Abstract
Background
Per-/polyfluoroalkyl substances (PFASs) are persistent organic pollutants and suspected endocrine disruptors.
Objective
The aim of this work was to conduct a systematic review with meta-analysis to summarise the associations between prenatal or childhood exposure to PFASs and childhood overweight/obesity.
Methods
The search was performed on the bibliographic databases PubMed and Embase with text strings containing terms related to prenatal, breastfeeding, childhood, overweight, obesity, and PFASs. Only papers describing a biomonitoring study in pregnant women or in children up to 18 years that assessed body mass index (BMI), waist circumference (WC), or fat mass in children were included. When the estimates of the association between a PFAS and an outcome were reported from at least 3 studies, a meta-analysis was conducted; moreover, to correctly compare the studies, we developed a method to convert the different effect estimates and made them comparable each other. Meta-analyses were performed also stratifying by sex and age, and sensitivity analyses were also performed.
Results
In total, 484 and 779 articles were retrieved from PubMed and Embase, respectively, resulting in a total of 826 articles after merging duplicates. The papers included in this systematic review were 49: 26 evaluating prenatal exposure to PFASs, 17 childhood exposure, and 6 both. Considering a qualitative evaluation, results were conflicting, with positive, negative, and null associations. 30 papers were included in meta-analyses (19 prenatal, 7 children, and 4 both). Positive associations were evidenced between prenatal PFNA and BMI, between PFOA and BMI in children who were more than 3 years, and between prenatal PFNA and WC. Negative associations were found between prenatal PFOS and BMI in children who were 3 or less years, and between PFHxS and risk of overweight. Relatively more consistent negative associations were evidenced between childhood exposure to three PFASs (PFOA, PFOS, and PFNA) and BMI, in particular PFOS in boys. However, heterogeneity among studies was high.
Conclusion
Even though heterogeneous across studies, the pooled evidence suggests possible associations, mostly positive, between prenatal exposure to some PFASs and childhood BMI/WC; and relatively stronger evidence for negative associations between childhood exposure to PFASs and childhood BMI.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12940-023-01006-6.
Keywords: Perfluoroalkyl substances, Fetal exposure, Early life exposure, Paediatric exposure, Childhood adiposity, Effect estimate conversion
Background
Childhood overweight and obesity are recognised worldwide issues. According to the World Health Organisation, 39 million children under 5 years were affected by overweight or obesity in 2020 and over 340 million aged 5–19 years were affected by overweight or obesity in 2016 [1]. The high prevalence of overweight and obesity is caused by a complex interaction between predisposing genetic factors and environmental factors [2, 3]. Unhealthy diet and physical inactivity are well-known causes leading to these problematic conditions [4]. However, other environmental factors that may contribute to the development of these conditions include exposure to endocrine-disrupting chemicals (EDCs) [5, 6]. Furthermore, the exposure to environmental pollutants may be critical, especially if it occurs in susceptible period of life, such as the prenatal period, or infancy [7], including lactation [8].
Per- and polyfluoroalkyl substances (PFASs) are a group of artificial compounds. Thanks to their surfactant, greaseproof, stain-proof, water repellent, and fire repellent properties, PFASs are used widely, including in food processing, medical articles, apparel, household products, electronics, and firefighting [9, 10]. PFAS pollution has been reported at global level since the beginning of this millennium, especially perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) [11–13]. Despite the use of some PFASs have been restricted [14], these persistent compounds are still widespread in the environment and in living organisms [15–17]. PFASs are often classified as EDCs, i.e., they can potentially interfere with the endocrine system [18–20]. Some in-vitro studies showed that PFASs can disrupt some hormone functions [21, 22], and adipocyte differentiation [23]; moreover, effects on body weight and adipogenesis were observed in animals [24, 25], highlighting the importance of investigating these compounds in human epidemiological studies.
Several observational studies in humans were conducted to assess the effects of PFASs, and many studied their effect on prenatal growth: indeed, previous systematic reviews evidenced that a prenatal exposure to PFOA and PFOS is associated with a lower birth weight [26–28]. Several epidemiological studies also investigated the adverse effects of the exposure to PFASs on childhood overweight and obesity, and the evidence deriving from these studies were partially summarised in some narrative reviews [6, 29, 30]. Vrijheid et al., in their narrative review, classified the evidence of the effects of PFASs on childhood growth and obesity as “insufficient”, due to the low number of studies and the inconsistent findings [31]. Conversely, according to the narrative review conducted by Braun, a prenatal exposure to PFASs may be related to increased adiposity and risk of childhood overweight or obesity [18]. Szilagyi et al., in their narrative review, stated that in utero exposure to PFAS is associated with increased incidence of childhood obesity [32]. Rappazzo and co-workers carried out a systematic review of the epidemiologic literature evaluating the exposure to PFASs and different health outcomes, among which outcomes of overweight and obesity were described within the cardio-metabolic category [33]. Lee and co-workers wrote a systematic review summarising the evidence of early-life exposure to several PFASs and different outcomes in children, including adiposity, and concluded that, although the evidence was inconsistent, prenatal PFASs mostly have negative associations with BMI in the first 2 years of life and positive associations with adiposity in childhood and adolescence, the latter observation is true for PFOA in particular [34]. Ribeiro and co-workers conducted a systematic review with meta-analysis considering the exposure to different EDCs after the age of 2 years and their association with anthropometric measures of obesity or body fat, but only one study assessing PFASs was included [35]. Liu and co-workers published a systematic review with meta-analysed focused exclusively to early life exposure to PFOA, in which they pooled the evidence from 10 prospective cohort studies and found a positive association with an increased risk for childhood adiposity [36]. Finally, a recent comprehensive systematic review with meta-analysis conducted by Stratakis and co-workers summarised the evidence of prenatal exposure to persistent organic pollutants and childhood obesity considering different outcomes such as childhood BMI-z, waist circumference, and overweight risk; for PFOS and PFOA, they found no overall significant associations for most of the considered outcomes [37]. However, in those previous meta-analyses, effect estimates were extracted from the studies and compared irrespectively from their differences: indeed, comparisons between continuous data and categorical data were found, as well as comparisons between data obtained from different log-transformations. Considering the importance of assessing both prenatal and childhood exposure to PFASs and to include a higher number of PFASs, the aim of this work was to conduct a systematic review of the literature to critically summarise the existing evidence of the effect of a prenatal and childhood exposure to as many PFASs as possible on childhood overweight and obesity, with also an effort to develop a methodology to convert data from different studies to make effect estimates comparable each other before conducting the meta-analysis.
The PECO statement [38] for the present study is the following: (P) in children, what is the effect of (E) higher PFASs exposure during pregnancy or during childhood versus (C) lowest PFASs exposure during pregnancy or during childhood on (O) childhood overweight and obesity.
Materials and methods
Search strategy and inclusion criteria
This review and its protocol were registered on PROSPERO, the International prospective register of systematic reviews [39, 40].
The papers were searched in the PubMed and Embase bibliographic databases. Search terms included: prenatal, children, adolescents, breastfeeding, and synonyms; overweight, obesity, BMI, waist circumference, fat mass, and similar; perfluorates and related terms. The PFASs considered for the research terms were the most spread and the most interesting PFASs on our knowledge, including both legacy and emerging compounds, and have been described on our previous work [41]. The complete text strings are reported in supplementary material (Supplementary text), and they were elaborated thanks to the help of the library system staff of the University of Milan. Only articles published from 1st January 2000 to 31st December 2022 and in English language were considered. Articles retrieved were collected in electronic databases (Table S01 and Table S02). Duplicates of papers were identified using the DOI number and merged in a single line using the R software [42] with the “tidyverse” package [43], and then exported to an Excel database (Table S03). The script developed to carry out this merge is reported in the supplementary material (Supplementary text, R script, Sect. 1).
Two reviewers (G.F. and C.M.F.) independently read the titles and abstracts to select suitable papers for inclusion, while blinded to each other’s decisions. At the end of the process, discrepancies were discussed and evaluated with the final decision made by a third person acting as a supervisor (S.F.). To be eligible for inclusion, a human biomonitoring study quantifying one or more PFASs in biological samples (such as serum, plasma, urine, or breastmilk) of pregnant women or in children/adolescent up to 18 years old had to be conducted. Furthermore, the measured outcomes had to include a measure of overweight/obesity in children (up to 18 years old) such as BMI, waist circumference (WC), or fat mass/body fat percentage (BFP), which can be obtained with different approaches such as Dual-Energy X-ray Absorptiometry (DXA) (considered the gold standard) or skinfold thickness [44, 45]. The measurement of weight alone was not considered an outcome suitable for inclusion; likewise, birthweight and any other parameters measured right after delivery were not considered suitable for inclusion, as they are indicator of prenatal growth, which is not the purpose of this review. Non-original works (such as reviews), non-full articles (such as conference abstracts or letters to editor), and studies considering only non-pregnant adults were excluded.
Afterwards, papers judged suitable after reading their title and abstract were further inspected in their entirety. Finally, included papers were grouped in three categories: studies considering only prenatal exposure to PFASs, studies considering only exposure in children/adolescents, and studies considering both prenatal and children exposure.
Data elaboration
Information from included papers were collected in the Excel database (Table S03). As for the paper selection, this elaboration was performed independently by G.F. and C.M.F. and discussed with S.F.. Information collected from the papers included: type of study, number of subjects, country or region, years of the first enrolment, measured PFASs and blood sampling period(s), outcome measured and period(s) of measurements, and if they were statistically positively or negatively associated with the considered outcomes.
Furthermore, each article was evaluated for the quality of its reporting: a list of 28 items was established, most of which following the STrengthening Reporting of Observational studies in Epidemiology-Molecular Epidemiology (STROBE-ME) [46], while others were created to specifically match the considered studies. The complete list of items is reported in the supplementary material (Table S03). For each item, either a 0 (not reported or not fulfilled), 0.5 (partially fulfilled) or 1 (fulfilled) was assigned. For each paper, the scores assigned to all the items were summed to evaluate the quality of reporting.
Meta-analyses
Inclusion criteria
Aside from the extraction of the information reported in the previous section and the qualitative evaluation of studies, meta-analyses were performed among a subset of the included papers. Studies were eligible for inclusion in meta-analyses if they reported the estimated beta coefficient of the association between PFAS concentrations and the considered outcomes (BMI, WC, fat mass), along with the 95% confidence intervals (CI); or the association between PFAS concentrations and the risk of overweight/obesity, along with 95% CI. For each study, the estimates were considered separately if they were calculated considering the entire population (both sexes combined), only boys, or only girls. If the same cohort of subjects was considered in more than one paper, only one study was included in the meta-analyses. For each PFAS, outcome, and sex-category, a meta-analysis was performed only if at least three studies reported suitable estimates.
Data extraction
The slopes of the continuous associations were considered; when only slopes from categories (such as percentiles) were reported, we considered the highest estimate reported (worst-case scenario) [47]. For risk of overweight/obesity, both odds ratio (OR) and risk ratios (RR) were considered. If multiple models were reported, we considered the adjusted estimates from the model with the highest number of considered confounding factors. When the estimates were reported for outcomes measured at different time periods during infancy, the one related to the latest measurement was considered. For each study, estimates from subgroups (e.g., region of the study) were considered only if there were not overall estimates reported. If necessary, corresponding author of the paper was contacted.
Data conversion of the effect estimates
A specific methodology was developed to ensure that the beta coefficients and the confidence intervals included for meta-analysis were comparable each other. The conversions were performed to have, for all the included studies, beta estimates and coefficient intervals that represent the mean increase of z-scores of the outcome for each unit (ng/mL) increase in the PFAS.
Firstly, the standard error was calculated from the 95% CI as follow:
Where is the calculated standard error, is the upper limit, and is the lower limit of the 95% coefficient interval.
Then, four different steps of conversion were performed:
If the concentrations of PFASs were log-transformed before performing the linear models, the beta was changed according to the following formula elaborated by Rodríguez-Barranco and co-workers [48].
While 95% CI was calculated as follows:
Where and are the converted beta and coefficient intervals, is the base of the logarithm used, is the mean (if not available, the median) of the PFAS concentration, is the slope reported by the study and is the standard error of the beta calculated as reported above.
-
2)
If also the outcome variable were log-transformed and the effect estimates were reported as percent change; first, the beta was calculated from the variation percentage (var%) with the following formula:
and the standard error was calculated from the 95% CI of the var% as follow:
then, beta and 95% CI were calculated with the following equation elaborated by Rodríguez-Barranco et. al [48].
While 95% coefficients intervals were calculated as follows:
Where and are the converted beta and coefficient intervals, is the base of the logarithm used for outcome data, is the base of the logarithm used for PFASs, is the mean (or median, if mean was not reported) of the PFAS concentration, is the mean (or median, if mean was not reported) of the outcome measure, is the slope reported by the study and is the standard error of the beta.
-
3)
If the effect estimate was reported by change in interquartile range, the slope was divided by the interquartile range in order to have a feasible beta for unit increase. Analogously, if the effect estimate was reported by change of a specific tercile versus the reference tercile, it was divided by the difference of means of tercile. The standard error was also divided accordingly, and the coefficient intervals calculated again.
-
4)
Finally, a further correction was carried out considering the type of data outcome used in each study. For BMI data, no change was made if the effect estimate was calculated from BMI z-score or BMI SDS, while if calculated from BMI expressed as kg/m2 the reported BMI mean (or median) was converted into BMI z-score using the Word Health Organization (WHO) references [49, 50], implementing the “anthro” R package for calculation [51], which uses the following formula [52]:
Where is the calculated z-score, is the mean (or median) of the BMI (in kg/m2) reported by the study; while the value used for , , and were chosen from the WHO references [49, 50] considering the overall age of children in each study and the sex of subjects: if the effect estimate of a study reported separately girls or boys, only one z-score was calculated, while if the study included both males and females, two different z-scores were calculated, and then the mean of the two was considered. Then, the beta estimates and 95%CI were calculated with a proportion:
Where , , and are the final beta, standard error, and coefficient intervals, respectively; is the z-score calculated as reported above, and are the beta and standard error as converted so far, is the reported mean (or median) of the BMI (in kg/m2) Analogously, estimates obtained from waist circumference (in cm) were transformed using the tables reported by Sharma et al. [53]. For body fat, if expressed as body fat percentage, the mean (or median) was firstly converted as total fat index (kg/m2) by dividing the percentage per 100 and multiplying by mean (or median) BMI, then as fat mass index z-score using tables from Weber et al. [54].
All the above calculations were performed on the beta coefficients considering the associations between PFASs and BMI or WC. Instead, in order to properly make comparable the data related to the risk of overweight, the following procedure was applied to:
If the data were reported as RR, they were converted as OR following a formula elaborated considering the one reported by [55], and explicating the OR:
Where OR is the odds ratio, RR is the risk ratio, P0 is the prevalence of overweight (expressed as proportion, so a number from 0 to 1) in the reference population (e.g: the first tercile or the first quartile) or in the total proportion (when data were reported as continuous). The same formula was applied to the lower and upper limit of the 95% CI.
-
b)
Since OR is not a symmetrical measure, OR data were converted as standardized mean difference following a formula reported by [56]. In particular:
Where d is the standardized mean difference and Log(OR) is the logarithm (base 10) of the OR. The same formula was applied to the lower and upper limit of the 95% CI.
Finally, the obtained standardized mean differences were further converted using the same methodology reported above in steps 1) and 3) to suitably consider together PFAS concentrations calculated with different log-transformations and the different ranges of concentrations considered in each study.
All these elaborations were carried out by developing a specific R-script, which is reported in the supplementary material (Supplementary text, R script, Sect. 2).
Performing the meta-analyses
The generic inverse variance method was used, both fixed and random pooled estimates were calculated, heterogeneity was measured with the I2 statistics [57] and the between-study variance was calculated using the restricted maximum-likelihood estimator (REML) (τ2) [58]. To assess for the possible publication bias, a funnel plot was plotted [59] and an Egger’s test was carried out [60]. All these functions were conducted in the R environment, using the R package “meta” [61]. Forest plots and funnel plots were created using functions from the “meta” package. Moreover, in further separated meta-analyses, the summary estimates were also calculated by weighting the studies by sample size, instead of using the inverse variance method. The latter were carried out using the R package “rmeta” [62] (Supplementary text, R script, Sect. 3). Furthermore, sensitivity analyses were performed by repeating the meta-analyses described above by excluding one study at a time (Supplementary text, R script, Sect. 4).
Finally, all of the above (generic inverse variance method analyses, analyses weighting by sample size, and the sensitivity analyses removing one study at a time) were also performed separately considering studies which measured the outcomes in children who were 3 or less years from those measuring the outcomes in children who were more than 3 years (Supplementary text, R script, Sect. 5 and 6).
For visualisation, innovative superimposed forest plots were ideated and set up by developing an R script, implementing functions from the packages “tidyverse”, “grid” and “gridExtra” [43, 63], which is reported in the supplementary material (Supplementary text, R script, Sect. 7).
Results
Search results
A total of 484 papers were retrieved from PubMed (Table S01) and 779 from Embase (Table S02), for a total of 1263 entries. Papers derived from both databases were 437, while 47 were uniquely retrieved from PubMed and 342 were unique from Embase; thus, after merging the duplicates, the papers considered for inclusion were 826. Titles and abstracts were evaluated and, in case, the papers were inspected in their full text. Several articles were not eligible for inclusion: 278 were out of scope and not relevant, 72 did not report original findings (such as reviews or project presentations), 27 were not full articles (such as letters or conference abstracts), 2 were pre-prints, and 1 was a retracted article. Furthermore, several studies did not meet the inclusion criteria for one or more reasons of the following: no humans involved (128); the outcomes were measured in human adults (61 of the remaining); while considering children or breastfeeding, the outcomes of interest were not measured (190 of the remaining); a biomonitoring study to measure exposure to PFASs was not performed (19). Hence, the total number of papers suitable for inclusion were 48. Later, an additional suitable paper was suggested by one of the external reviewers: it was not retrieved using the developed text string as it did not mention PFASs or related synonyms in title, abstract, or keywords, but suitable PFASs analyses were reported in the full text and in the supplementary material of the article [64]. Therefore, 49 papers were included in the systematic review, among which: 26 evaluated the prenatal exposure, 17 considered the exposure in children, and 6 both. A summary of the literature search results is given in Fig. 1, while the complete database is reported in the supplementary material (Table S03).
Fig. 1.
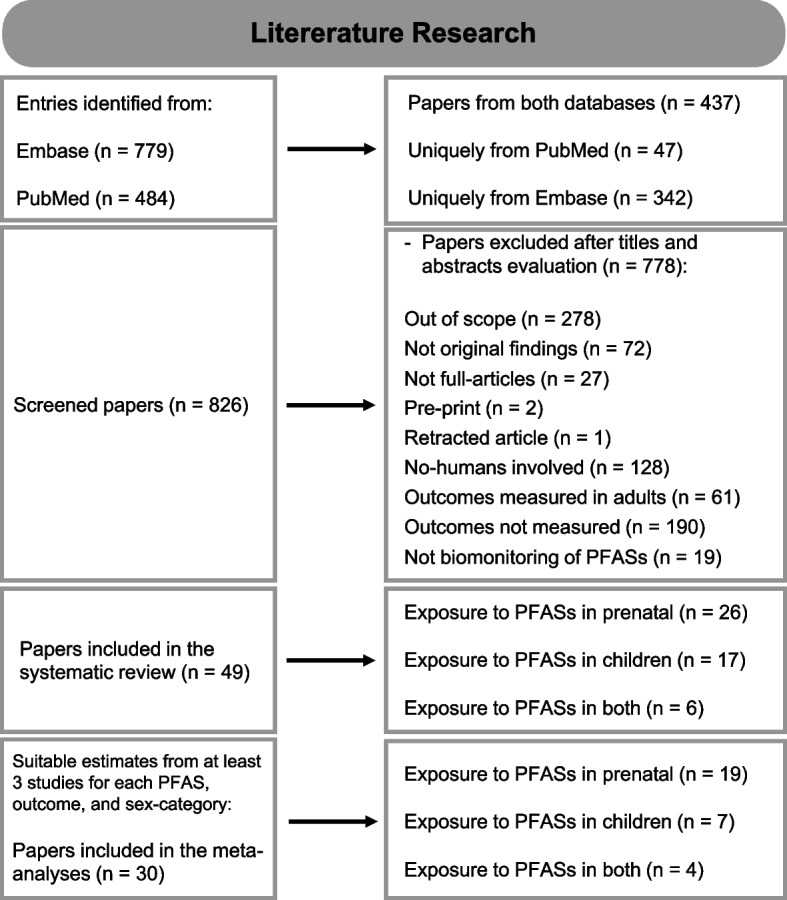
Summary of the literature search performed in this review
Studies included in this review
A summary of the included papers is reported in Table 1 and 2. Overall, the enrolment periods ranged from 1986 to 2022. Several studies were conducted in Europe (24), among which most in Northern Europe (14) (Norway, Sweden, Denmark, Greenland, and Finland). Other studies were conducted in the United States (17), and a few in Asia (8). The number of subjects considered for the analyses varied greatly among studies, from only 54 to 9362 subjects. Among considered PFASs, PFOA and PFOS were monitored in almost all studies; PFNA, PFHxS, and PFDA were measured in 35, 33, and 21 studies, respectively; other measured PFASs were PFHpA, PFBS, and PFOSA measured in 12, 11, and 10 studies, respectively. For studies evaluating the prenatal exposure to PFASs, they were quantified in the blood of mothers at different periods during gestation, at birth, or a couple of weeks after birth; only one study evaluated the prenatal exposure in blood samples of children at birth. For studies assessing PFASs during childhood, they were quantified in children blood across various ages: from 1 to 18 years. BMI was measured in almost all studies, while WC and FT/BFP were measured in less than half of the studies. Outcomes were measured at different ages, from few weeks after birth to 18 years old.
Table 1.
Summary of the studies included in this review considering a prenatal exposure to PFASs. For each study, information about the name of the study, country or region, year of the first enrolment, and number of subjects included in the statistical analyses were reported. Moreover, the information about PFASs (which ones monitored, when the samples were collected, and measured concentrations) is reported, as well as which outcomes were measured and when. The main associations found are also reported; in particular, each line represents a positive or a negative association between a compound and an outcome: an up arrow before the compound and an up arrow before the outcome indicates a positive association, while an up arrow before the compound and a downward arrow before the outcome indicates a negative association. Marginally significant associations are also reported (m). Finally, in the last column, it is reported if and which estimates were included in the meta-analyses
| Reference | Name of the study | Country or region | Years of first enrolment | Number of subjects | Exposure assessment in mothers | Outcomes measured in children | Significant or marginally significant associations between exposure and outcomes | Data included in the meta-analyses | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PFASs measured in blood | Sampling time |
PFAS concentrations (ng/mL) |
Type | Time | |||||||
|
Papadopoulou et al., 2021 [65] |
HELIX project | UK, France, Spain, Lithuania, Norway, Greece | from 1999 to 2010, across the 6 cohorts | 1101 | PFOA, PFNA, PFUnDA, PFHxS, PFOS | From mean (SD) week 14 (4) to 39 (1) |
Median PFOA: 2.22 PFNA: 0.69 PFHxS: 0.53 PFOS: 6.15 |
WC | 8 years (mean; range: 6 -12 years) |
↑ PFAS mixture—↓ WC ↑ PFNA—↑ WCm |
WC: PFOS, PFHxS, PFOA, PFNA (both sexes combined) |
|
Braun et al., 2021 [66] |
HOME study | Cincinnati, Ohio (U.S) | 2003–2006 | 345 | PFOA, PFOS, PFNA, PFHxS | 16 weeks of gestation (n = 294, 85.2%), 26 weeks of gestation (n = 34, 9.9%), or within 24 h of delivery (n = 17, 4.2%) |
Median PFOA: 5.5 PFOS: 13.8 PFNA: 0.9 PFHxS: 1.5 |
BMI | at 4 weeks and 1, 2, 3, 4, 5, 8, and 12 years |
↑ PFOA—↓ BMI (infancy and early childhood) (non-monotonic) ↑ PFOA—↑ BMI (mid-childhood and adolescence) (non-monotonic) ↑ PFOS—↓ BMI (infancy, childhood, adolescence) ↑ PFHxS—↓ BMI (infancy, childhood, adolescence) |
Data not used as, for the HOME study, data from Liu et al., 2020 were chosen for completeness |
|
Gao, et al. 2022 [67] |
Shanghai Birth Cohort | Shanghai, China | 2013–2016 | 1350 | PFOA, PFOS, PFNA, PFUA, PFDA, PFHxS, PFBS, PFDoA, PFHpA, PFOSA | From 9 and 16 weeks of gestation |
Median PFOS: 9.68 PFOA: 11.66 PFNA: 1.77 PFDA: 1.82 PFUA: 1.48 PFHxS: 0.54 PFHpA: 0.06 PFDoA: 0.15 PFBS: 0.02 PFOSA: 0.00 |
WL WA |
42 days, 6 months, 12 months, 24 months |
↑ PFAS mixture—↓ WL high-rising and low rising group ↑ PFHxS—↓ WL high-rising group ↑ PFHpA—↓ WL low rising group ↑ PFOA—↑ WA low-rising group ↑ PFNA—↑ WA low-rising group ↑ PFDA—↑ WA low-rising group ↑ PFHxS—↑ WA low-rising group ↑ PFUA—↑ WA low-rising group ↑ PFAS mixture—↑ WA low-rising group |
Not suitable data (WL not suitable) |
|
Romano et al., 2022 [68] |
New Hampshire Birth Cohort Study (NHBCS) | New England | 2009–2018 | 418 | PFHxS, n-PFOS, Sm-PFOS, n-PFOA, Sb-PFOA, PFNA, PFDA, PFUnDA, MeFOSAA, | ~ 24–28 weeks’ gestation |
Median PFOA: 1.44 PFOS: 4.00 MeFOSAA: < LOD PFDA: 0.20 PFHxS: 0.70 PFNA: 0.60 PFUnDA: < LOD |
BMI | 2 weeks, 1, 2, 4, 6, 9, and 12 months |
↑ PFOA—↑ BMI at 12 months for girls and boys m ↑ PFNA—↑ BMI at 6 months for girlsm ↑ PFHxS—↑ BMI at 12 months for boysm ↑ PFOS—↑ BMI at 6 months for girls |
BMI: PFOA, PFOS, PFHxS, PFNA, PFDA, PFUA (only boys and only girls) |
|
Zhang, Lei, et al., 2022 [69] |
LWBC | Shandong, China | 2010 – 2013 | 206 | PFOS, PFOA, PFBS, PFHxS, PFDA, PFDoA, PFHpA, PFOSA, PFNA, PFUA | within 3 days before delivery |
Median PFOA: 45.14 PFOS: 4.79 PFNA: 0.84 PFDA: 0.56 PFUA: 0.50 PFHxS: 0.33 PFDoA: 0.17 PFBS: 0.19 PFOSA: 0.13 PFHpA: 0.06 |
BMI WC FM/BFP WHtR |
7 years |
↑ PFHpA—↓ WC ↑ PFOSA—↓ FM/BFP ↑ PFHpA—↓ WC in boys ↑ PFHpA—↓ BMI in boys ↑ PFHpA—↓ FM in boys ↑ PFHpA—↓ WHtR in boys ↑ PFHpA—↑ BMI in girls ↑ PFOSA—↓ FM/BFP in boys ↑ PFOSA—↓ BMI in boys ↑ PFAS mixture—↓ FM/BFP ↑ PFAS mixture—↓ FM/BFP in boys ↑ PFAS mixture—↓ BMI in boys ↑ PFAS mixture—↓ WC in boys ↑ PFAS mixture—↓ WHtR in boys ↑ PFAS mixture—↑ FM in girls ↑ PFAS mixture—↑ BMI in girls ↑ PFAS mixture—↑ WC in girls ↑ PFAS mixture—↑ WHtR in girls |
BMI: PFOA, PFOS, PFNA, PFHxS, PFDA, PFUA, PFDoA, PFBS (both sexes combined, only boys, and only girls) WC: PFOA, PFOS, PFNA, PFHxS, PFDA, PFUA (both sexes combined, only boys, and only girls) |
|
Bloom et al., 2022 [70] |
Eunice Kennedy Shriver NICHD Fetal Growth ECHO-FGS |
U.S | 2009–2013 | 803 | PFHxS, PFOS, PFOSA, PFDS, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA | 8–13 weeks gestation |
Median PFHxS: 0.9 PFOS: 5.3 PFOA: 2.0 PFNA: 0.8 PFDA: 0.3 PFUnDA: 0.2 |
BMI WC FM/BFP |
4–8 years |
↑ PFUnDA—↑ WC, mother without obesity ↑ PFUnDA—↑ FM/BFP, mother without obesity ↑ PFOS—↑ BFP, mother without obesity m ↑ PFDA—↑ BMI, parous mother without obesity ↑ PFDA—↑ FM/BFP, parous mother without obesity ↑ PFOS—↓ BMI, mother with obesity ↑ PFDA—↓ BMI, mother with obesity ↑ PFOS—↓ FM/BFP, mother with obesity ↑ PFNA—↓ FM/BFP, mother with obesity ↑ PFDA—↓ FM/BFP, mother with obesity |
BMI: PFHxS, PFOS, PFOA, PFNA, PFDA, PFUA (both sexes combined, only boys, and only girls) WC: PFHxS, PFOS, PFOA, PFNA, PFDA, PFUA (both sexes combined, only boys, and only girls) Overweight risk: PFHxS, PFOS, PFOA, PFNA (both sexes combined) Included only data from mothers without obesity |
|
Martinsson et al., 2020 [71] |
Southern Sweden Maternity Cohort | Malmö, Sweden | 2003–2008 | 1048 | PFOS, PFOA, PFHxS, PFNA | 14 weeks of gestation |
Median PFOS: 16.6 PFOA: 3.1 PFHxS: 0.7 PFNA: 0.4 |
BMI | 4 years | No significant associations for the considered outcomes | Overweight risk: PFOS, PFOA, PFHxS, PFNA (both sexes combined) |
|
Li, Liu et al., 2021 [72] |
HOME Study | Cincinnati, Ohio (U.S) | 2003–2006 | 221 | PFOA, PFOS, PFNA, PFHxS |
16 weeks of gestation (86% of women) 26-week of gestation (9.5%) or at delivery (4.5%) |
Median PFOA: 5.3 PFOS: 12.9 PFNA: 0.9 PFHxS: 1.3 |
WC FM |
12 years |
↑ PFHxS—↑ WC ↑ PFOA—↑ WCm |
Data not used as, for the HOME study, data from Liu et al., 2020 were chosen for completeness |
|
Horikoshi et al., 2021 [73] |
HBC study | Hamamatsu, Japan | 2007 – 2012 | 597 | PFOS and PFOA | at birth |
Mean PFOS: 1.38 PFOA: 1.39 |
BMI | 1, 4, 10, 18, 24, 32, 40, 50, and 66 months |
↑ PFOS—↑ BMI increase while growing m ↑ PFOA—↑ BMI increase while growing |
Not suitable data (increas of BMI SDS while growing, and not numerical data indicating needed estimates) |
|
Lauritzen et al., 2018 [74] |
NICHD-SGA | Norway and Sweden | 1986–88 |
412 (254 Norway, 158 Sweden) |
PFOA, PFOS | 17–20 weeks of gestation |
Median: PFOA: 1.64 (Norwegian), 2.33 (Swedish) PFOS: 9.62 (Norwegian), 16.3 (Swedish) |
BMI FM/BFP |
5 years |
↑ PFOA—↑ BMI-for-age-and-sex z-score (only in Norway) ↑ PFOA—↑ FM Triceps skinfold z-score (only in Norway) ↑ PFOA—↑ OR Overweight (only in Norway) ↑ PFOS—↑ BMI BMI-for-age-and-sex z-score (all) ↑ PFOS—↑ FM Triceps skinfold z-score (all) ↑ PFOS—↑ OR Overweight (all) |
BMI: PFOA, PFOS (both sexes combined) Overweight risk: PFOA, PFOS (both sexes combined) |
|
Gyllenhammar et al., 2018 [75] |
POPUP study | Uppsala County, Sweden | 1996–2011 | 182–193 | PFOA, PFNA, PFDA, PFUnDA, PFBS, PFHxS, and PFOS | 3 weeks after delivery |
Mean: PFOA 2.4 PFNA 0.46 PFDA 0.23 PFUnDA 0.19 PFBS 0.03 PFHxS 3.6 PFOS 14 |
BMI | at 3, 4, and 5 years |
↑ PFOA—↑ BMI (3 and 4 years) ↑ PFNA—↑ BMI (3 and 4 years) ↑ PFHxS—↑ BMI (3 and 4 years) ↑ PFOS—↑ BMI (4 and 5 years) |
BMI: PFOA, PFOS, PFNA, PFHxS (both sexes combined) (data asked to the author) |
|
Zhang, Pan, et al., 2022 [76] |
Shanghai Birth Cohort | Shanghai, China | 2013–2016 | 2395 | PFOA, PFOS, PFNA, PFDA, PFUA, PFDoA, PFHxS, PFHpA, PFBS, PFOSA | median gestational age of 15 weeks [interquartile range (IQR): 13–17 weeks] |
Median PFOA: 11.62 PFOS: 9.38 PFNA: 1.68 PFDA: 1.72 PFUA: 1.40 PFHxS: 0.53 PFDoA: 0.17 PFBS: 0.03 PFHpA: 0.05 |
BMI WL |
42 days (± 2 days), 6 months (mean ± SD: 6.4 ± 0.7 months), and 12 months |
↑ PFBS—↓ WL ↑ PFBS—↓ BMI ↑ PFDoA—↑ WL ↑ PFDoA—↑ BMI |
BMI: PFOA, PFOS, PFNA, PFDA, PFUA, PFHxS, PFDoA, PFBS (both sexes combined, only boys, and only girls) |
|
Starling et al., 2019 [77] |
Healthy Start Study | Colorado, (U.S) | 2009–2014 | 415 | PFOSA, N-EtFOSAA, N-MeFOSAA, PFHxS, PFOA, PFOS, PFNA | 27 weeks of gestation, (median, range 20–34 weeks) |
Median PFOA: 1.0 PFOS: 2.2 PFNA: 0.4 PFDA: 0.1 PFHxS: 0.7 MeFOSAA: 0.1 |
WL WA FM/BFP |
5 months of age (average: 5.1 months, range: 2.8—9.4 months) |
↑ PFOA—↑ FM/BFP (males) ↑ PFNA—↑ FM/BFP (males) ↑ PFOS—↓ FM/BFP m (females) ↑ PFOS—↓ WA (females)↑ PFOS—↓ WL (females) ↑ PFHxS—↓ WA (females) ↑ PFHxS—↑ rapid growth in WL ↑ N-MeFOSAA—↑ WA (both sexes combined and females) ↑ N-MeFOSAA—↑ rapid growth in WA ↑ N-MeFOSAA—↑ rapid growth in WL |
Not suitable data (WL) |
|
Hartman et al. 2017 [78] |
ALSPAC | Avon, U.K | 1991–1992 | 359 girls | PFOA, PFOS, PFHxS, PFNA | 15 weeks of gestation (median; interquartile range: 10 and 28 weeks) |
Median: PFOA: 3.7 PFOS: 19.7 PFHxS: 1.6 PFNA: 0.5 |
BMI WC FM/BFP |
9 years |
↑ PFOS—↓ BMI ↑ PFOS—↓ WC ↑ PFOA—↓ WC ↑ PFOA—↑ BFP (girls with mothers in the middle education group) ↑ PFOA—↓ BFP (girls with mothers in the highest education group) ↑ PFOS—↓ BFP(girls with mothers in the highest education group) |
BMI: PFOA, PFOS, PFNA, PFHxS (only girls) WC: PFOA, PFOS, PFNA, PFHxS (only girls) |
|
Sevelsted et al., 2022 [79] |
COPSAC2010 birth cohort | Zealand, Denmark | 2009–2011 | 675 | PFOS and PFOA | pregnancy week 24 and 1 week postpartum |
Median [IQR] PFOS: 6.24 [4.96–7.73] PFOA: 1.08 [0.78–1.47] |
BMI BFP |
6, 8, and 10 years |
↑ PFOS—↓ BMI at 6 years in girls ↑ PFOS—↓ BFP at 6 years in girls ↑ PFOS—↑ BMI at 6 years in boys m ↑ PFOS—↑ BFP at 6 years in boys m |
BMI: PFOS, PFOA (both sexes combined) |
|
Shoaff et al., 2018 [80] |
HOME study | Cincinnati, Ohio (U.S) | 2003–2006 | 334 | PFOA, PFOS, PFNA, PHFxS | 16 (86%) and 26 weeks gestation (9%), or within 48 h from delivery (5%) |
Median: PFOA: 5.5 PFOS: 14 PFNA: 0.9 PFHxS: 1.5 |
BMI |
4 weeks, 1 and 2 years old |
↑ PFOA—↓ BMI ↑ PFOS—↓ BMI ↑ PFNA—↓ BMI m ↑ PFHxS—↓ BMI m |
Data not used as, for the HOME study, data from Liu et al., 2020 were chosen for completeness |
|
Liu et al., 2020 [81] |
HOME study | Cincinnati, Ohio (U.S) | 2003–2006 | 212 | PFOA, PFOS, PFNA, PFHxS | 16 or 26 weeks of gestation or within 48 h from delivery |
Median (during pregnancy): PFOA: 5.3 PFOS: 13.3 PFNA: 0.9 PFHxS: 1.3 |
BMI WC FM/BFP |
12 years |
↑ PFOA—↑ WtHr ↑ PFOA—↑ WCm (non-linear) ↑ PFOA—↑ visceral fat aream (non-linear) ↑ PFOA—↑ trunk fat percentm ↑ PFOA—↑ android fat percentm ↑ PFOA—↓ gynoid fat percentm ↑ PFOA—↑ WC in girls ↑ PFOA—↑ WtHr in girls ↑ PFOA—↑ BFP in girls ↑ PFHxS—↑ WtHr m ↑ PFHxS—↑ WC m ↑ PFHxS—↑ visceral fat area m ↑ PFHxS—↑ trunk fat percent m ↑ PFHxS—↑ android fat percent m ↑ PFHxS—↓ gynoid fat percent m ↑ PFHxS—↑ WtHr in girls ↑ PFHxS—↑ android/gynoid fat percent ratio in girls ↑ PFHxS—↑ risk of overweight/obesity |
BMI: PFOA, PFOS, PFNA, PFHxS (both sexes combined, only boys, and only girls) WC: PFOA, PFOS, PFNA, PFHxS (both sexes combined, only boys, and only girls) |
|
Andersen et al., 2013 [82] |
Danish National Birth Cohort | Denmark | 1996–2002 | 811 (BMI) 804 (WC) | PFOA, PFOS | 1st and 2nd trimesters of gestation, and at delivery |
Median: PFOS: 33.8 PFOA: 5.25 |
BMI WC |
7 years |
↑ PFOA—↓ BMI m ↑ PFOA—↓ WC m (in boys) ↑ PFOS—↓ BMIm ↑ PFOS—↓ WCm (in boys) |
BMI: PFOA, PFOS (only boys, and only girls) WC: PFOA, PFOS (only boys, and only girls) |
|
Mora et al., 2017 [83] |
Project Viva | Greater Boston, (U.S) | 1999–2002 | 1645 | PFOA, PFOS, PFHxS, PFNA | 9.6 weeks of gestation (median) |
Median: Children with early-childhood data PFOS: 24.8 PFOA: 5.6 PFHxS: 2.4 PFNA: 0.6 Median: Children with mid-childhood data PFOS: 24.7 PFOA: 5.6 PFHxS: 2.3 PFNA: 0.6 |
BMI WC FM/BFP |
3.2 years (median; range: 2.9–6.1) for 1006 children (61%) 7.7 years (median; range: 6.6–10.9) for 876 children (53%) |
↑ PFOS—↑ BMI (early childhood) ↑ PFHxS—↑ BFP (early childhood) ↑ PFOA—↑ WC (early childhood) ↑ PFOA—↑ WC (early childhood, boys) ↑ PFOS—↑ BMI (mid-childhood, girls) ↑ PFNA—↑ BMI (mid-childhood, girls) ↑ PFHxS—↑ BFP (mid-childhood, girls) ↑ PFNA—↑ BFP (mid-childhood) ↑ PFNA—↑ BFP (mid-childhood, girls) |
BMI: PFOS, PFOA, PFHxS, PFNA (both sexes combined, only boys, and only girls) WC: PFOS, PFOA, PFHxS, PFNA (both sexes combined, only boys, and only girls) Overweight risk: PFOS, PFOA, PFHxS, PFNA (both sexes combined) |
|
Braun et al., 2016 [84] |
HOME Study | Cincinnati, Ohio (U.S) | 2003–2006 | 204 | PFOA, PFOS, PFNA, PFHxS | 16 (n = 173, 87%) and 26 weeks of gestation (n = 19, 9%) and at delivery (n = 8, 4%) |
Median: PFOA: 5.3 PFOS: 13 PFNA 0.9 PFHxS 1.4 |
BMI WC BFP |
at 8 years and at 2, 3, 4, 5 years of age |
↑ PFOA—↑ WC at 8 years (non-linear) ↑ PFOA—↑ BMI at 8 years (non-linear) ↑ PFOA—↑ BFP at 8 years (non-linear) ↑ PFOA—↑ BMI gains from 2 to 8 years |
Overweight risk: PFOA, PFOS, PFNA, PFHxS (both sexes combined) Other data for the HOME study, taken from Liu et al., 2020 for completeness |
|
Marks et al., 2021 [85] |
ALSPAC | United Kingdom | 1991 – 1992 | 301 | PFOA, PFOS, PFHxS, PFNA, MeFOSAA, EtFOSAA, FOSA, PFDA | median of 15 (interquartile range (IQR): 10–28) weeks gestation |
Median PFOA: 3.7 PFOS: 19.6 PFHxS: 1.6 PFNA: 0.49 FOSA: 0.20 MeFOSAA: 0.35 EtFOSAA: 0.70 PFDA: < LOD |
BMI | 2, 9, and 19 months | ↑ overall endocrine disruptors—↓ BMI m |
Not suitable data (data were for weight-for-age z-score) |
|
Jensen et al., 2020 [86] |
OCC | Odense, Southern Denmark | 2010–2012 |
602 (3 months) 530 (18 months) |
PFOA, PFOS, PFHxS, PFNA, PFDA | before 16 weeks of gestation: median GA (IQR): 11.3 (9.9, 14.3) weeks |
Median PFHxS: 0.30 PFOS: 8.04 PFOA: 1.62 PFNA: 0.66 PFDA: 0.26 |
BMI WC FM/BFP |
3, and 18 months |
↑ PFNA—↑ BMI (3 and 18 months) ↑ PFNA—↑ BMI (in girls, 3 and 18 months) ↑ PFNA—↑ BFP (3 months) ↑ PFDA—↑ BMI (3 and 18 months) ↑ PFDA—↑ BMI m (in girls, 3 and 18 months) ↑ PFDA—↑ BFP (3 months) ↑ PFOS—↑ BMI m (3 and 18 months) |
BMI: PFOS, PFOA, PFHxS, PFNA, PFDA (both sexes combined, only boys, and only girls) WC: PFOS, PFOA, PFHxS, PFNA, PFDA (both sexes combined, only boys, and only girls) |
|
Chen et al., 2019 [87] |
Shanghai Prenatal Cohort | Shanghai, China | 2012–2017 | 404 | PFOA, PFOS, PFNA, PFDA, PFUnDA, PFHxS, PFOSA, PFDoA, PFBS, PFHpA | at birth |
Median PFOS: 2.44 PFOA: 6.74 PFNA: 0.64 PFDA: 0.36 PFUA: 0.40 PFDoA: 0.09 PFHxS: 0.16 PFBS: 0.05 |
BMI WC WtHr FM/BFP |
5 years |
↑ PFBS—↑ WC (girls) ↑ PFBS—↑ WtHe (girls) ↑ PFBS—↑ FM (girls) ↑ PFBS—↑ BFP (girls) ↑PFDoA—↓ WC (girls) ↑PFDoA—↓ FM (girls) ↑PFDoA—↓ BFP (girls) ↑ PFNA—↑ BFP (boys) |
BMI: PFOS, PFOA, PFHxS, PFNA, PFDA, PFUA, PFDoA, PFBS (both sexes combined, only boys, and only girls) WC: PFOS, PFOA, PFHxS, PFNA, PFDA, PFUA (both sexes combined, only boys, and only girls) |
|
Chen et al., 2017 [88] |
Taiwan Birth Panel Study | Taipei and New Taipei, Taiwan | 2004–2005 | 429 | PFOA, PFOS | At birth |
Median PFOA: 2.6 PFOS: 7.6 |
BMI | at 4, 6, 12, 24, 60, 84 and 108 months |
↑ PFOS—↓ BMI (for girls, time span from 6 to 12 months) ↑ PFOS—↓ BMI (for girls, time span from 12 to 24 months) ↑ PFOS—↑ BMI (for girls, time span from 60 to 108 months) |
BMI: PFOS, PFOA (both sexes combined, only boys, and only girls) |
|
Andersen et al., 2010 [89] |
Danish National Birth Cohort |
Denmark | 1996–2002 | 1010 | PFOS, PFOA | 1st and 2nd trimesters of gestation, and at delivery |
Median: PFOS 33.4 PFOA 5.21 |
BMI | 5 months 12 months |
↑ PFOS—↓ BMI m (5 months) ↑ PFOS—↓ BMI (12 months) ↑ PFOA—↓ BMI m (5 months) ↑ PFOA—↓ BMI m (12 months) ↑ PFOS—↓ BMI m (5 months, boys) ↑ PFOS—↓ BMI (12 months, boys) ↑ PFOA—↓ BMI (5 months, boys) ↑ PFOA—↓ BMI (12 months, boys) |
BMI: PFOS, PFOA (both sexes combined) Data separated for sexes were not included as for those data of the Danish National Birth Cohort from Andersen et al., 2013 were considered instead |
|
Karlsen et al., 2017 [90] |
Not specified | Faroe Islands, Denmark | 2007–2009 |
444 (at 18 months) 371 (at 5 years) |
PFOS, PFOA, PFHxS, PFNA, PFDA | 2 weeks after delivery |
Median PFOS: 8.25 PFOA: 1.40 PFHxS: 0.20 PFNA: 0.66 PFDA: 0.26 |
BMI | at 18 months and 5 years |
↑ PFOS—↑ BMI (18 months) ↑ PFOS—↑ RR overweight (18 months) ↑ PFOA—↑ overweight risk (5 years) ↑ PFOA—↑ BMIm (18 months) ↑ PFHxS—↑ BMI m (18 months) ↑ PFDA—↓ overweight risk (5 years) (non-linear) ↑ PFNA—↓ overweight risk m (18 months and 5 years) (non-linear |
BMI: PFOS, PFOA, PFHxS, PFNA, PFDA (both sexes combined) Risk overweight: PFOA, PFOS, PFHxS, PFNA (both sexes combined) |
|
Yeung et al., 2019 [91] |
Upstate KIDS Study | New York State (excluding New York City), (U.S) | 2008–2010 | 2920 (1,954 singletons and 966 twins) | PFOA, PFOS | at birth in children (dried blood spot) |
Mean PFOS 1.7 PFOA 1.1 |
BMI | 4, 8, 12, 18, 24, 30, and 36 months |
↑ PFOA—↓ BMI (in singletons) ↑ PFOS—↓ BMI (in singletons) ↑ PFOA—↓ BMI (in singleton girls) ↑ PFOS—↓ BMI (in singleton girls) ↑ PFOA—↓ BMI (in singleton boys, non-linearly) ↑ PFOA—↑ BMI (in twins, non-linearly) |
BMI: PFOS, PFOA (both sexes combined, only boys, and only girls) |
|
Manzano-Salgado et al., 2017 [92] |
INMA birth cohort study | three Spanish regions: Gipuzkoa, Sabadell, and Valencia | 2003–2008 |
1154 (6 months) 1230 (4 years) 1086 (7 years) |
PFHxS, PFOS, PFOA, PFNA | 1st trimester of gestation |
Geometric mean: PFHxS: 0.61 PFOS: 5.80 PFOA: 2.32 PFNA: 0.66 |
Weight gain from birth to 6 months BMI WC (only at 4 years for the Valencia and Sabadell subcohorts (n = 839, 68%)) |
6 months, 4, and 7 years |
↑ PFOA—↑ weight gain to 6 months (boys) ↑ PFHxS—↓ weight gain to 6 months m ↑ PFHxS—↓ BMI m (overall and boys) (at 4 and 7 years) ↑ PFHxS—↓ WC m (overall and boys) (at 4 and 7 years) ↑ PFHxS—↑ BMI m (girls) (at 4 and 7 years) ↑ PFHxS—↑ WC m (girls) (at 4 and 7 years) ↑ PFOS—↑ BMI m (overall and boys) (at 4 and 7 years) ↑ PFOA—↑ BMI m (overall and boys) (at 4 and 7 years) ↑ PFNA—↑ BMI m (overall and boys) (at 4 and 7 years) ↑ PFOS—↑ WC m (at 7 years) ↑ PFNA—↑ BMI m (at 7 years) ↑ PFNA—↑ WC m (at 7 years) |
BMI: PFOS, PFOA, PFHxS, PFNA (both sexes combined, only boys, and only girls) WC: PFOS, PFOA, PFHxS, PFNA (both sexes combined, only boys, and only girls) Risk overweight: PFOA, PFOS, PFHxS, PFNA (both sexes combined) |
|
Alkhalawi et al., 2016 [93] |
Duisburg Birth Cohort Study | North Rhine–Westphalia State (Germany) | 2000–2002 | 156 | PFOA, PFOS, PFHxS | 32 weeks of gestation and at delivery |
Geometric mean: PFOA: 2.43 PFOS: 9.04 PFHxS: 0.62 |
Ponderal index | 4–5 weeks, 3–4 months, 6–7 months, and 12 months | ↑ PFHxS—↑ Ponderal index (at 3–4 months) | Not suitable data (ponderal index not suitable) |
|
Høyer et al., 2015 [94] |
CLEAR and INUENDO studies | Greenland and Kharkiv (Ukraine) | 2002–2004 | 1022 | PFOA, PFOS | 24 ± 10 weeks of gestation (mean ± SD) |
Median PFOA: 1.3 PFOS: 10.8 Greenland: PFOA: 1.8 PFOS: 20.2 Ukraine: PFOA 1.0 PFOS 5.0 |
BMI WHtR |
between 5 and 9 years old |
↑ PFOA—↑ WtHe m ↑ PFOS—↑ WtHe ↑ PFOA—↑ overweight (girls, Greenland) ↑ PFOS—↑ WtHe (girls, ↑ PFOA—↑ BMI (Ukraine) ↑ PFOA—↑ WtHr (girls, Greenland) |
BMI: PFOS, PFOA Greenland and Ukraine separated (both sexes combined) Overweight risk: PFOA, PFOS (both sexes combined) |
|
Cai et al., 2023 [95] |
FLEHS | Belgium | 2002–2004 and 2008–2009 | 346 | PFOA, PFOS | Right after birth |
Median PFOA: 1.5 PFOS: 2.7 |
BMI | 4–8 years | No significant associations for the considered outcomes | Not suitable data (BMI change not suitable) |
|
Vrijheid et al., 2020 [64] |
HELIX | Europe | 2013 – 2016 | 1301 | PFOA, PFNA, PFUnDA, PFHxS, PFOS | During pregnancy |
Inter Quartile Ranges PFOA: 2.0 PFNA: 0.66 PFUnDA: 0.18 PFHxS: 0.6 PFOS: 5.5 |
BMI WC FM/BFP |
6–11 years | No significant associations for the considered outcomes |
BMI: PFOS, PFOA, PFHxS, PFNA, PFUA (both sexes combined) (mean concentrations of PFASs asked to the authors) |
Table 2.
Summary of the studies included in this review considering a childhood exposure to PFASs. For each study, information about the name of the study, country, year of the first enrolment, and number of subjects included in the statistical analyses were reported. Moreover, the information about PFASs (which ones monitored, when the samples were collected, and measured concentrations) is reported, as well as which outcomes were measured and when. The main associations found are also reported; in particular, each line represents a positive or a negative association between a compound and an outcome: an up arrow before the compound and an up arrow before the outcome indicates a positive association, while an up arrow before the compound and a downward arrow before the outcome indicates a negative association. Marginally significant associations are also reported (m). Finally, in the last column, it is reported if and which estimates were included in the meta-analyses
| Reference | Name of study | Country and region | Years of first enrolment | Number of subjects | Exposure assessment in children | Outcomes measured in children | Significant or marginally significant associations between exposure and outcomes | Data included in the meta-analyses | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PFASs measured in blood | Sampling time |
PFASs concentrations (ng/mL) |
Type | Time | |||||||
|
Papadopoulou et al., 2021 [65] |
HELIX project | UK, France, Spain, Lithuania, Norway, Greece | from 1999 to 2010, across the 6 cohorts | 1101 | PFOA, PFNA, PFUnDA, PFHxS, PFOS | From mean (SD) age 7 (0.5) to 11 (0.6) years |
Median PFOA: 1.53 PFNA: 0.47 PFUnDA: 0.06 PFHxS: 0.34 PFOS: 1.93 |
WC | 8 years (mean; range: 6 -12 years) |
↑ PFAS mixture—↓ WC ↑ PFOA—↓ WC |
Data not included as for the HELIX project, data from Vrijheid et al., 2020 considered a larger population |
|
Geiger et al., 2021 [96] |
NHANES | U.S | 1999–2000, 2003–2012 | 2473 | PFOA, PFOS | 12–18 years |
Mean PFOA: 3.79 PFOS: 15.66 |
BMI WC |
12–18 years |
↑ PFOA—↑ BMI m ↑ PFOS—↑ BMI (significant in the unadjusted model) ↑ PFOS—↑ WC (significant in the unadjusted model) |
Overweight risk: PFOA, PFOS (both sexes combined) |
|
Averina et al., 2021 [97] |
Fit Futures study | Northern Norway | 2010–2011 | 940 | PFBS, PFPS,PFHxS, PFHpS, PFOS, PFNS, PFDS, PFDoDS, PFOSA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA |
Mean (SD) 16.3 (1.1) – 16.5 (1.4) |
Mean (SD) PFBS: 0.005 (0.002) PFPS: 0.01 (0.007) PFHxS: 0.02 (0.01) PFHxA: 0.04 (0.04) PFHpS: 0.01 (0.01) PFHpS: 0.07 (0.06) PFOS: 0.04 (0.05) PFOA: 0.30 (0.13) PFNS: 0.02 (0.02) PFNA: 0.03 (0.01) PFDS: 0.02 (0.012) PFDA: 0.03 (0.01) PFUnDA: 0.03 (0.02) PFDoDS: 0.03 (0.035) PFDoDA: 0.02 (0.02) PFTrDA: 0.03 (0.030) PFTeDA: 0.08 (0.089) PFOSA: 0.01 (0.010) |
BMI |
Mean (SD) 16.3 (1.1) – 16.5 (1.4) |
↑ PFHxS—↑ obesity ↑ PFHpS—↑ obesity |
Not suitable data (not directly associating with BMI) |
|
Canova et al., 2021 [98] |
Veneto Region Health Surveillance Program | Italy | 2017–2019 |
9362 (6669 adolescents and 2693 children) |
PFOS, PFOA, PFHxS, PFNA, PFHpA, PFBS, PFHxA, PFBA, PFPeA, PFDeA, PFUnA, PFDoA | 14–19 years (adolescents) and 8–11 (children) |
Mean (SD) in adolescents: PFOA: 51.6 (47.2) PFOS: 4.1 (3.5) PFHxS: 3.6 (2.9) PFNA: 0.5 (0.3) Mean (SD) in children: PFOA: 26.2 (21.5) PFOS: 2.6 (2.5) PFHxS: 2.2 (1.5) PFNA: 0.4 (0.2) |
BMI | 14–19 years (adolescents) and 8–11 (children) |
↑ PFOS—↓ BMI (adolescents and children) ↑ PFOA—↓ BMI (children) ↑ PFHxS—↓ BMI (children) ↑ PFNA—↓ BMI (children) |
BMI: PFOS, PFOA, PFHxS (both sexes combined, only boys, and only girls) Included only data about adolescents (and not about children) |
|
Janis et al., 2021 [99] |
Project Viva | eastern Massachusetts, U.S | 1999–2022 | 537 | n-PFOA, total PFOA, n-PFOS, Sm-PFOS, total PFOS, PFDA, PFHxS, MeFOSAA, PFNA | 6–10 years |
Median (IQR) PFOA: 4.5 (3.0) PFOS: 6.4 (5.9) PFDA: 0.3 (0.3) PFHxS: 1.9 (2.4) MeFOSAA: 0.3 (0.5) PFNA: 1.5 (1.2) |
BMI FM |
6–10 years and 11–16 years |
↑ PFOS—↓ BMI increase from mid-childhood to early adolescents m ↑ PFOS—↓ FM increase from mid-childhood to early adolescents ↑ PFHxS—↓ BMI increase from mid-childhood to early adolescents m ↑ PFHxS—↓ FM increase from mid-childhood to early adolescents ↑ PFDA—↑ FM increase from mid-childhood to early adolescents m ↑ PFNA—↑ FM increase from mid-childhood to early adolescents m ↑ PFDA—↑ FM increase from mid-childhood to early adolescents in boys ↑ PFAS mixture—↓ lean mass increase from mid-childhood to early adolescents |
Not suitable data (data expressed as BMI change) |
|
Fassler et al., 2019 [100] |
BCERP |
Cincinnati (U.S) |
2004–2006 | 353 (only girls) | N-MeFOSAA, PFDA, PFNA, PFOA, PFOS, PFHxS | 6–8 years |
Median Me-PFOSA-AcOH: 0.8 PFDeA: 0.30 PFHxS: 5.20 PFNA: 1.40 PFOA: 7.30 PFOS: 13.60 |
BMI WtHe WtHi FM |
6–8 years |
↑ PFOA—↓ BMI ↑ N-MeFOSAA—↓ BMI ↑ PFDeA—↓ BMI ↑ PFOS—↓ BMI ↑ PFOA—↓ WtHe ↑ N-MeFOSAA—↓ WtHe ↑ PFDeA—↓ WtHe ↑ PFOS—↓ WtHe ↑ PFOA—↓ FM |
Not suitable data (no confidence intervals) |
|
Li, Liu, et al. 2021 [72] |
HOME Study | Cincinnati, Ohio (U.S) | 2003–2006 | 221 | PFOA, PFOS, PFNA, PFHxS | 3, 8, and 12 years |
Median PFOA: from 1.3 to 5.4 PFOS: from 2.4 to 6.2 PFNA: from 0.3 to 1.3 PFHxS: from 0.7 to 1.9 |
WC FM |
12 years | No significant associations for the considered outcomes | Data not used as, for the HOME study, data from Liu et al., 2020 were chosen for completeness |
|
Thomsen et al., 2021 [101] |
Copenhagen Mother–Child Cohort | Copenaghen, Denmark | 2009 | 109 | PFOA, PFOS, PFHxS, PFNA, and PFDA |
Median (25th-75th percentiles) 12.6 years (11.5–13.2) |
Median (25th-75th percentiles) PFOA: 2.79 (2.18–3.58) PFOS: 6.81 (5.85–9.47) PFHxS: 0.50 (0.40–0.65) PFNA: 0.92 (0.73–1.17) PFDA: 0.31 (0.25–0.37) |
BFP |
Median (25th-75th percentiles) 12.6 years (11.5–13.2) |
↑ PFOS—↓ BFP m ↑ PFDA—↓ BFP m ↑ PFNA—↓ BFP m ↑ PFOA—↑ BFP m ↑ PFHxS—↓ BFPm |
Not suitable data (associations with DXA body fat, not enough from other studies to perform a meta-analysis) |
|
Koponen et al., 2018 [102] |
birth cohort study LUKAS2 | Eastern Finland | 2005–2015 | 54 | PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFHxS, PFHpS, PFDS; N-MeFOSAA, N-EtFOSAA, 6:2 diPAP, 8:2 diPAP | 1, 6 and 10.5 years |
Median PFOS: 1.5—6.3 PFOA: 1.4—7.1 PFNA: 0.35—0.84 PFHxS: 0.20—0.49 |
BMI | 1, 6, and 10.5 years | No significant associations for the considered outcomes | Not suitable data (no suitable estimates reported) |
|
Harris et al., 2017 [103] |
Project Viva |
Boston—urban and suburban Eastern Massachusetts (U.S) |
2007–2010 | 653 |
PFOS, PFOA, PFHxS PFNA, N-EtFOSAA N-MeFOSAA, PFDA, PFOSA |
7.7 years (median; range: 6.6 − 10.6) |
Median PFOS: 6.2 PFOA: 4.4 PFHxS: 1.9 PFNA: 1.5 Et-PFOSA-AcOH:LOD Me-PFOSA-AcOH: 0.3 PFDeA: 0.3 FOSA: < LOD |
BMI | 7.7 years (median; range: 6.6 − 10.6) |
↑ PFOA—↓ BMI ↑ PFDeA—↓BMI |
Not suitable data (estimates as PFASs concentration change depending on the outcome, instead of the contrary) |
|
Scinicariello et al., 2020 [104] |
NHANES | U.S | 2013–2014 | 600 | PFOA, PFNA, PFHxS, PFOS | 3–11 years |
Geometric mean PFOA: 1.92 PFNA: 0.80 PFHxS: 0.85 PFOS: 3.90 |
BMI | 3–11 years |
↑ PFOA—↓ BMI m ↑ PFHxS—↓ BMI m ↑ PFOS—↓ BMI m ↑ PFOA—↓ BMI (boys) ↑ PFHxS—↓ BMI (boys) |
BMI: PFOS, PFOA, PFHxS, PFNA (both sexes combined) |
| Sevelsted et al., 2022 [79] | COPSAC2010 birth cohort | Zealand, Denmark | 2009–2011 | 533 | PFOS and PFOA | 6 and 18 months |
Median [IQR]: PFOS: 5.29 [4.05–6.94] PFOA 2.33 [1.40–3.56] |
BMI BFP |
6, 8, and 10 years | No significant associations for the considered outcomes | BMI: PFOS, PFOA (both sexes combined) |
|
Liu et al., 2020 [81] |
HOME Study | Cincinnati, Ohio (U.S) | 2003–2006 | 212 | PFOA, PFOS, PFNA, PFHxS | at birth and ages 3, 8 and 12 years |
Median PFOA: 5.4 (3 y), 2.5 (8 y), 1.3 (12 y) PFOS: 6.2 (3 y), 3.6 (8 y), 2.4 (12 y) PFNA: 1.3 (3 y), 0.7 (8 y), 0.3 (12 y) PFHxS: 1.9 (3 y), 1.2 (8 y), 0.7 (12 y) |
BMI WC FM/BFP |
12 years | No significant associations for the considered outcomes | BMI: PFOS, PFOA, PFHxS (both sexes combined, only boys, and only girls) PFNA (both sexes combined) |
|
Timmermann et al., 2014 [105] |
EYHS | Odense, Denmark | 1997 | 499 | PFOA, PFOS | 8–10-year-old children (third-grade students) |
Median PFOS: 41.5 PFOA: 9.3 |
BMI WC FM/BFP |
8–10-year-old children (third-grade students) |
↑ PFOS—↓ FM/BFP in girlsm ↑ PFOS—↓ FM/BFP in boysm |
BMI: PFOS, PFOA (both sexes combined) |
|
Li, Li et al., 2021 [106] |
- | Shanghai, China | 2019 | 189 | PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnA, PFBS, PFHxS, PFOS, PFDS | 8–12 years |
Mean PFHxA: 21.06 PFHpA: 129.57 PFOA: 117.34 PFNA: 28.78 PFDA: < LOD PFUnA: < LOD PFBS: 21.57 PFHxS: 27.66 PFOS: 38.54 PFDS: < LOD ΣPFAAs: 394.83 |
BMI | 8–12 years |
↑ PFHpA—↓ children with overweight/obesity ↑ PFBS—↓ children with overweight/obesity ↑ PFOS—↓ children with overweight/obesity |
Not suitable data (no estimates with BMI) |
|
Ye et al.; 2018 [107] |
NHANES | U.S | 2013–2014 | 639 | PFOSA, N-MeFOSAA, N-EtFOSAA, PFBS, PFHxS, PFHpA, PFNA, PFDA, PFUnDA, PFDoDA, n-PFOA, Sb-PFOA, n-PFOS, Sm-PFOS | 3–11 years |
Median ΣPFOS: 3.75 ΣPFOA: 1.94 PFHxS: 0.810 PFNA: 0.700 n-PFOS: 2.47 Sm-PFOS: 1.28 n-PFOA: 1.82 Sb-PFOA: < LOD PFBS: < LOD PFOSA: < LOD N-MeFOSAA: 0.110 N-EtFOSAA: < LOD PFHpA: < LOD PFDA: < LOD PFUnDA: < LOD PFDoDA: < LOD |
BMI | 3–11 years |
↑ ΣPFOA—↓ BMIm ↑ n-PFOA—↓ BMIm |
Not suitable data (no beta estimates, only geometric mean) |
|
Domazet et al., 2016 [108] |
EYHS | Odense, Denmark | 1997 and 2003 | 501 | PFOA, PFOS |
9 yeas 15 years |
Medians PFOS: from 20.8 to 44.5 PFOA: from 3.4 to 9.7 |
BMI WC FM/BFP |
9 yeas 15 years |
↑ PFOS (at 9 y)—↑ BMI (at 15y) ↑ PFOS (at 9 y)—↑ WC (at 15 y) ↑ PFOS (at 9 y)—↑ FM/BFP (at 15 y) |
Overweight risk: PFOS, PFOA (both sexes combined) |
|
Karlsen et al., 2017 [90] |
Not specified | Faroe Islands, Denmark | 2007–2009 | 444 | PFOS, PFOA, PFHxS, PFNA, PFDA | 5 years |
Median PFOS: 4.70 PFOA: 2.20 PFHxS: 0.33 PFNA: 1.13 PFDA: 0.34 |
BMI | 5 years |
↑ PFNA—↓ BMI ↑ PFDA—↓ BMI ↑ PFOA—↓ BMI ↑ PFOS—↓ BMI |
BMI: PFOS, PFOA, PFHxS, PFNA (both sexes combined) Overweight risk: PFOS, PFOA (both sexes combined) |
|
Kim et al., 2014 [109] |
Not specified | Dae-gu City, South Korea | 2012 | 120 | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFBS, PFHxS, PFHpS, PFOS, PFDS | 5—13 years |
Mean PFBA: 0.346 PFPeA: 0.497 PFHxA: 0.353 PFHpA: 0.312 PFOA: 5.15 PFNA: 1.72 PFDA: 0.604 PFUnDA: 0.748 PFDoDA: - PFTrDA: 0.306 PFTeDA: - PFBS: 0.105 PFHxS: 1.13 PFHpS: 0.203 PFOS: 6.58 PFDS: - |
BMI | 5—13 years |
↑ PFOA—↓ BMI ↑ PFHxS—↓ BMI ↑ total PFASs—↓ BMI |
Not suitable data (no beta estimates, only Pearson's correlation) |
|
Domazet et al., 2020 [110] |
Danish sub-study of the EYHS | Odense, Denmark | 1997 | 242 | PFOS, PFOA, PFNA, PFDA, PFHxS | 9 years |
Median PFOS: 42.0—42.9 PFOA: 9.5 PFNA: 0.41—0.44 PFDA: 0.11 PFHxS 0.89—0.95 |
BMI FM/BFP |
9 years |
↑ PFNA—↓ BMI ↑ PFDA—↓ BMI ↑ PFNA—↓ FM ↑ PFDA—↓ FM ↑ PFHxS—↓ FM |
Not suitable data (no beta estimates with BMI) |
|
Schillemans et al., 2023 [111] |
HBM4EU | Europe | 2014–2021 | 1957 | PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFBS, PFHxS, PFHpS, PFOS | 12–18 years old |
Median PFPeA: 0.10 PFHxA: 0.09 PFHpA: 0.05 PFOA: 0.99 PFNA: 0.32 PFDA: 0.14 PFUnDA: 0.08 PFHxS: 0.04 PFHpS: 0.03 PFOS: 2.1 |
BMI | 12–18 years old |
↑ PFPeA—↓ BMI ↑ PFHpA—↓ BMI ↑ PFOA—↓ BMI ↑ PFNA—↓ BMI ↑ PFOS—↓ BMI ↑ PFHxS—↑ BMIm ↑ PFHpS—↑ BMIm |
BMI: PFOS, PFOA, PFHxS (both sexes combined, only boys, and only girls), PFNA (both sexes combined) |
|
Pinney et al., 2019 [112] |
the female puberty cohort of the BCERP | San Francisco Bay Area and Cincinnati (U.S.) | 2004–2007 | 704 (only girls) | PFOA | 6–8 years at first enrolments |
Median: 6.4 |
BMI WtHe WtHi |
6–8 years at first enrolments Repeated measures up to 18 years |
↑ PFOA—↓ BMI ↑ PFOA—↓ WtHe |
BMI: PFOA (only girls) |
|
Vrijheid et al., 2020 [64] |
HELIX | Europe | 2013 – 2016 | 1301 | PFOA, PFNA, PFUnDA, PFHxS, PFOS | 6–11 years |
Inter Quartile Ranges PFOA: 0.78 PFNA: 0.43 PFUnDA: 0.08 PFHxS: 0.42 PFOS: 2.0 |
BMI WC FM/BFP |
6–11 years |
↑ PFNA—↓ BMI ↑ PFOA—↓ BMI ↑ PFUNDA—↓ BMI ↑ PFOA—↓ WC ↑ PFNA—↓ WC ↑ PFOS—↓ WC ↑ PFUNDA—↓ WC ↑ PFNA—↓ skinfold z-score/BFP ↑ PFOS—↓ skinfold z-score/BFP ↑ PFOA—↓ skinfold z-score/BFP |
BMI: PFOS, PFOA, PFHxS, PFNA (both sexes combined) Overweight risk: PFOA (both sexes combined) (mean concentrations of PFASs asked to the authors) |
The concentration of PFASs varied greatly in prenatal studies: for PFOA, mean or median concentrations ranged from 1 to 45.14 ng/mL; for PFOS, from 1.38 to 33.8; for PFHxS, from 0.33 to 3.6; for PFNA, from 0.4 to 1.77; for PFDA, from less than the limit of detection (LOD) to 1.82. Considering studies measuring PFASs in children, PFOA mean or median concentrations ranged from 0.3 to 117.34 ng/mL; PFOS from 0.04 to 41.5; PFHxS from 0.02 to 27.66; PFNA from 0.03 to 28.78; PFDA from less than the LOD to 0.604.
For prenatal studies, across all studies, most reported no significant associations between considered PFASs and outcomes, as well as some controversial results as both positive and negative associations were reported: for PFOA and BMI, 14 studies reported no associations, 4 a negative association, 8 a positive association, and 1 both a positive and a negative association; for PFOA and WC, 7 studies reported no associations, 2 a negative and 4 a positive; for PFOA and FM/BFP, 9 studies reported no associations, 4 a positive and 1 both; for PFOS and BMI 12 reported no associations, 6 a negative, 7 a positive, and 2 both; for PFOS and WC, 10 reported no associations, 2 a negative, and 1 a positive; for PFOS and FM/BFP, 9 no associations, 2 negative, 1 positive, and 2 both; for PFHxS and BMI, 11 no associations, 2 negative, 4 positive, and 1 both; for PFHxS and WC, 9 no associations, 2 positive, and 1 both; for PFHxS and FM/BFP, 10 no associations and 2 postive; for PFNA and BMI, 14 no associations, 2 negative, and 4 positive; for PFNA and WC, 10 no associations, 2 positive; for PFNA and FM/BFP, 8 no associations, 1 negative, and 3 positive; for PFDA and BMI, 6 no associations, 1 negative and 1 positive; for PFDA and WC, 4 no associations; for PFDA and FM/BFP, 2 no associations, 1 negative, and 1 positive.
For studies measuring PFASs in children, even though there were still several works reporting no significant associations, there were also several ones reporting a negative associations: in particular, for PFOA and BMI, 9 studies reported no associations, 10 a negative association, and 1 a positive association; for PFOA and WC, 5 studies reported no associations, and 2 a negative associations; for PFOA and FM/BFP, 7 studies reported no associations, 2 a negative; for PFOS and BMI 11 reported no associations, 7 a negative, and 1 a; for PFOS and WC, 11 reported no associations, 1 a negative, and 1 a positive; for PFOS and FM/BFP, 6 no associations, and 4 negative; for PFHxS and BMI, 9 no associations, 4 negative, 2 positive; for PFHxS and WC, just 4 no associations; for PFHxS and FM/BFP, 4 no associations and 3 negative; for PFNA and BMI, 10 no associations, and 5 negative; for PFNA and WC, 2 no associations, and 1 negative; for PFNA and FM/BFP, 4 no associations, and 3 negative; for PFDA and BMI, 7 no associations, and 3 negative; for PFDA and FM/BFP, 2 negative, and 1 positive.
Each study was evaluated for the quality of reporting, considering the guidelines of the STROBE-ME [46]. Most studies were judged of high quality: information needed were clearly reported, including selection criteria, possible follow-up, sample-size, collection of biological samples, methods and tools implemented for data collection and outcome measurements, demographic and clinical characteristics of subjects. Furthermore, most studies took into consideration several potential confounding factors in the statistical analysis. No particular bias was noted in the selection of study individuals. Even if it was not clearly stated in most studies, it is reasonable to assume that analytical measurements were blinded, in order to counter this possible bias. Considering the score of reporting, all the articles had score equal to or higher than 22/28, with a mean of 27.01 (Table S03), thus all of them were judged of high quality.
Meta-analyses
After collecting the estimates from the studies included in this systematic review, considering the requirement of having suitable estimates from at least 3 studies, we calculated the pooled estimates for the overall association between prenatal concentrations of PFOA, PFOS, PFHxS, PFNA, PFDA, PFUA, PFDoA, PFBS and BMI; between prenatal concentrations of PFOA, PFOS, PFHxS, PFNA and risk of overweight; and between prenatal concentrations of PFOA, PFOS, PFHxS, PFNA, PFDA, PFUA and WC. Furthermore, suitable data were available for calculating pooled estimates of the association between childhood concentrations of PFOA, PFOS, PFHxS, PFNA and childhood BMI; and between childhood concentrations of PFOA, PFOS and risk of overweight. For all of the above, we were able to pool the estimates for both sexes combined, for boys exclusively, and for girls exclusively, with the exception of childhood PFNA and childhood BMI, and for all the data related to risk of overweight, for which only the effect of both sexes combined were calculated. When more studies describing the same cohort were reported, only one was included in the meta-analysis, in particular: for the HOME study, data from Liu et al. [81] were chosen for completeness of information, while associations with overweight risks were taken from Braun et al. [84]; for the Danish National Birth Cohort, the paper from Andersen et al. (2010) [89] was considered for the data of both sexes combined while the sex-specific data were taken from Andersen et al., (2013) [82]; data from the HELIX project considering childhood exposure, were taken from Vrijheid et al., 2020 [64] and not from Papadopoulou et al., 2021 [65] as the population was greater in the first. Moreover, authors of Gyllenhammar et al. and Vrijheid et al. were contacted to obtain suitable numeric data for inclusion [64, 75]. Overall, all the information about the data included in the meta-analyses from each study included in the systematic review is reported in the last column of Table 1 and Table 2.
Altogether, out of the 32 studies included in this systematic review evaluating prenatal exposure to PFASs, 23 could be included for the meta-analyses, in particular: data from 17, 12, and 13 studies were suitable for the meta-analysis evaluating the associations between prenatal PFOA and BMI for both sexes combined, boys, and girls, respectively; similarly, 17, 12, and 13 studies were included for PFOS; 11, 9, and 10 studies for PFHxS; 11, 9, and 10 studies for PFNA; 6, 6, and 6 studies for PFDA; 5, 5, and 5 studies for PFUA; 3, 3, and 3 studies for PFDoA; 3, 3, and 3 studies for PFBS. Considering the association between prenatal exposure to PFASs and risk of overweight, data from 8 studies were included for the meta-analysis for PFOA for both sexes combined, 8 studies for PFOS, 6 studies for PFHxS, 6 studies for PFNA. Considering the association between prenatal exposure to PFASs and childhood WC, data from 8, 8, and 9 were included for the meta-analyses for PFOA for both sexes combined, boys, and girls, respectively; 8, 8, and 9 for PFOS; 8, 7, and 8 for PFHxS; 8, 7, and 8 for PFNA; 4, 4, and 4 for PFDA; 3, 3, and 3 for PFUA (Table S04).
Out of the 23 studies included in this systematic review evaluating childhood exposure to PFASs, 11 were included in the meta-analyses, in particular: considering the association with childhood BMI, data from 8, 3, and 4 studies were included for the meta-analyses for PFOA for both sexes combined, boys, and girls, respectively; 8, 3, and 3 for PFOS; 6, 3, and 3 for PFHxS; and 5 for PFNA (the latter only both sexes combined, as not enough suitable data for the analyses stratified by sexes). Finally, considering the association between childhood exposure to PFASs and risk of overweight, data from 4 studies were suitable for PFOA and 3 for PFOS, in both cases only for both sexes combined (Table S04).
Overall, considering all the 30 studies included in the meta-analyses, the effect estimates were calculated with different log-transformations of PFASs: in particular, 8 were reported as unit (non log-transformed), 12 as natural logarithm, 5 as base-10 logarithm and 5 as base-2 logarithm. Moreover, one study also natural log-transformed the outcome variable and reported the result as percentage change in the outcome. Among all the 30 studies, 19 calculated the estimates out of continuous data, 6 reported mean changes for interquartile range increase, 2 reported differences between terciles, 1 reported differences between quartiles, 1 reported mean differences for each standard deviation increase, and 1 reported percentage change in the outcome. Among the 25 studies considering BMI, 20 calculated the beta estimate from BMI z-scores or BMI SDS, while 5 from BMI as kg/m2. Among the 10 studies considering WC, 5 calculated the beta estimate from WC z-scores or WC SDS, while 5 from WC as cm. Among the 10 studies considering the risk of overweight, 5 reported the OR, 4 the RR, and 1 reported a linear association with a categorical variable “overweight—yes” (so was not modified considering steps a) nor b) reported in the “2.3.3” section). Thus, the data conversions described in the section “2.3.3” were useful to properly include all these studies in the meta-analyses ensuring that the effects estimates were comparable each other (Table S05).
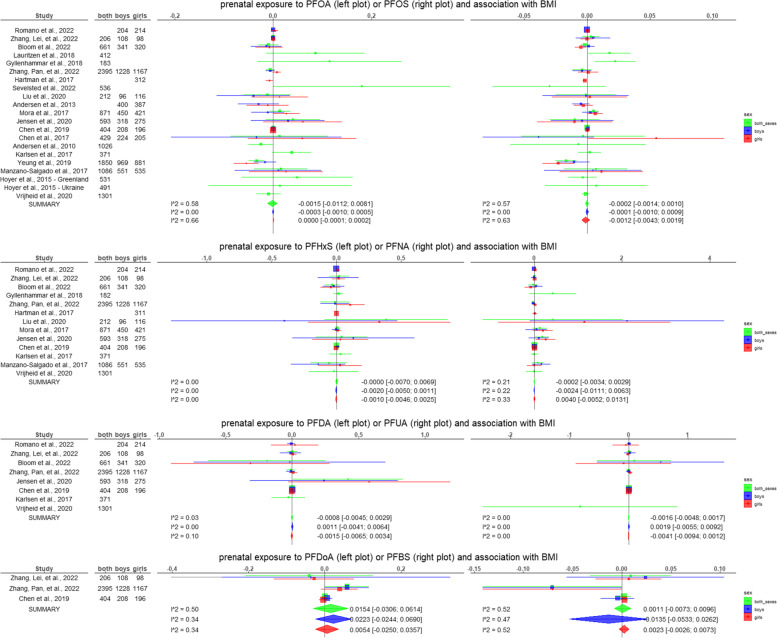
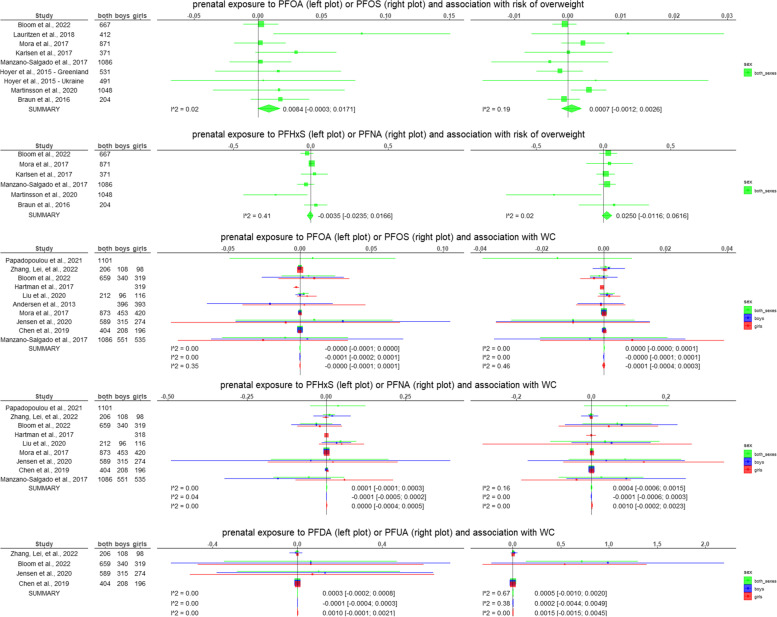
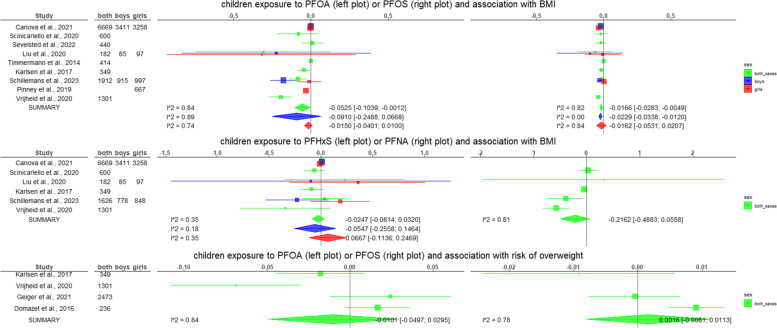
The results of all the meta-analyses performed are summarised in Table 3 and graphical representations are given in Fig. 2. The forest plots and funnel plots for each meta-analysis are reported in the supplementary material (Supplementary Figures).
Table 3.
Results of the meta-analyses performed on a subset of the studies included in the systematic review. For each combination of exposure and outcome, and for each sex category (boys and girls combined, only boys, and only girls) a meta-analysis was performed only if suitable information was reported from at least 3 studies. In this table, the pooled estimated of the random effects models (with the 95% confidence interval) from the generic invariance method, the I2 of the heterogeneity test, the p-value of the Egger’s test, and the pooled estimated of the random effects models (with the 95% confidence interval) by weighting per sample size are reported. The complete forest and funnel plots are reported in the supplementary figures
| Conditions assessed | Studies included | Random effects model [95% CI] Generic invariance method |
Heterogeneity (I2) | Egger’s test p-value | Random effects model [95% CI] Weighted per sample size |
|---|---|---|---|---|---|
| Prenatal PFOA and BMI | |||||
| Both sexes | [64, 69, 70, 74–76, 79, 81, 83, 86–92, 94] | -0.0015 [-0.0112; 0.0081] | 0.58 | 0.743 | 0.0099 [-0.0031; 0.0228] |
| Boys | [68–70, 76, 81–83, 86–88, 91, 92] | -0.0003 [-0.0010; 0.0005] | 0.00 | 0.027 | -0.0081 [-0.0229; 0.0068] |
| Girls | [68–70, 76, 78, 81–83, 86–88, 91, 92] | 0.0000 [-0.0001; 0.0002] | 0.66 | 0.814 | 0.0015 [-0.0104; 0.0134] |
| Prenatal PFOS and BMI | |||||
| Both sexes | [64, 69, 70, 74–76, 79, 81, 83, 86–92, 94] | -0.0002 [-0.0014; 0.0010] | 0.57 | 0.973 | -0.0035 [-0.0096; 0.0025] |
| Boys | [68–70, 76, 81–83, 86–88, 91, 92] | -0.0001 [-0.0010; 0.0009] | 0.00 | 0.299 | -0.0044 [-0.0103; 0.0014] |
| Girls | [68–70, 76, 78, 81–83, 86–88, 91, 92] | -0.0012 [-0.0043; 0.0019] | 0.63 | 0.588 | -0.0009 [-0.0064; 0.0045] |
| Prenatal PFHxS and BMI | |||||
| Both sexes | [64, 69, 70, 75, 76, 81, 83, 86, 87, 90, 92] | 0.0000 [-0.007; 0.0069] | 0.00 | 0.389 | -0.0008 [-0.0524; 0.0509] |
| Boys | [68–70, 76, 81, 83, 86, 87, 92] | -0.0020 [-0.0050; 0.0011] | 0.00 | 0.601 | -0.0268 [-0.0983; 0.0447] |
| Girls | [68–70, 76, 78, 81, 83, 86, 87, 92] | -0.0010 [-0.0046; 0.0025] | 0.00 | 0.080 | 0.0486 [-0.0013; 0.0986] |
| Prenatal PFNA and BMI | |||||
| Both sexes | [64, 69, 70, 75, 76, 81, 83, 86, 87, 90, 92] | -0.0002 [-0.0034; 0.0029] | 0.21 | 0.112 | 0.0481 [-0.013; 0.1091] |
| Boys | [68–70, 76, 81, 83, 86, 87, 92] | -0.0024 [-0.0111; 0.0063] | 0.22 | 0.232 | 0.0931 [0.0108; 0.1753] |
| Girls | [68–70, 76, 78, 81, 83, 86, 87, 92] | 0.0040 [-0.0052; 0.0131] | 0.33 | 0.040 | 0.0800 [0.0036; 0.1564] |
| Prenatal PFDA and BMI | |||||
| Both sexes | [69, 70, 76, 86, 87, 90] | -0.0008 [-0.0045; 0.0029] | 0.03 | 0.855 | 0.0201 [-0.0644; 0.1046] |
| Boys | [68–70, 76, 86, 87] | 0.0011 [-0.0041; 0.0064] | 0.00 | 0.753 | 0.0235 [-0.1035; 0.1504] |
| Girls | [68–70, 76, 86, 87] | -0.0015 [-0.0065; 0.0034] | 0.1 | 0.362 | 0.0329 [-0.0815; 0.1473] |
| Prenatal PFUA and BMI | |||||
| Both sexes | [64, 69, 70, 76, 87] | -0.0016 [-0.0048; 0.0017] | 0.00 | 0.618 | -0.2062 [-0.6457; 0.2333] |
| Boys | [68–70, 76, 87] | 0.0019 [-0.0055; 0.0092] | 0.00 | 0.343 | 0.0860 [-0.0910; 0.2630] |
| Girls | [68–70, 76, 87] | -0.0041 [-0.0094; 0.0012] | 0.00 | 0.994 | -0.0082 [-0.1418; 0.1254] |
| Prenatal PFDoA and BMI | |||||
| Both sexes | [69, 76, 87] | 0.0154 [-0.0306; 0.0614] | 0.50 | 0.680 | 0.0433 [-0.0221; 0.1088] |
| Boys | [69, 76, 87] | 0.0223 [-0.0244; 0.0690] | 0.34 | 0.677 | 0.0442 [-0.0187; 0.1070] |
| Girls | [69, 76, 87] | 0.0054 [-0.0250; 0.0357] | 0.34 | 0.732 | 0.0284 [-0.0188; 0.0757] |
| Prenatal PFBS and BMI | |||||
| Both sexes | [69, 76, 87] | 0.0011 [-0.0073; 0.0096] | 0.52 | 0.576 | -0.0555 [-0.1196; 0.0086] |
| Boys | [69, 76, 87] | -0.0135 [-0.0533; 0.0262] | 0.47 | 0.757 | -0.0552 [-0.1256; 0.0153] |
| Girls | [69, 76, 87] | 0.0023 [-0.0026; 0.0073] | 0.52 | 0.561 | -0.0557 [-0.1178; 0.0065] |
| Prenatal PFOA and risk of overweight | |||||
| Both sexes | [70, 71, 74, 83, 84, 90, 92, 94] | 0.0084 [-0.0003; 0.0171] | 0.02 | 0.063 | 0.0142 [-0.0006; 0.0289] |
| Prenatal PFOS and risk of overweight | |||||
| Both sexes | [70, 71, 74, 83, 84, 90, 92, 94] | 0.0007 [-0.0012; 0.0026] | 0.19 | 0.504 | 0.0016 [-0.0018; 0.0050] |
| Prenatal PFHxS and risk of overweight | |||||
| Both sexes | [70, 71, 83, 84, 90, 92] | -0.0035 [-0.0235; 0.0166] | 0.41 | 0.312 | -0.0627 [-0.1201; -0.0052] |
| Prenatal PFNA and risk of overweight | |||||
| Both sexes | [70, 71, 83, 84, 90, 92] | 0.0250 [-0.0116; 0.0616] | 0.02 | 0.338 | -0.0633 [-0.1611; 0.0344] |
| Prenatal PFOA and WC | |||||
| Both sexes | [65, 69, 70, 81, 83, 86, 87, 92] | 0.0000 [-0.0001; 0.0000] | 0.00 | 0.168 | 0.0017 [-0.0152; 0.0186] |
| Boys | [69, 70, 81–83, 86, 87, 92] | -0.0001 [-0.0002; 0.0001] | 0.00 | 0.467 | 0.0019 [-0.0177; 0.0214] |
| Girls | [69, 70, 78, 81–83, 86, 87, 92] | 0.0000 [-0.0001; 0.0001] | 0.35 | 0.884 | -0.0046 [-0.0207; 0.0114] |
| Prenatal PFOS and WC | |||||
| Both sexes | [65, 69, 70, 81, 83, 86, 87, 92] | 0.0000 [0.0000; 0.0001] | 0.00 | 0.405 | -0.0045 [-0.0117; 0.0027] |
| Boys | [69, 70, 81–83, 86, 87, 92] | 0.0000 [-0.0001; 0.0001] | 0.00 | 0.987 | -0.0024 [-0.0101; 0.0054] |
| Girls | [69, 70, 78, 81–83, 86, 87, 92] | -0.0001 [-0.0004; 0.0003] | 0.46 | 0.500 | 0.0003 [-0.0063; 0.0070] |
| Prenatal PFHxS and WC | |||||
| Both sexes | [65, 69, 70, 81, 83, 86, 87, 92] | 0.0001 [-0.0001; 0.0003] | 0.00 | 0.589 | -0.0050 [-0.0426; 0.0326] |
| Boys | [69, 70, 81, 83, 86, 87, 92] | -0.0001 [-0.0005; 0.0002] | 0.04 | 0.661 | -0.0516 [-0.1332; 0.0300] |
| Girls | [69, 70, 78, 81, 83, 86, 87, 92] | 0.0000 [-0.0004; 0.0005] | 0.00 | 0.620 | 0.0151 [-0.0300; 0.0601] |
| Prenatal PFNA and WC | |||||
| Both sexes | [65, 69, 70, 81, 83, 86, 87, 92] | 0.0004 [-0.0006; 0.0015] | 0.16 | 0.019 | 0.0467 [0.0063; 0.0872] |
| Boys | [69, 70, 81, 83, 86, 87, 92] | -0.0001 [-0.0006; 0.0003] | 0.00 | 0.042 | 0.0465 [-0.0123; 0.1053] |
| Girls | [69, 70, 78, 81, 83, 86, 87, 92] | 0.0010 [-0.0002; 0.0023] | 0.00 | 0.592 | 0.0137 [-0.0353; 0.0628] |
| Prenatal PFDA and WC | |||||
| Both sexes | [69, 70, 86, 87] | 0.0003 [-0.0002; 0.0008] | 0.00 | 0.607 | 0.0538 [-0.1353; 0.2429] |
| Boys | [69, 70, 86, 87] | -0.0001 [-0.0004; 0.0003] | 0.00 | 0.833 | 0.0632 [-0.2203; 0.3468] |
| Girls | [69, 70, 86, 87] | 0.0010 [-0.0001; 0.0021] | 0.00 | 0.728 | 0.0444 [-0.2168; 0.3056] |
| Prenatal PFUA and WC | |||||
| Both sexes | [69, 70, 87] | 0.0005 [-0.0010; 0.0020] | 0.67 | 0.345 | 0.3729 [0.0703; 0.6755] |
| Boys | [69, 70, 87] | 0.0002 [-0.0044; 0.0049] | 0.38 | 0.195 | 0.5132 [-0.1139; 1.1404] |
| Girls | [69, 70, 87] | 0.0015 [-0.0015; 0.0045] | 0.00 | 0.242 | 0.2805 [-0.1624; 0.7234] |
| Childhood PFOA and BMI | |||||
| Both sexes | [64, 79, 81, 90, 98, 104, 105, 111] | -0.0525 [-0.1039; -0.0012] | 0.84 | 0.023 | -0.0445 [-0.0604; -0.0286] |
| Boys | [81, 98, 111] | -0.0910 [-0.2488; 0.0668] | 0.89 | 0.394 | -0.0410 [-0.2285; 0.1464] |
| Girls | [81, 98, 111, 112] | -0.0150 [-0.0401; 0.0100] | 0.74 | 0.256 | -0.0134 [-0.0456; 0.0187] |
| Childhood PFOS and BMI | |||||
| Both sexes | [64, 79, 81, 90, 98, 104, 105, 111] | -0.0166 [-0.0283; -0.0049] | 0.82 | 0.011 | -0.0226 [-0.0412; -0.0039] |
| Boys | [81, 98, 111] | -0.0229 [-0.0338; -0.0120] | 0.00 | 0.359 | -0.0235 [-0.0354; -0.0116] |
| Girls | [81, 98, 111] | -0.0162 [-0.0531; 0.0207] | 0.84 | 0.972 | -0.0278 [-0.0719; 0.0164] |
| Childhood PFHxS and BMI | |||||
| Both sexes | [64, 81, 90, 98, 104, 111] | -0.0247 [-0.0814; 0.0320] | 0.35 | 0.260 | -0.0334 [-0.1157; 0.0489] |
| Boys | [81, 98, 111] | -0.0547 [-0.2558; 0.1464] | 0.18 | 0.444 | -0.0331 [-0.1620; 0.0957] |
| Girls | [81, 98, 111] | 0.0667 [-0.1136; 0.2469] | 0.35 | 0.079 | 0.0399 [-0.1249; 0.2047] |
| Childhood PFNA and BMI | |||||
| Both sexes | [64, 81, 90, 104, 111] | -0.2162 [-0.4883; 0.0558] | 0.81 | 0.383 | -0.3279 [-0.6121; -0.0438] |
| Childhood PFOA and risk of overweight | |||||
| Both sexes | [64, 90, 96, 108] | -0.0101 [-0.0497; 0.0295] | 0.84 | 0.413 | -0.0074 [-0.0554; 0.0407] |
| Childhood PFOS and risk of overweight | |||||
| Both sexes | [90, 96, 108] | 0.0016 [-0.0081; 0.0113] | 0.78 | 0.231 | -0.0007 [-0.0137; 0.0123] |
Fig. 2.
Superimposed forest plots of the meta-analyses performed with the inverse variance method considering separately both sexes, boys, and girls. The beta estimates represent the association between the increase of 1 z-score (of BMI or WC) per 1 ng/mL increase in the PFAS. For risk of overweight, the estimates represent transformed standardized mean differences (see section " 2.3.3 Data conversion of the effect estimates"). The squares are proportional to the weight assigned to each study in the random effects models, while horizontal lines represent the 95% confidence intervals reported. The diamonds at the bottom represent the pooled estimated of the random effects models (with the 95% confidence interval). The I2 is also reported as a measure of heterogeneity
Overall, when considering prenatal exposure to PFASs and BMI, risk of overweight, and WC, the results from the meta-analyses performed with the inverse variance method were all non-significant, while with the analyses weighted per sample size, a positive association was observed for prenatal PFNA and BMI in boys (0.0931; 95% CI: 0.0108, 0.1753) and in girls (0.08; 95% CI: 0.0036, 0.1564), for prenatal PFNA and WC in both sexes combined (0.0467; 95% CI: 0.0063, 0.0872), and for prenatal PFUA and WC in both sexes combined (0.3729; 95% CI: 0.0703, 0.6755) (Table 3), even though the latter was not confirmed in any of the sensitivity analyses performed by removing one study per time (Table S06). Conversely, a negative association was found between PFHxS and the risk of overweight (-0.0627; 95% CI: -0.1201, -0.0052) (Table 3). Heterogeneity across studies was high for some meta-analyses, in particular for prenatal PFOA, PFOS and BMI in both sexes combined (I2 = 0.58, 0.57) and in girls (I2 = 0.66, 0.63) (Table 3). In the sensitivity analyses performed by removing one study per time, some more significant positive associations were noted in the meta-analyses weighted per sample sizes for prenatal PFOA and BMI (both sexes combined), and for prenatal PFOA and risk of overweight (both sexes combined), the latter also for analyses performed with the generic invariance method. In a single analysis removing a study, a negative association between prenatal PFOS and BMI was found in boys and, similarly, a positive association between prenatal PFNA and BMI in both sexes combined was found (Table S06).
Considering the childhood exposure to PFASs and measurements of BMI, significant negative associations were found both in the meta-analyses performed with the inverse variance method and in those weighted per sample size for childhood PFOA and BMI both sexes (-0.0525, 95% CI: -0.1039, -0.0012 and -0.0445; 95% CI: -0.0604, -0.0286), PFOS and BMI both sexes (-0.0166; 95% CI: -0.0283, -0.0049 and -0.0226; 95% CI: -0.0412, -0.0039) and only boys (-0.0229; 95% CI: -0.0338, -0.0120 and -0.0235; 95% CI:-0.0354, -0.0116); a significant associations was found for childhood PFNA and BMI both sexes in the analyses weighted per samples sizes (-0.3279; 95% CI: -0.6121, -0.0438) (Table 3). The heterogeneity, though, was high in most of those cases (I2 = 0.84 for childhood PFOA and BMI both sexes, 0.82 for PFOS and BMI both sexes, 0.81 for PFNA and BMI both sexes), and the Egger’s test p-value evaluating was below 0.05 for PFOA and PFOS (0.023 and 0.011, respectively) (Table 3). Sensitivity analyses confirmed the negative associations in particularly for PFOS and found other negative associations also in sex stratified analyses considering PFOS and PFOA. Interestingly, in an inverse variance method sensitivity analysis for childhood PFHxS and BMI in boys, excluding one study, a significant positive association was found (Table S06).
In the meta-analyses performed stratifying by age (Table 4), a significant positive association was found for prenatal PFOA and BMI measured in children of both sexes who were more than 3 years, but only in analyses weighted per sample size (0.0275; 95% CI: 0.0059, 0.0491), although the Egger’s test p-value was 0.047. Conversely, an inverse association between prenatal PFOS and BMI measured in children of both sexes who were 3 or less years was found only in the invariance method analyses (-0.0101; 95% CI: -0.0189, -0.0013). Significant positive associations were also found also, only with the analyses weighted per sample size, between prenatal PFNA and boys who were more than 3 years (0.1851; 95% CI: 0.028, 0.3421). Significant negative associations were also found in the analyses weighted per sample size between prenatal PFHxS and risk of overweight in children who were more than 3 years and positive associations were found between prenatal PFUA and WC; significant negative associations, confirmed both with inverse variance and weighted per samples size analyses were also found between childhood PFOA and BMI in children of both sexes who were more than 3 years, and between childhood PFOS and BMI in children who were more than 3 years in both sexes and in boys only; finally, a negative association, exclusively in analyses weighted per sample size, was found for childhood PFNA and BMI in children of both sexes who were more than 3 years: all of these results were the same of the non-age-stratified analyses as all the included studies considered children who were more than 3 years.
Table 4.
Results of the meta-analyses performed on a subset of the studies included in the systematic review stratifying separately studies that measured the outcomes in children who were 3 or less years from studies that considered children who were more than 3 years. For each combination of exposure and outcome, and for each sex category (boys and girls combined, only boys, and only girls) a meta-analysis was performed only if suitable information was reported from at least 3 studies. In this table, the pooled estimated of the random effects models (with the 95% confidence interval) from the generic invariance method, the I2 of the heterogeneity test, the p-value of the Egger’s test, and the pooled estimated of the random effects models (with the 95% confidence interval) by weighting per sample size are reported. The complete forest and funnel plots are reported in the supplementary figures
| Conditions assessed | Studies included | Random effects model [95% CI] Generic invariance method |
Heterogeneity (I2) | Egger’s test p-value | Random effects model [95% CI] Weighted per sample size |
|---|---|---|---|---|---|
|
Prenatal PFOA and BMI in both sexes 0–3 years |
[76, 86, 89, 91] | -0.0140 [-0.0351; 0.0070] | 0.77 | 0.910 | -0.0133 [-0.0343; 0.0078] |
| 4–18 years | [64, 69, 70, 74, 75, 79, 81, 83, 87, 88, 90, 92, 94] | 0.0000 [-0.0008; 0.0009] | 0.24 | 0.047 | 0.0275 [0.0059; 0.0491] |
|
Prenatal PFOA and BMI in boys 0–3 years |
[68, 76, 86, 91] | -0.0022 [-0.0070; 0.0026] | 0.19 | 0.533 | -0.0051 [-0.0182; 0.0079] |
| 4–18 years | [69, 70, 81–83, 87, 88, 92] | 0.0000 [-0.0009; 0.0008] | 0.00 | 0.039 | -0.0115 [-0.0398; 0.0168] |
|
Prenatal PFOA and BMI in girls 0–3 years |
[68, 76, 86, 91] | -0.0036 [-0.0413; 0.0341] | 0.87 | 0.673 | -0.0087 [-0.0313; 0.0139] |
| 4–18 years | [69, 70, 78, 81–83, 87, 88, 92] | 0.0000 [-0.0001; 0.0002] | 0.23 | 0.672 | 0.0115 [-0.0084; 0.0313] |
|
Prenatal PFOS and BMI in both sexes 0–3 years |
[76, 86, 89, 91] | -0.0101 [-0.0189; -0.0013] | 0.38 | 0.965 | -0.0092 [-0.0210; 0.0026] |
| 4–18 years | [64, 69, 70, 74, 75, 79, 81, 83, 87, 88, 90, 92, 94] | 0.0002 [-0.0009; 0.0014] | 0.42 | 0.251 | 0.0008 [-0.0056; 0.0072] |
|
Prenatal PFOS and BMI in boys 0–3 years |
[68, 76, 86, 91] | -0.0024 [-0.0071; 0.0024] | 0.21 | 0.123 | -0.0060 [-0.0128; 0.0007] |
| 4–18 years | [69, 70, 81–83, 87, 88, 92] | 0.0002 [-0.0029; 0.0033] | 0.00 | 0.538 | -0.0026 [-0.0128; 0.0077] |
|
Prenatal PFOS and BMI in girls 0–3 years |
[68, 76, 86, 91] | -0.0065 [-0.0186; 0.0056] | 0.78 | 0.356 | -0.0086 [-0.0198; 0.0026] |
| 4–18 years | [69, 70, 78, 81–83, 87, 88, 92] | -0.0006 [-0.0033; 0.0021] | 0.56 | 0.883 | 0.0065 [-0.0019; 0.0150] |
|
Prenatal PFHxS and BMI in both sexes 4–18 years |
[64, 69, 70, 75, 81, 83, 87, 90, 92] | 0.0000 [-0.0071; 0.0071] | 0.00 | 0.435 | -0.0009 [-0.0600; 0.0582] |
|
Prenatal PFHxS and BMI in boys 0–3 years |
[68, 76, 86] | -0.0023 [-0.0054; 0.0009] | 0.00 | 0.659 | 0.0159 [-0.0998; 0.1317] |
| 4–18 years | [69, 70, 81, 83, 87, 92] | 0.0040 [-0.0108; 0.0188] | 0.00 | 0.051 | -0.0694 [-0.1534; 0.0146] |
|
Prenatal PFHxS and BMI in girls 0–3 years |
[68, 76, 86] | 0.0343 [-0.0360; 0.1046] | 0.33 | 0.463 | 0.0806 [-0.0197; 0.181] |
| 4–18 years | [69, 70, 78, 81, 83, 87, 92] | -0.0014 [-0.0050; 0.0021] | 0.00 | 0.387 | 0.0221 [-0.0348; 0.0790] |
|
Prenatal PFNA and BMI in both sexes 4–18 years |
[64, 69, 70, 75, 81, 83, 87, 90, 92] | -0.0001 [-0.0033; 0.0030] | 0.00 | 0.072 | 0.0656 [-0.0252; 0.1564] |
|
Prenatal PFNA and BMI in boys 0–3 years |
[68, 76, 86] | -0.0083 [-0.0183; 0.0016] | 0.00 | 0.831 | 0.0009 [-0.0495; 0.0513] |
| 4–18 years | [69, 70, 81, 83, 87, 92] | 0.0022 [-0.0052; 0.0097] | 0.17 | 0.120 | 0.1851 [0.0280; 0.3421] |
|
Prenatal PFNA and BMI in girls 0–3 years |
[68, 76, 86] | 0.0216 [-0.0031; 0.0463] | 0.52 | 0.119 | 0.0588 [-0.0023; 0.1198] |
| 4–18 years | [69, 70, 78, 81, 83, 87, 92] | -0.0002 [-0.0027; 0.0023] | 0.05 | 0.290 | 0.0976 [-0.0355; 0.2307] |
|
Prenatal PFDA and BMI in both sexes 4–18 years |
[69, 70, 87, 90] | -0.0007 [-0.003; 0.0015] | 0.00 | 0.001 | -0.0816 [-0.2605; 0.0973] |
|
Prenatal PFDA and BMI in boys 0–3 years |
[68, 76, 86] | -0.0069 [-0.0286; 0.0147] | 0.00 | 0.196 | 0.0365 [-0.0673; 0.1402] |
| 4–18 years | [69, 70, 87] | 0.0016 [-0.0038; 0.0070] | 0.00 | 0.410 | -0.0111 [-0.3852; 0.3630] |
|
Prenatal PFDA and BMI in girls 0–3 years |
[68, 76, 86] | 0.0111 [-0.0205; 0.0427] | 0.41 | 0.407 | 0.1055 [-0.0437; 0.2546] |
| 4–18 years | [69, 70, 87] | -0.0017 [-0.0077; 0.0043] | 0.00 | 0.829 | -0.1628 [-0.4729; 0.1474] |
|
Prenatal PFUA and BMI in both sexes 4–18 years |
[64, 69, 70, 87] | -0.0015 [-0.0048; 0.0017] | 0.00 | 0.749 | -0.3931 [-1.2412; 0.4549] |
|
Prenatal PFUA and BMI in boys 4–18 years |
[69, 70, 87] | 0.0021 [-0.0055; 0.0098] | 0.00 | 0.263 | 0.2827 [-0.2755; 0.8409] |
|
Prenatal PFUA and BMI in girls 4–18 years |
[69, 70, 87] | -0.0045 [-0.0098; 0.0008] | 0.00 | 0.325 | -0.0501 [-0.4704; 0.3701] |
|
Prenatal PFOA and risk of overweight 4–18 years |
[70, 71, 74, 83, 84, 90, 92, 94] | 0.0084 [-0.0003; 0.0171] | 0.02 | 0.063 | 0.0142 [-0.0006; 0.0289] |
|
Prenatal PFOS and risk of overweight 4–18 years |
[70, 71, 74, 83, 84, 90, 92, 94] | 0.0007 [-0.0012; 0.0026] | 0.19 | 0.504 | 0.0016 [-0.0018; 0.0050] |
|
Prenatal PFHxS and risk of overweight 4–18 years |
[70, 71, 83, 84, 90, 92] | -0.0035 [-0.0235; 0.0166] | 0.41 | 0.312 | -0.0627 [-0.1201; -0.0052] |
|
Prenatal PFNA and risk of overweight 4–18 years |
[70, 71, 83, 84, 90, 92] | 0.025 [-0.0116; 0.0616] | 0.02 | 0.338 | -0.0633 [-0.1611; 0.0344] |
|
Prenatal PFOA and WC in both sexes 4–18 years |
[65, 69, 70, 81, 83, 87, 92] | 0.0000 [-0.0001; 0.0000] | 0.00 | 0.232 | 0.0007 [-0.0171; 0.0184] |
|
Prenatal PFOA and WC in boys 4–18 years |
[69, 70, 81–83, 87, 92] | -0.0001 [-0.0002; 0.0001] | 0.00 | 0.199 | -0.0023 [-0.0218; 0.0173] |
|
Prenatal PFOA and WC in girls 4–18 years |
[69, 70, 78, 81–83, 87, 92] | 0.0000 [-0.0001; 0.0001] | 0.43 | 0.939 | -0.0040 [-0.0194; 0.0114] |
|
Prenatal PFOS and WC in both sexes 4–18 years |
[65, 69, 70, 81, 83, 87, 92] | 0.0000 [0.0000; 0.0001] | 0.00 | 0.675 | -0.0038 [-0.0115; 0.0039] |
|
Prenatal PFOS and WC in boys 4–18 years |
[69, 70, 81–83, 87, 92] | 0.0000 [-0.0001; 0.0001] | 0.00 | 0.492 | -0.0012 [-0.0093; 0.0068] |
|
Prenatal PFOS and WC in girls 4–18 years |
[69, 70, 78, 81–83, 87, 92] | -0.0001 [-0.0004; 0.0003] | 0.51 | 0.631 | 0.0015 [-0.0053; 0.0083] |
|
Prenatal PFHxS and WC in both sexes 4–18 years |
[65, 69, 70, 81, 83, 87, 92] | 0.0003 [-0.0014; 0.0020] | 0.13 | 0.621 | -0.0069 [-0.0420; 0.0281] |
|
Prenatal PFHxS and WC in boys 4–18 years |
[69, 70, 81, 83, 87, 92] | -0.0001 [-0.0041; 0.0039] | 0.19 | 0.726 | -0.0518 [-0.1069; 0.0032] |
|
Prenatal PFHxS and WC in girls 4–18 years |
[69, 70, 78, 81, 83, 87, 92] | 0.0000 [-0.0004; 0.0005] | 0.06 | 0.675 | 0.0144 [-0.0285; 0.0572] |
|
Prenatal PFNA and WC in both sexes 4–18 years |
[65, 69, 70, 81, 83, 87, 92] | 0.0004 [-0.0006; 0.0015] | 0.14 | 0.051 | 0.0411 [-0.0002; 0.0824] |
|
Prenatal PFNA and WC in boys 4–18 years |
[69, 70, 81, 83, 87, 92] | -0.0001 [-0.0006; 0.0003] | 0.00 | 0.070 | 0.0477 [-0.0105; 0.1059] |
|
Prenatal PFNA and WC in girls 4–18 years |
[69, 70, 78, 81, 83, 87, 92] | 0.0010 [-0.0002; 0.0023] | 0.00 | 0.779 | -0.0035 [-0.0509; 0.0438] |
|
Prenatal PFDA and WC in both sexes 4–18 years |
[69, 70, 87] | 0.0003 [-0.0002; 0.0008] | 0.00 | 0.956 | 0.0324 [-0.1791; 0.2438] |
|
Prenatal PFDA and WC in boys 4–18 years |
[69, 70, 87] | -0.0001 [-0.0004; 0.0003] | 0.00 | 0.738 | 0.0312 [-0.3097; 0.3721] |
|
Prenatal PFDA and WC in girls 4–18 years |
[69, 70, 87] | 0.0010 [-0.0001; 0.0021] | 0.00 | 0.963 | 0.0329 [-0.2441; 0.3100] |
|
Prenatal PFUA and WC in both sexes 4–18 years |
[69, 70, 87] | 0.0005 [-0.0010; 0.0020] | 0.67 | 0.345 | 0.3729 [0.0703; 0.6755] |
|
Prenatal PFUA and WC in boys 4–18 years |
[69, 70, 87] | 0.0002 [-0.0044; 0.0049] | 0.38 | 0.195 | 0.5132 [-0.1139; 1.1404] |
|
Prenatal PFUA and WC in girls 4–18 years |
[69, 70, 87] | 0.0015 [-0.0015; 0.0045] | 0.00 | 0.242 | 0.2805 [-0.1624; 0.7234] |
|
Childhood PFOA and BMI in both sexes 4–18 years |
[64, 79, 81, 90, 98, 104, 105, 111] | -0.0525 [-0.1039; -0.0012] | 0.84 | 0.023 | -0.0445 [-0.0604; -0.0286] |
|
Childhood PFOA and BMI in boys 4–18 years |
[81, 98, 111] | -0.0910 [-0.2488; 0.0668] | 0.89 | 0.394 | -0.0410 [-0.2285; 0.1464] |
|
Childhood PFOA and BMI in girls 4–18 years |
[81, 98, 111, 112] | -0.0150 [-0.0401; 0.0100] | 0.74 | 0.256 | -0.0134 [-0.0456; 0.0187] |
|
Childhood PFOS and BMI in both sexes 4–18 years |
[64, 79, 81, 90, 98, 104, 105, 111] | -0.0166 [-0.0283; -0.0049] | 0.82 | 0.011 | -0.0226 [-0.0412; -0.0039] |
|
Childhood PFOS and BMI in boys 4–18 years |
[81, 98, 111] | -0.0229 [-0.0338; -0.0120] | 0.00 | 0.359 | -0.0235 [-0.0354; -0.0116] |
|
Childhood PFOS and BMI in girls 4–18 years |
[81, 98, 111] | -0.0162 [-0.0531; 0.0207] | 0.84 | 0.972 | -0.0278 [-0.0719; 0.0164] |
|
Childhood PFHxS and BMI in both sexes 4–18 years |
[64, 81, 90, 98, 104, 111] | -0.0247 [-0.0814; 0.0320] | 0.35 | 0.260 | -0.0334 [-0.1157; 0.0489] |
|
Childhood PFHxS and BMI in boys 4–18 years |
[81, 98, 111] | -0.0547 [-0.2558; 0.1464] | 0.18 | 0.444 | -0.0331 [-0.1620; 0.0957] |
|
Childhood PFHxS and BMI in girls 4–18 years |
[81, 98, 111] | 0.0667 [-0.1136; 0.2469] | 0.35 | 0.079 | 0.0399 [-0.1249; 0.2047] |
|
Childhood PFNA and BMI in both sexes 4–18 years |
[64, 81, 90, 104, 111] | -0.2162 [-0.4883; 0.0558] | 0.81 | 0.383 | -0.3279 [-0.6121; -0.0438] |
|
Childhood PFOA and risk of overweight in both sexes 4–18 years |
[64, 90, 96, 108] | -0.0101 [-0.0497; 0.0295] | 0.84 | 0.413 | -0.0074 [-0.0554; 0.0407] |
|
Childhood PFOS and risk of overweight in both sexes 4–18 years |
[90, 96, 108] | 0.0016 [-0.0081; 0.0113] | 0.78 | 0.231 | -0.0007 [-0.0137; 0.0123] |
The sensitivity analyses stratified by age performed by excluding one study per time mostly confirmed the significant associations found, with an additional negative significant association in a single inverse variance method analysis between prenatal PFOA and BMI in children of both sexes who were than 3 years (Table S07).
Discussion
We conducted a systematic review aimed to summarise the available evidence about an early-life exposure to PFASs (including prenatal exposure, including exposure through breastfeeding, and exposure in children) and the association with childhood overweight and obesity. Forty-nine different papers were included, among which 26 assessing prenatal exposure to PFASs, 17 childhood exposure, and 6 both. Overall, most associations were conflicting and there was no clear pattern of unequivocal evidence, but a certain number of studies reported of a negative association between PAFSs measured during childhood and outcomes assessed during childhood. We developed a methodology to convert the effect estimates from the different studies to make them comparable to each other, and when data were available and suitable from at least three studies, considering separately both sexes combined, only boys, and only girls, we conducted meta-analyses to pool the estimates. In particular, meta-analyses were performed with 30 studies (19 evaluating prenatal exposure, 7 evaluating childhood exposure, and 4 both). Considering differences in the two methods performed for pooling the evidence (inverse variance and weighting per sample size) and in the sensitivity analyses (excluding one sample per time), no overall strong significant association was observed between prenatal exposure to PFASs and the outcomes. Otherwise, relatively more consistent significant negative associations were found between childhood concentrations of PFASs (PFOA and PFOS in particular) and BMI in children.
Concerning the PFASs measured in the included studies, PFOA and PFOS were always quantified and PFHxS and PFNA were measured in several studies. These are legacy compounds, whose presence is very persistent and ubiquitous in the environments despite having been restricted. Moreover, in the last years, other emerging PFASs have been introduced in the market; they include molecules with a shorter carbon chain such as PFBA and PFBS [10, 113, 114]. Eleven studies quantified PFBS, one of which found positive associations between prenatal levels of PFBS and adiposity in girls [87], one found negative association between prenatal concentrations and WL and BMI [76], and one a negative association between childhood levels and overweight/obesity in children [106]; and only two studies quantified PFBA [98, 109]. Other emerging PFASs, such as the fluorotelomers and per-/polyfluoroalkyl ether acids [114], were not quantified in any studies. Furthermore, most studies did not report information about the chain branching of PFASs; indeed, both linear and branched isomers can be found in the environment, with potentially different implication for human health [115], and they can be separately quantified, as performed in Ye et al. 2018, Romano et al. 2022, and Janis et al. 2021 [68, 99, 107]. Unfortunately, there were not enough suitable data for inclusion in meta-analyses for any of these non-legacy PFASs.
Concerning the analytical assay, PFASs were quantified using liquid chromatography coupled with tandem mass spectrometry, with isotopic dilution; this can be considered the gold standard approach to quantify PFASs in biological samples [116, 117]. Moreover, some studies also reported validation data, in particular the limits of determination or quantitation (LOD/LOQ). A further support to the validity of the analytical measures would be the participation in interlaboratory quality assessment studies, such as the German External Quality Assessment Scheme (G -EQUAS) [118], which would certify a better reproducibility of the data among laboratories. Moreover, PFASs were measured in both serum and plasma; this is not regarded as a source of bias as no difference in the quantitation of PFASs was reported [119]. According to the new definition by Organisation for Economic Co-operation and Development (OECD), more than 6 million molecules in PubChem can be defined as PFASs [120, 121], thus suggesting that, although targeted biomarker investigations remain the gold standard in terms of accuracy and precision, also non-targeted and suspect screening investigations are required to have a better picture of the exposure to this vast class of compounds [122–125].
The outcomes considered for this review were measures of overweight, obesity, or adiposity. Almost all studies measured BMI in children, and some of them also WC and/or FM/BFP. A possible source of bias is represented by how the anthropometric measures were collected: they were measured by experts in most studies (34), while in few studies they were only reported by questionnaires or in other indirect ways (6), or by a hybrid way of the two (2), while the information about the collection of anthropometric measures was not clear in a few other studies (4) (Table S03). Although the measurements of height and weight performed by experts can reduce the bias of measurements, previous investigations reported that there is a good correlation between measured and self-reported measures [126, 127]. Furthermore, the evaluation of FM/BFP (19) was performed with different approaches including skinfold thickness (7), bioelectrical impedance analysis (BIA) (5), dual-energy x-ray absorptiometry (DXA) (6), air displacement plethysmography (ADP) (1). While DXA can be considered the gold standard, other approaches such as skinfold thickness, are generally considered a good proxy for measuring adiposity [44]; however, some limitations in their use have been pointed out, especially in children and adolescents of particular population [128, 129]. As previously reported, BMI can be considered a suitable surrogate measure of adiposity in children, even though it has a strong correlation with adiposity measured with DXA at higher level [130].
Although the reporting of the studies was judged of good quality, some limitations in the study design can be pointed out. Studies evaluating the prenatal exposure to PFASs were longitudinal studies (i.e., PFASs were measured during pregnancy or at birth and the outcomes were measured later in the childhood), while mostly of those assessing the exposure to PFASs in children are cross-sectional studies. Results from longitudinal analyses are more likely to suggest a possible causal relationship than cross-sectional studies. Only 5 studies quantifying PFASs in children were conducted measuring outcomes some years later [72, 79, 81, 108, 112] (all of them reported repeated measures during childhood at different ages; also, among those five studies, three of them found no significant associations, one found positive associations between PFOS at 9 years and measures of adiposity at 15 years, and one reported negative association between PFOA at 6–8 years and BMI and waist to height ratio in girls, even though decreasing with age). Finally, while several confounders were taken into account in the considered studies, the concomitant exposure to other persistent organic pollutants may also play a role [37] but it was not considered in most studies.
The meta-analyses allowed to perform a quantitative evaluation of the association between PFASs exposure and the considered outcome for 30 of the studies included in the systematic review. In order to include in the systematic review as many studies as possible, while also ensuring the data compatibility, a specific methodology was developed to convert the effect estimates from different studies (section “ 2.3.3 Data conversion of the effect estimates”). Overall, this methodology for conversion of estimates can be applied to other similar future meta-analyses. The two previous meta-analyses that evaluated the association of prenatal exposure of PFOA [36], and of PFOS or PFOA [37] and outcome of overweight or obesity in children, considered the effect estimates from the different studies regardless of whether the data used to calculate the estimates were log-transformed, without performing any conversion. Likewise, estimates obtained from continuous associations or from differences of percentiles were combined as such. Furthermore, these other two meta-analyses considered only studies that expressed BMI as z-scores, thus missing the information from those studies that reported the data as kg/m2; while this was taken into consideration in the present work thanks to the conversions carried out; similarly in the meta-analyses of Stratakis and co-workers [37] only studies reporting WC as cm were included, not considering not calculating the WC z-scores, while in the present work the estimates were converted and meta-analyses were performed on WC z-scores. Moreover, considering risk, Stratakis and co-workers considered only studies with RR. Indeed, the method used for data conversion developed for the present meta-analysis allowed the inclusion of a greater number of studies, while suitably taking into account the different ways data were reported from the included works. A possible limitation of the present methodology applied to the studies included in this systematic review, is that the conversions carried out to estimate z-scores, instead of raw measurements, gave very low standard errors of the slopes, that led to some meta-analyses where a few studies had a predominant weight. Also, for this purpose, further meta-analyses by weighting per sample-size were carried out and sensitivity analyses were performed by repeating the meta-analyses excluding one study at a time.
Comparing the results of the present work with the two previously published meta-analyses, our results can be considered in agreement with the one performed by Liu and co-workers, as they found a positive association between early life exposure to PFOA and childhood BMI z-score [36]: while we found no overall significant associations, we did find a significant association in the meta-analyses weighted per sample size considering children of both sexes who were more than 3 years (Table 4); indeed, the studies included in the meta-analyses by Liu et al., considered only children who were 5 or more years [63, 75, 79, 108, 112, 131]. On the other hand, Liu et al. found a significant positive association between PFOA and risk of overweight, while we did not. A systematic review with meta-analysis conducted by Stratakis and co-workers investigated the association between prenatal exposure to persistent organic pollutants and childhood obesity [37]. Although some differences between that and our work, among which some additional studies included in the present work, the conversion of estimates that we carried out prior the meta-analyses, and our additional analyses stratified by sex, the overall conclusion about the absence of a clear overall association between prenatal exposure to PFOA and PFOS and childhood BMI was the same. Furthermore, both this review and the one conducted by Stratakis et al. considered waist circumference as an outcome and we both found no considerable significant associations with prenatal PFOA and PFOS. Other differences between our work and the one from Stratakis and co-workers include: other persistent organic pollutants considered by Stratakis, more PFASs assessed in our work (in particular, meta-analyses also for PFHxS, PFNA, PFDA, PFUA, PFBS), further meta-analyses evaluating childhood exposure to PFASs performed in our work, and the methodology for data conversion of estimates.
There are also some limitations in performing the meta-analyses, due to differences in the designs of the studies, in particular for the various times, or range of times, the PFASs were quantified during gestation, as well as the different ages, or range of ages, the PFASs were measured in children. Likewise, the outcomes were measured at several different ages, or range of ages, among the studies. The stratified analyses performed separately with studies that considered children who were 3 or less years from those considering children who were more than 3 years allowed to mitigate this limitation. Furthermore, considering the meta-analyses performed to pool the evidence about the risk of overweight, even though we mathematically made comparable RR with OR and data normalised with different logarithms or comparing different ranges of concentrations, criteria for defining overweight varied across studies. Moreover, for some of the studies, the measures of outcomes suitable for this review were the result of a secondary analysis. Finally, since only studies published in indexed journals were considered, there is the possibility that other unpublished studies were missed, in particular studies reporting no statistically significant associations [131]. We tried to assess this source of bias with the funnel plots and the Egger’s test (Supplementary Figures). Moreover, the low number of studies available for some associations does not allow a firm conclusion with the performed meta-analysis. As an additional limitation, we did not use a defined method to assess bias such as the one used within the Navigation Guide [27], but we implemented a score to evaluate the quality of each included study and all studies were considered suitable for inclusion. Finally, having considered exclusively papers in English as inclusion criteria is an additional limitation of the present review and a possible source bias [132].
To our knowledge, this is the first systematic review conducting meta-analyses evaluating the exposure to PFASs in children and their associations with childhood overweight and obesity, although this evidence comes mostly from cross sectional studies, thus limiting the causal relationship. The developed methodology for the conversion of effect estimates before performing the meta-analyses can be implemented in future meta-analyses in environmental epidemiology to ensure a better comparability of data among studies.
In conclusion, this systematic review with meta-analyses evidenced positive associations between prenatal concentrations of PFOA and BMI in children who were more than 3 years, and between prenatal PFNA and BMI (particularly in boys who were more than 3 years). Negative associations were found between prenatal PFOS and BMI in children who were 3 or less years, and between PFHxS and risk of overweight. Positive associations were also found between prenatal PFNA and WC, and between prenatal PFUA and WC (even though the latter not confirmed in sensitivity analyses). Nevertheless, these results were not consistent across meta-analyses performed with the generic invariance method or by pooling per sample size. The evidence pooled from studies measuring PFASs during childhood was more consistent as confirmed in both generic invariance and weighting per sample size analyses, and also in most sensitivity analyses: negative associations were found between PFOA and BMI, PFOS and BMI (in particular in boys), and between PFNA and BMI (the latter only weighting by sample size), even though heterogeneity among studies was high.
Supplementary Information
Additional file 1: Supplementary text. The supplementary text contains the text strings used for the search in PubMed and Embase and all the R scripts implemented prepared for this work
Additional file 2: Supplementary figures. In the supplementary figures file, for each meta-analysis, three figures are reported: a forest plot pooling the estimates with the generic invariance method, a forest plot pooling the estimates weighting by sample size, and a funnel plot evaluating the bias for included studies
Additional file 3: Supplementary Table S01. Table of entries retrieved from PubMed
Additional file 4: Supplementary Table S02. Table of entries retrieved from Embase
Additional file 5: Supplementary Table S03. Complete database of the considered studies, including: information retrieved from PubMed and Embase; whether the study was included or not in the systematic review and the category of inclusion or rejection; information collected from included articles; and score of reporting assigned to included articles
Additional file 6: Supplementary Table S04. Data extracted from articles used for performing the meta-analyses
Additional file 7: Supplementary Table S05. Data extracted transformed using the conversion factor developed
Additional file 8: Supplementary Table S06. Results of the meta-analyses performed with the generic invariance method (“inv_var”) and weighting by sample size (“wgtd”). The I2 as a measure of heterogeneity is also reported. Both the original meta-analyses are reported together with the sensitivity meta-analyses repeated by excluding one sample per time
Additional file 9: Supplementary Table S07. Results of the meta-analyses stratified by age (1-3 years or 4-18 years). Meta-analyses performed with the generic invariance method (“inv_var”) and weighting by sample size (“wgtd”) are reported. The I2 as a measure of heterogeneity is also reported. Both the original meta-analyses are reported together with the sensitivity meta-analyses repeated by excluding one sample per time
Acknowledgements
We thank the library system staff of the University of Milan, in particular Flavia Rampichini, for the valuable help in developing the text strings used for the literature search. We also thank Dr. Laura Campo for providing an initial Excel table for the quality of reporting. Moreover, we thank Ana Leticia Antonio Vital, University of Bayreuth, for the initial suggestions for the development of our graphs reported in Figures 2, 3 and 4. Finally, we thank Prof. Dr. Emma Schymanski for the helpful comments provided for improving the manuscript.
Abbreviations
- 6:2 diPAP
6:2 Polyfluoroalkyl phosphoric acid diesters
- 8:2 diPAP
8:2 Polyfluoroalkyl phosphoric acid diesters
- 95% CI
95% Confidence interval
- ALSPAC
Avon Longitudinal Study of Parents and Children
- BCERP
Breast Cancer and Environment Research Program
- BFP
Body fat percentage
- BMI
Body mass idex
- CI
Confidence interval
- CLEAR
Climate change, environmental contaminants and reproductive health
- COPSAC-2010
Copenhagen Prospective Studies of Asthma in Childhood 2010
- DXA
Dual-Energy X-ray Absorptiometry
- ECHOFGS
The Environmental Influences on Child Health Outcomes Study
- EDCs
Endocrine-disrupting chemicals
- EYHS
European Youth Heart Study
- FLEHS
Flemish Environment and Health Studies
- FM
Fat mass
- HBC
Hamamatsu Birth Cohort for Mothers and Children
- HBM4EU
The Human Biomonitoring for Europe initiative
- HELIX
Human Early Life Exposome project
- HOME
Health Outcomes and Measures of the Environment
- INMA
INfancia y Medio Ambiente, Environment and Childhood
- INUENDO
Biopersistent organochlorines in diet and human fertility
- LL
Lower limit of the 95% confidence interval
- LOD
Limit of detection
- LOQ
Limit of quantitation
- LWBC
Laizhou Wan (Bay) Birth Cohort
- N-EtFOSAA
N-Ethylperfluorooctane sulfonamidoacetic acid
- NHANES
National Health and Nutrition Examination Survey
- NICHD
National Institute of Child Health and Human Development
- NICHD-SGA
U.S. National Institute of Child Health and Human Development Scandinavian Successive Small-for-Gestational Age births study
- N-MeFOSAA
N-Methylperfluorooctane sulfonamidoacetic acid
- n-PFOA
Linear perfluorooctanoic acid
- n-PFOS
Linear perfluorooctanesulfonic acid
- NHBCS
New Hampshire Birth Cohort Study
- OCC
Odense Child Cohort
- PFASs
Per-/polyfluoroalkyl substances
- OR
Odds ration
- PFBA
Perfluorobutanoic acid
- PFBS
Perfluorobutanesulfonic acid
- PFDA
Perfluorodecanoid acid
- PFDoDA/PFDoA
Perfluorododecanoic acid
- PFDS
Perfluorodecane sulfonic acid
- PFHpA
Perfluoroheptanoic acid
- PFHpS
Perfluoroheptanesulfonic acid
- PFHxA
Perfluorohexanoic acid
- PFHxS
Perfluorohexanesulfonic acid
- PFNA
Perfluorononanoic acid
- PFOA
Perfluorooctanoic acid
- PFOS
Perfluorooctanesulfonic acid
- PFOSA
Perfluorooctanesulfonamide
- PFPeA
Perfluoropentanoic acid
- PFTeDA
Perfluorotetradecanoic acid
- PFTrDA
Perfluorotridecanoic acid
- PFUnDA/PFUA
Perfluoroundecanoic acid
- POPUP
Persistent Organic Pollutants in Uppsala Primiparas
- REML
Restricted maximum-likelihood estimator
- RR
Risk ratio
- Sb-PFOA
Sum of branched isomers of perfluorooctanoic acid
- Sm-PFOS
Sum of perfluoromethylheptane sulfonate isomers
- STROBE-ME
Strengthening Reporting of Observational studies in Epidemiology-Molecular Epidemiology
- UL
Upper limit of the 95% confidence interval
- WA
Weight-for-age
- WC
Waist circumference
- WHO
World Health Organization
- WL
Weight-for-length
- WtHe
Waist to height ratio
- WtHi
Waist to hip ratio
Authors’ contributions
S.F. and G.F. conceived the work; C.M.F. and G.F. conducted the literature search, the evaluation, and the collection of information from studies, with S.F. acting as supervisor; G.F. curated the database, extracted the estimates from the articles, developed the methodology for conversion of the estimates, performed the meta-analyses, designed the graphs, and wrote the paper; C.M.F. contributed to the paper preparation and revision; S.F. carried out major paper curation and revision.
Funding
G.F. is currently supported by the Luxembourg National Research Fund (FNR) for project A18/BM/12341006.
Availability of data and materials
All the data are reported in the supplementary material of the present article, including all the R-scripts developed for this work.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Obesity and overweight. 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight . (Accessed 4 August 2021).
- 2.Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91:1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalle Molle R, Fatemi H, Dagher A, et al. Gene and environment interaction: Is the differential susceptibility hypothesis relevant for obesity? Neurosci Biobehav Rev. 2017;73:326–339. doi: 10.1016/j.neubiorev.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 5.Darbre PD. Endocrine disruptors and obesity. Curr Obes Rep. 2017;6:18–27. doi: 10.1007/s13679-017-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang C, Lee HK, Kong APS, et al. Early-life exposure to endocrine disrupting chemicals associates with childhood obesity. Ann Pediatr Endocrinol Metab. 2018;23:182–195. doi: 10.6065/apem.2018.23.4.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.la Merrill M, Birnbaum LS. Childhood obesity and environmental chemicals. Mt Sinai J Med. 2011;78:22–48. doi: 10.1002/msj.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Beijsterveldt IALP, van Zelst BD, de Fluiter KS, et al. Poly- and perfluoroalkyl substances (PFAS) exposure through infant feeding in early life. Environ Int. 2022;164. 10.1016/J.ENVINT.2022.107274. Epub ahead of print [DOI] [PubMed]
- 9.ECHA. Perfluoroalkyl chemicals (PFAS). 2021. https://echa.europa.eu/hot-topics/perfluoroalkyl-chemicals-pfas, (Accessed 4 August 2021)
- 10.Buck RC, Franklin J, Berger U, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr Environ Assess Manag. 2011;7:513–541. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giesy JP, Kannan K. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ Sci Technol. 2001;35:1339–1342. doi: 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- 12.Hansen KJ, Clemen LA, Ellefson ME, et al. Compound-specific, quantitative characterization of organic Fluorochemicals in biological matrices. Environ Sci Technol. 2001;35:766–770. doi: 10.1021/es001489z. [DOI] [PubMed] [Google Scholar]
- 13.Kannan K, Corsolini S, Falandysz J, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol. 2004;38:4489–4495. doi: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- 14.Stockholm Convention. Overview. Per- and polyfluoroalkyl substances (PFASs) are chemicals that have partially or completely fluorinated carbon chains of varied lengths. These substances are used in almost all industry branches and many consumer products (Glüge et al. 2020). The most-studied PFASs are perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), and perfluorohexane sulfonic acid (PFHxS). 2022. http://chm.pops.int/Implementation/IndustrialPOPs/PFAS/Overview/tabid/5221/Default.aspx. (Accessed 10 August 2022).
- 15.CDC. Fourth National Report on Human Exposure to Environmental Chemicals. 2019. https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf. (Accessed 3 December 2020).
- 16.Pitter G, da Re F, Canova C, et al. Serum levels of perfluoroalkyl substances (PFAS) in adolescents and young adults exposed to contaminated drinking water in the Veneto region, Italy: A cross-sectional study based on a health surveillance program. Environ Health Perspect. 202;128. 10.1289/EHP5337. Epub ahead of print [DOI] [PMC free article] [PubMed]
- 17.Domingo JL, Nadal M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: a review of the recent scientific literature. Environmental Research. 2019;177. 10.1016/j.envres.2019.108648. Epub ahead of print October 2019 [DOI] [PubMed]
- 18.Braun JM. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017;13:161–173. doi: 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Q, Li H, Wen Z, et al. Perfluoroalkyl substances cause Leydig cell dysfunction as endocrine disruptors. Chemosphere. 2020;253. 10.1016/j.chemosphere.2020.126764. Epub ahead of print August 2020 [DOI] [PubMed]
- 20.Kahn LG, Philippat C, Nakayama SF, et al. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol. 2020;8:703–718. doi: 10.1016/S2213-8587(20)30129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjeldsen LS, Bonefeld-Jørgensen EC. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res. 2013;20:8031–8044. doi: 10.1007/s11356-013-1753-3. [DOI] [PubMed] [Google Scholar]
- 22.Long M, Ghisari M, Bonefeld-Jørgensen EC. Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ Sci Pollut Res. 2013;20:8045–8056. doi: 10.1007/s11356-013-1628-7. [DOI] [PubMed] [Google Scholar]
- 23.Watkins AM, Wood CR, Lin MT, et al. The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol Cell Endocrinol. 2015;400:90–101. doi: 10.1016/j.mce.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Hines EP, White SS, Stanko JP, et al. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol. 2009;304:97–105. doi: 10.1016/j.mce.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Shimpi P, Armstrong L, et al. PFOS induces adipogenesis and glucose uptake in association with activation of Nrf2 signaling pathway. Toxicol Appl Pharmacol. 2016;290:21–30. doi: 10.1016/j.taap.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bach CC, Bech BH, Brix N, et al. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit Rev Toxicol. 2015;45:53–67. doi: 10.3109/10408444.2014.952400. [DOI] [PubMed] [Google Scholar]
- 27.Johnson PI, Sutton P, Atchley DS, et al. The Navigation guide—evidence-based medicine meets environmental health: Systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014;122:1028–1039. doi: 10.1289/ehp.1307893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verner MA, Loccisano AE, Morken NH, et al. Assfociations of perfluoroalkyl substances (PFAS) with lower birth weight: An evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (PBPK) Environ Health Perspect. 2015;123:1317–1324. doi: 10.1289/ehp.1408837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalil N, Chen A, Lee M. Endocrine disruptive compounds and cardio-metabolic risk factors in children. Curr Opin Pharmacol. 2014;19:120–124. doi: 10.1016/j.coph.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Aris IM, Fleisch AF, Oken E. Developmental origins of disease: emerging prenatal risk factors and future disease risk. Curr Epidemiol Rep. 2018;5:293–302. doi: 10.1007/s40471-018-0161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrijheid M, Casas M, Gascon M, et al. Environmental pollutants and child health-A review of recent concerns. Int J Hyg Environ Health. 2016;219:331–342. doi: 10.1016/j.ijheh.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Szilagyi JT, Avula V, Fry RC. Perfluoroalkyl Substances (PFAS) and Their Effects on the Placenta, Pregnancy, and Child Development: a Potential Mechanistic Role for Placental Peroxisome Proliferator-Activated Receptors (PPARs) Curr Environ Health Rep. 2020;7:222–230. doi: 10.1007/s40572-020-00279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rappazzo KM, Coffman E, Hines EP. Exposure to Perfluorinated Alkyl Substances and Health Outcomes in Children: A Systematic Review of the Epidemiologic Literature. Int J Environ Res Public Health. 2017:14. 10.3390/IJERPH14070691. Epub ahead of print 1 July 2017 [DOI] [PMC free article] [PubMed]
- 34.Lee YJ, Jung HW, Kim HY, et al. Early-Life Exposure to Per- and Poly-Fluorinated Alkyl Substances and Growth, Adiposity, and Puberty in Children: A Systematic Review. Front Endocrinol (Lausanne). 2021;12. 10.3389/FENDO.2021.683297. Epub ahead of print 9 September 2021 [DOI] [PMC free article] [PubMed]
- 35.Ribeiro CM, Beserra BTS, Silva NG, et al. Exposure to endocrine-disrupting chemicals and anthropometric measures of obesity: A systematic review and meta-analysis. BMJ Open. 2020;10. 10.1136/bmjopen-2019-033509. Epub ahead of print June 2020 [DOI] [PMC free article] [PubMed]
- 36.Liu P, Yang F, Wang Y, et al. Perfluorooctanoic Acid (PFOA) Exposure in Early Life Increases Risk of Childhood Adiposity: A Meta-Analysis of Prospective Cohort Studies. Int J Environ Res Public Health. 2018;15. 10.3390/IJERPH15102070. Epub ahead of print 1 October 2018 [DOI] [PMC free article] [PubMed]
- 37.Stratakis N, Rock S, la Merrill MA, et al. Prenatal exposure to persistent organic pollutants and childhood obesity: A systematic review and meta-analysis of human studies. Obes Rev. 2022;23 Suppl 1. 10.1111/OBR.13383. Epub ahead of print 1 January 2022 [DOI] [PMC free article] [PubMed]
- 38.Morgan RL, Whaley P, Thayer KA, et al. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121:1027–1031. doi: 10.1016/j.envint.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.NIHR. PROSPERO - International prospective register of systematic reviews. 2021. https://www.crd.york.ac.uk/prospero/. (Accessed 30 July 2021).
- 40.Frigerio G, Ferrari CM, Fustinoni S. Exposure to per-/polyfluoroalkyl substances (PFASs) in children or during gestation and its effects on childhood overweight and obesity. PROSPERO 2021 CRD42021229964. 2021. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021229964. (Accessed 30 July 2021).
- 41.Frigerio G, Cafagna S, Polledri E, et al. Development and validation of an LC-MS/MS method for the quantitation of 30 legacy and emerging per- and polyfluoroalkyl substances (PFASs) in human plasma, including HFPO-DA, DONA, and cC6O4. Anal Bioanal Chem. 2022;414:1259–1278. doi: 10.1007/s00216-021-03762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.R Core Team. R: A Language and Environment for Statistical Computing. 2021 https://www.r-project.org/. (Accessed 7 July 2021)
- 43.Wickham H, Averick M, Bryan J, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4:1686. [Google Scholar]
- 44.Tuan NT, Wang Y. Adiposity assessments: Agreement between dual-energy X-ray absorptiometry and anthropometric measures in U.S. children. Obesity. 2014;22:1495–1504. doi: 10.1002/oby.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin-Calvo N, Moreno-Galarraga L, Martinez-Gonzalez MA. Association between body mass index, waist-to-height ratio and adiposity in children: A systematic review and meta-analysis. Nutrients. 2016;8. 10.3390/nu8080512. Epub ahead of print August 2016 [DOI] [PMC free article] [PubMed]
- 46.Gallo V, Egger M, McCormack V, et al. STrengthening the Reporting of OBservational studies in Epidemiology: Molecular Epidemiology STROBE-ME An extension of the STROBE statement. J Epidemiol Community Health (1978) 2012;66:844–854. doi: 10.1136/jech-2011-200318. [DOI] [PubMed] [Google Scholar]
- 47.Cano-Sancho G, Salmon AG, la Merrill MA. Association between Exposure to p,p’-DDT and Its Metabolite p,p’-DDE with Obesity: Integrated Systematic Review and Meta-Analysis. Environ Health Perspect. 2017;125. 10.1289/EHP527. Epub ahead of print 1 September 2017 [DOI] [PMC free article] [PubMed]
- 48.Rodríguez-Barranco M, Tobías A, Redondo D, et al. Standardizing effect size from linear regression models with log-transformed variables for meta-analysis. BMC Med Res Methodol. 2017;17. 10.1186/S12874-017-0322-8. Epub ahead of print 17 March 2017 [DOI] [PMC free article] [PubMed]
- 49.WHO. Body mass index-for-age (BMI-for-age). 2022. https://www.who.int/toolkits/child-growth-standards/standards/body-mass-index-for-age-bmi-for-age. (Accessed 30 December 2022).
- 50.WHO. BMI-for-age (5–19 years).2022. https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age. (Accessed 30 December 2022).
- 51.Schumacher D. anthro: Computation of the WHO Child Growth Standards. R package version 1.0.0.2021. https://CRAN.R-project.org/package=anthro (Accessed 30 December 2022).
- 52.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 53.Sharma AK, Metzger DL, Daymont C, et al. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5–19 y in NHANES III: association with cardio-metabolic risks. Pediatr Res. 2015;78:723–729. doi: 10.1038/pr.2015.160. [DOI] [PubMed] [Google Scholar]
- 54.Weber DR, Moore RH, Leonard MB, et al. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr. 2013;98:49–56. doi: 10.3945/ajcn.112.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 56.Polanin JR, Snilstveit B. Converting between effect sizes. Campbell Syst Rev. 2016;12:1–13. [Google Scholar]
- 57.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 58.Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat. 2005;30:261–293. [Google Scholar]
- 59.Light RJ, Pillemer DB. Summing Up: The science of reviewing research. Harvard University Press. 1986. 10.3102/0013189X015008016. Epub ahead of print 1 July 1986
- 60.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lumley T. rmeta: Meta-Analysis. R package version 3.0.2018 https://CRAN.R-project.org/package=rmeta (Accessed 18 June 2023).
- 63.Auguie B. gridExtra: Miscellaneous Functions for ‘Grid’ Graphics. R package version 2.3.2017 https://CRAN.R-project.org/package=gridExtra (Accessed 18 June 2023).
- 64.Vrijheid M, Fossati S, Maitre L, et al. Early-life environmental exposures and childhood obesity: an exposome-wide approach. Environ Health Perspect. 2020;128:1–14. doi: 10.1289/EHP5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Papadopoulou E, Stratakis N, Basagaña X, et al. Prenatal and postnatal exposure to PFAS and cardiometabolic factors and inflammation status in children from six European cohorts. Environ Int. 2021;157. 10.1016/J.ENVINT.2021.106853. Epub ahead of print 1 December 2021 [DOI] [PMC free article] [PubMed]
- 66.Braun JM, Eliot M, Papandonatos GD, et al. Gestational perfluoroalkyl substance exposure and body mass index trajectories over the first 12 years of life. Int J Obes (Lond) 2021;45:25–35. doi: 10.1038/s41366-020-00717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao Y, Luo J, Zhang Y, et al. Prenatal Exposure to Per- and Polyfluoroalkyl Substances and Child Growth Trajectories in the First Two Years. Environ Health Perspect, 2022;130. 10.1289/EHP9875. Epub ahead of print 1 March 2022 [DOI] [PMC free article] [PubMed]
- 68.Romano ME, Heggeseth BC, Gallagher LG, et al. Gestational per- and polyfluoroalkyl substances exposure and infant body mass index trajectory in the New Hampshire Birth Cohort Study. Environ Res. 2022;215. 10.1016/J.ENVRES.2022.114418. Epub ahead of print 1 December 2022 [DOI] [PMC free article] [PubMed]
- 69.Zhang S, Lei X, Zhang Y, et al. Prenatal exposure to per- and polyfluoroalkyl substances and childhood adiposity at 7 years of age. Chemosphere; 307. Epub ahead of print 1 November 2022. DOI: 10.1016/J.CHEMOSPHERE.2022.136077. [DOI] [PubMed]
- 70.Bloom MS, Commodore S, Ferguson PL, et al. Association between gestational PFAS exposure and Children’s adiposity in a diverse population. Environ Res. 2022;203. 10.1016/J.ENVRES.2021.111820. Epub ahead of print 1 January 2022 [DOI] [PMC free article] [PubMed]
- 71.Martinsson M, Nielsen C, Björk J, et al. Intrauterine exposure to perfluorinated compounds and overweight at age 4: A case-control study. PLoS One2020;15. 10.1371/journal.pone.0230137. Epub ahead of print 2020 [DOI] [PMC free article] [PubMed]
- 72.Li N, Liu Y, Papandonatos GD, et al. Gestational and childhood exposure to per- and polyfluoroalkyl substances and cardiometabolic risk at age 12 years. Environ Int. 2021;147.. 10.1016/J.ENVINT.2020.106344. Epub ahead of print 1 February 2021 [DOI] [PMC free article] [PubMed]
- 73.Horikoshi T, Nishimura T, Nomura Y, et al. Umbilical cord serum concentrations of perfluorooctane sulfonate, perfluorooctanoic acid, and the body mass index changes from birth to 5 1/2 years of age. Sci Rep. 2021;11. 10.1038/S41598-021-99174-3. Epub ahead of print 1 December 2021 [DOI] [PMC free article] [PubMed]
- 74.Lauritzen HB, Larose TL, Øien T, et al. Prenatal exposure to persistent organic pollutants and child overweight/obesity at 5-year follow-up: A prospective cohort study. Environ Health. 2018;17. 10.1186/s12940-017-0338-x. Epub ahead of print January 2018 [DOI] [PMC free article] [PubMed]
- 75.Gyllenhammar I, Diderholm B, Gustafsson J, et al. Perfluoroalkyl acid levels in first-time mothers in relation to offspring weight gain and growth. Environ Int. 2018;111:191–199. doi: 10.1016/j.envint.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Pan C, Ren Y, et al. Association of maternal exposure to perfluoroalkyl and polyfluroalkyl substances with infant growth from birth to 12 months: A prospective cohort study. Sci Total Environ2022;806. 10.1016/J.SCITOTENV.2021.151303. Epub ahead of print 1 February 2022 [DOI] [PubMed]
- 77.Starling AP, Adgate JL, Hamman RF, et al. Prenatal exposure to per- and polyfluoroalkyl substances and infant growth and adiposity: the Healthy Start Study. Environ Int, 2019;131. 10.1016/j.envint.2019.104983. Epub ahead of print October 2019 [DOI] [PMC free article] [PubMed]
- 78.Hartman TJ, Calafat AM, Holmes AK, et al. Prenatal exposure to perfluoroalkyl substances and body fatness in girls. Child Obes. 2017;13:222–230. doi: 10.1089/chi.2016.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sevelsted A, Gürdeniz G, Rago D, et al. Effect of perfluoroalkyl exposure in pregnancy and infancy on intrauterine and childhood growth and anthropometry. Sub study from COPSAC2010 birth cohort. EBioMedicine. 2022;83.. 10.1016/J.EBIOM.2022.104236. Epub ahead of print 1 September 2022 [DOI] [PMC free article] [PubMed]
- 80.Shoaff J, Papandonatos GD, Calafat AM, et al. Prenatal exposure to perfluoroalkyl substances. Environ Epidemiol. 2018;2:e010. doi: 10.1097/EE9.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y, Li N, Papandonatos GD, et al. Exposure to Per- And Polyfluoroalkyl Substances and Adiposity at Age 12 Years: evaluating periods of susceptibility. Environ Sci Technol. 2020;54:16039–16049. doi: 10.1021/acs.est.0c06088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andersen CS, Fei C, Gamborg M, et al. Prenatal exposures to perfluorinated chemicals and anthropometry at 7 years of age. Am J Epidemiol. 2013;178:921–927. doi: 10.1093/aje/kwt057. [DOI] [PubMed] [Google Scholar]
- 83.Mora AM, Oken E, Rifas-Shiman SL, et al. Prenatal exposure to Perfluoroalkyl substances and adiposity in early and mid-childhood. Environ Health Perspect. 2017;125:467–473. doi: 10.1289/EHP246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Braun JM, Chen A, Romano ME, et al. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity. 2016;24:231–237. doi: 10.1002/oby.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marks KJ, Howards PP, Smarr MM, et al. Prenatal exposure to mixtures of persistent endocrine disrupting chemicals and postnatal body size in British girls. Early Hum Dev. 2021;161.. 10.1016/J.EARLHUMDEV.2021.105450. Epub ahead of print 1 October 2021 [DOI] [PMC free article] [PubMed]
- 86.Jensen RC, Andersen MS, Larsen PV, et al. Prenatal exposures to perfluoroalkyl acids and associations with markers of adiposity and plasma lipids in infancy: An odense child cohort study. Environ Health Perspect. 2020;128:1–11. doi: 10.1289/EHP5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Q, Zhang X, Zhao Y, et al. Prenatal exposure to perfluorobutanesulfonic acid and childhood adiposity: a prospective birth cohort study in Shanghai. China Chemosphere. 2019;226:17–23. doi: 10.1016/j.chemosphere.2019.03.095. [DOI] [PubMed] [Google Scholar]
- 88.Chen MH, Ng S, Hsieh CJ, et al. The impact of prenatal perfluoroalkyl substances exposure on neonatal and child growth. Sci Total Environ. 2017;607–608:669–675. doi: 10.1016/j.scitotenv.2017.06.273. [DOI] [PubMed] [Google Scholar]
- 89.Andersen CS, Fei C, Gamborg M, et al. Prenatal exposures to perfluorinated chemicals and anthropometric measures in infancy. Am J Epidemiol. 2010;172:1230–1237. doi: 10.1093/aje/kwq289. [DOI] [PubMed] [Google Scholar]
- 90.Karlsen M, Grandjean P, Weihe P, et al. Early-life exposures to persistent organic pollutants in relation to overweight in preschool children. Reprod Toxicol. 2017;68:145–153. doi: 10.1016/j.reprotox.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yeung EH, Bell EM, Sundaram R, et al. Examining endocrine disruptors measured in newborn dried blood spots and early childhood growth in a prospective cohort. Obesity. 2019;27:145–151. doi: 10.1002/oby.22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, et al. Prenatal exposure to perfluoroalkyl substances and cardiometabolic risk in children from the spanish INMA birth cohort study. Environ Health Perspect. 2017;125. 10.1289/EHP1330. Epub ahead of print September 2017 [DOI] [PMC free article] [PubMed]
- 93.Alkhalawi E, Kasper-Sonnenberg M, Wilhelm M, et al. Perfluoroalkyl acids (PFAAs) and anthropometric measures in the first year of life: Results from the Duisburg Birth Cohort. J Toxicol Environ Health Part A Curr Issues. 2016;79:1041–1049. doi: 10.1080/15287394.2016.1219552. [DOI] [PubMed] [Google Scholar]
- 94.Høyer BB, Ramlau-Hansen CH, Vrijheid M, et al. Anthropometry in 5- to 9-year-old greenlandic and ukrainian children in relation to prenatal exposure to perfluorinated alkyl substances. Environ Health Perspect. 2015;123:841–846. doi: 10.1289/ehp.1408881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cai A, Portengen L, Govarts E, et al. Prenatal exposure to persistent organic pollutants and changes in infant growth and childhood growth trajectories. Chemosphere. 2023;314. 10.1016/J.CHEMOSPHERE.2022.137695. Epub ahead of print 1 February 2023 [DOI] [PubMed]
- 96.Geiger SD, Yao P, Vaughn MG, et al. PFAS exposure and overweight/obesity among children in a nationally representative sample. Chemosphere; 268. Epub ahead of print April 2021. DOI: 10.1016/j.chemosphere.2020.128852. [DOI] [PubMed]
- 97.Averina M, Brox J, Huber S, et al. Exposure to perfluoroalkyl substances (PFAS) and dyslipidemia, hypertension and obesity in adolescents. The Fit Futures study. Environ Res. 2021;195. 10.1016/J.ENVRES.2021.110740. Epub ahead of print 1 April 2021 [DOI] [PubMed]
- 98.Canova C, Di Nisio A, Barbieri G, et al. PFAS Concentrations and Cardiometabolic Traits in Highly Exposed Children and Adolescents. Int J Environ Res Public Health. 22118.. 10.3390/IJERPH182412881. Epub ahead of print 1 December 2021 [DOI] [PMC free article] [PubMed]
- 99.Janis JA, Rifas-Shiman SL, Seshasayee SM, et al. Plasma Concentrations of Per- and Polyfluoroalkyl Substances and Body Composition From Mid-Childhood to Early Adolescence. J Clin Endocrinol Metab. 2021;106:E3760–E3770. doi: 10.1210/clinem/dgab187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fassler CS, Pinney SE, Xie C, et al. Complex relationships between perfluorooctanoate, body mass index, insulin resistance and serum lipids in young girls. Environ Res. 2019;176. 10.1016/j.envres.2019.108558. Epub ahead of print September 2019 [DOI] [PMC free article] [PubMed]
- 101.Thomsen ML, Henriksen LS, Tinggaard J, et al. Associations between exposure to perfluoroalkyl substances and body fat evaluated by DXA and MRI in 109 adolescent boys. Environ Health. 2021;20. 10.1186/S12940-021-00758-3. Epub ahead of print 1 December 2021 [DOI] [PMC free article] [PubMed]
- 102.Koponen J, Winkens K, Airaksinen R, et al. Longitudinal trends of per- and polyfluoroalkyl substances in children’s serum. Environ Int. 2018;121:591–599. doi: 10.1016/j.envint.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 103.Harris MH, Rifas-Shiman SL, Calafat AM, et al. Predictors of Per- and Polyfluoroalkyl Substance (PFAS) Plasma Concentrations in 6–10 Year Old American Children. Environ Sci Technol. 2017;51:5193–5204. doi: 10.1021/acs.est.6b05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scinicariello F, Buser MC, Abadin HG, et al. Perfluoroalkyl substances and anthropomorphic measures in children (ages 3–11 years), NHANES 2013–2014. Environ Res. 2020;186. 10.1016/j.envres.2020.109518. Epub ahead of print July 2020 [DOI] [PMC free article] [PubMed]
- 105.Timmermann CAG, Rossing LI, Grøntved A, et al. Adiposity and glycemic control in children exposed to perfluorinated compounds. Journal of Clinical Endocrinology and Metabolism. 2014;99. 10.1210/jc.2013-3460. Epub ahead of print 2014 [DOI] [PubMed]
- 106.Li J, Li J, Ma Y, et al. Urine concentrations of perfluoroalkyl acids in children and contributions of dietary factors: a cross-sectional study from Shanghai. China Environ Sci Pollut Res Int. 2021;28:20440–20450. doi: 10.1007/s11356-020-12293-8. [DOI] [PubMed] [Google Scholar]
- 107.Ye X, Kato K, Wong LY, et al. Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. Int J Hyg Environ Health. 2018;221:9–16. doi: 10.1016/j.ijheh.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Domazet SL, GrØntved A, Timmermann AG, et al. Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: the European youth heart study. Diabetes Care. 2016;39:1745–1751. doi: 10.2337/dc16-0269. [DOI] [PubMed] [Google Scholar]
- 109.Kim DH, Lee MY, Oh JE. Perfluorinated compounds in serum and urine samples from children aged 5–13 years in South Korea. Environ Pollut. 2014;192:171–178. doi: 10.1016/j.envpol.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 110.Domazet SL, Jensen TK, Wedderkopp N, et al. Exposure to perfluoroalkylated substances (PFAS) in relation to fitness, physical activity, and adipokine levels in childhood: The european youth heart study. Environ Res. 2020;191. 10.1016/j.envres.2020.110110. Epub ahead of print December 2020 [DOI] [PubMed]
- 111.Schillemans T, Iszatt N, Remy S, et al. Cross-sectional associations between exposure to per- and polyfluoroalkyl substances and body mass index among European teenagers in the HBM4EU aligned studies. Environ Pollut. 2023;316. 10.1016/J.ENVPOL.2022.120566. Epub ahead of print 1 January 2023 [DOI] [PubMed]
- 112.Pinney SM, Windham GC, Xie C, et al. Perfluorooctanoate and changes in anthropometric parameters with age in young girls in the Greater Cincinnati and San Francisco Bay Area. Int J Hyg Environ Health. 2019;222:1038–1046. doi: 10.1016/j.ijheh.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olsen GW, Chang SC, Noker PE, et al. A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology. 2009;256:65–74. doi: 10.1016/j.tox.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 114.OECD. OECD/UNEP Global PFC Group, Synthesis paper on per- and polyfluorinated chemicals (PFCs), Environment, Health and Safety, Environment Directorate, OECD. 2013 https://www.oecd.org/env/ehs/risk-management/PFC_FINAL-Web.pdf (Accessed 3 December 2020).
- 115.Schulz K, Silva MR, Klaper R. Distribution and effects of branched versus linear isomers of PFOA, PFOS, and PFHxS: A review of recent literature. Science of the Total Environment. 2020;733. 10.1016/j.scitotenv.2020.139186. Epub ahead of print September 2020 [DOI] [PubMed]
- 116.al Amin M, Sobhani Z, Liu Y, et al. Recent advances in the analysis of per- and polyfluoroalkyl substances (PFAS)—A review. Environ Technol Innov. 2020;19:100879. [Google Scholar]
- 117.Ryu H, Li B, de Guise S, et al. Recent progress in the detection of emerging contaminants PFASs. Journal of Hazardous Materials. 2021;408. 10.1016/j.jhazmat.2020.124437. Epub ahead of print April 2021 [DOI] [PubMed]
- 118.Göen T, Schaller KH, Drexler H. External quality assessment of human biomonitoring in the range of environmental exposure levels. Int J Hyg Environ Health. 2012;215:229–232. doi: 10.1016/j.ijheh.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 119.Ehresman DJ, Froehlich JW, Olsen GW, et al. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ Res. 2007;103:176–184. doi: 10.1016/j.envres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 120.PubChem Classification Browser. PubChem: PFAS and Fluorinated Compounds in PubChem. 2022 https://pubchem.ncbi.nlm.nih.gov/classification/#hid=120 (Accessed 10 August 2022).
- 121.Schymanski E, Chirsir P, Zhang J, et al. How Open and FAIR Cheminformatics can Support the Discovery, Analysis and Assessment of PFAS. 2022. 10.5281/ZENODO.6461325. Epub ahead of print 13 May 2022
- 122.Jamari NLA, Dohmann JF, Raab A, et al. Novel non-targeted analysis of perfluorinated compounds using fluorine-specific detection regardless of their ionisability (HPLC-ICPMS/MS-ESI-MS) Anal Chim Acta. 2019;1053:22–31. doi: 10.1016/j.aca.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 123.Hallberg I, Plassmann M, Olovsson M, et al. Suspect and non-target screening of ovarian follicular fluid and serum - identification of anthropogenic chemicals and investigation of their association to fertility. Environ Sci Process Impacts. 2021;23:1578–1588. doi: 10.1039/d1em00211b. [DOI] [PubMed] [Google Scholar]
- 124.Miaz LT, Plassmann MM, Gyllenhammar I, et al. Temporal trends of suspect- and target-per/polyfluoroalkyl substances (PFAS), extractable organic fluorine (EOF) and total fluorine (TF) in pooled serum from first-time mothers in Uppsala, Sweden, 1996–2017. Environ Sci Process Impacts. 2020;22:1071–1083. doi: 10.1039/c9em00502a. [DOI] [PubMed] [Google Scholar]
- 125.Panagopoulos Abrahamsson D, Wang A, Jiang T, et al. A Comprehensive Non-targeted Analysis Study of the Prenatal Exposome. Environ Sci Technol. 2021;55:10542–10557. doi: 10.1021/acs.est.1c01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tuomela J, Kaprio J, Sipilä PN, et al. Accuracy of self-reported anthropometric measures — Findings from the Finnish Twin Study. Obes Res Clin Pract. 2019;13:522–528. doi: 10.1016/j.orcp.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Beleigoli A, Andrade A, Diniz M, et al. Validation of anthropometric measures self-reported in a randomized controlled trial of a web-based platform for weight loss. In: Studies in Health Technology and Informatics. Stud Health Technol Inform, pp. 30–36. [DOI] [PubMed]
- 128.Noradilah MJ, Ang YN, Kamaruddin NA, et al. Assessing body fat of children by skinfold thickness, bioelectrical impedance analysis, and dual-Energy X-Ray Absorptiometry: a validation study among Malay children aged 7 to 11 years. Asia Pac J Public Health. 2016;28:74S–84S. doi: 10.1177/1010539516641505. [DOI] [PubMed] [Google Scholar]
- 129.González-Ruíz K, Medrano M, Correa-Bautista JE, et al. Comparison of bioelectrical impedance analysis, slaughter skinfold-thickness equations, and dual-energy x-ray absorptiometry for estimating body fat percentage in colombian children and adolescents with excess of adiposity. Nutrients. 218;10. 10.3390/nu10081086. Epub ahead of print August 2018 [DOI] [PMC free article] [PubMed]
- 130.Boeke CE, Oken E, Kleinman KP, et al. Correlations among adiposity measures in school-aged children. BMC Pediatr. 2013;13. 10.1186/1471-2431-13-99. Epub ahead of print 24 June 2013 [DOI] [PMC free article] [PubMed]
- 131.Devito NJ, Goldacre B. Catalogue of bias: Publication bias. BMJ Evidence-Based Medicine. 2019;24:53–54. doi: 10.1136/bmjebm-2018-111107. [DOI] [PubMed] [Google Scholar]
- 132.Jüni P, Holenstein F, Sterne J, et al. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol. 2002;31:115–123. doi: 10.1093/ije/31.1.115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary text. The supplementary text contains the text strings used for the search in PubMed and Embase and all the R scripts implemented prepared for this work
Additional file 2: Supplementary figures. In the supplementary figures file, for each meta-analysis, three figures are reported: a forest plot pooling the estimates with the generic invariance method, a forest plot pooling the estimates weighting by sample size, and a funnel plot evaluating the bias for included studies
Additional file 3: Supplementary Table S01. Table of entries retrieved from PubMed
Additional file 4: Supplementary Table S02. Table of entries retrieved from Embase
Additional file 5: Supplementary Table S03. Complete database of the considered studies, including: information retrieved from PubMed and Embase; whether the study was included or not in the systematic review and the category of inclusion or rejection; information collected from included articles; and score of reporting assigned to included articles
Additional file 6: Supplementary Table S04. Data extracted from articles used for performing the meta-analyses
Additional file 7: Supplementary Table S05. Data extracted transformed using the conversion factor developed
Additional file 8: Supplementary Table S06. Results of the meta-analyses performed with the generic invariance method (“inv_var”) and weighting by sample size (“wgtd”). The I2 as a measure of heterogeneity is also reported. Both the original meta-analyses are reported together with the sensitivity meta-analyses repeated by excluding one sample per time
Additional file 9: Supplementary Table S07. Results of the meta-analyses stratified by age (1-3 years or 4-18 years). Meta-analyses performed with the generic invariance method (“inv_var”) and weighting by sample size (“wgtd”) are reported. The I2 as a measure of heterogeneity is also reported. Both the original meta-analyses are reported together with the sensitivity meta-analyses repeated by excluding one sample per time
Data Availability Statement
All the data are reported in the supplementary material of the present article, including all the R-scripts developed for this work.