Abstract
The consideration towards waste cooking oils is changing from hazardous waste to valuable raw material for industrial application. During the last 5 years, some innovative processes based on the employment of recycled waste cooking oil have appeared in the literature. In this review article, the most recent and innovative applications of recycled waste cooking oil are reported and discussed. These include the production of bioplasticizers, the application of chemicals derived from waste cooking oils as energy vectors and the use of waste cooking oils as a solvent for pollutant agents.
Keywords: Waste cooking oil, volatile organic compounds, plasticizers, recycle, syngas, biofuel
Introduction
Waste cooking oils (WCOs) are valuable by-products of the food chain, which can be employed as green raw materials for the production of chemicals. The amount of WCOs available worldwide is impressive, causing serious environmental, economic and social problems. It has been estimated that more than 15 million tonnes of waste vegetable oils are generated annually in the world, with European Union (EU) close to 1 million tonnes per year.1,2 Often, WCOs are discharged through public sewerages, making necessary extraordinary maintenances and increasing the water treatment costs. In fact, the presence of vegetable oil in the public conducts promotes the formation of foams and the flotation of sludge, and causes several mass transfer problems due to the adsorption of lipids onto the biomass. 3
WCOs are mainly composed of triglycerides, monoglycerides, diglycerides and variable quantities of free fatty acids (5%–20% w/w) generated during the frying process. 2 The main components of triglycerides are saturated and unsaturated fatty acids, which can be used as platform chemicals for the manufacturing of added-value products in various segments of industry. More common industrial applications of WCOs are related to energy production, by direct burning,4,5 or for the synthesis of biofuels.6,7 Two additional non-negligible market segments of recycled WCOs are represented by the biolubricants 8 and by the production of animal feed. 9 Many research papers, including review articles, have been reported on the abovementioned subjects during the last years.2,10–12 As the knowledge about the average composition and about the synthetic possibilities of WCOs increases, new applications of this green and biocompatible raw material have recently emerged. In this review article, a selection of recent and innovative applications of WCOs that appeared during the last 5 years is presented. The employment of WCOs in the field of biomaterials, for the design of biofuels of second generation, and in the area of biosorbents for pollutants will be discussed (Figure 1).
Figure 1.
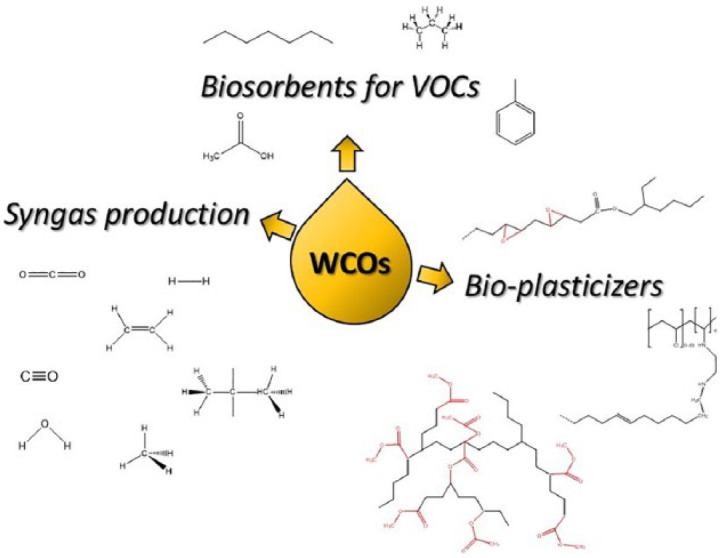
Overview of recent applications of WCOs.
Discussion
WCOs as plasticizers
The chemical composition of WCOs is mainly constituted by a mixture of three unsaturated fatty acids: oleic, linoleic and linolenic acids. 13 The carbon–carbon double bonds as well as the acidic moiety present in these substrates are amenable to several kinds of transformations and make this mixture particularly attractive as a source of building blocks for polymer chemistry. In particular, the employment of WCO as raw material for the production of bioplasticizers has been recently reported. Plasticizers are important polymer additives and have been used extensively for the production of plastics, rubbers and adhesives. Nevertheless, a number of notable controversies and concerns have been associated with the use of common plasticizers, namely, phthalate esters, since they exhibit a migration phenomenon from the polymer matrix to the surrounding media and they are suspected to produce bioaccumulation in the environment.14–16 Due to these potential harmful effects on human health and the environment, they have been banned in several countries as plasticizers in fields like fabrication of toys and packaging materials for food and medicines.15–18 Looking for alternative bio-based plasticizers, several kinds of modified edible oils have been considered. The syntheses of epoxidized soybean oil (ESO), acetylated derivatives of castor oil, methyl epoxy soyate, amyl epoxy soyate, tall-oil fatty esters, dicapryl sebacate and ESO fatty esters have been described.19–21 Unfortunately, the use of bioplasticizers from edible oils is still limited because of the high cost of the raw material and the negative impact on the withdrawal of resources from the food and feed chain. 22 Only recently, the synthesis of plasticizers for polyvinylchloride (PVC) from epoxidized WCO has been proposed.17,18,22 Nevertheless, after prolonged times, some degree of migration of the bioplasticizer was observed, as already reported for the classic phthalate esters.21–23 A solution to this issue has been reported by Jia et al., 14 who proposed the covalent bonding of the plasticizer to the PVC backbone. This target was reached by preparing a Mannich base of waste cooking oil methyl ester (WCOME), which was used as a non-migration plasticizer for self-plasticization PVC materials. The internally plasticized PVC film showed no migration in n-hexane, which is essential to ensure a long-term stability of the physical and chemical properties of PVC products. 14
Syngas production from WCOs
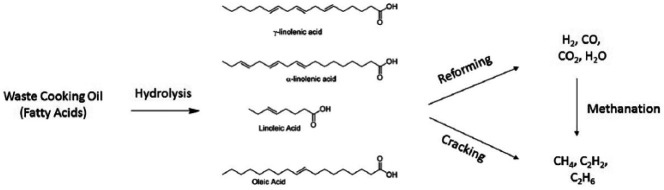
For many years, crude and used vegetable oils have been used as an ingredient for biodiesel or biofuels, or for heat production through direct burning (first-generation biofuels).24,25 Recently, some research activity has been dedicated to the development of second-generation biofuels derived from WCOs, more precisely hydrogen-rich synthesis gas (or syngas). Syngas can be directly burned or further converted into other chemicals using the Fischer–Tropsch process. It can for instance be transformed into liquid hydrocarbons, mostly diesel and kerosene or into dimethyl ether (DME). Bio-SNG (synthetic natural gas) and bio-DME are fuels that can be used in gasoline or diesel vehicles, respectively, with slight adaptations. 25 Several papers report on the use of lignocellulosic biomass as raw materials for second-generation of biofuels, more precisely for syngas production.25–27 On the contrary, limited literature is available on the utilization of WCO as a feedstock for syngas.28,29 The extraction of molecular hydrogen and carbon dioxide from WCOs has been discussed in the literature only very recently. The syngas produced can be employed as an energy carrier or as precursors of other chemicals. The production of hydrogen-rich syngas from Asian WCOs subjected to supercritical water gasification has been reported by Nanda et al. 28 The long-chain fatty acids contained in the WCO were subjected to C–C bond cleavage through thermal cracking to give short-chain fatty acids, converted into H2, CO and CO2 by the reforming process. Such conditions promote the water–gas shift reaction between CO and H2O to generate CO2 and H2, 26 and the dehydrogenation of saturated compounds to form H2. 30
From this mixture, it is possible to obtain methane, ethane, ethene and other gases. The same products can be generated directly from the mixture of fatty acids by increasing the temperature of the thermal treatment.
When crude WCO is employed, the feed concentration must be taken into account as it influences the production of H2, which decreases with the amount of feed dispersed in the oil. The process described by Nanda and co-workers from WCO is depicted in Figure 2.
Figure 2.
Schematic route to obtain energy carriers and building blocks for synthesis from waste cooking oils.
The selectivity of the reforming process can be tuned by employing alkali and metal catalysts as Na2CO3, K2CO3, Ru/Al2O3 and Ni/Si-Al2O3, in particular for enhancing the gas yield and the H2 concentration. Methanation reaction involving CO and CO2 leads to the production of CH4, which can arise also from the thermal decomposition of acetic acid, propionic acid and propionaldehyde. The latter compounds originate from the thermal decomposition of fatty acids in the presence of CO and CO2. Cracking of short-chain hydrocarbons produces also acetylene, ethylene, ethane and other light hydrocarbons (Figure 2).
Biosolvents for pollutants
Another exploitable property of the vegetable oil is the tendency to solubilize small organic molecules. When the vegetable oil arises from waste and the small organic molecules considered pertain to the family of the hazardous volatile organic compounds (VOCs), the matter becomes of general interest. Lhuissier et al. 31 developed a non-aqueous phase (NAP) bioreactor for the capturing and biodegradation of VOCs. The authors tested some mineral and vegetal (from food industries) WCOs as solvents for n-heptane, ethyl-acetate, 2-propanol, methyl isobutyl ketone (MIBK), toluene, m-xylene and 1,3,5-trimethylbenzene. The oleic phase enriched by the organic pollutants was then treated in a two-phase partitioning bioreactor (TPPB), where the VOC degradation was achieved by selected microorganisms.
Tarnpradab et al. 32 employed WCOs to treat the emission produced during the rice husk pyrolysis. In particular, WCO was able to reduce the content of organic hydrocarbon contaminants with a molecular weight higher than that of benzene, which are generically referred to with the term tar. In particular, the authors claimed that heavy tar was absorbed by WCO through a dissolution mechanism and several data about the saturation levels were reported. 32
The possibility to trap small molecules by vegetable oil was also exploited by Worthington et al. 33 in the realization of a mercury sorbent device, made through the co-polymerization of sulphur and unsaturated cooking oils. It is already known that mercury metal and inorganic mercury bind to reduced organic sulphur groups in dissolved organic matter (Hg-DOM),34,35 and sulphur-based polymers were recently shown to be able to remove Hg2+ from water.36–38 Searching for an abundant, inexpensive and easy-to-handle material, Worthington et al. exploited the double bonds present in the fatty acid fraction of WCOs as crosslinking points for an inverse vulcanization process of polysulphides. The authors described and characterized different polymers obtained by the combination of sulphur and canola, sunflower and olive oil, and demonstrated mercury removal from air, water and soil. With respect to previous studies,36–38 these new rubbers are effective not only in purifying water containing inorganic HgCl2, but also in capturing common forms of mercury pollution including liquid mercury metal, mercury vapour, inorganic mercury and organomercury compounds.
Conclusion
The availability of building blocks arising from the transformation of wastes for modern chemical applications represents one of the main research lines nowadays. The necessity to recycle WCOs combined with their specific chemical composition brings to the exploration of new applications in the fields of material science, energy and environmental chemistry. Some early results on these topics have been reviewed and discussed taking into consideration the bibliography of the last 5 years. Such results represent the starting point for further development of new technologies.
Author biographies
Alberto Mannu graduated in Organic Chemistry (Master Degree) from the University of Sassari (Italy) in 2005. He holds a PhD in Homogeneous Catalysis since 2011 from the University of Barcelona (Spain). Currently, his research line is targeted at the development of new process for biomass valorization, including the upgrade of existing industrial process in sustainable circular production.
Monica Ferro graduated in Chemistry at the University of Milan (Italy) in 2006 and received her Master Degree in ‘Chemical Sciences’ at the University of Milan in 2009. In 2018, she received her ‘PhD’ in ‘Industrial Chemistry and Chemical Engineering’ at the Politecnico of Milan (Italy). Currently, her research is focused on spectroscopic studies on poly- and oligosaccharidic systems from cellulose and cellulose derivatives and on HRMAS NMR investigation of small molecules of pharmaceutical interest dissolved in polymer–hydrogel systems.
Maria Enrica Di Pietro graduated in Pharmaceutical Chemistry and Technology in 2009. After a joint PhD project spent between the Università della Calabria (Italy) and the Université Paris-Sud (France), she worked as a post-doctoral researcher in Italy and Germany. Currently, her research activity is mainly focused on dynamic studies of different heterogeneous systems via NMR relaxometry and diffusometry, NMR pulse sequence development and methods for structure elucidation.
Andrea Mele is Full Professor of Chemistry at the Department of Chemistry, Materials and Chemical Engineering ‘Giulio Natta’ of Politecnico di Milano. He is in charge of the NMR and Mass Spectrometry facilities of the department. He has co-authored more than 180 papers in the field of physical organic chemistry and NMR spectroscopy.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1. Gui MM, Lee KT, Bhatia S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008; 33(11): 1646–1653. [Google Scholar]
- 2. Lin CSK, Pfaltzgraff LA, Herrero-Davila L, et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ Sci 2013; 6(2): 426–464. [Google Scholar]
- 3. Ortner ME, Müller W, Schneider I, et al. Environmental assessment of three different utilization paths of waste cooking oil from households. Resour Conserv Recycl 2016; 106: 59–67. [Google Scholar]
- 4. Namoco CS, Cloma JT, Rabago G, et al. Development of a mechanical dry corn picker. J Eng Appl Sci 2017; 12(2): 409–413. [Google Scholar]
- 5. Capuano D, Di Fraia S, Massarotti N, et al. Direct use of waste vegetable oil in internal combustion engines. Renew Sustain Energy Rev 2016; 69: 759–770. [Google Scholar]
- 6. Chrysikou LP, Dagonikou V, Dimitriadis A, et al. Waste cooking oils exploitation targeting EU 2020 diesel fuel production: environmental and economic benefits. J Clean Prod 2019; 219(2019): 566–575. [Google Scholar]
- 7. Karmee SK. Liquid biofuels from food waste: current trends, prospect and limitation. Renew Sustain Energy Rev 2016; 53: 945–953. [Google Scholar]
- 8. Karmakar G, Ghosh P, Sharma B. Chemically modifying vegetable oils to prepare green lubricants. Lubricants 2017; 5(4): 44. [Google Scholar]
- 9. Magrinyà N, Tres A, Codony R, et al. Use of recovered frying oils in chicken and rabbit feeds: effect on the fatty acid and tocol composition and on the oxidation levels of meat, liver and plasma. Animal 2012; 7(3): 505–517. [DOI] [PubMed] [Google Scholar]
- 10. Panadare DC, Rathod VK. Applications of waste cooking oil other than biodiesel: a review. Iran J Chem Eng 2015; 12(3): 55–76. [Google Scholar]
- 11. Talebian-Kiakalaieh A, Amin NAS, Mazaheri H. A review on novel processes of biodiesel production from waste cooking oil. Appl Energy 2013; 104: 683–710. [Google Scholar]
- 12. Hajjari M, Tabatabaei M, Aghbashlo M, et al. A review on the prospects of sustainable biodiesel production: a global scenario with an emphasis on waste-oil biodiesel utilization. Renew Sustain Energy Rev 2017; 72: 445–464. [Google Scholar]
- 13. McNutt J, He QS. Development of biolubricants from vegetable oils via chemical modification. J Ind Eng Chem 2016; 36: 1–12. [Google Scholar]
- 14. Jia P, Zhang M, Hu L, et al. A strategy for nonmigrating plasticized PVC modified with Mannich base of waste cooking oil methyl ester. Sci Rep 2018; 8(1): 1589–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bocqué M, Voirin C, Lapinte V, et al. Petro-based and bio-based plasticizers: chemical structures to plasticizing properties. J Polym Sci Part A Polym Chem 2016; 54(1): 11–33. [Google Scholar]
- 16. Greco A, Ferrari F, Maffezzoli A. UV and thermal stability of soft PVC plasticized with cardanol derivatives. J Clean Prod 2017; 164: 757–764. [Google Scholar]
- 17. Feng G, Hu L, Ma Y, et al. An efficient bio-based plasticizer for poly (vinyl chloride) from waste cooking oil and citric acid: synthesis and evaluation in PVC films. J Clean Prod 2018; 189: 334–343. [Google Scholar]
- 18. Zheng T, Wu Z, Xie Q, et al. Structural modification of waste cooking oil methyl esters as cleaner plasticizer to substitute toxic dioctyl phthalate. J Clean Prod 2018; 186: 1021–1030. [Google Scholar]
- 19. Jia P, Zhang M, Hu L, et al. Synthesis and application of environmental castor oil based polyol ester plasticizers for poly(vinyl chloride). ACS Sustain Chem Eng 2015; 3(9): 2187–2193. [Google Scholar]
- 20. Jia P, Hu L, Feng G, et al. Design and synthesis of a castor oil based plasticizer containing THEIC and diethyl phosphate groups for the preparation of flame-retardant PVC materials. RSC Adv 2017; 7(2): 897–903. [Google Scholar]
- 21. Li M, Li S, Xia J, et al. Tung oil based plasticizer and auxiliary stabilizer for poly(vinyl chloride). Mater Des 2017; 122(16): 366–375. [Google Scholar]
- 22. Suzuki AH, Botelho BG, Oliveira LS, et al. Sustainable synthesis of epoxidized waste cooking oil and its application as a plasticizer for polyvinyl chloride films. Eur Polym J 2017; 99: 142–149. [Google Scholar]
- 23. Chen J, Liu Z, Li X, et al. Thermal behavior of epoxidized cardanol diethyl phosphate as novel renewable plasticizer for poly(vinyl chloride). Polym Degrad Stab 2016; 126: 58–64. [Google Scholar]
- 24. Zhang Y, Dube MA, McLean DD, et al. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour Technol 2003; 89(1): 1–16. [DOI] [PubMed] [Google Scholar]
- 25. Naik SN, Goud VV, Rout PK, et al. Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev 2010; 14(2): 578–597. [Google Scholar]
- 26. Nanda S, Isen J, Dalai AK, et al. Gasification of fruit wastes and agro-food residues in supercritical water. Energy Convers Manag 2016; 110: 296–306. [Google Scholar]
- 27. Kulkarni MG, Dalai AK. Waste cooking oil – an economical source for biodiesel: a review. Ind Eng Chem Res 2006; 45(9): 2901–2913. [Google Scholar]
- 28. Nanda S, Rana R, Hunter HN, et al. Hydrothermal catalytic processing of waste cooking oil for hydrogen-rich syngas production. Chem Eng Sci 2019; 195: 935–945. [Google Scholar]
- 29. Ouerghi A, Baghdadi W, Naoui S, et al. Second generation biofuels production from waste cooking oil via pyrolysis process. Renew Energy 2018; 126: 888–896. [Google Scholar]
- 30. Youssef EA, Nakhla G, Charpentier PA. Oleic acid gasification over supported metal catalysts in supercritical water: hydrogen production and product distribution. Int J Hydrogen Energy 2011; 36(8): 4830–4842. [Google Scholar]
- 31. Lhuissier M, Couvert A, Amrane A, et al. Characterization and selection of waste oils for the absorption and biodegradation of VOC of different hydrophobicities. Chem Eng Res Des 2018; 138: 482–489. [Google Scholar]
- 32. Tarnpradab T, Unyaphan S, Takahashi F, et al. Tar removal capacity of waste cooking oil absorption and waste char adsorption for rice husk gasification. Biofuels 2016; 7(4): 401–412. [Google Scholar]
- 33. Worthington MJH, Kucera RL, Albuquerque IS, et al. Laying waste to mercury: inexpensive sorbents made from sulfur and recycled cooking oils. Chemistry 2017; 23(64): 16219–16230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haitzer M, Aiken GR, Ryan JN. Binding of mercury(II) to dissolved organic matter: the role of the mercury-to-DOM concentration ratio. Environ Sci Technol 2002; 36(16): 3564–3570. [DOI] [PubMed] [Google Scholar]
- 35. Pham ALT, Morris A, Zhang T, et al. Precipitation of nanoscale mercuric sulfides in the presence of natural organic matter: structural properties, aggregation, and biotransformation. Geochim Cosmochim Acta 2014; 133: 204–215. [Google Scholar]
- 36. Crockett MP, Evans AM, Worthington MJH, et al. Sulfur-limonene polysulfide: a material synthesized entirely from industrial by-products and its use in removing toxic metals from water and soil. Angew Chem Int Ed Engl 2016; 55(5): 1714–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hasell T, Parker DJ, Jones HA, et al. Porous inverse vulcanised polymers for mercury capture. Chem Commun (Camb) 2016; 52(31): 5383–5386. [DOI] [PubMed] [Google Scholar]
- 38. Thielke MW, Bultema LA, Brauer DD, et al. Rapid Mercury(II) removal by electrospun sulfur copolymers. Polymers (Basel) 2016; 8(7): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]