Abstract
The necessity of using petrochemicals for the development of polymers has been deteriorating because of the depletion in fossil fuels and environmental concerns such as the effect of greenhouse gases, global warming, and increasing population. Research has shown a shift from petroleum-based fuels to plant oil–based fuels in order to shift to renewable resources. Natural oils such as castor oil have shown competitive physical and chemical properties as compared to fossil fuels. The use of natural oils has gained a lot of research interest due to the fact that they are renewable, affordable, and environmentally friendly. Bio-oils are versatile because they have various derivatives and can be used in different grades based on the application in various industries such as agriculture, food, paper, and electronics. Bio-binders have been considered as the most promising materials for the different applications. In this review, the processes of chemical modifications of castor oil are discussed.
Keywords: Castor oil, bio-oil, castor oil industry, chemistry of castor oil, bio-binders
Introduction
The world that we are living in today has shown various areas of concern such as the effect of greenhouse gases, global warming, increasing population, and depletion of natural resources. As a result, scientists and engineers are looking for alternative ways to implement the use of renewable resources with required traits for applications in various industries 1 such as paints, cosmetics, coats, and lubricants. 2 These industries use different types of natural oils such as castor oil, olive oil, soybean oil, and linseed oil. Most technologies are concentrating on the use of castor oil because it is a cost-effective renewable resource; therefore, this then allows for research in the application of vegetable oils for the production of polymers that contain good chemical and physical properties for different applications. 3 The use of bio-based polymers and renewable resources was part of the first technology of polymers that were used; however, the use of fossil fuels gained more popularity due to the application of synthetic polymers. The growth in population, however, has led to the high demand of synthetic polymers and, as a result, the depletion of fossil fuels which leads to the return to biofuels as a source of renewable resources. 4
Most polymers such as polyurethane, epoxy, polyesteramide, and polyetheramide are produced from natural oils. Research has shown that these polymers are considered to be biodegradable, less volatile, non-toxic, and easy to modify, and the oils can be obtained in large quantities. These polymers are used for different applications such as binders and in different industries such as the automotive industry, 5 civil engineering,6,7 and the coating industry. 8
Castor oil overview
The castor oil industry
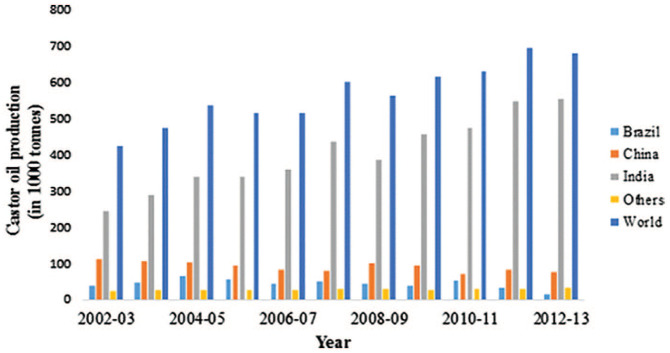
Previously, agricultural raw materials such as vegetable oils were mainly used for food purposes; however, their need for applications in non-food industries has increased. 9 Due to the high demand of renewable resources, especially from the Third World countries such as the United States of America, India, and Brazil; India has been found to be the biggest contributor to this industry and the trend has been seen to increase over the years. From 2003 to 2013 (Figure 1), the castor oil market all over the world has shown that it will grow to over 1.8 billion dollars by 2020. 10
Figure 1.
Production and percentage share of castor oil by major producing countries (1000 tons) during 2002–2013. 10
The use of biofuels has shown growth over the past two decades and hence has been of interest in the current research and new technology development. Currently, there is an increase in the bio-based economy as an alternative to the fossil fuel–based economy. Biofuels are used because they are renewable, cheap, and environmentally friendly and they come in two phases, that is, liquid and gas. 11 The price of crude oil also increased significantly in the past decade, that is, from US$150 to US$1000, hence increasing the demand to replace crude oil with vegetable oils such as castor oil. Annual production of castor oil is 271 million tons 12 and obtained in a pale color in the market and contains the properties that are outlined in Table 1.
Table 1.
| Non-edible oil seeds | Oil content (%) | Seed estimate (106 Tonnes/year | Oil yield (Tonnes/ Ha/ year) |
|---|---|---|---|
| Castor | 45–50 | 0.25 | 0.45–1.00 |
| Jatropha | 20–60 | 0.20 | 1.90–3.00 |
| Karanja | 25–50 | 0.06 | 2.00–4.00 |
| Mahau | 35–40 | 0.20 | 1.00–4.00 |
| Neem | 20–30 | 0.10 | 2.00–3.00 |
Table 1 shows the amounts of oil yields (tons/ha/year) and castor seeds give the lowest yield of oil as compared to the other seeds,13,14 though castor seeds are the highest each year and with a high oil content. 15 This is because of the difficulties involved in harvesting castor seeds, for example, it is easier to harvest Jatropha seeds as compared to castor seeds because they are less poisonous while harvesting. 1
Table 2 shows the physio-chemical properties of comparative non-edible vegetable oils that are applied in the lubricant industry.5,16 The densities of the oils are relatively similar, with castor oil having a density of 899 kg/m3. However, castor oil can be different from the other oils based on viscosity, oxidation stability, cloud point, and flash point. Castor oil has the highest viscosity of 15.25 (40°C, mm2 s−2), meaning that it is thicker than the other oils and therefore making it more stable at harsh temperatures. 17 Castor oil has the lowest oxidative stability (1.1 at 110°C, h), and the lower oxidative stability is obtained due to double bonds and hydroxyl functional groups that are present because they are likely to be broken into single bonds, therefore resulting in the formation of volatiles. The low oxidative stability is also due to the high fatty acid content; the more acidic the oil is, the more it is prone to oxidation. 18 Castor oil also has a very low cloud point, meaning that there is no presence of solidified waxes that thickens the oil and clog filters, pumps, and/or injectors in processing plants. The low cloud point also makes the castor oil suitable to be used in very cold environments for applications such as biodiesel. 19 And the highest flash point of castor oil means that the oil is not prone to ignition (less flammable) as compared to the others, 20 therefore, being safer to handle or transport at temperatures below 260°C.
Table 2.
| Vegetable oils | Density (Kg/m3) | Kinematic Viscosity ((40°C), mm2/s) | Oxidation Stability (110°C, h) | Cloud Point (°C) | Flash Point (°C) |
|---|---|---|---|---|---|
| Castor | 899–955 | 15.25 | 1.1 | –13.4 | 260 |
| Jatropha | 880–940 | 4.8 | 2.3 | 2.7 | 135–225 |
| Karanja | 920–936.5 | 4.8 | 6 | 9 | 150 |
| Mahau | 850–960 | 3.98 | – | – | 208–232 |
| Neem | 884–918.5 | 5.21 | 7.1 | 14.4 | 42 |
The vegetable oil plants in Table 2 can be grown and established in marginal and semi-marginal land under varied agro-climatic conditions. Therefore, edible oilseed usage for fuel and lubricant needs may not be able to meet the domestic requirements of the ever-increasing population unless more tropical environments get involved in the agriculture of farming vegetable oils. As an alternative, non-edible vegetable oil can prove to be worthwhile due to the depletion of fossil fuels and increasing populations.5,21
Bio-oils are obtained from both plants and animals, such as vegetables, plants (algae), and fish (Table 3). Plant oils, which are of interest to us, are considered to be lipids which contain both hydrophilic and lipophilic functional groups. The oil is a mixture of different triacylglycerols (or triglycerides). 4
Table 3.
| Properties | Bio-oil | |
|---|---|---|
| Ultimate analysis (W/W %) | Castor shell | Repseed |
| Carbon | 74.5 | 52.25 |
| Hydrogen | 10.76 | 8.06 |
| Nitrogen | 2.56 | 3.91 |
| Oxygen | 11.78 | 35.78 |
| H/C molar ratio | 1.76 | 1.85 |
| O/C molar ratio | 0.12 | 0.51 |
| Calorific value MJ/kg | 35.01 | 28.36 |
| Empirical formula | CH1.76O0.15N0.03 | CH1.85O0.51N0.06 |
The various applications have led to the new emerging markets that are looking at biopolymers such as polyurethanes and polyethylene. 24
The other reason for increasing interest in biofuels is that lignin-containing byproducts are obtained in biofuel production plants, therefore leading to various applications as well. 11 The plant history and the chemistry of castor oil enable the research into bio-binders and polymers to vary for different applications.
The origin of castor oil
Castor oil is derived from the seeds of a castor plant, also known as Ricinus communis, which forms part of the Euphorbiaceae family. The castor seeds usually contain up to 50% of the castor oil. 10 The castor plant is non-determinant, the seed grows at a different rate, hence resulting in harvesting challenges, and the fact that the plant grows in rather difficult environments do not help because it also contributes to harvesting challenges. The other challenge is that the seed produces lipids with toxic ricin that can be considered to be an allergen, therefore making conventional harvesting a challenge and poisonous to harvesters. However, the composition of fatty acids in castor oil makes it worthwhile to harvest. 25
The chemistry of castor oil
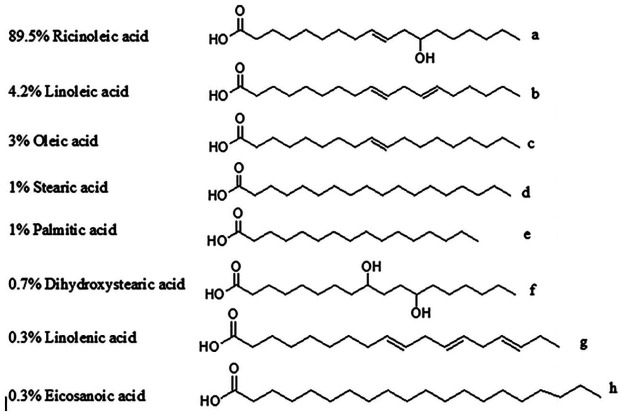
Castor oils contain both a hydroxyl group and an olefinic link. 26 The ricinoleic acid forms approximately 90% (Figure 2) of the total fatty acid content in castor oil 25 and the rest accounts for 10%. The ricinoleic structure contains most of the chemical and physical properties of castor oil as shown in Figure 2. 10 The hydroxyl group enhances the physical and chemical properties of castor oil. The physical properties are higher viscosity and higher solubility in most solvents. 27 The chemistry of castor oils allows the modification of its different functional groups such as the hydroxyl group, vinyl group, and the ester group.
Figure 2.
Composition of castor oil fatty acid. 10
Castor oil does not show any drying properties; however, it is still of interest in coating industries because it is stable. 28 It is available commercially in the form of natural hydroxylated triglycerides. The hydroxyl group that is contained in triglycerides has a significant impact on the physical properties such as high viscosity, stability, and miscibility in polar solvents. Castor oil can be used as a viscosity modifier in polymeric materials. The structure of castor oil such as the trifunctional nature contributes to the toughness of the oil and the long fatting acid chain to its flexibility for modification. The structural features of castor oil are high solubility, small hydrodynamic diameter, and lower melting point. 29 Most research on castor oil focuses on the hydroxyl group as discussed by Mutlu and Meier. 30 The well-defined composition of castor oil in terms of ricinoleic acid makes the processing much easier because the target is the largest component, whereas, with the other oils, they have two or more major components, for example, the soybean has oleic (23.26%) and linoleic (55.53%) acids, which makes it difficult to separate (Table 4). The other advantage of using castor oil is the fact that it is non-edible and therefore does not compete with food security.
Table 4.
| Vegetable oil | Fatty acid composition (% w/w) | ||||||
|---|---|---|---|---|---|---|---|
| Palmitic C16:0 | Stearic C18:0 | Oleic C18:1 | Ricinoleic C18:1 variety | Linoleic C18:2 | Linolenic C18:3 | Arachidic C20:0 | |
| Corn | 6.5–11.67 | 1.4–1.85 | 25.16–25.2 | 0.00 | 60.6–65.6 | 0.1–0.48 | 0.1–0.24 |
| Canola | 3.49 | 0.85 | 64.4 | 0.00 | 22.30 | 8.23 | 0.00 |
| Soybean | 11.3–11.75 | 3.15–3.6 | 23.26–24.9 | 0.00 | 53.0–55.53 | 6.16.31 | 0.00–0.3 |
| Sunflower | 6.08–6.2 | 3.26–3.7 | 16.93–25.2 | 0.00 | 63.1–73.73 | 0.00–0.2 | 0.00–0.3 |
| Castor | 0.70 | 0.90 | 2.80 | 90.20 | 4.40 | 0.20 | 0.00 |
Castor oil–based chemical processes
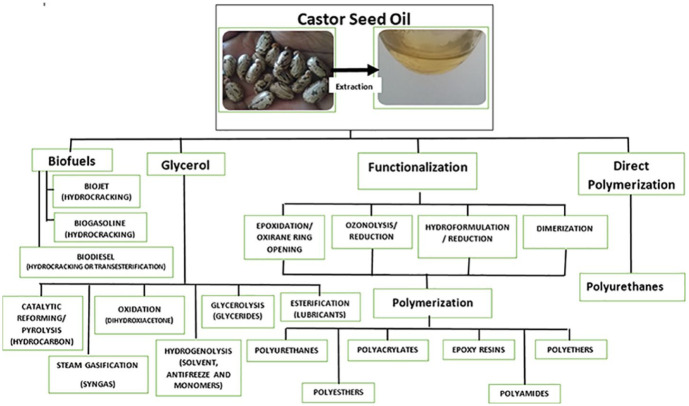
The unique properties of castor oil can be manipulated from different chemical reactions by exploiting the different functional groups such as the ester linkage, hydroxyl group, and the vinyl group. These functional groups can be modified through processes such as transesterification, epoxidation, dehydration, pyrolysis, hydrogenation, ozonolysis, sulfation, and hydrolysis. 34 The chemical modifications of castor oil are clearly illustrated below (Figure 3). From the versatile list of processes to follow, the process should be energy efficient, green, and less labor intensive. This is because the aspects of health, safety, and environment need to be taken into account.
Figure 3.
Routes for the synthesis of various products from vegetable oils.35–37
Hydrogenation
The process of hydrogenation involves the introduction of a hydrogen atom to the unsaturated fatty acid in the presence of a catalyst (e.g. nickel or palladium; Figure 4). The hydrogen converts the ricinoleic acid into a saturated 12-hydroxystearic acid that is semi-solid. The semi-solid material (wax-like) is highly used in the industry for polymer materials. 10 Catalytic transfer hydrogenation (CHT) is another route for the hydrogenation of castor oil. The advantage with CHT is that it can be used at ambient temperatures and pressures in organic solvents, therefore leading to less energy consumption. The CHT does not require distinct reactors and the solvent used can also be used as a hydrogen donor in the presence of a catalyst. The temperatures that are often used for hydrogenation range from 115°C to 180°C; temperatures are low due to the presence of the catalyst, and therefore less energy is required in the process. 38
Figure 4.
Hydrogenation of castor oil.
Hydrogenated castor oil is insoluble in water and organic solvents at ambient temperature; however, it is soluble at high temperatures and therefore highly recommended for industries. Hydrogenated castor oil has enhanced thermal and oxidative stability. 10 Hydrogenated castor oil also has a longer shelf life and it is odorless and tasteless.
The hydrogenated castor oil and its derivatives are used for plasticizers of nitrile rubbers because of optimum tensile strength, elongation at break, and the resistance of rubber to swelling due to other oils (e.g. motor oil) that it can interact with. In lubricant, paint, cosmetic, and miscellaneous industries, hydrogenated castor oil is used. 39
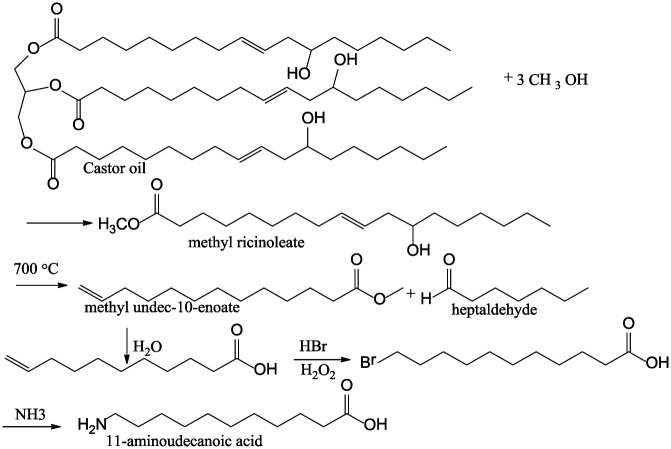
Pyrolysis
Pyrolysis is a highly recommended thermal method that is used for the conversion of biomass in the absence of oxygen into solid (char), liquid, or gaseous phases. 40 The pyrolysis of castor oil splits the molecule to produce undecylenic acid and heptaldehyde. In the presence of air, the biomass will undergo combustion and give off carbon dioxide (CO2) as a product. The process is conducted at high temperatures ranging from 345°C to 752°C, and the pyrolysis bio-oil is obtained 41 (Figure 5). The review of pyrolysis of various oils has been reported. 41 The presence or absence of a catalyst determines if the product will have a higher or lower molecular weight (i.e. more like diesel or gasoline). The pyrolysis of vegetable oils in the presence of a catalyst is much more common than direct thermal cracking and the main catalysts can be grouped into the following types: (a) molecular sieve catalysts, (b) activated alumina catalysts, (c) transition metal catalysts, and (d) sodium carbonate. 41
Figure 5.
Pyrolysis of castor oil.
Pyrolysis of castor oil usually takes place at 350°C for approximately 30 min in the pressure range of 1.47–1.96 MPa. 41
The castor oil can be converted into petrochemical feedstock (biodiesel) using thermal energy through a process called thermal cracking.41,42 However, an increase in temperature results in a decrease in density as indicated in Table 5. 43 This process is energy intensive due to extremely high temperatures, and therefore this might affect the cost of production. This is also a multi-step process that will require a large processing plant, hence very capital intensive to the commission.
Table 5.
Effect of temperature on density when using pyrolysis.
| Sample | Temperature (°C) | Density (g/mL) |
|---|---|---|
| 1 | 350 | 0.84 |
| 2 | 400 | 0.832 |
| 3 | 450 | 0.82 |
Fast pyrolysis produces up to 80 wt% liquid on dry feed. The remaining 20 wt% is from gaseous products consisting of aerosols, true vapors, non-condensable gases, and solid char. 44 The bio-oil from pyrolysis is dark brown in color. 45 Some of the major disadvantages of using the bio-oil as the diesel fuel are its low higher heating value (HHV) which is approximately 40% less than that of the fuel oil, high viscosity, and substantial amount of solid content. 45
Hydrolysis
The hydrolysis of castor oil is usually conducted through the addition of sodium hydroxide (80%) at approximately 250°C–360°C and results in the formation of glycerol and ricinoleic acid that can be further converted into sebacic acid and capryl alcohol (Figure 6). Sebacic acid is used as a monomer for the synthesis of nylon when reacted with hexamethylenediamine. The alcohol is often used as the solvent, dehydrator, floatation agent, and anti-bubbling agent. 46 The positive outcome of this process is that it is a green process because water is used in the process, and hence it is a more environmentally friendly alternative.
Figure 6.
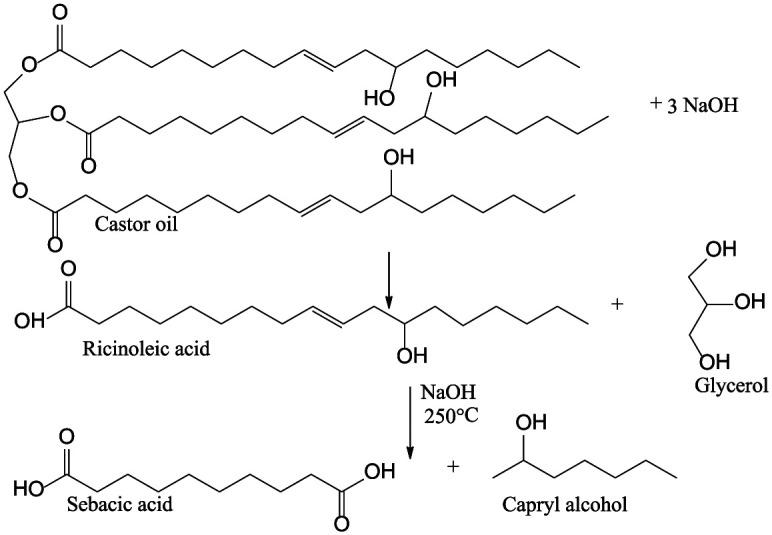
Hydrolysis of castor oil.
Dehydration
In this process, ricinoleic acid, which is a major component of the castor oil, is reacted with an acid, such as sulfuric acid or phosphoric acid, which acts as a catalyst, to remove the hydroxyl group and introduce a vinyl group (Figure 7). This process is performed at approximately 250°C under vacuum. 47 The removal of the hydroxyl group requires less energy due to the boiling point of water (100°C), and, at any temperature above the boiling point, already there should be a certain level of water removed.
Figure 7.
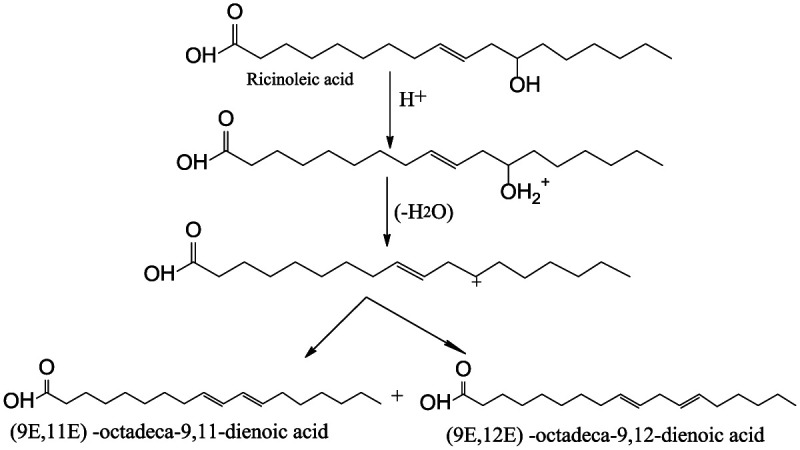
Dehydration of castor oil.
Castor oil has only one double bond in each fatty acid chain and so it is classified as non-drying oil. However, it can be dehydrated to give semi-drying or drying oil which is used extensively in paints and varnishes. Being a polyhydroxy compound, its hydroxyl functionality can be reduced through dehydration or increased by inter-esterification with a polyhydric alcohol. 10
Dehydrated castor oil is of significance because coatings that incorporate castor oil alone will never achieve complete cure through oxidative cross-linking as do coatings that contain oil with conjugated double bonds in their fatty acid components. The dehydrated castor oil is often used as a lubricant. 48
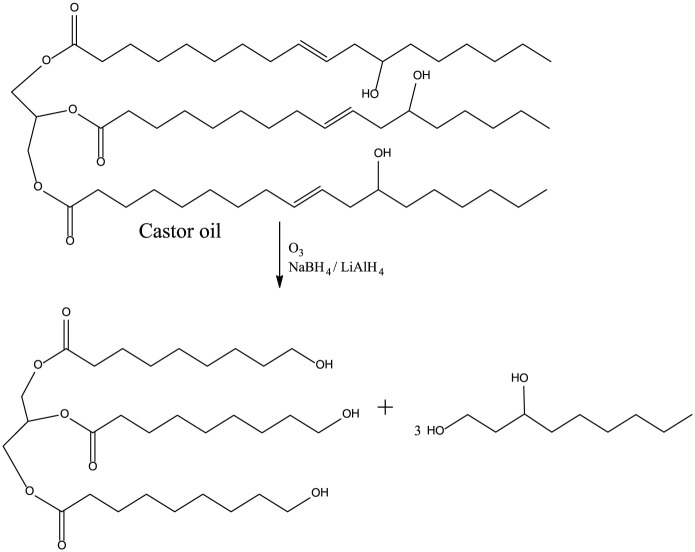
Ozonolysis
Ozonolysis is one of the methods used for the synthesis of polyols from bio-oils that are derived from plants (Figure 8). In this process, ozone is used as a strong oxidizing agent to cleave and oxidize alkenes that are then reduced to alcohols by a strong reducing agent such as sodium borohydride (NaBH4). 4 Ozonolysis is the only oxidative cleavage method that is currently used in industries because it is a clean reaction carried out at low temperatures (25°C–45°C) without catalyst and the decomposition occurs at 60°C–100°C. 49
Figure 8.
Ozonolysis of castor oil.
Ozonolysis of vegetable oils yields aldehyde functionalities that by posterior reduction yields a polyol with almost three hydroxyl groups per molecule, however not for saturated fatty acids in the oil. This ozonolysis procedure has been successfully applied to soybean oil, castor oil, canola oil, and triolein; these oils resulted in polyurethanes with improved mechanical properties and higher glass transition temperature compared to the polyurethanes produced from epoxidation. 50 The downside of this process is the fact that oxygen is highly reactive; therefore, this can result in many side reactions that can form multiple byproducts if the process does not take place in strictly controlled environments. However, the use of a catalyst means that less energy will be required (Figure 8).
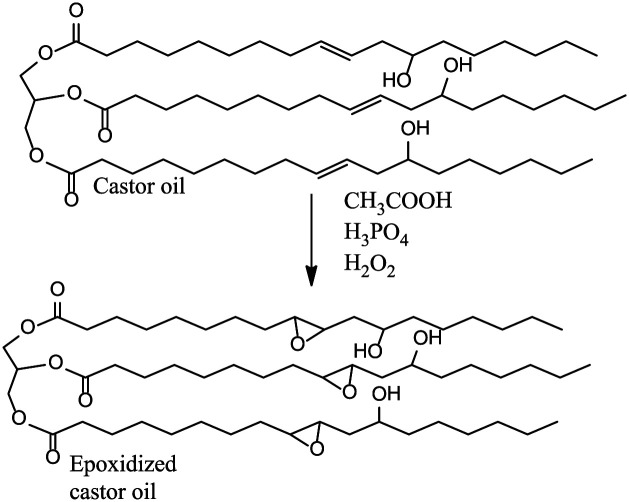
Epoxidation
Epoxidation reactions are usually conducted using a peracid (peroxy acid), either preformed or formed in situ, by reacting a carboxylic acid (usually acetic acid) as an oxygen carrier with hydrogen peroxide (H2O2) as an oxygen donor. Formic acid is preferred compared to acetic acid as the oxygen carrier due to its high reactivity and no need to use a catalyst in the formation of performic acid.51,52 Different kinds of catalysts such as enzymes, Ti(IV)-grafted silica catalysts, acid catalysts, tungsten-based catalyst, transition metal complexes, and ion exchange resin are used for epoxidation. 53 The products obtained from epoxidation are known as oxirane compounds or epoxides. Epoxidation of vegetable oils is a commercially important reaction because the epoxides obtained from vegetable oils and from their alkyl esters have their applications in such materials as plasticizers and polymer stabilizers. Moreover, the epoxides can be used as intermediates in the production of a variety of derivatives because of high reactivity of the strained epoxide ring. 53 Epoxidized castor oil is also applied in high-temperature lubricants, polyurethane dispersions, paints, coatings and adhesives, nanocomposites, surfactants, hydraulic oils, and biodiesel. 54 Figure 9 illustrates the epoxidation reaction. The process is versatile because it can use multiple catalysts that are both biological and inorganic. The presence of the catalyst makes the process less energy intensive.
Figure 9.
Epoxidation of castor oil.
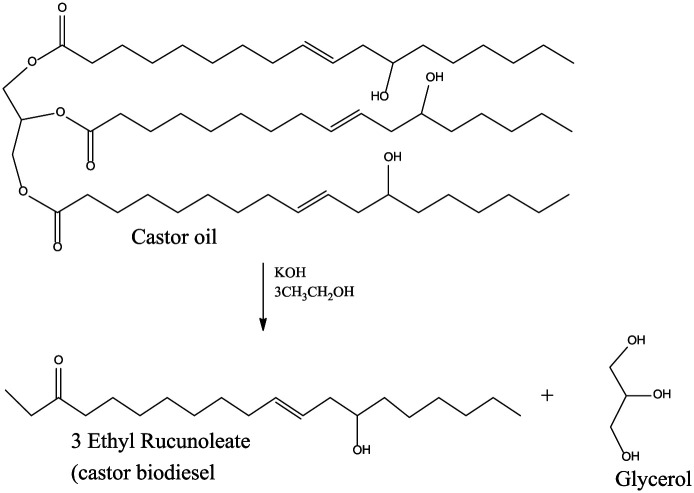
Transesterification
The process involves reacting an alcohol with an ester that is present in the castor oil. This process is performed in the presence of a catalyst that can be either an acid or a base though often acid catalysts are used (e.g. hydrochloric acid, sulfuric acid, etc.). This process is reversible and, to avoid this, excess alcohol is used to shift the reaction to completion. The products formed in this process are glycerol and biodiesel (methyl ricinoleate).10,43,55
The transesterification of castor oil can be achieved in the presence of a catalyst (e.g. KOH; Figure 10). 56 Deshpande et al. 43 varied the methanol ratio, reaction time, and concentration of the catalyst in their process; the concentration of the catalyst resulted in increased kinematic viscosity and specific gravity, however with decreasing acid value and Sap value, whereas Chakrabarti and Ahmad 31 also varied the same parameters as Deshpande et al. and obtained the same outcome.
Figure 10.
Transesterification of castor oil.
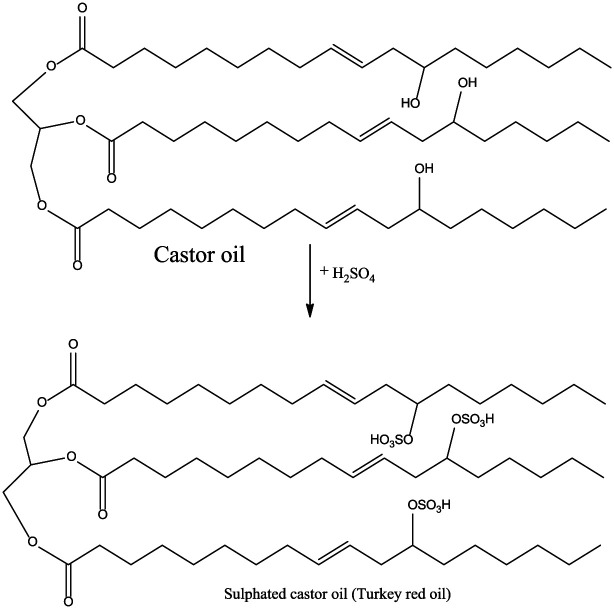
Sulfation
Sulfated castor oil, also known as Turkey Red Oil due to the sulfur reddish color, is one of the first chemical derivatives of castor oil. Sulfated castor oil is developed through the addition of concentrated sulfuric acid at the same rate to castor oil for a few hours with constant cooling and agitation at 25°C–30°C. After completion of the reaction, the product is neutralized using a base solution or an amine. This process uses room temperature and this is because of the application of a very strong acid that does not require high temperatures to react. The acid might need to be diluted in most instances to ensure that the process is not extremely hazardous (Figure 11).
Figure 11.
Sulfation of castor oil.
Bio-binders
The composition of plant oils such as castor oil allows for the development of bio-binders. Bio-binders also known as biopolymers are the compounds acquired from natural resources such as plants and contain monomers that are linked by covalent bonds to form polymeric chains. The castor oil–based biopolymers are usually in the form of polyurethanes, polyamides, polyethers, and polyesters. 10
Bio-binders are used as the glue in simple terms for different materials. Bio-binders are different based on their melt flow indices, impact properties, hardness, vapor transmission characteristics, coefficient of friction, and decomposition. There are other engineering properties that are also of significance in bio-binders such as tensile strength, tensile modulus, flexural strength, flexural modulus, and density. 57 The ability of bio-binders to absorb water is also an important parameter, especially during the synthesis of the binder because water is a green solvent and is cheaper. Bio-binders are used in multiple industries for different applications such as drug delivery system, wound healing, food containers and agricultural films, waste bags, soil retention sheeting, filtration, hygiene and protective clothing, 57 paper industry, wood-to-wood bonding, 58 and automotive industries. 59 Castor oil–based bio-binders have been reported to have been applied in launch vehicles and missile propellants 60 and also in the development of asphalt bio-binders for pavements in civil engineering.61,62 The properties are outlined below, which allow for the application of bio-binders in different industries.
Properties of bio-binders
The materials that are made from different composites are used for various applications and density is considered one of the most important properties as it determines where the binder will be applicable. Bio-binders are often classified according to their mechanical properties as illustrated in Table 6. The densities of the bio-binders that are available in the market normally range from 0.25 to 1.26 g/cm3. 63 The bio-binders that have low densities are mostly preferred in various industries because of various reasons such as energy efficiency, ease of handling, and relatively low costs. 57
Table 6.
Types of bio-binders and their mechanical properties. 57
| Types of bio-binders | Density (g/cm3) | Tensile strength (MPa) | Tensile modulus (MPa) | Elongation (%) |
|---|---|---|---|---|
| PLA | 1.26 | 5.9–7.2 | 1.08–3.61 | 2.1–30.7 |
| Polyhydroxybutyrate (PHB) | 1.2–1.5 | 24–40 | 3.5–7.7 | 1.56–6 |
| Poly-3-hydroxybutyrate (P-3-Hb) | 1.28 | 40 | 3.5 | 0.4–6 |
| Poly-3-hydroxybutyrate-co-3-hydroxyvalerate (P-3-Hb-Hv) | 0.22–0.25 | 23–40 | 3.5 | 1.6–20 |
| Poly-4-hydroxybutyrate (P-4-Hb) | 1.22 | 104 | 0 | 1000 |
| Polycaprolactone (PCL) | 1.13 | 16–23 | 0.4 | >700 |
PLA: polylactic acid.
The tensile strength of bio-binders is also one of the properties considered in the industry. The tensile strength of different biopolymers ranges between 5.9 and 72 MPa. 64 Tensile modulus is another important mechanical property of bio-binders because it is the measure of the stiffness of the polymer. The market-related tensile modulus of the bio-binders ranges between 0.4 and 7.7 GPa. 57
Elongation is another property of bio-binders that determines the amount of deformation that happens to the polymer when subjected to stress. The lowest elongation percentage reported is 1.0% and the highest was found to be 3.0%. 57 Research has shown that there is considerable variation in densities and elongation of different bio-binders and therefore there is a need to develop different synthetic methods for the development of new binders that will be considered for new technologies as research advances.
Conclusion
From the processes listed above, a process that uses less energy and does not result in many side reactions will be ideal. Castor oil is of benefit because it does not compete with edible oils and hence threatening food security. The various products that are obtained from castor oil have proved that the properties contained in the castor oil are worth exploring for various applications for the replacement of fossil fuel. These products are applicable in various industries such as agriculture, cosmetics, pharmaceuticals, and coatings with the starting material being castor oil. There is a limited amount of research reported with castor oil as a starting material for bio-binders to be applied in composite materials, especially in the automotive industry, and this opens a gap and an opportunity for advanced research in this field.
Acknowledgments
We are indebted to the Department of Science and Technology (DST), the Council for Scientific and Industrial Research (CSIR) South Africa, and Selokong Sa Dimelana (SSD) for supporting this work.
Author biographies
Ntsako Portia Chauke received her BSc Degree in Chemistry in 2013 and PGDip in Chemical Engineering (cum laude) in 2017 from the University of the Witwatersrand. She is an MSc Candidate working on a project that focuses on the development of bio-binders from locally produced castor oil for composite materials under the supervision of Dr Diakanua Nkazi, in the School of Chemical Engineering and Metallurgical Engineering of the same University. She is a career-driven professional with most of her experience in research and development, focusing mainly on environmental, energy and health studies.
Hembe Elie Mukaya received his BSc degree in Chemical Engineering from University of Lubumbashi, DRC, and his BSc Honours, MSc and PhD degrees in Chemistry from the University of the Witwatersrand, South Africa, working with Professor Eberhard W Neuse on polymeric drug delivery systems. For 5 years, he was a Postdoctoral Fellow in the research group of Professor Xavier Y Mbianda at University of Johannesburg. Currently, he is working with Dr Diakanua Nkazi at the University of the Witwatersrand. His interests are drug delivery systems and development of biodegradable and non-biodegradable polymers and nanocomposites materials for different applications.
Diakanua Bavon Nkazi is a Senior Lecturer in the School of Chemical and Metallurgical Engineering, University of the Witwatersrand, Johannesburg, South Africa. He received his PhD at the same University. He has authored 21 peer-reviewed papers and has two patents. One of his publications on rubber oil extraction and processing was rated as the most cited paper for the year 2016 and the University awarded him a certificate of recognition. Currently, he is undertaking and supervising research projects based on Renewables energy, Bio-oil processing, Biomaterials and Polymer synthesis and development. Since his joining the research group, he was able to develop biopolymers for fuel cell use and engineering tissue. He also develops bio-oil processing from natural rubber, castor and jatropha seeds.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received financial support for the research, authorship, and/or publication of this article: The authors received financial support for the research from DST and CRSI.
References
- 1. Gokdogan O, Eryilmaz T, Kadir Yesilyurt M. Thermophysical properties of castor oil (Ricinus communis L.) biodiesel and its blends. CT F-Cienc Tecn Fut 2015; 6: 95–128. [Google Scholar]
- 2. Shaik MR, Alam M, Alandis NM. Development of castor oil based poly (urethane-esteramide)/TiO 2 nanocomposites as anticorrosive and antimicrobial coatings. J Nanomater 2015; 16: 176. [Google Scholar]
- 3. NAYAK PL. Natural oil-based polymers: opportunities and challenges. J Macromol Sci C 2000; 40: 1–21. [Google Scholar]
- 4. Dotan A. Biobased thermosets. In: Dodiuk H, Goodman SH. (eds) Handbook of thermoset plastics. Amsterdam: Elsevier, 2014, pp. 577–622. [Google Scholar]
- 5. Suhane A, Rehman A, Khaira H. Potential of non edible vegetable oils as an alternative lubricants in automotive applications. Int J Eng Res Appl 2012; 2: 1330–1335. [Google Scholar]
- 6. Attia MI, Zoorob S, Hassan K, et al. Development of building blocks using vegetable oil and recycled aggregate. MATEC Web of Conf 2017; 12: 03006. [Google Scholar]
- 7. Forth JP, Zoorob S. Vegetable oil based construction materials. Google Patents, 2008. [Google Scholar]
- 8. Alam M, Akram D, Sharmin E, et al. Vegetable oil based eco-friendly coating materials: a review article. Arab J Chem 2014; 7: 469–479. [Google Scholar]
- 9. Derksen JT, Cuperus FP, Kolster P. Renewable resources in coatings technology: a review. Prog Org Coat 1996; 27: 45–53. [Google Scholar]
- 10. Mubofu EB. Castor oil as a potential renewable resource for the production of functional materials. Sustain Chem Process 2016; 4: 11. [Google Scholar]
- 11. Raouf MA, Metwally M, Williams RC. Development of non-petroleum based binders for use in flexible pavements. Report, Iowa Department of Transportation, October 2010. [Google Scholar]
- 12. Chauhan PS, Chhibber V. Non-edible oil as a source of bio-lubricant for industrial applications: a review. Int J Eng Sci Innov Technol 2013; 2: 299–305. [Google Scholar]
- 13. Banković-Ilić IB, Stamenković OS, Veljković VB. Biodiesel production from non-edible plant oils. Renew Sustain Energy Rev 2012; 16: 3621–3647. [Google Scholar]
- 14. Kumar A, Sharma S. Potential non-edible oil resources as biodiesel feedstock: an Indian perspective. Renewable and Sustainable Energy Reviews. 2011; 15: 1791–800. [Google Scholar]
- 15. Asadauskas S, Perez JM, Duda JL. Oxidative stability and antiwear properties of high oleic vegetable oils©. Lubr Eng 1996; 52: 877–882. [Google Scholar]
- 16. Karmakar A, Karmakar S, Mukherjee S. Properties of various plants and animals feedstocks for biodiesel production. Bioresour Technol 2010; 101: 7201–7210. [DOI] [PubMed] [Google Scholar]
- 17. Diamante LM, Lan T. Absolute viscosities of vegetable oils at different temperatures and shear rate range of 64.5 to 4835 s−1. J Food Process 2014; 2014: 234583. [Google Scholar]
- 18. Ali OMA, Zaini MAA, Danlami JM. Oxidation stability of castor oil in solvent extraction. Jurnal Teknologi 2016; 78: 239–244. [Google Scholar]
- 19. Forero CLB. Biodiesel from castor oil: a promising fuel for cold weather. Renew Energy Power Qual J 2005; 1: 59–62. [Google Scholar]
- 20. Patel VR, Dumancas GG, Viswanath LCK, et al. Castor oil: properties, uses, and optimization of processing parameters in commercial production. Lipid Insights 2016; 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dresel W, Mang T. Lubrication and lubricants. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA, 2007. [Google Scholar]
- 22. Mohammed TH, Lakhmiri R, Azmani A, et al. Bio-oil from pyrolysis of castor shell. Int J Basic Appl Sci 2014; 14: 1–5. [Google Scholar]
- 23. Şensöz S, Angın D, Yorgun S. Influence of particle size on the pyrolysis of rapeseed (Brassica napus L.): fuel properties of bio-oil. Biomass Bioenergy 2000; 19: 271–279. [Google Scholar]
- 24. Bergstra R. Emerging opportunities for natural oil based chemicals. In: MTN consulting associates, plant bio-industrial workshop, Saskatoon, SK, 27 February 2007. [Google Scholar]
- 25. Stohr T. Proteomic analysis of the endoplasmic reticulum of developing castor bean seeds—testing the feasibility of ER membrane topology elucidation using a sample of purified ER. Master’s Thesis, Durham University, Durham, 2015. [Google Scholar]
- 26. El-Adly R, Shoaib A. Optimum operating conditions for epoxidation reaction of Jojoba and castor oils. Int J Eng Res Appl 2014; 4: 816–822. [Google Scholar]
- 27. Gilbert E. The unique chemistry of castor oil. J Chem Educ 1941; 18: 338. [Google Scholar]
- 28. Tadesse MG. Synthesis of wetting agents from castor oil for the dyeing of cotton fabric. Appl Res J 2015; 1: 1–8. [Google Scholar]
- 29. Veronese VB, Menger RK, Forte MMDC, et al. Rigid polyurethane foam based on modified vegetable oil. J Appl Polym Sci 2011; 120: 530–537. [Google Scholar]
- 30. Mutlu H, Meier MA. Castor oil as a renewable resource for the chemical industry. Eur J Lipid Sci Tech 2010; 112: 10–30. [Google Scholar]
- 31. Chakrabarti MH, Ahmad R. Transesterification studies on castor oil as a first step towards its use in biodiesel production. Pak J Bot 2008; 40: 1153–1157. [Google Scholar]
- 32. Ramos MJ, Fernández CM, Casas A, et al. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 2009; 100: 261–268. [DOI] [PubMed] [Google Scholar]
- 33. Khan AK. Research into biodiesel kinetics and catalyst development. The University of Queensland, Department of Chemical Engineering. 2002; 17. [Google Scholar]
- 34. Desroches M, Auvergne R, Boutevin B, et al. Synthesis of bio-based building blocks from vegetable oils: a platform chemicals approach. Ol Corps Gras Li 2013; 20: 16–22. [Google Scholar]
- 35. Shah M, Dai J-J, Guo Q-X, et al. Products and production routes for the catalytic conversion of seed oil into fuel and chemicals: a comprehensive review. Sci China Chem 2015; 58: 1110–1121. [Google Scholar]
- 36. Sharmin E, Zafar F. Introductory Chapter: Flame Retardants. Flame Retardants. IntechOpen, 2019. [Google Scholar]
- 37. Wool RP. Polymers and composite resins from plant oils. Bio-Based Polymers and Composites. 2005; 1. [Google Scholar]
- 38. Alwaseem H, Donahue CJ, Marincean S. Catalytic transfer hydrogenation of castor oil. J Chem Educ 2014; 91: 575–578. [Google Scholar]
- 39. Yousef EA, Hussain AE, Shoeb ZE. Modification of castor oil by isomerization, halogenation and application of some modified products as plasticizer in nitrile rubber formulations. J Sci Ind Res 2001; 60:383–395. [Google Scholar]
- 40. Li H, Niu S, Lu C. Pyrolysis characteristics of castor oil through thermogravimetric coupled with Fourier transform infrared spectroscopy. Procedia Engineer 2017; 205: 3705–3710. [Google Scholar]
- 41. Chen G-L, Chen G-B, Li Y-H, et al. A study of thermal pyrolysis for castor meal using the Taguchi method. Energy. 2014; 71: 62–70. [Google Scholar]
- 42. Sánchez N, Encinar JM, Martínez G, et al. Biodiesel production from castor oil under subcritical methanol conditions. Int J Environ Sci Dev 2015; 6: 61. [Google Scholar]
- 43. Deshpande D, Haral S, Sarode P. Hydrocarbon liquid from castor oil. Res J Chem Sci 2013; 2231: 606X. [Google Scholar]
- 44. Bridgwater A, Peacocke G. Fast pyrolysis processes for biomass. Renew Sustain Energy Rev 2000; 4: 1–73. [Google Scholar]
- 45. Bridgwater AV. Renewable fuels and chemicals by thermal processing of biomass. Chem Eng J 2003; 91: 87–102. [Google Scholar]
- 46. Goswami D, De S Basu J. Effects of process variables and additives on mustard oil hydrolysis by porcine pancreas lipase. Braz J Chem Eng 2012; 29: 449–460. [Google Scholar]
- 47. Lligadas G, Ronda JC, Galià M, et al. Oleic and undecylenic acids as renewable feedstocks in the synthesis of polyols and polyurethanes. Polymers 2010; 2: 440–453. [Google Scholar]
- 48. Klein J, Bongardt F, Schmid K-H. Use of partially dehydrated castor oils as lubricants. Google Patents, 5468405A, USA, 1995. [Google Scholar]
- 49. Scrimgeour C. Chemistry of fatty acids, Bailey’s industrial oil and fat products, vol. 6. Hoboken, NJ: Wiley and Sons, 2005, pp. 1–43. [Google Scholar]
- 50. Nieto EDR. New polyurethanes from vegetable oil-based polyols. PhD Thesis, Universitat Rovira i Virgili, Tarragona, 2011. [Google Scholar]
- 51. Vaidya R, Chaudhary G, Raut N, et al. Synthesis of biocomposites from natural oils—a review. In: Proceedings of the 2012 international conference on environmental, biomedical and biotechnology (IPCBEE), Kuala Lumpur, Malaysia, 22–23 December 2012, pp. 55–60. Singapore: IACSIT Press. [Google Scholar]
- 52. Derawi D, Salimon J. Optimization on epoxidation of palm olein by using performic acid. J Chem 2010; 7: 1440–1448. [Google Scholar]
- 53. Patil H, Waghmare J. Catalyst for epoxidation of oils: a review. Discovery. 2013; 3: 10–4. [Google Scholar]
- 54. DeHonor Márquez E, Nieto Alarcón JF, Vigueras Santiago E, et al. Effective and fast epoxidation reaction of linseed oil using 50 wt% hydrogen peroxide. Am J Chem 2018; 8: 99–106. [Google Scholar]
- 55. Pena R, Romero R, Martinez SL, et al. Transesterification of castor oil: effect of catalyst and co-solvent. Ind Eng Chem Res 2008; 48: 1186–1189. [Google Scholar]
- 56. Mahla S. Performance evaluation and emissions characteristics of a compression ignition engine using castor oil based biodiesel. PhD Theses, Thapar University, Patiala, India, 2014. [Google Scholar]
- 57. Asokan P, Firdoous M, Sonal W. Properties and potential of bio fibres, bio binders, and bio composites. Rev Adv Mater Sci 2012; 30: 254–261. [Google Scholar]
- 58. Ibrahim S, Ahmad A, Mohamed N. Characterization of novel castor oil-based polyurethane polymer electrolytes. Polymers. 2015; 7: 747–759. [Google Scholar]
- 59. Akampumuza O, Wambua PM, Ahmed A, et al. Review of the applications of biocomposites in the automotive industry. Polym Composite 2017; 38: 2553–2569. [Google Scholar]
- 60. Krishnamurthy V, Thomas S. ISRO polyol—the versatile binder for composite solid propellants for launch vehicles and missiles. Defence Sci J 1987; 37: 29–37. [Google Scholar]
- 61. Xiu S, Shahbazi A. Bio-oil production and upgrading research: A review. Renew Sustain Energy Rev 2012; 16: 4406–4414. [Google Scholar]
- 62. Venema A. The potential of biobased materials in the civil engineering sector. Groningen: University of Groningen, 2012. [Google Scholar]
- 63. Oksman K, Skrifvars M, Selin J-F. Natural fibres as reinforcement in polylactic acid (PLA) composites. Compos Sci Technol 2003; 63: 1317–1324. [Google Scholar]
- 64. Chanprateep S, Kulpreecha S. Production and characterization of biodegradable terpolymer poly (3-hydroxybutyrate-co-3-hydroxyvalerate-co-4-hydroxybutyrate) by Alcaligenes sp. A-04. J Biosci Bioeng 2006; 101: 51–56. [DOI] [PubMed] [Google Scholar]