Abstract
Therapeutic interference with virus-cell surface receptor interactions represents a viable antiviral strategy. Here we demonstrate that cytoplasmic expression of the serine protease inhibitor (serpin), plasminogen activator inhibitor type 2 (PAI-2), affords a high level of protection from lytic infection by multiple human picornaviruses. The antiviral action of PAI-2 was mediated primarily through transcriptional down-regulation of the following virus receptors: intercellular adhesion molecule 1 (ICAM-1, a cellular receptor for the major group of rhinoviruses), decay-accelerating factor (a cellular receptor for echoviruses and coxsackieviruses), and to a lesser extent the coxsackie-adenovirus receptor protein (a cellular receptor for group B coxsackieviruses and group C adenoviruses). Expression of related cell surface receptors, including membrane cofactor protein and the poliovirus receptor, remained unaffected. These findings suggest that PAI-2 and/or related serpins may form the basis of novel antiviral strategies against picornavirus infections and also therapeutic interventions against ICAM-1-mediated respiratory inflammation.
Picornaviruses are small, spherical, naked viruses (20 to 25 nm) containing positive-sense genomic RNA and comprise a genus of the family Picornaviridae. The two largest subgroups are rhinoviruses and enteroviruses. There are ∼100 rhinovirus serotypes, which collectively constitute the most important cause of mild upper respiratory illnesses in adults. The human enteroviruses consist of polioviruses (3 serotypes), echoviruses (34 serotypes), group A coxsackieviruses (24 serotypes), and group B coxsackieviruses (6 serotypes). The human enteroviruses are ubiquitous; transmitted primarily by the oral-fecal route, they are capable of producing a wide range of clinical syndromes varying in severity. They are mainly found in the gastrointestinal tracts of infected individuals but also have the potential to infect the meninges, central nervous system, myocardium, pericardium, striated muscles, respiratory tract, and skin (23).
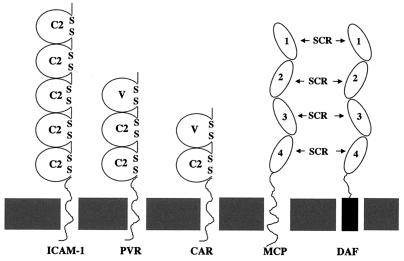
Identification and molecular characterization of virus-specific receptors and receptor-mediated processes are important not only because receptors determine host range, susceptibility to infection, and pathogenesis but also because virus receptors constitute potential targets for antiviral therapy. Three major groups of cell surface molecules have been shown to be involved with distinct stages of picornaviral cell attachment and membrane penetration: the immunoglobulin-like supergene family, complement regulatory proteins, and integrins. The poliovirus receptor (PVR) (24), intercellular adhesion molecule 1 (ICAM-1) (16), and coxsackievirus-adenovirus receptor (CAR) (8, 36) are all members of the immunoglobulin-like supergene family (Fig. 1) and are able to facilitate productive cell infection by polioviruses (24), a major group of rhinoviruses (16), and some group A (31) and group B (8, 33, 36) coxsackieviruses, respectively. In contrast, the complement regulatory protein, decay-accelerating factor (DAF) (28) (Fig. 1), is employed as an attachment or sequestering receptor by many enteroviruses (6, 7, 17, 30, 32, 41) but cannot by itself mediate entry of virus into the cell (7, 30) unless cross-linked by specific antibody (34, 34a). Additionally, a number of integrins have been shown to bind human picornaviruses, but their role in viral cell entry is uncertain (1, 5, 29). Recently, a number of picornaviruses have been shown to employ complexes of the above-mentioned receptors to enter cells, these receptor complexes consisting of distinct attachment and cell internalizing components (32, 33).
FIG. 1.
Schematic representation of structures of the virus receptors ICAM-1, PVR, CAR, MCP, and DAF. ICAM-1, PVR, and CAR belong to the immunoglobulin supergene family, and MCP and DAF are complement regulatory proteins. ICAM-1 is an internalization receptor for rhinoviruses and some group A coxsackieviruses. CAR is an internalization receptor for group B coxsackieviruses. DAF is a sequestration receptor for coxsackieviruses, echoviruses, and enteroviruses. C2, constant region 2; V, variable region; SCR, short consensus repeat.
The cellular expression of many picornavirus receptors is strongly influenced by the action of inflammatory cytokines (10, 26, 35, 37). Human rhinoviruses (HRVs) enhance their own dissemination by up-regulating the cell surface expression of ICAM-1 on neighboring cells through cytokine induction. ICAM-1 is highly susceptible to up-regulation via the inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) (37), and during initial stages of cellular lytic infection by HRVs, cytokines such as TNF-α and IL-1β are released by infected cells (35). Furthermore, the expression of CAR on the surface of colon carcinoma cells is markedly increased by the action of TNF-α (1a).
The expression of DAF, a major picornavirus attachment receptor, has been shown to be significantly up-regulated by the action of TNF-α (10, 26). In contrast, another member of the complement regulatory protein family, membrane cofactor protein (MCP; CD46), which is also a cellular receptor for measles viruses, is resistant to TNF-α-induced up-regulation (10, 26, 38). Thus, the ability to negate TNF-α-induced up-regulation of cellular virus receptors might be regarded a potential antiviral strategy for controlling human picornavirus infections.
TNF-α, in addition to up-regulating numerous cellular proteins, is also able to promote apoptosis in many cells (40). Recently, cells expressing plasminogen activator inhibitor type 2 (PAI-2) were shown to be protected from TNF-α-induced apoptosis (13, 21). PAI-2 is a serine proteinase inhibitor (serpin), which is a major product of macrophages in response to endotoxin and inflammatory cytokines (20). Under physiological conditions, PAI-2 expression is limited to a select number of cell types, which include differentiated keratinocytes, activated monocytes and macrophages, placental trophoblasts, and some tumor cell lines (20). PAI-2 is well characterized as an inhibitor of the extracellular proteinase urokinase-type plasminogen activator (20, 39). Recently, an additional, distinct, intracellular function for PAI-2 as a regulator of the intracellular signal transduction pathway(s) has been postulated (3, 13). Intracellular PAI-2 expression in HeLa cells was associated with resistance to TNF-α-mediated apoptosis (13) and with increased alpha/beta interferon (IFN-α/β) activity (3). PAI-2 is a member of the ovalbumin group of serpins (ov-serpins), which lack hydrophobic signal sequences. This results in inefficient secretion and translocation and thus a primarily cytoplasmic location of these proteins. In the case of HeLa cells expressing PAI-2, no secreted PAI-2 is detectable. Apart from PAI-2, a number of other ov-serpins have now been shown to have cytoplasmic functions; these include CrmA (15), protease inhibitor 9 (9), and protease inhibitor 6 (27).
Here we show that expression of PAI-2 in HeLa cells significantly reduced picornavirus infection through transcriptional down-regulation of the expression of TNF-α-inducible virus receptors. These findings may find application in the development of a novel serpin-based antiviral strategies for control of enteric picornavirus infections, and also for the therapeutic reduction of ICAM-1-mediated respiratory inflammations.
MATERIALS AND METHODS
Cells and viruses.
Echovirus type 7 (EH7; Wallace), poliovirus type 1 (PV1; Sabin), coxsackievirus A21 (CVA21; KuyKendall), coxsackievirus B3 (CVB3; Nancy), and HeLa B cells were obtained from Margery Kennett, Enterorespiratory Laboratory, Fairfield Hospital, Melbourne, Victoria, Australia. HRV type 14 (HRV14) was obtained from the American Type Culture Collection. All viruses were grown in HeLa B cells.
A panel of individual cloned HeLa cell lines expressing sense (S1a and S1b) and antisense (A2/7) PAI-2 cDNA was generated as previously described (13) by inserting a DNA fragment containing the entire PAI-2 coding sequence and the 3′ untranslated region in both orientations into the expression vector pRcCMV under control of the constitutive cytomegalovirus V promoter. Stable transfectants containing these constructs were selected by resistance to G418 and characterized by Northern blot, immunoblot, and immunofluorescence analyses (13, 14). HeLa and A2/7 cells express no detectable PAI-2 mRNA or protein. S1a and S1b cell lines each express cytoplasmic PAI-2 at levels similar to or less than those detected in activated monocytic cells (13).
Antibodies.
The anti-ICAM-1 monoclonal antibody (MAb) WEHI (11) was obtained from Andrew Boyd, Queensland Institute of Medical Research, Brisbane, Australia. The anti-DAF MAbs IA10, VIIIA7, and IIH6 (18) were generous gifts from Taroh Kinoshita, Department of Immunoregulation, Osaka University, Osaka, Japan; MAb IH4 and anti-MCP MAb E4.3 (12) were from Bruce Loveland, Austin Research Institute, Heidelberg, Victoria, Australia. The anti-PVR MAb 280 (25) was supplied by Philip Minor, National Institute for Biological Standards and Control, Potters Bar, United Kingdom, and the anti-CAR MAb (8) was from Jeffery Bergelson, Dana-Farber Cancer Institute, Boston, Mass.
Virus infectivity assay.
Cell monolayers in 96-well culture plates were challenged with 100 μl of 10-fold serial dilutions of the above-mentioned picornaviruses and then incubated at 37°C for 48 h. To quantitate cell survival, monolayers were stained with a crystal violet-methanol solution and washed with distilled water, and the plates were read at a wavelength of 540 nm. Results are expressed as mean percentages of cell lysis relative to the uninfected control cell monolayers of triplicate wells + standard deviation.
Radiolabeled virus binding assays.
Viruses were radiolabeled in Dulbecco’s modified essential medium containing [35S]methionine and purified by velocity centrifugation in 5 to 30% sucrose gradients as previously described (32). Monolayers of PAI-2-expressing S1a and S1b cells and parental HeLa cells were incubated with radiolabeled viruses (2 × 104 to 5 × 104 cpm) in serum-free Dulbecco’s modified essential medium for 1 h at 37°C. After three washes, the cell monolayers were dissolved in 200 μl of 0.2 M NaOH–1.0% sodium dodecyl sulfate (SDS). The amount of labeled virus bound was measured by liquid scintillation counting.
Flow cytometry.
Cells (5 × 105) were incubated with the appropriate MAbs (5.0 μg/ml) diluted in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (PBS-BSA) at 0°C for 30 min, after which the cells were washed with 5.0 ml of PBS-BSA. The cells were then pelleted at 1,000 × g for 5 min and resuspended in 100 μl of phycoerythrin-conjugated goat anti-mouse immunoglobulin G (heavy plus light chains) (Silenus, Melbourne, Victoria, Australia) diluted in PBS-BSA. After incubation at 0°C for 30 min, the cells were washed and pelleted as described above, resuspended in PBS-BSA, and analyzed with a FACStar analyzer (Becton Dickinson, Sydney, New South Wales, Australia).
Surface biotinylation and immunoprecipitation.
Cells (5 × 106) were detached from the surface of plastic tissue culture flasks by using an EDTA solution and washed once in PBS. Washed cells were resuspended into 3 ml of biotinylation buffer (10 nM sodium borate [pH 8.8], 150 nM NaCl). Biotin-amidocaproate N-hydroxysuccinimide ester was added to 50 μg/ml, and the sample stood at room temperature for 15 min. The reaction was terminated by the addition of NH4Cl to 10 mM. Cells were washed twice in PBS and resuspended into buffer A (138 mM NaCl, 2.9 mM KCl, 0.5 mM MgCl2, 12 mM NaHCO3, 0.3 mM NaH2PO4, 5.5 mM glucose, 10 mM HEPES [pH 7.4]). Cell lysates were placed on ice for 60 min and then centrifuged at 15,000 × g for 15 min to remove detergent-insoluble material. Soluble lysates were precleared with rabbit anti-mouse immunoglobulin G coupled to Sepharose 4B. Immunocomplexes were washed three times with lysis buffer and resolved (reduced) by SDS-polyacrylamide gel electrophoresis. Resolved proteins were electrophoretically transferred to nitrocellulose membranes. Streptavidin-biotin-horseradish peroxidase complexes were used to probe the membrane, and labeled proteins were visualized by using enhanced chemiluminescence (Amersham Life Sciences, Little Chalfont, United Kingdom).
Northern blot analysis.
Total RNA isolated from S1a, S1b, A2/7, and parental HeLa cells was separated by denaturing gel electrophoresis and transferred to Hybond-N nylon membranes as described elsewhere (2). The blot was hybridized with [32P]dCTP-labeled purified DNA fragments encoding cDNAs for ICAM-1 (30), DAF (30), CAR (8), and PVR (24). Blots were hybridized at 65°C and washed to a final stringency of 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS at 65°C. The level of 18S rRNA was used as a measure of RNA loading in each lane.
RESULTS
PAI-2 expression inhibits HeLa cell lytic infection by many picornaviruses.
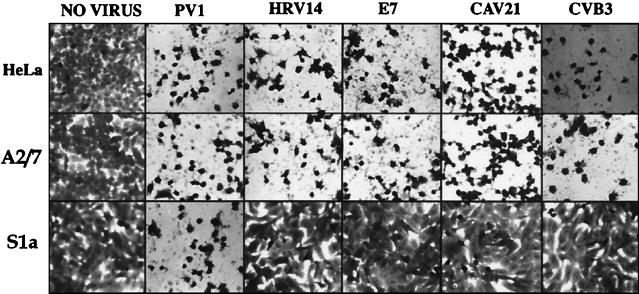
Monolayers of S1a, S1b, A2/7, and parental HeLa cells were challenged (105 PFU/well) with PV1, HRV14, E7, CAV21, and CVB3 and examined for cytopathic effect following a 48-h incubation period. The cell lines not expressing PAI-2 (HeLa and A2/7) were susceptible to lytic infection by all of these picornaviruses (Fig. 2). In contrast, PAI-2-expressing S1a cells were refractory to lytic infection by HRV14, E7, CAV21, and CVB3 but not PV1 (Fig. 2).
FIG. 2.
Picornavirus-induced lytic infection of PAI-2-expressing S1a cells and control A2/7 and parental HeLa cells. Monolayers of S1a, A2/7, and HeLa cells in 96-well culture plates were inoculated (approximately 105 PFU/well) with PV1, HRV14, E7, CAV21, and CVB3. Following incubation for 48 h at 37°C, the cell monolayers were inspected for signs of cell lysis and then photographed at an original magnification of ×20.
To determine whether infection of PAI-2-expressing cells was noncytopathic but still productive, monolayers of HeLa and S1a cells in six-well plates were infected with inocula of 105 and 103 PFU of CVB3. Following incubation for 24 h, cell lysis was evident only in the inoculated HeLa cells. Analysis of infectious virus production by plaque assay revealed that S1a cells yielded titers attributable to that of the input virus, whereas HeLa cell lysates yielded increases of 103- and 105-fold, respectively, over the input inoculum (data not shown).
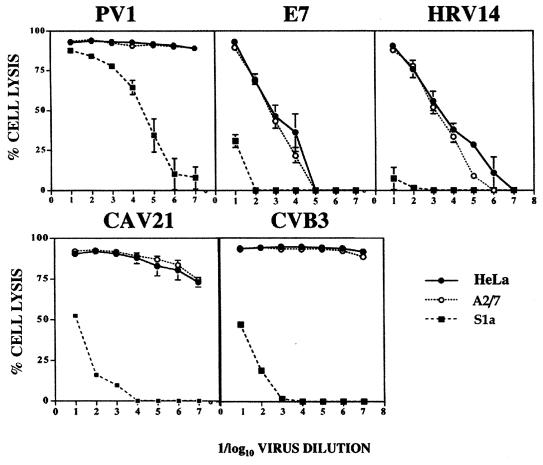
To further quantitate the antiviral effect of PAI-2 expression, monolayers of HeLa, A2/7, and S1a cells were infected with 10-fold serial dilutions of PV1, HRV14, E7, CAV21, and CVB3. HeLa and A2/7 cells showed higher levels of cell lysis than S1a cells for all dilutions of HRV14, E7, CAV21, and CVB3 (Fig. 3). S1b cells behaved similarly to S1a cells in these assays (data not shown). At low dilutions (or high multiplicity of infection [MOI]) of PV1, no difference between cytopathic effect of S1a cells and control cells was observed; however, at high dilutions of virus (low MOI), S1a cells showed increased survival (low percent lysis) relative to control lines (Fig. 3).
FIG. 3.
Dose-dependent picornavirus-induced lytic infection of S1a, A2/7, and HeLa cells, using a range of input multiplicities of PV1, HRV14, E7, CAV21, and CVB3.
The lower cell lysis values for E7 and HRV infection of control cell lines at high virus dilutions than for CAV21 and CVB3 infection reflect a lower replication competence of E7 and HRV in HeLa cells and thus less lysis within the 48-h assay period.
Thus, HeLa and A2/7 cells were substantially more susceptible than PAI-2-expressing cells to the cytopathic effects of HRV14, E7, CAV21, and CVB3 infection. Picornaviral replication appeared not to be modified by PAI-2 cytoplasmic expression, as HeLa and PAI-2-expressing S1a cells yielded comparable levels of infectious CAV21 and CVB3 when transfected with CAV21 and CVB3 viral RNAs, respectively (data not shown).
Reduced binding of picornaviruses to PAI-2-expressing cells.
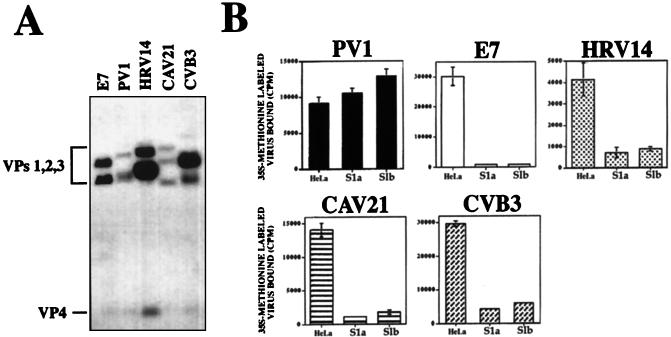
To determine whether inhibition of picornavirus lytic infection in S1a and S1b cells was due to interference with cellular attachment, preparations of gradient-purified picornaviruses were radiolabeled (Fig. 4A) and incubated with S1a, S1b, and HeLa cells to monitor relative levels of virus-cell surface binding. Cellular attachment of E7, HRV14, CAV21, and CVB3 was reduced by between 80 and 95% in S1a and S1b cells compared to HeLa controls (Fig. 4B). PV1 bound equally to S1a, S1b, and HeLa cell lines, albeit with a slight increase in binding to S1b cells compared with HeLa cells (Fig. 4B).
FIG. 4.
Picornavirus binding to S1a, A2/7, and parental HeLa cells. (A) 35S-labeled preparations of PV1, HRV14, E7, CAV21, and CVB3 were separated on a 15% slab SDS-polyacrylamide gel, and individual proteins identified by autoradiography. (B) Monolayers of PAI-2-expressing S1a and S1b cells and parental HeLa cells were incubated with radiolabeled viruses and washed, and the amount of labeled virus bound was measured by liquid scintillation counting. Results are expressed as the mean of triplicate wells + standard deviation.
The overall pattern of reduced virus binding to the panel of cells was similar to the pattern observed for inhibition of virus-induced lytic infection shown in Fig. 2 and 3, suggesting that the presence of PAI-2 reduced the surface expression of picornavirus cellular receptors.
PAI-2 reduces surface expression of picornavirus receptors.
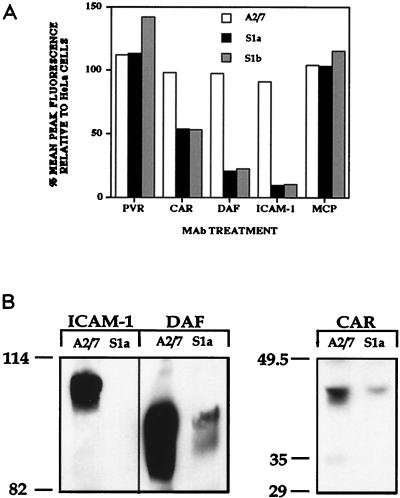
To determine whether PAI-2-transfected cells expressed lower levels of virus receptors, which would result in less virus binding (Fig. 4) and less cell infection (Fig. 2 and 3), the levels of the picornavirus receptor molecules PVR, CAR, DAF, and ICAM-1 were assessed by flow cytometry. The cellular receptor of the unrelated measles virus, MCP, was included as a control. Measurement of the mean peak fluorescence intensities showed that expression of CAR, DAF, and ICAM-1 on the surface of S1a and S1b cells compared to A2/7 cells was reduced by 50 to 90% (Fig. 5A). The surface expression of PVR and MCP was similar among all cell lines, indicating that these receptors were unaffected by PAI-2 expression. (Results for the parent HeLa cell line were similar to those for A2/7 cells in these assays [data not shown]). The slight increase in PV1 binding to S1b cells observed in Fig. 4B was mirrored by a slight increase in the level of PVR on S1b cells (Fig. 5A). The decreased picornavirus receptor expression on the surface of PAI-2-expressing HeLa cells was not due to an accident of the transfection and selection process, as the antisense PAI-2 clone A2/7 (Fig. 5) and a chloramphenicol acetyltransferase-expressing HeLa clone generated by the same protocol as used for the S1a and S1b cells showed no signs of reduced surface ICAM-1 expression (35a).
FIG. 5.
Analysis of picornavirus receptor expression on the surface of S1a, S1b, and HeLa cells. (A) Flow cytometric analysis of receptor surface expression. Suspensions of S1a, S1b, A2/7, and HeLa cells were incubated with MAbs against the virus receptors PVR, ICAM-1, DAF, CAR, and MCP. (B) Immunoprecipitation of biotinylated virus receptors. S1a and A2/7 cells were surface biotinylated and then immunoprecipitated with anti-DAF, anti-ICAM-1, and anti-CAR MAbs, as indicated. Sizes are indicated in kilodaltons.
To confirm that PAI-2 selectively reduced surface expression of CAR, DAF, and ICAM-1, S1a cells and A2/7 cells were surface biotinylated and immunoprecipitated with anti-DAF, anti-CAR, and anti-ICAM-1 MAbs. The level of surface expression of biotinylated DAF and CAR was significantly less on the surface of S1a cells than on the surface of A2/7 cells. ICAM-1 expression differed dramatically between the two cell lines, being undetectable on the surface of S1a cells (Fig. 5B). The reduction in CAR, DAF, and ICAM-1 expression on the surface of PAI-2-expressing S1a cells (Fig. 5B) mirrored that detected by flow cytometry (Fig. 5A).
These data clearly show a reduction in the expression of the picornavirus receptors CAR, DAF, and ICAM-1 in PAI-2 expressing cells, while PVR and MCP were unaffected.
PAI-2-mediated picornavirus receptor down-regulation occurs at the transcriptional level.
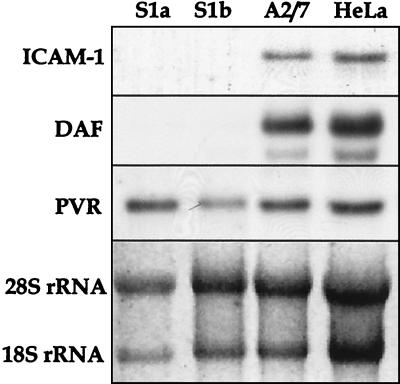
To ascertain whether PAI-2-induced picornavirus receptor down-regulation was mediated by transcriptional or posttranscriptional activity, mRNA levels for ICAM-1, DAF, and PVR were examined in PAI-2-expressing and control cell lines. Significant levels of PVR mRNA were detected in each cell line (S1a, S1b, A2/7, and HeLa) independent of expression of PAI-2 (Fig. 6). ICAM-1 and DAF mRNAs were present in HeLa and A2/7 cells, but no ICAM-1 or DAF mRNA was detected in PAI-2-expressing S1a and S1b cells (Fig. 6). A marked reduction in the CAR mRNA level was observed in the S1a cells but not in S1b cells (data not shown). This may be due to the finding that S1a cells express significantly higher levels of intracellular PAI-2 than S1b cells (3a), which raises the possibility that the intracellular threshold PAI-2 level required for CAR down-regulation is higher than that needed for DAF and ICAM-1 down-regulation. The levels of the loading control, 18S and 28S rRNAs, were comparable in all the samples. These findings demonstrated that the reduction in the expression of picornavirus receptors in PAI-2-expressing cells was due to a reduction in their gene transcription.
FIG. 6.
Northern blot analysis of virus receptor expression in PAI-2-expressing S1a and S1b cells and control A2/7 and HeLa cells. Total RNA was isolated from each cell line, separated by denaturing gel electrophoresis, and Northern blotted, and the membranes were probed with radiolabeled cDNA fragments encoding each receptor. Levels of 18S and 28S rRNAs are shown as a measure of RNA loading in each lane.
DISCUSSION
This study demonstrated that expression of PAI-2 protected the highly permissive HeLa cells against lytic infection by selected human picornaviruses (Fig. 2 and 3). The primary mode of action was a PAI-2-dependent transcriptional down-regulation of the surface expression of three picornavirus cellular receptors, DAF, CAR, and ICAM-1 (Fig. 6). The surface expression of other virus receptors, namely, PVR and MCP, although closely related to CAR/ICAM-1 and DAF (Fig. 1), respectively, were unaffected by cytoplasmic expression of PAI-2 (Fig. 5).
Cytoplasmic expression of PAI-2 has been shown to protect cells against TNF-α-mediated apoptosis (13, 21), and here we show that PAI-2 expression significantly reduced the surface expression of the virus receptor molecules DAF, CAR, and ICAM-1, which are all known to be TNF-α inducible (10, 26, 37). In contrast, PVR and MCP were not inducible by TNF-α, and their expression was largely unaffected by PAI-2 expression. The structural relatedness of PVR, CAR, and ICAM-1 and of DAF and MCP (Fig. 1) appeared to have no bearing on the PAI-2-mediated effects. Thus, PAI-2 appeared to selectively down-regulate TNF-α-inducible receptors, although the expression of the TNF-α-inducible HLA class I was not affected by PAI-2 expression (data not shown). These experiments suggest that PAI-2 has activity as a specific intracellular regulator of gene expression, consistent with previous reports of a PAI-2-mediated influence on the signal transduction pathway(s) (3, 13, 14).
Antalis et al. (3) recently described another phenotype of PAI-2-expressing HeLa cells whereby the cells were protected from the rapid cytopathic effects of alphavirus infection via a PAI-2-mediated induction of constitutive IFN-α/β production (3). The replication of many picornaviruses has been shown to be inhibited by the action of IFN-α/β (19, 22). The antiviral action of IFN-α/β may provide an explanation for the protection against poliovirus lytic infection seen in PAI-2-expressing cells at low MOI (Fig. 3), in the absence of receptor down-regulation or reduction in viral binding (Fig. 4 and 5). The antipicornaviral activity of PAI-2 may thus be twofold: (i) the major component via receptor down-regulation and reduction of viral binding and (ii) a minor component via inhibition of viral replication through the action of autocrine IFN-α/β.
Picornavirus infections are a major cause of morbidity in our community, with symptoms ranging from the common cold to pericarditis. For many of these viruses, the large number of serotypes makes vaccine development difficult. In recent years, much research effort has been directed at characterizing the cellular receptors used by viruses and the molecular basis of virus-receptor interactions, with the ultimate aim of developing generic antiviral strategies. Here we show that a specific serpin can transcriptionally down-regulate specific viral receptors, illustrating the potential for novel serpin based antiviral strategies for a large proportion of picornavirus infections.
ICAM-1 plays an important role in the pathogenesis of not only rhinovirus infection but also Plasmodium falciparum infection (4) and the exacerbations of asthma, chronic bronchitis, and cystic fibrosis (42). ICAM-1 was the most susceptible to the action of cytoplasmic PAI-2 expression, indicating that PAI-2-based therapy may also find application in the reduction of ICAM-1-mediated respiratory inflammations.
ACKNOWLEDGMENTS
We thank Margery Kennett for the stock picornaviral preparations, and we thank Rebecca Ingham and Simone Cross for excellent technical assistance.
This research was supported by grants from the National Health and Medical Research Council of Australia, Hunter Medical Research Foundation, Australian Center for International & Tropical Health & Nutrition, and the Australian Commonwealth AIDS Research Grants Program.
REFERENCES
- 1.Agrez M V, Shafren D R, Gu X, Cox K, Sheppard D, Barry R D. Integrin alpha v beta 6 enhances coxsackievirus B1 lytic infection of human colon cancer cells. Virology. 1997;239:71–77. doi: 10.1006/viro.1997.8831. [DOI] [PubMed] [Google Scholar]
- 1a.Agrez, M. V. Personal communication.
- 2.Antalis T M, Dickinson J L. Control of plasminogen-activator inhibitor type 2 gene expression in the differentiation of monocytic cells. Eur J Biochem. 1992;205:203–209. doi: 10.1111/j.1432-1033.1992.tb16769.x. [DOI] [PubMed] [Google Scholar]
- 3.Antalis T M, La Linn M, Donnan K, Mateo L, Gardner J, Dickinson J L, Buttigieg K, Suhrbier A. The serine proteinase inhibitor (serpin) plasminogen activation inhibitor type 2 protects against viral cytopathic effects by constitutive interferon alpha/beta priming. J Exp Med. 1998;187:1799–1811. doi: 10.1084/jem.187.11.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Antalis, T. M. Personal communication.
- 4.Berendt A R, McDowall A, Craig A G, Bates P A, Sternberg M J E, Marsh K, Newbold C I, Hogg N. The binding site on ICAM-1 for Plasmodium falciparum-infected erythrocytes overlaps, but is distinct from, the LFA-1-binding site. Cell. 1992;68:71–81. doi: 10.1016/0092-8674(92)90207-s. [DOI] [PubMed] [Google Scholar]
- 5.Bergelson J M, Shepley M P, Chan B M, Hemler M E, Finberg R W. Identification of the integrin VLA-2 as a receptor for echovirus 1. Science. 1992;255:1718–1720. doi: 10.1126/science.1553561. [DOI] [PubMed] [Google Scholar]
- 6.Bergelson J M, Chan B M, Solomon K R, St. John J N, Finberg R W. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc Natl Acad Sci USA. 1994;91:6245–6249. doi: 10.1073/pnas.91.13.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergelson J M, Mohanty J G, Crowell R L, St. John N F, Lublin D M, Finberg R W. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55) J Virol. 1995;69:1903–1906. doi: 10.1128/jvi.69.3.1903-1906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R C, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 9.Bird C H, Sutton V R, Sun J, Hirst C E, Novak A, Kumar S, Trapani J A, Bird P I. Selective regulation of apoptosis: the cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme B-mediated apoptosis without perturbing the Fas cell death pathway. Mol Cell Biol. 1998;18:6387–6398. doi: 10.1128/mcb.18.11.6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjorge L, Jensen T S, Matre R. Characterisation of the complement-regulatory proteins decay-accelerating factor (DAF, CD55) and membrane cofactor protein (MCP, CD46) on a human colonic adenocarcinoma cell line. Cancer Immunol Immunother. 1996;42:185–192. doi: 10.1007/s002620050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd A W, Wawryk S O, Burns G F, Fecondo J V. Intercellular adhesion molecule-1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci USA. 1988;85:3095–3099. doi: 10.1073/pnas.85.9.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christiansen D, Milland J, Thorley B R, McKenzie I F, Loveland B E. A functional analysis of recombinant soluble CD46 in vivo and a comparison with recombinant soluble forms of CD55 and CD35 in vitro. Eur J Immunol. 1996;26:578–585. doi: 10.1002/eji.1830260312. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson J L, Bates E J, Ferrante A, Antalis T M. Plasminogen activator inhibitor type 2 inhibits tumor necrosis factor alpha induced apoptosis. Evidence for an alternate biological function. J Biol Chem. 1995;270:27894–27904. doi: 10.1074/jbc.270.46.27894. [DOI] [PubMed] [Google Scholar]
- 14.Dickinson J L, Norris B J, Jensen P H, Antalis T M. The C-D interhelical domain of the serpin plasminogen activator inhibitor-type 2 is required for protection from TNFα-induced apoptosis. Cell Death Differ. 1997;5:163–171. doi: 10.1038/sj.cdd.4400324. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Calvo M, Peterson E P, Leiting B, Ruel R, Nicholson D W, Thornberry N A. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- 16.Greve J M, Davis G, Meyer A M, Forte C P, Connolly Yost S, Marlor C W, Kamark M E, McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–845. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 17.Karnauchow T M, Tolson D L, Harrison B A, Altman E, Lublin D M, Dimmock K. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55) J Virol. 1996;70:5143–5152. doi: 10.1128/jvi.70.8.5143-5152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinoshita T, Medof M E, Silber R, Nussenzweig V. Distribution of decay accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985;162:75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komatsu T, Bi Z, Reiss C S. Interferon-gamma induced type I nitric oxide synthase activity inhibits viral replication in neurons. J Neuroimmunol. 1996;68:101–108. doi: 10.1016/0165-5728(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 20.Kruithof E K O, Baker M S, Bunn C L. Biological and clinical aspects of plasminogen activator inhibitor type 2. Blood. 1995;86:4007–4024. [PubMed] [Google Scholar]
- 21.Kumar S, Baglioni C. Protection from tumour necrosis factor-mediated cytolysis by overexpression of plasminogen activator inhibitor type-2. J Biol Chem. 1991;26:20960–20964. [PubMed] [Google Scholar]
- 22.Langford M P, Crainic R, Vrijsen R, Wimmer E. Antibodies may act synergistically or additively with interferon to inhibit poliovirus. Microb Pathog. 1991;10:419–427. doi: 10.1016/0882-4010(91)90107-l. [DOI] [PubMed] [Google Scholar]
- 23.Melnick J L. Portraits of viruses: the picornaviruses. Intervirology. 1983;20:61–100. doi: 10.1159/000149376. [DOI] [PubMed] [Google Scholar]
- 24.Mendelsohn C L, Wimmer E, Racaniello V R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 25.Minor P D, Pipkin P A, Hockley D, Schild G C, Almond J W. Monoclonal antibodies which block cellular receptors of poliovirus. Virus Res. 1984;1:203–212. doi: 10.1016/0168-1702(84)90039-x. [DOI] [PubMed] [Google Scholar]
- 26.Moutabarrik A, Nakanishi I, Namiki M, Hara T, Matsumoto M, Ishibashi M, Okuyama A, Zaid T, Seya D. Cytokine-mediated regulation of the surface expression of complement regulatory proteins, CD46(MCP), CD55(DAF), and CD59 on human vascular endothelial cells. Lymphokine Cytokine. 1993;12:167–172. [PubMed] [Google Scholar]
- 27.Nakaya N, Nishibori M, Wang Z, Sakiyama J, Saeki K. The expression and localization of serine proteinase inhibitor PI-6 mRNA in developmental and ischemic mouse brain. Neurosci Res. 1998;3:221–230. doi: 10.1016/s0168-0102(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson-Weller A, Wang C E. Structure and function of decay accelerating factor CD55. J Lab Clin Med. 1994;123:485–491. [PubMed] [Google Scholar]
- 29.Roivainen M, Piirrainen L. Entry of coxsackievirus A9 into host cells: specific interactions with αvβ3 integrin, the vitronectin receptor. Virology. 1994;203:357–365. doi: 10.1006/viro.1994.1494. [DOI] [PubMed] [Google Scholar]
- 30.Shafren D R, Bates R C, Agrez M V, Herd R L, Burns G F, Barry R D. Coxsackieviruses B1, B3, and B5 use decay-accelerating factor as a receptor for cell attachment. J Virol. 1995;69:3873–3877. doi: 10.1128/jvi.69.6.3873-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shafren D R, Dohary D J, Greive S J, Burns G F, Barry R D. Mouse cells expressing human intercellular adhesion molecule-1 are susceptible to infection by coxsackievirus A21. J Virol. 1997;71:785–789. doi: 10.1128/jvi.71.1.785-789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shafren D R, Dorahy D J, Ingham R A, Burns G F, Barry R D. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J Virol. 1997;71:4736–4743. doi: 10.1128/jvi.71.6.4736-4743.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shafren D R, Williams D T, Barry R D. A decay-accelerating factor-binding strain of coxsackievirus B3 requires the coxsackievirus-adenovirus receptor protein to mediate lytic infection of rhabdomyosarcoma cells. J Virol. 1997;71:9844–9848. doi: 10.1128/jvi.71.12.9844-9848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shafren D R, Dorahy D J, Thorne R F, Kinoshita T, Barry R D, Burns G F. Antibody binding to individual short consensus repeats of decay-accelerating factor enhance enterovirus binding and cell infection. J Immunol. 1998;160:2318–2323. [PubMed] [Google Scholar]
- 34a.Shafren D R. Viral cell entry induced by cross-linked decay-accelerating factor. J Virol. 1998;72:9407–9412. doi: 10.1128/jvi.72.11.9407-9412.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subauste X C, Jacoby D B, Richards S M, Proud D. Infection of a human respiratory epithelial cell line with rhinovirus. Induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J Clin Investig. 1995;96:549–557. doi: 10.1172/JCI118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.Suhrbier, A. Personal communication.
- 36.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tosi M F, Stark J M, Smith C W, Hamedani A, Gruenert D C, Infeld M D. Induction of ICAM1 expression on human airway epithelial cells by inflammatory cytokines: effects on neutrophil-epithelial cell adhesion. Am J Respir Cell Mol Biol. 1992;7:214–221. doi: 10.1165/ajrcmb/7.2.214. [DOI] [PubMed] [Google Scholar]
- 38.Varsano S, Rashkovsky L, Shapiro H, Radnay I. Cytokines modulate expression of cell-membrane complement inhibitory proteins in human lung cancer cell lines. Am J Respir Cell Mol Biol. 1998;19:522–529. doi: 10.1165/ajrcmb.19.3.3181. [DOI] [PubMed] [Google Scholar]
- 39.Vassalli J D, Sappino A P, Belin D. The plasminogen activator/plasmin system. J Clin Investig. 1991;88:1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vassalli P. The pathophysiology of tumour necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 41.Ward T, Pipkin P A, Clarkson N A, Stone D M, Minor P D, Almond J W. Decay accelerating factor CD55 is identified as the receptor for echovirus 7 using CELICS, a rapid immuno-focal cloning method. EMBO J. 1994;13:5070–5074. doi: 10.1002/j.1460-2075.1994.tb06836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wegner C D, Gundel R H, Relly P, Haynes N, Letts L G, Rothlein R. Intercellular adhesion molecule-1 (ICAM1) in the pathogenesis of asthma. Science. 1990;247:456–459. doi: 10.1126/science.1967851. [DOI] [PubMed] [Google Scholar]