Abstract
Internationally renowned scientists gathered at the 2nd Human & Translational Immunology Conference in Kos, Greece, to discuss the latest advances in translational immunology, especially vaccinology, infectious diseases and tumor immunotherapy.
The new millennium has begun with great scientific and social challenges for humanity. The excessive administration of antibiotics has exacerbated antimicrobial resistance and has raised the need for new antimicrobial compounds and vaccines. Skepticism over the use of vaccines, the most successful medical intervention ever introduced for human health, has led to a resurgence of preventable infectious diseases. In contrast, battles have been won in the field of immunotherapy of chronic diseases, with several drugs getting approval for the targeting of immune checkpoints in cancer and of effector cytokines and their receptors in autoimmunity and allergic diseases. Thus, immunology stands once again at the frontier of providing solutions by translating scientific discoveries into new powerful therapies.
Vaccines for infectious diseases
The emergence of improved hygiene, the development of antibiotics and the application of an extended vaccination program directed against life-threatening viral and bacterial pathogens has resulted in a near doubling of life expectancy, particularly in Western countries and the developed world. This achievement is now under threat due to the resurgence of neglected pathogens and related infectious diseases, as the abuse of antibiotics, in both humans and livestock, has rendered many pathogenic microbial strains resistant to antibiotics; this has put pressure on researchers to find new solutions. Among these, vaccination still stands out as a very advantageous approach for combating infectious diseases, as was pointed out by Rino Rappuoli (GSK Vaccines)1. Over the centuries, vaccines have proven to be the most effective approach for disease prevention ever introduced. They have multiple targets and are thus not subject to resistance, and they confer long-lived, sometimes lifelong, protection. Several major classes of vaccines now exist; Rappuoli described both those known as ‘conjugate vaccines’, such as the pneumococcal vaccine in which bacterial capsular polysaccharides are linked to a strong protein antigen to boost the immune response, and those generated with a genetic approach through reverse vaccinology. The latter was pioneered by Rappuoli for the generation of a vaccine against meningococcal serogroup B, and it is based on the in silico prediction of immunogenic antigens, their expression by Escherichia coli and their analysis for antimicrobial efficacy and long-term protection. An evolution of this approach is structural vaccinology, in which structural data are additionally used for the design of antigens. This has been used for the development of a vaccine against respiratory syncytial virus now undergoing clinical trials. All vaccines have the advantage of combating antimicrobial resistance while preserving the host microbiome, which can be affected by antibiotics, with severe consequences for human health.
Surprisingly, although vaccines act to combat microbial diseases, they may actually need microbes in order to work efficiently2. Bali Pulendran and colleagues (Stanford University) used systems-vaccinology approaches to study immune responses to vaccination and identified early expression of the pattern-recognition receptor TLR5 as a correlate of antibody production in humans in response to vaccination against seasonal influenza. They subsequently used knockout mice to show that an efficient antibody response to vaccination against influenza requires TLR5-mediated sensing of the gut microbiota, which is also impaired by the use of antibiotics3. They then performed clinical trials to assess, in healthy adults, the effect of antibiotics on immunity to vaccination against seasonal influenza. The results showed that the administration of broad-spectrum antibiotics for 5 days resulted in a 10,000-fold, but transient, drop in gut bacteria and long-lasting restriction of bacterial diversity. This led to reduced influenza virus H1N1–specific immunoglobulin G1 (IgG1) and neutralization responses in subjects who had low baseline titers4. Antibiotic treatment also led to signatures of enhanced inflammation, including signaling via the transcription factor AP-1. In addition, there were significant changes in blood metabolites, including a 1000-fold reduction in secondary bile acids, a value inversely correlated with the enhanced inflammation and inflammasome activation; this suggests that secondary bile is a potent inhibitor of inflammasome activation in vivo in humans4.
Although the potency of a vaccine is evaluated by robust antibody titers, the generation of long-lasting memory also requires follicular helper T cell (TFH cell) responses. The use of an effective vaccine adjuvant should therefore potentiate B cell–TFH cell interactions to boost vaccination efficiency. For example, GLA-SE (glucopyranosyl lipid in a stable emulsion) has been used experimentally to replace alum (aluminum hydroxide), the traditionally used and thus far the most common adjuvant. In work presented by Michelle Linterman (Babraham Institute), GLA-SE was shown to be superior to alum in generating circulating TFH cells, which were shown to be transcriptionally similar to those present in lymph nodes. These circulating TFH cells can thus be used as a biomarker for the measurement of TFH cell responses after vaccination. Improving a vaccine’s efficiency by modulating the adjuvant used can provide great opportunities for pharmaceutical companies. Wivine Burny (Glaxo SmithKline, Belgium) discussed transcriptomics data on responses to vaccination with the hepatitis B virus surface A antigen achieved with the use of alternative adjuvants (instead of alum) and demonstrated that the early expression profile of blood cells was treatment based and was dependent on the adjuvant used. Interestingly, subjects vaccinated with vaccines adsorbed to the adjuvant AS01B, AS01E or AS03 were found to share common functional gene responses characterized by activation of the early innate cells natural killer (NK) cells and the interferon pathway. Moreover, Burny observed that early activation of genes encoding molecules of the innate immune system after the second dose was positively associated with antibody responses 2 weeks after vaccination. Rino Rappuoli also reported on how combining immunostimulants with adjuvants may potentiate vaccine efficacy. For example, AS01, the adjuvant system that combines the TLR4 ligand MPL (3-O-desacyl-4’monophosphoryl lipid A) and the saponin QS-21, was used in the first licensed vaccine against malaria, for a vaccine against herpes zoster and for a promising vaccine against tuberculosis (TB). AS01 was associated with increased early production of the cytokine IFN-γ by NK cells that was linked to its immunogenicity5.
Replacing alum with more-efficient adjuvants was discussed by Ken Ishii (University of Tokyo). Alum is thought to exert its adjuvant effects by inducing cell death and thus the release of damage-associated molecular patterns such as dsDNA. Dendritic cells (DCs) recruited to the area can stimulate TFH cell responses that additionally lead to potent production of IgE. That is the reason alum is commonly used for the induction of allergy in mouse models. Ishii showed that a cyclodextrin formulation was able to exert the same T cell–immunostimulatory effects as alum, but did not induce robust IgE responses6. Ishii also discussed the ‘adjuvantome’, a new transcriptome database that aims to collect all such information evaluating vaccine adjuvants. More than 20 adjuvants have now been evaluated after a single dose of each in mice, rats and humans, which via a machine-learning algorithm allows the prediction of any toxicity.
Alternatively, immunization efficacy may be improved by altering the route of administration. Ioanna Skoutzou (Emory University) showed data on immunization with three monovalent influenza vaccines, A/ChristChurch/16/2010 (H1N1), A/Texas/50/2012 (H3N2) and B/Massachessets/2/2012, via the skin versus an intramuscular route. Each type of immunization induced higher antibody titers in the vaccinated subjects than in the group given placebo. However, only immunization via the skin, delivered by a microneedle patch, led to sustained antibody titers at later time points analyzed. This was probably due to delivery of the vaccines into certain DC subsets in the skin7.
Another major obstacle to the design and generation of efficient T cell–inducing vaccines is their genetic restriction by highly polymorphic major histocompatibility complex (MHC) molecules and the selection of the proper antigens that are recognized in this context. In 2013, modified vaccinia Ankara antigen 85A (MVA85A) was evaluated as a new vaccine against TB designed to induce robust classical CD4+ type 1 helper T cell (TH1 cell)–mediated immunity both in animal models and in uninfected people primed with the anti-TB vaccine BCG (bacillus Calmette-Guérin)8. However, contrary to expectations, the MVA85A vaccine failed to confer any additional protection in clinical trials when given as a booster to BCG in infants. That finding inspired the search for alternative types of immunity to TB. One such approach is to mobilize donor-unrestricted T cells; these T cells are activated by antigens presented by alternative, non-polymorphic (thus donor-unrestricted) antigen-presenting molecules, including CD1, MR1, butyrophilin 3A1 and HLA-E9. HLA-E was highlighted by Tom Ottenhoff (Leiden University Medical Center) as a promising antigen-presentation pathway that could potentially be targeted by vaccines. Ottenhoff described the recognition of HLA-E-presented peptides by CD8+ T cells in humans and mice and provided evidence for a protective, HLA-E-restricted T cell response to Mycobacterium tuberculosis. γδ+ T cells, commonly found within epithelia, are a key cell population that recognize antigen in an MHC-independent manner. This includes non-peptidic lipid antigens presented by CD1 molecules. Adrian Hayday (The Francis Crick Institute) showed that the γδ T cell antigen receptors (TCRs) of such cells have additional binding sites for Btnl (butyrophilin-like) molecules, which are members of the B7 co-stimulator superfamily and are expressed by various epithelial cells. Thus, such γδ TCRs can use one site for Btnl-mediated sensing of their local epithelium while reserving their conventional, antigen-contacting CDR3 sites to engage clonotypic antigens. Because of this dual capacity of γδ+ TCRs for innate recognition and adaptive recognition, Hayday named them ‘adaptate’ T cells10. Hayday also discussed the potential relevance of this axis to inflammatory bowel disease.
Anne O’Garra (The Francis Crick Institute) gave an overview of her earlier work on the immune response in TB and the identification of a blood transcriptional signature dominated by type I interferon–inducible genes that discriminates patients with active TB from latently infected people and healthy control subjects. This signature has now been explored by O’Garra’s group to develop a diagnostic test that can be applicable in the clinic. O’Garra now showed that this type I interferon–inducible signature of active TB in humans could be recapitulated in susceptible mouse models of TB. In keeping with this, elevated and sustained levels of type I interferons resulted in exacerbation of TB in experimental mouse models, in part through induction of the suppressive cytokine IL-10 and the inhibition of IL-12. O’Garra also presented novel findings showing that the protective role of the cytokine GM-CSF includes control of type I interferon responses to inhibit TB pathogenesis. Overall, these findings provided data in support of the proposal of a role for type I interferons in the exacerbation of TB in both mouse models and human disease.
Computational tools may provide new parameters for assessing vaccination efficiency. For example, the effectiveness of vaccination against seasonal influenza is difficult to evaluate, as the virus changes rapidly due to antigenic drift, while influenza may often be confused with other influenza-like illnesses caused by other viruses. Adriana Tomic (Stanford University) introduced SIMON, a machine learning system that is used to address this problem. Through the processing of a large amount of clinical data obtained after vaccination against influenza, this analysis led to the identification of specific T cell subsets that positively correlated with a robust antibody response to such vaccination11. This could be used for determining the presence of an infection with influenza virus or vaccination against it in patients, which would facilitate the clinical development of new vaccines.
Like donor-unrestricted T cells, public antibodies (pathogen-specific clonotypes that are shared by many people) may be used for passive vaccination. Antonio Lanzavecchia (Institute for Research in Biomedicine) has identified such antibodies that target the hemagglutinin stem of influenza virus in people infected with this virus, isolated during 4 consecutive years12. Additionally, Lanzavecchia reported such protective antibodies in the response to Plasmodium falciparum13. This highlights the potential of using broadly neutralizing antibodies as a new therapeutic strategy for tackling infectious diseases.
It has long been observed that vaccination can induce non-specific protection against other pathogens that are not targeted by the vaccine. For example, BCG vaccination against M. tuberculosis not only can enhance protection against infection with Mycobacterium leprae (leprosy) or Mycobacterium ulcerans (Buruli ulcer) but also can decrease overall mortality in children from countries with a high infectious burden. It is now accepted that these non-specific protective effects are mediated at least in part by the ‘training’ of innate immunity. Trained immunity has long been described in lower organisms, such as plants and invertebrates, that lack adaptive immunity14. However, subsequent observations in humans and mouse models led to the realization that ‘trained’ memory exists in vertebrates as well. Mihai Netea (Radboud University Medical Center), one of the pioneers in the field, discussed the importance of epigenetic and metabolic rewiring in innate immunity training. Netea showed that the mevalonate and itaconate pathways are key to this process in monocytes and account for the greater production of pro-inflammatory cytokines and antimicrobial responses observed after secondary microbial challenge15–17. Of interest, BCG vaccination was reported by Simone Moorlag (Radboud UMC) to induce chromatin modification in hematopoietic stem and progenitor cells as late as 3 months after vaccination. Epigenetic imprints on the innate immune response were also reported by Bali Pulendran after trivalent influenza vaccination of humans. More specifically, Pulendran’s group used the mass-cytometry technique EpiTOF to study the epigenetic landscape of multiple subsets of immune cells at the single-cell level. The results indicated that vaccination against seasonal influenza induced striking changes in the epigenetic landscape of many cells, including myeloid DCs and monocytes, that lasted for 6 months. The functional consequences of these changes are being studied.
Understanding the complexity of the human T cell repertoire that is mounted against pathogens has been challenging. Federica Sallusto (Institute for Research in Biomedicine and Swiss Federal Institute of Technology in Zurich) has shown how combinations of the chemokine receptors CCR6, CXCR3 and CCR4 can be used to characterize the heterogeneity of the memory helper T cell response to Candida albicans in immunocompetent and immunodeficient patients with chronic mucocutaneous candidiasis18. Through adoptive transfer of helper T cells in an experimental mouse model of vaginal candidiasis, Sallusto’s group further identified the TH17 subset as the helper T cells responsible for the clearance of C. albicans, while at the same time showed that the TH2 subset suppressed the protective response of TH17 cells.
During untreatable chronic infection or in the tumor microenvironment, continuous stimulation of T cells leads to their exhaustion, which renders them dysfunctional. Understanding the mechanisms behind T cell exhaustion should pave the way for their re-activation, which would favor the eradication of a persistent pathogen or the tumor. Toward that objective, Peter Katsikis (Erasmus University Medical Center) has profiled microRNA signatures of CD8+ T cells in models of exhaustion, such as the infection of P14 mice (which have transgenic expression of a TCR specific for an epitope of the lymphocytic choriomeningitis virus glycoprotein) with lymphocytic choriomeningitis virus, in which he found that unique microRNA profiles characterize the various CD8+ cytotoxic T lymphocyte (CTL)–differentiation states and that a core microRNA expression signature is shared by exhausted CTLs during chronic infection and CTLs from tumors. Katsikis and colleagues also identified the microRNA miR-155 as a key determinant of the persistence of exhausted CTLs and the AP-1 member Fosl2 as a key transcription factor that antagonizes the effects of miR-15519.
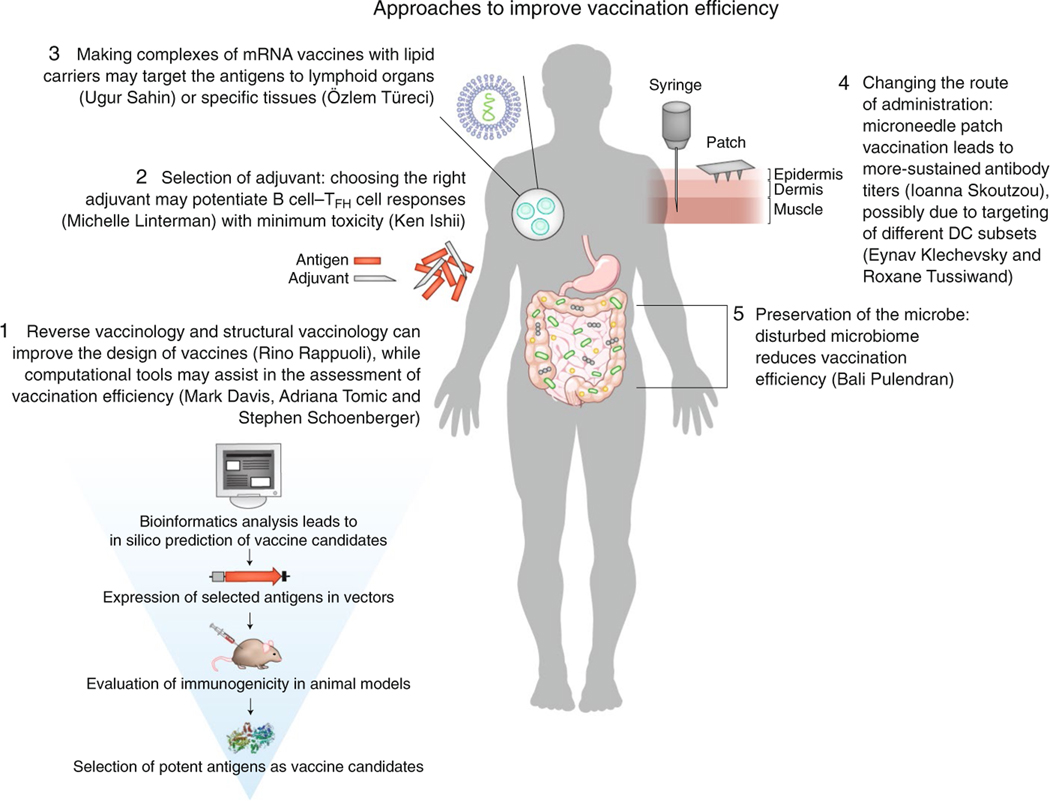
We have summarized various approaches for improving vaccination efficiency (Fig. 1). Alongside vaccination, immunotherapy has been used for over a century to complement the treatment of infectious diseases. For example, anti-tetanus serum directed against tetanus toxin is used to treat cases of insufficient vaccination. Also, the development of a therapeutic monoclonal antibody to respiratory syncytial virus (palivizumab, manufactured by AbbVie) has been approved, and the antibody is now in use for the prevention of infection with respiratory syncytial virus in newborns at risk. This is of particular importance due to the lack of an existing vaccine against respiratory syncytial virus. Immunotherapy, however, appears to be thriving for the treatment of cancer, with several promising therapies already approved, and many others in the pipeline, as discussed below.
Fig. 1 |. Approaches to improve vaccination efficiency presented at the 2nd Human & translational Immunology Conference in Kos, greece.
These approaches include new ways of improving vaccination design, administration, efficacy and evaluation (most of which are summarized here).
Cancer vaccines and immunotherapy
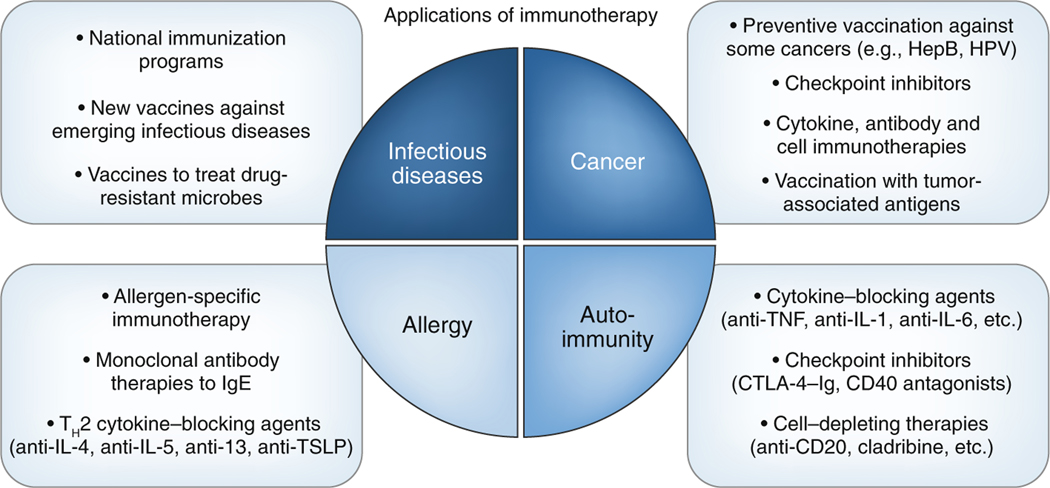
Vaccines and other immunotherapeutic approaches can be used to prevent or treat cancer. As certain types of cancer are caused by viruses, vaccines that prevent such viral infections can prevent cancer as well. For example, vaccination against hepatitis B virus prevents hepatocellular carcinoma, while the vaccine against human papillomavirus has already demonstrated efficacy in reducing cases of genital warts and precancerous lesions, the precursor of cervical cancer20. Given that the vaccine has been approved for only 10 years, the outcome for the prevention of cervical cancer will be evaluated in the coming decades, but it is anticipated to be positive. More commonly, cancer immunotherapy is used and has revolutionized cancer treatment. This includes (in addition to vaccines) antibody, cytokine and immune-cell therapies (Fig. 2). Several monoclonal antibodies that either target the tumor directly, such as the treatment of B cell leukemia with antibody to the B cell–specific surface antigen CD20, or target the immune system to potentiate the anti-tumor response, have already been approved and are being used to treat several types of cancer. In particular, the discovery of immune checkpoints and the generation of blocking antibodies to the immunoinhibitory receptor PD-1 and its ligand PD-L1 and to the immunomodulatory receptor CTLA-4 that unleash an adaptive T cell response to tumors has revolutionized the treatment of diverse malignancies21. However, although many tumors are recognized by adaptive immunity, only a proportion of patients with cancer achieve substantial responses to checkpoint-inhibitor immunotherapies. Interestingly, tumors with a greater mutation burden are more amenable to checkpoint-blockade immunotherapy22. This also comes at the cost of severe side effects due to activation of the immune system, collectively characterized as ‘immune-related adverse events’ that can range from a rash to severe autoimmune gastrointestinal, hepatic, endocrine and/or pulmonary problems23. New approaches thus aim to complement the current suboptimal treatments by stimulating new or exhausted adaptive antitumor responses in the local tumor microenvironment through the use of safer and more-targeted strategies.
Fig. 2 |. Clinical applications of immunotherapy currently in practice.
These applications aim at enhancing or suppressing the immune response in diverse disease areas, including infectious diseases, cancer, allergy and autoimmunity. HepB, hepatitis B; HPV, human papillomavirus.
As T cells are the main target for the therapies discussed above, it is critical to understand the properties of T cells that promote tumor progression and that are expanded in response to checkpoint-inhibitor treatment. Enrico Lugli (Humanitas Research Hospital) observed the presence of stem cell–like CXCR5+CD8+ T cells as the most functional T cell subset in human cancers. These cells are further lost with disease progression24 and act as a reservoir of anti-tumor responses after blockade of PD-1.
Zhi Zhang Yang (Mayo Clinic) used CyTOF analysis of follicular lymphoma to identify a subpopulation of T cells that lack CD27 and CD28, two essential co-stimulatory molecules required for the optimal stimulation and long-term maintenance of T cell immunity. The presence of these cells correlated with a worse disease outcome. Agnese Losurdo (Humanitas Research Hospital) performed single-cell RNA-sequencing analysis of breast-cancer tumor, normal tissue and blood and identified the presence of CD69+HLA-DR+CD127− T cells that expressed markers of T cell exhaustion and correlated with larger numbers of regulatory T cells. On the other hand, Thomas Duhen (Providence Cancer Institute) showed that human solid tumors contain a population of CD8+ T cells that co-express the ATP ectonucleotidase CD39 and the integrin CD103 (αEβ7). Those cells were cytolytic and responded to unique tumor antigens, and their higher frequency in patients with head and neck cancer was associated with better overall survival. Quiescent effectortype cells, which are marked by expression of the naive-cell marker CD45RA and the chemokine receptor CX3CR1, but lack expression of the chemokine receptor CCR7 and CD28, were unexpectedly found in the circulation by Rene van Lier (Sanquin). Manipulating specific properties of primary human T cells is critical for understanding their biology, as well as for potential future immunotherapeutic approaches. Sascha Rutz (Genentech) presented a new gene-editing method in primary T cells that combines the viral delivery of guide RNA with electroporation of the endonuclease Cas9 and thus allows the efficient manipulation of target genes by overcoming the toxicity that is accompanied by plasmid delivery in T cells and the low efficiency of viral delivery alone.
DCs in the skin should be considered the main target in the design and evaluation of vaccines, as they are the most potent antigen-presenting cells capable of taking up antigen and presenting it to T cells, especially in the context of a primary immune response. Eynav Klechevsky (Washington University School of Medicine) discussed the functional role of the terminally differentiated skin DC subset that expresses CD5, which she recently identified. These DCs, which are characterized by a much greater efficiency for T cell stimulation25, could be a therapeutic target for the treatment of not only inflammatory diseases but also cancer, for which the presence of such type of DC would be critical for tumor rejection and response to therapy. Klechevsky also showed that CD5 is present on mouse DCs, which will help in the evaluation of this in preclinical models. In addition to conventional DCs, plasmacytoid DCs (pDCs), which are characterized mainly by their potent production of type I interferons, also have antigen-presenting ability, due to their partial origin from the common DC progenitor. For this dual property, pDCs are currently in a clinical trial evaluating their efficacy against melanoma. Nevertheless, Roxane Tussiwand (National Institutes of Health) demonstrated, by analyzing spleen and bone marrow with single-cell RNA sequencing, that in mice only approximately 10% of pDCs originate from the common DC progenitor and thus possess antigen-presenting function, while most of pDCs originate from IL-7R+ lymphoid progenitor cells. Both subsets express the pDC lineage–defining molecules IRF8 and TCF4, and both are able to produce type I interferons26.
In addition to the type of DC, the type of antigens that are being targeted should be considered. Cancer vaccines generally aim to target two main groups of antigens: neoantigens that occur via somatic mutations, and non-mutant self tumor antigens with high expression by various types of cancers. Because the former type of antigen can be highly variable between people, these antigens are presently poorly exploitable for clinical use, while the latter type of antigen has an issue due to central tolerance. Ugur Sahin (BioNTech Pharmaceuticals) presented new technological approaches aimed at overcoming these obstacles. The first approach involves characterization of the ‘mutanome’ (the total set of mutations in a cancerous cell type) by exome sequencing, bioinformatics analysis to predict MHC class II–binding capacity and the generation of personalized mRNA vaccines that encode tumor antigens. Vaccination of mice with such a preparation against a single CD4+ T cell epitope has led to efficient tumor control of B16F10 mouse melanoma27. This therapeutic approach is currently in clinical trials of patients with stage III–IV melanoma, with the hope of delivering this personalized immunotherapy more widely for many types of cancer. To target shared tumor antigens and overcome central tolerance, Sahin and colleagues developed a strategy for delivering a vaccine composed of mRNA in complex with a liposomal formulation. This formulation was shown to target splenic CD11c+ DCs and to induce T cell responses to non-mutant tumor antigens. It is currently being tested in phase I clinical trial of patients with melanoma.
A lipid carrier was also used in vivo by Özlem Türeci (BioNTech Pharmaceuticals) to encapsulate and deliver mRNA encoding a bispecific antibody to the TCR invariant chain CD3 and the tight-junction protein CLDN6. Expression of CLDN6 is normally restricted to embryonic cells, but CLDN6 becomes aberrantly expressed in various human cancer types, including ovarian and uterine cancers, and can therefore be employed to target cancer cells through antibody- and cell-mediated cytotoxicity. In mice, this formulation proved to have pharmacokinetic properties and efficiency in eliminating tumors superior to those of the corresponding recombinant protein28.
Even after successful identification of tumor-associated antigens, it is not clear which would be more effective in inducing anti-tumor immune responses in vivo. Stephen Schoenberger (La Jolla Institute for Allergy and Immunology) presented a platform that takes advantage of neoantigens to generate personalized cancer vaccines but is not based on in silico prediction; instead, it is based on the patient’s spontaneous response to neoantigens. After a tumor biopsy is obtained, genomic sequencing is performed, followed by bioinformatics analysis that does not take into consideration HLA-binding prediction, as is the case with other similar approaches. In contrast, this platform verifies the prediction of neoantigens in functional T cell assays that monitor the production of IFN-γ and IL-5 with two-color ELISPOT technology as a marker for CD4+ T cell and CD8+ T cell responses, using the patient’s own T cells. This prediction method was used in a mouse model of spontaneous squamous-cell carcinoma (SCC VII) to vaccinate mice with a single neoantigen in combination with blockade of PD-1; this approach led to substantial tumor reduction, a process that was dependent on CD4+ T cells.
Cytokine therapy has also been used to revert the functional anergy of cytotoxic cells within the tumor. IL-15 is a cytokine important for the maintenance, proliferation and activation of lymphocytes. IL-15 was described as being cross-presented by its receptor (IL-15Rα) present on DCs and acting on lymphocytes29. George Pavlakis (National Cancer Institute) proposed that IL-15Rα is not actually a receptor chain but is a second chain of the IL-15 cytokine, forming a complex in the endoplasmic reticulum before being secreted from the cell30. The heterodimeric complex IL-15–IL-15Rα has been shown to exert potent anti-tumor activity in several pre-clinical mouse models, such as a models of breast cancer, melanoma and pancreatic cancer, in which IL-15–IL-15Rα was shown to increase the responses of NK cells and CD8+ T cells while reducing the number of regulatory T cells within the tumor. Of interest, macaques and humans treated with IL-15–IL-15Rα were also shown to have an increased abundance of intratumoral lymphocytes. IL-15–IL-15Rα is currently in clinical trials as a monotherapy or in combination therapy with antibody to PD-1.
Immunotherapy for other diseases
Allergic asthma is caused by the immune system’s response to otherwise innocuous environmental aeroallergens that leads to inappropriate TH2 cell responses that promote the production of IgE, the infiltration of lymphocytes and eosinophils, and the induction of airway inflammation and hyper-responsiveness. To this end, antibodies targeting the TH2 cell cytokines IL-4 and IL-13, cytokine receptors (IL-5Rα and IL-13Rα) or IgE have shown positive results in clinical trials, and many have been approved for the treatment of eosinophilic asthma. However, this list is expected to expand, with applications that target other asthma phenotypes and other diseases. Yong-Jun Liu (currently at Sanofi) gave an overview of his research over the years on the development of novel immunotherapeutic approaches. Liu’s team at DNAX was the first to describe TSLP (thymic stromal lymphopoietin), an epithelium-derived cytokine that acts on DCs to promote allergic responses31. This provided the scientific basis for the clinical development of agents that target TSLP for the treatment of asthma. Indeed, blockade of TSLP by tezepelumab, a monoclonal antibody to TSLP (manufactured by MedImmune and Amgen), led to a reduction of up to 70% in asthma exacerbations in a phase II clinical trial. Notably, this treatment was effective in both patients with high eosinophilia and those with low eosinophilia and was independent of TH2 cell inflammatory markers32, in contrast to previously existing immunotherapies. This drug is currently in a phase III clinical trial. As the IFN-λ family has been reported to skew the helper T cell balance from TH2 to TH133, it would be of interest to explore the potential beneficial effect of these interferons in asthma immunotherapy. This comes in light of findings presented by Evangelos Andreakos (Biomedical Research Foundation of the Academy of Athens) reporting that members of the IFN-λ family are a non-inflammatory and immunoregulatory form of interferons that lack the pro-inflammatory properties of type I interferons and are therefore more tolerable as therapeutic agents for an organism34. Interestingly, an increasing number of studies have shown that the IFN-λ family may also be therapeutically useful for diseases beyond infection, including allergic asthma35.
An emerging type of helper T cells that express IL-9, called ‘TH9 cells’, has been shown to contribute to immunopathology in allergy and autoimmunity and to facilitate immune responses to tumors and extracellular pathogens. Amit Awasthi (Translational Health Science and Technology Institute) discussed how IL-9 is regulated in TH9 cells as well as in other helper T cells.
In contrast to allergy, autoimmunity is driven mostly by TH1 cells and TH17 cells, often with mixed phenotypes. In multiple sclerosis, TH1-like TH17 cells (‘TH17.1 cells’; CCR6hiCXCR3hiCCR4neg or CCR6hiCXCR3hiCCR4dim) are key drivers of disease activity. Steven Koetzier (van Luijn group, Erasmus University Medical Center) revealed an unexpected mechanism by which brain-infiltrating TH17.1 cells become resistant to glucocorticoids, which are widely used to treat acute relapses but do not result in long-term recovery in patients with multiple sclerosis. Interestingly, this glucocorticoid-resistant phenotype was reversed by vitamin D, another steroid known to suppress pro-inflammatory TH17 cell responses.
Interestingly, a novel type of type 1 regulatory T cell–like regulatory CD4hiCD8αLo T cell with a specificity for the commensal bacteria Faecalibacterium prausnitzii, and whose suppressive function is driven through CD39’s activity, was found by Emmanuelle Godefroy (INSERM U1232, University of Nantes) to be specifically reduced in frequency in the blood (and colon) of patients with inflammatory bowel disease. Hence, these results provide new much-needed diagnostic, and possibly prognostic, tools for these disorders. Moreover, manipulating this cell subset could open the way for the development of therapeutic strategies for patients with inflammatory bowel disease.
In the era of new technologies
In the ‘-omics’ era, big data and multi-parametric analyses have expanded the ability to interpret biological processes. Developing a metric for something as complex as the immune system is difficult, as it is difficult to discern the implications of the large variation between people in various immune parameters. By monitoring a group of more than 100 subjects over 9 years and measuring their immune system at breadth from blood, Shai Shen-Orr (Technion-Israel Institute of Technology) and colleagues were able to identify a global pattern of change that the immune system of all older adults follows. From this they devised a score, IMM-AGE, that is measurable through a combination of 18 types of immune cells from blood36. These researchers are currently working on reducing this to a lower number of cells. From a clinical perspective, it seems that knowing one’s ‘immune age’ provides clinically meaningful information beyond what physicians can obtain and beyond what is available currently in the clinic. This underscores the importance of understanding variation in the immune system.
Mark Davis (Stanford University) and colleagues have developed a comprehensive pipeline for the analysis of T cells that allows the study of their specificity at the single-cell level, with applications for autoimmunity, infectious diseases and cancer. One part of the pipeline is the algorithm GLIPH (‘grouping of lymphocyte interactions by paratope hotspots’) that combines the analysis of TCR sequences with structural data37. As a proof of principle, Davis used this algorithm to analyze almost 6,000 TCR sequences from various people with latent infection with M. tuberculosis. GLIPH makes possible the grouping of TCRs on the basis of their specificity and predicts their HLA restriction. This should help in the study of T cell responses at the single-cell level and should also capture the breadth and diversity of human responses. Other parts of the pipeline were illustrated in work presented at the meeting, including the identification of suppressive CD8+ T cells in the experimental autoimmune encephalomyelitis model and intriguing similarities of that model to patients with multiple sclerosis38.
Concluding remarks
During the last century, vaccinology played a major role in the control and even eradication (as in the case of smallpox) of detrimental human infectious diseases. On the other hand, antimicrobial resistance due to over-prescription and social anti-vaccination movements constitutes a modern threat for the resurgence of pathogens. As the understanding of human diseases advances through novel approaches and powerful new technologies, vaccines will have a prevalent role in other disciplines as well, as a means of preventing non-infectious diseases such as cancer and neurodegenerative diseases. Current efforts aim to combine vaccination with other forms of immunotherapy, such as the therapeutic use of antibodies, cytokines and engineered immune cells to provide novel promising and more effective therapeutic strategies for non-infectious diseases. The data presented at the 2nd Human and Translational Immunology meeting showcased the latest advancements in the fields of vaccine technology and immunotherapy, including the host immune response and clinical evaluation, against both infectious diseases and non-infectious diseases such as cancer, autoimmunity and allergy. Inspired by the ancient Asklepieion in Kos, Greece, it was evident from this meeting that obtaining a deeper understanding of the human immune system and translating basic research and ideas into clinical applications is the right way forward to deliver tangible products in order to battle diseases in ways that were never imagined before.
Acknowledgements
We thank the organizers of the meeting (Peter Katsikis, Stephen Schoenberger, Bali Pulendran and Jamie D. K. Wilson) and the speakers for their useful feedback in preparing this Meeting Report. We also thank E. Alspach and X. Meshik (Washington University School of Medicine) for editorial input.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Bloom DE, Black S, Salisbury D & Rappuoli R. Proc. Natl Acad. Sci. USA 115, 12868–12871 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynn DJ & Pulendran BJ Leukoc. Biol 103, 225–231 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh JZ et al. Immunity 41, 478–492 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagan T. et al. Cell 178, 1313–1328.e1313 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penn-Nicholson A. et al. Vaccine 33, 4025–4034 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onishi M. et al. J. Immunol 194, 2673–2682 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klechevsky E. Adv. Exp. Med. Biol 850, 43–54 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Hawkridge T. et al. J. Infect. Dis 198, 544–552 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joosten SA et al. Vaccine 37, 3022–3030 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melandri D. et al. Nat. Immunol 19, 1352–1365 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomic A. et al. J. Immunol (2019). [Google Scholar]
- 12.Lee J. et al. Cell Host Microbe 25, 367–376.e365 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan J. et al. Nat. Med 24, 401–407 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Netea MG, Quintin J. & van der Meer JW Cell Host Microbe 9, 355–361 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Arts RJW et al. Cell Host Microbe 23, 89–100.e105 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Bekkering S. et al. Cell 172, 135–146.e139 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Dominguez-Andres J. et al. Cell Metab. 29, 211–220.e215 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Becattini S. et al. Science 347, 400–406 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Stelekati E. et al. Cell Rep. 23, 2142–2156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drolet M, Benard E, Perez N. & Brisson M. Lancet 394, 497–509 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma P. & Allison JP Science 348, 56–61 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Samstein RM et al. Nat. Genet 51, 202–206 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martins F. et al. Nat. Rev. Clin. Oncol 16, 563–580 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Brummelman J. et al. J. Exp. Med 215, 2520–2535 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korenfeld D. et al. JCI Insight 10.1172/jci.insight.96101 (2017). [DOI] [PMC free article] [PubMed]
- 26.Rodrigues PF et al. Nat. Immunol 19, 711–722 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreiter S. et al. Nature 520, 692–696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stadler CR et al. Nat. Med 23, 815–817 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Dubois S, Mariner J, Waldmann TA & Tagaya Y. Immunity 17, 537–547 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Bergamaschi C. et al. J. Biol. Chem 283, 4189–4199 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Soumelis V. et al. Nat. Immunol 3, 673–680 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Corren J. et al. N. Engl. J. Med 377, 936–946 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Koltsida O. et al. EMBO Mol. Med 3, 348–361 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galani IE et al. Immunity 46, 875–890.e876 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Andreakos E, Zanoni I. & Galani IE Curr. Opin. Immunol 56, 67–75 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alpert A. et al. Nat. Med. 25, 487–495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glanville J. et al. Nature 547, 94–98 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saligrama N. et al. Nature 572, 481–487 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]