Abstract
tRNAs are short noncoding RNAs responsible for decoding mRNA codon triplets, delivering correct amino acids to the ribosome, and mediating polypeptide chain formation. Due to their key roles during translation, tRNAs have a highly conserved shape and large sets of tRNAs are present in all living organisms. Regardless of sequence variability, all tRNAs fold into a relatively rigid three-dimensional L-shaped structure. The conserved tertiary organization of canonical tRNA arises through the formation of two orthogonal helices, consisting of the acceptor and anticodon domains. Both elements fold independently to stabilize the overall structure of tRNAs through intramolecular interactions between the D- and T-arm. During tRNA maturation, different modifying enzymes posttranscriptionally attach chemical groups to specific nucleotides, which not only affect translation elongation rates but also restrict local folding processes and confer local flexibility when required. The characteristic structural features of tRNAs are also employed by various maturation factors and modification enzymes to assure the selection, recognition, and positioning of specific sites within the substrate tRNAs. The cellular functional repertoire of tRNAs continues to extend well beyond their role in translation, partly, due to the expanding pool of tRNA-derived fragments. Here, we aim to summarize the most recent developments in the field to understand how three-dimensional structure affects the canonical and noncanonical functions of tRNA.
Keywords: tRNA, crystallography, NMR, cryo-EM, RNA modifications
The need of an adapter molecule that decodes genetic information into function was predicted by Francis Crick in 1958 (1). In eukaryotes, tRNAs are transcribed by RNA Polymerase III and undergo several steps of maturation before being charged with their cognate amino acids (2, 3). Approximately 1000 individual and unique tRNA sequences have been identified by different sequencing methods in over 400,000 analyzed genomes (4). The human genome contains over 500 genes that encode functional tRNAs, which are able to decode 62 codons, including selenocysteine (4) and pyrrolysine (5). As the redundant pool of tRNAs is produced from several genomic loci, the number of clinically relevant mutations in cytoplasmic tRNA genes is relatively low (6). Nonetheless, mutations in mitochondrial tRNA genes and altered expression levels of individual tRNAs are associated with various human diseases (7, 8). Numerous reports of patient-derived mutations in genes coding for tRNA maturation, processing, and modification enzymes directly highlight the importance of accurate tRNA biogenesis for human health and disease (9, 10, 11, 12, 13, 14). Recently, tRNAs have been rediscovered as potential therapeutics to readjust protein synthesis in specific diseases (15). This idea has sparked interest from various research groups and highlights the importance of revisiting the structure of these small RNAs.
The general structure and shape of tRNAs
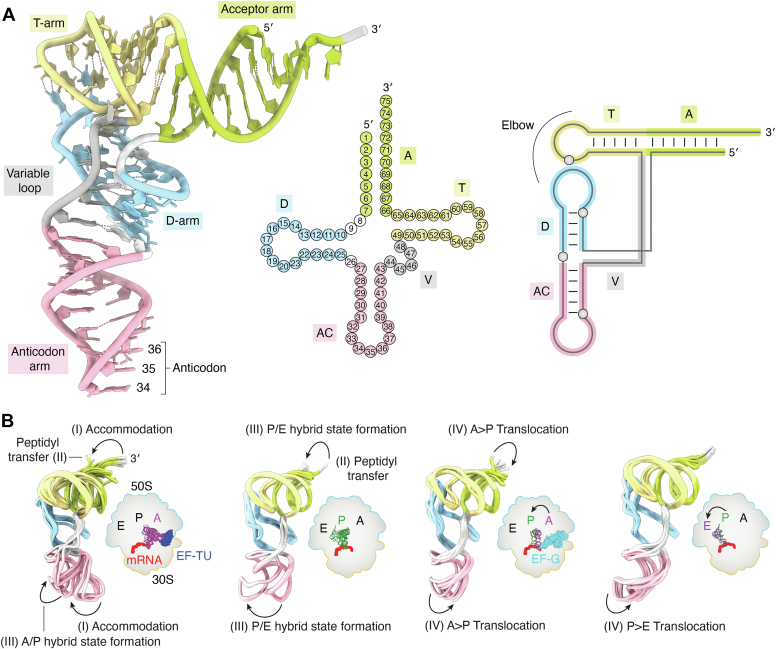
tRNA is a biopolymer composed of 76 to 90 RNA nucleotides (nt) linked together via 3ʹ-5ʹ phosphodiester bonds (16). Each nucleotide consists of a phosphate group attached via an ester bond to a five-carbon sugar (ribose), which is linked to one of the four nitrogenous bases, namely the purines (adenine and guanine) or the pyrimidines (uracil and cytosine). Specific hydrogen bonding networks between the bases define the characteristic clover-leaf secondary structure of tRNA, which was already predicted from the first sequence of yeast tRNAAlaAGC determined in 1965 by Holley et al. (17). The canonical secondary structure of tRNAs is divided into the following domains (Fig. 1A): (i) acceptor stem (also known as amino acid accepting stem (AAS) or acceptor arm) linked via (ii) the 2-nt “connector” to the (iii) D-stem loop (D-arm) closed by the D-loop, (iv) anticodon stem loop (ASL, anticodon arm), (v) variable region (variable loop, variable arm), and (vi) T-stem loop (T-arm or TΨC-arm) (18). The 3ʹ terminus includes a single-stranded CCA sequence (19), which constitutes the site of covalent amino acid attachment during aminoacylation (20). Nucleotides are numbered from 5ʹ to 3ʹ, and the posttranscriptionally added nucleotides are indicated by the number of the preceding nucleotide followed by a colon and a letter in alphabetical order. From extensive sequencing data, we know that U8, A14, G18, G19, A21, U33, G53, T54, Ψ55, C56, A58, C61, C74, C75, and A76 are the most conserved nucleotides within tRNAs. Y11, R15, R24, Y32, R37, Y48, R57, and Y60 (R = purine; Y = pyrimidine) are semiconserved nucleotides found in many tRNA sequences. Differences in tRNA length are mainly a consequence of the varying sequence length in the variable loop that can accommodate “extra” nucleotides, while preserving the overall domain organization and three-dimensional shape (21). Some exceptions to the clover-leaf secondary structure are found in mammalian mitochondrial tRNAs (22, 23), flagellates (24), and nematodes (25, 26). In these rare cases, deviations occur in the D-stem/loop or T-stem/loop architecture or the complete D-loop is lacking (18, 27), which will be described in the following sections.
Figure 1.
The structure of tRNA at different levels and during translation.A, left: specific regions are indicated on yeast tRNAPheGAA (PDB ID 1EHZ) as follows: 3ʹ and 5ʹ end; acceptor arm (light green); T-arm (yellow); D-arm (light blue); variable loop (gray); anticodon arm (pink); and 34, 35, 36 anticodon nt. The dimensions of folded tRNAs are approximately 60 × 70 × 25 Å. Middle: clover-leaf secondary structure representation of tRNA numbered according to the standard convention. Right: tertiary nucleotide interactions responsible for the L-shape tRNA structure. B, multiple structures of tRNA extracted from the context of elongating bacterial 70S ribosome determined by time-resolved cryo-EM. The schematic demonstrates local flexibility of the A-site (magenta), P-site (dark green), and E-site (purple) tRNA associated with the cognate mRNA (red) bound to the 70S ribosome [large 50S (blue) and small 30S (orange)]. The arrows highlight the bending of the 3ʹ end (silver) from the acceptor arm (light green) and the swinging of the anticodon arm (pink). The major sequential steps of the elongation cycle associated with the L-shape distortion are indicated with Roman numerals (I–IV). The two superimpositions on the left (A-site phenylalanine- and P-site methionine-tRNA) follow the steps and changes after cognate aminoacyl-tRNA delivery by elongation factor EF-TU (dark blue). The two superimpositions on the right depict AP proline- and PE methionine-tRNA translocation facilitated by elongation factor EF-G (cyan), which resets the system for the next elongation cycle. EF-Tu, Elongator factor-Tu.
The secondary structure of tRNAs not only depends on the RNA nucleotide sequence itself but also on the presence of other additional factors. For instance, coordination of magnesium ions is required for correct folding of tRNAGluUUC in Escherichia coli (28). Despite sequence variations, all tRNAs fold into the characteristic L-shaped three-dimensional tertiary structure (29) (Fig. 1A). This compact shape comprises two orthogonal helical branches, each containing several Watson-Crick base pairs (bp). In detail, the AAS is stacked over the T-stem loop to form the acceptor domain that is linked by the so-called “elbow” region (30) to the anticodon domain formed by ASL and D-stem loop. The L-shape is further stabilized by additional non-Watson-Crick interactions between D- and T-arms. This uniform L-shaped conformation was initially proposed in 1974 based on the first crystal structure of yeast tRNAPheGAA (31, 32, 33, 34) and then confirmed by other structural studies of yeast tRNAAspGUC (35, 36), bacterial (37) and yeast (38) initiator tRNAMetCAU, and human tRNALysUUU, the primer of HIV reverse-transcriptase (39). The correct folding, stability, and functionality of tRNAs are also influenced by the posttranscriptional incorporation of chemical modifications (40).
Individual regions of tRNAs
In general, the size of acceptor (AAS), anticodon, and T-arms are highly conserved among functional tRNAs. The AAS is composed of a stem that is formed between the first 7 nt (5ʹ end) and nt 66 to 72 (3ʹ end), creating a molecular zipper (Fig. 1A). It is completed by a 4-nucleotide long, single-stranded overhang, harboring the so-called discriminator base at position 73 (41) followed by the CCA-tail. The exceptions to this general principle are all tRNAHisGUG, which contain a posttranscriptionally added guanine nucleotide at the 5ʹ end, numbered “0” or “−1” (42). The discriminator base is one of the major identity elements that, in combination with several other nucleotides in the anticodon and the acceptor helix, ensures the very high specificity of aminoacyl-tRNA synthetases (43, 44). During the maturation process, 5ʹ leader sequences are excised by the endonuclease RNase P (45) prior to the 3ʹ trailer, although this order can be reversed for tRNAs harboring long 3ʹ trailer sequences (46). In the case of the 3ʹ end, the CCA sequence can be encoded in the tRNA gene (e.g., E. coli and related bacteria, certain Gram positive bacteria) or added to the tRNA during a separate maturation process in eukaryotes, certain Gram positive bacteria, and archaea (47). In the first scenario, 3ʹ trimming is primarily carried out by the exoribonucleases RNase T and RNase PH (48), but other exoribonucleases (e.g., RNase II and RNase D or RNase BN (49)) can act as a substitute, if needed. When the CCA is not encoded in the genomic sequence, the 3ʹ endonuclease RNase Z initially cleaves the overhang after the discriminator base and leaves a 3ʹ-OH group (50); subsequently the tRNA nucleotidyltransferase adds the 3′ terminal CCA residues without the need of a template (51). The fundamental role of the CCA sequence is to allow aminoacylation and therefore provide a charged tRNA to the ribosome. Moreover, inside the ribosome, the CCA sequence enables dynamic interactions with ribosomal proteins and rRNA elements as the tRNA is moved through the ribosome (52, 53) (Fig. 1B).
The 5ʹ end of the acceptor stem is connected to the D-arm by two nt (U8 and N9), which represents the second most variable region of tRNAs (Fig. 1A). The D-arm derives its name from the dihydrouridine (D) modification present at the 3ʹ and/or 5ʹ of conserved G18 and G19. Typically, the D-arm comprises a 4-bp stem (positions 10–13 pair with 22–25) that ends in a 7–11 nucleotide loop containing conserved nt A14, A21, G18, and G19. The 3ʹ part of the acceptor arm is directly connected to the T-arm, which is typically a 5-bp long stem (positions 61–65 pair with 49–53) with a loop composed of seven nt (positions 54–60). Due to the presence of a universally conserved motif [thymidine 54, pseudouridine (Ψ) 55, and cytosine 56], it is also called the “TΨC-arm”. C56 and Ψ55 form interactions with conserved residues G19 and G18 in the D-arm, which stabilizes the L-shaped form of tRNA (54). The D-arm is also responsible for interactions with the ribosome during all stages of translation (55).
The variable region, as its name indicates, can vary in length (56). In type I tRNAs, it usually consists of four or five nt, whereas in type II tRNAs (e.g., tRNASer, tRNALeu, tRNATyr), it consists of 10 to 24 nt. The longer variable regions are arranged in a double helical stem of 3 to 7 bp and a loop of 4 to 5 nt. Nucleotides in the variable region are located between position 45 and 46 and obey canonical base-pairing rules. The nt in the 5ʹ-strand and the 3ʹ-strand are numbered e11, e12, e13,... and e21, e22, e23,..., respectively. The prefix ‘e’ is specific for the variable region, and the second digit identifies the respective base-pair. In the case of a long variable region, the loop can be formed by up to 5 nt: e1, e2, e3, e4, and e5 (57).
The anticodon arm consists of a 5-bp stem (positions 27–31 pair with 39–43) and a 7-nt loop (positions 32–38), containing U33, R37, and the anticodon triplet N34, N35, N36. The anticodon, together with a discriminator base, is one of the main identity elements of tRNAs (58). It is responsible for reading codon triplets in mRNAs and 61 different sense codons encoding 20 standard amino acids. The 21st amino acid, selenocysteine, is recoded by the UGA stop codon if assisted by a specific selenocysteine insertion sequence elements in the mRNA and is directly synthetized on already charged tRNASerUCA (59, 60). The 22nd amino acid, pyrrolysine, was discovered in Methanosarcina barkeri and is encoded by an UAG codon in the methylamine methyltransferase genes (61). The number of tRNA species in different organisms varies between 23 and 45, clustered in 20 groups of so-called iso-acceptors. These are tRNAs that are charged with the same amino acid despite having different anticodons. Apart from methionine and tryptophan, all amino acids have multiple iso-acceptors. The anticodon does not even have to be fully complementary to recognize a specific codon triplet. The nature of the wobble base at position 34 enables the accommodation of noncanonical interactions between the codon and anticodon. Furthermore, the ASL is the most heavily modified region of tRNA, with these modifications either restricting or facilitating the decoding process (62).
The overall L-shape of tRNA is maintained even though conformational changes occur during the different steps of the ribosomal translation cycle (Fig. 1B). Advances in time-resolved cryo-EM (63) have allowed the structural visualization of distinct states of the ribosome and tRNAs during translation (64, 65). The characteristic L-shape of tRNAs is further substantiated by numerous examples of structural “mimicry”, where other RNAs or proteins adopt a tRNA-like shape (66). For instance, viruses use the tRNA-shaped internal ribosome entry site in order to recruit ribosomes to initiate translation at non-AUG codons (67, 68). Furthermore, Staphylococcus aureus uses a riboswitch (yjdF) that was found to mimic the overall shape of a tRNA to activate translation (69). The translational elongation and ribosome recycling factors (e.g., EF-G, EF2, or RRF) also use structural mimicry to specifically interact with the ribosome (70, 71, 72, 73).
RNA modifications and their influence on the folding/stability of tRNAs
tRNAs fold into an L-shaped three-dimensional structure despite their high sequence variation (33, 74). At first glance, one would simply assume that the available sequences provide sufficient constraints for all tRNA transcripts to adopt the L-shaped conformation. This notion is also supported by very similar crystal structures of modified and unmodified tRNAPheGAA from bacteria and eukaryotes (Fig. 2A) (74, 75, 76, 77). Several studies indeed have demonstrated that various “naked” in vitro–transcribed tRNAs are able to fold into functional L-shaped tRNAs that can be charged by aminoacyl-tRNA synthetases or used in in vitro translation systems (78, 79, 80, 81). However, other unmodified tRNA transcripts are found to dynamically switch between multiple conformations, and the equilibrium between misfolded and properly folded forms is regulated by additional factors, such as modifications or environmental conditions (82). For instance, unmodified human mitochondrial tRNALysUUU displays as an extended bulged-hairpin structure instead of the canonical cloverleaf structure. This finding is consistent with the observation that some hypomodified tRNAs are not functional in vivo and subject to degradation (83, 84, 85, 86, 87, 88, 89). Approximately 100 different RNA modifications are found in tRNAs, and on average ∼17% of nt are modified in each tRNA molecule (90, 91, 92). The occurrence of tRNA modifications can be divided into two groups, based on their functionality within the molecule: (i) modifications that are involved in the process of translational decoding and (ii) those that control tRNA folding and stability (91, 93, 94).
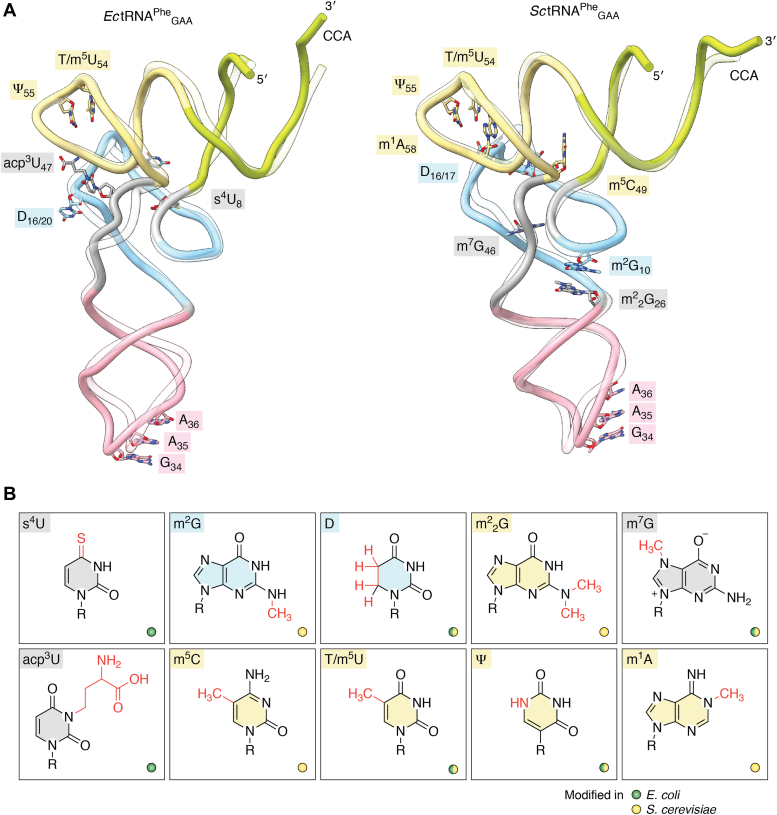
Figure 2.
Structure of unmodified and modified tRNAPhe.A, comparison of prokaryotic and eukaryotic tRNAPheGAA. Superimpositions of in vitro transcribed (transparent) and endogenous (opaque) tRNAPheGAA from Escherichia coli (Ec) and Saccharomyces cerevisiae (Sc) obtained by crystallography. These tRNAs are depicted as cartoons using the same color code for each region as in Figure 1. Modifications that are important for the tRNA core structure are represented in sticks and colored according to their position in the tRNA. The PDB IDs and resolution limits of each tRNA are as follows: unmodified EctRNA (PDB ID 3L0U at 3.0 Å) and SctRNA (PDB ID 5AXM at 2.2 Å) and modified EctRNA (PDB ID 6Y3G at 3.1 Å) and SctRNA (PDB ID 1EHZ at 1.9 Å). The m7G47 modification in EctRNA (PDB ID 6Y3G) is present in tRNAPheGAA but is not visible in the electron density map. B, chemical structures of modified nucleosides that are shown in (A). The modifications are indicated in red and the ribose moiety is annotated with R. Base colors correspond to the modified target positions in (A), while the color (green or yellow) of the small circle indicates the presence of modification in E. coli or S. cerevisiae. RMSD values for E. coli tRNAPhe and S. cerevisiae tRNAPhe were calculated with ChimeraX using the backbone atoms of nt 3 to 74. E. coli: overall RMSD 2.669 Å2, acceptor stem 4.426 Å2, D-arm 1.595 Å2, anticodon-loop 2.716 Å2, variable loop 2.362 Å2, T-arm 1.349 Å2; S. cerevisiae: overall RMSD 1.662 Å2, acceptor stem 2.132 Å2, D-arm 2.194 Å2, anticodon-loop 1.628 Å2, variable loop 0.801 Å2, T-arm 0.665 Å2.
In light of excellent reviews focusing on tRNA modifications (27, 40, 62, 95, 96, 97, 98, 99), we focus here on those modifications that seem important for the core structure of tRNAPheGAA in prokaryotes and eukaryotes. In general, modifications that affect the tertiary structure of tRNA are rather simple, such as reduction or isomerization of uridine to D or Ψ, methylation of the base and/or ribose sugar, replacement of oxygen on the base with sulfur, or the addition of small functional groups, like aminocarboxypropylation (acp) (Fig. 2B) (91, 93, 100). These modifications modulate the structural plasticity of the tRNA core, where some regions become rigid and others are more flexible (75, 87, 101, 102, 103). These changes are achieved through increased hydrophobicity, change of charge, and stabilization of a 2ʹ-endo or 3ʹ-endo sugar conformation (87, 103, 104, 105, 106, 107, 108). Depending on the position on the nucleotide, modifications can alter hydrogen bonding interactions on all three edges (Watson-Crick, Hoogsteen, and sugar-edge), which in turn can change the base-pairing properties, leading to local or global structural rearrangements of the tRNA (85, 107, 109, 110). The introduction of small chemical groups typically maximizes the stabilizing effect while minimizing the entropic penalty of attaching larger chemical groups, which are more common in the anticodon region.
One of the most prevalent tRNA modifications is methylation, which can occur on the oxygen of the 2ʹ-OH moiety (2ʹ-OMe), on nearly all nitrogen sites of the nucleobase (with the exception of the N7 and N3 position of adenosine) and on the C2/C5 atom of pyrimidines and adenosine (104, 111, 112). The presence of a 2ʹ-OMe group induces the 3ʹ-endo ribose conformation, through repulsion between the 2ʹ-OMe and 3ʹ-phosphate, resulting in the stabilization of an A-form helical region (104, 106, 112). This effect leads to an increased melting temperature of tRNA and decreased susceptibility to alkaline and enzymatic hydrolysis (109, 113, 114). Methylations on the Watson-Crick edge appear predominantly on the 5ʹ-side of the tRNA and prevent canonical base pairing (40, 104). The m1A modification at position 9 and 58 is common and has been linked to correct folding and structural stability of tRNA (85, 115). A prime example for global structural rearrangement is the well-studied m1A9 modification in human mitochondrial mt-RNALysUUU. It was shown that mt-RNALysUUU exists in an equilibrium between the classical L-shaped and an alternative extended hairpin conformation. Addition of the methyl group to N1 of adenosine disrupts A9-U64 base pairing, which supports the hairpin conformation and shifts the equilibrium towards the correctly folded structure through A50-U64 pairing (85, 115, 116). A similar effect was also described for mt-RNAAspGUC and mt-RNALeuUUR, demonstrating the importance of modifications for mitochondrial tRNA folding (117, 118, 119, 120, 121). Methylation of N1 at guanosine 9 (m1G9) in cytoplasmic and mitochondrial tRNAs also disrupts canonical base pairing and is believed to be involved in structural reorganization (122, 123). The N1-methylation of A58 does not disrupt the reverse-Hoogsteen base pairing with T54 but introduces a positive charge that stabilizes the base pairing and thus the tertiary structure (124, 125, 126, 127, 128).
Single or double methylation of N2 in guanosine (m2G, m22 G) occurs frequently in the junctions between acceptor stem and D-arm (position 9 and 10) as well as between the D-arm and anticodon loop (position 26 and 27) in some eukaryotic and some bacterial tRNAs (129, 130, 131, 132, 133, 134). m2G can also be found at position 6 and 7 in the acceptor stem of higher eukaryotes (133, 135). The single methyl group on N2 of G10 does not interfere with the triple base pairing between G10-C25-G45, but the hydrophobic character of the methyl group might stabilize the D-loop (132, 136). Dimethylation of G26 prevents base pairing with cytidine but still allows pairing with any other nucleotide (132, 137). Similar to m1A9, it was shown that m22G26 prevents an alternative folding of the human cytosolic tRNAAsnGUU (137). Interestingly, m22G26 prevents mispairing with C11 and controls pairing with A44, which contributes to the stabilization of the hinge region between the D-arm and anticodon loop (132, 134, 137, 138).
The most prominent methylation on the 3ʹ-half of tRNAs is m5U54/T or ribothymidine, and it occurs in the T-loop of nearly all tRNAs. m5U54 together with m1A58 forms a reverse-Hoogsteen bp, which stabilizes the T-arm through additional base stacking with the conserved G53-C61 pair (125, 127, 139, 140). m5C modifications between the variable loop and T-arm most likely contribute to stabilizing the tRNA core structure. In a subset of eukaryotic tRNAs, C48 in the Levitt bp (G15•C48) is methylated to m5C, which increases the hydrophobic character of the bp and has been suggested to promote base stacking (40, 128, 141). Another conserved modification in the variable loop is m7G46, which introduces a positive charge that facilitates a triple base pairing with C13 and G22 and stabilizes the tertiary structure of tRNA (124, 142).
Two highly abundant uridine modifications are Ψ and D, which engender opposite structural properties. Ψ is formed through isomerization of the glycosidic bond between N1-C1 into the C5-C1 carbon-carbon bond (143, 144, 145). This repositioning alters the chemical properties of the base, resulting in a free N1 atom at the Hoogsteen edge that increases base-stacking properties of Ψ with its neighboring bases through the coordination of a water molecule (143, 144, 145, 146, 147). Furthermore, Ψ favors the 3ʹ-endo sugar conformation that reinforces the local structural rigidity and stability of RNA helices (143, 144). Ψ is present at several positions in tRNA and is commonly found in position 55 in the T-loop, where it pairs with G18 and stabilizes the tRNA elbow region (30, 90, 148).
In contrast to Ψ and 2ʹ-OMe that confer rigidity, D promotes the more flexible 2ʹ-endo ribose conformation (103, 106, 143). This structural behavior originates from the nonplanar and nonaromatic nature of the D modification, where the double bond between C5-C6 of uridine is reduced to a single bond, shifting the atoms C5 and C6 on opposite sides of the N1, C2, N3, and C4 plane (149, 150). As D is no longer able to participate in stacking interactions with the neighboring aromatic residues, the modification increases the local flexibility of the D-loop. Moreover, D induces a 2ʹ-endo conformation in the adjacent 5ʹ-residue (74, 103, 151). The local flexibility of the D-loop may facilitate the interloop interaction of the conserved G18 and G19 residues with Ψ55 and C56, which are responsible for maintaining the structure of the elbow region (30, 103, 152). Because the lack of dihydrouridine in E. coli tRNASerGGA is associated with a lower melting temperature, the flexibility of the D-loop must somehow contribute to tRNA stability (110). The NMR-based analysis of an RNA hairpin derived from Schizosaccharomyces pombe tRNAiMetCAU confirmed that the presence of D can indeed promote the formation of a stable RNA hairpin (152).
There are two additional uridine-based modifications that show a positive contribution to the structural stability of tRNAs. Thiolation of C4 at uridine 8 and 9 (s4U) in the acceptor stem is conserved in bacteria and archaea but has not been reported in eukaryotes (92, 110, 153, 154). s4U8 is buried in the tRNA core and pairs with A14 of the D-loop. It has been suggested that s4U is involved in tRNA stabilization, as lack of the modification decreased the melting temperature of E. coli tRNASerGGA by 5 °C compared to the fully modified tRNA (110). The 3-(3-amino-3-carboxypropyl) uridine (acp3U) modification prevents Watson-Crick base pairing and is located in the core region of tRNA (90, 151, 155, 156). acp3U was initially detected in E. coli tRNAPheGAA and later on also found in several eukaryotic tRNAs (157, 158, 159). In E. coli, acp3U is located at position 47 in the variable loop (151, 160, 161). It was shown that the presence of acp3U47 in tRNAs of E. coli increases the melting temperature by 3 °C (160). However, in eukaryotes, acp3U occurs in the D-loop at position 20 and 20a of several cytosolic tRNAs and at position 47 in plastid and mitochondrial-encoded tRNAs of various land plants (151, 160). Interestingly, tRNALysUUU from Trypanosoma brucei has acp3U at position 20 and a dihydrouridine derivative at position 47 (acp3D). Despite its prevalence, the exact mechanistic and functional consequences of this acp3D modification are not completely understood. It has been proposed that the presence of acp3U in the variable loop might destabilize alternative tRNA conformations, as the acp3 group blocks Watson-Crick pairing similar to m1A9 (160). Furthermore, it was suggested that acp3U20a might stabilize the local structure through Mg2+ coordination and that acp3U47 could coordinate water or Mg2+ with the T-arm in a similar fashion to stabilize the local conformation (151, 160).
The various tRNA modifications can either happen as individual, independent events or as part of a complex modification circuitry that relies on a hierarchy of modifications (97, 98). These interdependencies between modification pathways are well described for the ASL, but recent work revealed the presence of a similar modification network also in the T-loop and tRNA core region (97, 98, 162, 163). In summary, the specific incorporation of tRNA modifications adds an additional layer of complexity that ensures the proper folding and structural stability of tRNAs. Foremost, the use of chemical modifications increases the number of RNA sequences that are able to fold into a functional tRNA and therefore the appearance of modification enzymes throughout evolution is directly correlated with the variation of tRNA sequences (164).
Recognition of the tRNA structure by modification enzymes and maturation factors
Although the shape of mature tRNAs must remain relatively rigid and stable to promote efficient translation of the ribosome, the structural characterization of precursor tRNAs that still need to undergo maturation suggests a very different scenario. In particular, analyses of tRNAs in complex with the proteins responsible for processing and modification provide insights into the dynamic nature of the tRNA structure itself, which could not be achieved by studying mature tRNAs alone or within the ribosome.
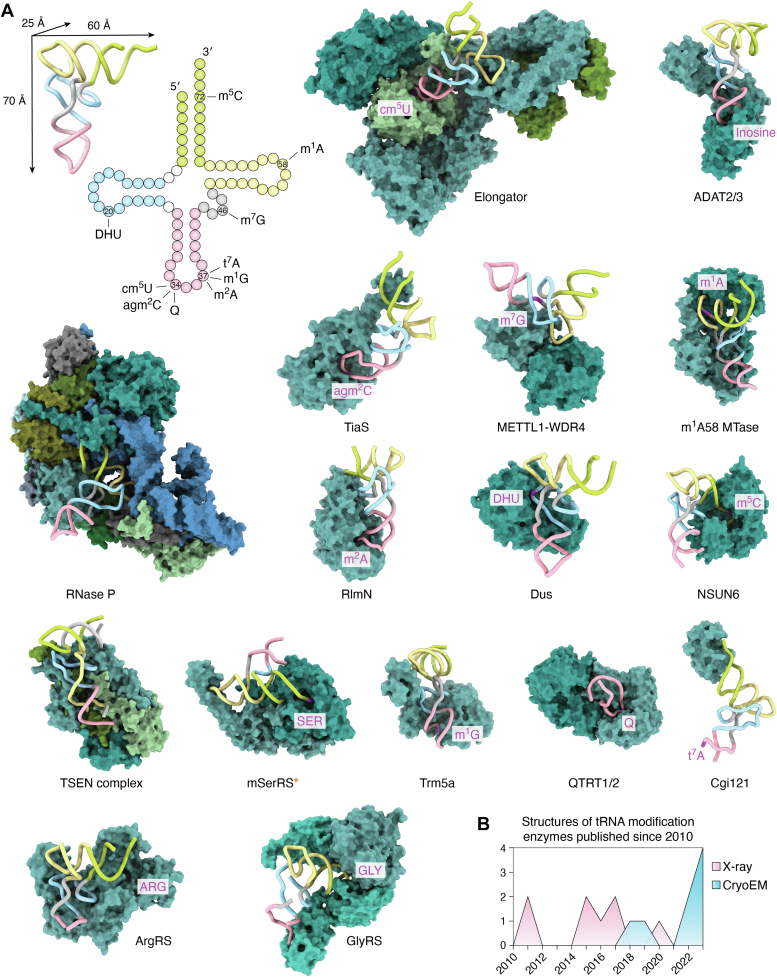
For several decades, X-ray crystallography and NMR have been the dominant methods for determining the structures of proteins bound to tRNAs. Numerous high-resolution structures of aminoacyl-tRNA-synthetases (type I aa-RS and type II aa-RS) in complex with their tRNA substrates have been reported and characteristic modes of tRNA recognition unveiled (Fig. 3A). Since 2010, eight crystal structures of tRNA-modifying enzymes bound to full-length tRNA have been reported, showing that these proteins use multiple approaches to recognize and bind substrate tRNAs. They can be either very specific to the target region or sense the global shape of tRNAs (Fig. 3A). Thermus thermophilus tRNA dihydrouridine synthase (TthDus) recognizes the elbow region of target tRNA and flips out the nucleotide at position 20 to present it to the catalytic active site (165). tRNA (cytosine (72)-C(5))-methyltransferase (NSUN6), responsible for 5-methylcytosine modification in C72 of the AAS, recognizes the specific consensus sequence on the acceptor arm (166) but also senses the overall shape of target tRNAs by additional contacts. Similarly, the heterodimeric tRNA m1A58 methyltransferase (m1A58 MTase) buries the whole tRNA and breaks the interaction between D- and T-arms to gain access and to methylate the N1 position of A58 (167). Although a tetrameric form of m1A58 MTase has been reported, it remains unclear if this represents a physiologically relevant oligomeric state of the complex.
Figure 3.
tRNA modification enzymes bind and recognize tRNAs using diverse strategies.A, gallery of available structures of tRNA modifying/processing enzymes solved with full-length tRNA (published since 2010). Precise modification sites are marked on the clover-leaf map of tRNA (left upper corner) and in the 3D structures (magenta). Elongator (PDB ID 8ASW), ADAT2/3 (PDB ID 8AW3), METTL1-WDR4 (PDB ID 8CTH), m1A58 MTase (PDB ID 5CD1), mitochondrial seryl-tRNA synthetase (PDB ID 7U2B), Dus (PDB ID 3B0V), Ribonuclease P (PDB ID 6AHU), TiaS (PDB ID 3AMT), Trm5a (PDB ID 5WT3), NSUN6 (PDB ID 5WWT), TSEN complex (PDB ID 7UXA/7ZRZ), RlmN (PDB ID 5HR6), QTRT1/2 (PDB ID 7NQ4), Cgi121 subunit of KEOPS (PDB ID 7KJT). All presented structures are cytoplasmic enzymes except for mitochondrial seryl-tRNA synthetase, which is marked by an orange asterisk. DusC from Escherichia coli (PDB ID 4YCP, 4YCO) is not shown as it is highly similar to TthDus (PDB ID 3B0V). The class I and class II aminoacyl tRNA synthetases are also included: arginyl-tRNA synthetase (ArgRS, PDB ID 5YYN) and glycyl-tRNA synthetase (GlyRS, PDB ID 7YSE). B, summary of the number of structures solved by different structural biology techniques together with full-length tRNA (2010–2022). KEOPS, Kinase, endopeptidase and other protein of small size complex.
Positions 34 and 37 on tRNAs, the modification hotspots in ASL, are modified with different moieties to guarantee a stringent decoding process. Despite these positions being in the same location on each tRNA, the mechanisms of binding and recognition for these positions differ dramatically. Such contextual recognition is essential for discrimination of nontarget from target tRNAs. For instance, tRNAIle-agm2C synthetase (TiaS) binds to the acceptor arm and the ASL and catalyzes the modification on C34. Contacts on the ASL stem are key substrate-specificity determinants of TiaS for tRNAIle (168). tRNA methyltransferase (Trm5a) in Pyrococcus abyssi undergoes a conformational change to bind tRNAs via their elbow as well as ASL and executes the bifunctional methyl-transfer at position G37 (169). The bacterial RNA methyltransferase (RlmN) modifies tRNA at A37. It binds to the concave surface of tRNA, like most tRNA-modifying enzymes, suggesting that recognition of the entire tRNA molecule is key for this modification machinery. Its tRNA-binding mode resembles those of aminoacyl-tRNA-synthetases, which contact the 3′-end and D-arm of tRNA substrates (170). The “Kinase, endopeptidase and other protein of small size complex” (KEOPS) contacts the 3′-CCA tail of tRNA via its Cgi121 subunit, which is essential for t6A37 modification (171). Details of how other subunits of the KEOPS complex orchestrate tRNA recognition is under investigation, but an initial tRNA–KEOPS complex model provides some insights into the potential reaction mechanism (172).
Because these tRNA-modifying enzymes act transiently and are highly dynamic, the chances of obtaining well-diffracting crystals are low thus scuttling structure determination. To overcome this problem, structural biologists have trimmed flexible regions of the proteins, crosslinked RNA to the complexes, or used point mutations to trap specific reaction intermediates. For instance, a crystal structure of heterodimeric human queuine tRNA-ribosyltransferase catalytic subunit 1 and 2 (QTRT1/2) crosslinked to a RNA stem-loop revealed details of substrate recognition and binding (173). These necessary compromises do raise concerns as to whether the obtained structures reflect the native architecture of the complexes. Foremost, the required quantities of homogenous tRNAs for macromolecular crystallography and NMR have complicated the determination of complexes with native tRNAs, reduced the variety of tRNA substrates used, and limited the number of structures of proteins in complex with full-length tRNA. For instance, out of the available repertoire of enzymes that modify tRNAs, the structures of only approximately 30% have been determined in complex with their tRNA target.
Recent developments in single-particle cryo-EM have boosted the field (Fig. 3B), allowing the structural characterization of enzymes in complex with tRNAs in their most natural conditions and requiring greatly reduced sample quantities. Moreover, advanced data analysis procedures allow delineation of different conformations and reaction states within the same sample, a feature that has the potential to provide a dynamic and more complete picture of the process of tRNA recognition and the underlying modification reaction itself. For example, the cryo-EM structure of the heterodimeric tRNA-specific adenosine deaminase 2/3 (ADAT2/3) complex bound to tRNA revealed that the anticodon loop, which harbors the modified nucleotide, is embedded in the catalytic site (174). The elbow region of the tRNA is recognized in a sequence-independent manner by distant regions of the protein located far from the catalytic core (174). Cryo-EM structures of the complex of methyltransferase 1-WD repeat–containing protein 4 (METTL1-WDR4) with a bound tRNA revealed that the noncatalytic subunit WDR4 interacts with the T-arm and is essential for the activity of METTL1, which further interacts with the elbow and modifies position 46 (175, 176).
Most of the known tRNA modification/processing enzymes exist as monomers or dimers and are usually relatively small (∼100 kDa) (62). One major exception is the 850-kDa eukaryotic Elongator complex, which is composed of two copies of its six individual subunits (e.g., Elp1, Elp2, Elp3, Elp4, Elp5, and Elp6). The Elongator complex binds tRNA via two contact points (177). The Elp1 subunit contacts T- and D-arms (178), while the catalytic Elp3 subunit interacts with the ASL and carboxymethylates uridines in position 34 (179). Elongator recognizes the overall tRNA shape and can bind target as well as nontarget tRNAs with similar binding affinities in vitro (177). Another family of tRNA-processing enzymes that have recently been studied using single particle cryo-EM are well-known aminoacyl-tRNA synthetases. For instance, the cryo-EM structure of mitochondrial seryl-tRNA synthetase (mt-SerRS) ultimately shows how this protein specifically recognizes this rather unusual tRNA that lacks the D-arm. These enzymes recognize the radically altered T-arm topology, which leads to the loss of the canonical L-shape (180). Very recently, the structures of arginyl-tRNA synthetase (ArgRS) (181) and glycyl-tRNA synthetase (GlyRS) (182, 183) from different organisms have been determined in the presence of full-length tRNA.
Several cryo-EM studies of human RNase P bound to a mature tRNA reveal how a catalytic RNA aided by 10 protein subunits recognizes the acceptor-T stem stack for accurate pre-tRNA processing (184). Recent cryo-EM structures of human tRNA splicing endonuclease complex together with a pre-tRNA in precleavage and postcleavage states revealed how the multisubunit complex coordinates substrate recognition and the two-step cleavage reaction (185, 186, 187). Three subunits of tRNA splicing endonuclease interact with the T-arm, D-arm, and the anticodon stem, which acts as a ruler to correctly orient the 5′-splice site and 3′-splice site at the catalytic pocket.
tRNA modification and processing enzymes alter the structure of tRNA
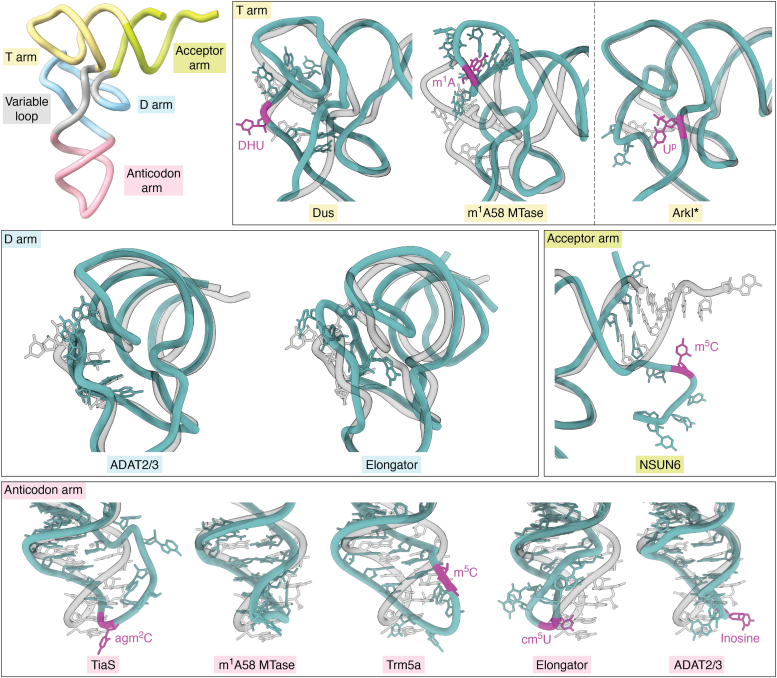
The structural studies mentioned above also illustrate that the bound tRNAs undergo clearly observable conformational changes while binding to the enzymes and during the intermediate steps of the modification reactions. These conformational changes aim to properly recognize and position the tRNAs and expose the specific target site (or region) to the active site of the modifying enzyme. The movements can be observed in almost every region of tRNA, either as local movements proximal to the target nt or large scale global conformational changes (Fig. 4). For example, NSUN6 methylates C72 in tRNACysGCA and tRNAThrUGU and distorts the conformation of the most distal 3ʹ region (166). Likewise, changes in the elbow region of tRNA can be observed upon binding of tRNA-dihydrouridine synthase (165, 188).
Figure 4.
Modification enzymes influence tRNA structure in various regions. Yeast tRNAPheGAA (PDB ID 1EHZ) is aligned on each structure by the matchmaker tool in ChimeraX and shown transparently. Structures are sorted by the region affected structurally, and the names of the modifying enzymes are colored, accordingly. Modification sites are marked in magenta. Structures of full length-tRNAs are shown without their respective protein partners - Dus (PDB ID 3B0V), m1A58 MTase (PDB ID 5CD1), Elongator (PDB ID 8ASW), ADAT2/3 (PDB ID 8AW3), TiaS (PDB ID 3AMT), Trm5a (PDB ID 5WT3), NSUN6 (PDB ID 5WWT). ArkI (PDB ID 7VNV) is a crystal structure of tRNA alone, modified by TkArkI.
In contrast, the binding of human m1A58 MTase to tRNA not only changes the canonical shape of the elbow region (167) but also alters the shape of the ASL, which is distant from the modification site. Similarly, distant structural changes of the tRNA elbow were also reported in the T. brucei ADAT2/3 deaminase complex (174) and the yeast Elongator complex (178). A more drastic tRNA conformation change is observed in a complex with archaeosine tRNA-guanine transglycosylase, which forms a “λ form” of bound tRNA to allow the target site to be accessible to the enzyme (102). The phosphorylation of tRNA in position 47 by Thermococcus kodakarensis Arkl also influences the shape of the elbow region by restricting local backbone rotation (94) (Fig. 4). In this specific case, the described structural changes could be the consequence of the modification itself, as the observed differences are visible in the crystal structure of the phosphorylated tRNA in the absence of the modification enzyme. As these structures were obtained after trapping early reaction intermediates, these structural rearrangements are directly induced by the contacts between the tRNA and the protein complexes.
In all the cases presented above, the target nucleotides of the modification reaction were specifically flipped out from the canonical tRNA structure, facilitating access to the enzymatic active site. Although the three bases of the anticodon are already flipped out and accessible, this phenomenon is also visible in tRNA structures in complex with enzymes modifying residue 34 within the ASL (168, 174, 189) (Fig. 4). In all three mentioned cases, the conformation of the ASL is still further distorted, which may be necessary to discriminate between the other accessible bases and different tRNAs with similar anticodon sequences. In summary, the distortion of the tRNAs seems to be a common feature of almost all tRNA modification enzymes. Therefore, the respective proteins and protein complexes have evolved features that go beyond providing the enzymatic requirements for the respective modification reaction. They also guarantee the proper exposure of the specific target site. On the one hand, these additional conditions may have impeded modifications in certain positions on the tRNA. On the other hand, they may have supported the development of modification hotspots in certain domains (e.g., ASL).
Unexpected appearance, function, and locations of tRNAs
In the previous sections, we summarized the specific three-dimensional features of tRNA molecules and described the fundamental determinants that influence their maturation and folding. In contrast to the conserved features, tRNAs sometimes do not obey their own rules and lack features that otherwise would appear crucial. Furthermore, some tRNAs are specifically cleaved and the obtained products can fulfill additional functions. Finally, as tRNAs are highly abundant, rigid building blocks present in all living cells, it is not surprising that they are also used in other cellular pathways and are even exploited by viruses and pathogens for their own benefit. In the following section, we summarize the most recently discovered and surprising examples of tRNAs, tRNA-like molecules, and tRNA-derived fragments.
Virus hijacking or mimicking host tRNAs
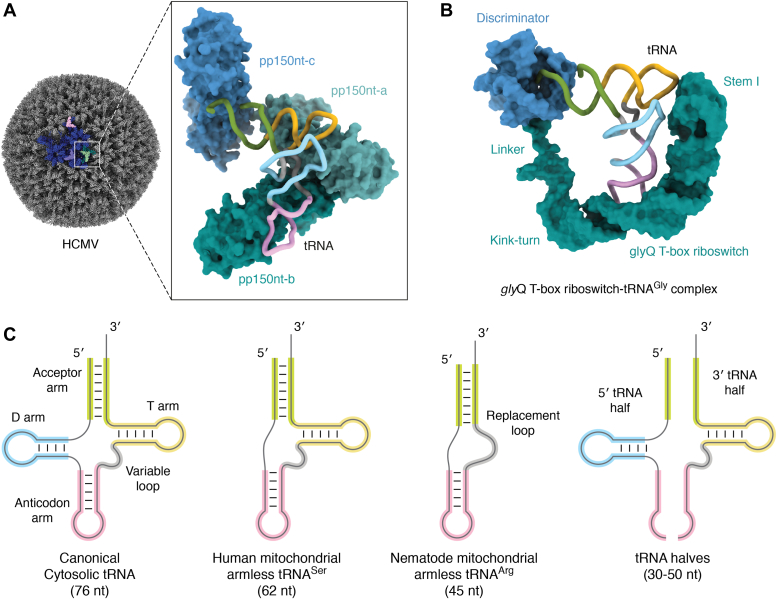
Viruses are known to hijack host molecules, such as rRNA and tRNAs, to enhance transcription, translation, and virion assembly (190). For instance, HIV type 1 retroviruses package host tRNAs during virion assembly and employ them as primers to initiate reverse transcription after infecting human cells. A recent study revealed a novel manner by which viruses recognize and utilize tRNAs from host cells. For instance, HIV type 1 virions are assembled by incorporation of multiple copies of the structural Gag protein, which selectively binds to several human tRNAs, including tRNALysCUU, tRNALysUUU, tRNAGluCUC, tRNAGluUUC, tRNAGlyGCC, tRNAGlyCCC, tRNAValAAC, and tRNAValCAC. The binding selectivity is achieved via the N-terminal matrix domain (MA) of Gag, which harbors four critical residues to track the characteristic tRNA elbow (191). The U-shape curvature and the invariant G19-C56 pairing in the T-loop also contributes to tRNA recognition and selection. Of note, the binding of tRNAs occludes the strong basic surface area of MA, which prevents the interaction of Gag with membranes and thus prevents premature virion assembly. In stark contrast to the highly regulatory role of host tRNAs in the HIV-1 life cycle, human cytomegalovirus is also known to associate with human host RNA but the details remained unclear. Recently, Liu et al. used a combination of cryo-EM and deep RNA-seq to confirm the presence of tRNAs bound via pp150, a tegument protein forming a net-like density layer between the nucleocapsid and virion envelope (192). They identified at least six different cytosolic tRNA species, with tRNAGluCUC appearing to be the most abundant bound tRNA. Structurally, one tRNA molecule bridges three tegument pp150 proteins (Fig. 5A). The interactions are mediated via the phosphate backbone instead of specific base-stacking, explaining the low sequence-specificity. Surprisingly, this way of incorporating host tRNAs directly into the capsid seems to be unique for human cytomegalovirus because the structures of β-herpesviruses murine cytomegalovirus and human herpesvirus 6B capsids reveal no sign of tRNA association between their pp150 counterparts (192).
Figure 5.
Examples of tRNAs with unexpected functions and atypical appearances.A, a cartoon representation of a cryo-EM structure of HCMV virion (PDB ID 5VKU) with tRNA bound to pp150 nt (inlet, PDB ID 7LJ3). The asymmetrical unit in the virion is colored in blue and the selected tRNAs are highlighted in pink, purple, and green. B, a cartoon representation of a cryo-EM structure of a full-length of Bacillus subtilis glyQ T-box-tRNAGlyGCC complex (PDB ID 6POM). The tRNA is illustrated as spaghetti representation, while the binding partners are shown with surface representation. C, two-dimensional representation of various tRNA folds, including the canonical and truncated forms. HCMV, human cytomegalovirus.
The term “tRNA-like structure” (TLS) refers to RNA motifs, which can fold into a tRNA fold. This type of a tRNA-mimicking motif was first discovered in the genome of positive-strand RNA Turnip yellow mosaic virus, and additional TLSs were identified later in other viruses and even mammals (193, 194). Apart from a predicted transcript with a sequence similar to tRNAs, several criteria need to be met to define a TLS. The potential TLS should (i) be able to fold into the full (or at least partial) shape of a tRNA (195), (ii) it should be able to interact with one or more tRNA-specific interactors (e.g., aminoacyl-tRNA synthetases, RNase P, RNase Z, CCA-NTase, or eEF1A). Several TLS that fulfill these requirements reside in viral genomic RNAs, mRNAs, and noncoding RNAs. Employing tRNA mimicry is a relatively simple strategy for viruses to hijack and to exploit the host’s cellular inventory for its own purposes. For instance, the Hepatitis C virus harbors a tRNA-like domain in its internal ribosome entry sites to facilitate binding to the human 80S ribosome (196). A tRNA-like element has also been identified in the 5′ untranslated region of the HIV-1 genome proximal to the primer-binding site. This U-rich element mimics the anticodon loop of human tRNALysUUU and competitively binds to lysyl-tRNA synthetase, thus increasing the availability of free tRNALysUUU, which serves as the primer for reverse transcription (197). As demonstrated in the crystal structure of Turnip yellow mosaic virus-TLS, the TLS folds into the characteristic L-shape, containing a pseudo-knotted acceptor stem (195). These TLSs can also be aminoacylated, like bona fide tRNAs. However, the selectivity of aminoacyl-tRNA synthetases limits aminoacylation of TLS to three amino acids, namely valine, histidine, and tyrosine. These various aminoacylated TLSs execute their function via different routes (198, 199). The TLS from Brome mosaic virus (BMV) has served as a model system to investigate the regulation of static and dynamic RNA structures for decades. For instance, the BMV TLS has been demonstrated to change conformation upon metal ion binding, which directly regulates viral replication (200). Recently, Bonilla et al. (201) presented the cryo-EM structures of the BMV TLS as well as the complex between TLS and tyrosyl-tRNA synthetase. Their work revealed how the BMV TLS undergoes conformational changes to interact with a host factor in a noncanonical fashion to achieve aminoacylation.
In stark contrast to the abundance of TLSs in viruses, only a limited number have been found in eukaryotic model organisms or humans (194). There are two known TLS in humans, namely mascRNA and Menβ, which are the cleavage products derived from two long noncoding RNAs, namely metastasis-associated long adenocarcinoma transcript 1 (MALAT1) and Menβ (also known as NEAT1). Both TLSs are CCA-edited at their 3ʹ-ends, but Menβ possesses an unstable acceptor stem which prompts the CCA-NTase to add tandem CCA motifs and trigger degradation (202). Although mascRNA has a 3ʹ-CCA, it is not aminoacylated. mascRNA has been linked to increased global protein synthesis and cell proliferation rates, as well as to several other cellular events, including antiviral and cardiovascular innate immunity regulation. A recent biochemical investigation demonstrated that mascRNA binds to glutaminyl-tRNA synthetase via its unique anticodon loop and upregulates glutaminyl-tRNA synthetase protein levels to promote protein translation (203). Although the aminoacyl-tRNA synthetase–enhanced protein translation is also observed in histidyl-tRNA synthetase (204), the details of this regulatory circuit remain elusive.
Y RNA-like A (YrlA) RNA represents a new class of tRNA mimicry, as it contains canonical tRNA motifs. YrlA RNA belongs to the family of Y RNAs, which are noncoding RNAs mostly found in bacteria and animal cells. The tRNA mimicry resembles the distinct tRNA L-shape (205), and these motifs are substrates of tRNA processing and modifying enzymes. The tRNA mimicry motif in Y RNA is believed to stabilize the structure and facilitate interaction with the Ro60 protein to regulate downstream cellular functions. Yet another kind of TLS is named nimtRNA for nuclear intronic mitochondrial-derived tRNAs. These nimtRNAs are transcribed from nuclear genomic DNA, which were originally derived from mitochondrial tRNA genes (206). These mitochondrial-tRNA look-alikes have been transferred and inserted into the human nuclear genome and are mainly found in the intronic regions of protein-coding or noncoding transcripts. Some of the sequences are identical to their mitochondrial predecessor, and others contain up to 25 mismatches while retaining the ability fold into the distinct tRNA structure (207). It has been shown that the presence of nimtRNAs in introns promotes pre-mRNA splicing and the tRNA-like motif seems crucial for the enhanced splicing efficiency.
tRNA sensing and metabolism
The T-box riboswitch is a regulatory noncoding RNA that binds to specifically uncharged tRNAs and thereby sense the availability of amino acids. This readout is used to attenuate downstream gene expression at the transcriptional or translational level (208). Over 23,000 T-box riboswitch domains are annotated according to the riboswitch database (209). These riboswitches are mainly found at the 5ʹ-leader regions of certain mRNAs. Meanwhile, a smaller number of T-box riboswitches can be found mostly in Actinobacteria, where they control translation. In general, riboswitches act in a two-step sensory and regulatory mechanism. In the past decade, many crystal and cryo-EM structures of T-box riboswitches with the appropriate tRNA target have been reported, but most structures only contain parts of the riboswitch. Therefore, we lack a complete picture of how each motif of the riboswitch orchestrates the conformational changes upon tRNA binding (210). Recently, an intermediate resolution cryo-EM structure of the complete Bacillus subtilis glyQ T-box riboswitch, which belongs to the class that controls transcription, revealed the overall conformation during tRNA recognition (211) (Fig. 5B). The overall U-shape architecture of the riboswitch “clamps” the cognate tRNAGlyGCC and the stem III, and discriminator domains sense the aminoacylation status (211). The regulatory domain can then switch between a terminator or antiterminator confirmation to govern the subsequent activation of transcription or translation (212). Overall, the recognition of cognate tRNA happens via the stem I domain of the riboswitch, which pairs the anticodon sequence of the tRNA with a “Specifier Sequence”. A crystal structure of the full-length of Mycobacterium tuberculosis ileS T-box, which belongs to the class that controls translation, shows that it recognizes the cognate tRNA similar to the glyQ T-box but does not contact the tRNA elbow (213). Another crystal structure of stem I and stem II domains of a Nocardia farcinica ileS translational T-box riboswitch in complex with its cognate tRNAIleGAU reveals a different picture of how stem II reinforces the interaction of stem I and tRNA (214). In summary, the rigid structure of tRNAs is used by a variety of biosensors to determine the aminoacylation levels of tRNA, which is directly related to the metabolic state of the cell.
Glycosylated tRNAs
tRNA is heavily decorated with various chemical moieties (90), including the attachment of sugar groups. Queuosine (Q), a 7-deazaguanosine nucleoside, is obtained from gut bacteria and incorporated in the anticodon wobble position of cytosolic tRNATyrGUA, tRNAHisGUG, tRNAAsnGUU, and tRNAAspGUC in humans (215). The Q-modified position 34 in tRNATyrGUA and tRNAAspGUC is further “sugar coated”. Unlike the polysaccharide conjugates on other noncoding RNAs, proteins, or lipid, tRNATyrGUA and tRNAAspGUC carry a monosaccharide. Two glycosylated derivatives exist, namely galactose-Q (galQ)-tRNATyrGUA and mannose-Q (manQ)-tRNAAspGUC. A method has been developed using an acid-denaturing gel and northern blot to detect and quantify the presence galQ-tRNATyrGUA and manQ-tRNAAspGUC (216). These glycosylated variants are found in all tested human organs without any tissue specificity, and the expression levels of all three modifications (galQ, manQ, and Q) seem to be correlated with age. These modified tRNAs are located in the cytosol and the nucleus. The enzyme responsible for this modification and the functional significance of this modification are yet to be identified. The chemical synthesis and the structure of galQ (217) and manQ (218) have been reported, while the biochemical pathways leading to them in living organisms still remains unknown. In addition to these known sugar modifications of tRNATyrGUA and tRNAAspGUC, a recent study employed a global labeling method and discovered that many tRNA species are attached to sialylated glycans in living cells (219). These so called “glyco-tRNAs” as well as other “glyco-RNAs” seem to be associated with membrane organelles and can also be detected on the cell surface. However, the modified sites on tRNAs and the detailed mechanism of RNA glycosylation are not known.
The atypical appearance of armless tRNAs
Despite the highly conserved L-shape structure of tRNA, there exists a group of structurally bizarre tRNAs (Fig. 5C) lacking D- and T-arms (220). They mostly exist in the mitochondria of nematodes, like Caenorhabditis elegans or Ascaris suum. Recently, it has also been found in the mitochondrial genome of arachnids (221). These structurally degenerate tRNAs are the consequences of the high mutations rates and genetic drift in the mitochondrial genome. Lack of either T-arm or D-arm (or both in extreme cases), as well as accumulation of weak base pairs and mismatches in stem regions, gives rise to the observed structural diversity (222, 223, 224). For instance, the smallest tRNA (with only 42 nt) has been identified in the mitochondria of Dermatophagoides farina, a mite. The predicted structure of two base-paired stems, connected by a variable bulge element, still resembles the dimensions and architecture of tRNAs (225). In the case of the T-armless tRNA, the canonical T-arm is replaced by a less structured 6- and 12-nt loop, named TV-replacement loop. Computational predictions show that some of these arm-less tRNAs retain the basic hairpin structure and adopt stable 3-D structures with “boomerang-like” shapes (224). Regardless of the missing parts, some of the miniaturized tRNAs have been proven to be functional, as they can be further edited by amino acid–specific aminoacyl-tRNA synthetases, CCA-NTase (226), and can be recognized by specific Elongator factor-Tu (EF-Tu) for mitochondrial protein translation (227). Of note, posttranscriptional modifications are also present on these arm-less tRNAs, including a methylation at position 9 (m1A9) (223). These modifications could stabilize mt-tRNA structure, enhance thermostability, and facilitate aminoacylation and translation. Correct aminoacylation of these arm-less tRNAs is crucial to ensure fidelity of mitochondrial translation. As the arm-less tRNA acts as an adapter (like the canonical tRNA), the mitochondrial EF-Tu1 and EF-Tu2 have undergone coevolutionary modulation to recognize features of these truncated tRNAs (228).
Mammals harbor mitochondrial tRNAs without D-arms, as exemplified by the mt-tRNASerGCU iso-acceptors that show this deviated architecture (229). Similar to armless tRNAs from nematodes, mammalian mt-tRNASerGCU also harbors RNA modifications (22), including three consecutive 5-methylcytosines (m5C) at positions C47, C49, and C50. A recent cryo-EM structure revealed the details of the armless mt-tRNASerGCU in complex with human mitochondrial seryl-tRNA synthetase (180). Due to the lack of D arm, the mt-tRNASerGCU loses its tRNA core domain and unexpectedly adopts an acute-angled “Y” shape. Furthermore, this drastically reshaped topology results in the loss of identity elements in mt-tRNASerGCU. As a consequence of this highly degenerated architecture, the human mitochondrial seryl-tRNA synthetase has also coevolved to bind to the specific acceptor stem of mt-tRNASerGCU via its distinct helical arm and to charge the tRNA. Moreover, the stability of mt-tRNASerGCU is sensitive to its tRNA charging status (230). The loss of mt-tRNASerGCU is linked to retinal disease and class II mitochondrial seryl-tRNA-synthetase–related diseases (231).
Suppressor tRNAs
A subgroup of tRNAs exist as “suppressor tRNAs” in some species. These tRNAs can read through stop codons and nonsense mutations during translation (232, 233). They have been postulated to have resulted from mutations in the anticodon sequence, which enabled pairing with stop codons and decoding nonsense mutations in mRNAs. Recently, Kachale et al. discovered a “reassignment” of an in-frame stop codon to tRNATrpCCA in a trypanosomatid, Blastocrithidia nonstop. A similar mechanism is also found in the ciliate Condylostoma magnum. Interestingly, tRNATrpCCA in both species harbors a shorter tRNA anticodon stem (from 5-bp to 4-bp). When a shorter anticodon stem version of tRNATrpCCA was engineered in yeast system, this specific tRNA indeed enhanced stop codon read-through (233). In principle, the simple engineering of the anticodon triplet of sense-coding tRNAs could transform them into suppressor tRNAs. However, only a few such mutant tRNAs have actually achieved high stop codon read-through activity and restored protein production (234). Various groups have taken different approaches (15, 235, 236) to understand what segments of tRNA are critical for suppression activity. Interestingly, redesigning the T-stem seems to be key strategy for suppressor tRNA to maintain a high decoding rate by strengthening the interaction with eEF1A.
The “+1-frameshift” (+1FS) suppressor tRNAs contain a single-nt insertion, which most often appears within the ASL (237, 238, 239). This extra nt generates a longer loop (8 nt), which differs from the canonical architecture. Nonetheless, the frameshift suppressor tRNA still decodes like a normal tRNA, as its anticodon is still defined by the universally conserved U33 and m1G37 (240). The local ASL structure still undergoes distortion by disrupting the interaction between position 32 and 38 (239, 241, 242). A +1-frameshift takes place during translocation, which is mediated by +1FS suppressor tRNA, ribosome, mRNA, and elongation factor EF-G (241, 243, 244). These “slippery” tRNAs, which facilitate frameshifting of codon triplets, could be exploited biotechnologically (245).
tRNA in biotechnological and pharmaceutical applications
tRNA is not only important in fundamental regulation but also has been used in biotechnological and pharmaceutical applications. Ponchon and Dardel developed a system based on a camouflage strategy to fuse a target RNA with a tRNA scaffold (246). Due to the rigid and highly structured features of tRNA, the chimeric RNA was able to escape cellular RNase digestion. The target RNA can then be produced, purified, and released by RNase H treatment for downstream applications. Recently, Lee et al. (247) have taken the “tRNA-scaffold” approach and successfully determined the crystal structure of the ZIKA virus stem-loop A-tRNA to provide insights into the mechanism of stem-loop A–mediated RNA synthesis. As suppressor tRNAs naturally exist in a few species, the frameshift-suppressor tRNA or suppressor tRNA that decodes nonsense mutation have been employed in orthogonal translation systems (245). This strategy allows refactoring codons and incorporating noncanonical amino acids to expand the genetic code for various designed functions. For instance, an engineered suppressor tRNA can be charged with the desired amino acid containing the glycan modification and this artificial product is incorporated into the target protein at the chosen position for further analyses (248). Moreover, decades ago, suppressor tRNAs have been proposed and engineered as a therapeutic tool to read through the premature codon termination. This method has been applied to several premature codon termination–related disease models, such as β-thalassemia (249) and cystic fibrosis transmembrane conductance regulator (234). The selected engineered tRNA indeed display promising effects on rescuing target protein production and restoring the responsible cellular function in cell and animal models (250, 251).
Future perspectives and directions
Despite the fact that tRNAs are highly structured and rigid RNA molecules, their dynamic and transient interactions with many processing factors and modification enzymes have complicated the structural analyses of the different maturation steps of tRNAs. Research on the dynamic structures of RNA domains has recently been changed fundamentally by the advent of high-resolution single particle cryo-EM (252). Recent examples (175, 176) have shown that cryo-EM facilitates the structural analyses of several different reaction intermediates without the need for identifying novel crystallization conditions for each individual conformation. We will most likely obtain whole galleries of structural snapshots for most tRNA modification reactions in the near future. Such an advance will fundamentally change our understanding of the sequential order of individual reaction steps and allow us to explore the dynamic transitions between them. Cryo-EM requires small amounts of sample, an attribute that will enable us to conduct structural studies on low-abundant RNAs isolated from endogenous sources or from sufficient quantities of in vitro–modified RNA samples. It is not only a new method to study previously known samples but it also allows us to rethink the list of RNA samples that can be visualized at high-resolution.
Recent advances in the field have essentially solved the fundamental problem of predicting three-dimensional structures of proteins from the primary amino acid sequence (253). The relatively small number of experimentally determined RNA and protein-RNA structures hinder the development of reliable models to predict the structure of these dynamic assemblies reliably in silico. Nonetheless, we are certain that the rapid advances in machine learning and molecular modeling will offer us significant support to understand the interaction between tRNAs and their cellular environment. Recently, an intermediate resolution cryo-EM structure of a DNA G-quadruplexes has demonstrated that we can reconstruct maps even for a 28-kDa biomolecule using electron microscopes (254). This advance suggests that RNAs with similar mass (e.g., tRNAs, armless tRNAs, Y RNAs, or tRNA-like molecule) can also be analyzed by cryo-EM. It remains to be shown whether one can obtain near-atomic resolution structures of RNAs that are more dynamic than proteins. As certain RNA modifications are known to increase the stability/rigidity of RNAs, these modifications may be exploited to achieve higher resolution structures.
Apart from studies on tRNA and tRNA-like molecules, numerous functional studies have identified subpopulations of small noncoding RNAs that are derived from tRNA, called tRNA halves or tRNA-derived fragments (Fig. 5C). These tRNA derivatives, either as separated tRNA halves or as a nicked tRNA complex, were reported to be involved in a plethora of cellular functions and pathways (255, 256, 257, 258).
As mentioned above, it still remains unclear if and how individual tRNA modification cascades are coupled or even depend on each other (97, 98, 162, 163). The first time-resolved NMR studies have tried to dissect the hierarchy of tRNA decoration and highlight the exciting complementary opportunities to record multiple modifications steps in parallel (162, 259). As tRNA modifications can directly influence the folding of tRNAs, the efficiency of the underlying modification reactions might provide another route to control cellular translation. As some of the modification cascades directly rely on basic metabolic pathways (260, 261, 262), tRNA-related processes might represent molecular switches between the metabolic state and the regulation of protein synthesis in a cell. Therefore, we need to fully understand tRNA processing and modification pathways at the cellular and organismal level in the future.
A major question that needs to be addressed soon is the actual role of the processing and modification enzymes for the functionality of tRNAs. We lack a fundamental mechanistic understanding of whether the tRNA-interacting proteins can contribute more than “just” attaching chemical groups or if they also act as molecular chaperones or regulators. Here, the biophysical and structural analyses of tRNAs before and after a specific modification reaction might provide direct insights. Furthermore, it remains to be shown whether tRNA splicing and processing activate or respond to specific tRNA modifications. Finally, modified nt of tRNAs, mRNAs, and ribosomal RNA are proximal to one another during ribosomal decoding. We need to understand whether these molecules and their modifications communicate with one another to reveal yet another regulatory layer of gene expression.
Finally, tRNA-based technologies seem to have a very bright future in the field of personalized biomedicine, especially if suppressor tRNAs break the current limitations of highly selective aminoacyl-tRNA synthetases. Here, natural variations of tRNAs and translation systems can and should inspire us to develop novel tRNA-based tools and technologies that could enable us to control, correct, or restore cellular protein synthesis.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We would like to thank all members of the Glatt lab for insightful discussion during the preparation of this manuscript and Jonathan G. Heddle for additional comments. We used ChimeraX for illustrating protein-tRNA structures and ChemSketch for depicting chemical modifications. Due to the vast number of published studies in the field, we were not able to refer to all the available publications. Therefore, we would apologize to all researchers in the community who were not directly cited or acknowledged.
Author contributions
A. B., A. H., I. K., M. W., L. K., T.-Y. L., and S. G. writing–original draft; A. B., A. H., I. K., M. W., L. K., T.-Y. L., and S. G. visualization; T.-Y. L. and S. G. conceptualization; A. B., A. H., I. K., M. W., L. K., T.-Y. L., and S. G. investigation.
Funding and additional information
This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No 101001394 to S. G.).
Reviewed by members of the JBC Editorial Board. Edited by Karin Musier-Forsyth
Contributor Information
Ting-Yu Lin, Email: ting-yu.lin@uj.edu.pl.
Sebastian Glatt, Email: sebastian.glatt@uj.edu.pl.
References
- 1.Crick F.H. On protein synthesis. Symp. Soc. Exp. Biol. 1958;12:138–163. [PubMed] [Google Scholar]
- 2.Roeder R.G., Rutter W.J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969;224:234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- 3.Weinmann R., Raskas H.J., Roeder R.G. Role of DNA dependent RNA polymerases II and III in transcription of the adenovirus genome late in productive infection. Proc. Natl. Acad. Sci. U. S. A. 1974;71:3426–3430. doi: 10.1073/pnas.71.9.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan P.P., Lowe T.M. GtRNAdb 2.0: an expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 2016;44:D184–D189. doi: 10.1093/nar/gkv1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baranov P.V., Gesteland R.F., Atkins J.F. Recoding: translational bifurcations in gene expression. Gene. 2002;286:187–201. doi: 10.1016/s0378-1119(02)00423-7. [DOI] [PubMed] [Google Scholar]
- 6.Lai L.B., Lai S.M., Szymanski E.S., Kapur M., Choi E.K., Al-Hashimi H.M., et al. Structural basis for impaired 50 processing of a mutant tRNA associated with defects in neuronal homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2022;119 doi: 10.1073/pnas.2119529119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott J.A., Francklyn C.S., Robey-Bond S.M. Transfer RNA and human disease. Front. Genet. 2014;5:158. doi: 10.3389/fgene.2014.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodarzi H., Nguyen H.C.B., Zhang S., Dill B.D., Molina H., Tavazoie S.F. Modulated expression of specific tRNAs drives gene expression and cancer progression. Cell. 2016;165:1416–1427. doi: 10.1016/j.cell.2016.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres A.G., Batlle E., Ribas de Pouplana L. Role of tRNA modifications in human diseases. Trends Mol. Med. 2014;20:306–314. doi: 10.1016/j.molmed.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Schaffer A.E., Pinkard O., Coller J.M. tRNA metabolism and neurodevelopmental disorders. Annu. Rev. Genomics Hum. Genet. 2019;20:359–387. doi: 10.1146/annurev-genom-083118-015334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirchner S., Ignatova Z. Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nat. Rev. Genet. 2015;16:98–112. doi: 10.1038/nrg3861. [DOI] [PubMed] [Google Scholar]
- 12.Hawer H., Hammermeister A., Ravichandran K.E., Glatt S., Schaffrath R., Klassen R. Roles of elongator dependent tRNA modification pathways in neurodegeneration and cancer. Genes (Basel) 2019;10:1–23. doi: 10.3390/genes10010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaik M., Kojic M., Stegeman M.R., Öncü-Öner T., Kościelniak A., Jones A., et al. Functional divergence of the two elongator subcomplexes during neurodevelopment. EMBO Mol. Med. 2022;14 doi: 10.15252/emmm.202115608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojic M., Wainwright B. The many faces of elongator in neurodevelopment and disease. Front. Mol. Neurosci. 2016;9:115. doi: 10.3389/fnmol.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albers S., Beckert B., Matthies M.C., Mandava C.S., Schuster R., Seuring C., et al. Repurposing tRNAs for nonsense suppression. Nat. Commun. 2021;12:1–10. doi: 10.1038/s41467-021-24076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharp S.J., Schaack J., Cooley L., Burke D.J., Soil D. Structure and transcription of eukaryotic tRNA gene. Crit. Rev. Biochem. Mol. Biol. 1985;19:107–144. doi: 10.3109/10409238509082541. [DOI] [PubMed] [Google Scholar]
- 17.Holley R.W., Apgar J., Everett G.A., Madison J.T., Marquisee M., Merrill S.H., et al. Structure of a ribonucleic acid. Science. 1965;147:1462–1465. doi: 10.1126/science.147.3664.1462. [DOI] [PubMed] [Google Scholar]
- 18.Giegé R., Jühling F., Pütz J., Stadler P., Sauter C., Florentz C. Structure of transfer RNAs: similarity and variability. Wiley Interdiscip. Rev. RNA. 2012;3:37–61. doi: 10.1002/wrna.103. [DOI] [PubMed] [Google Scholar]
- 19.Betat H., Rammelt C., Mörl M. tRNA nucleotidyltransferases: ancient catalysts with an unusual mechanism of polymerization. Cell. Mol. Life Sci. 2010;67:1447–1463. doi: 10.1007/s00018-010-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sprinzl M., Cramer F. The -C-C-A end of tRNA and its role in protein biosynthesis. Prog. Nucleic Acid Res. Mol. Biol. 1979;22:1–69. doi: 10.1016/s0079-6603(08)60798-9. [DOI] [PubMed] [Google Scholar]
- 21.Hamashima K., Tomita M., Kanai A. Expansion of noncanonical V-arm-containing tRNAs in eukaryotes. Mol. Biol. Evol. 2016;33:530–540. doi: 10.1093/molbev/msv253. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T., Yashiro Y., Kikuchi I., Ishigami Y., Saito H., Matsuzawa I., et al. Complete chemical structures of human mitochondrial tRNAs. Nat. Commun. 2020;11:4269. doi: 10.1038/s41467-020-18068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pütz J., Dupuis B., Sissler M., Florentz C. Mamit-tRNA, a database of mammalian mitochondrial tRNA primary and secondary structures. RNA. 2007;13:1184–1190. doi: 10.1261/rna.588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hafez M., Burger G., Steinberg S.V., Lang F. A second eukaryotic group with mitochondrion-encoded tmRNA. RNA Biol. 2013;10:1117–1124. doi: 10.4161/rna.25376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamashima K., Fujishima K., Masuda T., Sugahara J., Tomita M., Kanai A. Nematode-specific tRNAs that decode an alternative genetic code for leucine. Nucleic Acids Res. 2012;40:3653–3662. doi: 10.1093/nar/gkr1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jühling F., Pütz J., Florentz C., Stadler P.F. Armless mitochondrial tRNAs in Enoplea (Nematoda) RNA Biol. 2012;9:1161–1166. doi: 10.4161/rna.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorenz C., Lünse C.E., Mörl M. tRNA modifications: impact on structure and thermal adaptation. Biomolecules. 2017;7:35. doi: 10.3390/biom7020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madore E., Florentz C., Giege R., Lapointe J. Magnesium-dependent alternative foldings of active and inactive Escherichia coli tRNAGlu revealed by chemical probing. Nucleic Acids Res. 1999;27:3583–3588. doi: 10.1093/nar/27.17.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinberg S., Leclerc F., Cedergren R. Structural rules and conformational compensations in the tRNA L-form 1 1Edited by D. E. Draper. J. Mol. Biol. 1997;266:269–282. doi: 10.1006/jmbi.1996.0803. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J., Ferré-D'Amaré A. The tRNA elbow in structure, recognition and evolution. Life. 2016;6:3. doi: 10.3390/life6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertus J.D., Ladner J.E., Finch J.T., Rhodes D., Brown R.S., Clark B.F.C., et al. Structure of yeast phenylalanine tRNA at 3 Å resolution. Nature. 1974;250:546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- 32.Kim S.H., Suddath F.L., Quigley G.J., McPherson A., Sussman J.L., Wang A.H.J., et al. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974;185:435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- 33.Suddath F.L., Quigley G.J., McPherson A., Sneden D., Kim J.J., Kim S.H., et al. Three-dimensional structure of yeast phenylalanine transfer RNA at 3. 0Å resolution. Nature. 1974;248:20–24. doi: 10.1038/248020a0. [DOI] [PubMed] [Google Scholar]
- 34.Jovine L., Djordjevic S., Rhodes D. The crystal structure of yeast phenylalanine tRNA at 2.0 Å resolution: cleavage by Mg2+ in 15-year old crystals. J. Mol. Biol. 2000;301:401–414. doi: 10.1006/jmbi.2000.3950. [DOI] [PubMed] [Google Scholar]
- 35.Moras D., Comarmond M.B., Fischer J., Weiss R., Thierry J.C., Ebel J.P., et al. Crystal structure of yeast tRNAAsp. Nature. 1980;288:669–674. doi: 10.1038/288669a0. [DOI] [PubMed] [Google Scholar]
- 36.Westhof E., Dumas P., Moras D. Crystallographic refinement of yeast aspartic acid transfer RNA. J. Mol. Biol. 1985;184:119–145. doi: 10.1016/0022-2836(85)90048-8. [DOI] [PubMed] [Google Scholar]
- 37.Woo N.H., Roe B.A., Rich A. Three-dimensional structure of Escherichia coli initiator tRNAfMet. Nature. 1980;286:346–351. doi: 10.1038/286346a0. [DOI] [PubMed] [Google Scholar]
- 38.Basavappa R., Sigler P.B. The 3 A crystal structure of yeast initiator tRNA: functional implications in initiator/elongator discrimination. EMBO J. 1991;10:3105–3111. doi: 10.1002/j.1460-2075.1991.tb07864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bénas P., Bec G., Keith G., Marquet R., Ehresmann C., Ehresmann B., et al. The crystal structure of HIV reverse-transcription primer tRNA(Lys,3) shows a canonical anticodon loop. RNA. 2000;6:1347–1355. doi: 10.1017/s1355838200000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Väre V., Eruysal E., Narendran A., Sarachan K., Agris P. Chemical and conformational diversity of modified nucleosides affects tRNA structure and function. Biomolecules. 2017;7:29. doi: 10.3390/biom7010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crothers D.M., Seno T., Söll G. Is there a discriminator site in transfer RNA? Proc. Natl. Acad. Sci. U. S. A. 1972;69:3063–3067. doi: 10.1073/pnas.69.10.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooley L., Appel B., Söll D. Post-transcriptional nucleotide addition is responsible for the formation of the 5' terminus of histidine tRNA. Proc. Natl. Acad. Sci. U. S. A. 1982;79:6475–6479. doi: 10.1073/pnas.79.21.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giege R., Sissler M., Florentz C. Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res. 1998;26:5017–5035. doi: 10.1093/nar/26.22.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giegé R., Eriani G. The tRNA identity landscape for aminoacylation and beyond. Nucleic Acids Res. 2023;51:1528–1570. doi: 10.1093/nar/gkad007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frank D.N., Pace N.R. Ribonuclease P: unity and diversity in a tRNA processing ribozyme. Annu. Rev. Biochem. 1998;67:153–180. doi: 10.1146/annurev.biochem.67.1.153. [DOI] [PubMed] [Google Scholar]
- 46.Kerkhofs K., Garg J., Fafard-Couture É., Abou Elela S., Scott M.S., Pearlman R.E., et al. Altered tRNA processing is linked to a distinct and unusual La protein in Tetrahymena thermophila. Nat. Commun. 2022;13:1–17. doi: 10.1038/s41467-022-34796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deutscher M.P. Degradation of RNA in bacteria: comparison of mRNA and stable RNA. Nucleic Acids Res. 2006;34:659–666. doi: 10.1093/nar/gkj472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Z., Deutscher M.P. Maturation pathways for E. coli tRNA precursors: a random multienzyme process in vivo. Cell. 1996;86:503–512. doi: 10.1016/s0092-8674(00)80123-3. [DOI] [PubMed] [Google Scholar]
- 49.Ezraty B., Dahlgren B., Deutscher M.P. The RNase Z homologue encoded by Escherichia coli elaC gene is RNase BN. J. Biol. Chem. 2005;280:16542–16545. doi: 10.1074/jbc.C500098200. [DOI] [PubMed] [Google Scholar]
- 50.Pellegrini O. Endonucleolytic processing of CCA-less tRNA precursors by RNase Z in Bacillus subtilis. EMBO J. 2003;22:4534–4543. doi: 10.1093/emboj/cdg435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deutscher M.P. 7 tRNA nucleotidyltransferase. Enzymes. 1982;15:183–215. [Google Scholar]
- 52.Moazed D., Noller H.F. Sites of interaction of the CCA end of peptidyl-tRNA with 23S rRNA. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3725–3728. doi: 10.1073/pnas.88.9.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korostelev A., Trakhanov S., Laurberg M., Noller H.F. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell. 2006;126:1065–1077. doi: 10.1016/j.cell.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 54.Hou Y.-M. The tertiary structure of tRNA and the development of the genetic code. Trends Biochem. Sci. 1993;18:362–364. doi: 10.1016/0968-0004(93)90088-5. [DOI] [PubMed] [Google Scholar]
- 55.Graifer D., Karpova G. Interaction of tRNA with eukaryotic ribosome. Int. J. Mol. Sci. 2015;16:7173–7194. doi: 10.3390/ijms16047173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sigler P.B. An analysis of the structure of tRNA. Annu. Rev. Biophys. Bioeng. 1975;4:477–527. doi: 10.1146/annurev.bb.04.060175.002401. [DOI] [PubMed] [Google Scholar]
- 57.Sprinzl M. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1998;26:148–153. doi: 10.1093/nar/26.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giegé R., Frugier M. Madame Curie Bioscience Database [Internet] Bioscience; Austin (TX): Landes: 2000-2013. Transfer RNA structure and identity. [Google Scholar]
- 59.Chiba S., Itoh Y., Sekine S., Yokoyama S. Structural basis for the major role of O-phosphoseryl-tRNA kinase in the UGA-specific encoding of selenocysteine. Mol. Cell. 2010;39:410–420. doi: 10.1016/j.molcel.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 60.Hilal T., Killam B.Y., Grozdanović M., Dobosz-Bartoszek M., Loerke J., Bürger J., et al. Structure of the mammalian ribosome as it decodes the selenocysteine UGA codon. Science. 2022;376:1338–1343. doi: 10.1126/science.abg3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hao B., Gong W., Ferguson T.K., James C.M., Krzycki J.A., Chan M.K. A new UAG-encoded residue in the structure of a methanogen methyltransferase. Science. 2002;296:1462–1466. doi: 10.1126/science.1069556. [DOI] [PubMed] [Google Scholar]
- 62.Krutyhołowa R., Zakrzewski K., Glatt S. Charging the code — tRNA modification complexes. Curr. Opin. Struct. Biol. 2019;55:138–146. doi: 10.1016/j.sbi.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 63.Amann S.J., Keihsler D., Bodrug T., Brown N.G., Haselbach D. Frozen in time: analyzing molecular dynamics with time-resolved cryo-EM. Structure. 2023;31:4–19. doi: 10.1016/j.str.2022.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loveland A.B., Demo G., Korostelev A.A. Cryo-EM of elongating ribosome with EF-Tu•GTP elucidates tRNA proofreading. Nature. 2020;584:640–645. doi: 10.1038/s41586-020-2447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carbone C.E., Loveland A.B., Gamper H.B., Hou Y.M., Demo G., Korostelev A.A. Time-resolved cryo-EM visualizes ribosomal translocation with EF-G and GTP. Nat. Commun. 2021;12:7236. doi: 10.1038/s41467-021-27415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giegé R., Frugier M., Rudinger J. tRNA mimics. Curr. Opin. Struct. Biol. 1998;8:286–293. doi: 10.1016/s0959-440x(98)80060-2. [DOI] [PubMed] [Google Scholar]
- 67.Butcher S.E., Jan E. tRNA-mimicry in IRES-mediated translation and recoding. RNA Biol. 2016;13:1068–1074. doi: 10.1080/15476286.2016.1219833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Francisco-Velilla R., Embarc-Buh A., Abellan S., Martinez-Salas E. Picornavirus translation strategies. FEBS Open Bio. 2022;12:1125–1141. doi: 10.1002/2211-5463.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trachman R.J., Passalacqua L.F.M., Ferré-D'Amaré A.R. The bacterial yjdF riboswitch regulates translation through its tRNA-like fold. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.101934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Czworkowski J., Wang J., Steitz T.A., Moore P.B. The crystal structure of elongation factor G complexed with GDP, at 2.7 A resolution. EMBO J. 1994;13:3661–3668. doi: 10.1002/j.1460-2075.1994.tb06675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nyborg J., Nissen P., Kjeldgaard M., Thirup S., Polekhina G., Clark B.F.C., et al. Structure of the ternary complex of EF-Tu: macromolecular mimicry in translation. Trends Biochem. Sci. 1996;21:81–82. [PubMed] [Google Scholar]
- 72.Selmer M., Al-Karadaghi S., Hirokawa G., Kaji A., Liljas A. Crystal structure of Thermotoga maritima ribosome recycling factor: a tRNA mimic. Science. 1999;286:2349–2352. doi: 10.1126/science.286.5448.2349. [DOI] [PubMed] [Google Scholar]
- 73.Jørgensen R., Ortiz P.A., Carr-Schmid A., Nissen P., Kinzy T.G., Andersen G.R. Two crystal structures demonstrate large conformational changes in the eukaryotic ribosomal translocase. Nat. Struct. Mol. Biol. 2003;10:379–385. doi: 10.1038/nsb923. [DOI] [PubMed] [Google Scholar]
- 74.Shi H., Moore P.B. The crystal structure of yeast phenylalanine tRNA at 1.93 Å resolution: a classic structure revisited. RNA. 2000;6:1091–1105. doi: 10.1017/s1355838200000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Byrne R.T., Konevega A.L., Rodnina M.V., Antson A.A. The crystal structure of unmodified tRNAPhe from Escherichia coli. Nucleic Acids Res. 2010;38:4154–4162. doi: 10.1093/nar/gkq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kimura S., Suzuki T., Chen M., Kato K., Yu J., Nakamura A., et al. Template-dependent nucleotide addition in the reverse (3′-5′) direction by Thg1-like protein. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1501397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bourgeois G., Seguin J., Babin M., Gondry M., Mechulam Y., Schmitt E. Structural basis of the interaction between cyclodipeptide synthases and aminoacylated tRNA substrates. RNA. 2020;26:1589–1602. doi: 10.1261/rna.075184.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harrington K.M., Nazarenko I.A., Uhlenbeck O.C., Dix D.B., Thompson R.C. In vitro analysis of translational rate and accuracy with an unmodified tRNA. Biochemistry. 1993;32:7617–7622. doi: 10.1021/bi00081a003. [DOI] [PubMed] [Google Scholar]
- 79.Perret V., Garcia A., Puglisi J., Grosjean H., Ebel J.P., Florentz C., et al. Conformation in solution of yeast tRNAAsp transcripts deprived of modified nucleotides. Biochimie. 1990;72:735–743. doi: 10.1016/0300-9084(90)90158-d. [DOI] [PubMed] [Google Scholar]
- 80.Saks M.E., Sampson J.R., Abelson J.N. The transfer RNA identity problem: a search for rules. Science. 1994;263:191–197. doi: 10.1126/science.7506844. [DOI] [PubMed] [Google Scholar]
- 81.Sampson J.R., Uhlenbeck O.C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc. Natl. Acad. Sci. U. S. A. 1988;85:1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhaskaran H., Rodriguez-Hernandez A., Perona J.J. Kinetics of tRNA folding monitored by aminoacylation. RNA. 2012;18:569–580. doi: 10.1261/rna.030080.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Derrick W.B., Horowitz J. Probing structural differences between native and in vitro transcribed Escherichia coli valine transfer RNA: evidence for stable base modification-dependent conformers. Nucleic Acids Res. 1993;21:4948–4953. doi: 10.1093/nar/21.21.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dewe J.M., Whipple J.M., Chernyakov I., Jaramillo L.N., Phizicky E.M. The yeast rapid tRNA decay pathway competes with elongation factor 1A for substrate tRNAs and acts on tRNAs lacking one or more of several modifications. RNA. 2012;18:1886–1896. doi: 10.1261/rna.033654.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Helm M., Brulé H., Degoul F., Cepanec C., Leroux J.P., Giegé R., et al. The presence of modified nucleotides is required for cloverleaf folding of a human mitochondrial tRNA. Nucleic Acids Res. 1998;26:1636–1643. doi: 10.1093/nar/26.7.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Phizicky E.M., Alfonzo J.D. Do all modifications benefit all tRNAs? FEBS Lett. 2010;584:265–271. doi: 10.1016/j.febslet.2009.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vermeulen A., McCallum S.A., Pardi A. Comparison of the global structure and dynamics of native and unmodified tRNAval. Biochemistry. 2005;44:6024–6033. doi: 10.1021/bi0473399. [DOI] [PubMed] [Google Scholar]
- 88.Maglott E.J., Deo S.S., Przykorska A., Glick G.D. Conformational transitions of an unmodified tRNA: implications for RNA folding. Biochemistry. 1998;37:16349–16359. doi: 10.1021/bi981722u. [DOI] [PubMed] [Google Scholar]
- 89.Serebrov V., Vassilenko K., Kholod N., Gross H.J., Kisselev L. Mg2+ binding and structural stability of mature and in vitro synthesized unmodified Escherichia coli tRNA(Phe) Nucleic Acids Res. 1998;26:2723–2728. doi: 10.1093/nar/26.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boccaletto P., Stefaniak F., Ray A., Cappannini A., Mukherjee S., Zbieta Purta E., et al. MODOMICS: a database of RNA modification pathways. 2021 update. Nucleic Acids Res. 2021;50:D231–D235. doi: 10.1093/nar/gkab1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jackman J.E., Alfonzo J.D. Transfer RNA modifications: nature’s combinatorial chemistry playground. Wiley Interdiscip. Rev. RNA. 2013;4:35–48. doi: 10.1002/wrna.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jühling F., Mörl M., Hartmann R.K., Sprinzl M., Stadler P.F., Pütz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Motorin Y., Helm M. tRNA stabilization by modified nucleotides. Biochemistry. 2010;49:4934–4944. doi: 10.1021/bi100408z. [DOI] [PubMed] [Google Scholar]
- 94.Ohira T., Minowa K., Sugiyama K., Yamashita S., Sakaguchi Y., Miyauchi K., et al. Reversible RNA phosphorylation stabilizes tRNA for cellular thermotolerance. Nature. 2022;605:372–379. doi: 10.1038/s41586-022-04677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suzuki T. The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell Biol. 2021;22:375–392. doi: 10.1038/s41580-021-00342-0. [DOI] [PubMed] [Google Scholar]
- 96.Schaffrath R., Leidel S.A. Wobble uridine modifications–a reason to live, a reason to die?! RNA Biol. 2017;14:1209–1222. doi: 10.1080/15476286.2017.1295204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han L., Phizicky E.M. A rationale for tRNA modification circuits in the anticodon loop. RNA. 2018;24:1277–1284. doi: 10.1261/rna.067736.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sokolowski M., Klassen R., Bruch A., Schaffrath R., Glatt S. Cooperativity between different tRNA modifications and their modification pathways. Biochim. Biophys. Acta. 2018;1861:409–418. doi: 10.1016/j.bbagrm.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 99.Duechler M., Leszczyńska G., Sochacka E., Nawrot B. Nucleoside modifications in the regulation of gene expression: focus on tRNA. Cell. Mol. Life Sci. 2016;73:3075–3095. doi: 10.1007/s00018-016-2217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCown P.J., Ruszkowska A., Kunkler C.N., Breger K., Hulewicz J.P., Wang M.C., et al. Naturally occurring modified ribonucleosides. Wiley Interdiscip. Rev. RNA. 2020;11:e1595. doi: 10.1002/wrna.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cavarelli J., Rees B., Ruff M., Thierry J.C., Moras D. Yeast tRNAAsp recognition by its cognate class II aminoacyl-tRNA synthetase. Nature. 1993;362:181–184. doi: 10.1038/362181a0. [DOI] [PubMed] [Google Scholar]
- 102.Ishitani R., Nureki O., Nameki N., Okada N., Nishimura S., Yokoyama S. Alternative tertiary structure of tRNA for recognition by a posttranscriptional modification enzyme. Cell. 2003;113:383–394. doi: 10.1016/s0092-8674(03)00280-0. [DOI] [PubMed] [Google Scholar]
- 103.Dalluge J.J., Hashizume T., Sopchik A.E., McCloskey J.A., Davis D.R. Conformational flexibility in RNA: the role of dihydrouridine. Nucleic Acids Res. 1996;24:1073–1079. doi: 10.1093/nar/24.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Motorin Y., Helm M. RNA nucleotide methylation. Wiley Interdiscip. Rev. RNA. 2011;2:611–631. doi: 10.1002/wrna.79. [DOI] [PubMed] [Google Scholar]
- 105.Davis D.R., Dale Poulter C. H-15N NMR studies of Escherichia coli tRNAPhe from hisT mutants: a structural role for pseudouridine. Biochemistry. 1991;30:4223–4231. doi: 10.1021/bi00231a017. [DOI] [PubMed] [Google Scholar]
- 106.Kawai G., Yamamoto Y., Kamimura T., Masegi T., Sekine M., Hata T., et al. Conformational rigidity of specific pyrimidine residues in tRNA arises from posttranscriptional modifications that enhance steric interaction between the base and the 2'-hydroxyl group. Biochemistry. 1992;31:1040–1046. doi: 10.1021/bi00119a012. [DOI] [PubMed] [Google Scholar]
- 107.Nobles K.N., Yarian C.S., Liu G., Guenther R.H., Agris P.F. Highly conserved modified nucleosides influence Mg2+-dependent tRNA folding. Nucleic Acids Res. 2002;30:4751–4760. doi: 10.1093/nar/gkf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Agris P.F. The importance of being modified: roles of modified nucleosides and Mg2+ in RNA structure and function. Prog. Nucleic Acid Res. Mol. Biol. 1996;53:79–129. doi: 10.1016/s0079-6603(08)60143-9. [DOI] [PubMed] [Google Scholar]
- 109.Kowalak J.A., Dalluge J.J., McCloskey J.A., Stetter K.O. The role of posttranscriptional modification in stabilization of transfer RNA from hyperthermophiles. Biochemistry. 1994;33:7869–7876. doi: 10.1021/bi00191a014. [DOI] [PubMed] [Google Scholar]
- 110.Nomura Y., Ohno S., Nishikawa K., Yokogawa T. Correlation between the stability of tRNA tertiary structure and the catalytic efficiency of a tRNA-modifying enzyme, archaeal tRNA-guanine transglycosylase. Genes Cells. 2016;21:41–52. doi: 10.1111/gtc.12317. [DOI] [PubMed] [Google Scholar]
- 111.Hori H. Methylated nucleosides in tRNA and tRNA methyltransferases. Front. Genet. 2014;5:144. doi: 10.3389/fgene.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ayadi L., Galvanin A., Pichot F., Marchand V., Motorin Y. RNA ribose methylation (2′-O-methylation): occurrence, biosynthesis and biological functions. Biochim. Biophys. Acta Gene Regul. Mech. 2019;1862:253–269. doi: 10.1016/j.bbagrm.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 113.Kumagai I., Watanabe K., Oshima T. Thermally induced biosynthesis of 2'-O-methylguanosine in tRNA from an extreme thermophile, Thermus thermophilus HB27. Proc. Natl. Acad. Sci. U. S. A. 1980;77:1922–1926. doi: 10.1073/pnas.77.4.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McCloskey J.A., Graham D.E., Zhou S., Crain P.F., Ibba M., Konisky J., et al. Post-transcriptional modification in archaeal tRNAs: identities and phylogenetic relations of nucleotides from mesophilic and hyperthermophilic Methanococcales. Nucleic Acids Res. 2001;29:4699–4706. doi: 10.1093/nar/29.22.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Voigts-Hoffmann F., Hengesbach M., Kobitski A.Y., van Aerschot A., Herdewijn P., Nienhaus G.U., et al. A methyl group controls conformational equilibrium in human mitochondrial tRNA(Lys) J. Am. Chem. Soc. 2007;129:13382–13383. doi: 10.1021/ja075520+. [DOI] [PubMed] [Google Scholar]
- 116.Helm M., Giegé R., Florentz C. A Watson-Crick base-pair-disrupting methyl group (m1A9) is sufficient for cloverleaf folding of human mitochondrial tRNALys. Biochemistry. 1999;38:13338–13346. doi: 10.1021/bi991061g. [DOI] [PubMed] [Google Scholar]
- 117.Sohm B., Sissler M., Park H., King M.P., Florentz C. Recognition of human mitochondrial tRNALeu(UUR) by its cognate leucyl-tRNA synthetase. J. Mol. Biol. 2004;339:17–29. doi: 10.1016/j.jmb.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 118.Wittenhagen L.M., Kelley S.O. Impact of disease-related mitochondrial mutations on tRNA structure and function. Trends Biochem. Sci. 2003;28:605–611. doi: 10.1016/j.tibs.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 119.Wolfson A.D., Khvorova A.M., Sauter C., Florentz C., Giegé R. Mimics of yeast tRNAAsp and their recognition by aspartyl-tRNA synthetase. Biochemistry. 1999;38:11926–11932. doi: 10.1021/bi9908383. [DOI] [PubMed] [Google Scholar]
- 120.Sohm B., Frugier M., Brulé H., Olszak K., Przykorska A., Florentz C. Towards understanding human mitochondrial leucine aminoacylation identity. J. Mol. Biol. 2003;328:995–1010. doi: 10.1016/s0022-2836(03)00373-5. [DOI] [PubMed] [Google Scholar]
- 121.Messmer M., Pütz J., Suzuki T., Suzuki T., Sauter C., Sissler M., et al. Tertiary network in mammalian mitochondrial tRNAAsp revealed by solution probing and phylogeny. Nucleic Acids Res. 2009;37:6881–6895. doi: 10.1093/nar/gkp697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jackman J.E., Montange R.K., Malik H.S., Phizicky E.M. Identification of the yeast gene encoding the tRNA m1G methyltransferase responsible for modification at position 9. RNA. 2003;9:574–585. doi: 10.1261/rna.5070303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vilardo E., Amman F., Toth U., Kotter A., Helm M., Rossmanith W. Functional characterization of the human tRNA methyltransferases TRMT10A and TRMT10B. Nucleic Acids Res. 2020;48:6157–6169. doi: 10.1093/nar/gkaa353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Agris P.F., Sierzputowska-Gracz H., Smith C. Transfer RNA contains sites of localized positive charge: carbon NMR studies of [13C] methyl-enriched Escherichia coli and yeast tRNAPhe. Biochemistry. 1986;25:5126–5131. doi: 10.1021/bi00366a022. [DOI] [PubMed] [Google Scholar]
- 125.Romby P., Carbon P., Westhof E., Ehresmann C., Ebel J.P., Ehresmann B., et al. Importance of conserved residues for the conformation of the t-loop in trnas. J. Biomol. Struct. Dyn. 1987;5:669–687. doi: 10.1080/07391102.1987.10506419. [DOI] [PubMed] [Google Scholar]
- 126.Anderson J., Phan L., Cuesta R., Carlson B.A., Pak M., Asano K., et al. The essential Gcd10p-Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev. 1998;12:3650–3662. doi: 10.1101/gad.12.23.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saikia M., Fu Y., Pavon-Eternod M., He C., Pan T. Genome-wide analysis of N1-methyl-adenosine modification in human tRNAs. RNA. 2010;16:1317–1327. doi: 10.1261/rna.2057810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oliva R., Cavallo L., Tramontano A. Accurate energies of hydrogen bonded nucleic acid base pairs and triplets in tRNA tertiary interactions. Nucleic Acids Res. 2006;34:865–879. doi: 10.1093/nar/gkj491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dai Q., Zheng G., Schwartz M.H., Clark W.C., Pan T. Selective enzymatic demethylation of N2,N2-dimethylguanosine in RNA and its application in high-throughput tRNA sequencing. Angew. Chem. Int. Ed. Engl. 2017;56:5017–5020. doi: 10.1002/anie.201700537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Purushothaman S.K., Bujnicki J.M., Grosjean H., Lapeyre B. Trm11p and Trm112p are both required for the formation of 2-methylguanosine at position 10 in yeast tRNA. Mol. Cell. Biol. 2005;25:4359–4370. doi: 10.1128/MCB.25.11.4359-4370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Awai T., Kimura S., Tomikawa C., Ochi A., Ihsanawati, Bessho Y., et al. Aquifex aeolicus tRNA (N2,N2-guanine)-dimethyltransferase (Trm1) catalyzes transfer of methyl groups not only to guanine 26 but also to guanine 27 in tRNA. J. Biol. Chem. 2009;284:20467–20478. doi: 10.1074/jbc.M109.020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pallan P.S., Kreutz C., Bosio S., Micura R., Egli M. Effects of N2,N2-dimethylguanosine on RNA structure and stability: crystal structure of an RNA duplex with tandem m2 2G:A pairs. RNA. 2008;14:2125–2135. doi: 10.1261/rna.1078508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Menezes S., Gaston K.W., Krivos K.L., Apolinario E.E., Reich N.O., Sowers K.R., et al. Formation of m2G6 in Methanocaldococcus jannaschii tRNA catalyzed by the novel methyltransferase Trm14. Nucleic Acids Res. 2011;39:7641–7655. doi: 10.1093/nar/gkr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Edqvist J., Stråby K.B., Grosjean H. Enzymatic formation of N2,N2-dimethylguanosine in eukaryotic tRNA: importance of the tRNA architecture. Biochimie. 1995;77:54–61. doi: 10.1016/0300-9084(96)88104-1. [DOI] [PubMed] [Google Scholar]
- 135.Yang W.-Q., Xiong Q.-P., Ge J.-Y., Li H., Zhu W.-Y., Nie Y., et al. THUMPD3-TRMT112 is a m2G methyltransferase working on a broad range of tRNA substrates. Nucleic Acids Res. 2021;49:11900–11919. doi: 10.1093/nar/gkab927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nishida Y., Ohmori S., Kakizono R., Kawai K., Namba M., Okada K., et al. Required elements in tRNA for methylation by the eukaryotic tRNA (guanine-N2-) methyltransferase (Trm11-Trm112 complex) Int. J. Mol. Sci. 2022;23:4046. doi: 10.3390/ijms23074046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Steinberg S., Cedergren R. A correlation between N2-dimethylguanosine presence and alternate tRNA conformers. RNA. 1995;1:886–891. [PMC free article] [PubMed] [Google Scholar]
- 138.Sonawane K.D., Bavi R.S., Sambhare S.B., Fandilolu P.M. Comparative structural dynamics of tRNAPhe with respect to hinge region methylated guanosine: a computational approach. Cell Biochem. Biophys. 2016;74:157–173. doi: 10.1007/s12013-016-0731-z. [DOI] [PubMed] [Google Scholar]
- 139.Jantsch M.F., Quattrone A., O'Connell M., Helm M., Frye M., Macias-Gonzales M., et al. Positioning Europe for the EPITRANSCRIPTOMICS challenge. RNA Biol. 2018;15:829–831. doi: 10.1080/15476286.2018.1460996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davanloo P., Sprinzl M., Watanabe K., Albani M., Kersten H. Role of ribothymidine in the thermal stability of transfer RNA as monitored by proton magnetic resonance. Nucleic Acids Res. 1979;6:1571–1581. doi: 10.1093/nar/6.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Levitt M. Detailed molecular model for transfer ribonucleic acid. Nature. 1969;224:759–763. doi: 10.1038/224759a0. [DOI] [PubMed] [Google Scholar]
- 142.Alexandrov A., Chernyakov I., Gu W., Hiley S.L., Hughes T.R., Grayhack E.J., et al. Rapid tRNA decay can result from lack of nonessential modifications. Mol. Cell. 2006;21:87–96. doi: 10.1016/j.molcel.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 143.Davis D.R. Stabilization of RNA stacking by pseudouridine. Nucleic Acids Res. 1995;23:5020–5026. doi: 10.1093/nar/23.24.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Charette M., Gray M.W. Pseudouridine in RNA: what, where, how, and why. IUBMB Life. 2000;49:341–351. doi: 10.1080/152165400410182. [DOI] [PubMed] [Google Scholar]
- 145.Lin T.Y., Mehta R., Glatt S. Pseudouridines in RNAs: switching atoms means shifting paradigms. FEBS Lett. 2021;595:2310–2322. doi: 10.1002/1873-3468.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kierzek E., Malgowska M., Lisowiec J., Turner D.H., Gdaniec Z., Kierzek R. The contribution of pseudouridine to stabilities and structure of RNAs. Nucleic Acids Res. 2014;42:3492–3501. doi: 10.1093/nar/gkt1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hudson G.A., Bloomingdale R.J., Znosko B.M. Thermodynamic contribution and nearest-neighbor parameters of pseudouridine-adenosine base pairs in oligoribonucleotides. RNA. 2013;19:1474–1482. doi: 10.1261/rna.039610.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Westhof E., Auffinger P. eLS. Wiley; Hoboken, NJ: 2012. Transfer RNA structure. [Google Scholar]
- 149.Sundaralingam M., Rao S.T., Abola J. Molecular conformation of dihydrouridine: puckered base nucleoside of transfer RNA. Science. 1971;172:725–727. doi: 10.1126/science.172.3984.725. [DOI] [PubMed] [Google Scholar]
- 150.Suck D., Saenger W., Zechmeister K. Conformation of the tRNA minor constituent dihydrouridine. FEBS Lett. 1971;12:257–259. doi: 10.1016/0014-5793(71)80191-6. [DOI] [PubMed] [Google Scholar]
- 151.Stuart J.W., Basti M.M., Smith W.S., Forrest B., Guenther R., Sierzputowska-Gracz H., et al. Structure of the trinucleotide D-acp3U-A with coordinated Mg2+ demonstrates that modified nucleosides contribute to regional conformations of RNA. Nucleos. Nucleot. 1996;15:1009–1028. [Google Scholar]
- 152.Dyubankova N., Sochacka E., Kraszewska K., Nawrot B., Herdewijn P., Lescrinier E. Contribution of dihydrouridine in folding of the D-arm in tRNA. Org. Biomol. Chem. 2015;13:4960–4966. doi: 10.1039/c5ob00164a. [DOI] [PubMed] [Google Scholar]
- 153.Kimura S., Waldor M.K. The RNA degradosome promotes tRNA quality control through clearance of hypomodified tRNA. Proc. Natl. Acad. Sci. U. S. A. 2019;116:1394–1403. doi: 10.1073/pnas.1814130116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Davis D.R., Griffey R.H., Yamaizumi Z. 15N-labeled tRNA. Identification of dihydrouridine in Escherichia coli tRNAfMet, tRNALys, and tRNAPhe by 1H-15N two-dimensional NMR. J. Biol. Chem. 1986;261:3584–3587. [PubMed] [Google Scholar]
- 155.Friedman S., Li H.J., Nakanishi K., Van Lear G. 3-(3-amino-3-carboxypropyl)uridine. The structure of the nucleoside in Escherichia coli transfer ribonucleic acid that reacts with phenoxyacetoxysuccinimide. Biochemistry. 1974;13:2932–2937. doi: 10.1021/bi00711a024. [DOI] [PubMed] [Google Scholar]
- 156.Friedman S. The effect of chemical modification of 3-(3-amino-3-carboxypropyl)uridine on tRNA function. J. Biol. Chem. 1979;254:7111–7115. [PubMed] [Google Scholar]
- 157.Ohashi Z., Maeda M., McCloskey J.A., Nishimura S. 3-(3-Amino-3-Carboxypropyl)uridine. Novel modified nucleoside isolated from Escherichia coli phenylalanine transfer ribonucleic acid. Biochemistry. 1974;13:2620–2625. doi: 10.1021/bi00709a023. [DOI] [PubMed] [Google Scholar]
- 158.Johnson G.D., Pirtle I.L., Pirtle R.M. The nucleotide sequence of tyrosine tRNAQ∗ΨA from bovine liver. Arch. Biochem. Biophys. 1985;236:448–453. doi: 10.1016/0003-9861(85)90647-2. [DOI] [PubMed] [Google Scholar]
- 159.Barciszewska M., Dirheimer G., Keith G. The nucleotide sequence of methionine elongator tRNA from wheat germ. Biochem. Biophys. Res. Commun. 1983;114:1161–1168. doi: 10.1016/0006-291x(83)90684-8. [DOI] [PubMed] [Google Scholar]
- 160.Takakura M., Ishiguro K., Akichika S., Miyauchi K., Suzuki T. Biogenesis and functions of aminocarboxypropyluridine in tRNA. Nat. Commun. 2019;10:5542. doi: 10.1038/s41467-019-13525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meyer B., Immer C., Kaiser S., Sharma S., Yang J., Watzinger P., et al. Identification of the 3-amino-3-carboxypropyl (acp) transferase enzyme responsible for acp3U formation at position 47 in Escherichia coli tRNAs. Nucleic Acids Res. 2020;48:1435–1450. doi: 10.1093/nar/gkz1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Barraud P., Gato A., Heiss M., Catala M., Kellner S., Tisné C. Time-resolved NMR monitoring of tRNA maturation. Nat. Commun. 2019;10:3373. doi: 10.1038/s41467-019-11356-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schultz S.K.L., Kothe U. tRNA elbow modifications affect the tRNA pseudouridine synthase TruB and the methyltransferase TrmA. RNA. 2020;26:1131–1142. doi: 10.1261/rna.075473.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Novoa E.M., Pavon-Eternod M., Pan T., Ribas de Pouplana L. A role for tRNA modifications in genome structure and codon usage. Cell. 2012;149:202–213. doi: 10.1016/j.cell.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 165.Yu F., Tanaka Y., Yamashita K., Suzuki T., Nakamura A., Hirano N., et al. Molecular basis of dihydrouridine formation on tRNA. Proc. Natl. Acad. Sci. U. S. A. 2011;108:19593–19598. doi: 10.1073/pnas.1112352108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu R.-J., Long T., Li J., Li H., Wang E.-D. Structural basis for substrate binding and catalytic mechanism of a human RNA: m5C methyltransferase NSun6. Nucleic Acids Res. 2017;45:6684–6697. doi: 10.1093/nar/gkx473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Finer-Moore J., Czudnochowski N., O'Connell J.D., Wang A.L., Stroud R.M. Crystal structure of the human tRNA m1A58 methyltransferase-tRNA3Lys complex: refolding of substrate tRNA allows access to the methylation target. J. Mol. Biol. 2015;427:3862–3876. doi: 10.1016/j.jmb.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Osawa T., Kimura S., Terasaka N., Inanaga H., Suzuki T., Numata T. Structural basis of tRNA agmatinylation essential for AUA codon decoding. Nat. Struct. Mol. Biol. 2011;18:1275–1280. doi: 10.1038/nsmb.2144. [DOI] [PubMed] [Google Scholar]
- 169.Wang C., Jia Q., Zeng J., Chen R., Xie W. Structural insight into the methyltransfer mechanism of the bifunctional Trm5. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1700195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schwalm E.L., Grove T.L., Booker S.J., Boal A.K. Crystallographic capture of a radical S-adenosylmethionine enzyme in the act of modifying tRNA. Science. 2016;352:309–312. doi: 10.1126/science.aad5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Beenstock J., Ona S.M., Porat J., Orlicky S., Wan L.C.K., Ceccarelli D.F., et al. A substrate binding model for the KEOPS tRNA modifying complex. Nat. Commun. 2020;11:6233. doi: 10.1038/s41467-020-19990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Beenstock J., Sicheri F. The structural and functional workings of KEOPS. Nucleic Acids Res. 2021;49:10818–10834. doi: 10.1093/nar/gkab865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sievers K., Welp L., Urlaub H., Ficner R. Structural and functional insights into human tRNA guanine transgylcosylase. RNA Biol. 2021;18:382–396. doi: 10.1080/15476286.2021.1950980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dolce L.G., Zimmer A.A., Tengo L., Weis F., Rubio M.A.T., Alfonzo J.D., et al. Structural basis for sequence-independent substrate selection by eukaryotic wobble base tRNA deaminase ADAT2/3. Nat. Commun. 2022;13:6737. doi: 10.1038/s41467-022-34441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ruiz-Arroyo V.M., Raj R., Babu K., Onolbaatar O., Roberts P.H., Nam Y. Structures and mechanisms of tRNA methylation by METTL1–WDR4. Nature. 2023;613:383–390. doi: 10.1038/s41586-022-05565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li J., Wang L., Hahn Q., Nowak R.P., Viennet T., Orellana E.A., et al. Structural basis of regulated m7G tRNA modification by METTL1–WDR4. Nature. 2023;613:391–397. doi: 10.1038/s41586-022-05566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kojic M., Gawda T., Gaik M., Begg A., Salerno-Kochan A., Kurniawan N.D., et al. Elp2 mutations perturb the epitranscriptome and lead to a complex neurodevelopmental phenotype. Nat. Commun. 2021;12:19. doi: 10.1038/s41467-021-22888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jaciuk M., Scherf D., Kaszuba K., Gaik M., Rau A., Kościelniak A., et al. Cryo-EM structure of the fully assembled Elongator complex. Nucleic Acids Res. 2023;51:2011–2032. doi: 10.1093/nar/gkac1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lin T.-Y., Abbassi N.E.H., Zakrzewski K., Chramiec-Głąbik A., Jemioła-Rzemińska M., Różycki J., et al. The Elongator subunit Elp3 is a non-canonical tRNA acetyltransferase. Nat. Commun. 2019;10:1–12. doi: 10.1038/s41467-019-08579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kuhle B., Lander G.C., Hirschi M., Doerfel L.K., Lander G.C., Schimmel P. Structural basis for shape-selective recognition and aminoacylation of a D-armless human mitochondrial tRNA. Nat. Commun. 2022;13:1–12. doi: 10.1038/s41467-022-32544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stephen P., Ye S., Zhou M., Song J., Zhang R., Wang E.D., et al. Structure of Escherichia coli arginyl-tRNA synthetase in complex with tRNAArg: pivotal role of the D-loop. J. Mol. Biol. 2018;430:1590–1606. doi: 10.1016/j.jmb.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 182.Yu Z., Wu Z., Li Y., Hao Q., Cao X., Blaha G.M., et al. Structural basis of a two-step tRNA recognition mechanism for plastid glycyl-tRNA synthetase. Nucleic Acids Res. 2023;51:4000–4011. doi: 10.1093/nar/gkad144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Han L., Luo Z., Ju Y., Chen B., Zou T., Wang J., et al. The binding mode of orphan glycyl-tRNA synthetase with tRNA supports the synthetase classification and reveals large domain movements. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adf1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wu J., Niu S., Tan M., Huang C., Li M., Song Y., et al. Cryo-EM structure of the human ribonuclease P holoenzyme. Cell. 2018;175:1393–1404.e11. doi: 10.1016/j.cell.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 185.Zhang X., Yang F., Zhan X., Bian T., Xing Z., Lu Y., et al. Structural basis of pre-tRNA intron removal by human tRNA splicing endonuclease. Mol. Cell. 2023;83:1328–1339.e4. doi: 10.1016/j.molcel.2023.03.015. [DOI] [PubMed] [Google Scholar]
- 186.Sekulovski S., Sušac L., Stelzl L.S., Tampé R., Trowitzsch S. Structural basis of substrate recognition by human tRNA splicing endonuclease TSEN. Nat. Struct. Mol. Biol. 2023;30:834–840. doi: 10.1038/s41594-023-00992-y. [DOI] [PubMed] [Google Scholar]
- 187.Hayne C.K., Butay K.J.U., Stewart Z.D., Krahn J.M., Perera L., Williams J.G., et al. Structural basis for pre-tRNA recognition and processing by the human tRNA splicing endonuclease complex. Nat. Struct. Mol. Biol. 2023;30:824–833. doi: 10.1038/s41594-023-00991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Byrne R.T., Jenkins H.T., Peters D.T., Whelan F., Stowell J., Aziz N., et al. Major reorientation of tRNA substrates defines specificity of dihydrouridine synthases. Proc. Natl. Acad. Sci. U. S. A. 2015;112:6033–6037. doi: 10.1073/pnas.1500161112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dauden M.I., Jaciuk M., Weis F., Lin T.Y., Kleindienst C., Abbassi N.E.H., et al. Molecular basis of tRNA recognition by the Elongator complex. Sci. Adv. 2019;5:1–14. doi: 10.1126/sciadv.aaw2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang J. Interplay between host trnas and hiv-1: a structural perspective. Viruses. 2021;13:1–14. doi: 10.3390/v13091819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bou-Nader C., Muecksch F., Brown J.B., Gordon J.M., York A., Peng C., et al. HIV-1 matrix-tRNA complex structure reveals basis for host control of Gag localization. Cell Host Microbe. 2021;29:1421–1436.e7. doi: 10.1016/j.chom.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu Y., Strugatsky D., Liu W., Zhou Z.H. Structure of human cytomegalovirus virion reveals host tRNA binding to capsid-associated tegument protein pp150. Nat. Commun. 2021;12:1–9. doi: 10.1038/s41467-021-25791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lezzhov A.A., Atabekova A.K., Tolstyko E.A., Lazareva E.A. Plant science RNA phloem transport mediated by pre-miRNA and viral tRNA-like structures. Plant Sci. 2019;284:99–107. doi: 10.1016/j.plantsci.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 194.Khan S.Y., Hackett S.F., Riazuddin S.A. Non-coding RNA profiling of the developing murine lens. Exp. Eye Res. 2016;145:347–351. doi: 10.1016/j.exer.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Colussi T.M., Costantino D.A., Hammond J.A., Ruehle G.M., Nix J.C., Kieft J.S. The structural basis of transfer RNA mimicry and conformational plasticity by a viral RNA. Nature. 2014;511:366–369. doi: 10.1038/nature13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Boehringer D., Thermann R., Ostareck-Lederer A., Lewis J.D., Stark H. Structure of the hepatitis C virus IRES bound to the human 80S ribosome: remodeling of the HCV IRES. Structure. 2005;13:1695–1706. doi: 10.1016/j.str.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 197.Jones C.P., Cantara W.A., Olson E.D., Musier-Forsyth K. Small-angle X-ray scattering-derived structure of the HIV-1 5' UTR reveals 3D tRNA mimicry. Proc. Natl. Acad. Sci. U. S. A. 2014;111:3395–3400. doi: 10.1073/pnas.1319658111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tsai C.H., Dreher T.W. Turnip yellow mosaic virus RNAs with anticodon loop substitutions that result in decreased valylation fail to replicate efficiently. J. Virol. 1991;65:3060–3067. doi: 10.1128/jvi.65.6.3060-3067.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Matsuda D., Dunoyer P., Hemmer O., Fritsch C., Dreher T.W. The valine anticodon and valylatability of peanut clump virus RNAs are not essential but provide a modest competitive advantage in plants. J. Virol. 2000;74:8720–8725. doi: 10.1128/jvi.74.18.8720-8725.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vieweger M., Holmstrom E.D., Nesbitt D.J. Single-molecule FRET reveals three conformations for the TLS domain of Brome mosaic virus genome. Biophys. J. 2015;109:2625–2636. doi: 10.1016/j.bpj.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bonilla S.L., Sherlock M.E., MacFadden A., Kieft J.S. A viral RNA hijacks host machinery using dynamic conformational changes of a tRNA-like structure. Science. 2021;374:955–960. doi: 10.1126/science.abe8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kuhn C., Wilusz J.E., Zheng Y., Beal P.A., Joshua L. On-enzyme refolding permits small RNA and tRNA surveillance by the CCA-adding enzyme. Cell. 2015;160:644–658. doi: 10.1016/j.cell.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lu X., Huang J., Wu S., Zheng Q., Fu H., Xi Q., et al. The tRNA-like small noncoding RNA mascRNA promotes global protein translation. EMBO Rep. 2020;21:1–17. doi: 10.15252/embr.201949684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Levi O., Arava Y. mRNA association by aminoacyl tRNA synthetase occurs at a putative anticodon mimic and autoregulates translation in response to tRNA levels. PLoS Biol. 2019;17:1–24. doi: 10.1371/journal.pbio.3000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang W., Chen X., Wolin S.L., Xiong Y. Structural basis for tRNA mimicry by a bacterial Y RNA. Structure. 2018;26:1635–1644.e3. doi: 10.1016/j.str.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Telonis A.G., Loher P., Kirino Y., Rigoutsos I. Nuclear and mitochondrial tRNA-lookalikes in the human genome. Front. Genet. 2014;5:1–11. doi: 10.3389/fgene.2014.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hoser S.M., Hoffmann A., Meindl A., Gamper M., Fallmann J., Bernhart S.H., et al. Intronic tRNAs of mitochondrial origin regulate constitutive and alternative splicing. Genome Biol. 2020;21:299. doi: 10.1186/s13059-020-02199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kreuzer K.D., Henkin T.M. The T-box riboswitch: tRNA as an effector to modulate gene regulation. Microbiol. Spectr. 2018;6:10–1128. doi: 10.1128/microbiolspec.rwr-0028-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Marchand J.A., Pierson Smela M.D., Jordan T.H.H., Narasimhan K., Church G.M. TBDB: a database of structurally annotated T-box riboswitch:tRNA pairs. Nucleic Acids Res. 2021;49:D229–D235. doi: 10.1093/nar/gkaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang J. Unboxing the T-box riboswitches—A glimpse into multivalent and multimodal RNA–RNA interactions. Wiley Interdiscip. Rev. RNA. 2020;11:1–21. doi: 10.1002/wrna.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li S., Su Z., Lehmann J., Stamatopoulou V., Giarimoglou N., Henderson F.E., et al. Structural basis of amino acid surveillance by higher-order tRNA-mRNA interactions. Nat. Struct. Mol. Biol. 2019;26:1094–1105. doi: 10.1038/s41594-019-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang J., Chetnani B., Cormack E.D., Alonso D., Liu W., Mondragón A., et al. Specific structural elements of the t-box riboswitch drive the two-step binding of the tRNA ligand. Elife. 2018;7:1–22. doi: 10.7554/eLife.39518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Battaglia R.A., Grigg J.C., Ke A. Structural basis for tRNA decoding and aminoacylation sensing by T-box riboregulators. Nat. Struct. Mol. Biol. 2019;26:1106–1113. doi: 10.1038/s41594-019-0327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Suddala K.C., Zhang J. High-affinity recognition of specific tRNAs by an mRNA anticodon-binding groove. Nat. Struct. Mol. Biol. 2019;26:1114–1122. doi: 10.1038/s41594-019-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kasai H., Nakanishi K., Macfarlane R.D., Torgerson D.F., Ohashi Z., McCloskey J.A., et al. The structure of Q∗ nucleoside isolated from rabbit liver transfer ribonucleic acid. J. Am. Chem. Soc. 1976;98:5044–5046. doi: 10.1021/ja00432a071. [DOI] [PubMed] [Google Scholar]
- 216.Zhang W., Xu R., Matuszek Z.A., Cai Z., Pan T. Detection and quantification of glycosylated queuosine modified tRNAs by acid denaturing and APB gels. RNA. 2020;26:1291–1298. doi: 10.1261/rna.075556.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Thumbs P., Ensfelder T.T., Hillmeier M., Wagner M., Heiss M., Scheel C., et al. Synthesis of galactosyl-queuosine and distribution of hypermodified Q-nucleosides in mouse tissues. Angew. Chem. Int. Ed. Engl. 2020;59:12352–12356. doi: 10.1002/anie.202002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hillmeier M., Wagner M., Ensfelder T., Korytiakova E., Thumbs P., Müller M., et al. Synthesis and structure elucidation of the human tRNA nucleoside mannosyl-queuosine. Nat. Commun. 2021;12:7123. doi: 10.1038/s41467-021-27371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Flynn R.A., Pedram K., Malaker S.A., Batista P.J., Smith B.A.H., Johnson A.G., et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell. 2021;184:3109–3124.e22. doi: 10.1016/j.cell.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Okimoto R., Wolstenholme D.R. A set of tRNAs that lack either the TψC arm or the dihydrouridine arm: towards a minimal tRNA adaptor. EMBO J. 1990;9:3405–3411. doi: 10.1002/j.1460-2075.1990.tb07542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pons J., Bover P., Bidegaray-batista L., Arnedo M.A. Arm-less mitochondrial tRNAs conserved for over 30 millions of years in spiders. BMC Genomics. 2019;20:665. doi: 10.1186/s12864-019-6026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sprinzl M., Vassilenko K.S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 2005;33:D139–D140. doi: 10.1093/nar/gki012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sakurai M., Ohtsuki T., Watanabe K. Modification at position 9 with 1-methyladenosine is crucial for structure and function of nematode mitochondrial tRNAs lacking the entire T-arm. Nucleic Acids Res. 2005;33:1653–1661. doi: 10.1093/nar/gki309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jühling T., Duchardt-Ferner E., Bonin S., Wöhnert J., Pütz J., Florentz C., et al. Small but large enough: structural properties of armless mitochondrial tRNAs from the nematode Romanomermis culicivorax. Nucleic Acids Res. 2018;46:9170–9180. doi: 10.1093/nar/gky593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wende S., Platzer E.G., Jühling F., Pütz J., Florentz C., Stadler P.F., et al. Biochimie biological evidence for the world’s smallest tRNAs. Biochimie. 2014;100:151–158. doi: 10.1016/j.biochi.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 226.Hennig O., Philipp S., Bonin S., Rollet K., Kolberg T., Jühling T., et al. Adaptation of the romanomermis culicivorax CCA-adding enzyme to miniaturized armless tRNA substrates. Int. J. Mol. Sci. 2020;21:9047. doi: 10.3390/ijms21239047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sakurai M., Watanabe Y., Watanabe K., Ohtsuki T. A protein extension to shorten RNA : elongated elongation factor-Tu recognizes the D-arm of T-armless tRNAs in nematode mitochondria. Biochem. J. 2006;256:249–256. doi: 10.1042/BJ20060781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Suematsu T., Sato A., Sakurai M., Watanabe K., Ohtsuki T. A unique tRNA recognition mechanism of Caenorhabditis elegans mitochondrial EF-Tu2. Nucleic Acids Res. 2005;33:4683–4691. doi: 10.1093/nar/gki784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.de Bruijn M.H., Klug A. A model for the tertiary structure of mammalian mitochondrial transfer RNAs lacking the entire “dihydrouridine” loop and stem. EMBO J. 1983;2:1309–1321. doi: 10.1002/j.1460-2075.1983.tb01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tuppen H.A.L., Naess K., Kennaway N.G., Al-Dosary M., Lesko N., Yarham J.W., et al. Mutations in the mitochondrial tRNA Ser(AGY) gene are associated with deafness, retinal degeneration, myopathy and epilepsy. Eur. J. Hum. Genet. 2012;20:897–904. doi: 10.1038/ejhg.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yu T., Zhang Y.Y., Zheng W.-Q., Wu S., Li G., Zhang Y.Y., et al. Selective degradation of tRNASer(AGY) is the primary driver for mitochondrial seryl-tRNA synthetase-related disease. Nucleic Acids Res. 2022;50:11755–11774. doi: 10.1093/nar/gkac1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Eggertsson G., Söll D. Transfer ribonucleic acid-mediated suppression of termination codons in Escherichia coli. Microbiol. Rev. 1988;52:354–374. doi: 10.1128/mr.52.3.354-374.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kachale A., Pavlíková Z., Nenarokova A., Roithová A., Durante I.M., Miletínová P., et al. Short tRNA anticodon stem and mutant eRF1 allow stop codon reassignment. Nature. 2023;613:751–758. doi: 10.1038/s41586-022-05584-2. [DOI] [PubMed] [Google Scholar]
- 234.Lueck J.D., Yoon J.S., Perales-Puchalt A., Mackey A.L., Infield D.T., Behlke M.A., et al. Engineered transfer RNAs for suppression of premature termination codons. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-019-08329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ogawa A., Hayami M., Sando S., Aoyama Y. A concept for selection of codon-suppressor tRNAs based on read-through ribosome display in an in vitro compartmentalized cell-free translation system. J. Nucleic Acids. 2012;2012 doi: 10.1155/2012/538129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lin T.-Y.Y., Glatt S. ACEing premature codon termination using anticodon-engineered sup-tRNA-based therapy. Mol. Ther. Nucleic Acids. 2022;29:368–369. doi: 10.1016/j.omtn.2022.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cummins C.M., Culbertson M.R., Knapp G. Frameshift suppressor mutations outside the anticodon in yeast proline tRNAs containing an intervening sequence. Mol. Cell. Biol. 1985;5:1760–1771. doi: 10.1128/mcb.5.7.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Atkins J.F., Björk G.R. A gripping tale of ribosomal frameshifting: extragenic suppressors of frameshift mutations spotlight P-site realignment. Microbiol. Mol. Biol. Rev. 2009;73:178–210. doi: 10.1128/MMBR.00010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Maehigashi T., Dunkle J.A., Miles S.J., Dunham C.M. Structural insights into +1 frameshifting promoted by expanded or modification-deficient anticodon stem loops. Proc. Natl. Acad. Sci. U. S. A. 2014;111:12740–12745. doi: 10.1073/pnas.1409436111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Qian Q., Li J.N., Zhao H., Hagervall T.G., Farabaugh P.J., Björk G.R. A new model for phenotypic suppression of frameshift mutations by mutant tRNAs. Mol. Cell. 1998;1:471–482. doi: 10.1016/s1097-2765(00)80048-9. [DOI] [PubMed] [Google Scholar]
- 241.Hong S., Sunita S., Maehigashi T., Hoffer E.D., Dunkle J.A., Dunham C.M. Mechanism of tRNA-mediated +1 ribosomal frameshifting. Proc. Natl. Acad. Sci. U. S. A. 2018;115:11226–11231. doi: 10.1073/pnas.1809319115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fagan C.E., Maehigashi T., Dunkle J.A., Miles S.J., Dunham C.M. Structural insights into translational recoding by frameshift suppressor tRNASufJ. RNA. 2014;20:1944–1954. doi: 10.1261/rna.046953.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gamper H., Mao Y., Masuda I., McGuigan H., Blaha G., Wang Y., et al. Twice exploration of tRNA +1 frameshifting in an elongation cycle of protein synthesis. Nucleic Acids Res. 2021;49:10046–10060. doi: 10.1093/nar/gkab734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Demo G., Gamper H.B., Loveland A.B., Masuda I., Carbone C.E., Svidritskiy E., et al. Structural basis for +1 ribosomal frameshifting during EF-G-catalyzed translocation. Nat. Commun. 2021;12:1–12. doi: 10.1038/s41467-021-24911-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wang K., Schmied W.H., Chin J.W. Reprogramming the genetic code: from triplet to quadruplet codes. Angew. Chem. Int. Ed. Engl. 2012;51:2288–2297. doi: 10.1002/anie.201105016. [DOI] [PubMed] [Google Scholar]
- 246.Ponchon L., Dardel F. Recombinant RNA technology: the tRNA scaffold. Nat. Methods. 2007;4:571–576. doi: 10.1038/nmeth1058. [DOI] [PubMed] [Google Scholar]
- 247.Lee E., Bujalowski P.J., Teramoto T., Gottipati K., Scott S.D., Padmanabhan R., et al. Structures of flavivirus RNA promoters suggest two binding modes with NS5 polymerase. Nat. Commun. 2021;12:1–12. doi: 10.1038/s41467-021-22846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Fahmi N.E., Dedkova L., Wang B., Golovine S., Hecht S.M., Charlottes V. Site-specific incorporation of glycosylated serine and tyrosine derivatives into proteins. J. Am. Chem. Soc. 2007;129:3586–3597. doi: 10.1021/ja067466n. [DOI] [PubMed] [Google Scholar]
- 249.Porter J.J., Heil C.S., Lueck J.D. Therapeutic promise of engineered nonsense suppressor tRNAs. Wiley Interdiscip. Rev. RNA. 2021;12:1–27. doi: 10.1002/wrna.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ko W., Porter J.J., Sipple M.T., Edwards K.M., Lueck J.D. Efficient suppression of endogenous CFTR nonsense mutations using anticodon-engineered transfer RNAs. Mol. Ther. Nucleic Acids. 2022;28:685–701. doi: 10.1016/j.omtn.2022.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang J., Zhang Y., Mendonca C.A., Yukselen O., Muneeruddin K., Ren L., et al. AAV-delivered suppressor tRNA overcomes a nonsense mutation in mice. Nature. 2022;604:343–348. doi: 10.1038/s41586-022-04533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kappel K., Zhang K., Su Z., Watkins A.M., Kladwang W., Li S., et al. Accelerated cryo-EM-guided determination of three-dimensional RNA-only structures. Nat. Methods. 2020;17:699–707. doi: 10.1038/s41592-020-0878-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Monsen R.C., Chua E.Y.D., Hopkins J.B., Chaires J.B., Trent J.O. Structure of a 28.5 kDa duplex-embedded G-quadruplex system resolved to 7.4 Å resolution with cryo-EM. Nucleic Acids Res. 2023;51:1–17. doi: 10.1093/nar/gkad014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kaymak E., Rando O.J. Staying together after the breakup: tRNA halves in extracellular fluids. Proc. Natl. Acad. Sci. U. S. A. 2023;120:10–12. doi: 10.1073/pnas.2300300120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chen X., Wolin S.L. Transfer RNA halves are found as nicked tRNAs in cells: evidence that nicked tRNAs regulate expression of an RNA repair operon. RNA. 2023;29:620–629. doi: 10.1261/rna.079575.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Chen L., Xu W., Liu K., Jiang Z., Han Y., Jin H., et al. 5' Half of specific tRNAs feeds back to promote corresponding tRNA gene transcription in vertebrate embryos. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abh0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bayazit M.B., Jacovetti C., Cosentino C., Sobel J., Wu K., Brozzi F., et al. Small RNAs derived from tRNA fragmentation regulate the functional maturation of neonatal β cells. Cell Rep. 2022;40 doi: 10.1016/j.celrep.2022.111069. [DOI] [PubMed] [Google Scholar]
- 259.Catala M., Gato A., Tisné C., Barraud P. 1H, 15N chemical shift assignments of the imino groups of yeast tRNAPhe: influence of the post-transcriptional modifications. Biomol. NMR Assign. 2020;14:169–174. doi: 10.1007/s12104-020-09939-6. [DOI] [PubMed] [Google Scholar]
- 260.Laxman S., Sutter B.M., Wu X., Kumar S., Guo X., Trudgian D.C., et al. Sulfur amino acids regulate translational capacity and metabolic homeostasis through modulation of tRNA thiolation. Cell. 2013;154:416–429. doi: 10.1016/j.cell.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Tuorto F., Legrand C., Cirzi C., Federico G., Liebers R., Müller M., et al. Queuosine-modified tRNAs confer nutritional control of protein translation. EMBO J. 2018;37 doi: 10.15252/embj.201899777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Liu F., Clark W., Luo G., Wang X., Fu Y., Wei J., et al. ALKBH1-mediated tRNA demethylation regulates translation. Cell. 2016;167:816–828.e16. doi: 10.1016/j.cell.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]