Abstract
Obesity and male sex are main risk factors for sleep-disordered breathing (SDB). We have shown that male diet-induced obesity (DIO) mice develop hypoventilation, sleep apnea, and sleep fragmentation. The effects of DIO on breathing and sleep architecture in females have not been investigated. We hypothesized that female mice are less susceptible to the detrimental effects of DIO on sleep and SDB compared to males. Female DIO-C57BL/6J and lean C57BL/6J mice underwent 24-hour metabolic studies and were exposed to 8% CO2 to measure the hypercapnic ventilatory response (HCVR), and sleep studies. Ventilatory response to arousals was calculated as ratio of the average and peak minute ventilation (VE) during each arousal relative to the baseline VE. Breathing stability was measured with Poincaré plots of VE. Female obesity was associated with decreased metabolism, indicated by reduced oxygen consumption (VO2) and CO2 production (VCO2). VE in 8% CO2 and HCVR were significantly attenuated during wakefulness. NREM sleep duration was reduced in DIO mice, but REM sleep was preserved. Ventilation during NREM and REM sleep was augmented compared to lean mice. Arousal frequency was similar between groups. Obesity increased the frequency of spontaneous arousals, whereas the apnea index was 4-fold reduced in DIO compared to lean mice. Obesity decreased pre- and post-apnea arousals. Obese mice had more stable breathing with reduced ventilatory response to arousals, compared to lean females. We conclude that obese female mice are protected against SDB, which appears to be related to an attenuated CO2 responsiveness, compared to the lean state.
Keywords: Females, obesity, diet-induced obesity, sleep-disordered breathing, arousals
Graphical abstract
Graphical Abstract.
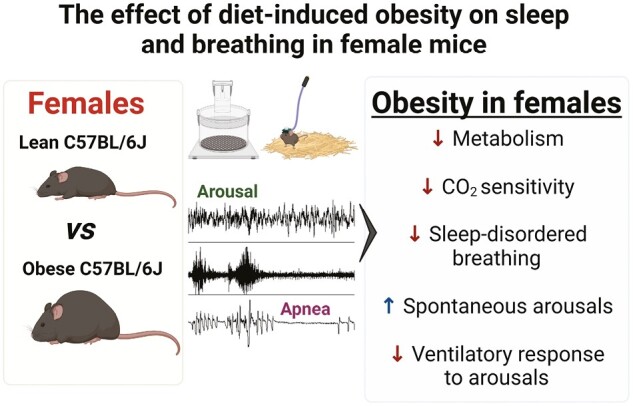
Statement of Significance.
Diet-induced obesity (DIO) in male mice is associated with sleep-disordered breathing (SDB) and sleep fragmentation. The effects of DIO on sleep and breathing of female mice have not been investigated. We showed that female mice were less susceptible to the detrimental effects of DIO on sleep and SDB. Female DIO mice showed reduced apnea severity and more stable breathing during sleep compared to lean mice. Obesity in females did not exacerbate sleep fragmentation, but it shifted the etiology of arousals as obese mice had more spontaneous arousals compared to lean females. CO2 sensitivity and ventilatory responses to arousals were attenuated in obese females. Our data suggested potential sex differences in the effects of DIO on sleep and SDB in mice.
Introduction
Obesity is a public health problem and a major risk factor for several chronic diseases, including sleep-disordered breathing (SDB) [1–3]. SDB in patients with obesity is frequently manifested as obstructive sleep apnea (OSA). OSA is characterized by recurrent episodes of upper airway collapse and hypoventilation during sleep. OSA affects approximately 41%–50% of men and 23%–26% of women in the general adult population [4, 5] with the prevalence exceeding 80% in obese men and 50% in obese women [4]. Female sex appears to protect against SDB development in obesity, although clinical and epidemiological data are confounded by multiple variables, including higher prevalence of cardiometabolic disorders and visceral type of fat distribution in obese males [6–8].
Our group has extensively studied a mouse model of obesity-induced SDB. We have shown that male diet-induced obesity (DIO) mice develop SDB [9, 10]. Male DIO mice have higher partial pressure of arterial CO2 (PaCO2), sleep hypoventilation, increased apnea severity, unstable breathing, and more frequent arousals from sleep compared to lean males. These findings resemble the effects of obesity on sleep and SDB in obese men. The effect of DIO on sleep and breathing in female mice is unknown.
In this study, our main goal was to examine whether DIO female mice develop SDB. We hypothesized that female mice are less susceptible to the detrimental effects of DIO on breathing and sleep compared to males. We analyzed breathing patterns and sleep architecture in female DIO and lean mice on the same genetic background, using four approaches. First, we evaluated metabolism and respiratory CO2 sensitivity. Second, we examined the macrostructure of sleep and sleep fragmentation. Third, we quantified ventilation during sleep and analyzed SDB severity using conventional apnea metrics and an objective measurement of breathing stability. Fourth, we examined the relationship between arousals and ventilation by assessing the time relationships between arousals and respiratory events.
Methods
Animals and study design
Female C57BL/6J mice were purchased at 8–10 weeks of age from Jackson Laboratory (#000664, Bar Harbor, MA). Twenty-four mice were fed with regular chow diet (3.0 kcal/g, 13% kcal from fat) and 30 mice were fed a high-fat diet (60% of kcal from fat, D12492, Research Diets, New Brunswick, NJ) for 12 weeks. Out of the 30 females kept on high-fat diet, 19 mice showed significant weight gain and DIO (63.3%), and were included in the protocols. The remaining 11 females were excluded from the study. When mice reached 20 weeks of age, they underwent the following protocols: (1) 24-hour metabolic studies, (2) acute exposure to 8% CO2 to examine the hypercapnic sensitivity during wakefulness, and (3) full-polysomnography to assess sleep architecture and breathing during sleep. Water and food were provided ad libitum to both groups. Mice were housed under thermoneutral conditions (~28–30°C room temperature) in a 12:12-hour light/dark cycle with lights-on at 07:00 am. Mice were euthanized by anesthetic overdose and cervical dislocation following the completion of the protocols. All experimental procedures were approved by the Johns Hopkins University Animal Care and Use Committee (#MO21M165) and complied with the policies of American Physiological Society Guidelines for Animal Studies and ARRIVE guidelines.
Estrous cycle assessment
All mice were subjected to vaginal lavages at the day of the experiments to determine their estrous phase. All vaginal smears were performed in the morning between 09:00 am and 10:00 am. Samples were collected using a micropipette and sterile pipette tips filled with 100 µL of autoclaved distilled water. Mice were restrained and the pipette tips were gently placed on the opening of vaginal canal. Smears were obtained by releasing the distilled water into the vagina and pushing it back up to the pipette 3–4 times. The 100 µL samples were spread on Superfrost plus slides (Thermo Scientific, Waltham, MA) and stained with 0.1% crystal violet solution [11]. Slides were covered with glycerin and glass cover slips, and the smears were examined under light microscopy using 10x magnification. The phases of estrous cycle were determined based on the type of cells predominating on the slides as previously reported [12]. Briefly, proestrus was determined by the predominance of nucleated epithelial cells. Estrus was characterized by the presence of cornified epithelial cells with no noticeable nucleus. Metestrus was defined as a stage with predominance of leukocytes and the presence of cornified epithelial cells. Diestrus was determined by the predominance of leukocytes and few epithelial cells.
Metabolic studies
Ten mice (5 mice per group) underwent 24-hour metabolic studies as previously reported [13–15]. The percentage of fat/lean mass was assessed in all animals by placing each mouse into a nuclear magnetic resonance spectroscopy system (Echo MRI 3-in-1 analyzer, Houston, TX). Mice were individually housed in Comprehensive Laboratory Animal Monitoring System (CLAMS) units and metabolism was measured by indirect calorimetry. Mice were placed in the CLAMS units at 11:00 am and the following 24 hours were used as acclimation. Recordings started at 11:00 am of the next day and lasted for 24 hours. CLAMS units were set at ~30°C (thermoneutral) and airflow of 600mL/min. All mice received water and food ad libitum during the entire study. Daily food intake was estimated by subtracting the weight of the food holder before and after the studies, and dividing it by 2 to account for the 48 hours that the mice were housed in the CLAMS units. Oxygen consumption (VO2), CO2 production (VCO2), and respiratory exchange ratio (RER) were sampled every 11 minutes of recording and averaged by light-phase and dark-phase. VO2 and VCO2 were normalized by body weight. Infrared beam sensors in the CLAMS units were used to estimate total locomotor activity.
Ventilatory responses to CO2
We analyzed hypercapnic sensitivity during wakefulness in five DIO mice (same mice from metabolic studies) and nine lean females (five from metabolic studies) as described by our group [13, 16]. Measurements were performed in a whole-body plethysmography (WBP) chamber using LabChart 7 Pro (ADInstruments, CO) after one day of acclimation. Mice were exposed to three cycles of hypercapnia (8% CO2 at 20.9% FiO2 balanced in N2) of 5 minutes/each. CO2 exposures were alternated with 20 minutes of recordings under room air conditions. Breaths were selected from sections of quiet wakefulness, which were defined as epochs in the absence of moving artifacts in the flow channel. Tidal volume (VT) and respiratory rate (RR) were measured and VE was calculated as VE = VT × RR. VE was normalized by body weight. Hypercapnic ventilatory response (HCVR) was calculated by the slope between VE and percentage of CO2via a linear least-squares regression analysis.
EEG and EMG electrodes implantation
Fourteen DIO and 15 lean females were implanted with EEG electrodes and EMG leads as previously described [9, 10, 13–15, 17]. Animals were initially weighted and anesthetized with 2% isoflurane. Subsequently, mice were placed in a stereotaxic frame and received 1.0%–1.5% isoflurane during the surgery through a nasal mask at 2L/min flow. All surgical procedures were performed under aseptic conditions. Mice had their head shaved and cleaned up with betadyne scrub solution. A midline incision was performed and connective tissues were gently removed from the surface of the skull using sterile cotton tips. A four-pin EEG headmount (Pinnacle Technology, Lawrence, KS) was glued above the bregma and 2 holes were gently made into the skull in the parietal and frontal areas, bilaterally, using a 22G needle. Two pairs of EEG electrodes were securely screwed to the holes coated with silver conductive epoxy. One insulated EMG lead was inserted over the nuchal muscle, bilaterally, and secured using 6–0 silk suture. The area surrounding the headmount was closed with dental acrylic. The animals received 0.05mg/kg buprenorphine (s.c.) at the end of the surgery and for at least 3 days post-surgery or until no signs of pain were observed. Mice recovered for 2 weeks until the sleep studies.
Full-polysomnography
Mice underwent full-polysomnographies in a WBP chamber as extensively reported by our group [9, 10, 13–15, 17]. Oxyhemoglobin saturation (SpO2) was measured using a pulse oximetry collar (STARR Life Sciences Corp., PA). All mice were acclimated to the WBP and to the SpO2 collar for at least 1 day before the sleep studies. Sleep studies were performed during light-phase, totalizing 6 hours of recording (from 10:00 am to 04:00 pm). Measurements were conducted under thermoneutral condition (~28–30°C) and at a humidity of 90% inside of the WBP chamber. Rectal body temperature was measured before and after the studies. Temperature inside of the WBP chamber was assessed at the end of the recordings. Studies were recorded in LabChart 7 Pro (ADInstruments, CO) and sampled at a rate of 1000 Hz. The WBP tidal volume was obtained from the pressure signals inside of the WBP chamber using the Drorbaugh and Fenn equation [18]. Required parameters for this equation included mice body temperature, chamber temperature, barometric and water pressures, and chamber gas constant. Respiratory effort was assessed with sensor bladders placed above and under the platform of the WBP chamber. The sensor bladders were attached to pressure transducers and estimated respiratory efforts based on mechanical deformations of the mice’s torso [15, 19].
Sleep–wake stages were scored in 5 second-epochs using visual analysis of the EEG signals. Stages were assigned when they comprised ≥50% of each epoch (≥2.5 second). NREM sleep was determined as the predominance of high amplitude and low-frequency band (~2–5 Hz) in the EEG. REM sleep was defined as the predominance of low amplitude and mixed frequencies in the EEG (~5–10 Hz) [10, 13, 14]. Sleep efficiency was calculated as the ratio between the total sleep time and time after sleep onset.
Sleep ventilatory analysis
We performed breath detection analyses to assess ventilation during sleep as previously described [9, 10, 13–17]. Briefly, stretches of ~20 seconds of breaths during NREM sleep were manually sampled every 30 minutes of sleep recording. Considering the short duration of REM episodes in mice within the 6 hours of recording [9, 10], all breaths during REM sleep were examined. RR and maximal inspiratory airflow (VImax) were measured. VT was calculated by integrating the flow over the duration of each inspiration. VE was calculated as VE = VT/TTot where Ttot is the duration of breath from inspiration to end expiration. VE was normalized by body weight. Analyses were performed in R version 4.2.2.
SDB parameters
SDB in female mice was examined using the following parameters: (1) apnea index, (2) mean levels of SpO2, (3) percentage of flow limitation, and (4) breathing stability during sleep. Apneas during sleep were manually scored as breathing interruptions (≥90% flow reduction) for at least 2 breath cycles or ≥0.7 seconds. Apnea index was calculated by the total number of apneas divided by the total sleep time in hours. Mean SpO2 values were calculated in NREM and REM sleep. Inspiratory flow limitation (IFL) was assessed based on an algorithm developed by our group using airflow characteristics and respiratory effort signals [15, 19]. IFL was defined as breaths with early inspiratory plateau detected in the flow associated with continued increase in the respiratory effort. Percentage of IFL was determined by dividing the flow-limited breaths by the total number of breaths obtained from the breath detection analysis. Breathing stability was examined using Poincaré method as previously described by our group [10]. In order to examine variability in breathing across the entire sleep recording, we assessed VE in all NREM and REM sleep epochs. VE was calculated by the rectified moving average of the airflow using Matlab (Mathworks, Natick, MA, USA). We applied a commonly used method to analyze periodic signals called demodulation. In this method, the ventilatory flow signal is rectified then smoothed. Integrating flow over a time interval and dividing by that duration mathematically equivalent to the mean of the flow over this window. Since rectifying the ventilatory signal counts both inspiration and expiration, we divided the value by 2. VE was averaged every 0.5 seconds to provide individual data points throughout sleep and normalized by the mice’s body weight on the day of the sleep study. As previously reported [10], the 0.5 seconds duration was arbitrarily chosen for being in a range of ~2 breaths in mice, allowing to analyze the associations between respiratory stability and apneas defined as breathing interruptions for ≥2 breath cycles. The individual 0.5 seconds VE data points were plotted against the preceding VE data point using a scatterplot graph. Breathing variability during sleep was estimated by calculating the standard deviation of the distance perpendicular to the line of identity (short-term variability, SD1) or in parallel to the line of identity (long-term variability, SD2). SD1 estimates fast breathing oscillations (e.g. “breath-to-breath”), while SD2 represents ventilatory variations across sleep. Increased breathing instability was defined as higher SD1 and SD2 values.
Sleep fragmentation and ventilatory responses to arousals
Arousals from sleep were manually scored as abrupt increases in the frequency of EEG signals for at least 0.5 seconds and less than 2.5 seconds (<50% of each sleep epoch). Arousals were marked in one of the EEG channels when they were preceded by at least 2.5 seconds of stable sleep. In REM sleep, EEG arousals were scored when associated with increases in EMG activity. Arousal index was calculated as the total number of arousals divided by the total sleep time in hours.
We examined the etiology of the arousals based on whether they were preceded by an apnea, which could indicate that the arousal was provoked by a respiratory disturbance. As previously reported by our group [10], the probability of the onset of an apnea was increased for approximately 5 seconds after the onset of an arousal until falling back to baseline values. Mice exhibited a 2.5 seconds interval between the peak of VE during arousal (time from arousal 0 seconds) and the return to the baseline VE values. Based on these intervals, we classified arousals as pre-apnea, post-apnea (i.e. respiratory arousal), or spontaneous. Pre-apnea and post-apnea arousals were defined as events in which apneas were following or preceding an arousal, respectively, within a window of 2.5 seconds from the onset of the apnea. Arousals not associated with apneas were classified as spontaneous. We calculated the peak VE at an arousal and the average VE during an arousal (0.0-2.5 seconds after the arousal) for each arousal in all mice from the rectified moving average of the flow (as described for the Poincaré analysis). We used this approach in order to analyze the temporal relationship between arousals and VE across the entire recording. Because of the high number of arousals, it was infeasible to apply breath-by-breath methods to calculate the RR. Ventilatory responses to arousals were estimated by calculating two VE ratios in each mouse: (1) ratio between the average VE during arousal (2.5 seconds duration) relative to baseline VE; and (2) ratio between the averaged peak VE at an arousal and the baseline VE. Baseline VE was calculated from all breaths (NREM and REM sleep) selected in our breath detection analysis as described above. VE ratio values >1 indicated increases in ventilation relative to baseline. Analyses were performed in Matlab (Mathworks, Natick, MA, USA).
Leptin levels
Blood samples were collected after the completion of the protocols and immediately centrifuged at 3500 rpm for 15 minutes at 4°C. Plasma samples were stored at −80°C. Circulating levels of leptin were measured by ELISA (Millipore, MA) in 8 DIO and 9 lean females.
Statistical analyses
All analyses were performed using R version 4.2.2. Data were tested for normality and homogeneity of variance using Shapiro–Wilk test and F-test, respectively. Independent t-test and Mann–Whitney U test were used to compared normally and non-normally distributed variables, respectively. Distribution of estrous cycle stages was analyzed by chi-squared test. Data were plotted using boxplots (median ± 1.5*IQR). Descriptive statistics were presented as mean ± standard error of the mean (SEM). p < 0.05 was considered statistically significant.
Results
Obesity reduces metabolism in female mice
Body weight in female DIO mice was on average 63% greater than in lean mice (Table 1), which was similar to the levels of obesity observed in our male DIO model [10]. Obesity in females was also characterized by increased percentage of body fat mass and robust hyperleptinemia (leptin levels >30 ng/mL). Food consumption by weight was similar between groups, but the daily intake of calories was significantly higher in DIO mice (Table 1). No significant differences were observed in the body temperature and the distribution of estrous cycle stages between lean and obese mice. Based on vaginal smears, the majority of obese and lean females were in proestrus and estrus on the day of the experimental procedures. Figure 1 depicts the results from the 24-hour metabolic studies. Compared to lean females, DIO females showed significant reduction in metabolism. During light phase, VO2 and VCO2 were decreased by 16% and 26%, respectively, and RER was also reduced (Figure 1A). During dark-phase, VO2 and VCO2 were decreased by 33% and 45% respectively, but the differences in RER did not reach statistical significance (Figure 1B). No significant differences were observed in total locomotor activity. We performed additional analyses normalizing VO2 and VCO2 by lean body mass instead of body weight. Overall, no significant differences were observed in VO2 during dark-phase and VCO2 during both light- and dark-phase. However, VO2 during light-phase was significantly increased in DIO mice compared to lean females (3.4 ± 0.2 vs. 2.8 ± 0.1 mL/kg/h; p = 0.016). Thus, relative hypometabolism in female DIO mice appears to be related to increased fat body mass.
Table 1.
Basic Characteristics of Female DIO and Lean C57BL/6J Mice.
| Lean C57BL/6J mice | DIO mice |
P-value | |
|---|---|---|---|
| N | 15 | 14 | |
| Body weight (g) | 23.3 ± 0.4 | 38.0 ± 1.0 | <0.001 |
| Body temperature (oC) | 35.9 ± 0.2 | 35.9 ± 0.3 | 0.818 |
| Estrous cycle stage (%) | |||
| Proestrus | 6 (40.0%) | 4 (28.6%) | 0.277 |
| Estrus | 8 (53.3%) | 5 (35.7%) | |
| Metestrus | 0 (0.0%) | 1 (7.1%) | |
| Diestrus | 1 (6.7%) | 4 (28.6%) | |
| N | 5 | 5 | |
| Fat mass (% of body weight) | 16.2 ± 3.7 | 42.5 ± 1.5 | <0.001 |
| Lean mass (% of body weight) | 75.5 ± 3.1 | 54.0 ± 1.4 | 0.008 |
| Daily food intake (g) | 1.4 ± 0.2 | 1.7 ± 0.4 | 0.833 |
| Daily calories intake (kcal) | 4.2 ± 0.4 | 8.6 ± 2.3 | 0.035 |
| N | 9 | 8 | |
| Plasma leptin (ng/mL) | 3.4 ± 0.6 | 34.5 ± 4.7 | <0.001 |
Figure 1.
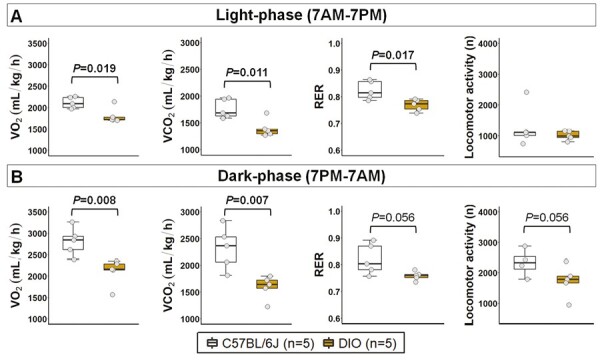
Metabolism over 24 hours in female DIO and lean C57BL/6J mice. Obesity significantly reduced metabolism during (A) light-phase and (B) dark-phase. Data are shown as median ± 1.5*IQR. Mann–Whitney U test. VO2: oxygen consumption; VCO2: CO2 production.
Obesity attenuates CO2 sensitivity in females
We examined ventilatory responses to 8% CO2 in awake females. Normalized VE under room air was similar between groups, but significantly reduced at 8% CO2 in DIO mice compared to lean females (Figure 2A). However, VT and RR were significantly higher in DIO under room air and at 8% CO2 (Figure 2B-C), which suggests that increases in ventilation are proportional to weight gain in females. HCVR defined by the slope of VE was reduced in obese females compared to lean mice (Figure 2D), suggesting that obesity in females is associated with an attenuated CO2 sensitivity.
Figure 2.
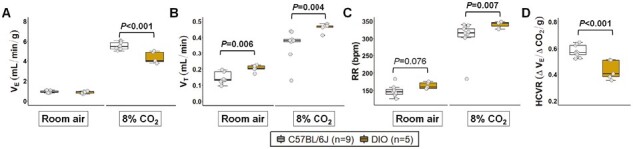
Obesity reduced CO2 sensitivity during wakefulness in female mice. (A) Normalized minute ventilation (VE) under room air condition was similar between DIO and lean C57BL/6J mice. However, normalized VE at 8% CO2 + 20.9% O2 was significantly reduced in DIO mice. (B) Tidal volume (VT) and (C) RR were significantly increased in obese females compared to lean mice under room air and at 8% CO2, which may suggest that increases in ventilation are proportional to weight gain in females. (D) DIO mice showed a reduced hypercapnic ventilatory response (HCVR) compared to lean mice. Data are shown as median ± 1.5*IQR. Independent t-test and Mann–Whitney U test.
Female obesity reduces sleep duration, but does not cause sleep fragmentation
Total sleep duration was significantly decreased in DIO mice compared to lean C57BL/6J group (Figure 3A), which was attributed to a reduction in NREM sleep (Figure 3B), but not in REM sleep (Figure 3C). Overall, obesity was not associated with major changes in macrostructure of sleep indicated by similar values of sleep efficiency (Figure 3D), number of sleep bouts (Figure 3E-F), and averaged duration of bouts (Figure 3G-H) between groups. The level of sleep fragmentation was also similar between DIO and lean animals as both groups showed an arousal index of approximately 78 events/hour (Figure 3I). We performed additional analyses removing the mice in diestrus and metestrus phases on the day of the sleep study and the arousal index remained unchanged between groups. These findings may suggest that obesity leads to shorter sleep duration, but does not induce sleep fragmentation in female mice.
Figure 3.
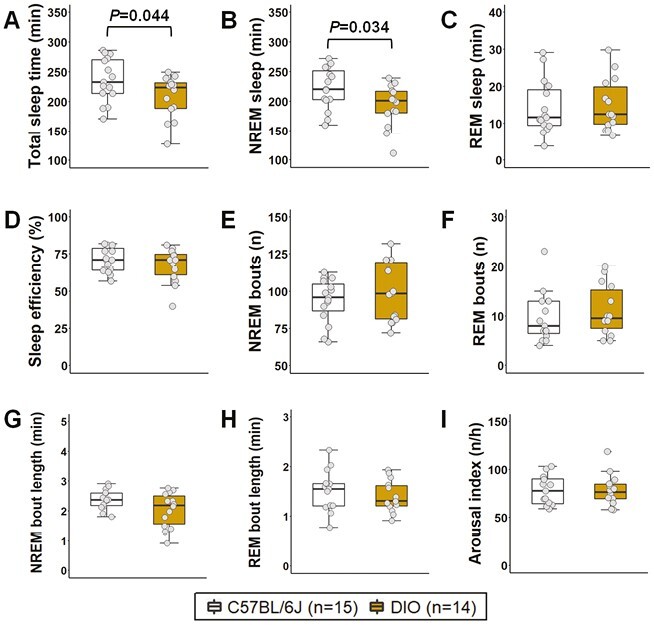
Sleep architecture of female DIO and lean C57BL/6J mice. Obesity significantly decreased (A) total sleep time, which was attributed to a reduction in the duration of (B) NREM sleep, but not (C) REM sleep. Overall quality of sleep was similar between groups since no significant differences were observed in (D) sleep efficiency, (E-F) number of sleep bouts, and (G-H) average duration of sleep bouts. (I) Arousal index was similar between groups, which suggests that obesity did not affect sleep fragmentation in female mice. Data are shown as median ± 1.5*IQR. Independent t-test and Mann–Whitney U test.
Obesity is associated with augmented breathing during sleep and decreased SDB severity in female mice
Female DIO mice showed an increase in their VE during sleep, which was proportional to weight gain in both NREM and REM sleep (Figure 4A-B), similar to the results observed in the HCVR measurements (Figure 2A-C). Augmented VE was associated with increases in both RR and VT in DIO mice during NREM and REM sleep (Figure 4A-B). In obese females compared to lean C57BL/6J mice, VImax was 27% and 41% higher in NREM and REM sleep, respectively (Figure 4A-B). DIO in females was associated with a decreased SDB severity. DIO mice showed >4-fold reduction in the apnea index compared to lean mice (14.7 ± 3.9 vs. 74.2 ± 11.9 events/hour; Figure 5A) with apneas being significantly shorter (Figure 5B). We performed additional analyses removing the mice in diestrus and metestrus phases on the day of the sleep study and the apnea index remained unchanged between groups. The degree of upper airway obstruction did not differ between DIO and lean mice, indicated by similar percentage of IFL breaths during NREM and REM sleep in both groups (Figure 5C-D). Mean SpO2 levels did not also differ between groups (Figure 5E-F). Compared to lean mice, DIO females had more stable breathing during sleep indicated by lower values of short-term (SD1) and long-term (SD2) VE variability in the Poincaré analysis during NREM (Figure 6A) and REM sleep (Figure 6B). Thus, these findings suggest that DIO in female mice stabilizes breathing during sleep and leads to a reduced SDB severity.
Figure 4.
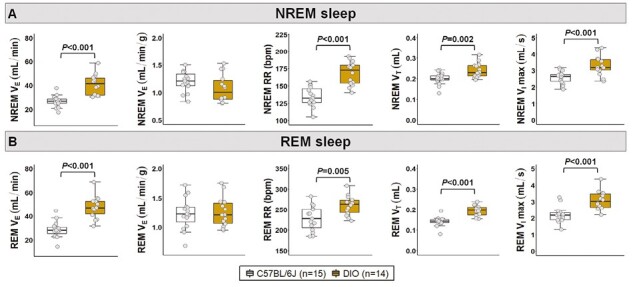
Ventilation during sleep in female DIO and lean C57BL/6J mice. Obesity augmented breathing during both (A) NREM and (B) REM sleep. Increases in minute ventilation (VE) were proportional to the levels of obesity indicated by similar values after body weight normalization between groups. VT: tidal volume; VImax: maximal inspiratory airflow. Data are shown as median ± 1.5*IQR. Independent t-test.
Figure 5.
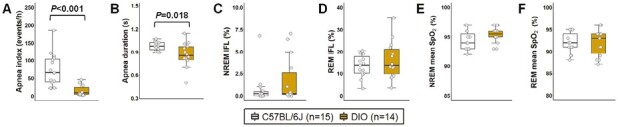
Obesity reduced the severity of SDB in female mice. DIO mice showed decreased (A) apnea index and (B) average apnea duration compared to lean C57BL/6J group. No significant differences were observed in (C-D) percentage of IFL and (E-F) mean oxyhemoglobin saturation (SpO2) during sleep between groups. Data are shown as median ± 1.5*IQR. Independent t-test and Mann–Whitney U test.
Figure 6.
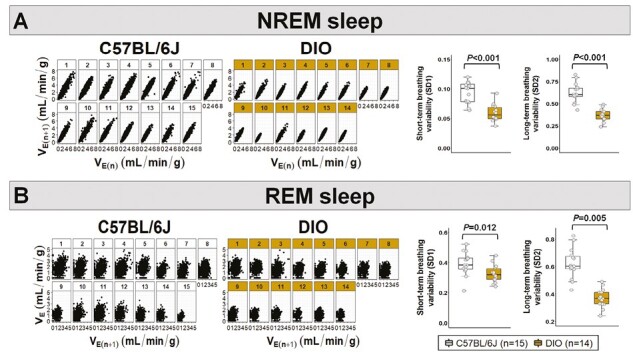
Obesity was associated with a more stable breathing during sleep. DIO mice showed reduced short- (SD1) and long-term (SD2) breathing variability in the Poincaré plots during both (A) NREM and (B) REM sleep. VE: minute ventilation. Data are shown as median ± 1.5*IQR. Independent t-test and Mann–Whitney U test.
Ventilatory response to arousals is attenuated in obese females and sleep fragmentation is mainly attributed to non-respiratory arousals
As shown in Figure 7A, we classified arousals as spontaneous, pre-apnea, and post-apnea depending on the presence or absence of apneas preceding and following the arousals. Overall, frequency of arousals was similar in both groups (Figure 3I). In both groups, sleep fragmentation was mainly attributed to spontaneous arousals, accounting for 66.5% and 89.4% of all arousals in lean and DIO mice, respectively. However, we observed a shift in the etiology of arousals and the frequency of spontaneous arousals was significantly increased in DIO mice (Figure 7B). The proportion of arousals related to apneas was elevated in lean females due to higher apnea frequency (Figure 7C-D). These findings suggest that the increased apnea severity in lean females was more likely driven by arousals. Next, we examined the ventilatory response to arousals in both groups. Baseline VE, calculated from breaths sampled in both NREM and REM sleep, was similar between DIO and lean females (Figure 8A). VE during arousals (2.5 seconds window) and peak VE at arousals were both significantly reduced in obese mice compared to lean females (Figure 8B-C). Averaged ventilatory response to arousals across sleep was reduced in DIO females, indicated by a 30% decreased VE ratio (during arousal VE relative to baseline) in the obese group compared to lean mice (Figure 8D). A reduction of 39% in the ventilatory response to arousals of DIO females was observed when calculating VE ratio based on the peak VE during arousal (Figure 8E). Data remained the same after removing mice in diestrus and metestrus phases at the day of the sleep study. Thus, changes in ventilation elicited by arousals are attenuated in DIO females, which could be associated with the augmented breathing stability and decreased apnea frequency.
Figure 7.
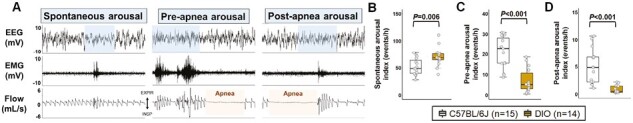
Sleep fragmentation was mainly attributed to non-respiratory arousals in females. (A) Representative trace shows the types of arousals analyzed in female mice. Blue boxes represent arousals from sleep. Orange boxes represent apneas. The frequency of (B) spontaneous arousals was significantly increased, while the (C-D) index of apnea-related arousals was reduced in DIO mice compared to lean group. Data are shown as median ± 1.5*IQR. Independent t-test and Mann–Whitney U test.
Figure 8.
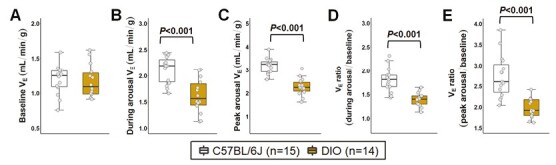
Obesity attenuated ventilatory responses to arousals in females. (A) Baseline minute ventilation (VE), averaged from both NREM and REM sleep, did not differ between groups. Ventilation during arousal was analyzed within a 2.5-second window from the arousal onset. (B) VE during arousal and (C) peak VE at an arousal were decreased in DIO mice. (D-E) Ventilatory response to arousals was significantly reduced in DIO mice compared to lean C57BL/6J group. Data are shown as median ± 1.5*IQR. Independent t-test and Mann–Whitney U test.
Discussion
We have previously shown that DIO in male mice leads to SDB and sleep fragmentation [9, 10]. We now examined the effects of obesity on breathing and sleep of female mice. In contrast to male mice, DIO did not lead to SDB in females. Overall, our study had four major findings. First, obese females had reduced metabolism and showed decreased CO2 sensitivity during wakefulness. Second, unlike males [10], DIO did not increase arousal frequency in females, but shifted the etiology such that sleep fragmentation was mainly attributed to non-respiratory arousals. Third, surprisingly, compared to lean females, obese females were able to protect their ventilation, had decreased apnea severity, and more stable breathing during sleep. Fourth, obesity attenuated the ventilatory response to arousals.
Sex differences in DIO have been previously shown. Female mice manifest a delay in weight gain and resistance to metabolic dysfunctions associated with DIO [20–23]. Nevertheless, female mice eventually develop obesity after chronic high-fat feeding [21, 22]. In our study, 63% of females showed significant weight gain on a high-fat diet, reaching similar levels of obesity to male DIO mice [10]. Female DIO mice showed a robust increase in the percentage of body fat mass and severe hyperleptinemia compared to lean females, which are key features of human obesity. DIO in females significantly decreased metabolism, indicated by reduced VO2 and VCO2. However, overall metabolism in female DIO mice was higher than the values previously observed in male DIO [13]. Huang et al. [22] observed similar findings, in which female DIO-C57BL/6J mice showed increased energy expenditure compared to obese males. Taken together, these results suggest that females are relatively protected from the detrimental effects of DIO on metabolism compared to males.
Our findings suggest potential sex differences in the effects of DIO on sleep architecture and SDB in mice on the C57BL/6J background. In humans, obesity is associated with a poor quality of sleep, sleep fragmentation, and increased SDB severity [2, 24]. We have previously shown that male DIO mice manifest the same features. DIO in male mice induced a 45% increase in the arousal frequency, augmented apnea severity, and breathing instability during sleep [10]. In females, we showed that obesity did not exacerbate sleep fragmentation and was associated with a decreased SDB severity. Arousal frequency was similar between DIO and lean females (~78 arousals/hour). However, arousal frequency was much higher and sleep fragmentation was more severe in female mice compared to males, regardless of the obese state [10]. These findings suggest that female sex was associated with a hyperarousal state.
Arousals from sleep lead to transient increases in ventilation, which predispose to augmented breathing instability and central apneas [25–27]. Hyperventilation during arousals can be induced by an increased PaCO2 during sleep and differences in chemosensitivity between sleep–wake states [25]. However, Horner et al. [27] have shown that >50% of ventilatory increases related to arousals are driven by neural mechanisms associated with arousals per se (“waking reflex”). We have previously reported that sleep fragmentation in male DIO mice was mainly attributed to non-respiratory events as only 15% of arousals were preceded by reductions in ventilation [10]. Here, we had similar findings in obese females with only ~11% of total arousals associated with apneas. In fact, most of the apneas followed the arousals, which suggests that the primary cause of SDB in mice is sleep fragmentation rather than vice versa.
We leveraged our arousals analyses [10] to examine the ventilatory responses to arousals from sleep. To the best of our knowledge, this is the first study to examine the temporal relationship between arousals and ventilation in DIO females. We showed that obesity attenuated the ventilatory response to arousals by 30%–39% compared to lean females. Reduced ventilatory responses to arousals were associated with a more stable breathing, preserved ventilation, and reduced number of apneas during sleep in obese females. Thus, the reduced SDB severity observed in female DIO mice could be in part explained by the attenuated ventilatory response to arousals.
Mechanisms related to the effects of DIO on sleep and SDB of female mice are still unknown. Here, we showed that obesity in females was associated with a decreased CO2 sensitivity during wakefulness. CO2 is the main stimulus to breathe during sleep and a potent activator of arousal neural circuitry [28–30]. Previous studies have shown conflicting results regarding sex differences in the respiratory responses to hypercapnia, and data on the effects of ovarian hormones on CO2 sensitivity is still inconclusive [31]. In this sense, differences in the sex hormones alone may not account for all changes in sleep and breathing during sleep in females. Kaur et al. [29, 32, 33] have shown that glutamatergic neurons in the external lateral parabrachial nucleus (PBel) can directly sense CO2 levels and extensively projects to areas involved in arousals regulation. However, the underlying mechanisms associated with the CO2-induced arousals, as well as the influence of female sex in this pathway remain unclear. Orexin may play a role in the effects of DIO on sleep and breathing of female mice. Orexin neurons promote arousals and wakefulness, and are potent stimulators of breathing [34–36]. Previous studies have indicated sex differences in the expression and activity of orexin in humans and rodents. Prepro-orexin mRNA levels and the expression of orexin receptors OX1R and OX2R in the hypothalamus, as well as the levels of orexin A in the cerebrospinal fluid are significantly higher in females compared to males [37–41]. Orexin also plays a critical role in the regulation of metabolism and pathogenesis of obesity. Stimulation of orexin signaling induces resistance to DIO in rodents by increasing energy expenditure and improving leptin sensitivity [42–44]. Circulating levels of orexin A are significantly reduced in obese and morbidly obese patients [45]. Orexin deficiency is associated with weight gain and obesity is more prominent in female mice [42]. Suppression of orexin signaling reduces HCVR during wakefulness [46]. Modulation of CO2 sensitivity by orexin neurons appears to be regulated by ovarian hormones [47]. Thus, orexin signaling could be involved in the metabolic and sleep changes associated with DIO in female mice. Attenuated orexin activity could be linked to the reduced CO2 sensitivity during wakefulness in obese females, decreasing the ventilatory responses to arousals and breathing instability during sleep. However, suppression of orexin pathway cannot entirely explain the hyperarousal state in female mice and, therefore, other mechanisms should be also involved in the CO2-related arousals in female DIO, such as serotonin neurons in the dorsal raphe [30].
Our study has limitations to be considered. First, we only analyzed female mice in this study, which could have impaired the analysis of sex differences in DIO-induced SDB and sleep fragmentation. We have previously reported data on sleep and SDB in male DIO mice in a similar experimental design [9, 10], which allows us to compare the overall results to female mice. Second, we were not able to fully examine the effects of estrous cycle on sleep and SDB of obese mice due to a limited number of mice that developed DIO. However, we performed vaginal smears on the days of the studies and found no differences in the frequency of estrous cycle phase between lean and obese females. In both groups, the majority of mice were in proestrus and estrus phases, and the removal of mice in diestrus and metestrus did not affect the outcomes. Chronic high-fat feeding can induce disruption in the female reproductive cycle, characterized by elongation, and skipping of phases [48]. Thus, the analysis of estrous cycle can be compromised by DIO. Third, we did not measure ovarian hormone levels in obese and lean females. It is known that DIO increases estrogen levels in female mice [48]. However, the literature on the effects of ovarian hormones on breathing and SDB is mixed [31] and may not explain the differences observed in the present study. Fourth, we performed sleep studies for only 6 hours during light-phase. Longer recordings could have provided a more comprehensive analysis of sleep architecture and sleep fragmentation of female mice. Fifth, CO2 sensitivity was only analyzed during wakefulness. In our experience, HCVR measurements during sleep are difficult, since mice are more likely to wake up with the 8% CO2 flush.
Conclusions
Female sex appears to be associated with a hyperarousal state. Female mice are more resistant to the detrimental effects of DIO on SDB and sleep fragmentation. The mechanisms by which obesity attenuates SDB severity in females are unknown, but an attenuated CO2 responsiveness related to a reduced ventilatory response to arousals may be a candidate.
Contributor Information
Lenise J Kim, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Anesthesiology and Critical Care Medicine, School of Medicine and Health Sciences, George Washington University, Washington, DC, USA.
Huy Pho, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Frederick Anokye-Danso, Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Rexford S Ahima, Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Luu V Pham, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Vsevolod Y Polotsky, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Anesthesiology and Critical Care Medicine, School of Medicine and Health Sciences, George Washington University, Washington, DC, USA; Department of Pharmacology and Physiology, School of Medicine and Health Sciences, George Washington University, Washington, DC, USA.
Funding
This research was supported by National Heart, Lung, and Blood Institute grant R01 HL128970, R01 HL133100, and R01 HL13892 (to V.Y.P.), American Academy of Sleep Medicine Foundation 238-BS-20, and American Thoracic Society Unrestricted Award (to L.V.P.), and American Heart Association Postdoctoral Fellowship Award 828142 (to L.J.K.).
Disclosure Statement
All authors report no support from commercial entities or other financial or non-financial conflicts of interest.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
- 1. Flegal KM, Carroll MD, Ogden CL, Curtin LR.. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014 [DOI] [PubMed] [Google Scholar]
- 2. Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK.. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711–719. doi: 10.1378/chest.09-0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Young T, Shahar E, Nieto FJ, et al.; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162(8):893–900. doi: 10.1001/archinte.162.8.893 [DOI] [PubMed] [Google Scholar]
- 4. Tufik S, Santos-Silva R, Taddei JA, Bittencourt LRA.. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 5. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin CM, Davidson TM, Ancoli-Israel S.. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12(6):481–496. doi: 10.1016/j.smrv.2007.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ye L, Pien GW, Weaver TE.. Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Med. 2009;10(10):1075–1084. doi: 10.1016/j.sleep.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 8. Shepertycky MR, Banno K, Kryger MH.. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28(3):309–314. doi: 10.1093/sleep/28.3.309 [DOI] [PubMed] [Google Scholar]
- 9. Fleury Curado T, Pho H, Berger S, et al. Sleep-disordered breathing in C57BL/6J mice with diet-induced obesity. Sleep. 2018;41(8). doi: 10.1093/sleep/zsy089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim LJ, Alexandre C, Pho H, Latremoliere A, Polotsky VY, Pham LV.. Diet-induced obesity leads to sleep fragmentation independently of the severity of sleep-disordered breathing. J Appl Physiol (1985). 2022;133(6):1284–1294. doi: 10.1152/japplphysiol.00386.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McLean AC, Valenzuela N, Fai S, Bennett SAL.. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012;(67):e4389. doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antunes IB, Andersen ML, Baracat EC, Tufik S.. The effects of paradoxical sleep deprivation on estrous cycles of the female rats. Horm Behav. 2006;49(4):433–440. doi: 10.1016/j.yhbeh.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 13. Kim LJ, Shin MK, Pho H, et al. TRPM7 channels regulate breathing during sleep in obesity by acting peripherally in the carotid bodies. J Physiol. 2022;600(23):5145–5162. doi: 10.1113/JP283678 [DOI] [PubMed] [Google Scholar]
- 14. Pho H, Berger S, Freire C, et al. Leptin receptor expression in the dorsomedial hypothalamus stimulates breathing during NREM sleep in db/db mice. Sleep. 2021;44(6). doi: 10.1093/sleep/zsab046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pho H, Hernandez AB, Arias RS, et al. The effect of leptin replacement on sleep-disordered breathing in the leptin-deficient ob/ob mouse. J Appl Physiol. 2016;120(1):78–86. doi: 10.1152/japplphysiol.00494.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pho H, Amorim MR, Qiu Q, et al. The effect of brain serotonin deficiency on breathing is magnified by age. Physiol Rep. 2022;10(10):e15245. doi: 10.14814/phy2.15245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim LJ, Shin MK, Pho H, et al. Leptin receptor blockade attenuates hypertension, but does not affect ventilatory response to hypoxia in a model of polygenic obesity. Front Physiol. 2021;12:688375. doi: 10.3389/fphys.2021.688375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drorbaugh JE, Fenn WO.. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16(1):81–87. [PubMed] [Google Scholar]
- 19. Hernandez AB, Kirkness JP, Smith PL, et al. Novel whole body plethysmography system for the continuous characterization of sleep and breathing in a mouse. J Appl Physiol. 2012;112(4):671–680. doi: 10.1152/japplphysiol.00818.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pettersson US, Waldén TB, Carlsson PO, Jansson L, Phillipson M.. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One. 2012;7(9):e46057. doi: 10.1371/journal.pone.0046057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maric I, Krieger JP, van der Velden P, et al. Sex and species differences in the development of diet-induced obesity and metabolic disturbances in rodents. Frontiers in Nutrition. 2022;9:828522. doi:10.3389/fnut.2022.828522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang KP, Ronveaux CC, Knotts TA, Rutkowsky JR, Ramsey JJ, Raybould HE.. Sex differences in response to short-term high fat diet in mice. Physiol Behav. 2020;221:112894. doi: 10.1016/j.physbeh.2020.112894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Casimiro I, Stull ND, Tersey SA, Mirmira RG.. Phenotypic sexual dimorphism in response to dietary fat manipulation in C57BL/6J mice. J Diabetes Complications. 2021;35(2):107795. doi: 10.1016/j.jdiacomp.2020.107795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Sousa AGP, Cercato C, Mancini MC, Halpern A.. Obesity and obstructive sleep apnea-hypopnea syndrome. Obes Rev. 2008;9(4):340–354. doi: 10.1111/j.1467-789X.2008.00478.x [DOI] [PubMed] [Google Scholar]
- 25. Phillipson EA, Bowes G.. Control of breathing during sleep. In: Cherniack NS, Widdicombe JG, Editors. The Respiratory System. Vol 2. Bethesda: American Physiological Society; 1986:649–89. [Google Scholar]
- 26. Khoo MC, Koh SS, Shin JJ, Westbrook PR, Berry RB.. Ventilatory dynamics during transient arousal from NREM sleep: implications for respiratory control stability. J Appl Physiol (1985). 1996;80(5):1475–1484. doi: 10.1152/jappl.1996.80.5.1475 [DOI] [PubMed] [Google Scholar]
- 27. Horner RL, Rivera MP, Kozar LF, Phillipson EA.. The ventilatory response to arousal from sleep is not fully explained by differences in CO2 levels between sleep and wakefulness. J Physiol. 2001;534(Pt 3):881–890. doi: 10.1111/j.1469-7793.2001.00881.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gothe B, Altose MD, Goldman MD, Cherniack NS.. Effect of quiet sleep on resting and CO2-stimulated breathing in humans. J Appl Physiol. 1981;50(4):724–730. doi: 10.1152/jappl.1981.50.4.724 [DOI] [PubMed] [Google Scholar]
- 29. Kaur S, Wang JL, Ferrari L, et al. A Genetically Defined Circuit for Arousal from Sleep during Hypercapnia. Neuron. 2017;96(5):1153–1167.e5. doi: 10.1016/j.neuron.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith HR, Leibold NK, Rappoport DA, et al. Dorsal Raphe Serotonin Neurons Mediate CO2-Induced Arousal from Sleep. J Neurosci. 2018;38(8):1915–1925. doi: 10.1523/JNEUROSCI.2182-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gargaglioni LH, Marques DA, Patrone LGA.. Sex differences in breathing. Comp Biochem Physiol A Mol Integr Physiol. 2019;238:110543. doi: 10.1016/j.cbpa.2019.110543 [DOI] [PubMed] [Google Scholar]
- 32. Kaur S, Saper CB.. Neural circuitry underlying waking up to hypercapnia. Front Neurosci. 2019;13:401. doi: 10.3389/fnins.2019.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaur S, Pedersen NP, Yokota S, et al. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J Neurosci. 2013;33(18):7627–7640. doi: 10.1523/JNEUROSCI.0173-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saper CB, Scammell TE, Lu J.. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284 [DOI] [PubMed] [Google Scholar]
- 35. Alexandre C, Andermann ML, Scammell TE.. Control of arousal by the orexin neurons. Curr Opin Neurobiol. 2013;23(5):752–759. doi: 10.1016/j.conb.2013.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams RH, Burdakov D.. Hypothalamic orexins/hypocretins as regulators of breathing. Expert Rev Mol Med. 2008;10:e28. doi: 10.1017/S1462399408000823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmidt FM, Kratzsch J, Gertz HJ, et al. Cerebrospinal fluid melanin-concentrating hormone (MCH) and hypocretin-1 (HCRT-1, Orexin-A) in alzheimer’s disease. PLoS One. 2013;8(5):e63136. doi: 10.1371/journal.pone.0063136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taheri S, Mahmoodi M, Opacka-Juffry J, Ghatei MA, Bloom SR.. Distribution and quantification of immunoreactive orexin A in rat tissues. FEBS Lett. 1999;457(1):157–161. doi: 10.1016/s0014-5793(99)01030-3 [DOI] [PubMed] [Google Scholar]
- 39. Jöhren O, Neidert SJ, Kummer M, Dominiak P.. Sexually dimorphic expression of prepro-orexin mRNA in the rat hypothalamus. Peptides. 2002;23(6):1177–1180. doi: 10.1016/s0196-9781(02)00052-9 [DOI] [PubMed] [Google Scholar]
- 40. Grafe LA, Cornfeld A, Luz S, Valentino R, Bhatnagar S.. Orexins Mediate Sex Differences in the Stress Response and in Cognitive Flexibility. Biol Psychiatry. 2017;81(8):683–692. doi: 10.1016/j.biopsych.2016.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grafe LA, Bhatnagar S.. The contribution of orexins to sex differences in the stress response. Brain Res. 2020;1731:145893. doi: 10.1016/j.brainres.2018.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fujiki N, Yoshida Y, Zhang S, Sakurai T, Yanagisawa M, Nishino S.. Sex difference in body weight gain and leptin signaling in hypocretin/orexin deficient mouse models. Peptides. 2006;27(9):2326–2331. doi: 10.1016/j.peptides.2006.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kotz C, Nixon J, Butterick T, Perez-Leighton C, Teske J, Billington C.. Brain orexin promotes obesity resistance. Ann N Y Acad Sci. 2012;1264(1):72–86. doi: 10.1111/j.1749-6632.2012.06585.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Funato H, Tsai AL, Willie JT, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9(1):64–76. doi: 10.1016/j.cmet.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adam JA, Menheere P, van Dielen FMH, Soeters PB, Buurman WA, Greve JWM.. Decreased plasma orexin-A levels in obese individuals. Int J Obes. 2002;26(2):274–276. doi: 10.1038/sj.ijo.0801868 [DOI] [PubMed] [Google Scholar]
- 46. Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T.. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol. 2007;102(1):241–248. doi: 10.1152/japplphysiol.00679.2006 [DOI] [PubMed] [Google Scholar]
- 47. Tenorio-Lopes L, Fournier S, Henry MS, Bretzner F, Kinkead R.. Disruption of estradiol regulation of orexin neurons: a novel mechanism in excessive ventilatory response to CO2 inhalation in a female rat model of panic disorder. Transl Psychiatry. 2020;10:394. doi: 10.1038/s41398-020-01076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chakraborty TR, Donthireddy L, Adhikary D, Chakraborty S.. Long-term high fat diet has a profound effect on body weight, hormone levels, and estrous cycle in mice. Med Sci Monit. 2016;22:1601–1608. doi: 10.12659/msm.897628 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


