Abstract
Mitochondrial calcium ion (Ca2+) uptake is important for buffering cytosolic Ca2+ levels, for regulating cell bioenergetics, and for cell death and autophagy. Ca2+ uptake is mediated by a mitochondrial Ca2+ uniporter (MCU) and the discovery of this channel in trypanosomes has been critical for the identification of the molecular nature of the channel in all eukaryotes. However, the trypanosome uniporter, which has been studied in detail in Trypanosoma cruzi, the agent of Chagas disease, and T. brucei, the agent of human and animal African trypanosomiasis, has lineage-specific adaptations which include the lack of some homologues to mammalian subunits, the presence of unique subunits, and different functional roles of the common subunits. Here, we review newly emerging insights into the role of mitochondrial Ca2+ homeostasis in trypanosomes, the composition of the uniporter, its functional characterization, and its role in general physiology.
Keywords: Acidocalcisome, calcium, cell bioenergetics, inositol phosphate, mitochondria, mitochondrial calcium uniporter, polyphosphate, trypanosomatids
1. INTRODUCTION
Trypanosomatids are the etiologic agents of several neglected tropical diseases that cause significant morbidity and mortality in millions of people and animals in the world. Trypanosoma cruzi is the agent of Chagas disease, which is endemic in the Americas. The Trypanosoma brucei group causes human African trypanosomiasis or sleeping sickness, and nagana in cattle, and is endemic in Sub-Saharan Africa. Leishmania spp. cause cutaneous, mucocutaneous and visceral leishmaniasis in several continents. T. cruzi has four main life cycle stages, epimastigotes and metacyclic trypomastigotes in the triatomine vector, and bloodstream trypomastigote and intracellular amastigote in the mammalian host. T. brucei has two best studied stages, procyclic trypomastigotes in the tsetse fly vector and bloodstream trypomastigotes in the mammalian host. Leishmania spp. has two well-studied forms, the promastigote in the sand fly vector and the intracellular amastigote in the mammalian host.
Trypanosomatids belong to the eukaryotic supergroup Excavata, which is distantly related to the supergroup Opisthokonta, which includes animals and fungi (Adl et al., 2012). However, like animals and fungi, trypanosomatids possess mitochondria, although with lineage-specific adaptations. They have only one mitochondrion per cell that is characterized for the presence of the kinetoplast. The kinetoplast is formed by thousands of concatenated DNA minicircles and a few DNA maxicircles encoding a few mitochondrial proteins and rRNA (Shlomai, 2004). Several mitochondrial mRNA are edited by a complex mechanism, first discovered in these cells (Benne et al., 1986). As the mitochondrial genome does not possess tRNA genes, these molecules have to be imported into the mitochondrion (Seidman et al., 2012). Some respiratory complexes are incomplete (Surve et al., 2012; van Hellemond et al., 2005), or absent in some stages of these parasites, like in the bloodstream form of T. brucei, which possesses an alternative oxidase (Clarkson et al., 1989) and also lacks the tricarboxylic acid cycle.
Despite these distinct characteristics, mitochondrial Ca2+ homeostasis mechanisms are conserved in trypanosomatids and will be the subject of this review.
2. THE MITOCHONDRIAL Ca2+ UNIPORTER: DISCOVERY
The mitochondrial Ca2+ uniporter (MCU) was discovered almost 60 years ago in rat kidney mitochondria (Deluca, Engstrom, 1961; Vasington, Murphy, 1962). Ca2+ transport was found to be energized by coupled respiration, blocked by respiratory chain inhibitors and oxidative phosphorylation uncouplers, and resulted in large amounts of Ca2+ taken up (Vasington, Murphy, 1962). Further work revealed that the process does not require ATP, except when the respiratory chain is inhibited and, in this case, it is sensitive to oligomycin because it is driven by the ATP synthase working in reverse as an ATPase (Lehninger et al., 1963). Phosphate is also needed for Ca2+ uptake (Lehninger et al., 1963) and the uniporter is inhibited by ruthenium red (Moore, 1971), or its derivative, Ru360 (Ying et al., 1991). The uniporter is a Ca2+-selective channel (Kirichok et al., 2004), although other cations, such as Mn2+ (Bartley, Amoore, 1958) and Sr2+ (Greenawalt, Carafoli, 1966) can also be taken up.
The finding that MCU was absent in Saccharomyces cerevisiae mitochondria (Carafoli et al., 1970) led to the proposal that this process was absent in non-animal species (Carafoli, Lehninger, 1971; McCormack, 1986). However, functional evidence of a mitochondrial Ca2+ uniporter with similar properties to the animal MCU was found in T. cruzi (Docampo, Vercesi, 1989a, b). T. cruzi mitochondrial Ca2+ transport has all the characteristics of the animal MCU: it is electrogenic, has high capacity and low affinity for Ca2+, and is inhibited by ruthenium red (Docampo, Vercesi, 1989a, b).
Other trypanosomatids were later shown to possess an MCU, like several Leishmania spp. (Benaim et al., 1990; Vercesi, Docampo, 1992; Vercesi et al., 1990), and T. brucei (Moreno et al., 1992; Vercesi et al., 1992; Vercesi et al., 1993; Xiong et al., 1997). Even the mitochondria of the bloodstream form of T. brucei, which lacks a respiratory chain and oxidative phosphorylation is able to transport Ca2+, but in this case using the electrochemical gradient generated by the ATP synthase working in reverse, as an ATPase. This Ca2+ transport is inhibited by oligomycin (Vercesi et al., 1992).
Interestingly, the lack of MCU in yeast (Carafoli et al., 1970) and its presence in trypanosomes (Docampo, Vercesi, 1989a) together with the availability of several eukaryotic genomes, led to the identification, first of the gene encoding a modulator of MCU in animals, the mitochondrial calcium uptake 1 (MICU1) (Perocchi et al., 2010), and then of the gene encoding the MCU pore subunit (Baughman et al., 2011; De Stefani et al., 2011; Docampo, Lukes, 2012).
3. THE MITOCHONDRIAL Ca2+ UNIPORTER OF TRYPANOSOMES: THE PORE SUBUNITS
Following the discovery of the molecular nature of the MCU pore subunit, other components of the MCU complex (known as uniplex or holocomplex) were described in mammals, such as MCU regulator 1 (MCUR1) (Mallilankaraman et al., 2012a), MICU2 and MICU3 (Plovanich et al., 2013), MCUb (Raffaello et al., 2013), and essential MCU regulator or EMRE (Sancak et al., 2013).
The trypanosomatid MCU complex differs from the mammalian one in several aspects. First, some subunits, like MCUR1, MICU3, and EMRE, are absent (Docampo et al., 2014). Second, additional subunits, named MCUc, and MCUd form part of the complex and together with the MCU and MCUb subunits, have Ca2+-transporting roles (Chiurillo et al., 2019; Huang, Docampo, 2018).
The MCU subunit was the first MCU complex component described in trypanosomes (Huang et al., 2013b). The gene is single copy in trypanosomes and the encoded protein in T. brucei (TbMCU) has only 20% identity and 33% similarity with the human MCU. In contrast, the orthologues in T. cruzi (TcMCU) and Leishmania major (LmMCU) share 49 and 41% identity, respectively, with the T. brucei protein. All these MCU subunits have two transmembrane domains and a mitochondrial targeting signal and localize to the inner mitochondrial membrane. The processed proteins have an apparent molecular weight of ~30 kDa.
Knockdown of TbMCU by RNAi significantly affects the growth of procyclic (insect form) and bloodstream (mammalian form) forms, reduces mitochondrial Ca2+ uptake, and their ability to accumulate large Ca2+ quantities (Huang et al., 2013b). The mitochondrial membrane potential (ΔΨm) is not affected, indicating specific MCU inhibition. Procyclic forms in which TbMCU is downregulated by RNAi have a higher AMP/ATP ratio and increased autophagy, as revealed by the increase in the number of autophagosomes per cell and the increase in the autophagy marker Atg8.2-II, orthologue to LC3-II in mammalian cells (Huang et al., 2013b). In contrast, overexpression of TbMCU in procyclic forms leads to increased mitochondrial Ca2+ uptake and mitochondrial Ca2+ overload, changes that make the cells more sensitive to proapoptotic agents like C2-ceramide and H2O2, increases production of reactive oxygen species (ROS), and cell death (Huang et al., 2013b).
Conditional knockdown of TbMCU in the bloodstream stage greatly affects its growth, mitochondrial Ca2+ uptake, and virulence in mice, demonstrating its essentiality (Huang et al., 2013b). The bloodstream form of T. brucei uses glucose as the main source of energy, as it does not have an active tricarboxylic acid cycle or respiratory chain. The end product of glycolysis is pyruvate, that is mostly excreted with protons to maintain their intracellular pH (Vanderheyden et al., 2000). However, some pyruvate is needed in the mitochondria where a pyruvate dehydrogenase (PDH) catalyzes its conversion into acetyl-CoA (Huang et al., 2013b; Zhuo et al., 2017). Acetyl-CoA is used for intramitochondrial fatty acid synthesis (FAS II) to generate lipoic acid and myristic acid (Stephens et al., 2007). Alternatively, acetyl-CoA is used to generate acetate (Van Hellemond et al., 1998) that is transferred to the cytosol where it is converted back to acetyl-CoA by acetyl-CoA synthetase (Millerioux et al., 2012) and can be used for fatty acid synthesis. Pyruvate dehydrogenase is one of the mitochondrial dehydrogenases that has been demonstrated to be regulated by Ca2+ (McCormack, 1986). Ca2+ stimulates a PDH phosphatase (PDP) that dephosphorylates the E1α subunit of PDH stimulating its activity, which explains the partial rescue of the lethal effect of TbMCU downregulation by addition of threonine to the culture medium (Huang et al., 2013b). Bloodstream forms have a threonine dehydrogenase (Linstead et al., 1977) able to bypass the need of a Ca2+-stimulated step for generation of acetyl-CoA. More recent work demonstrated that Ca2+ directly stimulate the PDH phosphatase of T. brucei (Lander et al., 2018). Fig. 1 shows a scheme of the reactions that occur in these cells.
Figure 1.
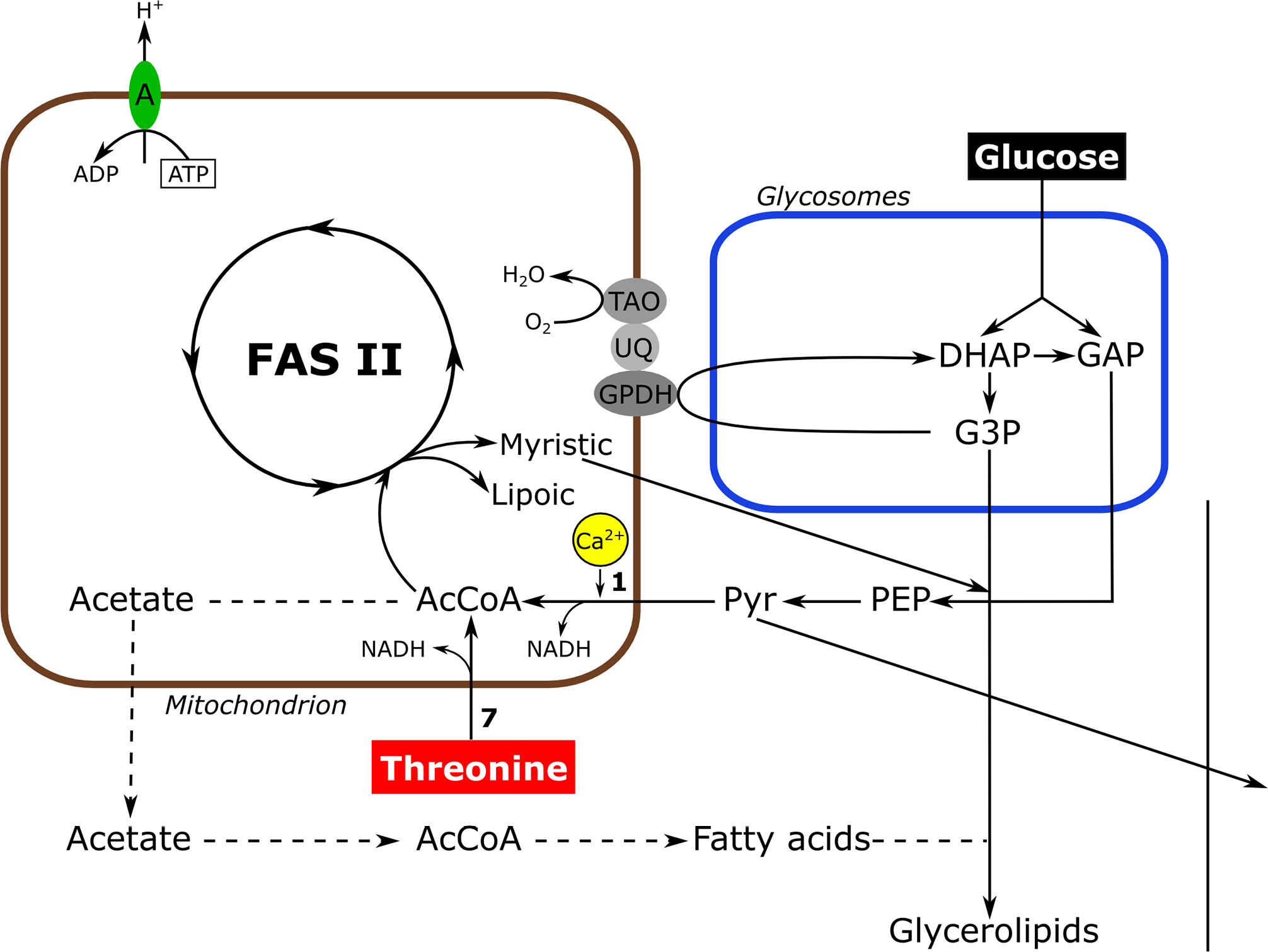
Scheme of metabolic pathway in T. brucei bloodstream forms. Rectangles indicate steps of glucose and threonine metabolism; dashed arrows indicate steps for which no evidence of flux is available. A, ATPase; AcCoA, acetyl-CoA; FAS II, type II fatty-acid biosynthesis pathway; DHAP, dihydroxyacetone phosphate; GAP, glyceraldehyde 3-phosphate; G3P, glycerol 3-phosphate; GPDH, glycerol 3-phosphate dehydrogenase; PEP, phosphoenolpyruvate; Pyr, pyruvate; UQ, ubiquinone; TAO, trypanosome alternative oxidase. Enzymes are: 1. Pyruvate dehydrogenase; 7, threonine dehydrogenase. Activity stimulated by Ca2+ is in yellow. Figure 2. Phylogenetic tree of trypanosomatid and human MCU complex subunits. Thee scale bar corresponds to a distance of 20 changes per 100 amino acid positions. Reproduced with permission from reference (Huang et al., 2013b).
Screening for genes encoding TbMCU orthologs led to the identification of three putative proteins, each with two transmembrane domains (TMD) that were designated TbMCUb, TbMUCc, and TbMCUd. The open reading frames predict proteins of 254, 249, and 214 amino acids, with apparent molecular weights of 28.4, 27.8, and 24.7 kDa, respectively. TbMCUb, TbMCUc, and TbMCUd have 16 to 19% identity and 28 to 34% similarity with TbMCU and, in addition to two TMD, they have a modified putative Ca2+ selectivity filter (WDXXEPXXY) and belong to the MCU family (Pfam: PF04678) (Huang, Docampo, 2018).
The T. cruzi ortholog TcMCUb was studied first, together with TcMCU (Chiurillo et al., 2017). Both genes were knocked out using the CRISPR/Cas9 system (Lander et al., 2015) and the mutants lost their mitochondrial capacity to take up Ca2+ without any alteration in their mitochondrial membrane potential. Complementation of TcMCU-KO cells with an exogenous TcMCU gene, but not with the human orthologue (HsMCU) or a TcMCU gene mutated in the critical Asp (D) and Glu (E) amino acids of the putative pore region, was able to restore mitochondrial Ca2+ uptake.
Overexpression of both genes (TcMCU-OE and TcMUCb-OE) led to increased mitochondrial Ca2+ uptake, also without alterations in ΔΨm. The results suggest that both proteins are Ca2+ transporting subunits. This is in contrast with the mammalian ortholog of TcMCUb, which encodes a dominant negative subunit that inhibits mitochondrial Ca2+ transport when overexpressed (Raffaello et al., 2013). Both proteins, TcMCU and TcMCUb, co-immunoprecipitate indicating that they form oligomers. Growth of both mutants was affected by varying degrees. Growth was slightly slower in TcMCU-KO epimastigotes (vector form), especially in a glucose-deficient medium, but recovered during stationary phase, suggesting an alternative source of energy. In agreement with these results, TcMCU-KO epimastigotes have more lipid droplets, which suggests that they might use fatty acids as alternative source of energy in the stationary phase. These mutants have higher ability to differentiate into the infective forms (metacyclic trypomastigotes) and trypomastigotes were able to infect host cells and replicate normally as intracellular amastigotes. In contrast, TcMCUb-KO epimastigotes were difficult to differentiate to infective metacyclic trypomastigotes and it was not possible to obtain infected host cells, indicating the relevance of this subunit for infection. Concurrently with the higher mitochondrial Ca2+ uptake and overload, TcMCU-OE and TcMCUb-OE also caused oxidative stress. On the other hand, TcMCUb-KO, but not TcMCU-KO epimastigotes, had decreased respiratory rate, lower mitochondrial mass, and increased autophagy, suggesting a better adaptation of TcMCU-KO parasites to the lower mitochondrial ability to take up Ca2+ (Chiurillo et al., 2017).
The two other subunits identified in the trypanosome proteome, MCUc and MCUd, have only orthologues in trypanosomatids (Chiurillo et al., 2019; Huang, Docampo, 2018) (Fig. 2). Knockdown of all T. brucei proteins (TbMCU, TbMCUb, TbMCUc, and TbMCUd) by RNAi decreased mitochondrial Ca2+ uptake without affecting ΔΨm (Huang, Docampo, 2018) and their overexpression enhanced Ca2+ uptake. Therefore, TbMCUb is not a dominant negative subunit as it occurs with the animal MCUb (Raffaello et al., 2013). In addition, knockout of each of the T. cruzi subunits by CRISPR/Cas9 suppressed, while their overexpression increased, mitochondrial Ca2+ uptake (Chiurillo et al., 2019). Taken together the results indicate that MCU, MCUb, MCUc, and MCUd are all Ca2+-transporting subunits. All the T. brucei subunits co-immunoprecipitate and exist in a large protein complex with a net molecular weight of ~380 kDa, suggesting that the complex is a hetero-oligomer (Huang, Docampo, 2018). Further evidence of its hetero-oligomeric structure was provided using the split-ubiquitin membrane-based yeast two hybrid (MYTH) and by co-immunoprecipitation assays. Combining mutagenesis analysis with MYTH assays determined that the transmembrane helices (TMH) of the subunits are involved in these interactions and that the subunits form a hetero-hexamer (Fig. 3A). Mutagenesis of TM helix 1 (TMH1) and especially 2 (TMH2) of each of the four subunits of the complex showed that they are required for their interactions (Huang, Docampo, 2018). These results are apparently different from those reported in animals (Baradaran et al., 2018) and fungi (Baradaran et al., 2018; Fan et al., 2018; Nguyen et al., 2018; Yoo et al., 2018) in which structural studies proposed that the MCU subunit forms homo-tetramers. However, these studies were done using recombinant MCU and how MCU interacts with its membrane partners, like MCUb, and which is their oligomeric structure in vivo remains to be investigated.
Figure 2.
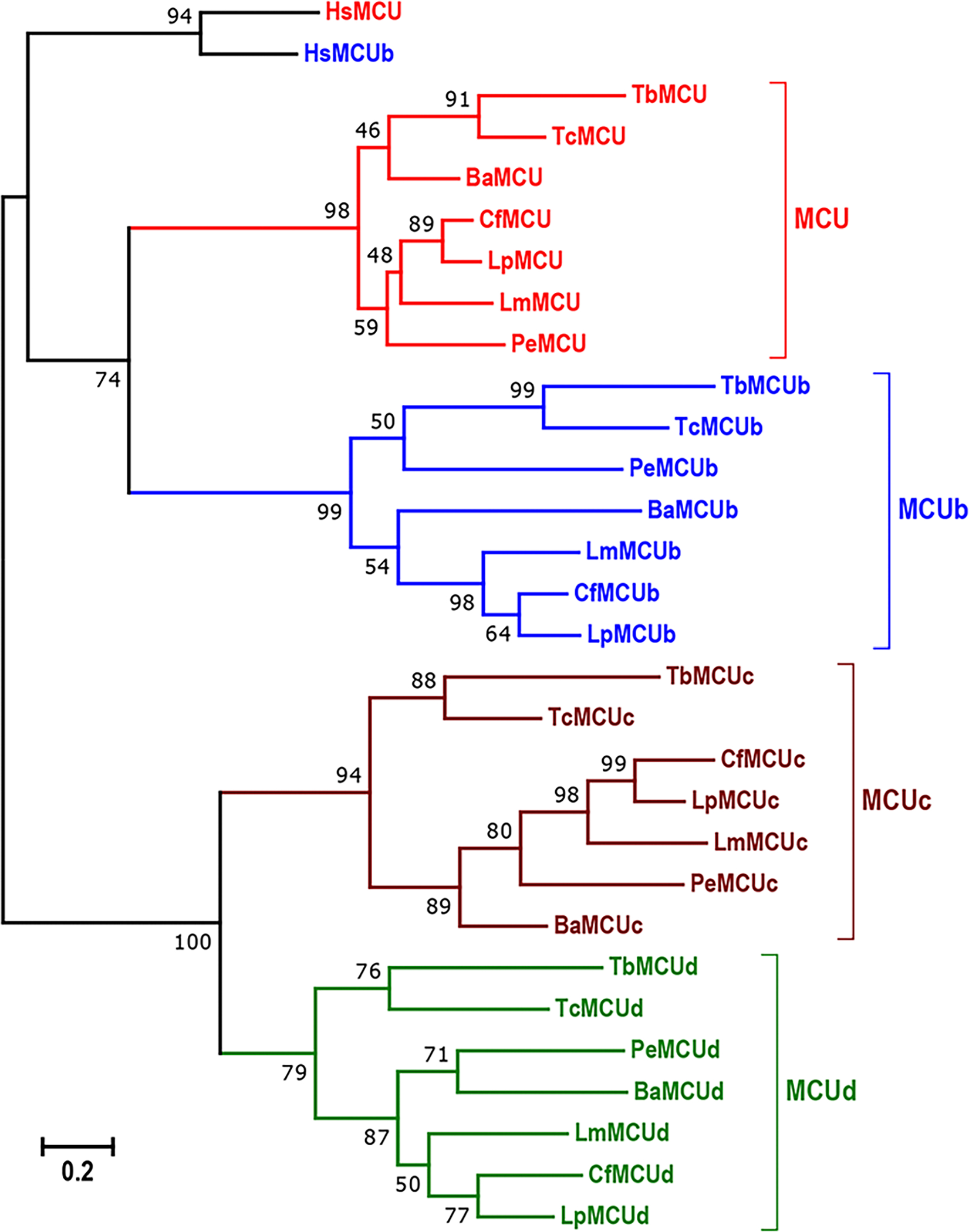
Phylogenetic tree of trypanosomatid and human MCU complex subunits. The TriTrpDB and GenBank accession numbers for 30 MCUC subunits were described in (Huang, Docampo, 2018). The scale bar corresponds to a distance of 20 changes per 100 amino acid positions. Reproduced with permission from reference (Huang, Docampo, 2018).
Figure 3.
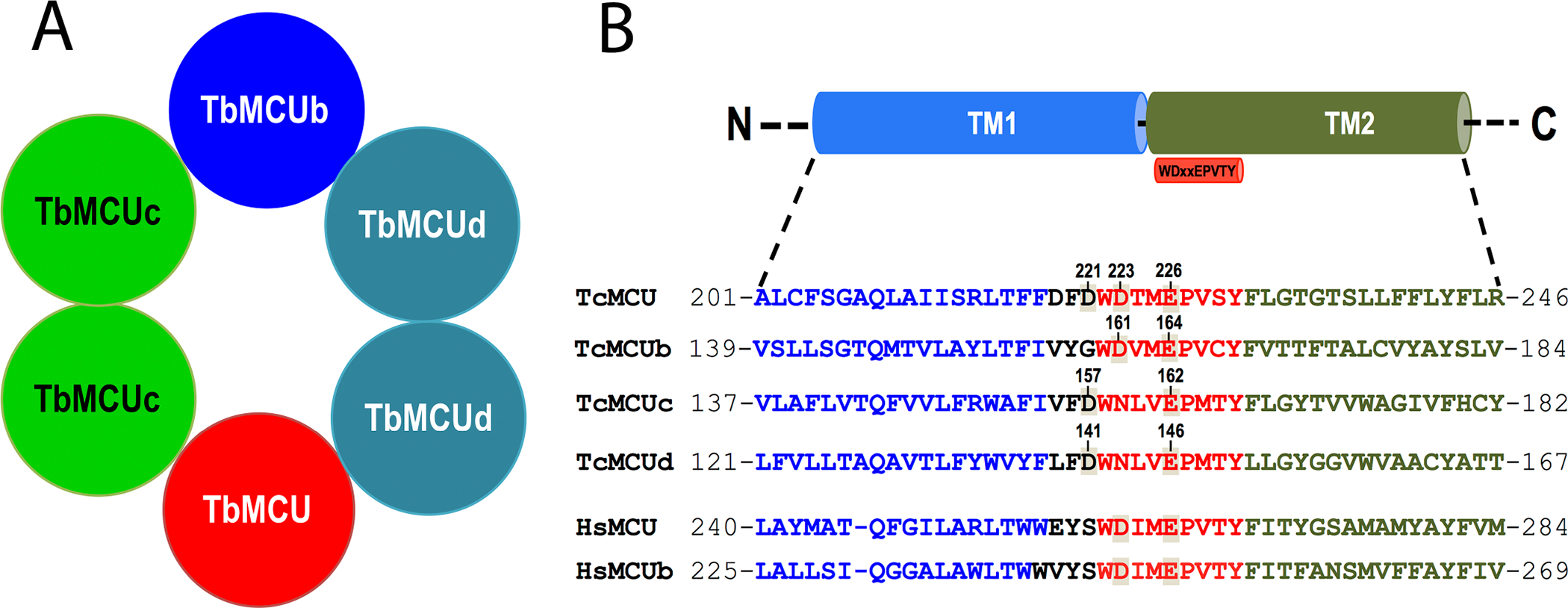
A. Scheme depicting the putative organization and composition of a hetero-hexameric TbMCU complex. Reproduced with permission from reference (Huang, Docampo, 2018). B. conserved WDXXEPXTY motif in TcMCU complex subunits. Alignment of the C-terminal fragment of the first transmembrane domain (TM1, in blue) and the N-terminal fragment of the second transmembrane domain (TM2, in green), including conserved WDXXEPXTY motif (in red), of TcMCU complex subunits with human MCU and MCUb. Conserved putative critical acidic residues in or near the WDXXEPXTY selectivity filter are indicated. TcMCU, TcMCUb, TcMCUc and TcMCUd exhibit a substitution of the Ru360-sensitive residue S to D/G in the pore region. Residues in each TcMCU complex subunit that were subjected to substitutions are indicated with the corresponding number in their sequence. Reproduced with permission from reference (Chiurillo et al., 2019).
Knockdown of TbMCUc and TbMCUd reduced procyclic trypomastigotes growth in glucose-deficient media (Huang, Docampo, 2018), and increased the AMP/ATP ratio (Huang, Docampo, 2020), in agreement with the importance of mitochondrial metabolism for these stages (Lamour et al., 2005). Similar results were observed after ablation of TcMCUc or TcMCUd by CRISPR/Cas9 in epimastigotes (Chiurillo et al., 2019). TcMCUc-KO and TcMCUd-KO epimastigotes have also alterations in their respiratory rate, citrate synthase activity, and AMP/ATP ratio, but they normally differentiate into metacyclic trypomastigotes, while trypomastigotes are less infective and amastigotes have a reduced replication rate. All these results are in agreement with the relevance of mitochondrial metabolism for T. cruzi invasion of host cells (Schenkman et al., 1991) and for replication of intracellular amastigotes (Dumoulin, Burleigh, 2018).
Complementation of the knockout of each MCU subunit of T. cruzi with mutant genes in which the Glu (E) and Asp (D) of the pore motif (WDXXEPXXY) were mutated revealed that the Glu is essential and the Asp is important for Ca2+ uptake (Chiurillo et al., 2019) (Fig. 3B). The results are in agreement with structural studies that have shown that the selectivity filter is formed by symmetrical arrangement of WDXXEPXXY sequences in TMH2 from each monomer around the pore. The Glu residues form an acid mouth (Site 1), and the Asp residues form a second acidic ring (Site 2) (Baradaran et al., 2018). Site 2 is a high-affinity binding site for Ca2+ in MCU. Mutations of the Asp residues located in the filter region of TcMCU (D223) or TcMCUb (D161), or the Asp residues located N-terminal to the selectivity filter of TcMCU (D221), TcMCUc (D157) or TcMCUd (D141), reduced but did not completely abolished mitochondrial Ca2+ transport (Chiurillo et al., 2019). In contrast, mutations of the Glu residues of each monomer suppressed mitochondrial Ca2+ transport. Since mitochondrial Ca2+ uptake in TcMCU-KO cells can be restored with TcMCUR214W/D219V mutant, these other residues are not important for Ca2+ transport in T. cruzi (Chiurillo et al., 2017) (Fig. 3B). These residues are located in TMH1 and loop region near the WDXXEPXXY motif but outside the channel entrance (Baradaran et al., 2018; Fan et al., 2018; Nguyen NX, 2018; Oxenoid et al., 2016; Yoo et al., 2018).
Complementation of TcMCU-KO epimastigotes by co-expression of HsMCU and HsEMRE were unsuccessful, possibly because the proteins did not insert properly in the inner membrane or with the right topology, failed to interact, or did not form part of the MCU complex (Chiurillo et al., 2019).
All T. cruzi MCU complex monomers lack the serine amino acid (Ser259 in the human MCU) (Fig. 3B) that has been proposed to be responsible for the sensitivity of the uniporter to the ruthenium red derivative Ru360 (Baughman et al., 2011). As mitochondrial Ca2+ transport in T. cruzi is sensitive to Ru360, this suggest that there is another target for Ru360 besides Ser259 (Chiurillo et al., 2019).
In conclusion, four pore subunits MCU, MCUb, MCUc, and MCUd form a hetero-oligomer, probably a hetero-hexamer, required for mitochondrial Ca2+ uptake in trypanosomes (Chiurillo et al., 2019; Huang, Docampo, 2018). The selectivity filter is formed by symmetrical arrangement of WDXXEPXXY sequences in TMH2 from each subunit around the pore. The lack of any of the subunits abolishes Ca2+ uptake. MCUb is not a dominant negative subunit of the uniporter as in animal cells (Chiurillo et al., 2017; Huang, Docampo, 2018). MCUc and MCUd are subunits present only in trypanosomatids (Chiurillo et al., 2019; Huang, Docampo, 2018). All subunits are required for growth under glucose-limited conditions, revealing the importance of the uniporter in mitochondrial metabolism (Chiurillo et al., 2019; Huang, Docampo, 2018). Complementation studies of all the pore subunits with mutant subunits found that the Glu and Asp of the selectivity filter are essential, and important, respectively, for Ca2+ uptake (Chiurillo et al., 2019). Complementation of MCU-KO cells with human MCU (Chiurillo et al., 2017) or human MCU and EMRE (Chiurillo et al., 2019) or attempts to reconstitute the uniporter in yeast transforming them with TcMCU (Chiurillo et al., 2017) failed, suggesting that exogenous subunits could not form part of the trypanosome uniporter and that probably all the endogenous subunits of the pore would be required for reconstitution of its function in yeast. The different function of the MCUb subunit and the presence of two additional Ca2+-transporting subunits (MCUc and MCUs) in trypanosomes reflect the parallel evolution of the uniporter in different supergroups of eukaryotes (Pittis et al., 2020).
4. THE MITOCHONDRIAL Ca2+ UNIPORTER OF TRYPANOSOMES: THE GATEKEEPER SUBUNITS
MICU1 was reported to act as a gatekeeper of the uniporter by inhibiting Ca2+ uptake at low cytosolic Ca2+ concentration ([Ca2+]cyt), thus preventing mitochondrial Ca2+ overload under physiological ([Ca2+]cyt (Csordas et al., 2013; Mallilankaraman et al., 2012b). Later work suggested that MICU2, which binds covalently to MICU1,was the most important inhibitor (Patron et al., 2014). Subsequently either MICU1 or MICU2 or both were considered more relevant gatekeepers in a variety of cells (Kamer et al., 2017; Liu et al., 2016; Matesanz-Isabel et al., 2016; Paillard et al., 2017; Payne et al., 2017).
T. cruzi and T. brucei possess orthologs to MICU1 and MICU2, but orthologs to MICU2 are absent in Leishmania spp. (Docampo et al., 2014). The T. cruzi proteins have been studied in more detail (Bertolini et al., 2019). TcMICU1 and TcMICU2 have estimated molecular masses of 46.7 and 53.2 kDa, respectively, have 20% identity and 38% similarity between them, possess mitochondrial targeting signals, and two canonical and two noncanonical EF-hand domains. TcMICU1 and TcMICU2 have only 22% and 23.9% overall sequence identity (44.4% and 40% of similarity), respectively, to their human orthologs (Bertolini et al., 2019).
Ablation of TcMICU1 or TcMICU2 reduces mitochondrial Ca2+ uptake and increases the Ca2+ concentration needed for opening the uniporter without affecting the ΔΨm. Interestingly, these mitochondria are less efficient in taking up Ca2+ across a wide range of Ca2+ concentrations and the threshold for Ca2+ uptake is elevated (Bertolini et al., 2019). These results indicate that although these proteins have a role in Ca2+ sensing in the intermembrane space, they have no role as gatekeepers at low Ca2+ concentrations, as it occurs with the mammalian proteins. Ca2+ transport at low Ca2+ concentrations is already inhibited in the absence of either protein. Both proteins are required for normal growth and respiration of epimastigotes, for normal metacyclogenesis, for trypomastigote invasion of host cells, and for intracellular replication of amastigotes, indicating their relevance for parasite survival (Bertolini et al., 2019). However, these cells do not show changes in mitochondrial mass, AMP/ATP ratio, or autophagy. The lower Ca2+ transport correlates with the increase in phosphorylation of the PDH, reflecting the lower stimulation of the T. cruzi PDH phosphatase (TcPDP) by Ca2+ (Bertolini et al., 2019).
Overexpression of MICU1 or MICU2 in HeLa cells increases or decreases, respectively, mitochondrial Ca2+ accumulation (Patron et al., 2014). However, this does not occur in T. cruzi where no changes are observed when TcMICU1 or TcMICU2 are overexpressed. Overexpression does not affect epimastigote growth, and these overexpressed proteins do not form covalently bound oligomeric complexes (Bertolini et al., 2019).
In conclusion, trypanosomes appear to also differ from mammalian cells in the ability of either MICU1 or MICU2 to act as gatekeepers of the uniporter at low Ca2+ concentrations, in their formation of covalently-bound dimers, and in changes in mitochondrial Ca2+ accumulation by their overexpression, although they are still able to sense changes in Ca2+ concentration in the mitochondrial intermembrane space and are important for parasite survival and infectivity.
5. THE MITOCHONDRIAL Ca2+ UNIPORTER OF TRYPANOSOMES: INTERACTION WITH THE ATP SYNTHASE
Recent work has indicated that the MCU complex of trypanosomes physically interacts with the subunit c of the ATP synthase (Huang, Docampo, 2020). Tandem affinity purification using overexpressed TbMCU, combined with mass spectrometry (MS), resulted in the identification of 19 subunits of the ATP synthase together with the voltage dependent anion channel (VDAC), the adenine nucleotide translocator (ANT), the phosphate carrier (PiC), and the MCU complex components MCU, MCUb and MCUc. The ATP synthase, the ANT, and the PiC constitute what is known as ATP synthasome in mammals (Ko et al., 2003). Similar results were obtained by immunoprecipitation of tagged TbMCU combined with MS. When two subunits of the ATP synthase (TbATPβ, TbATPp18) were in situ tagged they were also able to immunoprecipitate TbMCU. In situ tagged ANT and PiC were not able to do the same, suggesting that TbMCU is closely associated with the ATP synthase but only loosely associated with ANT and PiC.
The split-ubiquitin membrane-based yeast two-hybrid (MYTH) assay was used to validate the interaction of TbMCU with ten of the ATP synthase subunits but only subunit c (TbATPc) was shown to specifically physically interact with TbMCU. Each TbMCU subunit, except TbMCUb, (TbMCU, TbMCUc, and TbMCUd), T. cruzi MCU, as well as human MCU, were also able to physically interact with the subunit c of the corresponding species. Expression of truncated or substituted forms of the different TbMCU subunits (TbMCU, TbMCUc, and TbMCUd) or of the TbATPc subunit (without the C- or N-terminal regions, with mutations in conserved residues of the TMHs or with substitutions of TMHs with artificial helices) indicated that TMH1s of each TbMCU complex subunit (except for TbMCUb) specifically interacts with TMH1 of TbATPc via the conserved motifs of the TMH1s. TbMCU subunits (TbMCU, TbMCUc, and TbMCUd) also co-immunoprecipitated with TbATPc. Blue native PAGE and immunodetection analyses showed that the TbMCU complex physically interacts with TbATPc in a large protein complex of ~900 kDa. Further evidence of this interaction was the co-immunoprecipitation of TbMCU and TbMCUc with TbATPb and TbATPp18.
Interestingly, MYTH assays using the human MCU (HsMCU) and ATPc (HsATPc) validated that their specific physical interaction is also through their respective TMH1s. Reciprocal co-immunoprecipitations of HsMCU and HsATPc using HEK-293T and HeLa cells confirmed this interaction in vivo, which was not detected when HsMCU-KO HEK-293T cells were used as control.
In conclusion, three of the T. brucei MCU subunits (TbMCU, TbMCUc, and TbMCUd) physically interact with mitochondrial ATP synthase subunit c (TbATPc) when expressed in yeast membranes. These interactions are also observed with the T. cruzi (TcMCU) or human MCU (HsMCU) and the corresponding ATP synthase subunit c (TcATPc, HsATPc), and in all cases these results were confirmed by co-immunoprecipitations, and by their co-localizations, as studied by immunofluorescence microscopy. These interactions were also confirmed in trypanosomes or human cells in vivo by their co-immunoprecipitations from cell lysates and in the case of T. brucei by blue native PAGE and immunodetection analysis. As a result of these interactions it was possible to pull down the ATP synthase complex together with the ANT and PiC by TbMCU, suggesting the presence of a ATP synthasome megacomplex that includes the TbMCU complex (Huang, Docampo, 2020).
These results suggest several intriguing possibilities: 1) As these interactions involve the TMH1s of both the TbMCU subunits and the TbATPc, the results suggest that, if this interaction occurs in situ, the TbMCU complex would be within the c-ring of the T. brucei ATP synthase (Fig. 4) (Huang, Docampo, 2020). In this context, the presence of a protein within the c-ring of the porcine ATP synthase, as studied in situ by cryo-electron microscopy, has been reported (Gu et al., 2019), and further work is needed to investigate whether it corresponds to a MCU component; 2) the MCU complex could have a potential role in the formation of the mitochondrial permeability transition pore (mPTP) (Huang, Docampo, 2020). This is because the c-ring of the ATP synthase (Alavian et al., 2014; Bonora et al., 2013), a channel inside the ATP synthase dimers (Bonora et al., 2013; Bonora et al., 2017), or the purified ATP synthase itself (Urbani et al., 2019), have been proposed to form the pore of this channel. 3) the formation of a megacomplex including the ATP synthase, the MCU complex, the ANT, and the PiC suggest the coupling between ADP and Pi transport with ATP synthesis, which is stimulated by Ca2+ (Huang, Docampo, 2020). In this regard several reports indicate that the ATP synthase binds or is stimulated by Ca2+ (Giorgio et al., 2017; Hubbard, McHugh, 1996; Territo et al., 2001; Territo et al., 2000; Zakharov et al., 1993).
Figure 4.
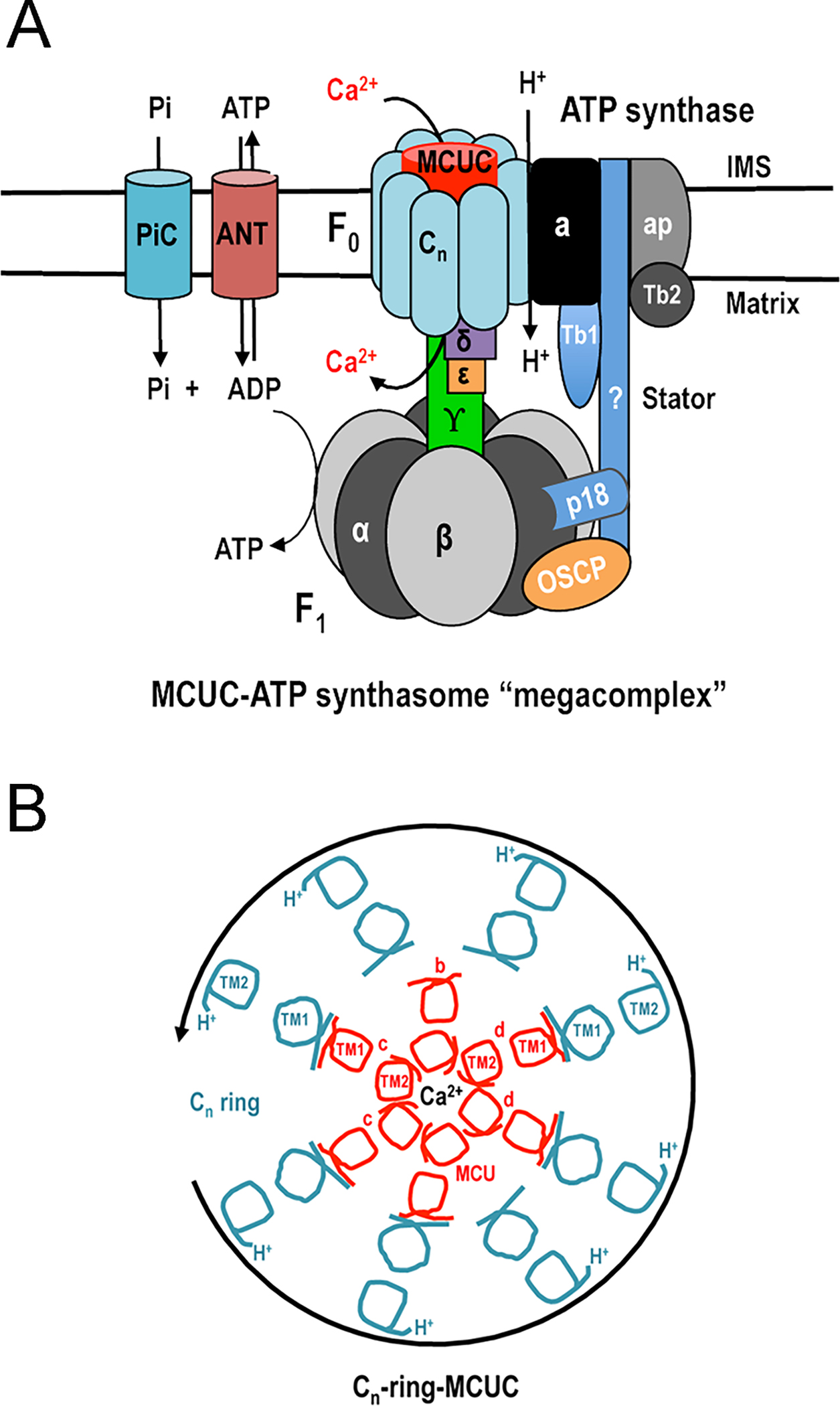
Models showing organization of putative MCU complex-ATP synthasome megacomplex in trypanosomes. A. The MCU complex physically interacts with the ATP synthasome (ATP synthase, ANT, and PiC) via the c-ring of the F0 ATP synthase. In trypanosomes, ATP synthase consists of F1 region with the central stalk (α3β3, γ, δ and ε) for ATP synthesis, F0 region with the putative stator (cn, a, p18, Tb1, Tb2, and OSCP) for proton (H+) translocation, and trypanosome-specific associated proteins (ap), while the molecular identity of the peripheral stalk is unknown. OSCP, oligomycin sensitivity-conferring protein; ANT, adenine nucleotide translocator; PiC, phosphate carrier. B. Cross section model to hypothetical T. brucei cn-ring-MCU complex. The T. brucei heterohexameric MCU complex consisting of 4 different subunits (MCU, MCUb, MCUc, and MCUd), with a molecular weight of approximately 145 kDa, is within the c-ring of ATP synthase. TMH1 of each MCU complex subunit (excluding MCUb) interacts with TMH1 of ATPc. The c-ring rotates in counterclockwise direction and translocates H+ from the intermembrane space to matrix during ATP synthesis. Reproduced with permission from reference (Huang, Docampo, 2020).
6. MECHANISM OF MITOCHONDRIAL Ca2+ EFFLUX IN TRYPANOSOMES
In mammalian cells mitochondrial Ca2+ is extruded by either a H+- or a Na+-coupled exchanger. While the activity of the Na+/H+ exchanger is found in most cell types and is especially important in excitable cells, a Ca2+/H+ exchanger is mainly found in non-excitable cells (Palty et al., 2010). Early work described the activity of the Na+/H+ exchanger (Carafoli et al., 1974) but the molecular identity of the exchanger was discovered more recently (Palty et al., 2010). Trypanosomes lack orthologues to this gene. In contrast, evidence of the presence of a Ca2+/H+ exchanger was found as judged by the response of T. cruzi mitochondria to the additions of Ca2+ and EGTA (Docampo, Vercesi, 1989b). A mitochondrial Ca2+/H+ exchanger named Letm1 was identified in mammalian cells (Jiang et al., 2009; Tsai et al., 2014), and trypanosomes do have orthologues to this gene. Studies in T. brucei concluded that TbLETM1 is involved in maintaining mitochondrial volume via K+/H+ exchange across the inner membrane (Hashimi et al., 2013) but its function in Ca2+ transport was not investigated. Further work is needed to investigate the role of trypanosome Letm1 in Ca2+ influx and/or efflux.
7. ROLE OF MITOCHONDRIAL Ca2+ UPTAKE
Mitochondrial Ca2+ uptake in mammalian cells is important as cytosolic Ca2+-buffering system for regulation of spatially confined cytosolic Ca2+ rises, for regulation of mitochondrial metabolism, and for cell survival (Rizzuto et al., 2012). Some of these functions are conserved in trypanosomes.
Cytosolic Ca2+ buffering system.
Experiments with T. brucei expressing the genetically-encoded Ca2+ indicator aequorin targeted to the mitochondria found that intramitochondrial Ca2+ concentrations can reach values much higher than cytosolic Ca2+ levels when Ca2+ influx through the plasma membrane or Ca2+ release from acidocalcisomes are stimulated (Xiong et al., 1997). Mitochondrial Ca2+ uptake can be induced at both nano- and micromolar Ca2+ concentrations (Xiong, Ruben, 1998), suggesting a very close proximity of these organelles and the presence of microdomains of high Ca2+ concentration in the vicinity of the plasma membrane or acidocalcisomes (Xiong et al., 1997). Such membrane contact sites between acidocalcisomes and mitochondria were reported in both T. brucei (Ramakrishnan et al., 2018) and T. cruzi (Miranda et al., 2000). The inositol 1,4,5-trisphosphate receptor is located in acidocalcisomes of both trypanosome species (Huang et al., 2013a; Lander et al., 2016) and close contacts between these organelles facilitate the mitochondrial Ca2+ transfer to the mitochondria when the IP3 receptor is activated (Chiurillo et al., 2020).
Regulation of mitochondrial metabolism.
Mitochondrial Ca2+ in vertebrate cells is important for regulation of the activity of several mitochondrial dehydrogenases (McCormack, 1986) and the ATP synthase (Territo et al., 2001). Only one of the dehydrogenases stimulated by Ca2+ in vertebrates has been studied in detail in trypanosomatids (Lander et al., 2018). The pyruvate dehydrogenase is activated by dephosphorylation of the E1α subunit catalyzed by a PDH phosphatase (PDP), which is stimulated by Ca2+. Both T. brucei and T. cruzi recombinant PDPs catalyze the dephosphorylation of a synthetic phosphopeptide from either species containing the phosphorylated sites that regulate PDH activity (Lander et al., 2018). TcPDP and TbPDP exhibit maximal activity at 100 nM and 1 μM Ca2+, respectively, suggesting a physiological response. Interestingly, although the binding site for mammalian PDP is formed in the presence of PDH E2 subunit (Turkan et al., 2004), the parasite enzymes are able to directly dephosphorylate E1α phosphopeptides (Lander et al., 2018), in agreement with earlier studies with the mammalian enzyme that identified an E1α binding site (Teague et al., 1982). Knockout of TcPDP results in reduced growth of epimastigotes, defective metacyclogenesis, and reduced host cell invasion by trypomastigotes (Lander et al., 2018). The epimastigotes have a respiratory deficiency, lower citrate synthase activity, higher AMP/ATP ratio and increased autophagy. These cells have a compensatory increase in amino acid metabolism, as revealed by the increased ammonia production (Lander et al., 2018).
The stimulation by Ca2+ of two other dehydrogenases that are stimulated by Ca2+ in animals, isocitrate dehydrogenase and the α-ketoglutarate dehydrogenase, has not been studied in trypanosomes. However, in contrast to the Ca2+-regulated mammalian NAD-dependent enzyme, the mitochondrial isocitrate dehydrogenase present in trypanosomatids is NADP-dependent (Leroux et al., 2011). Another dehydrogenase regulated by Ca2+, the glycerol phosphate dehydrogenase (Denton, 2009) is devoid of the Ca2+-binding EF-hands domains in trypanosomes and presumably insensitive to Ca2+. The aspartate-glutamate carrier (AGC) and the ATP-Mg-Pi carriers (SCaMCs) are known to be regulated by Ca2+ in mammalian cells (Satrustegui et al., 2007). However, the trypanosomatid homologues lack EF-hand domains and are therefore potentially Ca2+ insensitive. Finally, regulation of the ATP synthase by Ca2+ has not been studied in trypanosomes.
Regulation of cell survival.
Mitochondrial Ca2+ is important for programmed cell death (PCD), or apoptosis, in trypanosomatids. Early work on the effects of naphthoquinones on T. cruzi demonstrated changes in their morphology that can be attributed to PCD, such as shrinking, membrane blebbing, mitochondrial alterations and chromatin condensation (Docampo et al., 1977). However, trypanosomes lack some key regulatory or effector molecules involved in apoptosis in mammalian cells, such as the tumor necrosis factor (TNF)-related family of receptors, Bcl-2 family members, and caspases (Kaczanowski et al., 2011; Smirlis et al., 2010). Mitochondrial Ca2+ overload affects the mitochondrial membrane potential, induces the generation of reactive oxygen species (ROS) generation and releases cytochrome c in trypanosomatids (Smirlis, Soteriadou, 2011). The production of ROS in T. brucei impairs mitochondrial Ca2+ uptake, and leads to its accumulation in the nucleus, resulting in cell death (Ridgley et al., 1999). A mitochondrial endonuclease G is released and translocated to the nucleus in Leishmania spp. (Gannavaram et al., 2008) and this change stimulates a caspase-independent, apoptosis-like cell death (reviewed in (Smirlis, Soteriadou, 2011)). T. cruzi is highly resistant to mitochondrial permeability transition (Docampo, Vercesi, 1989b), and apoptosis-like death upon mitochondrial Ca2+ overload is dependent on superoxide anion generation (Irigoin et al., 2009).
In conclusion, mitochondrial Ca2+ uptake in trypanosomatids has a role in shaping the amplitude of cytosolic Ca2+ increases after influx through the plasma membrane or release from acidocalcisomes, in the regulation of ATP production, and in apoptosis-like death.
8. CONCLUSIONS AND OPEN QUESTIONS
The mitochondria of trypanosomes possess a Ca2+ uniporter for Ca2+ uptake and a putative Ca2+/H+ exchanger for Ca2+ release. The finding of a Ca2+ transporting mechanism in trypanosomes with similar characteristics to those of the mammalian uniporter and its absence in yeast, together with the elucidation of the genomes of these and mammalian organisms, led to the discovery of the molecular nature of MICU1 and MCU subunits. However, the MCU complex of trypanosomes has lineage-specific adaptations not seen in the vertebrate uniporter complex. For example, some subunits present in vertebrate cells, such and MCUR1, MICU3, and EMRE, are absent in trypanosomatids. The homologous to vertebrate subunit MCUb does not have a dominant negative effect but has Ca2+-transporting activity. Trypanosomes possess four Ca2+-transporting subunits (MCU, MCUb, MCUc, and MCUd) that form hetero-oligomers where each subunit contributes to the formation of the pore of the channel, interacting through their TMHs. Interestingly, the MCUc and MCUd subunits are exclusive components of the MCU complex of trypanosomatids. In addition, MICU1 and MICU2 do not form covalently-bound dimers and do not act as gatekeepers of the channel at low Ca2+ concentrations. Concerning trypanosome biology, the MCU complex is essential for normal growth in vitro and in vivo. Downregulation of the uniporter expression leads to increase in the AMP/ATP ratio and autophagy while overexpression leads to Ca2+ overload, reactive oxygen species (ROS) generation, and cell death. The MCU complex interacts with the subunit c of the ATP synthase and contributes to the formation of a megacomplex including the phosphate carrier (PiC) and the adenine nucleotide translocator (ANT), which couples ADP and Pi uptake with ATP synthesis stimulated by Ca2+. Finally, Ca2+ has a direct role in the stimulation of the mitochondrial PDH activity by dephosphorylation of the E1α subunit catalyzed by a Ca2+-sensitive PDH phosphatase (PDP).
It has not been possible to reconstitute the trypanosome uniporter in yeast, or to complement the lack of the MCU subunit in trypanosomes by either HsMCU alone or together with HsEMRE, suggesting that the four Ca2+-transporting subunits would be needed for reconstitution and that the human MCU subunit is not compatible with the trypanosome-specific subunits. Further work is needed to identify whether Letm1 is the Ca2+/H+ exchanger in trypanosomes and to determine how MICU1/MICU2 interact with the pore of the channel, given the apparent absence of an EMRE ortholog. It will also be important to confirm, by structural studies, whether the Ca2+-transporting subunits form hetero-hexamers in situ, and the nature of the megacomplex involving the ATP synthase in situ. The regulation of the activity of the MCU complex in vivo also needs to be investigated.
ACKNOWLEDGEMENTS
This work was funded by the U.S. National Institutes of Health (grants AI140421 and AI108222) and the São Paulo Research Foundation (FAPESP, Brazil, grant 2013/50624–0). N.L. is a postdoctoral trainee supported by the U.S. National Institutes of Health under award number K99-AI137322.
REFERENCES
- Adl SM, Simpson AG, Lane CE, Lukes J, Bass D, Bowser SS, Brown MW, Burki F, Dunthorn M, Hampl V, Heiss A, Hoppenrath M, Lara E, Le Gall L, Lynn DH, McManus H, Mitchell EA, Mozley-Stanridge SE, Parfrey LW, Pawlowski J, Rueckert S, Shadwick L, Schoch CL, Smirnov A,Spiegel FW, 2012. The revised classification of eukaryotes. J Eukaryot Microbiol 59, 429–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr.,Jonas EA, 2014. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 111, 10580–10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baradaran R, Wang C, Siliciano AF,Long SB, 2018. Cryo-EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature 559, 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley W, Amoore JE, 1958. The effects of manganese on the solute content of rat-liver mitochondria. Biochem J 69, 348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V,Mootha VK, 2011. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaim G, Bermudez R,Urbina JA, 1990. Ca2+ transport in isolated mitochondrial vesicles from Leishmania braziliensis promastigotes. Mol Biochem Parasitol 39, 61–68. [DOI] [PubMed] [Google Scholar]
- Benne R, Van den Burg J, Brakenhoff JP, Sloof P, Van Boom JH,Tromp MC, 1986. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell 46, 819–826. [DOI] [PubMed] [Google Scholar]
- Bertolini MS, Chiurillo MA, Lander N, Vercesi AE,Docampo R, 2019. MICU1 and MICU2 Play an Essential Role in Mitochondrial Ca(2+) Uptake, Growth, and Infectivity of the Human Pathogen Trypanosoma cruzi. mBio 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, Wieckowski MR, Kroemer G, Galluzzi L,Pinton P, 2013. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle 12, 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora M, Morganti C, Morciano G, Pedriali G, Lebiedzinska-Arciszewska M, Aquila G, Giorgi C, Rizzo P, Campo G, Ferrari R, Kroemer G, Wieckowski MR, Galluzzi L,Pinton P, 2017. Mitochondrial permeability transition involves dissociation of F1FO ATP synthase dimers and C-ring conformation. EMBO Rep 18, 1077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E, Balcavage WX, Lehninger AL,Mattoon JR, 1970. Ca2+ metabolism in yeast cells and mitochondria. Biochim Biophys Acta 205, 18–26. [DOI] [PubMed] [Google Scholar]
- Carafoli E, Lehninger AL, 1971. A survey of the interaction of calcium ions with mitochondria from different tissues and species. Biochem J 122, 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E, Tiozzo R, Lugli G, Crovetti F,Kratzing C, 1974. The release of calcium from heart mitochondria by sodium. J Mol Cell Cardiol 6, 361–371. [DOI] [PubMed] [Google Scholar]
- Chiurillo MA, Lander ES, Vercesi AE,Docampo R, 2020. IP3 Receptor-Mediated Ca2+ Release from Acidocalcisomes Regulates Mitochondrial Bioenergetics and Prevents Autophagy in Trypanosoma cruzi Cell Calcium. [DOI] [PubMed] [Google Scholar]
- Chiurillo MA, Lander N, Bertolini MS, Storey M, Vercesi AE,Docampo R, 2017. Different Roles of Mitochondrial Calcium Uniporter Complex Subunits in Growth and Infectivity of Trypanosoma cruzi. mBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurillo MA, Lander N, Bertolini MS, Vercesi AE,Docampo R, 2019. Functional analysis and importance for host cell infection of the Ca(2+)-conducting subunits of the mitochondrial calcium uniporter of Trypanosoma cruzi. Mol Biol Cell 30, 1676–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AB Jr., Bienen EJ, Pollakis G,Grady RW, 1989. Respiration of bloodstream forms of the parasite Trypanosoma brucei brucei is dependent on a plant-like alternative oxidase. J Biol Chem 264, 17770–17776. [PubMed] [Google Scholar]
- Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S., Bogorad R., Koteliansky V., Adijanto J., Mootha VK.,Hajnoczky., 2013. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca(2)(+) uniporter. Cell Metab 17, 976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I,Rizzuto R, 2011. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca HF, Engstrom GW, 1961. Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci U S A 47, 1744–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton RM, 2009. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787, 1309–1316. [DOI] [PubMed] [Google Scholar]
- Docampo R, Lopes JN, Cruz FS,Souza W, 1977. Trypanosoma cruzi: ultrastructural and metabolic alterations of epimastigotes by beta-lapachone. Exp Parasitol 42, 142–149. [DOI] [PubMed] [Google Scholar]
- Docampo R, Lukes J, 2012. Trypanosomes and the solution to a 50-year mitochondrial calcium mystery. Trends Parasitol 28, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, Vercesi AE, 1989a. Ca2+ transport by coupled Trypanosoma cruzi mitochondria in situ. J Biol Chem 264, 108–111. [PubMed] [Google Scholar]
- Docampo R, Vercesi AE, 1989b. Characteristics of Ca2+ transport by Trypanosoma cruzi mitochondria in situ. Arch Biochem Biophys 272, 122–129. [DOI] [PubMed] [Google Scholar]
- Docampo R, Vercesi AE,Huang G, 2014. Mitochondrial calcium transport in trypanosomes. Mol Biochem Parasitol 196, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin PC, Burleigh BA, 2018. Stress-Induced Proliferation and Cell Cycle Plasticity of Intracellular Trypanosoma cruzi Amastigotes. mBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Fan M, Orlando BJ, Fastman NM, Zhang J, Xu Y, Chambers MG, Xu X, Perry K, Liao M,Feng L, 2018. X-ray and cryo-EM structures of the mitochondrial calcium uniporter. Nature 559, 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannavaram S, Vedvyas C,Debrabant A, 2008. Conservation of the pro-apoptotic nuclease activity of endonuclease G in unicellular trypanosomatid parasites. J Cell Sci 121, 99–109. [DOI] [PubMed] [Google Scholar]
- Giorgio V, Burchell V, Schiavone M, Bassot C, Minervini G, Petronilli V, Argenton F, Forte M, Tosatto S, Lippe G,Bernardi P, 2017. Ca(2+) binding to F-ATP synthase beta subunit triggers the mitochondrial permeability transition. EMBO Rep 18, 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenawalt JW, Carafoli E, 1966. Electron microscope studies on the active accumulation of Sr++ by rat-liver mitochondria. J Cell Biol 29, 37–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Zhang L, Zong S, Guo R, Liu T, Yi J, Wang P, Zhuo W,Yang M, 2019. Cryo-EM structure of the mammalian ATP synthase tetramer bound with inhibitory protein IF1. Science 364, 1068–1075. [DOI] [PubMed] [Google Scholar]
- Hashimi H, McDonald L, Stribrna E,Lukes J, 2013. Trypanosome Letm1 protein is essential for mitochondrial potassium homeostasis. J Biol Chem 288, 26914–26925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Bartlett PJ, Thomas AP, Moreno SN,Docampo R, 2013a. Acidocalcisomes of Trypanosoma brucei have an inositol 1,4,5-trisphosphate receptor that is required for growth and infectivity. Proc Natl Acad Sci U S A 110, 1887–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Docampo R, 2018. The Mitochondrial Ca(2+) Uniporter Complex (MCUC) of Trypanosoma brucei Is a Hetero-oligomer That Contains Novel Subunits Essential for Ca(2+) Uptake. mBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Docampo R, 2020. The Mitochondrial Calcium Uniporter Interacts with Subunit c of the ATP Synthase of Trypanosomes and Humans. mBio 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Vercesi AE,Docampo R, 2013b. Essential regulation of cell bioenergetics in Trypanosoma brucei by the mitochondrial calcium uniporter. Nat Commun 4, 2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard MJ, McHugh NJ, 1996. Mitochondrial ATP synthase F1-beta-subunit is a calcium-binding protein. FEBS Lett 391, 323–329. [DOI] [PubMed] [Google Scholar]
- Irigoin F, Inada NM, Fernandes MP, Piacenza L, Gadelha FR, Vercesi AE,Radi R, 2009. Mitochondrial calcium overload triggers complement-dependent superoxide-mediated programmed cell death in Trypanosoma cruzi. Biochem J 418, 595–604. [DOI] [PubMed] [Google Scholar]
- Jiang D, Zhao L,Clapham DE, 2009. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326, 144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczanowski S, Sajid M,Reece SE, 2011. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasit Vectors 4, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer KJ, Grabarek Z,Mootha VK, 2017. High-affinity cooperative Ca(2+) binding by MICU1-MICU2 serves as an on-off switch for the uniporter. EMBO Rep 18, 1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G,Clapham DE, 2004. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360–364. [DOI] [PubMed] [Google Scholar]
- Ko YH, Delannoy M, Hullihen J, Chiu W,Pedersen PL, 2003. Mitochondrial ATP synthasome. Cristae-enriched membranes and a multiwell detergent screening assay yield dispersed single complexes containing the ATP synthase and carriers for Pi and ADP/ATP. J Biol Chem 278, 12305–12309. [DOI] [PubMed] [Google Scholar]
- Lamour N, Riviere L, Coustou V, Coombs GH, Barrett MP,Bringaud F, 2005. Proline metabolism in procyclic Trypanosoma brucei is down-regulated in the presence of glucose. J Biol Chem 280, 11902–11910. [DOI] [PubMed] [Google Scholar]
- Lander N, Chiurillo MA, Bertolini MS, Storey M, Vercesi AE,Docampo R, 2018. Calcium-sensitive pyruvate dehydrogenase phosphatase is required for energy metabolism, growth, differentiation, and infectivity of Trypanosoma cruzi. J Biol Chem 293, 17402–17417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander N, Chiurillo MA, Storey M, Vercesi AE,Docampo R, 2016. CRISPR/Cas9-mediated endogenous C-terminal Tagging of Trypanosoma cruzi Genes Reveals the Acidocalcisome Localization of the Inositol 1,4,5-Trisphosphate Receptor. J Biol Chem 291, 25505–25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander N, Li ZH, Niyogi S,Docampo R, 2015. CRISPR/Cas9-Induced Disruption of Paraflagellar Rod Protein 1 and 2 Genes in Trypanosoma cruzi Reveals Their Role in Flagellar Attachment. mBio 6, e01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger AL, Rossi CS,Greenawalt JW, 1963. Respiration-dependent accumulation of inorganic phosphate and Ca ions by rat liver mitochondria. Biochem Biophys Res Commun 10, 444–448. [DOI] [PubMed] [Google Scholar]
- Leroux AE, Maugeri DA, Cazzulo JJ,Nowicki C, 2011. Functional characterization of NADP-dependent isocitrate dehydrogenase isozymes from Trypanosoma cruzi. Molecular and biochemical parasitology 177, 61–64. [DOI] [PubMed] [Google Scholar]
- Linstead DJ, Klein RA,Cross GA, 1977. Threonine catabolism in Trypanosoma brucei. J Gen Microbiol 101, 243–251. [DOI] [PubMed] [Google Scholar]
- Liu JC, Liu J, Holmstrom KM, Menazza S, Parks RJ, Fergusson MM, Yu ZX, Springer DA, Halsey C, Liu C, Murphy E,Finkel T, 2016. MICU1 Serves as a Molecular Gatekeeper to Prevent In Vivo Mitochondrial Calcium Overload. Cell Rep 16, 1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, Miller R, Kolesar JE, Molgo J, Kaufman B, Hajnoczky G, Foskett JK,Madesh M, 2012a. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol 14, 1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, Hoffman NE, Gandhirajan RK, Molgo J, Birnbaum MJ, Rothberg BS, Mak DO, Foskett JK,Madesh M, 2012b. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell 151, 630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matesanz-Isabel J, Arias-del-Val J, Alvarez-Illera P, Fonteriz RI, Montero M,Alvarez J, 2016. Functional roles of MICU1 and MICU2 in mitochondrial Ca(2+) uptake. Biochim Biophys Acta 1858, 1110–1117. [DOI] [PubMed] [Google Scholar]
- McCormack JG, Denton RM, 1986. Ca2+ as a second messenger within mitochondria. Trends in Biochemical Sciences 11, 258–262. [Google Scholar]
- Millerioux Y, Morand P, Biran M, Mazet M, Moreau P, Wargnies M, Ebikeme C, Deramchia K, Gales L, Portais JC, Boshart M, Franconi JM,Bringaud F, 2012. ATP synthesis-coupled and -uncoupled acetate production from acetyl-CoA by mitochondrial acetate:succinate CoA-transferase and acetyl-CoA thioesterase in Trypanosoma. The Journal of biological chemistry 287, 17186–17197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K, Benchimol M, Docampo R,de Souza W, 2000. The fine structure of acidocalcisomes in Trypanosoma cruzi. Parasitol Res 86, 373–384. [DOI] [PubMed] [Google Scholar]
- Moore CL, 1971. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun 42, 298–305. [DOI] [PubMed] [Google Scholar]
- Moreno SN, Docampo R,Vercesi AE, 1992. Calcium homeostasis in procyclic and bloodstream forms of Trypanosoma brucei. Lack of inositol 1,4,5-trisphosphate-sensitive Ca2+ release. The Journal of biological chemistry 267, 6020–6026. [PubMed] [Google Scholar]
- Nguyen NX AJ-P, Lee C, Yang Y, Zeng W, Mootha VK, Cheng Y, Bai X-c, a, 2018. Cryo-EM structure of a fungal mitochondrial calcium uniporter. Nature 559, 570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NX, Armache JP, Lee C, Yang Y, Zeng W, Mootha VK, Cheng Y, Bai XC,Jiang Y, 2018. Cryo-EM structure of a fungal mitochondrial calcium uniporter. Nature 559, 570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenoid K, Dong Y, Cao C, Cui T, Sancak Y, Markhard AL, Grabarek Z, Kong L, Liu Z, Ouyang B, Cong Y, Mootha VK,Chou JJ, 2016. Architecture of the mitochondrial calcium uniporter. Nature 533, 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard M, Csordas G, Szanda G, Golenar T, Debattisti V, Bartok A, Wang N, Moffat C, Seifert EL, Spat A,Hajnoczky G, 2017. Tissue-Specific Mitochondrial Decoding of Cytoplasmic Ca(2+) Signals Is Controlled by the Stoichiometry of MICU1/2 and MCU. Cell Rep 18, 2291–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D,Sekler I, 2010. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A 107, 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabo I, De Stefani D,Rizzuto R, 2014. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell 53, 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R, Hoff H, Roskowski A,Foskett JK, 2017. MICU2 Restricts Spatial Crosstalk between InsP3R and MCU Channels by Regulating Threshold and Gain of MICU1-Mediated Inhibition and Activation of MCU. Cell Rep 21, 3141–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE,Mootha VK, 2010. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature 467, 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittis AA, Goh V, Cebrian-Serrano A, Wettmarshausen J, Perocchi F,Gabaldon T, 2020. Discovery of EMRE in fungi resolves the true evolutionary history of the mitochondrial calcium uniporter. Nat Commun 11, 4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, Taneja N, Oshea J, Koteliansky V,Mootha VK, 2013. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS One 8, e55785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabo I,Rizzuto R, 2013. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. The EMBO journal 32, 2362–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan S, Asady B,Docampo R, 2018. Acidocalcisome-Mitochondrion Membrane Contact Sites in Trypanosoma brucei. Pathogens 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgley EL, Xiong ZH,Ruben L, 1999. Reactive oxygen species activate a Ca2+-dependent cell death pathway in the unicellular organism Trypanosoma brucei brucei. Biochem J 340 ( Pt 1), 33–40. [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Raffaello A,Mammucari C, 2012. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13, 566–578. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, Goldberger O,Mootha VK, 2013. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342, 1379–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satrustegui J, Pardo B,Del Arco A, 2007. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev 87, 29–67. [DOI] [PubMed] [Google Scholar]
- Schenkman S, Robbins ES,Nussenzweig V, 1991. Attachment of Trypanosoma cruzi to mammalian cells requires parasite energy, and invasion can be independent of the target cell cytoskeleton. Infect Immun 59, 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman D, Johnson D, Gerbasi V, Golden D, Orlando R,Hajduk S, 2012. Mitochondrial membrane complex that contains proteins necessary for tRNA import in Trypanosoma brucei. J Biol Chem 287, 8892–8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomai J, 2004. The structure and replication of kinetoplast DNA. Curr Mol Med 4, 623–647. [DOI] [PubMed] [Google Scholar]
- Smirlis D, Duszenko M, Ruiz AJ, Scoulica E, Bastien P, Fasel N,Soteriadou K, 2010. Targeting essential pathways in trypanosomatids gives insights into protozoan mechanisms of cell death. Parasit Vectors 3, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirlis D, Soteriadou K, 2011. Trypanosomatid apoptosis: ‘Apoptosis’ without the canonical regulators. Virulence 2, 253–256. [DOI] [PubMed] [Google Scholar]
- Stephens JL, Lee SH, Paul KS,Englund PT, 2007. Mitochondrial fatty acid synthesis in Trypanosoma brucei. J Biol Chem 282, 4427–4436. [DOI] [PubMed] [Google Scholar]
- Surve S, Heestand M, Panicucci B, Schnaufer A,Parsons M, 2012. Enigmatic presence of mitochondrial complex I in Trypanosoma brucei bloodstream forms. Eukaryot Cell 11, 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague WM, Pettit FH, Wu TL, Silberman SR,Reed LJ, 1982. Purification and properties of pyruvate dehydrogenase phosphatase from bovine heart and kidney. Biochemistry 21, 5585–5592. [DOI] [PubMed] [Google Scholar]
- Territo PR, French SA, Dunleavy MC, Evans FJ,Balaban RS, 2001. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mVO2, NADH, AND light scattering. J Biol Chem 276, 2586–2599. [DOI] [PubMed] [Google Scholar]
- Territo PR, Mootha VK, French SA,Balaban RS, 2000. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am J Physiol Cell Physiol 278, C423–435. [DOI] [PubMed] [Google Scholar]
- Tsai MF, Jiang D, Zhao L, Clapham D,Miller C, 2014. Functional reconstitution of the mitochondrial Ca2+/H+ antiporter Letm1. The Journal of general physiology 143, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkan A, Hiromasa Y,Roche TE, 2004. Formation of a complex of the catalytic subunit of pyruvate dehydrogenase phosphatase isoform 1 (PDP1c) and the L2 domain forms a Ca2+ binding site and captures PDP1c as a monomer. Biochemistry 43, 15073–15085. [DOI] [PubMed] [Google Scholar]
- Urbani A, Giorgio V, Carrer A, Franchin C, Arrigoni G, Jiko C, Abe K, Maeda S, Shinzawa-Itoh K, Bogers JFM, McMillan DGG, Gerle C, Szabo I,Bernardi P, 2019. Purified F-ATP synthase forms a Ca(2+)-dependent high-conductance channel matching the mitochondrial permeability transition pore. Nat Commun 10, 4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hellemond JJ, Opperdoes FR,Tielens AG, 1998. Trypanosomatidae produce acetate via a mitochondrial acetate:succinate CoA transferase. Proceedings of the National Academy of Sciences of the United States of America 95, 3036–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hellemond JJ, Opperdoes FR,Tielens AG, 2005. The extraordinary mitochondrion and unusual citric acid cycle in Trypanosoma brucei. Biochem Soc Trans 33, 967–971. [DOI] [PubMed] [Google Scholar]
- Vanderheyden N, Wong J,Docampo R, 2000. A pyruvate-proton symport and an H+-ATPase regulate the intracellular pH of Trypanosoma brucei at different stages of its life cycle. Biochem J 346 Pt 1, 53–62. [PMC free article] [PubMed] [Google Scholar]
- Vasington FD, Murphy JV, 1962. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem 237, 2670–2677. [PubMed] [Google Scholar]
- Vercesi AE, Docampo R, 1992. Ca2+ transport by digitonin-permeabilized Leishmania donovani. Effects of Ca2+, pentamidine and WR-6026 on mitochondrial membrane potential in situ. The Biochemical journal 284 ( Pt 2), 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercesi AE, Docampo R,Moreno SN, 1992. Energization-dependent Ca2+ accumulation in Trypanosoma brucei bloodstream and procyclic trypomastigotes mitochondria. Mol Biochem Parasitol 56, 251–257. [DOI] [PubMed] [Google Scholar]
- Vercesi AE, Macedo DV, Lima SA, Gadelha FR,Docampo R, 1990. Ca2+ transport in digitonin-permeabilized trypanosomatids. Mol Biochem Parasitol 42, 119–124. [DOI] [PubMed] [Google Scholar]
- Vercesi AE, Moreno SN, Bernardes CF, Meinicke AR, Fernandes EC,Docampo R, 1993. Thapsigargin causes Ca2+ release and collapse of the membrane potential of Trypanosoma brucei mitochondria in situ and of isolated rat liver mitochondria. The Journal of biological chemistry 268, 8564–8568. [PubMed] [Google Scholar]
- Xiong ZH, Ridgley EL, Enis D, Olness F,Ruben L, 1997. Selective transfer of calcium from an acidic compartment to the mitochondrion of Trypanosoma brucei. Measurements with targeted aequorins. The Journal of biological chemistry 272, 31022–31028. [DOI] [PubMed] [Google Scholar]
- Xiong ZH, Ruben L, 1998. Trypanosoma brucei: the dynamics of calcium movement between the cytosol, nucleus, and mitochondrion of intact cells. Exp Parasitol 88, 231–239. [DOI] [PubMed] [Google Scholar]
- Ying WL, Emerson J, Clarke MJ,Sanadi DR, 1991. Inhibition of mitochondrial calcium ion transport by an oxo-bridged dinuclear ruthenium ammine complex. Biochemistry 30, 4949–4952. [DOI] [PubMed] [Google Scholar]
- Yoo J, Wu M, Yin Y, Herzik MA Jr., Lander GC,Lee SY, 2018. Cryo-EM structure of a mitochondrial calcium uniporter. Science 361, 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharov SD, Ewy RG,Dilley RA, 1993. Subunit III of the chloroplast ATP-synthase can form a Ca(2+)-binding site on the lumenal side of the thylakoid membrane. FEBS Lett 336, 95–99. [DOI] [PubMed] [Google Scholar]
- Zhuo Y, Cordeiro CD, Hekmatyar SK, Docampo R,Prestegard JH, 2017. Dynamic nuclear polarization facilitates monitoring of pyruvate metabolism in Trypanosoma brucei. J Biol Chem 292, 18161–18168. [DOI] [PMC free article] [PubMed] [Google Scholar]


