).[3]
Continuous glucose monitors (CGMs) are non-invasive devices which measure subcutaneous interstitial glucose for every 5–10 minutes and provide 288 readings per day and also the blood glucose trend and variability over the day. There are limited studies done with professional CGM in the paediatric population. Further, there are conflicting results regarding the beneficial effect of professional CGM in children, as seen in few studies previously done.[4-8]
The role of CGMs in achieving a target glycaemic control in Indian children with type 1 diabetes has not been studied. This study will help us to understand the utility and feasibility of such intervention in a resource-limited setting.
STUDY PROCEDURE
This is an open-label randomised controlled trial conducted between January 2020 and June 2021, one and half years. The study group consists of children with type 1 diabetes mellitus attending tertiary health care diabetes OPD.
A) Inclusion criteria
Children between 3 and 18 years with a diagnosis of type 1 DM for atleast one year.
On multiple daily insulin injections; taking atleast 3–4 times per day.
Doing self-monitored blood glucose atleast 4–5 times per day, three days a week.
Children with HbA1c levels of more than 9%.
B) Exclusion criteria
Children with known poor compliance and HbA1c level of >15%.
Children with a history of DKA in the past two months.
Children with other co-morbidities like celiac disease, subclinical, and overt hypothyroidism.
Subjects who have not given informed written and signed consent for the study
Randomisation
The study population were divided into an intervention group (CGMs + SMBG) and a control group (SMBG). Simple randomisation was done based on a computer-generated number list using RAND function in MS excel.
All the children who met the inclusion criteria of age group 3–18 years attending the diabetes OPD were enlisted into the study, informed written consent and assent (children above seven years) were taken from parents/guardians. Patients were randomly assigned to the intervention arm (CGMs + SMBG) or control (SMBG alone) as shown in Figure 1. Pre-structured proforma was used to record the relevant information. Both the groups were given diabetes management education, they were given log sheets and asked to record their insulin dose and timing of insulin administration, food intake, exercise and any critical event (hypoglycaemia, fever).
Figure 1.
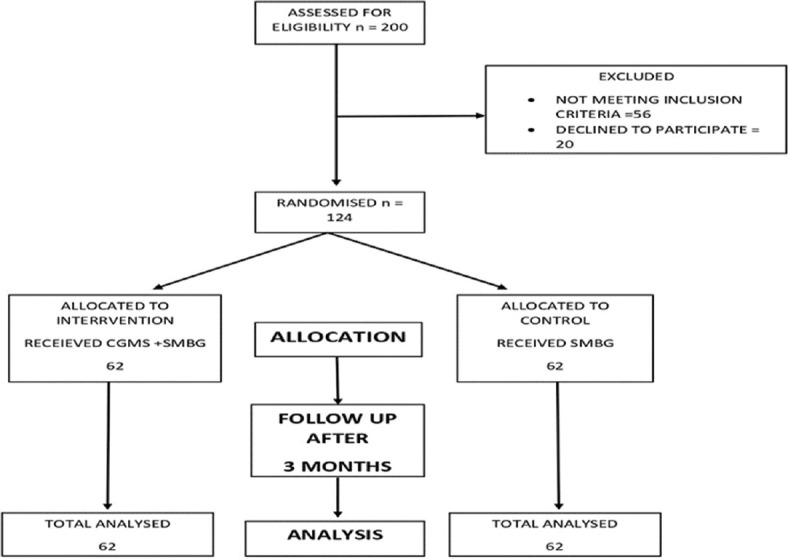
Study procedure
Subjects in the intervention group (CGMs + SMBG) were placed on CGM for 14 days along with regular SMBG. The hospital-owned CGM Abbott freestyle libre pro, an intermittently scanned CGM was used. Patients were given instructions on how to take care of and maintain sensors, reader was with the health care professionals and patients were reviewed after 14 days, and CGM reports were downloaded. Patterns of glucose trends were analysed and insulin dose was adjusted according to the CGM data in intervention group.
In the control group, glucometer was used for the SMBG and asked to record atleast four readings (fasting, pre-lunch, pre-dinner and at bed time) in a day, three days a week and reviewed after 14 days. Insulin adjustment was made based on SMBG recordings. Patients in the intervention group were asked to do SMBG at home similar to the control group after the intervention period. They were followed up after three months, SMBG recording of three months was documented along with the HbA1c levels. Patients from both the groups were on basal bolus regimen with regular insulin and NPH before and after the intervention. Same physician was involved in titrating insulin dose in both the group. On follow up patients were asked about the side effects of wearing CGMs and any interference in daily activities like sleeping, bathing, during exercise.
Hypoglycaemia is defined as any blood glucose level less than 70 mg/dl; asymptomatic hypoglycaemia is defined as blood glucose less than 70 mg/dl without any symptoms. Symptomatic is defined as blood glucose less than 70 mg/dl with symptoms of hypoglycaemia like tremor, blurring of vision, headache, sweating, mood changes like irritability.
Hyperglycaemia is defined as any blood glucose more than 180 mg/dl.
Sample size estimation
According to the published article’s reduction in HbA1c results,[4] assuming 80% power and 5% level of significance the total required sample size was 112 assuming 10% attrition sample size was 124 which will be divided into two groups 62 in each group.
Method of statistical analysis
Descriptive statistics of the explanatory and outcome variables were calculated by the mean, standard deviation for quantitative variables, frequency, and proportions for qualitative variables. Inferential statistics like paired t test was applied to check the statistical difference of continuous variables within the same group, Chi-square test was applied for categorical variables and the unpaired t-test was applied to check the statistical difference of continuous variables between the two groups. P value less than 0.05 is considered significant.
Ethical clearance statement
The study was approved by Indira Gandhi Institute of child Health Ethical committee vide letter no IGICH/ACA/EC/P116-05/06/2022-21 on 21/11/2019. Written informed consent was obtained for participation in the study and use of the patient data for research and educational purposes. The procedures follow the guidelines laid down in the Declaration of Helsinki 2008.
RESULTS
There were 62 children in the intervention group who were included in the final analysis. Out of them 30 were boys and 32 were girls. There were 62 children analysed in control group. Out of which 20 were boys and 42 were girls as shown in Table 1.
Table 1.
Baseline characteristics of type 1 DM patients in intervention (CGMs+SMBG) and control (SMBG only) group (independent t test)
| Baseline parameters | CGMs+SMBG (intervention) (n=62) | SMBG alone (controls) (n=62) | P | |
|---|---|---|---|---|
| Age in years (Mean±SD) | 11.44±3.3 | 11.28±3.34 | 0.39 | |
| Gender | Male | 30 (48.38) | 20 (32.26) | |
| number (%) | Female | 32 (51.62) | 42 (67.74) | 0.067 (Chi square test) |
| Duration of diabetes | Mean±SD | 4.87±3.11 | 5.09±3.16 | 0.35 |
| Baseline average HbA1c % | Mean±SD | 11.23±1.53 | 11.62±1.62 | 0.08 |
| Baseline total insulin (units/Kg/day) | Mean±SD | 1.02±0.21 | 0.88±0.28 | 0.53 |
| Baseline % hypoglycemic records | Mean±SD | 3.12±0.22 | 3.09±0.21 | 0.29 |
| Baseline % hyperglycemic records | Mean±SD | 61.42±24.35 | 59.92±24.64 | 0.28 |
In the intervention group, baseline average HbA1c was 11.23% ± 1.53%and follow up HbA1c was 10.14% ± 1.99%with a P value of 0.01 which was significant. Baseline average total insulin units was 1.02 ± 0.21 units/kg/day and at follow up was 1.09 ± 0.11 units/kg/day. Baseline percentage of hypoglycaemic records was 3.12 ± 0.22and at follow up was 3.02 ± 0.96. Baseline percentage of hyperglycaemic records was 61.42 ± 24.35 and at follow up was 50.51 ± 19.14 as mentioned in Table 2.
Table 2.
Baseline and follow-up (three months) parameters in intervention group (CGMs+SMBG) (paired t test)
| Parameters | Baseline | Follow up at three months | P |
|---|---|---|---|
| HbA1c % (Mean±SD) | 11.23±1.53 | 10.14±1.99 | 0.01 |
| Total insulin units/kg/day (Mean±SD) | 1.02±0.21 | 1.09±0.11 | 0.09 |
| Percentage hypoglycemic records (Mean±SD) | 3.12±0.22 | 3.02±0.96 | 0.23 |
| Percentage hyperglycemic records (Mean±SD) | 61.42±24.35 | 50.51±19.14 | 0.04 |
In the control group, baseline average HbA1c was 11.62% ± 1.62% and follow up HbA1c was 11.32% ± 1.57%. Baseline average total insulin units was 0.88 ± 0.28 units/kg/day and at follow up was 0.96 ± 0.35 units/kg/day, Baseline percentage of hypoglycaemic records was 3.09 ± 0.01and at follow up was 3.01 ± 0.97. Baseline percentage of hyperglycaemic records was 59.92 ± 24.64 and at follow up was 52.33 ± 25.03 as mentioned in Table 3.
Table 3.
Baseline and follow-up (three months) parameters in control group (SMBG alone) (paired t test)
| Parameters | Baseline | Follow up at three months | P |
|---|---|---|---|
| HbA1c % (Mean±SD) | 11.62±1.62 | 11.32±1.57 | 0.37 |
| Total insulin units/kg/day (Mean±SD) | 0.88±0.28 | 0.96±0.35 | 0.16 |
| Percentage hypoglycemic records | 3.09±0.01 | 3.01±0.97 | 0.48 |
| Percentage hyperglycemic records (Mean±SD) | 59.92±24.64 | 52.33±25.03 | 0.09 |
In our study, there was a fall in HbA1c level in intervention group and control group by − 1.09% ± 0.31% and − 0.3% ± 0.04%, respectively, this fall in percentage of HbA1c level is significant in intervention group as compared to control group (p value < 0.0001). There was decrease in hypoglycaemic records in intervention group − 0.1 ± 0.77 and in control group − 0.08 ± 0.96, the decrease in hypoglycemia records are not significant when compared between the groups. There was decrease in hyperglycaemic episodes − 10.91 ± 5.21in intervention group as compared to control group − 7.59 ± 2.39 which was significant (p value < 0.001). There was increase in insulin dosage in both groups, but it is not significant (p value = 0.14) as shown in Table 4.
Table 4.
Comparison of difference in outcome parameters in two groups (independent t test)
| Parameters | Intervention group (CGMs + SMBG) (Mean±SD) | Control group (SMBG only) (Mean±SD) | P |
|---|---|---|---|
| Change in HbA1c % | −1.09±0.31 | −0.3±0.04 | <0.0001 |
| Change in % of hypoglycemic record | −0.1±0.77 | −0.08±0.96 | 0.89 |
| Change in % of hyperglycemic record | −10.91±5.21 | −7.59±2.39 | <0.001 |
| Change in total insulin units/kg/day | 0.06±0.03 | 0.08±0.05 | 0.14 |
Out of 62 patients, six had an accidental removal, and two had fixing issues (these patients were inserted with a new CGM and data were included in the results), five complained of local pain, three complained of redness, 15 patients complained of irritation, two patients with swelling, for ten patients it caused sleep disturbance, six patients had an interference in daily activity as shown in Table 5.
Table 5.
Problems with CGMs
| Problem | Number |
|---|---|
| 1.Accidental removal | 6 |
| 2.Fixing/stability issue | 2 |
| 3.Local pain | 5 |
| 4.Local redness | 12 |
| 5.Local irritation | 15 |
| 6.Swelling | 2 |
| 7.Sleep disturbance | 10 |
| 8.Interference in daily activity | 6 |
DISCUSSION
In our study, children were followed up for three months after the intervention was made, there was a fall in HbA1c level in intervention group − 1.09% ± 0.31% as compared to control group − 0.3% ± 0.04% and this fall in HbA1c level is significant (p-value < 0.0001). The fall in HbA1c levels in our study is more as compared to pilot study done by Chase et al.[9] where the fall in HbA1c level was − 0.36% ± 0.07% (Mean ± SD) and it is less when compared to an Indian study by Raviteja et al.,[4] where fall in HbA1 was − 1.27% ± 1.46% in subgroup of population with HbA1c of more than seven.
The fall in HbA1c levels in our study was due to availability of adequate information regarding glycaemic excursion obtained through CGMs like significant pre-meal hyperglycaemia in 39 (63.93%) children, post-meal hyperglycaemia in 50 (81.97%) children and nocturnal hyperglycaemia in 23 (37%) children. Based on these observations, more interventions were made in the study group. Also, there was good compliance by the patient; parents were counselled regarding the timing of insulin administration and about the glycaemic excursion by using CGMs data. Hence, all these factors contributed to the significant fall in HbA1C level, Although not in the recommended target ranges as per guidelines.[2]
There was a reduction in the number of hyperglycaemic records in the intervention group compared to baseline by − 10.91 ± 5.21 which is significant, this finding is similar to a study done by Laffel et al.[10] where there was decrease in hyperglycaemic records by − 4.2 ± 2.3.
There was a decrease in hypoglycaemia records in intervention group − 0.1 + 2.27 and also in control group − 0.08 + 0.96 but they were not significant when compared to the baseline and also between the two groups (p value = 0.89). The reduction in hypoglycaemia episodes are similar compared to studies of Raviteja et al.[4] and Laffel et al.[10]
Baseline parameters were likely high due to the pandemic and lockdown, which resulted in children missing school, lack of physical activity, and inconsistent carbohydrate intake which may have contributed in not achieving the target HbA1c.
In this study, with use of CGMs along with SMBG for 14 days showed significant reduction in HbA1c, further there was fall in hyperglycaemic records and a statistically non-significant decrease in hypoglycaemic episodes and a statistically non-significant change in insulin requirement. Hence, the fall in HbA1c is due to reduction in hyperglycaemic records brought by the intervention made on the basis of CGM data.
The cost of this new technology is high but CGMs provides information which are valuable in improving the management of diabetes, its burden and constraints and also serves as an educational tool, which outweigh the cost involved especially in economically poor condition. Real time CGM has got a vital role in reducing hypoglycaemia episodes as it provides alerts for early intervention.
The limitations of this study are that professional CGMs were used and that it was used only once. More frequent follow up during the CGM period was not possible due to family financial constraints in frequent hospital visits and a lack of digital means with the patients to share data. The baseline HbA1c was high, and the target HbA1c was not achieved after the intervention, most likely due to the COVID pandemic, which disrupted the lifestyle.
CONCLUSION
Our study results show that the use of continuous glucose monitors along with the standard SMBG for a period of 14 days leads to improvement in glycaemic control for three months as noticed by a reduction in Hba1c levels and hyperglycaemia,
In conclusion, the use of CGMs with standard SMBG will help in understanding the glucose variation pattern in a day, and with appropriate therapeutic, lifestyle modification, it helps to reduce the HbA1c levels and achieve a better glycaemic control. In a resource limited setting use of professional CGMs once in two to three months will help in understanding glycaemic pattern throughout the day, the factors affecting glycaemic variability and can be used as educational tool for family in management of the diabetes. Hence more studies are required to find out the accuracy and efficacy of CGMs in paediatric age groups which has been deemed as emerging standard of care in coming years.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.International Diabetes Federation. IDF Diabetes Atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017. [[Last accessed on 2022 Aug 08]]. Available from: http://www.diabetesatlas.org . [Google Scholar]
- 2.DiMeglio LA, Acerini CL, Codner E, Craig ME, Hofer SE, Pillay K, et al. ISPAD clinical practice consensus guidelines 2018:Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018:123–25. doi: 10.1111/pedi.12737. [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–8. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raviteja KV, Kumar R, Dayal D, Sachdeva N. Clinical efficacy of professional continuous glucose monitoring in improving glycemic control among children with type 1 diabetes mellitus:Open label randomised control trial. Sci Rep. 2019;9:6120. doi: 10.1038/s41598-019-42555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yates K, Hasnat Milton A, Dear K, Ambler G. Continuous glucose monitoringguided insulin adjustment in children and adolescents on near-physiological insulin regimens:A randomized controlled trial. Diabetes Care. 2006;29:1512–7. doi: 10.2337/dc05-2315. [DOI] [PubMed] [Google Scholar]
- 6.Langendam M, Luijf YM, Hooft L, Devries JH, Mudde AH, Scholten RJ. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2012:101–4. doi: 10.1002/14651858.CD008101.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szypowska A, Ramotowska A, Dzygalo K, Golicki D. Beneficial effect of real-time continuous glucose monitoring system on glycemic control in type 1 diabetic patients:Systematic review and meta-analysis of randomized trials. Eur J Endocrinol. 2012;166:567–74. doi: 10.1530/EJE-11-0642. [DOI] [PubMed] [Google Scholar]
- 8.Yeh HC, Brown TT, Maruthur N, Ranasinghe P, Berger Z, Suh YD, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus:a systematic review and meta-analysis. Ann Intern Med. 2012;157:336–47. doi: 10.7326/0003-4819-157-5-201209040-00508. [DOI] [PubMed] [Google Scholar]
- 9.Chase HP, Kim LM, Owen SL, MacKenzie TA, Klingensmith GJ, Murtfeldt R, et al. Continuous sub cutaneous glucose monitoring in children with type 1 diabetes. Pediatrics. 2001;107:222–6. doi: 10.1542/peds.107.2.222. [DOI] [PubMed] [Google Scholar]
- 10.Laffel LM, Kanapka LG, Beck RW, Bergamo K, Clements MA, Criego A, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes:A randomized clinical trial. JAMA. 2020;323:2388–6. doi: 10.1001/jama.2020.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]


