Abstract
Excessive temporal discounting undergirds addiction, and the quantitative relationships of changes in discounting have yet to be investigated. The quantitative relationship between pre- and post-treatment discount rates was examined using data from 5 of our studies with diverse interventions across different groups of substance users. Discounting and treatment outcome drug use data from 222 drug-dependent individuals were analyzed. The primary measures are discounting of delayed reinforcers and objective measures of drug use. Results revealed that change in discounting was inversely related to baseline rates of discounting, such that participants with low discount rates showed little change in discounting with treatment, while those participants with high discount rates showed large reductions in discounting. Finally, those treatments that produced the largest gains in drug abstinence had the largest effects on discount rates. Temporal discounting changes with the specific quantitative signature of rate dependence and more efficacious treatments remediate high discounting rates.
Keywords: Self-control, addiction, rate dependent, temporal discounting, neurobehavioral, biomarker
INTRODUCTION
The inability to delay gratification along with the excessive devaluation of delayed reinforcers constitutes important aspects of self-control failure evident in substance dependence disorders (Bickel, Koffarnus, Moody, & Wilson, 2013). This failure of self-control appears persistent, raising questions of its modifiability by treatment and how treatment interacts across the diversity of that bias evident among those with substance-dependence (Odum, 2011). At one extreme, the more trait-like this bias, the more likely that treatment will have positive outcomes among those exhibiting less severe form of this bias. Alternatively, this bias may function as an enduring but changeable state, alterable by treatment with the degree of change perhaps proportional to the extent of this bias. Importantly, how the relationships of change to extent of dysfunction interact with treatments of differing efficacy are unknown.
To examine these issues, we re-analyzed five of our prior studies examining different treatments where patients with different forms of addiction were randomly assigned to study conditions (in 4 of the 5 studies) to ascertain if and to what extent measures of this immediate bias changes. All five studies measured temporal discounting at the beginning and end of the intervention. Also, four of these studies collected biological measures of drug use, permitting a measure of treatment efficacy that will allow an assessment of the impact of treatment efficacy on immediacy bias.
The approach we take here is generally consistent with novel approaches being developed to investigate psychopathology (see Bickel, Jarmolowicz, Mueller, Koffarnus, & Gatchalian, 2012b; Redish, 2013, for a review). Those approaches seek to obtain new empirical insight into the psychopathology, by discerning cross-cross cutting processes shared across psychopathologies, identifying previously unrecognized subtypes among individuals with a particular disorder, and identifying processes that distinguish individuals who do and do not respond to a given intervention.
To accomplish these aims, we employ methods developed in behavioral economics and neuroeconomics that have successfully identified dysfunctional decisions strongly evident in addiction (Bickel, Jarmolowicz, Mueller, & Gatchalian, 2011). As a measure of immediacy bias, these economic approaches use a specific procedure referred to as temporal discounting (also referred to in various literatures as delay discounting, discounting of delayed reinforcers, inter-temporal choice, time preference or the continuum ranging from impulsivity to self-control). Temporal discounting refers to the decreased value of a reinforcer as a function of its temporal distance (Mazur, 1987). Excessive discounting seems strongly related to substance dependence (Bickel et al., 2013): excessive discounting predicts the start of drug use (Audrain-McGovern et al., 2009a), increases with amount of drugs used (Johnson, Bickel, & Baker, 2007; Ohmura, Takahashi, & Kitamura, 2005; Takahashi, Ohmura, Oono, & Radford, 2009; Vuchinich & Simpson, 1998), and distinguishes addicted individuals from those without addiction (see Bickel et al., 2012b; MacKillop et al., 2011, for a review).
Although discounting at the group level robustly distinguishes those who are substance dependent from those who are not (MacKillop et al., 2011), overlap between the groups is observed when the distributions of discounting from individual subjects are examined (Bickel, Yi, Kowal, & Gatchalian, 2008) . Those substance-dependent individuals who discount in the normal range may not substantively change following intervention, perhaps suggesting that their dependence results from a different dysfunction (Redish, Jensen, & Johnson, 2008). In this case, an orderly relationship may exist between the extent of discounting at baseline (e.g., how close or far away from the normal range) and the extent of change following an intervention. Such an orderly relationship may reveal a signature of change between pre-intervention temporal discounting and post-treatment discounting.
Quantitative signatures of change in decision tasks have not previously been reported among the drug dependent. However, a signature of change, referred to as rate dependence, has been observed in the behavioral pharmacology and may be applicable to temporal discounting Rate dependence generally refers to an inverse relationship between baseline rates of responding and rates of responding following an intervention (Witkin & Katz, 1990). Rate-dependent effects have also been observed in both drug and non-drug interventions (Bickel, Higgins, Kirby, & Johnson, 1988; Koffarnus, Jarmolowicz, Mueller, & Bickel, 2013), and have been posited as a basis of the therapeutic effects of stimulant medication seen among individuals with hyperactivity (Bowers, Winett, & Frederiksen, 1987). We, therefore, set out to ascertain if temporal discounting changed in a rate-dependent manner and to examine if those changes were systematically related to treatment efficacy.
METHODS
Participants:
Across the five studies, 222 of 514 initial participants contributed usable temporal discounting data for this analysis. One-hundred sixty-five (165) subjects dropped out of their respective studies consistent with retention outcomes reported in a variety of studies in addiction (see Dutra et al., 2008, for a meta-analysis); 70 were excluded according to criteria of Johnson & Bickel (2008) for the identification of non-systematic discounting performance; and data from 44 individuals were lost in a computer crash, and data from 13 were not available for unknown reasons; also see Table 1. Below we describe each study and the number of participants associated with each (see Table 1). All of the studies were approved by an Institutional Review Board and written informed consent was collected from all participants before any data were collected or treatment was administered.
Table 1.
Description of each study, the number of participants in each study contributing to the results and the types of discounting tasks and the times of their administration within each study. “H” and “R” indicate hypothetical and real rewards, respectively. Numbers are the amount of the reward in USD. Unless otherwise indicated, all rewards are in the future. Those supplying usable discounting data at both pre- and post-treatment are compared to those supplying only baseline discounting.
| Study-group Abbreviation | Sample Size | Brief Description |
|---|---|---|
| WMTraining WMTc |
14 11 |
Working Memory Trial (WMT). Two treatment arms (WMTraining and WMTc). WMTc is considered a separate study group because it was a true sham. Abstinence was not monitored. Originally 27 entered treatment which lasted 25 days on average (Bickel, Yi, et al., 2011). All but two (both WMTc) supplied usable pre- and post-treatment discounting measures. Discounting Tasks: R100, H100, and H1000 administered at baseline and treatment-end. |
| ODT1 | 39 | Opioid Dependence Trial (ODT1). Three treatment arms. Abstinence monitored thrice weekly. Originally 120 entered the 12-week treatment (Chopra et al., 2009; Murphy, MacKillop, Vuchinich, & Tucker, 2011). Discounting data were lost on 44 subjects in a computer crash, leaving 76 who supplied pre-treatment discounting measure(s), of which 57 supplied both pre- and post-treatment measures, of which 39 supplied usable pre- and post-treatment measures. Discounting Tasks: H1000 administered at baseline and treatment-end. Completers’ mean pre-treatment discounting was −4.35. Non-completers’ mean was 1.17 higher; CI for the difference: (−0.41, 2.76), t[73]=1.47, p=.15. |
| ODT2 | 83 | Opioid Dependence Trial 2 (ODT2). Two treatment arms. Abstinence monitored thrice weekly. Originally 152 entered the 12-week treatment (Christensen et al., under review), of which 150 supplied pre-treatment discounting measure(s), of which 108 supplied both pre- and post-treatment measures, of which 83 supplied usable pre- and post-treatment measures. Discounting Tasks: H1000 and H10,000 administered at baseline, mid-treatment, and treatment-end. Completers’ mean pre-treatment discounting was −6.11. Non-completers’ mean was 0.04 lower; CI for the difference: (−0.90, 0.98), t[141]=0.08, p=.93. |
| SRS | 35 | Smoking Relapse Study (SRS). One treatment arm. Abstinence monitored weekly starting in week 4. Originally 80 subjects entered the 8-week treatment (Murphy et al., 2011; Sheffer et al., 2012), of which 72 supplied pre-treatment discounting measure(s), of which 37 supplied both pre- and post-treatment measures, of which 35 supplied usable pre- and post-treatment measures. Discounting Tasks: R100, H1000 (future & past) administered at baseline. H100 (future & past) administered at baseline and weekly thereafter. Completers’ mean pre-treatment discounting was −5.39. Non-completers’ mean was 0.56 higher; CI for the difference: (−0.77, 1.90), t[70]=0.84, p=.40. |
| SDT | 40 | Stimulant Dependent Trial (SDT). Two treatment arms. Abstinence monitored thrice weekly. One hundred and thirty five stimulants dependent participants in 12-week treatment, of which 132 supplied pre-treatment discounting measure(s), of which 63 supplied both pre- and post-treatment measures, of which 40 supplied usable pre- and post-treatment measures. Discounting Tasks: H1000 administered at baseline and treatment-end. Completers’ mean pre-treatment discounting was −2.43. Non-completers’ mean was 0.84 lower; CI for the difference: (−0.41, 2.09), t[122]=1.33, p=.19. |
Working Memory Trial (WMT) had two treatment arms, one receiving a working memory training (WMTraining) and the other a control training (Bickel, Yi, Landes, Hill, & Baxter, 2011). These two arms are treated as separate study groups. Twenty-seven patients at a residential treatment facility for stimulant dependence and abuse were randomized to working memory training (WMTraining, N=14) or control training (WMTc, N=13). Usable discounting data were obtained from all 14 WMTraining and 11 WMTc participants at the start and end of treatment. No objective measure of drug use was reported in this study; however, randomly tested urine samples had to be negative for participants to continue.
Opioid Dependence Trial 1 (ODT1) had three treatment arms, all receiving the opioid replacement medication, buprenorphine: (i) received standard counseling, (ii) received abstinence-contingent modifications of buprenorphine dosing, (iii) received abstinence-contingent vouchers for services or goods from local businesses and/or money details (see Chopra et al., 2009, for full details). One-hundred twenty (120) entered treatment, 88 completed. Some discounting data were lost in a computer crash. Thirty-nine (39) participants had usable discounting data from baseline and post-treatment.
Opioid Dependence Trial 2 (ODT2) had two treatment arms, both were identical to (iii) in ODT1, with one arm adding a web-based behavior therapy intervention (see Christensen et al., under review; Everly et al., 2011, for full details). One-hundred seventy (170) participants entered treatment with 111 completing. Of the completers, 83 had usable discounting data from both pre- and post-treatment.
Smoking Relapse Study (SRS) consisted of only one treatment and was designed to examine predictors of treatment outcome; participants received group cognitive behavior treatment and nicotine replacement therapy was not provided (American Cancer Society, 2010). Eighty (80) participants started treatment. Of 58 completers, 35 provided usable discounting data at pre- and post-treatment.
Stimulant Dependent Trial (SDT) had two treatment arms, both receiving attendance-contingent vouchers for local businesses and/or cash, and one also receiving web-based therapy intervention (see Bickel unpublished observations for full details2). One hundred fifty-two (152) entered treatment, and 73 completed treatment. Forty (40) participants supplied usable (Koffarnus et al., 2013) discounting data at pre- and post-treatment.
Procedures
Discounting Tasks & Rate Estimation.
Two adjusting-amount discounting tasks were used among the 5 studies: one based on the double limit algorithm (Johnson & Bickel, 2002) and the other on a decreasing adjustment algorithm (Du, Green, & Myerson, 2002). These two tasks have been statistically compared and found to produce comparable outcomes (Kowal, Yi, Erisman, & Bickel, 2007). The former was used in ODT1 and ODT2, and the latter in WMT, SRS and SDT. The studies had participants discount a variety of temporal outcomes; these outcomes, along with the times at which discounting tasks were administered, are given in Table 1.
Participants in each study completed discounting tasks at the beginning of treatment (baseline) and were scheduled to complete discounting tasks at the end of treatment. Not all participants completed treatment. Non-completers could not be evaluated in the primary analyses for this work since we had no measure of treatment-end discounting. We compared mean pre-treatment discounting between non-completers and completers: the largest observed difference in baseline discounting came from the ODT1 study where completers were 1.17 [95% CI: (−0.41, 2.76); t73 =1.47, p=.15] lower than non-completers (see Table 1). Any discounting task in which the subject provided no variability (i.e., always gave the same response) across time frames was excluded from analyses; i.e., were not usable (Johnson & Bickel, 2008).
Measures
The discounting tasks used in these studies presented the participant with series of “smaller now vs. larger later” choices between an immediate or delayed reward. These choices are summarized into a single measure of discounting that quantifies the extent to which the participant discounts a delayed reward. We used the method described in Johnson and Bickel (2002) to compute a normalized measure of discounting based on Mazur’s hyperbolic function (Mazur, 1987):
| Equation 1 |
where Y is the expected indifference point for a reward delayed by D days and discounted at a “rate” of k days−1. The natural logarithm of estimated k values is approximately normal in distribution. For this reason, we used ln(k) as our discounting measure for a particular task. We were also interested in how much a person changed his or her discounting between pre- and post-treatment; thus, for same-type discounting tasks, we captured this change with . For studies having more than one type of discounting task, an individual’s average , and were used as his or her data points.
Biochemical Measures of Substance Use. Biochemical measures of drug use were obtained in 4 of the studies. Specifically, during ODT1, ODT2 and SDT, urines were collected under observation at pre- and post-treatment study visits and three times per week throughout the treatment phases. Each urine specimen was tested on-site using a Siemens V-Twin® drug testing diagnostic system with Syva® EMIT reagents for methadone, opioids, propoxyphene, cocaine, and benzodiazepines. In addition, Oxycontin was tested using a single-panel CLIA-waved Oxycontin dipstick (see Chopra et al., 2009). For SRS, exhaled CO was measured weekly during the treatment phase of the study (see Sheffer et al., 2012).
Statistical Methods
Rate Dependence and Regression to the Mean:
When the magnitude and direction of a change measure (here, ) depend on the initial starting point (here, ), the change is said to be “rate dependent.” Regressing the change, , on the initial measure, , evaluates rate dependence. The regression slope of measures the magnitude and direction of that dependence; and, when is centered about its mean, the regression intercept measures the expected change in regardless of .
It is possible, though, for there to be evidence of rate dependence, but on average, no difference in the distributions of initial discounting, , and treatment-end discounting, ; this is known as “regression to the mean.” Regression to the mean (RtM) is when more extreme measures converge toward the mean when resampled (Koffarnus & Katz, 2011). Mathematically, this occurs when the mean and variance of and are equal (i.e., mean is 0), and the expectation of given is , where is the mean of and is the correlation of and . If RtM is true, then the difference should equal 0 on average. Because of the mathematical relationship between correlation () and simple linear regression and the relationship among , , and , RtM is estimated with the aforementioned regression intercept.
To examine rate dependence within each of the 6 study groups, we regressed on centered about its mean; see Figure 1 and Table 2. Residuals of had homogenous variance and no substantial violations of normal errors. In addition to original regression coefficients, we report standardized regression coefficients to allow easier comparison of effect sizes among the studies. Confidence intervals (CIs) for the intercept from these regressions also provide a goodness-of-fit test for RtM; CIs containing 0 imply RtM is a plausible explanation of any rate-dependent effect. We also used a bootstrap method (using 1,000 bootstrapped samples per study group) to obtain a nonparametric verification of RtM’s goodness-of-fit and RtM’s effect size to compare among studies (Efron & Tibshirani, 1993); see Table 2.
Fig. 1.

For each study, individuals’ change in discounting (loge scale) are plotted against baseline discounting (loge scale, centered about the sample mean). Lower values of both baseline discounting and change in discounting indicate more self-control and becoming more self-controlled. Solid lines are linear regressions with standard errors; horizontal lines are at 0, the null hypothesized regression. Panels (A)-(F) are for the indicated studies.
Table 2.
Regression coefficients of change in discounting at treatment-end on baseline discounting, and tests of goodness-of-fit from Regression to the Mean. Intervals (CI) constructed with 95% confidence. CIs not containing 0 indicate statistical significance at the level. Baseline discounting was centered about the sample mean.
| Regression of Discounting Change on Baseline Discounting | Regression to the Mean Goodness of Fit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intercept | Slope | H0: E(dRoM) = 0 | |||||||
| Study | N | Estimate | CI | Estimate | CI | Standardized Estimate | Estimate | CI | Standardized Estimate |
| WMTraining | 14 | −1.53 | (−3.30, +0.24) | −0.52 | (−1.07, +0.03) | −0.51 | −1.53 | (−3.34, +0.13) | −0.52 |
|
| |||||||||
| WMTc | 11 | +0.75 | (−0.24, +1.74) | +0.03 | (−0.35, +0.42) | +0.07 | +0.75 | (−0.08, +1.47) | +0.53 |
|
| |||||||||
| ODT1 | 39 | −1.52 | (−2.55, −0.50) | −0.69 | (−1.04, −0.35) | −0.56 | −1.52 | (−2.72, −0.38) | −0.49 |
|
| |||||||||
| ODT2 | 83 | −1.23 | (−1.69, −0.76) | −0.85 | (−1.03, −0.67) | −0.72 | −1.23 | (−1.94, −0.58) | −0.58 |
|
| |||||||||
| SRS | 35 | −0.78 | (−1.96, +0.39) | −1.06 | (−1.47, −0.64) | −0.67 | −0.78 | (−2.32, +0.61) | −0.23 |
|
| |||||||||
| SDT | 40 | −0.56 | (−1.56, +0.44) | −0.16 | (−0.54, +0.21) | −0.14 | −0.56 | (−1.56, +0.31) | −0.18 |
Biochemical Measures of Abstinence:
For each individual within a study, we computed the percent of documented abstinence out of the total number of originally scheduled visits: 36 visits for ODT1, ODT2, SDT and 4 visits for SRS. We plotted within-study means of these percentages by mean change in discounting.
We used for all tests and present 95% confidence intervals.
RESULTS
Rate-dependent effects
On average, subjects in the active-treatment study groups (excluding WMTc) had improved discount rates at treatment-end relative to pre-treatment levels (Table 2, intercepts), and patients with higher discount rates tended to improve (decrease) more than their counterparts having closer-to-normal discounting (Table 2, slopes), though not statistically improved in all cases. Examining relationships between baseline discounting and changes in discounting at treatment-end revealed a common pattern in three of the six study groups (ODT1, ODT2, WMTraining), but not in the other three (SRS, SDT, WMTc): that substantially more participants than expected (i.e., half) decreased their discount rates (improved) from baseline to treatment-end. Further, improvements in discounting were more robust relative to the other studies (Table 2, standardized estimates).
Starting with the study examining WMT, we found that 9/14 (64%) WMTraining participants showed improvement in discounting rate from baseline to treatment-end. Those who discounted most at baseline showed the greatest change, implying a rate-dependent effect (, ). Though regression to the mean (RtM) cannot be ruled out, it was only marginally plausible as a parsimonious explanation of how extreme discounters change over treatment; thus implying some other process may have been driving the change. (We note that the standardized RtM effect, RtMst, was −0.52, and a 90% CI for the RtM effect did not include 0.) In contrast, only 3/11 (27%) of the WMTc participants decreased their discounting from baseline levels to the end-of-treatment test, and the relationship between baseline discounting and change in discounting was near 0 (, ). See Figure 1 and Table 2.
Among the clinical studies, 26/39 (67%) of ODT1 participants decreased their treatment-end discounting from baseline levels. Plotting treatment-end change as a function of baseline discounting revealed a rate-dependent effect such that those who discounted most at baseline showed larger improvements (decreases) than those who initially discounted less (, ). Likewise, 55/83 (66%) of ODT2 participants decreased their treatment-end discounting from baseline, and ODT2 participants who discounted most at baseline showed the greatest improvement (, ). For both ODT1 and ODT2, the distributions of treatment-end discounting differed from baseline distributions; thus RtM failed to explain the observed rate dependence (, for ODT1 and ODT2, respectively). See Table 2 and Figure 1.
In contrast, only 16/35 (46%) of SRS participants discounted less at treatment-end than at baseline. As with the previous three active-treatment groups, SRS participants showed a rate-dependent effect (, ). Though rate dependence was clearly evident, the changes individuals experienced may have been RtM, with a standardized effect size less than half the magnitude of that for WMTraining, ODT1 and ODT2 (). Similarly, only 21/40 (53%) of SDT participants exhibited a decrease in treatment-end discounting compared to baseline levels. The relationship between change and baseline discounting was not significantly different from 0 (, ), nor was there any compelling evidence to suggest anything beyond RtM (). See Table 2 and Figure 1.
Relationship of changes in discounting to treatment outcomes
The four random-assignment clinical studies documented abstinence from the substance of abuse targeted for cessation. We measured treatment efficacy within each study group by averaging the participants’ percentages of their total number of scheduled visits that were negative (i.e., drug-free) samples. Plotting the change in discounting (adjusted for baseline discounting) by percent of drug-free samples revealed that study groups with the largest decreases in discounting rates also documented the most abstinence (Figure 2). Among the four study groups, ODT1 participants exhibited the largest average decrease in treatment-end discounting (, ), and 79% of the scheduled urines were negative for paneled drugs. Similarly, ODT2 participants showed decreased discounting (, ), and 91% drug-negative samples. In contrast, the decrease in discounting experienced by SRS participants was attenuated and failed to significantly differ from no change (, ). Further, only 44% of scheduled breath samples had CO≤8ppm. SDT participants showed the least decrease in baseline discounting , ), and only 30% of scheduled urines were drug-negative (See Table 2 and Figure 2).
Fig. 2.
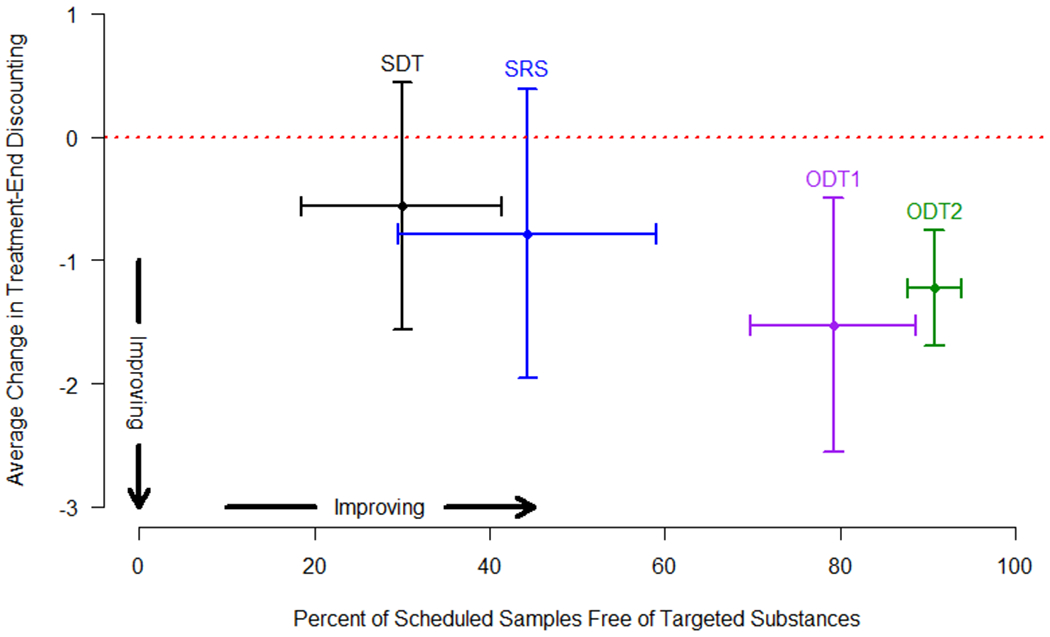
For each study having clinical verification of abstinence, mean change in discounting, adjusted for baseline discounting, is plotted against the percent of scheduled visits that were documented abstinences within the particular study. Vertical error bars are 95% CIs for the mean change in discounting, horizontal error bars are 95% CIs for the true percent of documented abstinences.
DISCUSSION
In this study, we report for the first time that temporal discount rates changed in a rate-dependent fashion following highly efficacious treatments and working memory training. Less efficacious treatments and the control condition for working memory training did not produce rate-dependent effects. These findings extend the understanding of how temporal discounting as a measure of self-control can change as a function of interventions. Koffarnus and colleagues (2013) have recently reviewed 30 studies that have attempted to change temporal discounting. An overwhelming number of these studies were conducted in non-patient populations (e.g., college students) and used transitory interventions (e.g., examining acute doses of alcohol or framing the choices differently) and none examined the relationship between baseline rate of discounting and the rate observed following the intervention. Moreover, only 4 of the research reports reviewed were therapeutic interventions. Two of those four were not re-analyzed here. One reported a decrease in monetary and cigarette discounting from pre to post and relative to a control group following a 5-day contingency management procedure reinforcing reductions in carbon monoxide among cigarette smokers (Yi et al., 2008). The other reported a decrease in temporal discounting among alcohol- and cocaine-dependent individuals after receiving an extensive monetary management training program (Black & Rosen, 2011). Thus, the finding from the present report that temporal discounting showed a systematic relationship between pre and post temporal discounting across subjects and the relationship between that effect and the efficacy of the treatment constitute a novel finding in this research domain. Whether rate dependence relates to the transitory interventions remains to be determined. Below we address 5 aspects of our findings.
First, our results suggest a provocative hypothesis that effects of an intervention on temporal discounting may be a marker of its efficacy. The only treatments that changed discounting were highly efficacious. Working memory training was not examined as a clinical treatment, but it also changed discounting in a rate-dependent fashion. Therefore, if that change is a mark of efficacy, then working memory training should be an efficacious treatment. Studies of working memory have shown it to decrease alcohol consumption (Houben, Wiers, & Jansen, 2011), to help sustain weight loss among obese adolescents (Verbeken, Braet, Goossens, & van der Oord, 2013) and to improve symptoms in ADHD children (Beck, Hanson, Puffenberger, Benninger, & Benninger, 2010). These studies did not measure discounting, but these disorders have been shown to be associated with excessive discounting (Bickel et al., 2012b) . Moreover, the money management intervention referred to above that changed temporal discounting also has been shown to be efficacious as a treatment (Rosen, Rounsaville, Ablondi, Black, & Rosenheck, 2010). Future studies examining other putative treatments may be able to use the effects of those treatments on temporal discounting, in those disorders associated with excessive discounting, as a marker of efficacy.
Second, the rate-dependent effects observed here may also be interpreted from the contemporary neuro-economic perspectives in addiction. Specifically, neuro-economics has suggested that addiction results from an imbalance between two neurobehavioral decision systems (Bechara, 2005; Bickel et al., 2007); that is, the impulsive decision system, embodied in the limbic and paralimbic brain regions and associated with an immediacy bias, is relatively stronger than the executive decision systems, embodied in aspects of the prefrontal cortices and associated with the valuation of delayed outcomes. These two systems also have been shown to contribute to discounting, and the excessive discounting exhibited by those with addiction is consistent with greater control by impulsive decision system. These rate-dependent effects we observed may suggest that the efficacious treatment appears to render these two decision systems into something approximating regulatory balance. Of course, this inference will await neuroimaging studies to confirm this speculation.
Third, the fact that a portion of substance-dependent participants did not exhibit excessive discounting suggests that self-control failure is only one of several possible processes that can contribute in drug dependence (Bickel, Jarmolowicz, Mueller, Koffarnus, & Gatchalian, 2012a; Koffarnus & Katz, 2011; Redish et al., 2008). This view suggests that addiction results from multiple endophenotypes (MacKillop, 2013) and suggests that these trans-disease processes will be exhibited across different addicted populations, with subsets of patients exhibiting different profiles of dysfunctional processes (Redish et al., 2008). Moreover this view suggests that when the specific processes are identified, treatment can be organized to target the specific dysfunction exhibited by a given patient. For example, the effects of treatment on normalizing impulsivity in the participants with greatest discounting suggests that these interventions may be useful with other impulsive patient groups such as those with ADHD (Barkley, 1997), problem gambling (Petry, 2001), and obesity (Weller, Cook III, Avsar, & Cox, 2008). Additionally and importantly, the heterogeneity of the extent of discounting suggests that replication of this rate-dependent effect will depend on the extent and range of temporal discounting exhibited by the target population. Specifically, recruitment of a sample with a restricted baseline discount rate should result in limited or no changes in discounting.
Fourth, there are at least two substantive weaknesses associated with this report. One potential weakness is that the majority of studies reported here used hypothetical monetary rewards in temporal discounting procedures and, perhaps, hypothetical outcomes are not reflective of temporal discounting with real money. Fortunately, a substantial body of literature demonstrates that real and hypothetical outcomes produce comparable behavioral choices (Johnson & Bickel, 2002; Lawyer, Schoepflin, Green, & Jenks, 2011; Madden, Begotka, Raiff, & Kastern, 2003; Madden et al., 2004), brain activations (Bickel, Pitcock, Yi, & Angtuaco, 2009), and that hypothetical rewards are predictive of real monetary behavior (Bickel et al., 2010); therefore, this concern should be minimal here. Second, is a problem that often occurs in random assignment studies in clinical populations, generally, and among those with addiction, in particular. That is, namely, that participant attrition limited the data for analyses. Additionally, some participants exhibited a pattern of behavior that suggests they failed to understand or attend to the task, thus making their data difficult to interpret. These limitations are in some ways offset by the fact that our observations were obtained in a diverse and overall large number of individuals with different addiction disorders.
Fifth, and perhaps most importantly, these data set the occasion for two lines of additional research that would have importance for improving the treatment for substance dependence and abuse and in understanding why those with excessive discounting have worse outcomes is treatments of moderate efficacy. The first line of research would be to explore the possibility of empirically defining those temporal discount rates that are modifiable. If so, then temporal discounting could be used as a means to cost-effectively personalize treatments. Consider that several moderately efficacious treatments have reported that baseline discounting rates are predictive of therapeutic outcomes (e.g., MacKillop & Kahler, 2009; Sheffer et al., 2012; Stanger et al., 2012; Yoon et al., 2007); that is, those that discount the most have the worst treatment outcomes. Perhaps, instead of providing everyone with a costly, highly efficacious treatment, only those with changeable discount rates who might not respond to a moderately efficacious treatment could receive a supplemental intervention (e.g., working memory training) that would improve their discount rates and, if discount rates are causally related to therapeutic outcome, improve their treatment response.
The second research line could test whether treatment attenuates the greater control by the immediate environment that we speculate is evident among those who excessively discount delayed events. If individuals who discount excessively exhibit greater subjective response to drug-related cues and/or to internal stimuli resulting from drug withdrawal compared to those who discount future rewards less, then this may be a reason why those who excessively discount do poorly in moderately efficacious treatments, as we noted above. However, if highly efficacious treatments change discounting and produce the greatest change in discounting among those who discount the most, as we have shown here, then those individuals, following the intervention, should be less under the control of the immediate environment relative to baseline. This “stimulus bound” hypothesis of excessive discounting could be tested using either highly efficacious treatment in clinical trials or working memory training in more experimental settings. Indeed, if this hypothesis is valid, then a gradient of change should be obtained where the most stimulus bound individuals show the greatest proportional change in that dimension. Importantly, given that excessive discounting may function as a trans-disease process (Bickel et al., 2012b), such a demonstration could be relevant to a variety of disorders.
FUNDING
This work was supported by the National Institutes of Health [grant numbers R01DA024080, R01DA024080 (NIAAA ARRA Supplement), R01DA030241, and 1U19CA157345].
Footnotes
DECLARATION OF CONFLICTING INTERESTS
Warren Bickel is a principal in HealthSim, LLC which specializes in the research and development of prevention and therapeutic-educational software. The other authors have no financial interests to disclose.
This trial did not demonstrate efficacy.
REFERENCES
- American Cancer Society. (2010). Cancer Facts and Figures. Atlanta. [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, & Wileyto EP (2009a). Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug and alcohol dependence, 103(3), 99–106. doi: doi: 10.1016/j.drugalcdep.2008.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA (1997). ADHD and the Nature of Self-Control. New York: Guilford Press. [Google Scholar]
- Bechara A (2005). Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience, 8(11), 1458–1463. doi: nn1584 [pii]; 10.1038/nn1584 [DOI] [PubMed] [Google Scholar]
- Beck SJ, Hanson CA, Puffenberger SS, Benninger KL, & Benninger WB (2010). A controlled trial of working memory training for children and adolescents with ADHD. Journal of Clinical Child and Adolescent Psychology, 39(6), 825–836. doi: 10.1080/15374416.2010.517162 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Higgins ST, Kirby KN, & Johnson MW (1988). An inverse relationship between baseline fixed-interval response rate and the effects of a tandem response requirement. Journal of the Experimental Analysis of Behavior, 50(2), 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, & Gatchalian KM (2011). The behavioral economics and neuroeconomics of reinforcer pathologies: Implications for etiology and treatment of addiction. Current psychiatry reports, 13(5), 406–415. doi: 10.1007/s11920-011-0215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, & Gatchalian KM (2012a). Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: Emerging evidence. Pharmacology and Therapeutics, 134(3), 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, & Gatchalian KM (2012b). Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: Emerging evidence. Pharmacology and Therapeutics, 134(3), 287–297. doi: 10.1016/j.pharmthera.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jones BA, Landes RD, Christensen DR, Jackson L, & Mancino M (2010). Hypothetical intertemporal choice and real economic behavior: Delay discounting predicts voucher redemptions during contingency-management procedures. Experimental and clinical psychopharmacology, 18(6), 546–552. doi: 10.1037/a0021739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, & Wilson AG (2013). The behavioral- and neuro-economic process of temporal discounting as a candidate biomarker for addiction. Neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, & Pitcock JA (2007). Behavioral and neuroeconomics of drug addiction: Competing neural systems and temporal discounting processes. Drug and alcohol dependence, 90S, S85–S91. doi: doi: 10.1016/j.drugalcdep.2006.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Pitcock JA, Yi R, & Angtuaco EJC (2009). Congruence of BOLD response across intertemporal choice conditions: Fictive and real money gains and losses. Journal of Neuroscience, 29(27), 8839–8846. doi: doi: 10.1523/jneurosci.5319-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Kowal BP, & Gatchalian KM (2008). Cigarette smokers discount past and future rewards symmetrically and more than controls: Is discounting a measure of impulsivity? Drug and alcohol dependence, 96(3), 256–262. doi: doi: 10.1016/j.drugalcdep.2008.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, & Baxter C (2011). Remember the Future: Working memory training decreases delay discounting among stimulant addicts. Biological psychiatry, 69(3), 260–265. doi: 10.1016/j.biopsych.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AC, & Rosen MI (2011). A money management-based substance use treatment increases valuation of future rewards. Addictive behaviors, 36(1-2), 125–128. doi: 10.1016/j.addbeh.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers TG, Winett RA, & Frederiksen LW (1987). Nicotine fading, behavioral contracting, and extended treatment: Effects on smoking cessation. Addictive behaviors, 12(2), 181–184. [DOI] [PubMed] [Google Scholar]
- Chopra MP, Landes RD, Gatchalian KM, Jackson LC, Buchhalter AR, Stitzer ML, … Bickel WK (2009). Buprenorphine medication versus voucher contingencies in promoting abstinence from opioids and cocaine. Experimental and clinical psychopharmacology, 17(4), 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen D, Landes RD, Jackson L, Marsch LA, Mancino M, Chopra MP, & Bickel WK (under review). Does a web-based behavior therapy intervention enhance treatment outcomes over and above motivational incentives and buprenorphine dosing for opioid dependence in a randomized trial? [Google Scholar]
- Du W, Green L, & Myerson J (2002). Cross-cultural comparisons of discounting delayed and probabilistic rewards. The Psychological Record, 52, 479–492. [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, & Otto MW (2008). A meta-analytic review of psychosocial interventions for substance use disorders. The American journal of psychiatry, 165(2), 179–187. doi: 10.1176/appi.ajp.2007.06111851 [DOI] [PubMed] [Google Scholar]
- Efron B, & Tibshirani RJ (1993). An Introduction to the Bootstrap. London; New York: Chapman & Hall Ltd. [Google Scholar]
- Everly JJ, DeFulio A, Koffarnus MN, Leoutsakos JM, Donlin WD, Aklin WM, … Silverman K (2011). Employment-based reinforcement of adherence to depot naltrexone in unemployed opioid-dependent adults: a randomized controlled trial. Addiction, 106(7), 1309–1318. doi: 10.1111/j.1360-0443.2011.03400.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben K, Wiers RW, & Jansen A (2011). Getting a grip on drinking behavior: training working memory to reduce alcohol abuse. Psychological Science, 22(7), 968–975. doi: 10.1177/0956797611412392 [DOI] [PubMed] [Google Scholar]
- Johnson MW, & Bickel WK (2002). Within-subject comparison of real and hypothetical money rewards in delay discounting. Journal of the Experimental Analysis of Behavior, 77(2), 129–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, & Bickel WK (2008). An algorithm for identifying nonsystematic delay-discounting data. Experimental and clinical psychopharmacology, 16(3), 264–274. doi: doi: 10.1037/1064-1297.16.3.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, & Baker F (2007). Moderate drug use and delay discounting: A comparison of heavy, light, and never smokers. Experimental and clinical psychopharmacology, 15(2), 187–194. doi: 10.1037/1064-1297.15.2.187 [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Jarmolowicz DP, Mueller ET, & Bickel WK (2013). Changing delay discounting in the light of the competing neurobehavioral decision systems theory: a review. Journal of the Experimental Analysis of Behavior, 99(1), 32–57. doi: 10.1002/jeab.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, & Katz JL (2011). Response requirement and increases in accuracy produced by stimulant drugs in a 5-choice serial reaction-time task in rats. Psychopharmacology, 213(4), 723–733. doi: 10.1007/s00213-010-2027-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal BP, Yi R, Erisman AC, & Bickel WK (2007). A comparison of two algorithms in computerized temporal discounting procedures. Behavioural processes, 75(2), 231–236. doi: doi: 10.1016/j.beproc.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Lawyer SR, Schoepflin F, Green R, & Jenks C (2011). Discounting of hypothetical and potentially real outcomes in nicotine-dependent and nondependent samples. Experimental and clinical psychopharmacology, 19(4), 263–274. doi: 10.1037/a0024141 [DOI] [PubMed] [Google Scholar]
- MacKillop J (2013). Integrating behavioral economics and behavioral genetics: delayed reward discounting as an endophenotype for addictive disorders. Journal of the Experimental Analysis of Behavior, 99(1), 14–31. doi: 10.1002/jeab.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, & Munafo MR (2011). Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology, 216(3), 305–321. doi: 10.1007/s00213-011-2229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, & Kahler CW (2009). Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug and alcohol dependence, 104(3), 197–203. doi: doi: 10.1016/j.drugalcdep.2009.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Begotka AM, Raiff BR, & Kastern LL (2003). Delay discounting of real and hypothetical rewards. Experimental and clinical psychopharmacology, 11(2), 139–145. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Raiff BR, Laforio CH, Begotka AM, Mueller AM, Hehli DJ, & Wegener AA (2004). Delay discounting of potentially real and hypothetical rewards: Il. Between-and within-subject comparisons. Experimental and clinical psychopharmacology, 12(4), 251–256. [DOI] [PubMed] [Google Scholar]
- Mazur JE (1987). An adjusting procedure for studying delayed reinforcement. In Commons ML, Mazur JE, Nevin JA & Rachlin H (Eds.), Behavior (Vol. 5, pp. 55–73). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Murphy JG, MacKillop J, Vuchinich RE, & Tucker JA (2011). The behavioral economics of substance abuse. In Walters ST, Rotgers F (Ed.), Treating Substance Abuse: Theory and Technique (Third ed., pp. 48–80). New York: Guilford Publications, Inc. [Google Scholar]
- Odum AL (2011). Delay discounting: Trait variable? Behavioral Processes, 87, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, & Kitamura N (2005). Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology, 182(4), 508–515. doi: doi: 10.1007/s00213-005-0110-8 [DOI] [PubMed] [Google Scholar]
- Petry NM (2001). Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. Journal of Abnormal Psychology, 110(3), 482–487. [DOI] [PubMed] [Google Scholar]
- Redish AD (2013). The Mind within the Brain: How We Make Decisions and How those Decisions Go Wrong. New York: Oxford University Press. [Google Scholar]
- Redish AD, Jensen S, & Johnson A (2008). A unified framework for addiction: Vulnerabilities in the decision process. Behavioral and Brain Sciences, 31(4), 415–437. doi: 10.1017/S0140525X0800472X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MI, Rounsaville BJ, Ablondi K, Black AC, & Rosenheck RA (2010). Advisor-Teller Money Manager (ATM) therapy for substance use disorders. Psychiatric services, 61(7), 707–713. doi: 10.1176/appi.ps.61.7.707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffer C, MacKillop J, McGeary J, Landes RD, Carter L, Yi R, … Bickel WK (2012). Delay discounting, locus of control, and cognitive impulsiveness independently predict tobacco dependence treatment outcomes in a highly dependent, lower socioeconomic group of smokers. American Journal on Addictions, 21(3), 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, Bickel WK, & Budney AJ (2012). Delay discounting predicts adolescent substance abuse treatment outcome. Experimental and clinical psychopharmacology. doi: 10.1037/a0026543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Ohmura Y, Oono H, & Radford M (2009). Alcohol use and discounting of delayed and probabilistic gain and loss. Neuroendocrinology Letters, 30(6), 749–752. [PubMed] [Google Scholar]
- Verbeken S, Braet C, Goossens L, & van der Oord S (2013). Executive function training with game elements for obese children: a novel treatment to enhance self-regulatory abilities for weight-control. Behaviour research and therapy, 51(6), 290–299. doi: 10.1016/j.brat.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, & Simpson CA (1998). Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and clinical psychopharmacology, 6(3), 292–305. [DOI] [PubMed] [Google Scholar]
- Weller RE, Cook EW III, Avsar KB, & Cox JE (2008). Obese women show greater delay discounting than healthy-weight women. Appetite, 51(3), 563–569. [DOI] [PubMed] [Google Scholar]
- Witkin JM, & Katz JL (1990). Analysis of behavioral effects of drugs. Drug Dev Res, 20, 389–409. doi: 10.1002/ddr.430200312 [DOI] [Google Scholar]
- Yi R, Johnson MW, Giordano LA, Landes RD, Badger GJ, & Bickel WK (2008). The effects of reduced cigarette smoking on discounting future rewards: An initial evaluation. Psychol Rec, 58(2), 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, & Badger GJ (2007). Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Experimental and clinical psychopharmacology, 15(2), 176–186. doi: doi: 10.1037/1064-1297.15.2.186 [DOI] [PubMed] [Google Scholar]


