Abstract
Background
Laboratory studies have indicated that a cholesterol metabolite and selective estrogen receptor modulator, 27-hydroxycholesterol (27HC), may be important in breast cancer etiology and explain associations between obesity and postmenopausal breast cancer risk. Epidemiologic evidence for 27HC in breast cancer risk is limited, particularly in multiethnic populations.
Methods
In a nested case–control study of 1470 breast cancer cases and 1470 matched controls within the Multiethnic Cohort Study, we examined associations of pre-diagnostic circulating 27HC with breast cancer risk among African American, Japanese American, Native Hawaiian, Latino, and non-Latino White postmenopausal females. We used multivariable logistic regression adjusted for age, education, parity, body mass index, and smoking status. Stratified analyses were conducted across racial and ethnic groups, hormone receptor (HR) status, and use of lipid-lowering drugs. We assessed interactions of 27HC with steroid hormones.
Results
27HC levels were inversely related to breast cancer risk (odds ratio [OR] 0.80; 95% confidence interval [CI] 0.58, 1.12), but the association was not statistically significant in the full model. Directions of associations differed by racial and ethnic group. Results suggested an inverse association with HR-negative breast cancer (OR 0.46; 95% CI 0.20, 1.06). 27HC interacted with testosterone, but not estrone, on risk of breast cancer; 27HC was only inversely associated with risk among those with the highest levels of testosterone (OR 0.46; 95% CI 0.24, 0.86).
Conclusion
This is the first US study to examine circulating 27HC and breast cancer risk and reports a weak inverse association that varies across racial and ethnic groups and testosterone level.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-023-01693-6.
Keywords: 27-Hydroxycholesterol, Lipids, Steroid hormones, Breast cancer risk, Race and ethnicity
Background
Breast cancer is the most common cancer and the second leading cause of cancer death among US females [1]. Obesity and body fat distribution are important risk factors for breast cancer [2–6]. Dyslipidemia accompanies obesity, and adipose tissue is a key source of circulating estrogen after menopause [7, 8]. At the same time, observational studies have reported associations of low high-density lipoprotein cholesterol (HDL-C) levels, high total cholesterol levels, and high triglyceride levels with increased breast cancer risk, although with some variability across studies [9–15]. Thus, hypothesized mechanisms for the association between obesity and postmenopausal breast cancer implicate higher levels of cholesterol and estradiol.
27-Hydroxycholesterol (27HC) is a cholesterol metabolite, the most-abundant circulating oxysterol, and an endogenous selective estrogen receptor modulator (SERM) [16–21]. 27HC levels co-vary with cholesterol and hypercholesterolemia results in commensurately high levels of 27HC (82). In mouse models, 27HC has been shown to promote breast cancer formation and growth by binding to the estrogen receptor (ER) and to increase the metastatic potential of breast tumors through the activation of the liver X receptor (LXR) [18–21]. In breast cancer cell lines, the role of 27HC in cell growth was modulated by levels of exogenous estradiol, so the impact of 27HC on breast cancer development may vary with concomitant levels of steroid hormones [17]. Thus, while 27HC is a potential molecular target for breast cancer prevention [20, 22–24], there is a need for epidemiologic studies that assess the influence of 27HC on breast cancer risk, especially in the presence of steroid hormones that also modulate estrogen signaling [25–31].
There are substantial racial and ethnic disparities in breast cancer risk in the US; breast cancer incidence rates range from a high of 172.1 per 100,000 person years among Native Hawaiian to a low of 92.1 among Hispanic females [32, 33]. Additionally, total cholesterol levels vary by racial and ethnic group in the US from a high of 13.1% among non-Hispanic White, females to 9.2% among Hispanic females [34]. These national prevalence estimates, however, report Asian American and Pacific Islander groups as a single aggregate group, masking the prevalence of high cholesterol that has been observed to be as high as 48% among Native Hawaiian and Pacific Islander females [35]. Thus, racial and ethnic disparities in hypercholesterolemia appear to track with disparities in breast cancer risk. However, in 2019, a nested case–control study within the European EPIC-Heidelberg cohort showed that higher levels of circulating 27HC were associated with a decreased risk of postmenopausal breast cancer among 193 cases and 200 controls within a largely White, European study population [36] (relative risk = 0.56 95% CI 0.34, 0.95). Therefore, we hypothesized that associations between 27HC and breast cancer risk may differ across racial and ethnic groups and that these differences may shed light on racial and ethnic disparities in breast cancer risk.
To our knowledge, there is no epidemiologic study that has assessed the role of circulating 27HC and breast cancer risk across groups defined by race and ethnicity or examined the interaction of 27HC with lipid or steroid hormone levels. Thus, we conducted a nested case–control study within the Multiethnic Cohort to examine associations of pre-diagnostic, circulating 27HC with breast cancer risk among African American, Japanese American, Latino, Native Hawaiian, and non-Latino White females (1470 cases and 1470 controls). Our primary aim was to assess differences in associations across groups defined by race and ethnicity and to examine interactions of 27HC with lipids and steroid hormones. We also examined associations of 27HC with breast cancer according to hormone receptor (HR) status and use of lipid-lowering drugs. Our secondary aim was to assess differences in associations of lipids and steroid hormones with breast cancer risk across racial and ethnic groups.
Methods
Study population and outcome assessment
The Multiethnic Cohort (MEC) is a prospective cohort of 215,251 individuals (male and female) 45–75 years of age at enrollment between 1993 and 1996 [37]. Participants are from five racial and ethnic groups categorized as African American, Japanese American, Latino, Native Hawaiian, and non-Latino White and live in Hawaiʻi or California (primarily Los Angeles County). Participants completed a baseline questionnaire that assessed demographics, lifestyle, diet, and anthropometrics, and for females, menstrual and reproductive history, and hormone therapy use. In 2001–2006, a prospective biorepository was established that collected blood specimens from approximately 70,000 healthy participants. A short questionnaire was administered at biospecimen collection that assessed age, smoking status, weight, and hormone therapy use. In 2003–2008, a follow-up questionnaire was administered that ascertained information on use of lipid-lowering drugs.
Participants diagnosed with invasive breast cancer were identified through linkage to the Hawaiʻi and California tumor registries of the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. Case and control selection are illustrated in Additional file 1: Fig. 1. This nested case–control study included 1470 females diagnosed with incident invasive breast cancer who were postmenopausal or age > 50 years at cohort entry and provided sufficient pre-diagnostic blood (plasma) sample (cases). Controls were female participants who provided blood samples and were alive and breast cancer free at the age of the matched case’s diagnosis. Controls were matched 1:1 to cases on geographic area (Hawaiʻi or California), race and ethnicity (African American, Japanese American, Latino, Native Hawaiian, and non-Latino White), age at blood draw (± 1 year), birth year (± 1 year), time of blood draw (± 2 h), and hours fasting prior to blood draw (groups: 0 to < 6, 6 to < 8, 8 to < 10, 10 + hours). Cases that did not match with these criteria (n = 192, 15%) were matched by sequentially relaxing criteria to comprise age at blood draw and birth year ± 3 years (6.6%), time of blood draw ± 4 h and < / = 1 adjacent fasting group (4.8%), age at blood draw and birth year ± 5 years (3.0%), time of blood draw ± 6 years and < / = 2 fasting groups adjacent (0.4%), and finally, time of blood draw ± 8 years and < / = 4 fasting groups adjacent (0.2%). Thus, the final study population consisted of 1470 matched pairs.
Exposures of interest—blood plasma analytes
Our main exposure of interest was circulating 27HC. We also assessed circulating lipids (i.e., high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], total cholesterol, and triglycerides), steroid hormones (i.e., total estradiol, estrone, and testosterone), and sex hormone binding globulin (SHBG).
Biomarker assays were conducted at the University of Hawaiʻi Cancer Center Analytical Biochemistry Shared Resource under the supervision of Dr. Adrian Franke. Plasma samples were prepared according to Honda et al. with slight modifications [38] (Additional file 1). 27HC and steroid hormone levels were measured with liquid chromatography-orbitrap mass spectrometry (LC/MS) (Additional file 1) [39]. One matched pair was missing for steroid biomarker assays. Lipid assays were conducted as part of a previous study, so not all cases had lipid assay measurements available (4 of 1470 matched pairs missing). HDL-C, total cholesterol, and triglyceride levels were assayed by an automated chemical analyzer (Cobas Mira Plus, Roche Diagnostics, Switzerland). LDL-C was derived by applying the Friedewald formula, except for participants whose fasting triglyceride was over 400 mg/dl, which resulted in exclusion of an additional 11 matched pairs for LDL-C [40, 41]. Plasma SHBG was assayed by double-antibody enzyme-linked immunosorbent-assay according to manufacturer’s specifications (R&D Systems, Minneapolis, MN) [42].
Laboratory personnel were blinded to case–control status. Matched pairs were assayed in the same batch; quality control pairs were included within each batch, and duplicate pairs were included between batches. All coefficients of variation (CVs) are available in Additional file 1; within-batch CVs were all < 7%, except for estradiol and testosterone which were 16% and 14%, respectively. Analytes below the lower limit of detection (LLOD) were assigned a value of half of the LLOD. For 27HC, no samples were below LLOD. For estradiol, 49.9% of control and 44.7% of case samples were below LLOD. For estrone, 0.4% of control and 0.2% of case samples were below LLOD. For testosterone, 0.4% of control and 0.3% of case samples were below LLOD.
Covariates
Covariates included socioeconomic, behavioral, and reproductive factors assessed at baseline or blood collection and consisted of factors that were hypothesized confounders of associations between 27HC and breast cancer risk or previously observed to be associated with breast cancer risk (Additional file 1: Fig. 2). Hypothesized confounders were age at blood draw, education, body size, smoking status, alcohol, and diet. Reproductive factors previously observed to be associated with breast cancer risk (i.e., parity, age at menarche, oral contraception, and hormone replacement therapy) were considered since inclusion of these covariates if associated with breast cancer risk in our sample would confer increased precision in our estimates.
Baseline measures were individual-level education (less than high school, some college, college graduate, graduate school, missing), alcohol consumption (yes, no, missing), Alternate Mediterranean Diet total score [43] (quartiles based on distribution of scores in the study population), age at menarche (≤ 12 years, 13–14 years, > 14 years, missing), parity (0,1,2–3, ≥ 4, missing), oral contraceptive use (yes, no), and hormone replacement therapy (never estrogen, past estrogen, current estrogen without progesterone, current estrogen with progesterone, missing).
Covariates collected at the time of blood collection were age at blood draw, body mass index (BMI; underweight and normal weight, < 25.0; overweight, 25.0–29.9; and obese, > 30.0 kg/m2, missing), smoking status and pack-years (never smoker, former smoker/ < 20 pack-years, former smoker/ ≥ 20 pack-years, current smoker/ < 20 pack-years, current smoker/ ≥ 20 pack-years, missing), hormone replacement therapy (yes, no, missing), and lipid-lowering drug use (yes, no, missing).
For breast cancer cases, hormone receptor (HR) status was obtained from cancer registries (HR-positive, estrogen receptor- [ER] or progesterone receptor- [PR] positive; HR-negative, ER- and PR-negative, missing).
Statistical analysis
We calculated the mean, SD, and range of each analyte among control and case populations in the overall study population and across racial and ethnic groups. Distributions of raw values of biomarkers were right skewed; thus, the log-transformed values of biomarkers were used for analysis. We generated frequency distributions of race and ethnicity, HR status, lipid-lowering drug use, and covariates among cases and controls.
We used the quasi-Newton method to determine optimal parameters for multivariable conditional logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between log-transformed levels of circulating analytes and breast cancer risk [44]. We examined each circulating analyte in the overall study population and according to race and ethnicity, HR status, and lipid-lowering drug use. 27HC was also analyzed according to levels of testosterone (described below). The frequencies of matched pairs that have the same category of stratifying variables within each model are noted in figures.
Potential covariates were determined with a directed acyclic graph (DAG) involving 27HC, lipid levels, and steroid hormones to breast cancer diagnosis (Additional file 1: Fig. 2). Minimal models were adjusted for age at blood draw. We tested associations between each potential covariate with breast cancer diagnosis; potential covariates with type 3 P-values < 0.1 (Additional file 1: Table 1) were included in fully adjusted models. Full models were adjusted for baseline education and parity as well as age, BMI, and smoking status at blood draw. Spline models with tests for polynomial forms did not indicate substantial nonlinearity, so we present linear model forms with analytes of interest modeled as continuous. ORs represent the increase in odds of breast cancer diagnosis for every unit increase in log-transformed analyte concentration.
To assess joint effects between 27HC and other analytes, an interaction term for 27HC and each lipid, steroid hormone, and SHBG were assessed separately in the full model. Using the Wald test, only the model with the interaction term for 27HC and testosterone levels was significant at p < 0.05 (p for interaction = 0.013). Thus, the association between 27HC and breast cancer risk was assessed across four quartiles of testosterone based on levels among controls (quartile 1, 5 to < 72.5; quartile 2, 72.5 to < 105.6; quartile 3, 105.6 to < 152.5; quartile 4, ≥ 152.46 mg/dL). Only matched pairs with testosterone levels within the same quartile of testosterone were used in this analysis.
To assess whether 27HC associations with breast cancer risk were independent of steroid hormones, full models were additionally adjusted for estrone and testosterone. We also assessed whether associations between 27HC and breast cancer diagnosis differed by BMI status (low BMI, underweight/normal; high BMI, overweight/obese).
To summarize our results on the association between 27HC and breast cancer risk with those of the mostly White, European EPIC-Heidelberg Cohort [36], we used random effects meta-analysis to calculate pooled ORs from study-specific ORs (this study) or relative risks (EPIC-Heidelberg) available for (1) the total study population of this study with postmenopausal females of the EPIC-Heidelberg study, (2) the White female population of this study with postmenopausal females of the EPIC-Heidelberg study, and (3) HR-positive breast cancer. Forest plots were generated to visualize study-specific and pooled estimates.
All p-values were two-sided; p-values < 0.05 were considered statistically significant. All analyses except for meta-analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). Meta-analyses were conducted with Stata v17 (StataCorp, College Station, TX).
Results
Levels of 27HC, lipids, steroid hormones, and SHBG among controls and cases are presented in Table 1. Mean 27HC was 83.9 pg/mL (SD, 22.5 pg/mL) among controls and 82.6 pg/mL (SD, 21.58) among cases. Table 2 shows levels of 27HC across racial and ethnic groups; African American and Native Hawaiian females (both cases and controls) had higher 27HC levels than the mean among the overall study sample. Additional file 1: Table 2 provides Spearman correlation coefficients for biomarkers. 27HC was weakly correlated with total cholesterol (ρ = 0.14) and triglycerides (ρ = 0.28). Given that estrone and estradiol were correlated (ρ = 0.72) and over 45% of estradiol observations were below the LLOD, while less than 0.5% of estrone observations were below the LLOD, we restricted further analysis to the estrone biomarker. Frequency distributions of subgroups are presented in Table 3. One-third (35%) of the study population were Japanese American, 19% were non-Latino White, 19% were Latino, 15% were African American, and 12% were Native Hawaiian.
Table 1.
Circulating levels of biomarkers of interest according to case–control status among postmenopausal females, the Multiethnic Cohort
| Biomarkers of interest | Matched pairs | Controls | Breast cancer cases | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Min | Max | Mean | SD | Min | Max | |
| 27HC (ng/mL) | 1470 | 83.89 | 22.45 | 33.08 | 261.9 | 82.63 | 21.58 | 0.57 | 200.46 |
| Lipidsa | |||||||||
| HDL-C (mg/dL) | 1466 | 51.96 | 19.04 | 1.00 | 146 | 44.49 | 17.17 | 2.64 | 131.05 |
| LDL-C (mg/dL) | 1455 | 131.96 | 39.25 | 25.20 | 346.4 | 135.31 | 37.95 | 25.00 | 307.20 |
| Triglycerides (mg/dL) | 1466 | 120.46 | 73.27 | 20.00 | 1000 | 118.84 | 79.41 | 21.00 | 995.00 |
| Total cholesterol (mg/dL) | 1466 | 207.69 | 41.68 | 49.70 | 500 | 203.58 | 38.79 | 77.00 | 438.14 |
| Steroid hormonesb | |||||||||
| Estrone (pg/mL) | 1469 | 563.16 | 1472.85 | 2.50 | 27,713 | 633.25 | 1477.97 | 2.50 | 19,708.89 |
| Testosterone (pg/mL) | 1469 | 129.78 | 171.65 | 5.00 | 5300.3 | 150.19 | 240.96 | 5.00 | 8056.94 |
| SHBG (nM) | 1470 | 77.13 | 53.51 | 6.36 | 404 | 68.23 | 51.01 | 6.00 | 404.00 |
aLipid assays were conducted as part of a previous study, so not all cases had lipid assay measurements available (4 of 1470 matched pairs missing). An additional 11 matched pairs are missing for LDL-C, because LDL-C levels are derived from fasting triglyceride levels and samples with fasting triglyceride levels over 400 mg/dl were excluded from this derivation procedure
bOne sample missing from steroid hormone assays
27HC 27-hydroxy cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol (calculated), SHBG sex hormone binding globulin, SD standard deviation
Table 2.
Circulating levels of 27HC according to case–control status and racial and ethnic group among postmenopausal females, the Multiethnic Cohort
| 27HC (ng/mL) | Matched pairs | Controls | Breast cancer cases | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Min | Max | Mean | SD | Min | Max | |
| Overall | 1470 | 83.89 | 22.45 | 33.08 | 261.90 | 82.63 | 21.58 | 0.57 | 200.46 |
| Race and ethnicity | |||||||||
| African American | 220 | 85.64 | 23.91 | 33.08 | 171.71 | 86.37 | 22.17 | 36.70 | 156.67 |
| Japanese American | 517 | 83.53 | 22.01 | 36.73 | 203.88 | 81.74 | 20.67 | 0.57 | 166.97 |
| Latino | 272 | 82.68 | 20.88 | 33.29 | 175.09 | 80.04 | 21.61 | 31.76 | 200.46 |
| Native Hawaiian | 177 | 86.30 | 23.19 | 43.58 | 261.90 | 84.26 | 22.78 | 36.20 | 188.00 |
| Non-Latino White | 284 | 82.86 | 23.03 | 35.21 | 236.53 | 82.83 | 21.60 | 33.18 | 168.26 |
27HC 27-hydroxycholesterol, SD standard deviation
Table 3.
Frequency distribution of covariates among the study population according to case–control status among postmenopausal females, the Multiethnic Cohort
| Total (N = 2940) | Controls (N = 1470) | Breast cancer cases | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Race and ethnicity | |||
| African American | 440 (15.0%) | 220 (15.0%) | 220 (15.0%) |
| Japanese American | 1034 (35.2%) | 517 (35.2%) | 517 (35.2%) |
| Latino | 544 (18.5%) | 272 (18.5%) | 272 (18.5%) |
| Native Hawaiian | 354 (12.0%) | 177 (12.0%) | 177 (12.0%) |
| Non-Latino White | 568 (19.3%) | 284 (19.3%) | 284 (19.3%) |
| HR status | |||
| HR + (ER + or PR +) | 2410 (82.0%) | 1205 (82.0%) | 1205 (82.0%) |
| HR− (ER− and PR− ) | 434 (14.8%) | 217 (14.8%) | 217 (14.8%) |
| Lipid-lowering drug use | |||
| No | 2070 (70.4%) | 1060 (72.1%) | 1010 (68.7%) |
| Yes | 854 (29.0%) | 404 (27.5%) | 450 (30.6%) |
| Age at blood draw, mean years (SD) | 66.4 (8.0) | 66.4 (7.9) | 66.3 (8.0) |
| Education | |||
| < = High school | 980 (33.3%) | 462 (31.4%) | 518 (35.2%) |
| Some college | 939 (31.9%) | 470 (32.0%) | 469 (31.9%) |
| College graduate | 493 (16.8%) | 254 (17.3%) | 239 (16.3%) |
| Graduate school | 512 (17.4%) | 276 (18.8%) | 236 (16.1%) |
| Missing | 16 (0.5%) | 8 (0.5%) | 8 (0.5%) |
| Parity | |||
| None | 351 (11.9%) | 152 (10.3%) | 199 (13.5%) |
| 1 child | 310 (10.5%) | 161 (11.0%) | 149 (10.1%) |
| 2–3 children | 1449 (49.3%) | 746 (50.7%) | 703 (47.8%) |
| 4 + children | 813 (27.7%) | 402 (27.3%) | 411 (28.0%) |
| Missing | 17 (0.6%) | 9 (0.6%) | 8 (0.5%) |
| BMI at blood draw, kg/m2 | |||
| Underweight/normal | 1140 (38.8%) | 641 (43.6%) | 499 (33.9%) |
| Overweight | 990 (33.7%) | 479 (32.6%) | 511 (34.8%) |
| Obese | 796 (27.1%) | 347 (23.6%) | 449 (30.5%) |
| Missing | 14 (0.5%) | 3 (0.2%) | 11 (0.7%) |
| Smoking status at blood draw | |||
| Never smoking | 1728 (58.8%) | 918 (62.4%) | 810 (55.1%) |
| Former smoker | 1044 (35.5%) | 492 (33.5%) | 552 (37.6%) |
| Current smoker | 165 (5.6%) | 57 (3.9%) | 108 (7.3%) |
| Missing | 3 (0.1%) | 3 (0.2%) | 0 (0.0%) |
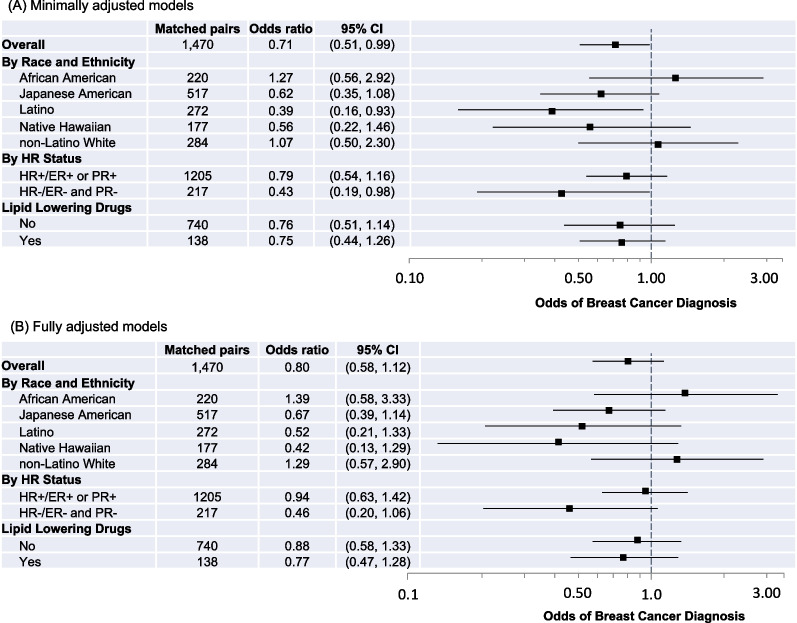
Results from minimally adjusted models are presented in Fig. 1A; 27HC was significantly inversely associated with risk among the overall population (OR 0.71; 95% CI 0.51–0.99). In analyses stratified by racial and ethnic group, 27HC was inversely associated with risk among Latino females (OR 0.39; 95% CI 0.16–0.93) and Japanese American females, although the estimate among Japanese American females was not statistically significant (OR 0.62; 95% CI 0.35–1.08). 27HC was inversely associated with risk among those with HR-negative breast cancer (OR 0.43; 95% CI 0.19–0.98) but not HR-positive breast cancer. Of potential covariates (see DAG in Additional file 1: Fig. 2), education, BMI, parity, and smoking status were associated with breast cancer risk at p < 0.1 (Additional file 1: Table 1), so these covariates were included in fully adjusted models.
Fig. 1.
Odds ratios and 95% confidence intervals (CIs) for associations between 27HC (continuous) and breast cancer diagnosis overall and according to joint race and ethnicity, HR status, and lipid-lowering drug use. A Minimally adjusted models are adjusted for age at blood draw. B Fully adjusted models are adjusted for age at blood draw, education, parity, BMI at blood draw, and smoking status at blood draw. Odds ratios are indicated with black squares; 95% CIs are indicated with solid lines; the x-axis is on the log scale. For each model, controls are the reference group; the reference odds ratio (1.0) is indicated by the vertical dashed line
Results from fully adjusted models of the association between 27HC and breast cancer risk are presented in Fig. 1B. There were no statistically significant associations among the overall population or in stratified analyses. However, 27HC may be inversely associated with risk for certain groups; effect sizes among Japanese American, Latino, and Native Hawaiian females suggest weak inverse associations (Japanese American, OR 0.67, 95% CI 0.39–1.14; Latino, OR 0.52, 95% CI 0.21–1.33; Native Hawaiian, OR 0.42, 95% CI 0.13–1.29). A test for heterogeneity across racial and ethnic groups was null (p = 0.26). Results also suggest a weak inverse association of 27HC for HR-negative breast cancer (HR- OR 0.46, 95% CI 0.20–1.06). The p-value for a test of heterogeneity across HR status was p = 0.14.
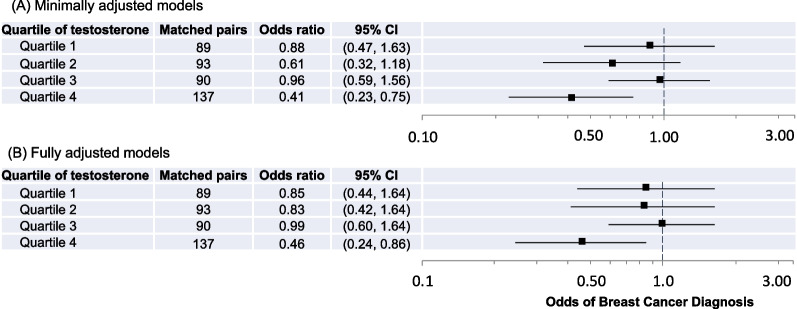
We observed a significant interaction between 27HC and testosterone levels (p = 0.013). Thus, we examined associations between 27HC and breast cancer risk in the overall study population across quartiles of testosterone (Fig. 2). 27HC was associated with breast cancer risk only among females with the highest levels of testosterone (OR 0.46, 95% CI 0.24–0.86). A test for trend was null (p-trend = 0.22). We did not observe interactions between 27HC and estrone, SHBG, or lipid biomarkers.
Fig. 2.
Odds ratios and 95% confidence intervals (CIs) for associations between 27HC and breast cancer diagnosis according to quartile of testosterone level. A Minimally adjusted models are adjusted for age at blood draw. B Fully adjusted models are adjusted for age at blood draw, education, parity, BMI at blood draw, and smoking status at blood draw. Odds ratios are indicated with black squares; 95% CIs are indicated with solid lines; the x-axis is on the log scale. For each model, controls are the reference group; the reference odds ratio (1.0) is indicated by the vertical dashed line
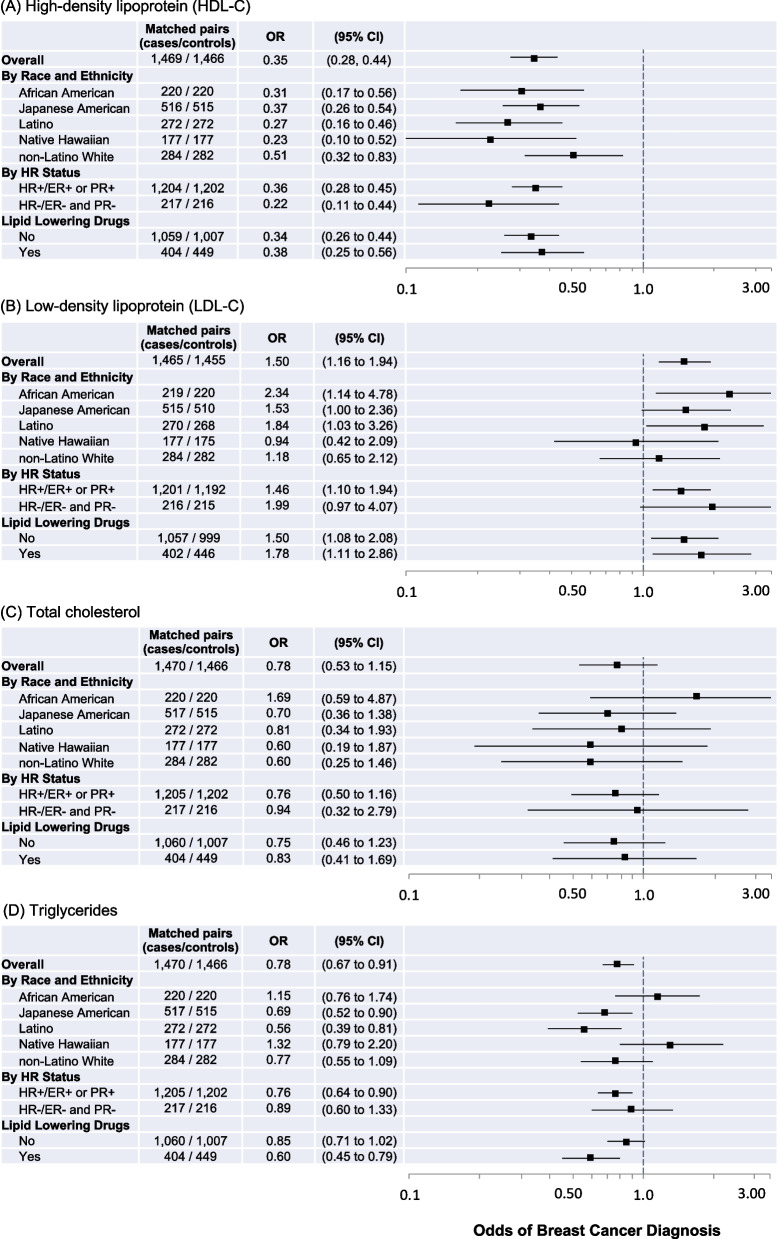
Figure 3 shows results from fully adjusted models of associations between lipids and breast cancer risk. HDL-C was strongly inversely associated with breast cancer risk among the overall study population (OR 0.35, 95% CI 0.28–0.44) and across all groups in stratified analyses. LDL-C was associated with higher breast cancer risk among the overall study population (OR 1.50, 95% CI 1.16–1.94), but effect sizes differed across racial and ethnic groups. For total cholesterol, an inverse association among the overall study population was suggested but not statistically significant (OR 0.78, 95% CI 0.53–1.15); African American females were the only racial and ethnic group for which this direction of association for total cholesterol was positive (OR 1.69, 95% CI 0.59–4.87). Triglyceride levels were inversely associated with risk among the overall study population (OR 0.78, 95% CI 0.67–0.91), for Japanese American and Latino females, and for HR-positive breast cancer. Among lipids, only associations between triglyceride and breast cancer risk had significant statistical heterogeneity across racial and ethnic groups (p = 0.02) and for lipid-lowering drug use (p = 0.02).
Fig. 3.
Fully adjusted models estimating odds ratios and 95% confidence intervals (CIs) of breast cancer diagnosis associated with A high-density lipoprotein (HDL-C), B low-density lipoprotein (LDL-C), C total cholesterol, and D triglyceride levels overall and according to joint race/ethnicity, HR status, and lipid-lowering drug use. All models are adjusted for age at blood draw, education, parity, BMI at blood draw, and smoking status at blood draw. Odds ratios are indicated with black squares, 95% CIs indicated with solid lines; the x-axis is on the log scale. For each model, controls are the reference group; the reference odds ratio (1.0) is indicated by the vertical dashed line
Additional file 1: Table 3 shows results from fully adjusted models of associations of steroid hormones and SHBG with breast cancer diagnosis. Estrone level was positively associated with breast cancer risk among the overall study population (OR 1.13, 95% CI 1.07–1.21), but associations varied across racial and ethnic group. Testosterone level was positively associated with risk among the overall study population (OR 1.39, 95% CI 1.22–1.58) as well as for the African American (OR 1.57, 95% CI 1.13–2.17), the Japanese American (OR 1.66, 95% CI 1.31–2.09), and the non-Latino White groups (OR 1.28, 95% CI 0.96–1.71) and for HR-positive breast cancers (OR 1.44, 95% CI 1.25–1.67). SHBG levels were inversely associated overall (OR 0.75, 95% CI 0.66–0.86) and generally across all groups in stratified analyses, although not all estimates are statistically significant.
Estimates of associations between 27HC and breast cancer risk did not change with further adjustment for levels of estrone and testosterone (Additional file 1: Table 4). While not statistically significant, the inverse association between 27HC and breast cancer risk may be specific to those with a BMI status of underweight/normal (OR 0.65, 95% CI 0.40, 1.06) (Additional file 1: Table 5).
The overall odds ratio summarizing results from the Multiethnic Cohort and the postmenopausal population of the EPIC-Heidelberg cohort indicates a significant inverse association between 27HC and breast cancer risk (summary OR 0.72, 95% CI 0.54–0.94), with a similar but not statistically significant association among non-Latino White females within the MEC and postmenopausal females within EPIC-Heidelberg (summary OR 0.70, 95% CI 0.46–1.06) and among HR-positive cases within the Multiethnic Cohort and ER-positive postmenopausal cases within EPIC-Heidelberg (summary OR 0.83, 95% CI 0.60–1.16) (Additional file 1: Fig. 3).
Discussion
Our study of the Multiethnic Cohort is the first in the US to examine the association between circulating 27HC and breast cancer risk and suggests an inverse association between 27HC and postmenopausal breast cancer risk that varies across racial and ethnic groups. This inverse association also seems to be restricted to HR-negative breast cancer in fully adjusted models. In addition, the inverse association of 27HC with breast cancer risk is apparent only among those with the highest levels of testosterone. Associations of 27HC with breast cancer risk were otherwise independent of steroid hormone and lipid levels.
A 2019 study of circulating 27HC and breast cancer risk among the EPIC-Heidelberg Cohort reported an inverse association between 27HC levels and breast cancer risk among postmenopausal females within the predominantly White, European cohort (193 cases, 200 controls) [36]. Results from our study of 1470 cases and 1470 controls in the Multiethnic Cohort also suggest an inverse association for 27HC but indicate that results from the EPIC-Heidelberg Cohort may not be generalizable to a multiracial and multiethnic US population. In fact, we found a positive, but not-statistically significant, association between 27HC levels and breast cancer risk among non-Latino White females (284 matched pairs), the group presumably most like the EPIC-Heidelberg Cohort in terms of self-identified race and ethnicity. A non-significant summary OR was observed in a meta-analysis comparing results from this population with results among postmenopausal females of the EPIC-Heidelberg study.
Notably, our results did not confirm those from animal studies that suggest 27HC would confer increased risk for breast cancer [18–21]. It is likely that multilevel influences on lipid and steroid hormone levels and complex functional interactions among lipids and steroid hormones require larger prospective studies with deep exposure assessment. This includes assessing whether the inverse association of 27HC with breast cancer is dependent on exposures or factors not measured in the current study. Moreover, even if 27HC levels do not confer observable risk to breast cancer formation, it is possible that, after cancer formation, 27HC levels could, as animal studies suggest, promote tumor growth and metastasis and thus worse prognosis. Future studies should examine this possibility.
While most of our estimates of the association between 27HC and breast cancer risk across racial and ethnic groups were not statistically significant, they do suggest differences in the association between 27HC and risk across racial and ethnic groups. Specifically, higher levels of 27HC may be inversely associated with breast cancer risk among Japanese American, Latino, and Native Hawaiian females and positively associated among White and African American postmenopausal females. However, while this suggests that our hypothesis that associations would vary across racial and ethnic groups may be valid, our hypothesis that high 27HC levels would shed light on racial and ethnic disparities in breast cancer risk is not supported by our results. For example, 27HC is inversely associated with risk among Native Hawaiian females who have the highest breast cancer incidence rate as well as Latino females who have the lowest breast cancer incidence rate. Given the complexity of endocrine signaling, it may be that the influence of 27HC on breast cancer development may be modified by other endocrine molecules; our observation of an interaction between 27HC and testosterone in breast cancer risk supports this possibility.
Testosterone levels have been linked to higher breast cancer risk [25, 26, 28–30, 45]; testosterone may promote cancer cell proliferation directly or by increasing estrogen levels. We also observed associations between higher testosterone levels and breast cancer. In addition, we observed an inverse association between 27HC and breast cancer risk only among individuals with the highest levels of testosterone, suggesting potential interaction between testosterone and 27HC. There is evidence that 27HC can mediate androgen receptor (AR) transcriptional activity and may facilitate ER and AR crosstalk activity [31]. However, the mechanism underlying testosterone modification of 27HC as a protective factor for breast cancer remains to be elucidated. Testosterone levels were also positively associated with HR-positive breast cancer [46], but we had insufficient sample sizes to stratify 27HC analyses simultaneously by race and ethnicity, HR subtype, and testosterone levels to further interrogate these interactions.
A 2015 meta-analysis of prospective cohort studies reported an inverse association between HDL-C and risk among postmenopausal females (RR = 0.45; 0.64, 0.93); these studies included only White females [47]. Our study of the racially and ethnically diverse Multiethnic Cohort confirms a consistent inverse association between prospective HDL-C and breast cancer. The same 2015 meta-analysis reported null results for total cholesterol with substantial heterogeneity across available estimates; our estimate of the association between total cholesterol and risk was non-significant but suggested an inverse association that was consistent across racial and ethnic groups except for African American females. Thus, our results are consistent with prior cohort studies to the extent that our study population can be compared with those of prior studies.
Notably, ours is the first study of 27HC among a multiracial, multiethnic population and thereby sheds light on differences in the mechanism of the impact of cholesterol on breast cancer risk across groups. There has only been one prospective cohort study among African American females that examined total cholesterol and found no association, but that study included females across a wide age range and used self-reported total cholesterol (high, not high) [48]. On the other hand, a 2013 case–control study of African American females in Washington DC reported a positive association of total cholesterol and LDL-C with breast cancer risk [49]. In our study, African American females were the only racial and ethnic group for which a positive association for total cholesterol was suggested and one of the groups for which a positive association for LDL-C was observed. Prospective cohort studies of lipids and breast cancer risk among postmenopausal Japanese American, Native Hawaiian, or Latino females are not, to our knowledge, available. Overall, our observations illustrate that future studies of the impact of cholesterol and cholesterol metabolites on breast cancer risk should consider modification of these processes by race and ethnicity and that the inverse association observed in White study populations may not be generalizable [47].
Our study of breast cancer risk within the Multiethnic Cohort with prospective assessment of 27HC, lipids, and steroid hormones and information on other covariates (e.g., health behaviors, breast cancer risk factors) offers substantial data to evaluate hypotheses regarding 27HC as a risk factor for breast cancer. However, we note some limitations. Despite being the largest and most diverse cohort population in the US to date to examine 27HC and breast cancer risk, assessing the mechanisms behind associations between 27HC or lipid levels and breast cancer risk may require larger populations, particularly in consideration of groups for which these associations differ (i.e., racial and ethnic groups, lipid-lowering drug use, levels of steroid hormones) and in consideration of the potential for differences in risk across breast cancer subtypes.
Conclusion
Ours is the first US study to examine associations between 27HC and breast cancer risk and suggests a weak inverse association that varied across groups within a multiethnic population. The inverse association of 27HC with breast cancer risk was strongest in females with the highest levels of testosterone. Results from this study extend our understanding of the role of lipids and their metabolites in breast cancer risk as well as racial and ethnic differences in risk of postmenopausal breast cancer.
Supplementary Information
Additional file 1. Supplemental methods, tables, and figures.
Acknowledgements
We acknowledge Xingnan Li for plasma analyses and Jennifer Lai for her assistance preparing the manuscript.
Abbreviations
- 27HC
27-Hydroxycholesterol
- AR
Androgen receptor
- BMI
Body mass index
- CI
Confidence interval
- CV
Coefficient of variation
- DAG
Directed acyclic graph
- ER
Estrogen receptor
- HDL-C
High-density lipoprotein cholesterol
- HR
Hormone receptor
- LDL-C
Low-density lipoprotein cholesterol
- LXR
Liver X receptor
- OR
Odds ratio
- PR
Progesterone receptor
- SD
Standard deviation
- SERM
Selective estrogen receptor modulator
- SHBG
Sex hormone binding globulin
- US
United States
Author contributions
The work reported in the paper has been performed by the authors, unless clearly specified in the text. MCD contributed to methodology, investigation, visualization, writing of the original draft, and reviewing and editing the manuscript. JY contributed to methodology and formal analysis. YL contributed to formal analysis and reviewing and editing the manuscript. AF contributed to methodology, data curation, investigation, reviewing and editing the manuscript, and supervision. AT contributed to data curation, formal analysis, and reviewing and editing of the manuscript. KW and BYH contributed to data curation and reviewing and editing of the manuscript. YS contributed to methodology and reviewing and editing of the manuscript. VS and AW contributed to investigation and reviewing and editing of the manuscript. LW contributed to methodology, resources, investigation, and reviewing and editing of the manuscript. LLM contributed to resources, investigation, and reviewing and editing of the manuscript. LWML and IC contributed to conceptualization, methodology, investigation, writing of the original draft, reviewing and edit of the manuscript, project administration, supervision, and funding acquisition.
Funding
This work was supported by the National Cancer Institute (R01 CA229815-01 to IC and LWML; U01 CA164973-10 and P30 CA71789 to the University of Hawaiʻi Cancer Center). The collection of cancer incidence data used in this study was supported by the NCI Surveillance, Epidemiology, and End Results Contract Award Nos. HHSN261201300009I to the Hawaiʻi Tumor Registry and HHSN2612018000321 to University of California, San Francisco, HHSN2612018000151 to the University of Southern California, and HHSN2612018000091 to the Public Health Institute (California Cancer Registry). Funders were not involved in the designing of the study; in the collection, analysis, and interpretation of data; in writing this report; or in the decision to submit this paper for publication.
Data availability
The Multiethnic Cohort investigators and institutions affirm their intention to share the research data consistent with all relevant NIH resource/data sharing policies. Data requests should be submitted through Multiethnic Cohort online data request system at https://www.uhcancercenter.org/for-researchers/mec-data-sharing. Further information is available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
This study was approved by the IRBs of University of California San Francisco, the University of Hawaii, and the University of Southern California. Return of the baseline survey was consent to enroll in the Multiethnic Cohort Study. Written informed consent was obtained from participants who provided biospecimens.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lenora W. M. Loo and Iona Cheng contributed equally to senior authorship.
Contributor Information
Mindy C. DeRouen, Email: mindy.hebert-derouen@ucsf.edu
Iona Cheng, Email: iona.cheng@ucsf.edu.
References
- 1.American Cancer Society Cancer Facts & Figures. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html.
- 2.Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women's Health Initiative (United States) Cancer Causes Control. 2002;13(8):741–751. doi: 10.1023/a:1020239211145. [DOI] [PubMed] [Google Scholar]
- 3.Ahn J, Schatzkin A, Lacey JV, Jr, et al. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med. 2007;167(19):2091–2102. doi: 10.1001/archinte.167.19.2091. [DOI] [PubMed] [Google Scholar]
- 4.Ballard-Barbash R, Schatzkin A, Carter CL, et al. Body fat distribution and breast cancer in the Framingham Study. J Natl Cancer Inst. 1990;82(4):286–290. doi: 10.1093/jnci/82.4.286. [DOI] [PubMed] [Google Scholar]
- 5.Folsom AR, Kaye SA, Prineas RJ, et al. Increased incidence of carcinoma of the breast associated with abdominal adiposity in postmenopausal women. Am J Epidemiol. 1990;131(5):794–803. doi: 10.1093/oxfordjournals.aje.a115570. [DOI] [PubMed] [Google Scholar]
- 6.Friedenreich CM. Review of anthropometric factors and breast cancer risk. Eur J Cancer Prev. 2001;10(1):15–32. doi: 10.1097/00008469-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and inflammation: new insights into breast cancer development and progression. Am Soc Clin Oncol Educ Book. 2013;33:46–51. doi: 10.1200/EdBook_AM.2013.33.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard BV, Ruotolo G, Robbins DC. Obesity and dyslipidemia. Endocrinol Metab Clin North Am. 2003;32(4):855–867. doi: 10.1016/s0889-8529(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 9.Agnoli C, Berrino F, Abagnato CA, et al. Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: a nested case–control study. Nutr Metab Cardiovasc Dis. 2010;20(1):41–48. doi: 10.1016/j.numecd.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furberg A-S, Veierød MB, Wilsgaard T, et al. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst. 2004;96(15):1152–1160. doi: 10.1093/jnci/djh216. [DOI] [PubMed] [Google Scholar]
- 11.Kabat GC, Kim M, Chlebowski RT, et al. A longitudinal study of the metabolic syndrome and risk of postmenopausal breast cancer. Cancer Epidemiol Biomark Prev. 2009;18(7):2046–2053. doi: 10.1158/1055-9965.EPI-09-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha M, Sung J, Song Y-M. Serum total cholesterol and the risk of breast cancer in postmenopausal Korean women. Cancer Causes Control. 2009;20:1055–1060. doi: 10.1007/s10552-009-9301-7. [DOI] [PubMed] [Google Scholar]
- 13.Gaard M, Tretli S, Urdal P. Risk of breast cancer in relation to blood lipids: a prospective study of 31,209 Norwegian women. Cancer Causes Control. 1994;5:501–509. doi: 10.1007/BF01831377. [DOI] [PubMed] [Google Scholar]
- 14.Kucharska-Newton AM, Rosamond WD, Mink PJ, et al. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann Epidemiol. 2008;18(9):671–677. doi: 10.1016/j.annepidem.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosato V, Bosetti C, Talamini R, et al. Metabolic syndrome and the risk of breast cancer in postmenopausal women. Ann Oncol. 2011;22(12):2687–2692. doi: 10.1093/annonc/mdr025. [DOI] [PubMed] [Google Scholar]
- 16.Nelson ER, Chang CY, McDonnell DP. Cholesterol and breast cancer pathophysiology. Trends Endocrinol Metab. 2014;25(12):649–655. doi: 10.1016/j.tem.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DuSell CD, Umetani M, Shaul PW, et al. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22(1):65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson ER, Wardell SE, Jasper JS, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342(6162):1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Q, Ishikawa T, Sirianni R, et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013;5(3):637–645. doi: 10.1016/j.celrep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baek AE, Nelson ER. The contribution of cholesterol and its metabolites to the pathophysiology of breast cancer. Horm Cancer. 2016;7(4):219–228. doi: 10.1007/s12672-016-0262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asghari A, Umetani M. Obesity and cancer: 27-hydroxycholesterol, the missing link. Int J Mol Sci. 2020;21(14):4822. doi: 10.3390/ijms21144822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonnell DP, Chang CY, Nelson ER. The estrogen receptor as a mediator of the pathological actions of cholesterol in breast cancer. Climacteric. 2014;17(02):60–5. doi: 10.3109/13697137.2014.966949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma L, Cho W, Nelson ER. Our evolving understanding of how 27-hydroxycholesterol influences cancer. Biochem Pharmacol. 2022;196:114621. doi: 10.1016/j.bcp.2021.114621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.González-Ortiz A, Galindo-Hernández O, Hernández-Acevedo GN, et al. Impact of cholesterol-pathways on breast cancer development, a metabolic landscape. J Cancer. 2021;12(14):4307–4321. doi: 10.7150/jca.54637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasui T, Matsui S, Tani A, et al. Androgen in postmenopausal women. J Med Invest. 2012;59(1–2):12–27. doi: 10.2152/jmi.59.12. [DOI] [PubMed] [Google Scholar]
- 26.Hankinson SE, Eliassen AH. Endogenous estrogen, testosterone and progesterone levels in relation to breast cancer risk. J Steroid Biochem Mol Biol. 2007;106(1–5):24–30. doi: 10.1016/j.jsbmb.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sieri S, Krogh V, Bolelli G, et al. Sex hormone levels, breast cancer risk, and cancer receptor status in postmenopausal women: the ORDET cohort. Cancer Epidemiol Biomark Prev. 2009;18(1):169–176. doi: 10.1158/1055-9965.EPI-08-0808. [DOI] [PubMed] [Google Scholar]
- 28.Key T, Appleby P, Barnes I, et al. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 29.Missmer SA, Eliassen AH, Barbieri RL, et al. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96(24):1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 30.Woolcott CG, Shvetsov YB, Stanczyk FZ, et al. Plasma sex hormone concentrations and breast cancer risk in an ethnically diverse population of postmenopausal women: the Multiethnic Cohort Study. Endocr Relat Cancer. 2010;17(1):125–134. doi: 10.1677/ERC-09-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raza S, Meyer M, Schommer J, et al. 27-Hydroxycholesterol stimulates cell proliferation and resistance to docetaxel-induced apoptosis in prostate epithelial cells. Med Oncol. 2016;33(2):12. doi: 10.1007/s12032-015-0725-5. [DOI] [PubMed] [Google Scholar]
- 32.Pike MC, Kolonel LN, Henderson BE, et al. Breast cancer in a multiethnic cohort in Hawaii and Los Angeles: risk factor-adjusted incidence in Japanese equals and in Hawaiians exceeds that in whites. Cancer Epidemiol Biomark Prev. 2002;11(9):795–800. [PubMed] [Google Scholar]
- 33.Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review, 1975-2012. Bethesda: National Cancer Institute; 2015. [Google Scholar]
- 34.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 35.Moy KL, Sallis JF, David KJ. Health indicators of native Hawaiian and pacific islanders in the United States. J Commun Health. 2010;35:81–92. doi: 10.1007/s10900-009-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu DL, Le Cornet C, Sookthai D, et al. Circulating 27-Hydroxycholesterol and breast cancer risk: results from the EPIC-Heidelberg cohort. J Natl Cancer Inst. 2019;111(4):365–371. doi: 10.1093/jnci/djy115. [DOI] [PubMed] [Google Scholar]
- 37.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(4):346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honda A, Yamashita K, Hara T, et al. Highly sensitive quantification of key regulatory oxysterols in biological samples by LC-ESI-MS/MS. J Lipid Res. 2009;50(2):350–357. doi: 10.1194/jlr.D800040-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Franke AA. Improved profiling of estrogen metabolites by orbitrap LC/MS. Steroids. 2015;99(Pt A):84–90. doi: 10.1016/j.steroids.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morimoto Y, Conroy SM, Ollberding NJ, et al. Erythrocyte membrane fatty acid composition, serum lipids, and non-Hodgkin's lymphoma risk in a nested case-control study: the multiethnic cohort. Cancer Causes Control. 2012;23(10):1693–1703. doi: 10.1007/s10552-012-0048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 42.Rinaldi S, Geay A, Déchaud H, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomark Prev. 2002;11(10):1065–1071. [PubMed] [Google Scholar]
- 43.Shvetsov YB, Harmon BE, Ettienne R, et al. The influence of energy standardisation on the alternate Mediterranean diet score and its association with mortality in the Multiethnic Cohort. Br J Nutr. 2016;116(9):1592–1601. doi: 10.1017/S0007114516003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dennis JJE, Moré JJ. Quasi-newton methods, motivation and theory. SIAM Rev. 1977;19(1):46–89. [Google Scholar]
- 45.Sieri S, Krogh V, Bolelli G, et al. Sex hormone levels, breast cancer risk, and cancer receptor status in postmenopausal women: the ORDET cohort. Cancer Epidemiol Biomark Prev. 2009;18(1):169–176. doi: 10.1158/1055-9965.EPI-08-0808. [DOI] [PubMed] [Google Scholar]
- 46.Cummings SR, Lee JS, Lui LY, et al. Sex hormones, risk factors, and risk of estrogen receptor-positive breast cancer in older women: a long-term prospective study. Cancer Epidemiol Biomark Prev. 2005;14(5):1047–1051. doi: 10.1158/1055-9965.EPI-04-0375. [DOI] [PubMed] [Google Scholar]
- 47.Ni H, Liu H, Gao R. Serum lipids and breast cancer risk: a meta-analysis of prospective cohort studies. PLoS One. 2015;10(11):e0142669. doi: 10.1371/journal.pone.0142669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bosco JLF, Palmer JR, Boggs DA, et al. Cardiometabolic factors and breast cancer risk in U.S. black women. Breast Cancer Res Treat. 2012;134(3):1247–1256. doi: 10.1007/s10549-012-2131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Llanos AA, Makambi KH, Tucker CA, et al. Cholesterol, lipoproteins, and breast cancer risk in African-American women. Ethn Dis. 2012;22(3):281. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplemental methods, tables, and figures.
Data Availability Statement
The Multiethnic Cohort investigators and institutions affirm their intention to share the research data consistent with all relevant NIH resource/data sharing policies. Data requests should be submitted through Multiethnic Cohort online data request system at https://www.uhcancercenter.org/for-researchers/mec-data-sharing. Further information is available from the corresponding author upon request.